Achieving Permanent Male Infertility by Magnetic Nanoparticle Hyperthermia: A Breakthrough in Animal Fertility Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Magnetic Fluid Characteristics
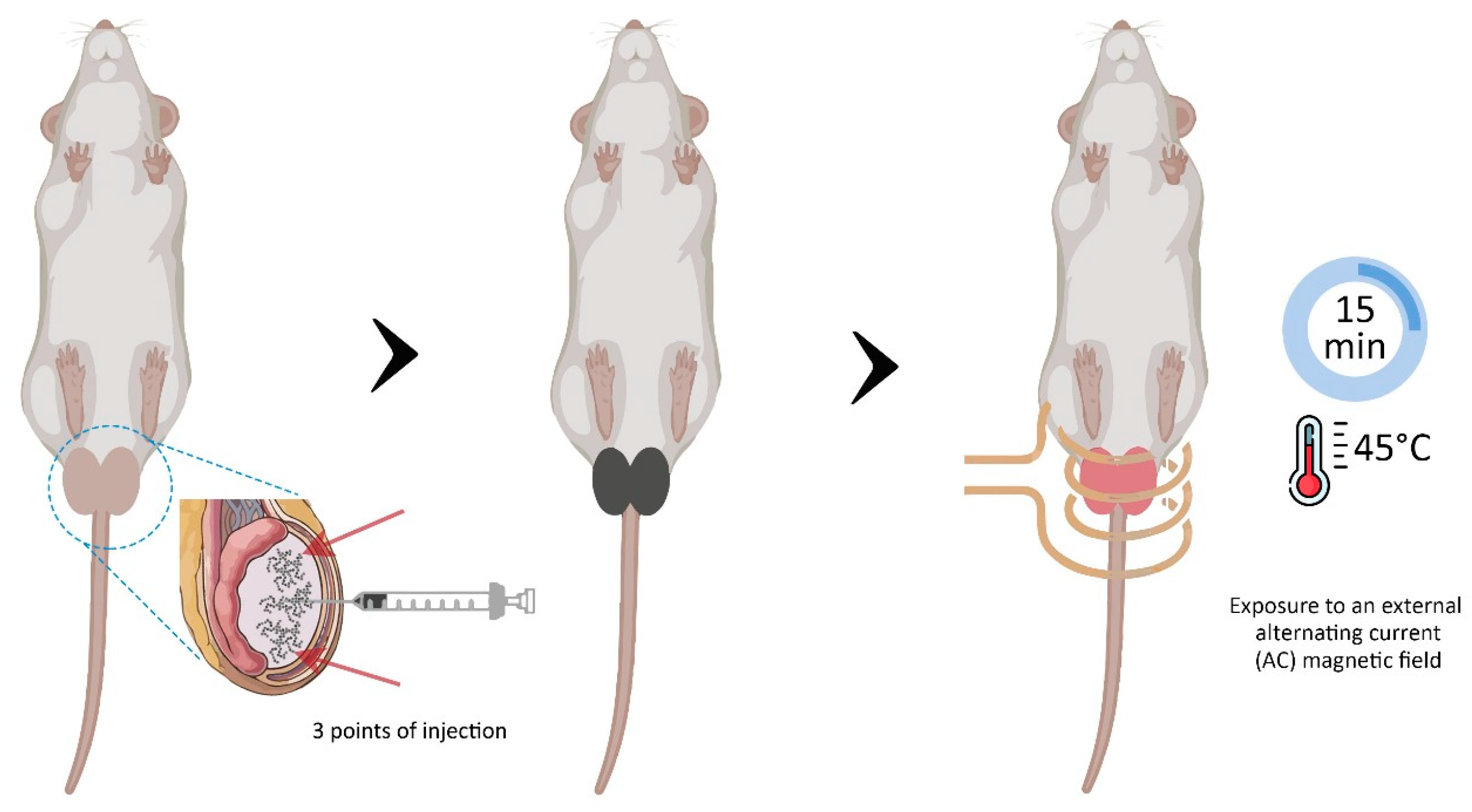
2.3. Experimental Protocol
2.4. Ultrasound Examination
2.5. Blood Analysis and Serum Testosterone Test
2.6. Sperm Analysis
2.7. Histopathological Evaluation
2.8. Detection and Quantification of Magnetic Nanoparticles (MNP) by Ferromagnetic Resonance (FMR)
2.9. Statistical Analysis
3. Results
3.1. Effect of Testicular MNH on General Animal Condition
3.2. Reproductive Parameters
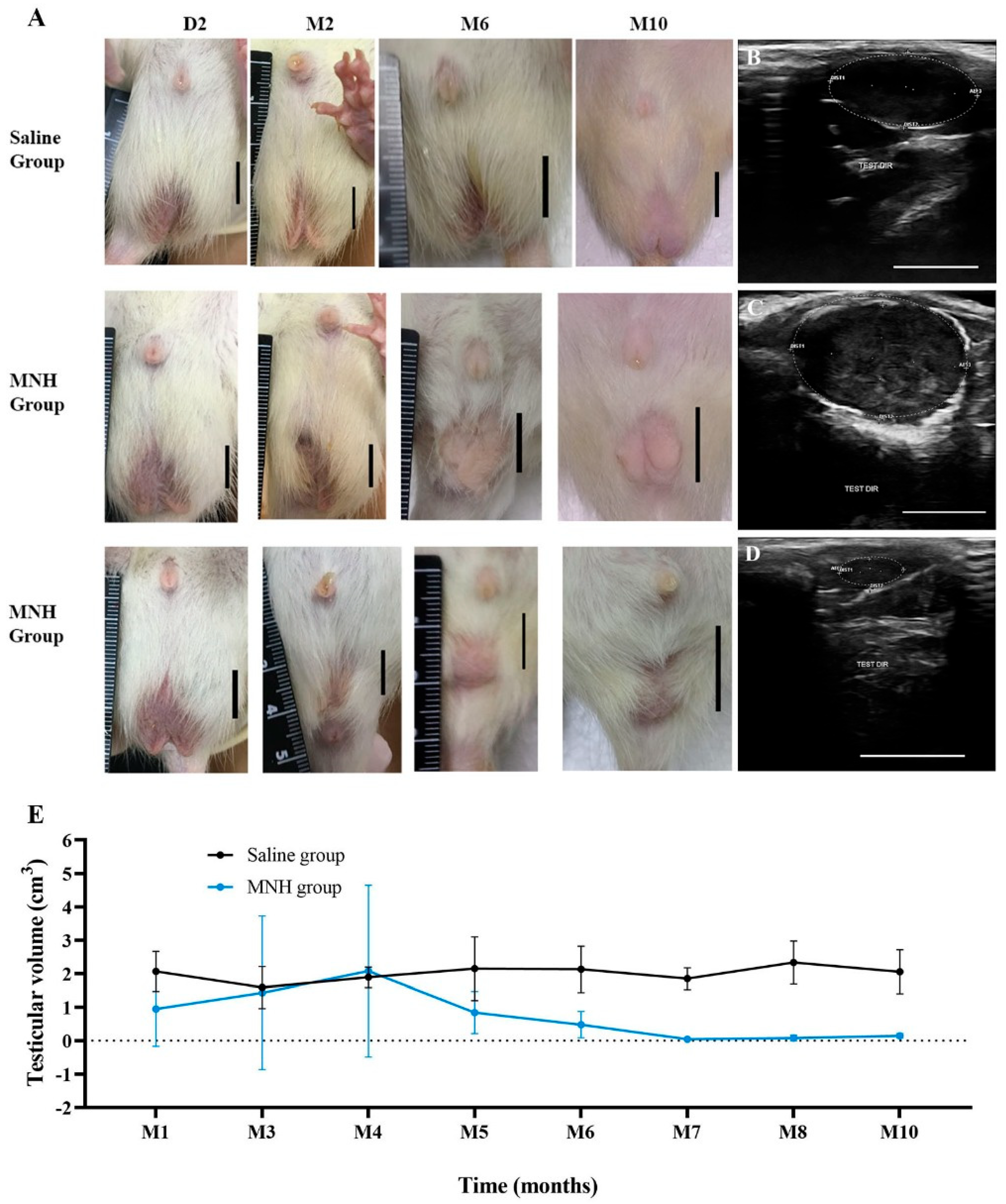
3.2.1. Testicular Appearance and Volume
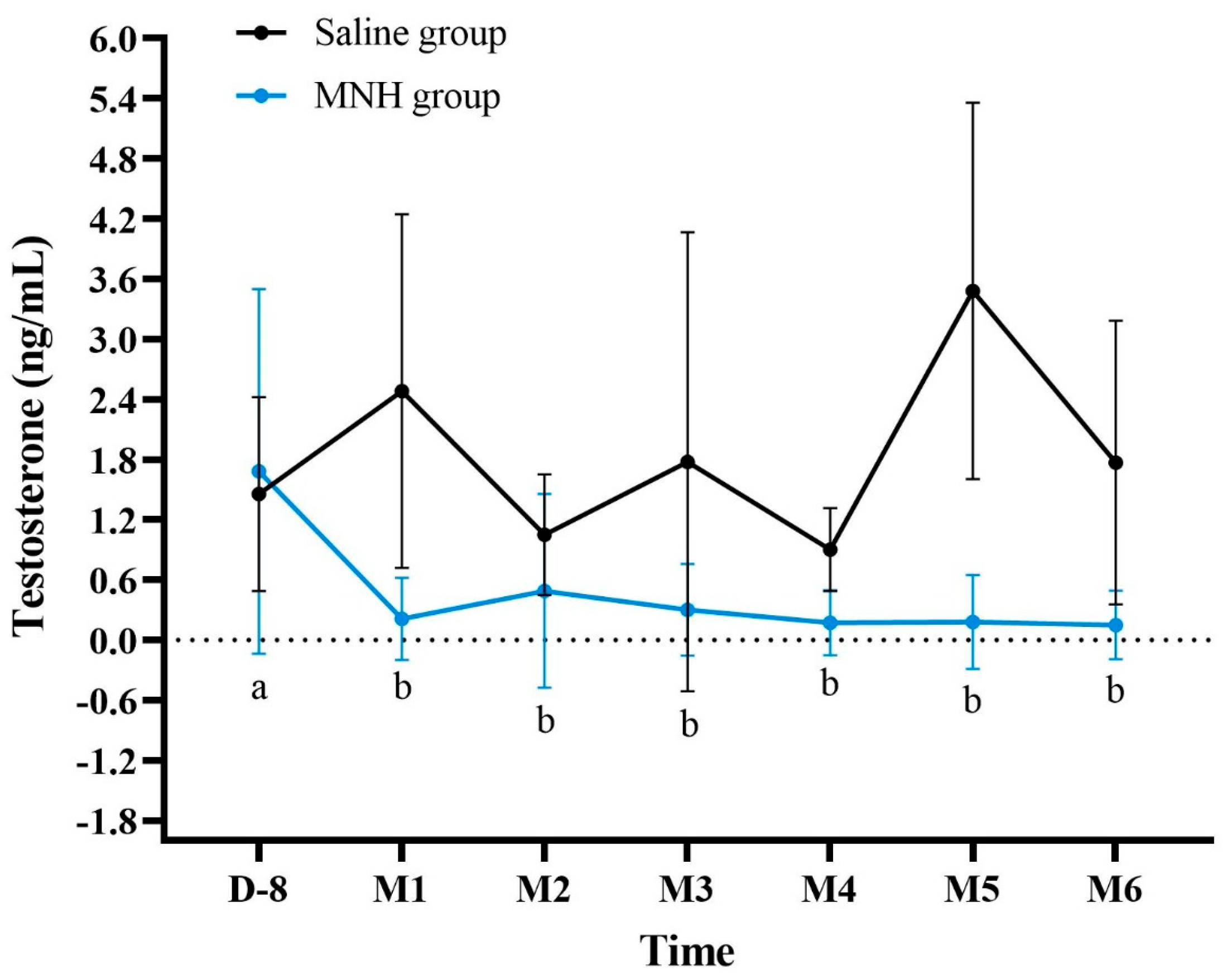
3.2.2. Serum Testosterone Levels After MNH
3.2.3. Sperm Analysis After MNH
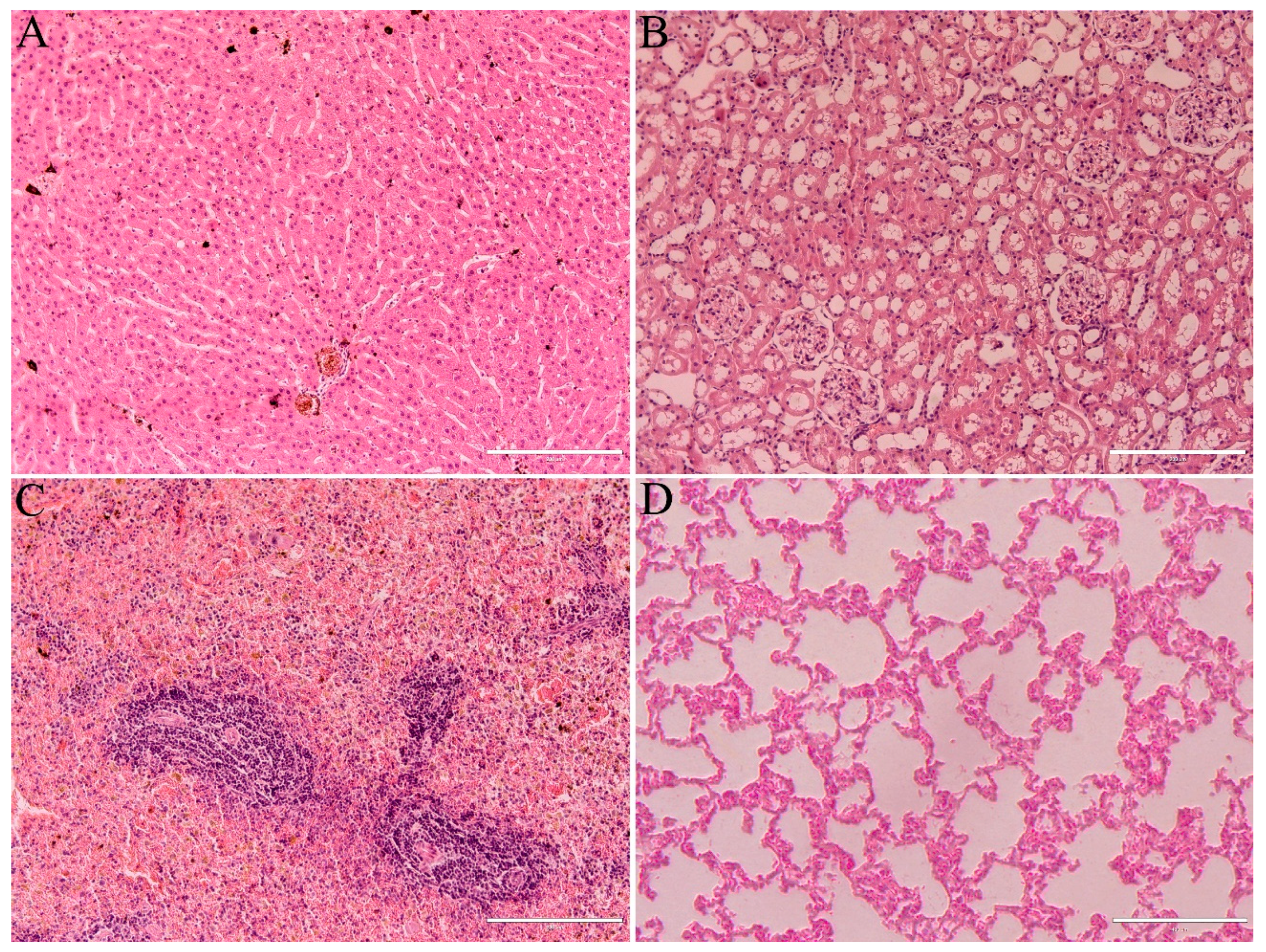
3.2.4. Histological Analysis of Testicles and Epididymis
3.3. Health Parameters
