The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus
Abstract
1. Introduction
2. Hair Structure and Cycle
3. Current Strategies in Alopecia Treatment
3.1. Non-Therapeutic Treatment
3.1.1. Hair Transplantation
3.1.2. Platelet-Rich Plasma (PRP)
3.1.3. Stem Cells
3.1.4. Exosomes
3.1.5. Low-Level Light Therapy (LLLT)
3.1.6. Microneedling
3.2. Therapeutic Treatment
3.2.1. Topical Corticosteroids (TCs)
3.2.2. Intralesional Corticosteroids
3.2.3. Systemic Corticosteroids
3.2.4. Contact Immunotherapy
3.2.5. Topical Minoxidil
3.2.6. Finasteride
3.2.7. Dutasteride
3.2.8. Sodium Valproate
3.3. Phytochemical Treatment
3.3.1. Mechanism of Phytochemicals in Alopecia
Aloe Vera (Aloe barbadensis)
Amla (Phyllanthus emblica)
Onion (Allium cepa)
Garlic (Allium sativam)
Bhringraj (Eclipta Alba)
Tea (Thea sinesis)
Fenugreek (Trigonella foenum graecum)
Coconut (Cocos nucifera L.)
Almond (Prunus amygdalus)
Tulsi (Oscimum sanctum)
3.3.2. Limitations of Phytochemicals
3.3.3. Safety and Regulatory Concerns of Phytochemicals
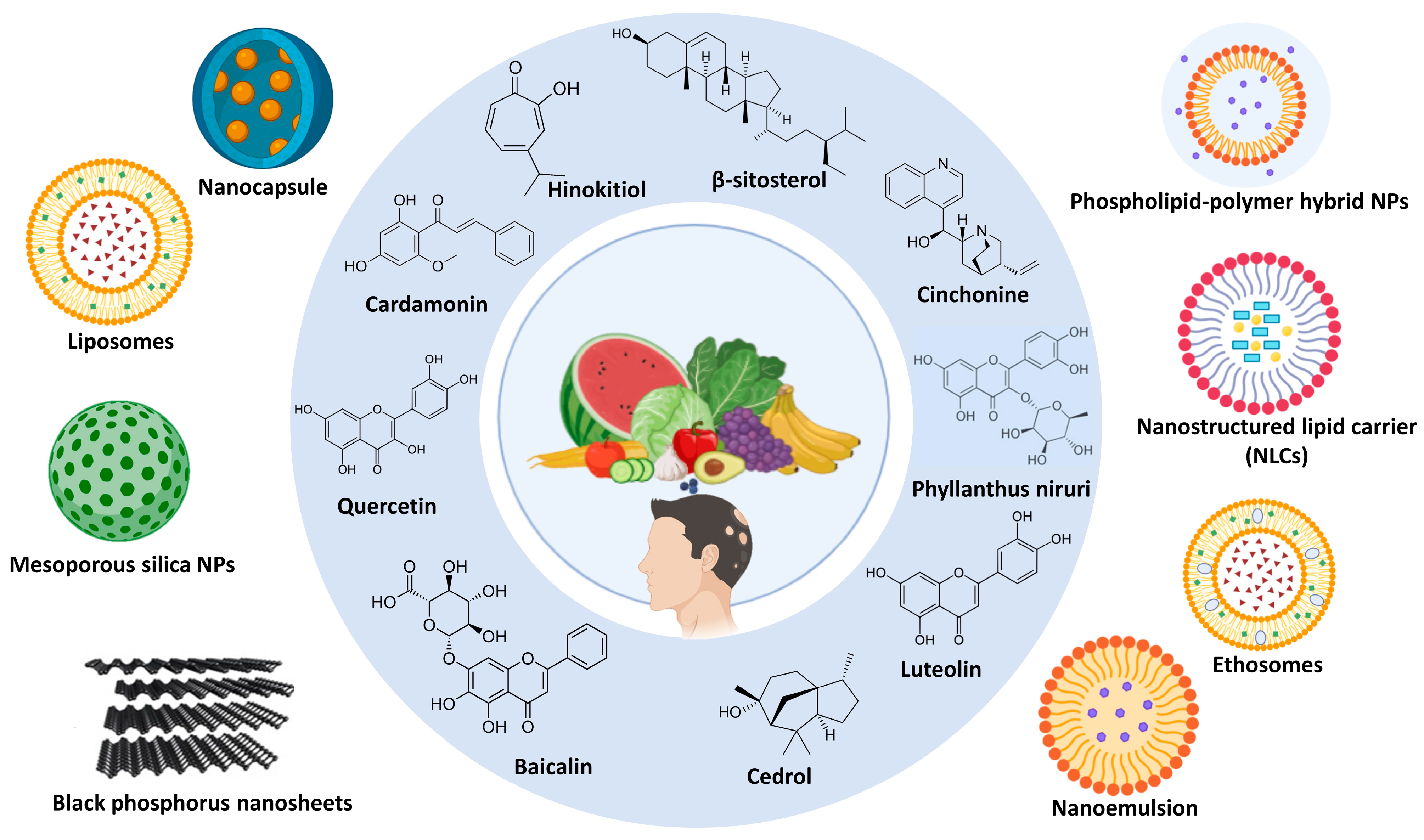
4. Nano-Drug Delivery Systems in Loading Phytochemicals for Alopecia
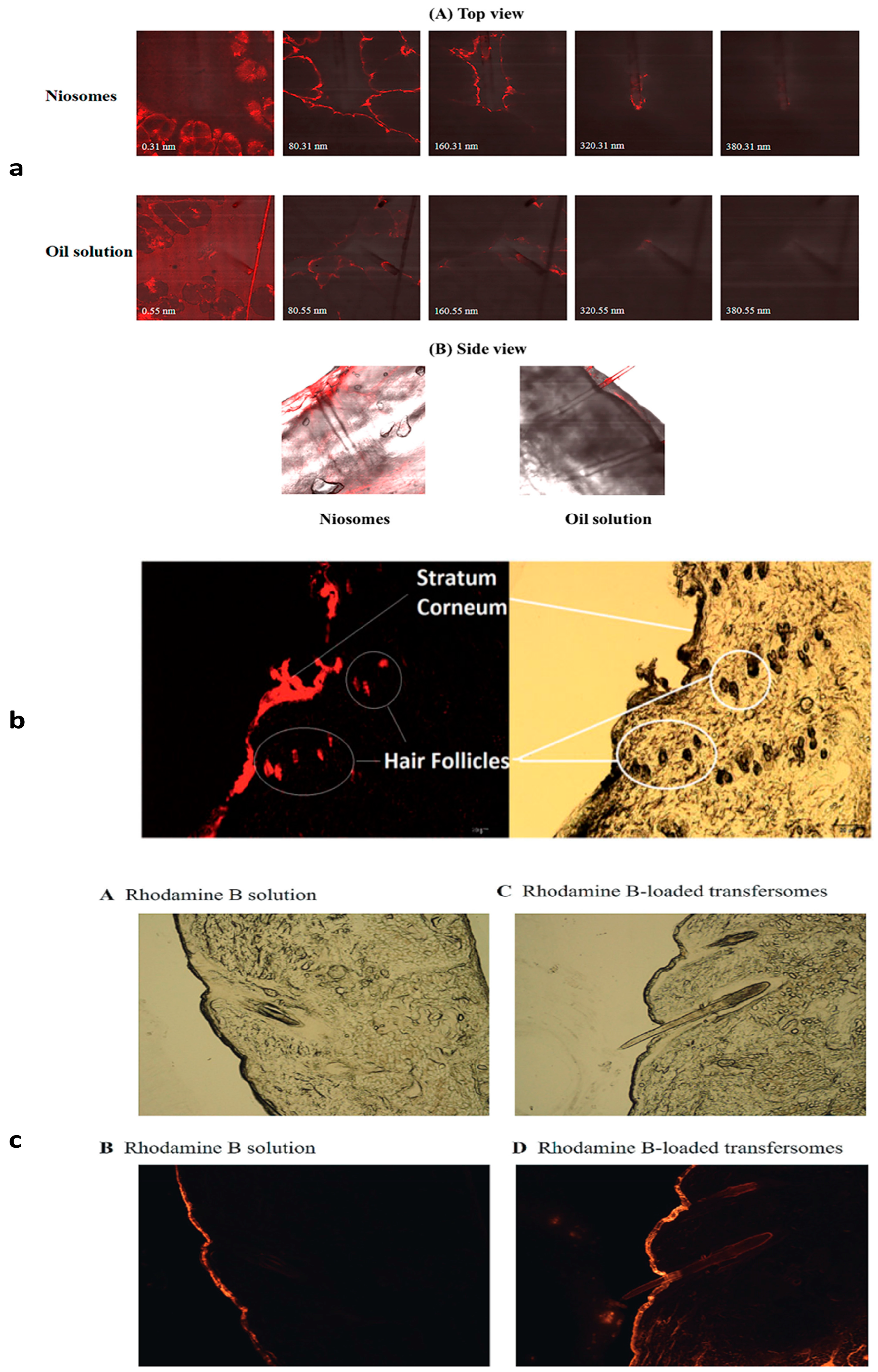
4.1. Niosomes
4.2. Zinc Mesoporous Silica Nanoparticles
4.3. Phospholipid–Polymer Hybrid Nanoparticles
| Phytomedicine | Source from Plant | Nanovesicle | Materials | Method | Animal | In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Pumpkin Seed Oil (PSO) | Seeds | Niosomes | Tween 20, cholesterol | Ethanol injection method | Pig ear skin |
| [121] |
| Quercetin (Qu) | Skins, peels, outer leaves, and flowers that are found in red onions(skin), capers, berries, kale, and buckwheat | Zinc mesoporous silica | Zinc nitrate hexahydrate, sodium alginate, cetyltrimethylammonium bromide, tetraethyl orthosilicate, copper nitrate trihydrate, ammonium fluoride, and copper nitrate trihydrate | Sol–gel method | C57BL/6 mice |
| [123] |
| Phospholipid–polymer Hybrid nanoparticles | Polyvinyl alcohol (PVA), Ethyl acetate, 1, 2-Dipalmitoylsn-glycero-3-phosphocholine (DPPC) | Double emulsification Solvent evaporation | Sprague–Dawley male rats |
| [126] | ||
| Cinchonine (CN) | Cinchona bark | NLCs | Stearic acid, oleic acid, polysorbate 80, and glycerin | Combination of microemulsification and ultra-sonification | Swiss Webster male mice |
| [127] |
| Carthamus tinctorius florets extract | Safflower florets | Monostearin, capric/caprylic triglycerides, Brij-L4, span 60, Tween 60, and Pluronic F-68 | Hot high-pressure homogenization | C56BL/6Mlac male mice |
| [128] | |
| β-vulgaris L. Extract (BVEN) | Roots | Nanoparticles incorporated into a gel | Chitosan, sodium alginate, calcium chloride, acetic acid, sodium hydroxide, carbopol 934, methyl paraben, propyl paraben, and propylene glycol | Ionic gelation | Male Swiss albino mice |
| [129] |
| β-sitosterol | Seeds, nuts, and oily fruits, or the vegetable oils derived from them | NLCs | Glyceryl mono stearate, virgin coconut oil, and Tween 80 | High-speed homogenization | Male Wistar rats |
| [130] |
| Phyllanthus niruri | Root | Ethosomes | Ethanol, Propylene glycol, Soya lecithin | Hot method | Male Wistar rats |
| [131] |
| Hinokitiol (HKL) | The heartwood of certain trees belonging to the Cupressaceae family (cypress family). | Nanocapsule | Poly(ε-caprolactone), cetyltrimethylamonium chloride, and octyl salicylate | Emulsion–diffusion method | C57BL/6 mouse |
| [132] |
| Poly (γ-Glutamic Acid) | Chitosan Hydrogel Nanoparticles | Acetic acid and chitosan | Ionic gelation | C57BL/6N female telogenic mice |
| [133] | |
| Luteolin | Leaves, flowers, fruits, vegetables (stalks/roots), and seeds/hulls | Nanoemulsions | Lipoid P75-3, poly (ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) | Phase inversion composition | C57BL/6 male mice |
| [134] |
| Cedrol | Wood | Medium chain oil Span 80 | _ | C57BL/6 Mice |
| [135] | |
| Cardamonin (CAR) | Several plant parts, primarily from plants belonging to the Ginger family (Zingiberaceae) | Liposomes | Phospholipid and cholesterol | Thin-film hydration | Rat |
| [136] |
| Baicalin (BA) | Root and bark | Black phosphorus nanosheets encapsulated MN | NH2-PEG and black phosphorus | Liquid-phase exfoliation | SD rats |
| [137] |
4.4. Solid Lipid Nanoparticles (SLNs)
4.5. Nanostructured Lipid Carriers (NLCs)
4.6. Transfersomes
4.7. Ethosomes
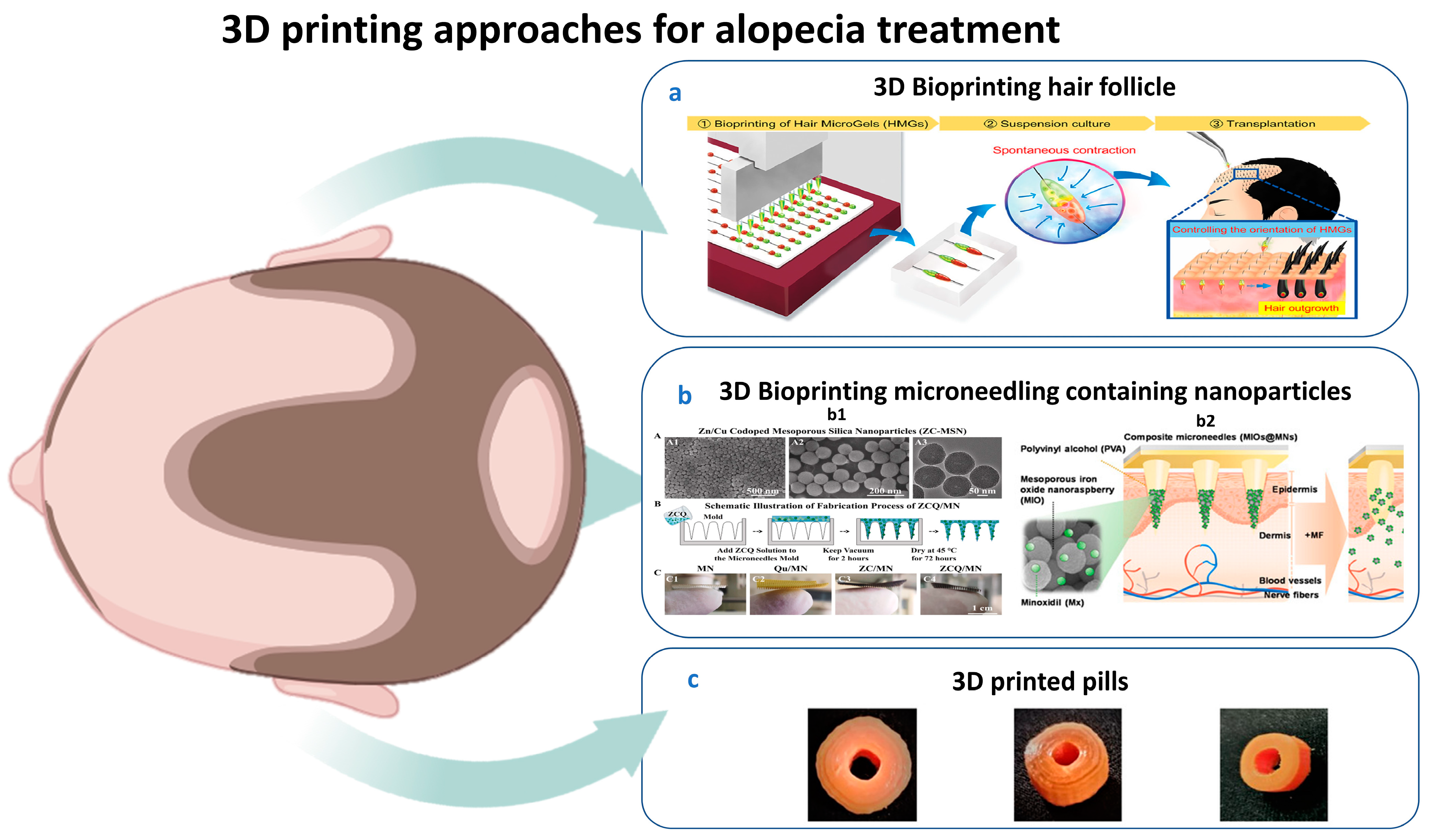
5. 3D Printing for Alopecia
5.1. History of Dermal 3D Bioprinting
