Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases
Abstract
1. Introduction
2. Therapeutic Enzymes
3. Biotechnological Strategies to Enhance Enzyme Therapeutics
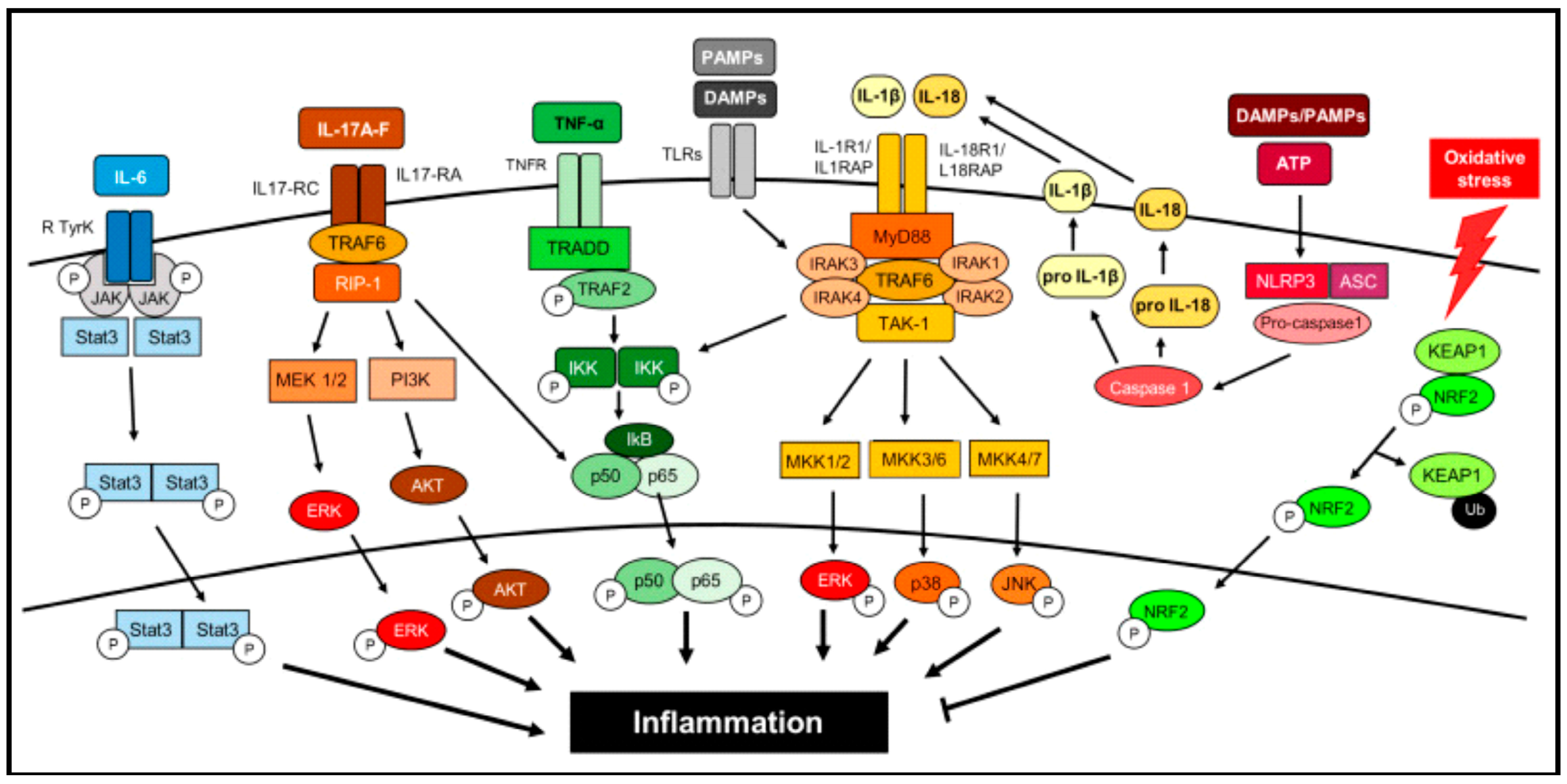
4. Inflammation
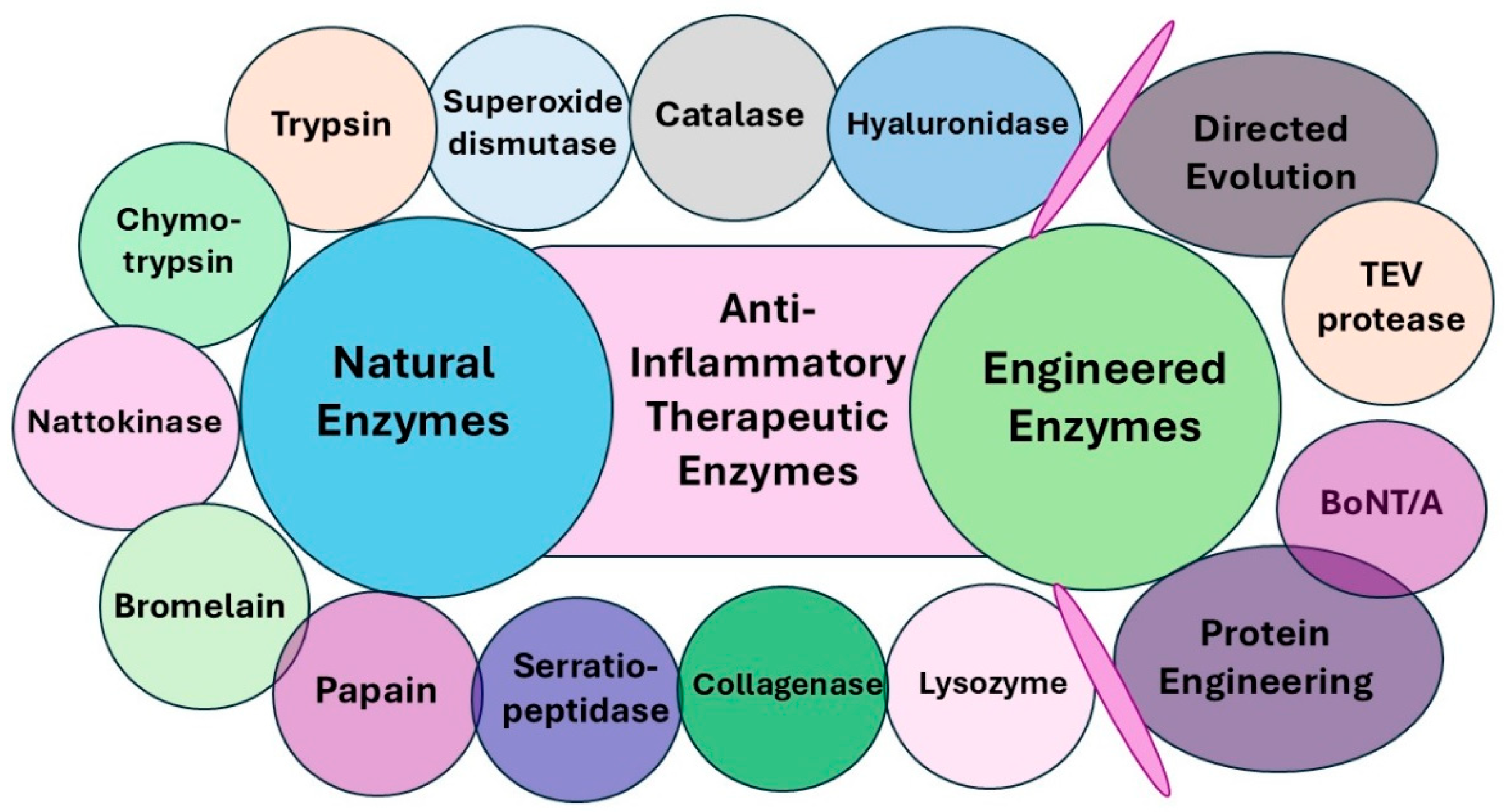
5. Anti-Inflammatory Therapeutic Enzymes
6. Anti-Inflammatory Natural Enzymes
6.1. Oxidoreductases
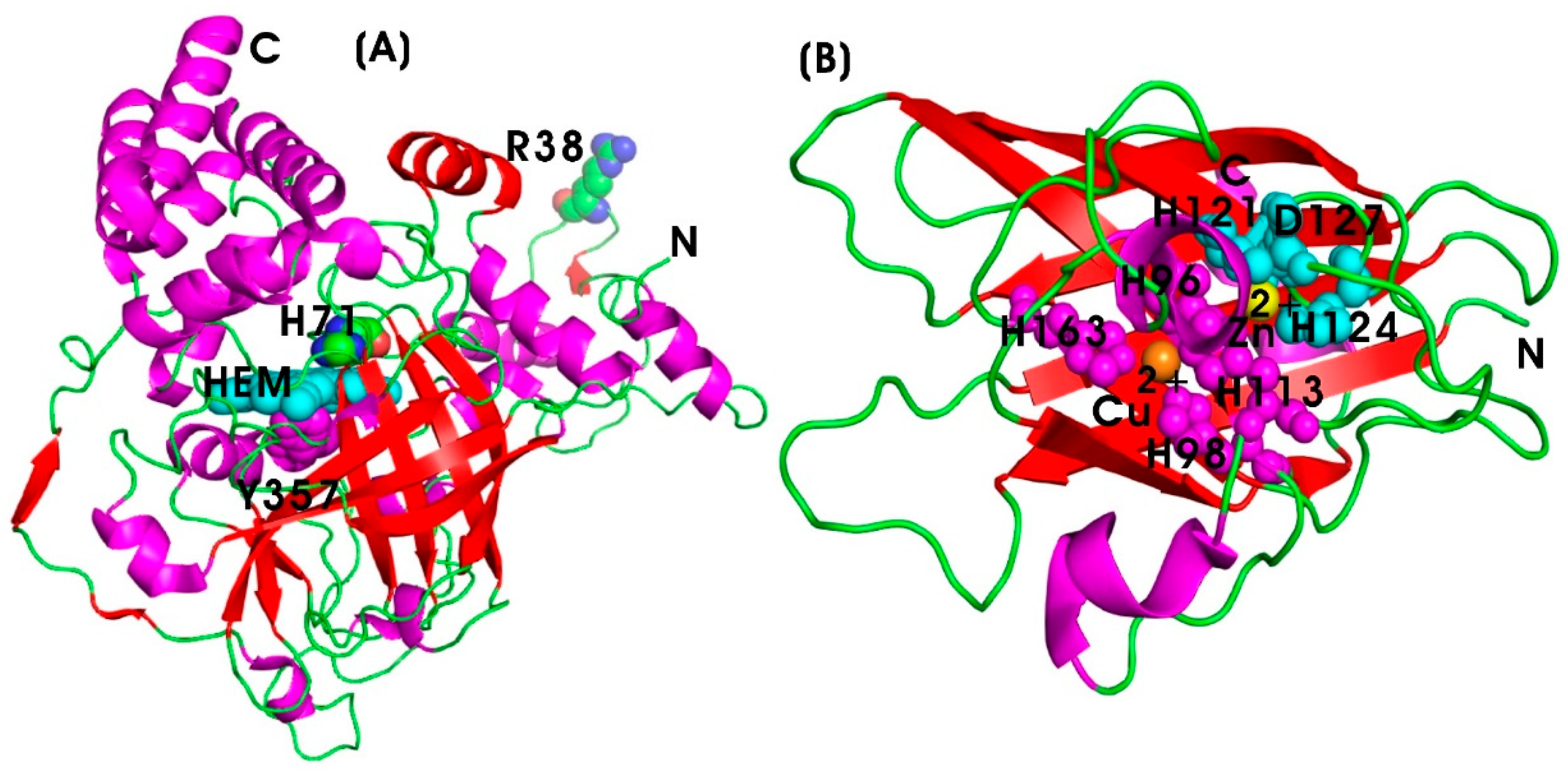
6.1.1. Catalase (CAT)
6.1.2. Superoxide Dismutase (SOD)
6.2. Hydrolases
6.2.1. Serine and Cysteine Proteases
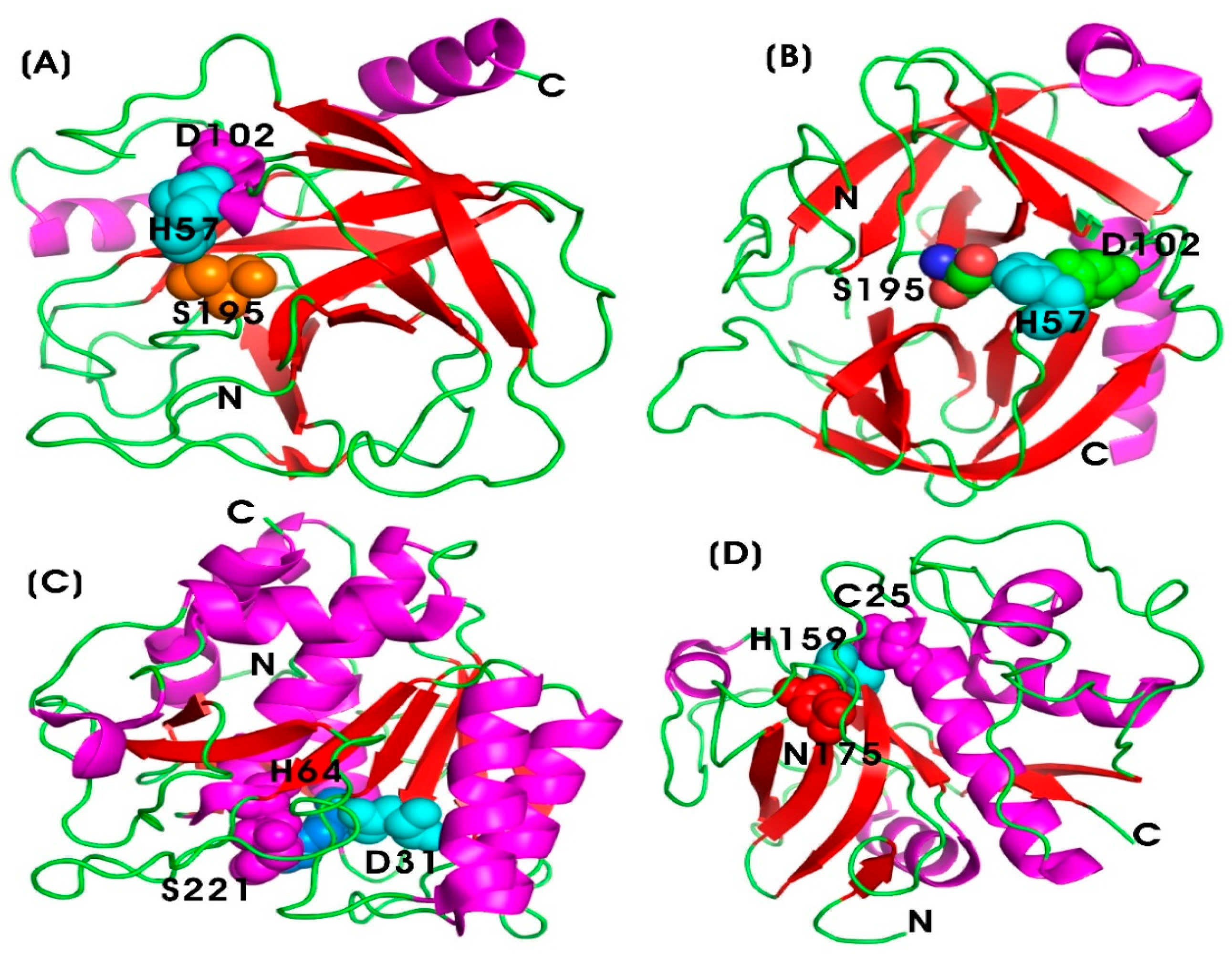
Trypsin (TRYP) and Chymotrypsin (CHYMO)
Nattokinase (NK)
Bromelain (BROM)
Papain (PAP)
6.2.2. Metalloprotease
Serratiopeptidase (SERR)
Collagenase (COLL)
6.2.3. Glycoside Hydrolase
Hyaluronidase (HYAL)
Lysozyme (LYS)
7. Anti-Inflammatory Engineered Enzymes
7.1. Directed Evolution of Tobacco Etch Virus (TEV) Protease
7.2. Protein Engineering of Botulinum Neurotoxin Type A (BoNT/A)
8. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Agarwal, P.K. Enzymes: An integrated view of structure, dynamics and function. Microb. Cell Factories 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.G.; Tipton, K.F. Enzyme nomenclature and classification: The state of the art. FEBS J. 2023, 290, 2214–2231. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.