3.4. MNP Detection by FMR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MNH | Magnetic Nanoparticle Hyperthermia |
| MNP | Magnetic Nanoparticles |
| FMR | Ferromagnetic Resonance |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| RBC | Red Blood Cell Count |
| HB | Hemoglobin Concentration |
| HTC | Hematocrit |
| PLT | Platelets |
| WBC | White Blood Cell Count |
| LP | Lymphocyte Percentage |
References
- Gerhold, R.W.; Jessup, D.A. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health 2013, 60, 189–195. [Google Scholar] [CrossRef]
- Woolley, C.K.; Hartley, S. Activity of free-roaming domestic cats in an urban reserve and public perception of pet-related threats to wildlife in New Zealand. Urban. Ecosyst. 2019, 22, 1123–1137. [Google Scholar] [CrossRef]
- Kumar, S. Stray dogs are a growing threat to public health. BMJ 2002, 325, 66. [Google Scholar] [CrossRef] [PubMed Central]
- Robertson, S.A. A review of feral cat control. J. Feline Med. Surg. 2008, 10, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.N.; Johnston, S.D. New developments in small animal population control. J. Am. Vet. Med. Assoc. 1993, 202, 904–909. [Google Scholar] [CrossRef]
- Jana, K.; Samanta, P.K. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception 2006, 73, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Mendes-de-Almeida, F.; Remy, G.L.; Gershony, L.C.; Rodrigues, D.P.; Chame, M.; Labarthe, N.V. Reduction of feral cat (Felis catus Linnaeus 1758) colony size following hysterectomy of adult female cats. J. Feline Med. Surg. 2011, 13, 436–440. [Google Scholar] [CrossRef]
- Sternheim, I. How Holland Became Free of Stray Dogs, 1st ed.; Isis: Amsterdam, The Netherlands, 2012; pp. 1–9. Available online: https://stray-afp.org/nl/wp-content/uploads/sites/2/2015/11/DR_Dutch_Straydogs1.pdf (accessed on 12 February 2025).