| Features | 2D Bioprinting HF | 3D Bioprinting HF | Refs. |
|---|---|---|---|
| Structure | Two-dimensional single-layer simple structure. | The 3D layer-by-layer complex structure resembles native HFs. | [159] |
| Cellular and biomaterials | Graphene oxide, cellulose, chitin, and proteins. | Dermal papilla cells, HUVECs, keratinocytes, and melanocytes in collagen–dermatan sulfate matrices, gelatin–alginate hydrogel. | [158,160,161] |
| Function | Supports basic cell studies. | Creates more realistic HF constructs that closely resemble the native HF structure. | [162] |
| Applications | Investigate signaling pathways of skin illnesses, such as psoriasis, or melanoma wound healing and test the efficacy of safe therapies. | Facilitates cell migration, mimicking a native-like microenvironment essential for angiogenesis, neurogenesis, proliferation, and differentiation. | [163,164] |
5.2. Applications of 3D Printing for HF Generation
5.3. 3D Bioprinting Assisted by Artificial Intelligence and Machine Learning
5.4. Limitations of 3D Bioprinting
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2023, 25, 87–100. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Review, E. Clinical and Genetic Aspects of Alopecia Areata: A Cutting. Genes 2023, 14, 1362. [Google Scholar] [CrossRef] [PubMed]
- Kassira, S.; Korta, D.Z.; Chapman, L.W.; Dann, F. Review of treatment for alopecia totalis and alopecia universalis. Int. J. Dermatol. 2017, 56, 801–810. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, F.; Tao, N.; Wang, X.; Jin, X.; Zhang, C.; Xu, C. An androgenetic alopecia remedy based on marine collagen peptide-incorporated dissolving microneedles. Int. J. Pharm. 2024, 650, 123629. [Google Scholar] [CrossRef] [PubMed]
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of Hair Loss: Stress and the Underestimated Psychosocial Impact of Telogen Effluvium and Androgenetic Alopecia. J. Investig. Dermatol. 2004, 123, 455–457. [Google Scholar] [CrossRef]
- Aukerman, E.L.; Jafferany, M. The psychological consequences of androgenetic alopecia: A systematic review. J. Cosmet. Dermatol. 2023, 22, 89–95. [Google Scholar] [CrossRef]
- Ntshingila, S.; Oputu, O.; Arowolo, A.T. REVIEWS/META—ANALYSES Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A. Pathogenesis of androgenetic alopecia. J. Am. Acad. Dermatol. 2004, 50, 777–779. [Google Scholar] [CrossRef]
- Guo, H.; Gao, W.V.; Endo, H.; McElwee, K.J. Experimental and early investigational drugs for androgenetic alopecia. Expert Opin. Investig. Drugs 2017, 26, 917–932. [Google Scholar] [CrossRef]
- Saumya, P.; Shyam, V. The menace of dermatophytosis in India: The evidence that we need. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 6–15. [Google Scholar] [CrossRef]
- Hibberts, N.A.; Howell, A.E.; Randall, V.A. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. 1998, 156, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, P.S.; Dave, P.A.; Rohit, R.K.; Tibrewal, C.; Modi, N.S.; Gandhi, S.K.; Patel, P. Comparing Current Therapeutic Modalities of Androgenic Alopecia: A Literature Review of Clinical Trials. Cureus 2023, 15, e42768. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J. Alopecia Areata of the Beard: A Review of the Literature. Am. J. Clin. Dermatol. 2017, 18, 789–796. [Google Scholar] [CrossRef]
- Ibler, E.; Silverberg, J.I. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J. Am. Dermatol. 2019, 82, 675–682. [Google Scholar] [CrossRef]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Robbibs, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642256103. [Google Scholar]
- Paus, R. Principles of hair cycle control. J. Dermatol. 1998, 25, 793–802. [Google Scholar] [CrossRef]
- Breehl, L.; Caban, O. Physiology, Puberty; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Piccolo, M.; Ferraro, M.G.; Maione, F.; Maisto, M.; Stornaiuolo, M.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Induction of Hair Keratins Expression by an Annurca Apple-Based Nutraceutical Formulation in Human Follicular Cells. Nutrients 2019, 11, 3041. [Google Scholar] [CrossRef]
- Rose, P.T. Hair restoration surgery: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2015, 8, 361–370. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Sharma, R.; Ranjan, A. Follicular Unit Extraction (FUE) Hair Transplant: Curves Ahead. J. Maxillofac. Oral Surg. 2019, 18, 509–517. [Google Scholar] [CrossRef]