-E.; Liese, A.; Syldatk, C. Introduction to enzyme technology. In Introduction to Enzyme Technology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–16. [Google Scholar]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K. Enzymes as Targets for Drug Development II. Int. J. Mol. Sci. 2023, 24, 3258. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. An overview of three biocatalysts of pharmaceutical importance synthesized by microbial cultures. AIMS Microbiol. 2021, 7, 124. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
- Deckers, M.; Deforce, D.; Fraiture, M.-A.; Roosens, N.H. Genetically modified micro-organisms for industrial food enzyme production: An overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef]
- Mark, J.K.K.; Lim, C.S.Y.; Nordin, F.; Tye, G.J. Expression of mammalian proteins for diagnostics and therapeutics: A review. Mol. Biol. Rep. 2022, 49, 10593–10608. [Google Scholar] [CrossRef]
- Tandon, S.; Sharma, A.; Singh, S.; Sharma, S.; Sarma, S.J. Therapeutic enzymes: Discoveries, production and applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102455. [Google Scholar] [CrossRef]
- Kanhere, M. Therapeutic Enzymes Market Report 2025 (Global Edition). Available online: https://www.cognitivemarketresearch.com/therapeutic-enzymes-market-report?srsltid=AfmBOoqh2N1IMe4m0Fp-l5Dgm2wjmIqIj0fwqkN7BPs539NtI_GIXhtU (accessed on 23 January 2025).