- Spehar, D.D.; Wolf, P.J. The Impact of an Integrated Program of Return-to-Field and Targeted Trap-Neuter-Return on Feline Intake and Euthanasia at a Municipal Animal Shelter. Animals 2018, 8, 55. [Google Scholar] [CrossRef]
- Spehar, D.D.; Wolf, P.J. Integrated Return-To-Field and Targeted Trap-Neuter-Vaccinate-Return Programs Result in Reductions of Feline Intake and Euthanasia at Six Municipal Animal Shelters. Front. Vet. Sci. 2019, 6, 77. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Moura, M.R.; de Sá, M.J.; Silva, V.A., Jr.; Kastelic, J.P.; Douglas, R.H.; Marques, A.P., Jr. Permanent contraception of dogs induced with intratesticular injection of a Zinc Gluconate-based solution. Theriogenology 2012, 77, 1056–1063. [Google Scholar] [CrossRef]
- Eugster, S.; Schawalder, P.; Gaschen, F.; Boerlin, P. A prospective study of postoperative surgical site infections in dogs and cats. Vet. Surg. 2004, 33, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.; Wood, A. Non-surgical methods of contraception and sterilization. Theriogenology 2006, 66, 514–525. [Google Scholar] [CrossRef]
- Kustritz, M.V.R. Effects of surgical sterilization on canine and feline health and on society. Reprod. Domest. Anim. 2012, 47, 214–222. [Google Scholar] [CrossRef]
- Levy, J.K.; Bard, K.M.; Tucker, S.J.; Diskant, P.D.; Dingman, P.A. Perioperative mortality in cats and dogs undergoing spay or castration at a high-volume clinic. Vet. J. 2017, 224, 11–15. [Google Scholar] [CrossRef]
- Jana, K.; Samanta, P.K. Sterilization of male stray dogs with a single intratesticular injection of calcium chloride: A dose-dependent study. Contraception 2007, 75, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.G.; Setchell, B.P. Selecting Merino rams for ability to withstand infertility caused by heat. 2. The effect of heat on scrotal and testicular blood flow. Aust. J. Exp. Agric. 1971, 11, 143–147. [Google Scholar] [CrossRef]
- Fahim, M.S.; Fahim, Z.; Der, R.; Hall, D.G.; Harman, J. Heat in male contraception (hot water 6o”c, infrared, microwave and ultrasound). Contraception 1975, 11, 549–562. [Google Scholar] [CrossRef]
- Bedford, J.M. Effects of elevated temperature on the epididymis and testis: Experimental studies. Adv. Exp. Med. Biol. 1991, 286, 19–32. [Google Scholar] [CrossRef]
- Setchell, B.P.; Bergh, A.; Widmark, A.; Damber, J.E. Effect of testicular temperature on vasomotion and blood flow. Int. J. Androl. 1995, 18, 120–126. [Google Scholar] [CrossRef]
- Setchell, B.P. The effects of heat on the testes of mammals. Anim. Reprod. 2006, 3, 81–91. [Google Scholar]
- Roberts, W.W.; Wright, E.J.; Fried, N.M.; Nicol, T.; Jarrett, T.W.; Kavoussi, L.R.; Solomon, S.B. High-intensity focused ultrasound ablation of the epididymis in a canine model: A potential alternative to vasectomy. J. Endourol. 2002, 16, 621–625. [Google Scholar] [CrossRef]
- Aktas, C.; Kanter, M. A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J. Mol. Hist. 2009, 40, 31–39. [Google Scholar] [CrossRef]
- Leoci, R.; Aiudi, G.; Salvati, A.S.; Silvestre, F.; Binetti, F.; Lacalandra, G.M. Ultrasound as a mechanical method for male dog contraception. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 326–328. [Google Scholar] [CrossRef]
- Tsuruta, J.K.; Dayton, P.A.; Gallippi, C.M.; O’Rand, M.G.; Streicker, M.A.; Gessner, R.C.; Gregory, T.S.; Silva, E.J.; Hamil, K.G.; Moser, G.J.; et al. Therapeutic ultrasound as a potential male contraceptive: Power, frequency and temperature required to deplete rat testes of meiotic cells and epididymides of sperm determined using a commercially available system. Reprod. Biol. Endocrinol. 2012, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.S.; Sonu; Dhaked, R. K.; Badar, A.; Khilwani, B.; Lohiya, N.K. Reproductive Functions and Toxicology Following Scrotal Ultrasound Therapy in Rats. Int. J. Sci. Res. Biol. Sci. 2020, 7, 15–24. [Google Scholar]
- Yostawonkul, J.; Surassmo, S.; Namdee, K.; Khongkow, M.; Boonthum, C.; Pagseesing, S.; Saengkrit, N.; Ruktanonchai, U.R.; Chatdarong, K.; Ponglowhapan, S.; et al. Nanocarrier-mediated delivery of α-mangostin for non-surgical castration of male animals. Sci. Rep. 2017, 7, 16234. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.L.M.; Lima, V.N.; Ansa, D.O.; Moya, S.E.; Morais, P.C.; Azevedo, R.B.; Lucci, C.M. Acute reproductive toxicology after intratesticular injection of silver nanoparticles (AgNPs) in Wistar rats. Nanotoxicology 2020, 14, 893–907. [Google Scholar] [CrossRef]