- Girijala, R.L.; Riahi, R.R.; Cohen, P.R. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol. Online J. 2018, 24, 1–13. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Griggs, J.W.; Tosti, A. Platelet-Rich Plasma in the Treatment of Alopecia Areata: A Review. J. Investig. Dermatol. Symp. Proc. 2020, 20, S45–S49. [Google Scholar] [CrossRef]
- Egger, A.; Tomic-Canic, M.; Tosti, A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4-Repair Replace. Regen. Reprogram. 2020, 8, e2894. [Google Scholar]
- Master, Z.; McLeod, M.; Mendez, I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson’s disease. J. Med. Ethics 2007, 33, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Zhang, J.; Shen, Y.; Hou, X.; Su, L.; Chen, W.; Chen, J.; Guo, X.; Song, H. Treatment of androgenetic alopecia by exosomes secreted from hair papilla cells and the intervention effect of LTF. J. Cosmet. Dermatol. 2023, 22, 2996–3007. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-L.; Shi, Y.-R.; Hu, X.-E.; Yu, D.-J.; Chen, B.-Y.; Wang, L.-J.; Xu, X.-L.; Zhu, M.-L. The role of exosomes in follicle regeneration of androgenic alopecia. J. Drug Deliv. Sci. Technol. 2023, 90, 105126. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef]
- Sondagar, D.M.; Mehta, H.H.; Agharia, R.S.; Jhavar, M.K. Efficacy of Low-Level Laser Therapy in Androgenetic Alopecia—A Randomized Controlled Trial. Int. J. Trichol. 2023, 15, 25–32. [Google Scholar] [CrossRef]
- Eells, J.T.; Wong-Riley, M.T.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Schwartzberg, L.; Gold, M.H. Complications seen with the use of lasers for cosmetic applications. Dermatol. Rev. 2020, 1, 63–70. [Google Scholar] [CrossRef]
- Faghihi, G.; Nabavinejad, S.; Mokhtari, F.; Fatemi Naeini, F.; Iraji, F. Microneedling in androgenetic alopecia; comparing two different depths of microneedles. J. Cosmet. Dermatol. 2021, 20, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, M.; Ahn, H.; Kang, B.M.; Yang, H.; Kang, G.; Kwon, O.; Jung, H. Novel treatment of alopecia areata with shooting-type candlelit-dissolving microneedle. Appl. Mater. Today 2023, 35, 101946. [Google Scholar] [CrossRef]
- Alsantali, A. Alopecia areata: A new treatment plan. Clin. Cosmet. Investig. Dermatol. 2011, 4, 107–115. [Google Scholar] [CrossRef]
- Das, S.; Ghorami, R.C.; Chatterjee, T.; Banerjee, G. Comparative assessment of topical steroids, topical tretenoin (0.05%) and dithranol paste in alopecia areata. Indian J. Dermatol. 2010, 55, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Stacey, S.K.; Mceleney, M. Topical Corticosteroids: Choice and Application. Am. Fam. Physician 2021, 103, 337–343. [Google Scholar]
- Sardana, S.; Goyal, T.; Kushwaha, P.; Jha, P. A Prospective Study to Compare the Efficacy of Cryotherapy Versus Intralesional Steroid in Alopecia Areata. J. Cutan. Aesthet. Surg. 2022, 15, 175–178. [Google Scholar] [CrossRef]
- Malhotra, K.; Madke, B. An Updated Review on Current Treatment of Alopecia Areata and Newer Therapeutic Options. Int. J. Trichol. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gupta, M.; Rani, R. Delineating Injectable Triamcinolone-Induced Cutaneous Atrophy and Therapeutic Options in 24 Patients-A Retrospective Study. Indian Dermatol. Online J. 2022, 13, 199–206. [Google Scholar] [CrossRef]
- Alkhalifah, A. Alopecia Areata Update. Dermatol. Clin. 2013, 31, 93–108. [Google Scholar] [CrossRef]
- Burton, J.L.; Shuster, S. Large doses of glucocorticoid in the treatment of alopecia areata. Acta Derm. Venereol. 1975, 55, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, M. Intralesional Steroids for Alopecia Areata. Int. J. Trichol. 2010, 2, 63–65. [Google Scholar] [CrossRef]
- Kirchner, J.T. Medical Treatments for Patients with Alopecia Areata. Am. Fam. Physician 2000, 61, 1162–1164. [Google Scholar]
- Singh, G.; Lavanya, M. Topical immunotherapy in alopecia areata. Int. J. Trichol. 2010, 2, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Keen, A. Management of alopecia areata: An update. Br. J. Med. Pract. 2012, 5, 530. [Google Scholar]
- Lee, S.; Kim, B.J.; Lee, Y.B.; Lee, W.-S. Hair Regrowth Outcomes of Contact Immunotherapy for Patients with Alopecia Areata: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1145–1151. [Google Scholar] [CrossRef]
- Sica, D.A. Minoxidil: An underused vasodilator for resistant or severe hypertension. J. Clin. Hypertens. 2004, 6, 283–287. [Google Scholar] [CrossRef]