- Ashok, A.; Doriya, K.; Rao, J.V.; Qureshi, A.; Tiwari, A.K.; Kumar, D.S. Microbes producing L-asparaginase free of glutaminase and urease isolated from extreme locations of Antarctic soil and moss. Sci. Rep. 2019, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Biswas, M.; Gandhi, K.A.; Gupta, S.K.; Gera, P.B.; Gota, V.; Sonawane, A. Preclinical evaluation of engineered L-asparaginase variants to improve the treatment of Acute Lymphoblastic Leukemia. Transl. Oncol. 2024, 43, 101909. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Biswas, S.; Ghosh, R.; Modak, R. Recent advances in L-Asparaginase enzyme production and formulation development for acrylamide reduction during food processing. Food Chem. X 2024, 25, 102055. [Google Scholar] [CrossRef]
- Chakraborty, M.; Shivakumar, S. Bioprospecting of the agaricomycete Ganoderma australe GPC191 as novel source for L-asparaginase production. Sci. Rep. 2021, 11, 6192. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.; Subathra Devi, C. In vitro thrombolytic activity of purified streptokinase extracted from Streptococcus equinus VIT_VB2 isolated from bovine milk. J. Thromb. Thrombolysis 2015, 39, 71–78. [Google Scholar] [CrossRef]
- Warwick, D.; Arandes-Renú, J.M.; Pajardi, G.; Witthaut, J.; Hurst, L.C. Collagenase Clostridium histolyticum: Emerging practice patterns and treatment advances. J. Plast. Surg. Hand surgery 2016, 50, 251–261. [Google Scholar] [CrossRef]
- Lauritzson, A.; Atroshi, I. Collagenase injections for Dupuytren’s disease: Prospective cohort study assessing 2-year treatment effect durability. BMJ Open 2017, 7, e012943. [Google Scholar] [CrossRef]
- Lauková, L.; Konečná, B.; Janovičová, Ľ.; Vlková, B.; Celec, P. Deoxyribonucleases and their applications in biomedicine. Biomolecules 2020, 10, 1036. [Google Scholar] [CrossRef]
- Lazarus, R.A.; Wagener, J.S. Recombinant human deoxyribonuclease I. In Pharmaceutical Biotechnology: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 471–488. [Google Scholar]
- Nair, S.R. Serratiopeptidase: An integrated view of multifaceted therapeutic enzyme. Biomolecules 2022, 12, 1468. [Google Scholar] [CrossRef]
- Ding, W. Expression of Glucocerebrosidase in Humanized Pichia Pastoris Expression System and Protein Purification. Doctoral Dissertation, University of Victoria, Victoria, BC, Canada, 2007. [Google Scholar]
- Sinclair, G.; Choy, F.Y. Synonymous codon usage bias and the expression of human glucocerebrosidase in the methylotrophic yeast, Pichia pastoris. Protein Expr. Purif. 2002, 26, 96–105. [Google Scholar] [CrossRef]
- Jawed, A.; Singh, G.; Kohli, S.; Sumera, A.; Haque, S.; Prasad, R.; Paul, D. Therapeutic role of lipases and lipase inhibitors derived from natural resources for remedies against metabolic disorders and lifestyle diseases. S. Afr. J. Bot. 2019, 120, 25–32. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Kondratyev, M.S.; Artyukhov, V.G. Complexation of Bromelain, Ficin, and Papain with the Graft Copolymer of Carboxymethyl Cellulose Sodium Salt and N-Vinylimidazole Enhances Enzyme Proteolytic Activity. Int. J. Mol. Sci. 2023, 24, 11246. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, U.; Trimarco, V.; Manzi, M.V.; Cervi, E.; Mone, P.; Santulli, G. Exploring the Therapeutic Potential of Bromelain: Applications, Benefits, and Mechanisms. Nutrients 2024, 16, 2060. [Google Scholar] [CrossRef]
- Brennan, G.T.; Saif, M.W. Pancreatic enzyme replacement therapy: A concise review. JOP J. Pancreas 2019, 20, 121. [Google Scholar]
- Sikkens, E.C.; Cahen, D.L.; Kuipers, E.J.; Bruno, M.J. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 337–347. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Henry, R.A.; Andrews, A.J. Measuring specificity in multi-substrate/product systems as a tool to investigate selectivity in vivo. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 70–76. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gómez, G. Enzyme therapy: Current challenges and future perspectives. Int. J. Mol. Sci. 2021, 22, 9181. [Google Scholar] [CrossRef]
- Vairo, F.; Netto, C.; Dorneles, A.; Mittelstadt, S.; Wilke, M.; Doneda, D.; Michelin, K.; Ribeiro, C.B.; Quevedo, A.; Vieira, T. Enzyme replacement therapy in a patient with Gaucher disease type III: A paradigmatic case showing severe adverse reactions started a long time after the beginning of treatment. JIMD Rep. 2013, 11, 1–6. [Google Scholar]
- Wang, S.; Guo, X.; Xiu, W.; Liu, Y.; Ren, L.; Xiao, H.; Yang, F.; Gao, Y.; Xu, C.; Wang, L. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 2020, 6, eaaz8204. [Google Scholar] [CrossRef]
- Yang, Y.-h.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L.-q. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-f.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.U.; Kharwar, A.; Ibrahim, M.A.; Singh, S.B.; Bajaj, P. Enzymes as indispensable markers in disease diagnosis. Bioanalysis 2024, 16, 485–497. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Jt. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Gray, D. Overview of protein expression by mammalian cells. Curr. Protoc. Protein Sci. 1997, 10, 5.9.1–5.9.18. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sun, Z.; Qu, G. Enzyme Engineering: Selective Catalysts for Applications in Biotechnology, Organic Chemistry, and Life Science; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Griswold, K.E.; Bailey-Kellogg, C. Design and engineering of deimmunized biotherapeutics. Curr. Opin. Struct. Biol. 2016, 39, 79–88. [Google Scholar] [CrossRef]
- Andrady, C.; Sharma, S.K.; Chester, K.A. Antibody–enzyme fusion proteins for cancer therapy. Immunotherapy 2011, 3, 193–211. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bagshawe, K.D. Antibody directed enzyme prodrug therapy (ADEPT): Trials and tribulations. Adv. Drug Deliv. reviews 2017, 118, 2–7. [Google Scholar] [CrossRef]
- Veronese, F.M.; Caliceti, P.; Schiavon, O.; Sergi, M. Polyethylene glycol–superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002, 54, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Padhy, A.; Gupta, M.; Das, A.; Farook, I.; Dutta, T.; Datta, S.; Datta, R.; Gupta, S.S. Lysosome-Specific Delivery of β-Glucosidase Enzyme Using Protein-Glycopolypeptide Conjugate via Protein Engineering and Bioconjugation. Bioconjug. Chem. 2025, 36, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Luo, L.; Chhay, B.; Zhong, L.; Wei, Y.; Yao, L.; Yu, W.; Li, J.; Nelson, C.L.; Tsourkas, A. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials 2022, 283, 121437. [Google Scholar] [CrossRef]
- Grabowski, G.A.; Golembo, M.; Shaaltiel, Y. Taliglucerase alfa: An enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 2014, 112, 1–8. [Google Scholar] [CrossRef]
- Bondy, S.C.; Campbell, A. Inflammation, Aging, and Oxidative Stress; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Wang, C.-R.; Yang, C.-C. Adalimumab therapy in hepatitis B virus-negative polyarteritis nodosa: A case report. Medicine 2018, 97, e11053. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Wen, Y.; Zhang, H.; Zhao, G.N.; Gao, Q. Signaling pathways in macrophages: Molecular mechanisms and therapeutic targets. MedComm 2023, 4, e349. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fu, S.; Yang, R.; Yang, K.; Lei, W.; Yang, Y.; Zhang, Q.; Zhao, Y.; Yu, J.; Yu, L. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J. Inflamm. 2023, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Martínez, M.S.; Chávez-Castillo, M.; Núñez, V.; Añez, R.; Torres, Y.; Toledo, A.; Chacín, M.; Silva, C.; Pacheco, E. C-reactive protein: An in-depth look into structure, function, and regulation. Int. Sch. Res. Not. 2014, 2014, 653045. [Google Scholar] [CrossRef] [PubMed]