- Coimbra, J.L.P.; Dantas, G.P.F.; de Andrade, L.M.; Brener, M.R.G.; Viana, P.I.M.; Lopes, R.A.; Gontijo, D.O.G.; Ervilha, L.O.G.; Assis, M.Q.; Barcelos, L.S.; et al. Gold nanoparticle intratesticular injections as a potential animal sterilization tool: Long-term reproductive and toxicological implications. Toxicology 2023, 492, 153543. [Google Scholar] [CrossRef]
- Yi, L.; Zhu, S.; Wu, P.; Zhang, Y.; Wang, M.; Xu, P.; Zeng, J.; Wang, G.; Luo, L.; Li, W. Catalysis-Mediated Male Contraception through Black Phosphorus Nanosheets. ACS Appl. Mater. Interfaces 2023, 15, 42284–42292. [Google Scholar] [CrossRef]
- Li, W.Q.; Sun, C.Y.; Wang, F.; Wang, Y.C.; Zhai, Y.W.; Liang, M.; Liu, W.J.; Liu, Z.M.; Wang, J.; Sun, F. Achieving a new controllable male contraception by the photothermal effect of gold nanorods. Nano Lett. 2013, 13, 2477–2484. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ran, X.; Ju, E.; Ren, J.; Qu, X. Single-layer tungsten oxide as intelligent photo-responsive nanoagents for permanent male sterilization. Biomaterials 2015, 69, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Du, Y.; Ren, J.; Qu, X. Using Plasmonic Copper Sulfide Nanocrystals as Smart Light-Driven Sterilants. ACS Nano 2015, 27, 10335–10346. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Scholz, R.; Tesche, B.; Fähling, H.; Mitrovics, T.; Vogl, T.; Cervós-Navarro, J.; Felix, R. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int. J. Hyperth. 1996, 12, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanopart Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Golneshan, A.A.; Lahonian, M. The effect of magnetic nanoparticle dispersion on temperature distribution in a spherical tissue in magnetic fluid hyperthermia using the lattice Boltzmann method. Int. J. Hyperth. 2011, 27, 266–274. [Google Scholar] [CrossRef]
- Rytov, R.A.; Bautin, V.A.; Usov, N.A. Towards optimal thermal distribution in magnetic hyperthermia. Sci. Rep. 2022, 12, 3023. [Google Scholar] [CrossRef]
- Jivago, J.L.P.R.; Brito, J.L.M.; Capistrano, G.; Vinícius-Araújo, M.; Verde, E.L.; Bakuzis, A.F.; Souza, P.E.N.; Azevedo, R.B.; Lucci, C.M. New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia. Pharmaceutics 2021, 13, 1465. [Google Scholar] [CrossRef]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia. Sci. Rep. 2013, 3, 2887, Erratum in Sci. Rep. 2014, 10, 3637. [Google Scholar] [CrossRef]
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In vivo magnetic nanoparticle hyperthermia: A review on preclinical studies, low-field nano-heaters, noninvasive thermometry and computer simulations for treatment planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011, 7, 55. [Google Scholar] [CrossRef]
- Louvandini, H.; Pimentel, C.M.M.; Martins, R.D.; Lucci, C.M.; Corrêa, P.S. Características biométricas testiculares em carneiros Santa Inês submetidos a diferentes regimes de suplementação protéica e tratamentos anti-helmínticos. Ciência Anim. Bras./Braz. Anim. Sci. 2008, 9, 638–647. [Google Scholar]
- Cubas, Z.S.; Silva, J.C.R.; Catão-Dias, J.L. Tratado de Animais Selvagens: Medicina Veterinária, 2nd ed.; Editora GEN/Roca: São Paulo, Brazil, 2014; pp. 137–168. [Google Scholar]
- Quesenberry, K.E.; Carpenter, J.W. Ferrets, Rabbits, and Rodents Clinical Medicine and Surgery, 3rd ed.; Elsevier: St. Louis, MI, USA, 2012; pp. 345–349. [Google Scholar]
- Weir, M.P.; Peters, T.J.; Gibson, J.F. Electron spin resonance studies of splenic ferritin and haemosiderin. Biochim. Biophys. Acta 1985, 828, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, E.; El-Jaick, L.J.; Linhares, M.P.; Esquivel, D.M. Ferromagnetic resonance of horse spleen ferritin: Core blocking and surface ordering temperatures. J. Magn. Reson. 2001, 153, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y.; Harvey, S.C. Duration of the Cycle of the Seminiferous Epithelium of Normal, Hypophysectomized and Hypophysectomized-Hormone Treated Albino Rats. Endocrinology 1965, 76, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-Level Laser (Light) Therapy (LLLT) in Skin: Stimulating, Healing, Restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Ding, W.; Chen, Z.; Gu, Y.; Chen, Z.; Zheng, Y.; Sun, F. Magnetic Testis Targeting and Magnetic Hyperthermia for Noninvasive, Controllable Male Contraception via Intravenous Administration. Nano Lett. 2021, 21, 6289–6297. [Google Scholar] [CrossRef]