- Fiedler-Weiss, V.C. Topical minoxidil solution (1% and 5%) in the treatment of alopecia areata. J. Am. Acad. Dermatol. 1987, 16, 745–748. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F. Alginate-based hydrogel containing minoxidil/hydroxypropyl-β-cyclodextrin inclusion complex for topical alopecia treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.F.M.; Verbinnen, A.; Bakst, A.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. An update on nanocarriers for follicular-targeted drug delivery for androgenetic alopecia topical treatment. Expert Opin. Drug Deliv. 2025, 22, 367–381. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z. Interventions for Female Pattern Hair Loss. JAMA Dermatol. 2017, 153, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Takeda, A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia. J. Dermatol. 2012, 39, 27–32. [Google Scholar] [CrossRef]
- Caserini, M.; Radicioni, M.; Leuratti, C.; Terragni, E.; Iorizzo, M.; Palmieri, R. Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. Int. J. Clin. Pharmacol. Ther. 2016, 54, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hajheydari, Z.; Akbari, J.; Saeedi, M.; Shokoohi, L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 47–51. [Google Scholar] [CrossRef]
- Arif, T.; Dorjay, K.; Adil, M.; Sami, M. Dutasteride in Androgenetic Alopecia: An Update. Curr. Clin. Pharmacol. 2017, 12, 31–35. [Google Scholar] [CrossRef]
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184. [Google Scholar] [CrossRef]
- Andrade, J.F.M.; Andrew, V.; Andrew, B.; Marcílio, C.-F.; Guilherme, M.G.; and Gratieri, T. Topical dutasteride for androgenic alopecia: Current state and prospects. Ther. Deliv. 2025, 16, 271–283. [Google Scholar] [CrossRef]
- Herz-Ruelas, M.E.; Álvarez-Villalobos, N.A.; Millán-Alanís, J.M.; de León-Gutiérrez, H.; Ocampo-Garza, S.S.; Gómez-Flores, M.; Grimalt, R. Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Ski. Appendage Disord. 2020, 6, 338–345. [Google Scholar] [CrossRef]
- Kakunje, A.; Prabhu, A.; Sindhu Priya, E.S.; Karkal, R.; Kumar, P.; Gupta, N.; Rahyanath, P.K. Valproate: It’s Effects on Hair. Int. J. Trichol. 2018, 10, 150–153. [Google Scholar] [CrossRef]
- Badria, F.A.; Fayed, H.A.; Ibraheem, A.K.; State, A.F.; Mazyed, E.A. Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics 2020, 12, 866. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.D.; Kim, B.J.; Kim, M.N.; Han, D.H. A case of androgenetic alopecia treated with valproic acid. Int. J. Dermatol. 2014, 53, e214–e215. [Google Scholar] [CrossRef]
- Khandagale, S.S.; Ratnaparkhe, C.L.; Sayyad, S.R.; Shelar, V.D.; Supekar, A.V.; Sarukh, V.S. A Review of Herbal Medications for the Treatment of Alopecia. Int. J. Ayurveda Pharma Res. 2023, 11, 5–10. [Google Scholar] [CrossRef]
- Jain, P.K.; Das, D. The wonder of herbs to treat-Alopecia. Innov. J. Med. Sci. 2016, 4, 1–6. [Google Scholar]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W. The molecular mechanism of natural products activating Wnt/β-catenin signaling pathway for improving hair loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef]
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T. Rosemary Oil vs Minoxidil 2 % for the Treatment of Androgenetic Alopecia: A Randomized Comparative Trial. Ski. Med. 2015, 13, 15–21. [Google Scholar]
- Rashid, K.; Raj, V.B.; Kumar, P.S.; Nishad, K.M. Hair care promising herbs: A review. Pharm. Res. 2020, 10, 677–688. [Google Scholar]
- Gonc, B.; Elaine, S.; Belo, D.; Gaspar, L.R. skin bioengineering techniques. Ski. Res. Technol. 2006, 12, 241–246. [Google Scholar]
- Bartere, S.A.; Malode, L.L.; Malode, G.P.; Nimbalwar, M.G.; Gulhane, C.A.; Manwar, J.V.; Bakal, R.L. Exploring the potential of herbal drugs for the treatment of hair loss. GSC Biol. Pharm. Sci. 2021, 16, 212–223. [Google Scholar] [CrossRef]
- Akhbari, M.; Firooz, A.; Rahimi, R.; Shirzad, M.; Esmaealzadeh, N.; Shirbeigi, L. The effect of an oral product containing Amla fruit (Phyllanthus emblica L.) on female androgenetic alopecia: A randomized controlled trial. J. Ethnopharmacol. 2024, 318, 116958. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Al-Obaidi, H.K. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar] [CrossRef]
- Tkachenko, E.; Okhovat, J.-P.; Manjaly, P.; Huang, K.P.; Senna, M.M.; Mostaghimi, A. Complementary and alternative medicine for alopecia areata: A systematic review. J. Am. Acad. Dermatol. 2023, 88, 131–143. [Google Scholar] [CrossRef]