- Bränn, E.; Edvinsson, Å.; Rostedt Punga, A.; Sundström-Poromaa, I.; Skalkidou, A. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early postpartum. Sci. Rep. 2019, 9, 1863. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and novel markers for measuring inflammation and oxidative stress ex vivo in research and clinical practice—Which to use regarding disease outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Devi, P.R.; Kumari, S.K.; Kokilavani, C. Effect of Vitex negundo leaf extract on the free radicals scavengers in complete Freund’s adjuvant induced arthritic rats. Indian J. Clin. Biochem. 2007, 22, 143–147. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide dismutase and neurological disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, C.; Lai, L. Specificity of trypsin and chymotrypsin: Loop-motion-controlled dynamic correlation as a determinant. Biophys. J. 2005, 89, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Mital, K. The role of trypsin: Chymotrypsin in tissue repair. Adv. Ther. 2018, 35, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Abji, F.; Rasti, M.; Gómez-Aristizábal, A.; Muytjens, C.; Saifeddine, M.; Mihara, K.; Motahhari, M.; Gandhi, R.; Viswanathan, S.; Hollenberg, M.D. Proteinase-mediated macrophage signaling in psoriatic arthritis. Front. Immunol. 2021, 11, 629726. [Google Scholar] [CrossRef]
- Grimsey, N.J.; Trejo, J. Integration of endothelial protease-activated receptor-1 inflammatory signaling by ubiquitin. Curr. Opin. Hematol. 2016, 23, 274–279. [Google Scholar] [CrossRef]
- Urano, T.; Ihara, H.; Umemura, K.; Suzuki, Y.; Oike, M.; Akita, S.; Tsukamoto, Y.; Suzuki, I.; Takada, A. The profibrinolytic enzyme subtilisin NAT purified frombacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2001, 276, 24690–24696. [Google Scholar] [CrossRef]
- Yatagai, C.; Maruyama, M.; Kawahara, T.; Sumi, H. Nattokinase-promoted tissue plasminogen activator release from human cells. Pathophysiol. Haemost. Thromb. 2007, 36, 227–232. [Google Scholar] [CrossRef]
- Ren, N.; Chen, H.; Li, Y.; Mcgowan, G.; Lin, Y. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Zhonghua Yi Xue Za Zhi 2017, 97, 2038–2042. [Google Scholar]
- Jang, J.-Y.; Kim, T.-S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.S.; Park, S.K.; Lee, S.-P.; Choi, E.-K. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim. Res. 2013, 29, 221–225. [Google Scholar] [CrossRef]
- Huang, Z.; Ng, T.K.; Chen, W.; Sun, X.; Huang, D.; Zheng, D.; Yi, J.; Xu, Y.; Zhuang, X.; Chen, S. Nattokinase attenuates retinal neovascularization via modulation of Nrf2/HO-1 and glial activation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Errasti, M.E.; Caffini, N.O.; Pelzer, L.E.; Rotelli, A.E. Anti-inflammatory activity of Bromelia hieronymi: Comparison with bromelain. Planta Medica 2013, 79, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Kambala, R.; Bhola, N.; Jadhav, A. Comparative efficacy of bromelain and aceclofenac in limiting post-operative inflammatory sequelae in surgical removal of lower impacted third molar: A randomized controlled, triple blind clinical trial. J. Dent. Anesth. Pain Med. 2022, 22, 29. [Google Scholar] [CrossRef]
- Shoham, Y.; Gasteratos, K.; Singer, A.J.; Krieger, Y.; Silberstein, E.; Goverman, J. Bromelain-based enzymatic burn debridement: A systematic review of clinical studies on patient safety, efficacy and long-term outcomes. Int. Wound J. 2023, 20, 4364–4383. [Google Scholar] [CrossRef]
- Silva-López, R.; Gonçalves, R. Therapeutic proteases from plants: Biopharmaceuticals with multiple applications. J. Appl. Biotechnol. Bioeng. 2019, 6, 101–109. [Google Scholar] [CrossRef]
- Kamphuis, I.; Kalk, K.; Swarte, M.; Drenth, J. Structure of papain refined at 1.65 Å resolution. J. Mol. Biol. 1984, 179, 233–256. [Google Scholar] [CrossRef]
- Mansfield, L.; Ting, S.; Haverly, R.; Yoo, T. The incidence and clinical implications of hypersensitivity to papain in an allergic population, confirmed by blinded oral challenge. Ann. Allergy 1985, 55, 541–543. [Google Scholar]
- Maeda, H.; Morihara, K. Serralysin and related bacterial proteinases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 248, pp. 395–413. [Google Scholar]
- Steiger, S.; Harper, J.L. Mechanisms of spontaneous resolution of acute gouty inflammation. Curr. Rheumatol. Rep. 2014, 16, 392. [Google Scholar] [CrossRef]
- Martín-Vázquez, E.; Cobo-Vuilleumier, N.; López-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus. Int. J. Biol. Sci. 2023, 19, 4157. [Google Scholar] [CrossRef]
- Rajinikanth, B.; Venkatachalam, V.; Manavalan, R. Investigations on the potential of serratiopeptidase–a proteolytic enzyme, on acetic acid induced ulcerative colitis in mice. Int. J. Pharm. Pharm. Sci. 2014, 6, 525–531. [Google Scholar]
- Wang, Z.-Z.; Wang, K.; Xu, L.-F.; Su, C.; Gong, J.-S.; Shi, J.-S.; Ma, X.-D.; Xie, N.; Qian, J.-Y. Unlocking the potential of collagenases: Structures, functions, and emerging therapeutic horizons. BioDesign Res. 2024, 6, 0050. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D.; Young, D.A. Collagenase gene regulation by pro-inflammatory cytokines in cartilage. Front. Biosci. 2007, 12, 536–550. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Peak, T.C.; Mitchell, G.C.; Yafi, F.A.; Hellstrom, W.J. Role of collagenase clostridium histolyticum in Peyronie’s disease. Biol. Targets Ther. 2015, 9, 107–116. [Google Scholar]
- Girish, K.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; d’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1170–1181. [Google Scholar] [CrossRef]
- Meszaros, M.; Kis, A.; Kunos, L.; Tarnoki, A.D.; Tarnoki, D.L.; Lazar, Z.; Bikov, A. The role of hyaluronic acid and hyaluronidase-1 in obstructive sleep apnoea. Sci. Rep. 2020, 10, 19484. [Google Scholar] [CrossRef]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gerdol, M.; Pallavicini, A.; Greco, S.; Schepens, I.; Hamelin, R.; Armand, F.; Dyson, P.J.; Sava, G. Lysozyme-induced transcriptional regulation of TNF-α pathway genes in cells of the monocyte lineage. Int. J. Mol. Sci. 2019, 20, 5502. [Google Scholar] [CrossRef] [PubMed]
- Habte, M.L.; Beyene, E.A. Biological application and disease of oxidoreductase enzymes. In Oxidoreductase; IntechOpen: London, UK, 2020. [Google Scholar]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Xu, P.; Huang, Z.; Xu, Y.; Liu, H.; Liu, Y.; Wang, L. Antioxidants and inflammatory immune-related diseases. Front. Immunol. 2024, 15, 1476887. [Google Scholar] [CrossRef]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Zhigmitova, E.B.; Postnov, A.Y.; Orekhov, A.N. Potential use of antioxidants for the treatment of chronic inflammatory diseases. Front. Pharmacol. 2024, 15, 1378335. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Pleiotropic functions of antioxidant nanoparticles for longevity and medicine. Adv. Colloid Interface science 2013, 201, 30–42. [Google Scholar] [CrossRef]
- Filippi, A.; Liu, F.; Wilson, J.; Lelieveld, S.; Korschelt, K.; Wang, T.; Wang, Y.; Reich, T.; Pöschl, U.; Tremel, W. Antioxidant activity of cerium dioxide nanoparticles and nanorods in scavenging hydroxyl radicals. RSC Adv. 2019, 9, 11077–11081. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Razzaq, H.; Saira, F.; Yaqub, A.; Qureshi, R.; Mumtaz, M.; Saleemi, S. Interaction of gold nanoparticles with free radicals and their role in enhancing the scavenging activity of ascorbic acid. J. Photochem. Photobiol. B Biol. 2016, 161, 266–272. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Singh Dhanjal, D.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Kunjan, F.; Shanmugam, R.; Govindharaj, S. Evaluation of Free Radical Scavenging and Antimicrobial Activity of Coleus amboinicus-Mediated Iron Oxide Nanoparticles. Cureus 2024, 16, e55472. [Google Scholar] [CrossRef] [PubMed]
- Marin-Flores, C.A.; Rodríguez-Nava, O.; García-Hernández, M.; Ruiz-Guerrero, R.; Juárez-López, F.; de Jesús Morales-Ramírez, A. Free-radical scavenging activity properties of ZnO sub-micron particles: Size effect and kinetics. J. Mater. Res. Technol. 2021, 13, 1665–1675. [Google Scholar] [CrossRef]
- Suliasih, B.A.; Budi, S.; Katas, H. Synthesis and application of gold nanoparticles as antioxidants. Pharmacia 2024, 71, 1–19. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Venkidasamy, B.; Samynathan, R.; Govindasamy, R.; Thiruvengadam, M.; Kim, J.H. Association of nanoparticles and Nrf2 with various oxidative stress-mediated diseases. Chem.-Biol. Interact. 2023, 380, 110535. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, H.; Lin, Y.; Hu, Y.; An, H.; Zhang, D.; Feng, S.; Hu, H.; Wang, R.; Li, X. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [Google Scholar] [CrossRef]
- Ren, S.-X.; Zhang, B.; Lin, Y.; Ma, D.-S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 991. [Google Scholar] [CrossRef]