- Liu, Y.X. Temperature control of spermatogenesis and prospect of male contraception. Front. Biosci. 2010, 2, 730–755. [Google Scholar] [CrossRef]
- Li, Z.; Tian, J.; Cui, G.; Wang, M.; Yu, D. Effects of local testicular heat treatment on Leydig cell hyperplasia and testosterone biosynthesis in rat testes. Reprod. Fertil. Dev. 2016, 28, 202–209. [Google Scholar] [CrossRef]
- Rizzoto, G.; Ferreira, J.C.P.; Codognoto, V.M.; Oliveira, K.C.; Mogollón García, H.D.; Pupulim, A.G.R.; Teixeira-Neto, F.J.; Castilho, A.; Nunes, S.G.; Thundathil, J.C.; et al. Testicular hyperthermia reduces testosterone concentrations and alters gene expression in testes of Nelore bulls. Theriogenology 2020, 152, 64–68. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A.; El-Gindi, O.D. Free serum testosterone level in male rats treated with Tribulus alatus extracts. Int. Braz. J. Urol. 2007, 33, 554–558. [Google Scholar] [CrossRef]
- Ebomoyi, M.I.; Ahumibe, K.C. Serum testosterone and morphology of the testes in wistar rats following chronic garlic feeding. J. Physiol. Pathophysiol. 2010, 1, 39–43. [Google Scholar]
- Carvalho, R.P.R.; Lima, G.D.A.; Ribeiro, F.C.D.; Ervilha, L.O.G.; Oliveira, E.L.; Viana, A.G.A.; Machado-Neves, M. Eugenol reduces serum testosterone levels and sperm viability in adult Wistar rats. Reprod. Toxicol. 2022, 113, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Whalen, R.R. Testicular Hormones Reduce Individual Differences in the Aggressive Behavior of Male Mice: A Theory of Hormone Action. Neurosci. Biobehav. Rev. 1988, 12, 93–99. [Google Scholar] [CrossRef]
- Albert, D.J.; Walsh, M.L.; Gorzalka, B.B.; Siemens, Y.; Louie, H. Testosterone removal in rats results in a decrease in social aggression and a loss of social dominance. Physiol. Behav. 1986, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.J.; Jonik, R.H.; Watson, N.V.; Gorzalka, B.B.; Walsh, M.L. Hormone-dependent aggression in male rats is proportional to serum testosterone concentration but sexual behavior is not. Physiol. Behav. 1990, 48, 409–416. [Google Scholar] [CrossRef]
- Giammanco, M.; Tabacchi, G.; Giammanco, S.; Di Majo, D.; La Guardia, M. Testosterone and aggressiveness. Med. Sci. Monit. 2005, 11, 136–145. [Google Scholar]
- Kanter, M.; Aktas, C.; Erboga, M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: An immunohistochemical and ultrastructural study. Toxicol. Ind. Health 2011, 29, 99–113. [Google Scholar] [CrossRef]
- Dholakia, U.; Clark-Price, S.C.; Keating, S.C.J.; Stern, A.W. Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD-1 mice. PLoS ONE 2017, 12, e0184911. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 23, 1431–1445. [Google Scholar] [CrossRef]
- Hawkins, P. Recognizing and assessing pain, suffering and distress in laboratory animals: A survey of current practice in the UK with recommendations. Lab. Anim. 2002, 36, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B. A Systematic Approach for Establishing Humane Endpoints. ILAR J. 2000, 41, 80–86. [Google Scholar] [CrossRef]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining body-weight reduction as a humane endpoint: A critical appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Hamilton, K.H.; Henderson, E.R.; Toscano, M.; Chanoit, G.P. Comparison of postoperative complications in healthy dogs undergoing open and closed orchidectomy. J. Small Anim. Pract. 2014, 55, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Crawford, P.C.; Appel, L.D.; Clifford, E.L. Comparison of intratesticular injection of zinc gluconate versus surgical castration to sterilize male dogs. Am. J. Vet. Res. 2008, 69, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, A.K.; Oliveira, E.C.; Tenorio, B.M.; Melo, C.C.; Nery, L.T.; Santos, F.A.; Alves, L.C.; Douglas, R.H.; Silva, V.A., Jr. Injection of a chemical castration agent, zinc gluconate, into the testes of cats results in the impairment of spermatogenesis: A potentially irreversible contraceptive approach for this species? Theriogenology 2014, 81, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Kalhori, M.R.; Hadjighassem, M.R.; Shaterabadi, Z.; Alizadeh, A.M. Effects of multiple injections on the efficacy and cytotoxicity of folate-targeted magnetite nanoparticles as theranostic agents for MRI detection and magnetic hyperthermia therapy of tumor cells. Sci. Rep. 2020, 10, 1695. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size Dependent Biodistribution and Toxicokinetics of Iron Oxide Magnetic Nanoparticles in Mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef]