- Almasri, R.S.; Bedir, A.S.; Al Raish, S.M. Comprehensive Ethnopharmacological Analysis of Medicinal Plants in the UAE: Lawsonia inermis, Nigella sativa, Ziziphus spina-christi, Allium cepa, Allium sativum, Cymbopogon schoenanthus, Matricaria aurea, Phoenix dactylifera, Portulaca oleracea, Reichardia tingitana, Salvadora persica, Solanum lycopersicum, Trigonella foenum-graecum, Withania somnifera, and Ziziphus lotus. Nutrients 2025, 17, 411. [Google Scholar] [CrossRef]
- Ezekwe, N.; King, M.; Hollinger, J.C. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: A systematic review. J. Clin. Aesthet. Dermatol. 2020, 13, 23. [Google Scholar]
- Hosny, K.M.; Rizg, W.Y.; Alhakamy, N.A.; Alamoudi, A.J.; Mushtaq, R.Y.; Safhi, A.Y. Utilization of nanotechnology and experimental design in development and optimization of Aloe vera gel loaded with Finasteride–Garlic Oil–Nanotransfersomes. J. Drug Deliv. Sci. Technol. 2022, 68, 103130. [Google Scholar] [CrossRef]
- Maluki, A.H.; DDV, F. Treatment of alopecia areata with topical garlic extract. Kufa Med. J. 2009, 12, 1. [Google Scholar]
- Hajheydari, Z.; Akbari, J.; Saidi, M.; Jamshidi, M.; Khalilian, A.R.; Maboodi, M. The effects of garlic topical gel (5%) in the treatment of alopecia areata. J. Maz. Univ. Med. Sci. 2006, 16, 9–15. [Google Scholar]
- Hajheydari, Z.; Jamshidi, M.; Akbari, J.; Mohammadpour, R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: A double-blind randomized controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 29. [Google Scholar] [CrossRef]
- Roy, R.K.; Thakur, M.; Dixit, V.K. Hair growth promoting activity of Eclipta alba in male albino rats. Arch. Dermatol. Res. 2008, 300, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Lee, M.R.; Gu, L.J.; Hossain, M.J.; Kim, H.K.; Sung, C.K. Comparative hair restorer efficacy of medicinal herb on nude (Foxn1nu) mice. BioMed Res. Int. 2014, 2014, 319795. [Google Scholar] [CrossRef]
- Hosking, A.-M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and alternative treatments for alopecia: A comprehensive review. Ski. Appendage Disord. 2019, 5, 72–89. [Google Scholar] [CrossRef]
- Ashique, S.; Sandhu, N.K.; Haque, S.N.; Koley, K. A systemic review on topical marketed formulations, natural products, and oral supplements to prevent androgenic alopecia: A review. Nat. Prod. Bioprospect. 2020, 10, 345–365. [Google Scholar] [CrossRef]
- Semwal, B.C.; Agrawal, K.K.; Singh, K.; Tandon, S.; Sharma, S. Alopecia: Switch to herbal medicine. J. Pharm. Res. Opin. 2011, 1, 101–104. [Google Scholar]
- Won, H.J.; Kim, T.M.; An, I.; Bae, H.J.; Park, S.Y. Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties. Biomimetics 2023, 8, 296. [Google Scholar] [CrossRef]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 2288. [Google Scholar] [CrossRef]
- Yu, J.Y.; Gupta, B.; Park, H.G.; Son, M.; Jun, J.-H.; Yong, C.S.; Kim, J.A.; Kim, J.O. Preclinical and Clinical Studies Demonstrate That the Proprietary Herbal Extract DA-5512 Effectively Stimulates Hair Growth and Promotes Hair Health. Evid. Based. Complement. Alternat. Med. 2017, 2017, 4395638. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Development and clinical evaluation of green tea hair tonic for greasy scalp treatment. J. Cosmet. Sci. 2016, 67, 161–166. [Google Scholar]
- Rambwawasvika, H.; Dzomba, P.; Gwatidzo, L. Alopecia types, current and future treatment. J. Dermatol. Cosmetol. 2021, 5, 93–99. [Google Scholar] [CrossRef]
- Imtiaz, F.; Islam, M.; Saeed, H.; Saleem, B.; Asghar, M.; Saleem, Z. Impact of Trigonella foenum-graecum leaves extract on mice hair growth. Pak. J. Zool. 2017, 49, 1405–1412. [Google Scholar] [CrossRef]
- Yusharyahya, S.N. Potential role of fenugreek (Trigonella foenumgraecum) in the prevention of skin aging. J. Med. Sci. 2020, 53, 78–86. [Google Scholar]
- Ghosh, B.; Chandra, I.; Chatterjee, S. Fenugreek (Trigonella foenum-graecum L.) and its necessity. Fire J. Eng. Technol. 2015, 1, 60–67. [Google Scholar]
- Schulz, C.; Bielfeldt, S.; Reimann, J. Fenugreek+ micronutrients: Efficacy of a food supplement against hair loss. Kosmet. Med. 2006, 27, 176. [Google Scholar]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Neupane, P.; Shah, K. Clinical Study to Evaluate the Efficacy and Safety of a Hair Serum Product in Healthy Adult Male and Female Volunteers with Hair Fall. Clin. Cosmet. Investig. Dermatol. 2020, 13, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Vaidyanathan, P. News from Sabinsa on Hair Care. Euro Cosmet. 2014, 10, 23–24. [Google Scholar]