- Ismaila, M.S.; Sanusi, K.O.; Iliyasu, U.; Imam, M.U.; Georges, K.; Sundaram, V.; Jones, K.R. Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model. Antioxidants 2024, 13, 75. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.; Lee, D.Y. Aurozyme: A Revolutionary Nanozyme in Colitis, Switching Peroxidase-Like to Catalase-Like Activity. Small 2023, 19, 2302331. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Bang, B.W.; Park, D.; Kwon, K.S.; Lee, D.H.; Jang, M.-J.; Park, S.K.; Kim, J.-Y. BST-104, a water extract of Lonicera japonica, has a gastroprotective effect via antioxidant and anti-inflammatory activities. J. Med. Food 2019, 22, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-W.; Cao, M.-W.; Qiao, J.-Y.; Li, Q.-R.; Zhang, X.-Z. Integrated cascade catalysis of microalgal bioenzyme and inorganic nanozyme for anti-inflammation therapy. Nanoscale Horiz. 2023, 8, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Jung, J.; Sahu, A.; Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Release 2022, 344, 160–172. [Google Scholar] [CrossRef]
- Riccardi, C.M.; Cole, K.S.; Benson, K.R.; Ward, J.R.; Bassett, K.M.; Zhang, Y.; Zore, O.V.; Stromer, B.; Kasi, R.M.; Kumar, C.V. Toward “stable-on-the-table” enzymes: Improving key properties of catalase by covalent conjugation with poly (acrylic acid). Bioconjug. Chem. 2014, 25, 1501–1510. [Google Scholar] [CrossRef]
- Santos, J.H.; Oliveira, C.A.; Rocha, B.M.; Carretero, G.; Rangel-Yagui, C.O. Pegylated catalase as a potential alternative to treat vitiligo and UV induced skin damage. Bioorg. Med. Chem. 2021, 30, 115933. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Dunaevskaya, S.; Sergeeva, E.Y.; Titova, N.; Fefelova, Y.A.; Deulina, V. Role of superoxide dismutase in acute pancreatitis: From antioxidant protection to gene regulation. Khirurgiia 2024, 112–117. [Google Scholar] [CrossRef]
- Antonyuk, S.V.; Strange, R.W.; Marklund, S.L.; Hasnain, S.S. The structure of human extracellular copper–zinc superoxide dismutase at 1.7 Å resolution: Insights into heparin and collagen binding. J. Mol. Biol. 2009, 388, 310–326. [Google Scholar] [CrossRef]
- Lin, W.-C.; Fessler, M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021, 78, 4095–4124. [Google Scholar] [CrossRef]
- Riaz, B.; Sohn, S. Neutrophils in inflammatory diseases: Unraveling the impact of their derived molecules and heterogeneity. Cells 2023, 12, 2621. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88. [Google Scholar]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Caputi, A.P.; Aston, K.; Riley, D.P.; Salvemini, D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001, 132, 19–29. [Google Scholar] [CrossRef]
- Eun, J.C.; Moore, E.E.; Banerjee, A.; Kelher, M.R.; Khan, S.Y.; Elzi, D.J.; McLaughlin, N.J.; Silliman, C.C. Leukotriene b4 and its metabolites prime the neutrophil oxidase and induce proinflammatory activation of human pulmonary microvascular endothelial cells. Shock 2011, 35, 240–244. [Google Scholar] [CrossRef]
- Pooja, G.; Shweta, S.; Patel, P. Oxidative stress and free radicals in disease pathogenesis: A review. Discov. Med. 2025, 2, 104. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Lin, S.-L.; Lee, S.-T.; Huang, S.-E.; Chang, T.-H.; Geng, Y.-J.; Sulistyowati, E.; Yeh, J.-L. Delivery of Superoxide Dismutase 3 Gene with Baculoviruses Inhibits TNF-α Triggers Vascular Smooth Muscle Cell Proliferation and Inflammation. Curr. Gene therapy 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps–myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Kim, M.-B.; Park, S.-M.; Lim, G.-H.; Oh, Y.-H.; Seo, K.-W.; Youn, H.-Y. Neuroprotective and immunomodulatory effects of superoxide dismutase on SH-SY5Y neuroblastoma cells and RAW264. 7 macrophages. PLoS ONE 2024, 19, e0303136. [Google Scholar]
- Riedl, C.R.; Sternig, P.; Gallé, G.; Langmann, F.; Vcelar, B.; Vorauer, K.; Wagner, A.; Katinger, H.; Pflüger, H. Liposomal recombinant human superoxide dismutase for the treatment of Peyronie’s disease: A randomized placebo-controlled double-blind prospective clinical study. Eur. Urol. 2005, 48, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Kiseleva, R.Y.; Arguiri, E.; Villa, C.H.; Muro, S.; Christofidou-Solomidou, M.; Stan, R.V.; Muzykantov, V.R. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J. Control. Release 2018, 272, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.R.; Williams, L.D.; Sobczyk, M.A.; Michaels, S.J.; Saifer, M.G. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjugate Chem. 2012, 23, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A. Modifying superoxide dismutase for improved biopharmaceutical properties. Biotecnol. Apl. 2006, 23, 17–21. [Google Scholar]
- Zhao, N.; Feng, Z.; Shao, M.; Cao, J.; Wang, F.; Liu, C. Stability profiles and therapeutic effect of cu/zn superoxide dismutase chemically coupled to o-quaternary chitosan derivatives against dextran sodium sulfate-induced colitis. Int. J. Mol. Sci. 2017, 18, 1121. [Google Scholar] [CrossRef]
- Valdivia, A.; Perez, Y.; Dominguez, A.; Caballero, J.; Gomez, L.; Schacht, E.H.; Villalonga, R. Improved anti-inflammatory and pharmacokinetic properties for superoxide dismutase by chemical glycosidation with carboxymethylchitin. Macromol. Biosci. 2005, 5, 118–123. [Google Scholar] [CrossRef]
- Perez, Y.; Valdivia, A.; Gomez, L.; Simpson, B.K.; Villalonga, R. Glycosidation of Cu, Zn-Superoxide Dismutase with End-Group Aminated Dextran. Pharmacological and Pharmacokinetics Properties. Macromol. Biosci. 2005, 5, 1220–1225. [Google Scholar] [CrossRef]
- Hangaishi, M.; Nakajima, H.; Taguchi, J.-i.; Igarashi, R.; Hoshino, J.; Kurokawa, K.; Kimura, S.; Nagai, R.; Ohno, M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia-reperfusion injury in rat hearts in vivo. Biochem. Biophys. Res. Commun. 2001, 285, 1220–1225. [Google Scholar] [CrossRef]
- Tong, J.; Yi, X.; Luxenhofer, R.; Banks, W.A.; Jordan, R.; Zimmerman, M.C.; Kabanov, A.V. Conjugates of superoxide dismutase 1 with amphiphilic poly (2-oxazoline) block copolymers for enhanced brain delivery: Synthesis, characterization and evaluation in vitro and in vivo. Mol. Pharm. 2013, 10, 360–377. [Google Scholar] [CrossRef]
- Yi, X.; Zimmerman, M.C.; Yang, R.; Tong, J.; Vinogradov, S.; Kabanov, A.V. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic. Biol. Med. 2010, 49, 548–558. [Google Scholar] [CrossRef]
- Stick, R.; Williams, S. Enzymatic cleavage of glycosides: Mechanism, inhibition and synthetic applications. In Carbohydrates: The Essential Molecules of Life, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 253–284. [Google Scholar]
- Peach, C.J.; Edgington-Mitchell, L.E.; Bunnett, N.W.; Schmidt, B.L. Protease-activated receptors in health and disease. Physiol. Rev. 2023, 103, 717–785. [Google Scholar] [CrossRef] [PubMed]
- Shah, R. Protease-activated receptors in cardiovascular health and diseases. Am. Heart J. 2009, 157, 253–262. [Google Scholar] [CrossRef]
- Shokhen, M.; Traube, T.; Vijayakumar, S.; Hirsch, M.; Uritsky, N.; Albeck, A. Differentiating serine and cysteine proteases mechanism by new covalent QSAR descriptors. Chembiochem Eur. J. Chem. Biol. 2011, 12, 1023. [Google Scholar] [CrossRef]
- Zamani, A.; Khajavi, M.; Abedian Kenari, A.; Haghbin Nazarpak, M.; Solouk, A.; Esmaeili, M.; Gisbert, E. Physicochemical and biochemical properties of trypsin-like enzyme from two sturgeon species. Animals 2023, 13, 853. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, Y.; Lu, L. Low-dose trypsin accelerates wound healing via protease-activated receptor 2. ACS Pharmacol. Transl. Sci. 2023, 7, 274–284. [Google Scholar] [CrossRef]
- Ceuleers, H.; Van Spaendonk, H.; Hanning, N.; Heirbaut, J.; Lambeir, A.-M.; Joossens, J.; Augustyns, K.; De Man, J.G.; De Meester, I.; De Winter, B.Y. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases. World J. Gastroenterol. 2016, 22, 10275. [Google Scholar] [CrossRef]
- De Bruyn, M.; Ceuleers, H.; Hanning, N.; Berg, M.; De Man, J.G.; Hulpiau, P.; Hermans, C.; Stenman, U.-H.; Koistinen, H.; Lambeir, A.-M. Proteolytic cleavage of bioactive peptides and protease-activated receptors in acute and post-colitis. Int. J. Mol. Sci. 2021, 22, 10711. [Google Scholar] [CrossRef]