- Eaton, J.W.; Qian, M. Molecular bases of cellular iron toxicity. Free Radic. Biol. Med. 2002, 32, 833–840. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, M.; Yang, F.; Mao, Y.; Xie, H.; Zhou, Q. Iron overload by Superparamagnetic Iron Oxide Nanoparticles is a High Risk Factor in Cirrhosis by a Systems Toxicology Assessment. Sci. Rep. 2016, 30, 29110. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 16, 204. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]



| Saline Group | D8 | D15 | D30 | D60 | D90 | D120 | D150 | D180 | D210 | D240 | D270 | D300 | D330 | D345 | Normal Limits * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC (106/µL) | 7 ± 3 (2–9) | 9 ± 1 (8–10) | 7 ± 1 (6–9) | 9 ± 6 (1–18) | 9 ± 1 (8–9) | 9 ± 0 (9–9) | 7 ± 3 (1–10) | 10 ± 0 (9–10) | 9 ± 0 (8–9) | 9 ± 0 (8–9) | 9 ± 0 (9–9) | 9 ± 0 (9–9) | 9 ± 0 (8–9) | 8 ± 0 (7–9) | 5–10 |
| HB (g/dL) | 14 ± 6 (3–18) | 17 ± 1 (16–18) | 13 ± 2 (10–17) | 17 ± 2 (14–18) | 17 ± 1 (16–18) | 18 ± 0 (18–19) | 14 ± 7 (2–18) | 19 ± 0 (19–19) | 17 ± 0 (17–18) | 17 ± 1 (15–18) | 17 ± 1 (16–18) | 17 ± 0 (17–18) | 16 ± 0 (16–17) | 15 ± 1 (14–16) | 11–19 |
| HTC (%) | 37 ± 16 (10–47) | 48 ± 4 (45–53) | 37 ± 6 (29–47) | 39 ± 19 (6–49) | 47 ± 3 (43–50) | 49 ± 1 (48–50) | 38 ± 18 (7–51) | 51 ± 1 (51–53) | 45 ± 1 (44–47) | 45 ± 2 (42–49) | 48 ± 0 (47–48) | 47 ± 1 (46–48) | 45 ± 1 (44–45) | 44 ± 3 (40–47) | 35–57 |
| PLT (103/µL) | 520 ± 285 (133–805) | 583 ± 444 (97–966) | 168 ± 175 (51–470) | 694 ± 376 (37–956) | 496 ± 248 (310–920) | 932 ± 33 (877–962) | 416 ± 397 (59–1022) | 915 ± 74 (823–984) | 537 ± 323 (171–839) | 835 ± 298 (316–1035) | 793 ± 180 (627–1008) | 965 ± 53 (885–1025) | 988 ± 58 (913–1067) | 718 ± 193 (425–928) | 200–1500 |
| WBC (103/µL) | 8 ± 3 (3–10) | 10 ± 3 (7–12) | 6 ± 2 (3–8) | 10 ± 3 (5–13) | 8 ± 1 (7–10) | 9 ± 2 (8–11) | 6 ± 4 (1–11) | 7 ± 1 (7–8) | 7 ± 1 (6–9) | 7 ± 1 (6–8) | 6 ± 1 (5–8) | 7 ± 1 (6–9) | 8 ± 2 (6–11) | 5 ± 2 (3–9) | 3–17 |
| LP (%) | 67 ± 11 (48–78) | 68 ± 2 (66–71) | 72 ± 4 (66–77) | 71 ± 2 (69–73) | 69 ± 5 (61–73) | 68 ± 7 (57–74) | 68 ± 7 (57–74) | 70 ± 3 (67–73) | 64 ± 4 (60–71) | 54 ± 10 (39–62) | 63 ± 5 (57–70) | 62 ± 6 (56–71) | 55 ± 6 (49–63) | 55 ± 14 (45–78) | 65–85 |
| MNH Group | D8 | D15 | D30 | D60 | D90 | D120 | D150 | D180 | D210 | D240 | D270 | D300 | D330 | D345 | Normal Limits * |
| RBC (106/µL) | 8 ± 1 (6–9) | 7 ± 1 (4–8) | 8 ± 1 (6–9) | 8 ± 2 (3–9) | 8 ± 1 (7–9) | 9 ± 1 (7–10) | 8 ± 1 (7–9) | 9 ± 0 (9–9) | 9 ± 0 (8–9) | 7 ± 3 (3–9) | 8 ± 1 (6–9) | 9 ± 0 (8–9) | 8 ± 1 (7–9) | 8 ± 1 (6–9) | 5–10 |
| HB (g/dL) | 16 ± 2 (12–18) | 13 ± 3 (8–16) | 15 ± 2 (11–18) | 15 ± 4 (5–18) | 16 ± 1 (13–17) | 18 ± 2 (14–20) | 16 ± 2 (13–18) | 17 ± 1 (16–18) | 18 ± 1 (16–19) | 13 ± 6 (5–18) | 16 ± 2 (12–18) | 17 ± 1 (16–19) | 16 ± 1 (13–18) | 15 ± 2 (12–18) | 11–19 |
| HTC (%) | 43 ± 6 (31–49) | 37 ± 7 (21–43) | 41 ± 5 (31–48) | 41 ± 10 (15–49) | 44 ± 4 (36–49) | 47 ± 5 (35–52) | 46 ± 4 (38–50) | 48 ± 2 (44–51) | 47 ± 2 (44–51) | 35 ± 16 (14–49) | 48 ± 11 (33–75) | 47 ± 2 (44–52) | 44 ± 3 (39–48) | 43 ± 5 (34–49) | 35–57 |
| PLT (103/µL) | 505 ± 256 (133–857) | 493 ± 561 (61–1463) | 572 ± 352 (56–998) | 559 ± 334 (11–1095) | 579 ± 348 (69–1117) | 822 ± 283 (163–1144) | 712 ± 185 (337–870) | 734 ± 328 (135–1260) | 805 ± 198 (434–1071) | 524 ± 403 (91–957) | 750 ± 425 (51–1296) | 955 ± 196 (536–1233) | 982 ± 376 (226–1697) | 676 ± 247 (185–948) | 200–1500 |
| WBC (103/µL) | 6 ± 2 (3–9) | 10 ± 6 (5–23) | 10 ± 2 (7–13) | 13 ± 9 (2–34) | 12 ± 6 (7–26) | 14 ± 7 (6–26) | 11 ± 7 (5–26) | 10 ± 4 (4–20) | 10 ± 5 (5–22) | 7 ± 4 (2–12) | 9 ± 5 (4–21) | 11 ± 4 (7–19) | 11 ± 3 (7–15) | 6 ± 2 (2–9) | 3–17 |
| LP (%) | 73 ± 10 (47–81) | 62 ± 12 (45–77) | 59 ± 13 (40–78) | 65 ± 14 (39–84) | 67 ± 11 (43–82) | 60 ± 14 (40–80) | 67 ± 8 (52–75) | 63 ± 11 (43–75) | 62 ± 10 (47–73) | 64 ± 9 (51–76) | 57 ± 11 (38–70) | 54 ± 9 (36–65) | 49 ± 12 (24–62) | 53 ± 7 (41–65) | 65–85 |