- Dulal, M.S.R.; Sheikh, H.; Taher, M.A.; Rahaman, M.S.U.; Rahman, Z.; Malek, M.A. Formulation and finding out the efficacy of the herbal hair oil over simple coconut oil (purified)-A formulation and clinical study in Bangladesh. Int. J. Pharm. Sci. Res. 2014, 5, 1801. [Google Scholar]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Cohen, D.E.; Klaassen, C.D.; Rettie, A.E.; Ross, D.; Slaga, T.J.; Snyder, P.W.; Tilton, S. Prunus Amygdalus Dulcis (Sweet Almond) Seed Meal. Int. J. Toxicol. 2023, 42, 93S–95S. [Google Scholar] [CrossRef]
- Singhal, P.; Vyas, V.; Chhayani, P.; Patel, M.; Gupta, S.N. Ayurvedic management of alopecia areata: A case report. J. Ayurveda Integr. Med. 2022, 13, 100604. [Google Scholar] [CrossRef]
- Sajikumar, S.; Rajeshkumar, A.; Sundaram, M.; Ramasamy, K.M.S. Effectiveness, safety and tolerability of dheedhi herbal shampoo against alopecia and seborrheic dermatitis-a clinical perspective. J. Ayurvedic Herb. Med. 2020, 6, 145–148. [Google Scholar] [CrossRef]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Mishra, Y.; Amin, H.I.M.; Mishra, V.; Vyas, M.; Prabhakar, P.K.; Gupta, M.; Kanday, R.; Sudhakar, K.; Saini, S.; Hromić-Jahjefendić, A.; et al. Application of nanotechnology to herbal antioxidants as improved phytomedicine: An expanding horizon. Biomed. Pharmacother. 2022, 153, 113413. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; De Luise, A.; Petillo, O.; Calarco, A.; Peluso, G. New therapeutic potentials of nanosized phytomedicine. J. Nanosci. Nanotechnol. 2016, 16, 8176–8187. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Health Part C 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Suzuki, K.; Derek, T.; Ozeki, M.; Okubo, T. Clinical evaluation of Emblica Officinalis Gatertn (Amla) in healthy human subjects: Health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study. Contemp. Clin. Trials Commun. 2020, 17, 100499. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; White, I.R.; Rycroft, R.J.G. Allergic contact dermatitis from garlic. Contact Dermat. 1992, 27, 333. [Google Scholar] [CrossRef]
- Borrelli, F.; Capasso, R.; Izzo, A.A. Garlic (Allium sativum L.): Adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 2007, 51, 1386–1397. [Google Scholar] [CrossRef]
- Qadri, N.M.; Ahmad, S.; Qureshi, S.; Badar, Y. Acute toxicological evaluation of the aqueous extract of Eclipta alba Hassk. Biol. Sci. 2001, 44, 38–41. [Google Scholar]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef]
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological properties of fenugreek (Trigonella foenum graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Heidari-Soureshjani, S.; Asadi-Samani, M.; Yang, Q. A systematic review of phytochemical and phytotherapeutic characteristics of bitter almond. Int. J. Pharm. Phytopharm. Res. 2017, 7, 1–9. [Google Scholar]
- Soundran, V.; Namagiri, T.; Manonayaki, S.; Vanithakumari, G. Hepatotoxicity of eugenol. Anc. Sci. Life 1994, 13, 213–217. [Google Scholar]
- Shaikh, Z.S.; Patel, B.A.; Patil, S.G.; Maniyar, A.R. Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia. Mater. Proc. 2023, 14, 57. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Butler, K.S.; Durfee, P.N.; Theron, C.; Ashley, C.E.; Carnes, E.C.; Brinker, C.J. Protocells: Modular mesoporous silica nanoparticle-supported lipid bilayers for drug delivery. Small 2016, 12, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.; Udupa, N. Formulation and Evaluation of Oral and Transdermal Preparations of Flurbiprofen and Piroxicam Incorporated with Different Carriers. Drug Dev. Ind. Pharm. 1993, 19, 843–852. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Parichatikanond, W.; Junyaprasert, V.B.; Morakul, B. Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects. Pharmaceuticals 2022, 15, 930. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Chang, D.; Wei, Z.; Wang, E.; Yu, J.; Xu, Y.; Que, Y.; Chen, Y.; Fan, C.; et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact. Mater. 2023, 24, 81–95. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Lim, S.K.; De Hoog, H.-P.; Parikh, A.N.; Nallani, M.; Liedberg, B. Hybrid, Nanoscale Phospholipid/Block Copolymer Vesicles. Polymers 2013, 5, 1102–1114. [Google Scholar] [CrossRef]
- Lenin, D.; Monika, K.; Shankar, P.R. Phospholipid-polymer hybrid nanoparticles mediated transfollicular delivery of Quercetin: Prospective implement for the treatment of androgenic alopecia. Drug Dev. Ind. Pharm. 2019, 45, 1654–1663. [Google Scholar]