- Katona, G.; Berglund, G.I.; Hajdu, J.; Gráf, L.; Szilágyi, L. Crystal structure reveals basis for the inhibitor resistance of human brain trypsin. J. Mol. Biol. 2002, 315, 1209–1218. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Chatake, T.; Chiba-Kamoshida, K.; Naito, S.; Ohsugi, T.; Sumi, H.; Yasuda, I.; Morimoto, Y. Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1670–1673. [Google Scholar] [CrossRef]
- Mousavi Ghahfarrokhi, S.S.; Mahdigholi, F.S.; Amin, M. Collateral beauty in the damages: An overview of cosmetics and therapeutic applications of microbial proteases. Arch. Microbiol. 2023, 205, 375. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, J.; Yang, Y.; Xia, Y.; Liu, L.; Liu, L.; Wang, Q.; Gao, X. Nattokinase enhances the preventive effects of Escherichia coli Nissle 1917 on dextran sulfate sodium-induced colitis in mice. World J. Microbiol. Biotechnol. 2023, 39, 8. [Google Scholar] [CrossRef] [PubMed]
- Granito, M.; Alvarenga, L.; Ribeiro, M.; Carvalhosa, P.; Andrade, T.; Mesquita, C.T.; Stockler-Pinto, M.B.; Mafra, D.; Cardozo, L.F. Nattokinase as an adjuvant therapeutic strategy for non-communicable diseases: A review of fibrinolytic, antithrombotic, anti-inflammatory, and antioxidant effects. Expert Rev. Cardiovasc. Ther. 2024, 22, 565–574. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukuyama, R.; Fujita, M. Effect of nattokinase on the pathological conditions in streptozotocin induced diabetic rats. Heliyon 2024, 10, e28835. [Google Scholar] [CrossRef]
- Cahill, C.M.; Rogers, J.T. Interleukin (IL) 1β induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IκB kinase α pathway targeting activator protein-1. J. Biol. Chem. 2008, 283, 25900–25912. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Klein, G.; Kullich, W.; Schnitker, J.; Schwann, H. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin. Exp. Rheumatol. 2006, 24, 25. [Google Scholar]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Hu, P.-A.; Chen, C.-H.; Guo, B.-C.; Kou, Y.R.; Lee, T.-S. Bromelain confers protection against the non-alcoholic fatty liver disease in male c57bl/6 mice. Nutrients 2020, 12, 1458. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Knäuper, V.; Atkinson, S.; Butler, G.; English, W.; Hutton, M.; Stracke, J.; Clark, I. Matrix metalloproteinases in arthritic disease. Arthritis Res. Ther. 2002, 4, S39. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Straub, R.H. Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 244. [Google Scholar] [CrossRef]
- Fattahi, M.J.; Mirshafiey, A. Prostaglandins and rheumatoid arthritis. Arthritis 2012, 2012, 239310. [Google Scholar] [CrossRef]
- Youssef, M.E.; Abdel-Reheim, M.A.; Morsy, M.A.; El-Daly, M.; Atwa, G.M.; Yahya, G.; Cavalu, S.; Saber, S.; Ahmed Gaafar, A.G. Ameliorative effect of dabigatran on CFA-induced rheumatoid arthritis via modulating kallikrein-kinin system in rats. Int. J. Mol. Sci. 2022, 23, 10297. [Google Scholar] [CrossRef]
- Rooney, T.; Scherzer, R.; Shigenaga, J.K.; Graf, J.; Imboden, J.B.; Grunfeld, C. Levels of plasma fibrinogen are elevated in well-controlled rheumatoid arthritis. Rheumatology 2011, 50, 1458–1465. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Koch, A.E. Endothelial cells and immune cell migration. Arthritis Res. Ther. 2000, 2, 368. [Google Scholar] [CrossRef]
- Kargutkar, S.; Brijesh, S. Anti-rheumatic activity of Ananas comosus fruit peel extract in a complete Freund’s adjuvant rat model. Pharm. Biol. 2016, 54, 2616–2622. [Google Scholar] [CrossRef]
- Madkhali, J.Y.; Hussein, R.H.; Alnahdi, H.S. Therapeutic effect of bromelain and papain on intestinal injury induced by indomethacin in male rats. Int. J. Health Sci. 2023, 17, 23. [Google Scholar]
- Edwin, F.; Jagannadham, M. Single disulfide bond reduced papain exists in a compact intermediate state. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2000, 1479, 69–82. [Google Scholar] [CrossRef]
- Venetikidou, M.; Lykartsi, E.; Adamantidi, T.; Prokopiou, V.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare. Appl. Sci. (2076-3417) 2025, 15, 2367. [Google Scholar] [CrossRef]
- Mamboya, E.A.F.; Amri, E. Papain, a plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H. Beneficial role of Carica papaya extracts and phytochemicals on oxidative stress and related diseases: A mini review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kang, Y.-M.; Lee, M.; An, H.-J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. [Google Scholar] [CrossRef]
- Fei, X.; Yuan, W.; Zhao, Y.; Wang, H.; Bai, S.; Huang, Q. Papain Ameliorates the MPAs Formation-Mediated Activation of Monocytes by Inhibiting Cox-2 Expression via Regulating the MAPKs and PI3K/Akt Signal Pathway. BioMed Res. Int. 2018, 2018, 3632084. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, C.; Zhao, Y.; Huang, Q.; Yuan, W.; Wu, Y.; Fei, X. Papain ameliorates monocyte-platelet aggregate formation-mediated inflammatory responses in monocytes by upregulating miRNA-146a transcription. PLoS ONE 2022, 17, e0278059. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Anil, C.S.; Kashinath, M.A. Production, characterization & optimization of potent protease (serratiopeptidase) from Serratia marcescens e 15. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 95–98. [Google Scholar]
- Bhagat, S.; Agarwal, M.; Roy, V. Serratiopeptidase: A systematic review of the existing evidence. Int. J. Surg. 2013, 11, 209–217. [Google Scholar] [CrossRef]
- Gupte, V.; Luthra, U. Analytical techniques for serratiopeptidase: A review. J. Pharm. Anal. 2017, 7, 203–207. [Google Scholar] [CrossRef]
- Baumann, U. Crystal structure of the 50 kDa metallo protease from Serratia marcescens. J. Mol. Biol. 1994, 242, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jadav, S.P.; Patel, N.H.; Shah, T.G.; Gajera, M.V.; Trivedi, H.R.; Shah, B.K. Comparison of antiinflammatory activity of serratiopeptidase and diclofenac in albino rats. J. Pharmacol. Pharmacother. 2010, 1, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Zapotoczna, M.; Stevens, N.; Humphreys, H.; O’Gara, J.; O’Neill, E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J. Hosp. Infect. 2017, 96, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Seo, H.; Oyama, Y.; Sakata, M.; Tomoda, K.; Takahama, K.; Hitoshi, T.; Okano, Y.; Miyata, T. A new method for evaluating mucolytic expectorant activity and its application. II. Application to two proteolytic enzymes, serratiopeptidase and seaprose. Arzneimittel-forschung 1982, 32, 374–378. [Google Scholar]
- Nair, S.R.; Subathra Devi, C. Bioprospecting of serratiopeptidase-producing bacteria from different sources. Front. Microbiol. 2024, 15, 1382816. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The role of matrix metalloproteinase in inflammation with a focus on infectious diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Lovejoy, B.; Hassell, A.M.; Luther, M.A.; Weigl, D.; Jordan, S.R. Crystal structures of recombinant 19-kDa human fibroblast collagenase complexed to itself. Biochemistry 1994, 33, 8207–8217. [Google Scholar] [CrossRef]
- Murphy, G.; Allan, J.A.; Willenbrock, F.; Cockett, M.I.; O’Connell, J.P.; Docherty, A. The role of the C-terminal domain in collagenase and stromelysin specificity. J. Biol. Chem. 1992, 267, 9612–9618. [Google Scholar] [CrossRef]
- Lovejoy, B.; Cleasby, A.; Hassell, A.M.; Longley, K.; Luther, M.A.; Weigl, D.; McGeehan, G.; McElroy, A.B.; Drewry, D.; Lambert, M.H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science 1994, 263, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Sheets, A.R.; Demidova-Rice, T.N.; Shi, L.; Ronfard, V.; Grover, K.V.; Herman, I.M. Identification and characterization of novel matrix-derived bioactive peptides: A role for collagenase from Santyl® ointment in post-debridement wound healing? PLoS ONE 2016, 11, e0159598. [Google Scholar] [CrossRef] [PubMed]
- Galperin, R.C.; Lange, D.L.; Ramsay, S.J.; Shi, L.; Weedon, K.A.; Hudson, N.M.; Dickerson, J.E.; Cargill, D.I.; Slade, H.B. Anti-inflammatory effects of clostridial collagenase: Results from in vitro and clinical studies. J. Am. Podiatr. Med. Assoc. 2015, 105, 509–519. [Google Scholar] [CrossRef]
- Frederick, R.E.; Bearden, R.; Jovanovic, A.; Jacobson, N.; Sood, R.; Dhall, S. Clostridium collagenase impact on zone of stasis stabilization and transition to healthy tissue in burns. Int. J. Mol. Sci. 2021, 22, 8643. [Google Scholar] [CrossRef]