| Saline Group | D−8 | D15 | D30 | D60 | D90 | D120 | D150 | D180 | D210 | D240 | D270 | D300 | D330 | D345 | Normal Limits * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT (U/L) | 33 ± 16 (21–59) | 57 ± 0 (57–57) | 27 ± 5 (20–30) | 34 ± 4 (28–40) | 34 ± 11 (22–50) | 33 ± 5 (28–41) | 44 ± 6 (35–50) | 56 ± 6 (51–66) | 61 ± 7 (52–69) | 58 ± 7 (49–64) | 61 ± 3 (56–64) | 69 ± 7 (58–78) | 71 ± 11 (61–86) | 62 ± 2 (59–65) | 17–224 |
| AST (U/L) | 99 ± 26 (73–136) | 249 ± 0 (249–249) | 178 ± 50 (129–243) | 93 ± 26 (64–127) | 78 ± 6 (71–85) | 90 ± 17 (77–120) | 106 ± 8 (94–116) | 102 ± 25 (82–142) | 176 ± 33 (122–208) | 130 ± 41 (100–201) | 144 ± 43 (106–203) | 118 ± 20 (98–148) | 156 ± 42 (118–205) | 105 ± 98 (0–217) | 63–175 |
| CREA (mg/dL) | 0.9 ± 0.2 (0.7–1.1) | 0.9 ± 0.0 (0.9–0.9) | 0.7 ± 0.0 (0.7–0.7) | 0.7 ± 0.0 (0.6–0.7) | 0.7 ± 0.1 (0.6–0.8) | 0.6 ± 0.1 (0.5–0.7) | 0.7 ± 0.0 (0.7–0.7) | 0.8 ± 0.1 (0.7–1.0) | 0.8 ± 0.1 (0.7–0.9) | 0.7 ± 0.1 (0.7–0.8) | 0.7 ± 0.0 (0.7–0.7) | 0.7 ± 0.1 (0.6–0.8) | 0.8 ± 0.1 (0.7–1.0) | 0.8 ± 0.0 (0.7–0.8) | 0.2–0.8 |
| UREA (mg/dL) | 51 ± 5 (44–59) | 44 ± 0 (44–44) | 42 ± 4 (38–46) | 40 ± 4 (35–43) | 43 ± 1 (42–45) | 52 ± 4 (46–56) | 48 ± 4 (45–54) | 57 ± 3 (54–60) | 53 ± 4 (48–58) | 47 ± 5 (41–52) | 47 ± 2 (45–49) | 50 ± 6 (42–55) | 44 ± 3 (40–47) | 41 ± 3 (37–44) | 26–58 |
| MNH Group | D−8 | D15 | D30 | D60 | D90 | D120 | D150 | D180 | D210 | D240 | D270 | D300 | D330 | D345 | Normal Limits * |
| ALT (U/L) | 32 ± 10 (22–49) | 53 ± 24 (28–103) | 32 ± 10 (18–50) | 47 ± 19 (31–98) | 39 ± 8 (25–48) | 45 ± 6 (34–53) | 68 ± 22 (34–92) | 51 ± 12 (38–70) | 61 ± 10 (45–76) | 71 ± 7 (63–82) | 62 ± 4 (57–68) | 78 ± 16 (59–113) | 78 ± 12 (65–99) | 74 ± 6 (67–86) | 17–224 |
| AST (U/L) | 96 ± 29 (65–156) | 190 ± 42 (116–248) | 139 ± 32 (102–222) | 162 ± 54 (110–298) | 128 ± 42 (66–196) | 105 ± 32 (58–174) | 190 ± 56 (112–291) | 153 ± 35 (84–200) | 117 ± 17 (93–138) | 124 ± 16 (104–149) | 138 ± 45 (77–223) | 117 ± 28 (89–170) | 126 ± 39 (80–219) | 125 ± 70 (0–259) | 63–175 |
| CREA (mg/dL) | 1.0 ± 0.7 (0.6–3.0) | 0.8 ± 0.6 (0.0–1.9) | 0.8 ± 0.3 (0.6–1.7) | 0.8 ± 0.2 (0.5–1.4) | 0.7 ± 0.4 (0.1–1.5) | 0.9 ± 0.5 (0.6–2.4) | 0.9 ± 0.5 (0.7–2.2) | 0.9 ± 0.4 (0.7–1.9) | 0.9 ± 0.4 (0.7–2.1) | 0.9 ± 0.4 (0.7–2.0) | 0.9 ± 0.4 (0.7–2.0) | 0.8 ± 0.5 (0.5–2.1) | 0.8 ± 0.4 (0.6–1.9) | 0.9 ± 0.6 (0.6–2.4) | 0.2–0.8 |
| UREA (mg/dL) | 42 ± 5 (33–49) | 50 ± 5 (42–56) | 44 ± 6 (31–53) | 46 ± 7 (38–64) | 44 ± 6 (34–51) | 52 ± 12 (39–79) | 44 ± 5 (40–55) | 47 ± 5 (41–55) | 47 ± 7 (34–59) | 42 ± 4 (33–47) | 39 ± 4 (32–46) | 42 ± 5 (33–48) | 41 ± 4 (32–47) | 39 ± 5 (30–50) | 26–58 |
| Organ | Saline Group | MNH Group |
|---|---|---|
| Liver | 2.8 ± 0.1 | 2.5 ± 0.4 |
| Kidney | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Spleen | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Lungs | 0.4 ± 0.1 | 0.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, J.L.M.; Lima, V.N.; Jivago, J.L.P.R.; Marangon, A.R.M.; Vinícius-Araújo, M.; Bakuzis, A.F.; Santos, J.d.A.R.d.; Souza, P.E.N.; Azevedo, R.B.; Lucci, C.M. Achieving Permanent Male Infertility by Magnetic Nanoparticle Hyperthermia: A Breakthrough in Animal Fertility Management. Pharmaceutics 2025, 17, 602. https://doi.org/10.3390/pharmaceutics17050602
Brito JLM, Lima VN, Jivago JLPR, Marangon ARM, Vinícius-Araújo M, Bakuzis AF, Santos JdARd, Souza PEN, Azevedo RB, Lucci CM. Achieving Permanent Male Infertility by Magnetic Nanoparticle Hyperthermia: A Breakthrough in Animal Fertility Management. Pharmaceutics. 2025; 17(5):602. https://doi.org/10.3390/pharmaceutics17050602
Chicago/Turabian StyleBrito, Juliana Lis Mendes, Vanessa Nicolau Lima, José Luiz P. R. Jivago, Aline R. M. Marangon, Marcus Vinícius-Araújo, Andris Figueiroa Bakuzis, Juliana dos Anjos Ribeiro dos Santos, Paulo E. N. Souza, Ricardo Bentes Azevedo, and Carolina Madeira Lucci. 2025. "Achieving Permanent Male Infertility by Magnetic Nanoparticle Hyperthermia: A Breakthrough in Animal Fertility Management" Pharmaceutics 17, no. 5: 602. https://doi.org/10.3390/pharmaceutics17050602
APA StyleBrito, J. L. M., Lima, V. N., Jivago, J. L. P. R., Marangon, A. R. M., Vinícius-Araújo, M., Bakuzis, A. F., Santos, J. d. A. R. d., Souza, P. E. N., Azevedo, R. B., & Lucci, C. M. (2025). Achieving Permanent Male Infertility by Magnetic Nanoparticle Hyperthermia: A Breakthrough in Animal Fertility Management. Pharmaceutics, 17(5), 602. https://doi.org/10.3390/pharmaceutics17050602