- Hariyanti, H.; Mauludin, R.; Sumirtapura, Y.C.; Kurniati, N.F. Activity and Safety of Cinchonine Nanostructured Lipid Carriers as a Hair Growth Stimulant in Mice Model of Androgenetic Alopecia. Sains Malays. 2023, 52, 1671–1683. [Google Scholar] [CrossRef]
- Kumar, N.; Chaiyasut, C. Hair growth promoting activity of Carthamus tinctorius florets extract-loaded nanostructured lipid carriers. Int. J. Pharm. Pharm. Sci. 2015, 7, 252–257. [Google Scholar]
- Singh, S.; Sindhu, R.K.; Alsayegh, A.A.; Batiha, G.E.; Alotaibi, S.S.; Albogami, S.M.; Conte-junior, C.A. Formulation Development and Investigations on Therapeutic Potential of Nanogel from Beta vulgaris L. extract in testosterone-induced alopecia. BioMed Res. Int. 2023, 2023, 1777631. [Google Scholar] [CrossRef] [PubMed]
- Prabahar, K.; Udhumansha, U.; Elsherbiny, N.; Qushawy, M. Microneedle mediated transdermal delivery of β-sitosterol loaded nanostructured lipid nanoparticles for androgenic alopecia. Drug Deliv. 2022, 29, 3022–3034. [Google Scholar] [CrossRef]
- Madhunithya, E.; Venkatesh, G.; Shyamala, G.; Manjari, V. Development of ethosome comprising combined herbal extracts and its effect on hair growth. Adv. Tradit. Med. 2021, 21, 131–141. [Google Scholar] [CrossRef]
- Hwang, S.L.; Kim, J. In vivo hair growth promotion effects of cosmetic preparations containing hinokitiol-loaded poly (e-caprolacton) nanocapsules. J. Microencapsul. 2008, 25, 351–356. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Fu, L.; Wang, H.; Qi, F.; Jiang, Y.; Meng, F.; Wu, M.; Shi, Z.; Yang, G. Glutamic acid-loaded separable microneedle composite for long-acting hair regeneration treatment. Adv. Compos. Hybrid Mater. 2025, 8, 187. [Google Scholar] [CrossRef]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. ACS Biomater. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, F.; Wang, J.; Zhang, Y.; Zhang, Y.; Su, G.; Zhao, Y. Hair growth promoting activity of cedrol nanoemulsion in c57bl/6 mice and its bioavailability. Molecules 2021, 26, 1795. [Google Scholar] [CrossRef]
- Liu, Z.; He, Z.; Ai, X.; Guo, T.; Feng, N. Cardamonin-loaded liposomal formulation for improving percutaneous penetration and follicular delivery for androgenetic alopecia. Drug Deliv. Transl. Res. 2024, 14, 2444–2460. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Usmani, S.; Sufiyan, M.; Singh, P. Innovating alopecia treatment: Nanostructured lipid carriers as advanced delivery platforms. In Naunyn-Schmiedeberg’s Arch Pharmacol; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Ghanbarzadeh, S.; Sepehran, S.; Javadzadeh, Y.; Adib, Z.M.; Kouhsoltani, M. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: Potential tool for the treatment of androgenic alopecia. Drug Dev. Ind. Pharm. 2016, 42, 846–853. [Google Scholar] [CrossRef]
- Daneshmand, S.; Niazi, M.; Fazeli-Nasab, B.; Asili, J.; Golmohammadzadeh, S.; Sayyed, R.Z. Solid Lipid Nanoparticles of Platycladus orientalis L. possessing 5-alpha Reductase Inhibiting Activity for Treating Hair Loss and Hirsutism. J. Med. Plants By-Prod. 2024, 13, 233–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Chen, S.-S.; Guan, J.; Qu, F.-Z.; Zhao, Y.-Q. Hair growth promoting activity of cedrol isolated from the leaves of Platycladus orientalis. Biomed. Pharmacother. 2016, 83, 641–647. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-L.; A Al-Suwayeh, S.; Fang, J.-Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef]
- Chamel, S.; Mishra, A.; Gull, A. Transferosomes as innovative drug delivery systems for enhanced antifungal therapy: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105545. [Google Scholar] [CrossRef]
- Rahman, M.; Indabawa, A.H.; Alam, K.; Beg, S.; Sahoo, A. Chapter 15—Transferosomes drug delivery in topical infectious disorders. In Nanostructured Drug Delivery Systems in Infectious Disease Treatment; Beg, S., Shukla, R., Handa, M., Rahman, M., Dhir, A., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 351–367. ISBN 978-0-443-13337-4. [Google Scholar]
- Opatha, S.A.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, A.; Leanpolchareanchai, J.; Morakul, B.; Parichatikanond, W.; Teeranachaideekul, V. Phyllanthus emblica Extract-loaded Transfersomes for Hair Follicle Targeting: Phytoconstituents, Characterization, and Hair Growth Promotion. J. Oleo Sci. 2022, 71, 1085–1096. [Google Scholar] [CrossRef]