- Mickelson, D.T.; Noland, S.S.; Watt, A.J.; Kollitz, K.M.; Vedder, N.B.; Huang, J.I. Prospective randomized controlled trial comparing 1-versus 7-day manipulation following collagenase injection for Dupuytren contracture. J. Hand Surg. 2014, 39, 1933–1941. [Google Scholar] [CrossRef]
- Van Nuffel, M.; Reyniers, P.; Warlop, J.; De Smet, L.; Degreef, I. Adjuvant Treatment with Celecoxib after Collagenase Injection for Dupuytren Contracture: A Double-Blind Randomised Controlled Trial. J. Hand Surg. (Asian-Pac. Vol.) 2024, 29, 309–320. [Google Scholar] [CrossRef]
- Fischer, S.; Hirsch, T.; Diehm, Y.; Kiefer, J.; Bueno, E.M.; Kueckelhaus, M.; Kremer, T.; Hirche, C.; Kneser, U.; Pomahac, B. The collagenase of the bacterium clostridium histolyticum for the treatment of capsular fibrosis after silicone implants. Plast. Reconstr. Surg. 2015, 136, 981–989. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Q.; Zhao, Y.; Yang, Y. Clinical observation of low temperature plasma ablation combined with collagenase injection in lumbar disc herniation. Medicine 2024, 103, e39739. [Google Scholar] [CrossRef]
- Bassetto, F.; Maschio, N.; Abatangelo, G.; Zavan, B.; Scarpa, C.; Vindigni, V. Collagenase from Vibrio alginolyticus cultures: Experimental study and clinical perspectives. Surg. Innov. 2016, 23, 557–562. [Google Scholar] [CrossRef]
- Hoppe, I.J.; Brandstetter, H.; Schönauer, E. Biochemical characterisation of a collagenase from Bacillus cereus strain Q1. Sci. Rep. 2021, 11, 4187. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.-P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Wang, X.; Keswani, S.G. Hyaluronidases in human diseases. Int. J. Mol. Sci. 2021, 22, 3204. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef]
- Zhang, L.; Bharadwaj, A.G.; Casper, A.; Barkley, J.; Barycki, J.J.; Simpson, M.A. Hyaluronidase activity of human Hyal1 requires active site acidic and tyrosine residues. J. Biol. Chem. 2009, 284, 9433–9442. [Google Scholar] [CrossRef]
- Kaya, G.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.-H. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006, 3, e493. [Google Scholar] [CrossRef]
- Washington, P.M.; Lee, C.; Dwyer, M.K.R.; Konofagou, E.E.; Kernie, S.G.; Morrison III, B. Hyaluronidase reduced edema after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2020, 40, 2026–2037. [Google Scholar] [CrossRef]
- Fronza, M.; Caetano, G.F.; Leite, M.N.; Bitencourt, C.S.; Paula-Silva, F.W.; Andrade, T.A.; Frade, M.A.; Merfort, I.; Faccioli, L.H. Hyaluronidase modulates inflammatory response and accelerates the cutaneous wound healing. PLoS ONE 2014, 9, e112297. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Gorges, K.; Reiners, O.; Bölke, E.; Fischer, J.W.; Homey, B.; Gerber, P.A. Dose-and time-dependent effects of hyaluronidase on structural cells and the extracellular matrix of the skin. Eur. J. Med. Res. 2020, 25, 60. [Google Scholar] [CrossRef]
- Kuroki, R.; Yutani, K. Structural and thermodynamic responses of mutations at a Ca2+ binding site engineered into human lysozyme. J. Biol. Chem. 1998, 273, 34310–34315. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, X.; Hang, T.; Chen, J.; Wang, Z.; Mosselhy, D.A.; Xu, J.; Wang, S.; Zheng, Y. Fabrication of Cu2+-loaded phase-transited lysozyme nanofilm on bacterial cellulose: Antibacterial, anti-inflammatory, and pro-angiogenesis for bacteria-infected wound healing. Carbohydr. Polym. 2023, 309, 120681. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, A.; Nishi, K.; Matsumoto, S.; Sugahara, T. Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages. Cytotechnology 2018, 70, 929–938. [Google Scholar] [CrossRef]
- Tagashira, A.; Nishi, K.; Sugahara, T. Lysozyme from hen egg white ameliorates lipopolysaccharide-induced systemic inflammation in mice. Cytotechnology 2019, 71, 497–506. [Google Scholar] [CrossRef]
- Carrillo, W.; Spindola, H.; Ramos, M.; Recio, I.; Carvalho, J.E. Anti-inflammatory and anti-nociceptive activities of native and modified hen egg white lysozyme. J. Med. Food 2016, 19, 978–982. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Hamasaki, K.; Miyata, T. Novel peptide motifs from lysozyme suppress pro-inflammatory cytokines in macrophages by antagonizing toll-like receptor and LPS-scavenging action. Eur. J. Pharm. Sci. 2017, 107, 240–248. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Tatsumi, K.; Inoue, H.; Sakata, Y.; Shibata, K.; Miyagishi, H.; Marukawa, Y.; Ichinose, M. Prevention of COPD exacerbation by lysozyme: A double-blind, randomized, placebo-controlled study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 831–838. [Google Scholar] [CrossRef]
- Dong, B.; Sun, C. Production of an invertebrate lysozyme of Scylla paramamosain in E. coli and evaluation of its antibacterial, antioxidant and anti-inflammatory effects. Protein Expr. Purif. 2021, 177, 105745. [Google Scholar] [CrossRef]
- Bastamy, M.; Raheel, I.; Elbestawy, A.; Diab, M.; Hammad, E.; Elebeedy, L.; El-Barbary, A.M.; Albadrani, G.M.; Abdel-Daim, M.M.; Abdel-Latif, M.A. Postbiotic, anti-inflammatory, and immunomodulatory effects of aqueous microbial lysozyme in broiler chickens. Anim. Biotechnol. 2024, 35, 2309955. [Google Scholar] [CrossRef]
- Yang, Q.-W.; Wang, J.-Z.; Li, J.-C.; Zhou, Y.; Qi-Zhong; Lu, F.-L.; Xiang, J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 243–254. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Na, D.H.; Bae, J.-S. Anti-inflammatory effects of lysozyme against HMGB1 in human endothelial cells and in mice. Inflammation 2015, 38, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, M.; Sanford, B.L.; Kurtzberg, J.; DeOliveira, D.; Frankel, S.R.; Powell, B.L.; Kolitz, J.E.; Bloomfield, C.D.; Larson, R.A. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood 2007, 109, 4164–4167. [Google Scholar] [CrossRef] [PubMed]
- Keating, M.J.; Holmes, R.; Lerner, S.; Ho, D.H. L-asparaginase and PEG asparaginase—Past, present, and future. Leuk. Lymphoma 1993, 10, 153–157. [Google Scholar] [CrossRef]
- Nunn, C.M.; Jeeves, M.; Cliff, M.J.; Urquhart, G.T.; George, R.R.; Chao, L.H.; Tscuchia, Y.; Djordjevic, S. Crystal structure of tobacco etch virus protease shows the protein C terminus bound within the active site. J. Mol. Biol. 2005, 350, 145–155. [Google Scholar] [CrossRef]
- Packer, M.S.; Rees, H.A.; Liu, D.R. Phage-assisted continuous evolution of proteases with altered substrate specificity. Nat. Commun. 2017, 8, 956. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, B.; Jung, Y.; Park, J.-B.; Oh, J.-M.; Hwang, J.; Cho, J.Y.; Kweon, D.-H. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor-derived peptides for regulation of mast cell degranulation. Front. Immunol. 2018, 9, 725. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.; Assi, M.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.; Hezmee, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine induces vascular hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Lorentz, A.; Baumann, A.; Vitte, J.; Blank, U. The SNARE machinery in mast cell secretion. Front. Immunol. 2012, 3, 143. [Google Scholar] [CrossRef]
- Hennigan, J.N.; Lynch, M.D. The past, present, and future of enzyme-based therapies. Drug Discov. Today 2022, 27, 117–133. [Google Scholar] [CrossRef]
- Zuniga, J.E.; Hammill, J.T.; Drory, O.; Nuss, J.E.; Burnett, J.C.; Gussio, R.; Wipf, P.; Bavari, S.; Brunger, A.T. Iterative structure-based peptide-like inhibitor design against the botulinum neurotoxin serotype A. PLoS ONE 2010, 5, e11378. [Google Scholar] [CrossRef] [PubMed]
- Sikorra, S.; Litschko, C.; Müller, C.; Thiel, N.; Galli, T.; Eichner, T.; Binz, T. Identification and characterization of botulinum neurotoxin A substrate binding pockets and their re-engineering for human SNAP-23. J. Mol. Biol. 2016, 428, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Blum, T.R.; Liu, H.; Packer, M.S.; Xiong, X.; Lee, P.-G.; Zhang, S.; Richter, M.; Minasov, G.; Satchell, K.J.; Dong, M. Phage-assisted evolution of botulinum neurotoxin proteases with reprogrammed specificity. Science 2021, 371, 803–810. [Google Scholar] [CrossRef]
- Zhao, H.; Brooks, S.A.; Eszterhas, S.; Heim, S.; Li, L.; Xiong, Y.Q.; Fang, Y.; Kirsch, J.R.; Verma, D.; Bailey-Kellogg, C. Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing. Sci. Adv. 2020, 6, eabb9011. [Google Scholar] [CrossRef]
- Palleon Pharmaceuticals. Available online: https://palleonpharma.com/pipeline/ (accessed on 23 January 2025).


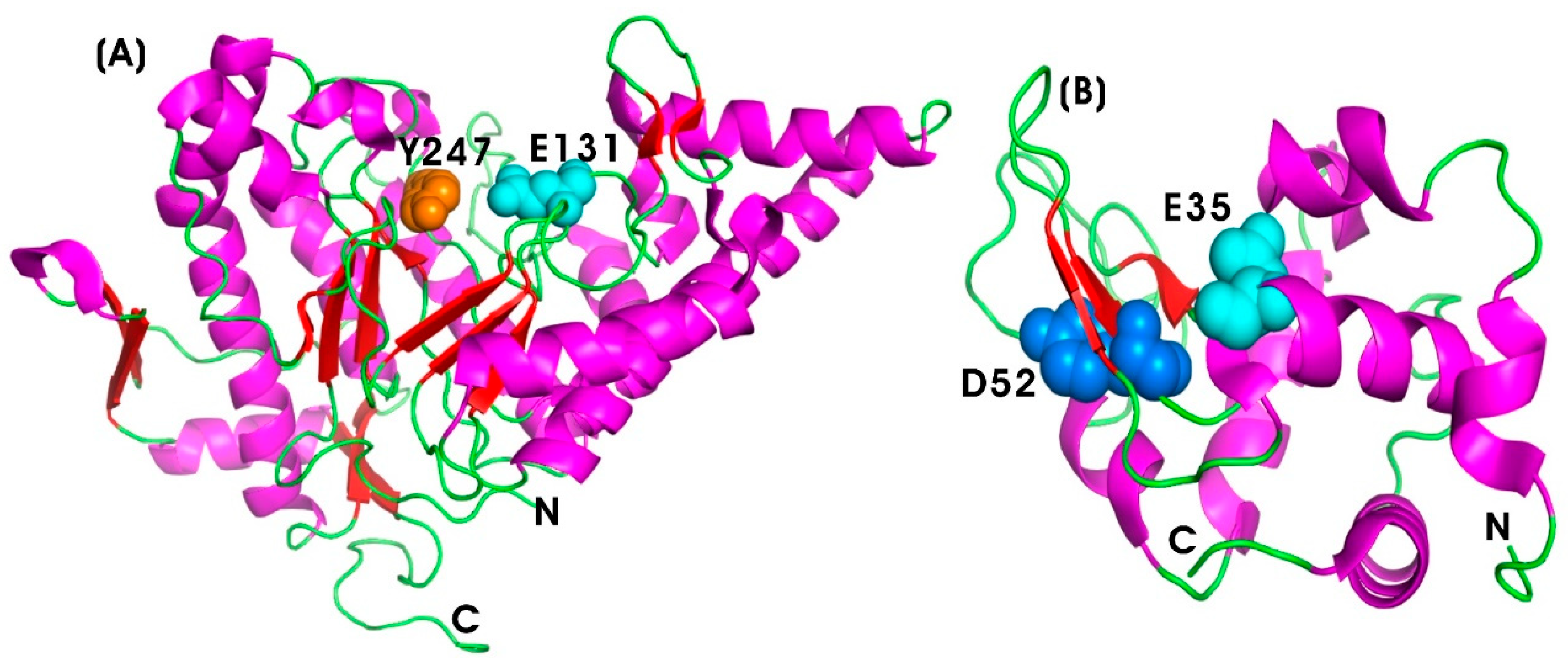
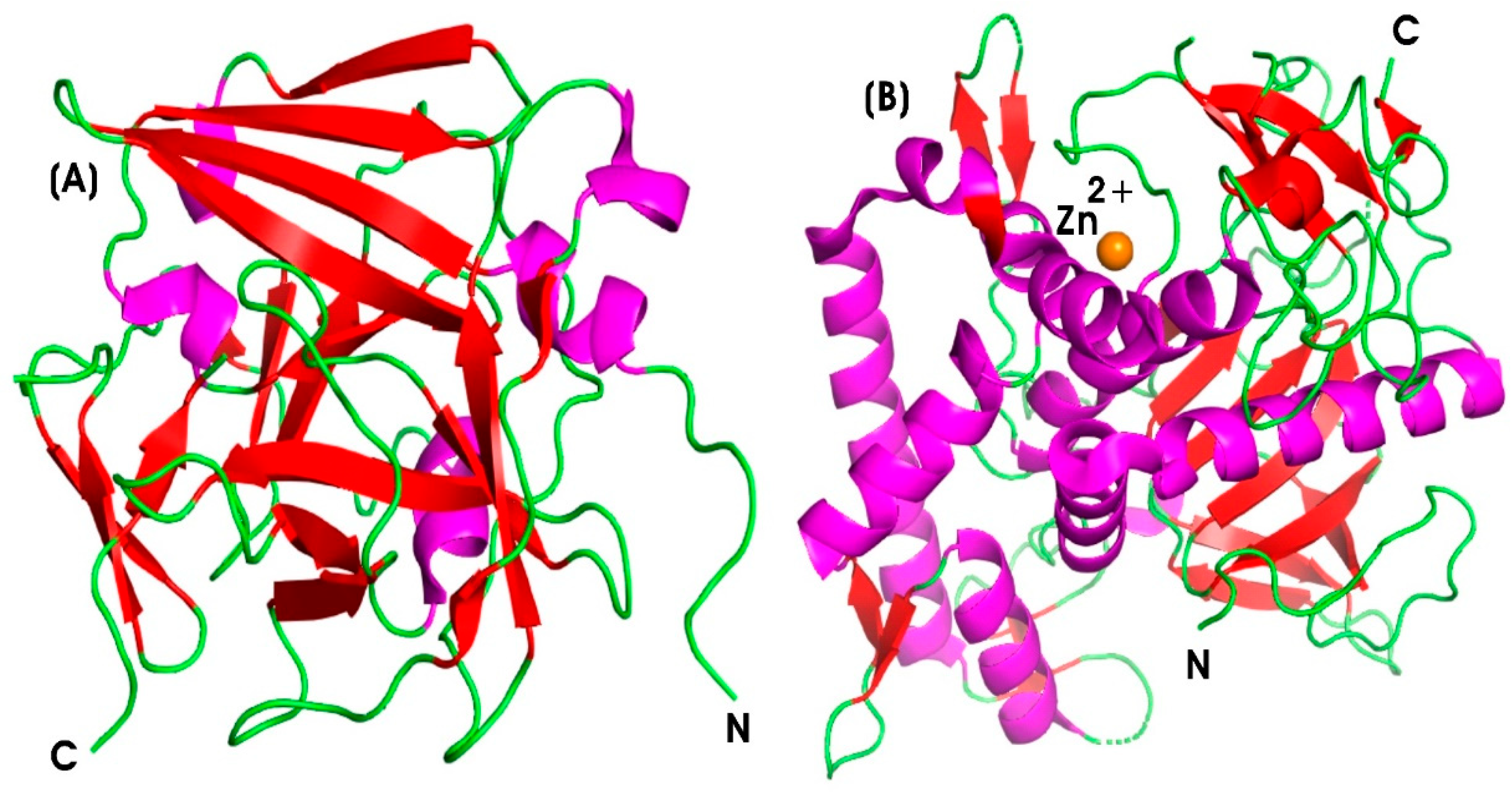
| S. No. | Enzyme | Brand Name | Manufacturer | Anti-Inflammatory Mechanisms | References |
|---|---|---|---|---|---|
| 1 | Catalase | 1. Catalase-250, 2. CAT-200, 3. CATzyme | 1. Creative Enzymes, Shirley, NY, USA, 2. Megazyme International Ireland Ltd., Wicklow, Ireland, 3. MP Biomedicals, LLC, Santa Ana, CA, USA | Converts ROS species (hydrogen peroxide) into water and oxygen, reduces oxidative stress in inflamed tissues, inhibits NF-κB activation, and decreases pro-inflammatory cytokines. | [64,65] |
| 2 | Superoxide dismutase | GliSODin, Orgotein | ISOCELL SA, Paris, France | Neutralizes ROS, reduces stress-induced inflammation, modules cytokine production, and inhibits NF-κB pathway. | [66,67] |
| 3 | Trypsin-Chymotrypsin | Enzomac, Combizyme, | Macleods Pharmaceuticals, Mumbai, India | Degrades pro-inflammatory mediators like fibrin at injury sites, promotes fibrinolysis, and aids in clearing necrotic tissues for faster healing. | [68,69,70,71] |
| 4 | Chymotrypsin | Orthomol Chondro | Macleods Pharmaceuticals, Mumbai, India | Works synergistically with trypsin to reduce tissue inflammation, clear inflammatory debris, and reduce edema and swelling. | [69,71] |
| 5 | Nattokinase | NSK-SD, Doctor’s Best Natto-Serra | Japan Bio Science Laboratory, Tokyo, Japan (NSK-SD) & Doctor’s Best, San Clemente, CA, USA (Natto-Serra) | Degrades fibrin clots, improves circulation, reduces inflammation, suppresses pro-inflammatory cytokines (IL-6 and TNF-α), and reduces oxidative stress and tissue damage. | [72,73,74,75,76] |
| 6 | Bromelain | Bromelain, Ananas | Nature’s’ way, Green Bay, WI, USA (Bromelain), NOW Foods, Bloomingdale, IL, USA (Ananas) | Inhibits COX-2 and reduces prostaglandin production, modulates cytokine levels (TNF-α, IL-1β), suppresses inflammation, reduces swelling and edema, and promotes fibrinolysis. | [16,77,78,79,80,81] |
| 7 | Papain | Accuzyme | Smith & Nephew, Inc., Andover, MA, USA | Facilitates wound debridement, treats chronic ulcers and burns, and reduces inflammation by breaking down necrotic tissues. | [82,83,84] |
| 8 | Serratiopeptidase | 1. Serracore-NK, 2. Danzen, 3. Serra | 1. Arthur Andrew Medical, Tucson, AZ, USA, 2. Takeda Pharmaceuticals, Tokyo, Japan, 3. Vitalzym, World Nutrition, Inc., Mesa, AZ, USA | Reduces inflammation and swelling, enhances tissue repair, reduces scarring, improves cardiovascular health, and inhibits bradykinin, which promotes inflammation. | [85,86,87,88] |
| 9 | Collagenase | Santyl (ointment) | Smith & Nephew, Andover, MA, USA & Worthington Biochemical Corporation, Lakewood, NJ, USA | Breaks down collagen in damaged tissue, reducing fibrosis and promoting tissue remodeling, clears necrotic tissues, aids in inflammation resolution, and facilitates the reduction of pro-inflammatory cytokines. | [89,90,91,92,93] |
| 10 | Hyaluronidase | 1. Hylase 2. Hyalumax 3. Wydase | 1. Riemser Pharma GmbH, Greifswald, Germany 2. Wockhardt Ltd., Mumbai, India 3. Wyeth Pharmaceuticals, New York, NY, USA | Breaks down hyaluronic acid in the ECM matrix, reduces tissue swelling, and enhances fluid drainage from inflamed areas. | [94,95,96,97,98] |
| 11 | Lysozyme | 1. Lyso-6 2. Enzylex 3. Neuzym | 1. Kora Biomedicine, Gyeonggi-do, Republic of Korea, 2. Omnix International, Dubai, UAE, 3. Nippon Zoki Pharmaceutical Co., Ltd., Osaka, Japan | Breaks down bacterial cell walls, reduces infection-driven inflammation, enhances phagocytic activity of immune cells, and modulates immune responses to decrease chronic inflammation markers. | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, K.B. Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases. Pharmaceutics 2025, 17, 606. https://doi.org/10.3390/pharmaceutics17050606
Narayanan KB. Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases. Pharmaceutics. 2025; 17(5):606. https://doi.org/10.3390/pharmaceutics17050606
Chicago/Turabian StyleNarayanan, Kannan Badri. 2025. "Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases" Pharmaceutics 17, no. 5: 606. https://doi.org/10.3390/pharmaceutics17050606
APA StyleNarayanan, K. B. (2025). Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases. Pharmaceutics, 17(5), 606. https://doi.org/10.3390/pharmaceutics17050606