- Mohanty, D.; Mounika, A.; Bakshi, V.; Haque, M.A.; Sahoo, C.K. Ethosomes: A novel approach for transdermal drug delivery. Int. J. ChemTech Res. 2018, 11, 219–226. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef]
- Ainbinder, D.; Paolino, D.; Fresia, M.; Touitou, E. Drug delivery applications with ethosomes. J. Biomed. Nanotechnol. 2010, 6, 558. [Google Scholar] [CrossRef]
- Satyam, G.; Shivani, S.; Garima, G. Ethosomes: A novel tool for drug delivery through the skin. J. Pharm. Res. 2010, 3, 688–691. [Google Scholar]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Elnady, R.E.; Amin, M.M.; Zakaria, M.Y. Phytomedicinal flavonoid loaded phospholipid sheathed lipidic nano-carriers as a platform with boosted oral anti-mycobacterium activity. J. Drug Deliv. Sci. Technol. 2023, 87, 104775. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Yao, B.; Hu, T.; Li, Z.; Huang, S.; Fu, X. Beyond 2D: 3D bioprinting for skin regeneration. Int. Wound J. 2019, 13, 134–138. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, H.; Liu, B.; Xu, T.; Zhang, Y. Adaptive multi-degree-of-freedom in situ bioprinting robot for hair-follicle-inclusive skin repair: A preliminary study conducted in mice. Bioeng. Transl. Med. 2022, 7, e103036. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Gao, T.; Zhao, W.; Xu, T.; Liu, Z. Robot-assisted in situ bioprinting of gelatin methacrylate hydrogels with stem cells induces hair follicle-inclusive skin regeneration. Biomed. Pharmacother. 2023, 158, 114140. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhou, Z.; Qian, X.; Shen, H.; Cheng, H.; Zhang, J. Functional regeneration strategies of hair follicles: Advances and challenges. Stem Cell Res. Ther. 2025, 16, 77. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, X.; Lv, S.; Yang, C.; Wang, Z.; Liao, M.; Zhou, B.; Zhang, Y.; Sun, S.; Chen, P.; et al. 3D bioprinting of prefabricated artificial skin with multicomponent hydrogel for skin and hair follicle regeneration. Theranostics 2025, 15, 2933–2950. [Google Scholar] [CrossRef]
- Kang, Y.; Yeo, M.; Derman, I.D.; Ravnic, D.J.; Singh, Y.P.; Alioglu, M.A.; Wu, Y.; Makkar, J.; Driskell, R.R.; Ozbolat, I.T. Intraoperative bioprinting of human adipose-derived stem cells and extra-cellular matrix induces hair follicle-like downgrowths and adipose tissue formation during full-thickness craniomaxillofacial skin reconstruction. Bioact. Mater. 2024, 33, 114–128. [Google Scholar] [CrossRef]
- Motter Catarino, C.; Cigaran Schuck, D.; Dechiario, L.; Karande, P. Incorporation of hair follicles in 3D bioprinted models of human skin. Sci. Adv. 2023, 9, eadg0297. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Kim, J.; Jeon, J.S.; Park, J.; Yi, H. Bioprinting Methods for Fabricating In Vitro Tubular Blood Vessel Models. Cyborg Bionic Syst. 2023, 4, 43. [Google Scholar] [CrossRef]
- Aliyazdi, S.; Frisch, S.; Neu, T.; Veldung, B.; Karande, P.; Schaefer, U.F.; Loretz, B.; Vogt, T.; Lehr, C.-M. A Novel 3D Printed Model of Infected Human Hair Follicles to Demonstrate Targeted Delivery of Nanoantibiotics. ACS Biomater. Sci. Eng. 2024, 10, 4947–4957. [Google Scholar] [CrossRef]
- Nanmo, A.; Yan, L.; Asaba, T.; Wan, L.; Kageyama, T.; Fukuda, J. Bioprinting of hair follicle germs for hair regenerative medicine. Acta Biomater. 2023, 165, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Fatima, F.; Alnami, A.; Alsenaidy, M.; Aodah, A.H.; Aldawsari, M.F.; Almutairy, B.; Anwer, M.K.; Jafar, M. Design and Characterization of Baricitinib Incorporated PLA 3D Printed Pills by Fused Deposition Modeling: An Oral Pill for Treating Alopecia Areata. Polymers 2023, 15, 1825. [Google Scholar] [CrossRef]
- Wu, J.; Ma, J.; Zhuang, H.; Ma, H.; Wu, C. 3D bioprinting of calcium molybdate nanoparticles-containing immunomodulatory bioinks for hair regrowth. Nano Today 2023, 51, 101917. [Google Scholar] [CrossRef]
- Kim, B.R.; Min Jae, K.; Jieun, K.; Hwa-Jung, C.; Kyung Ho, P.; Soon Hyo, K.; Hye-Ryung, C.; Chang Hun, H.; Jung Won, S.; Dong-sun, P.; et al. Artificial intelligence-based prescription of personalized scalp cosmetics improved the scalp condition: Efficacy results from 100 participants. J. Dermatol. Treat. 2024, 35, 2337908. [Google Scholar] [CrossRef]
- Farooq, S.A.; Ali, A.; Bashir, A. The prediction of hairfall pattern in a person using artificial intelligence for better care and treatment. In Proceedings of the 2024 4th International Conference on Innovative Practices in Technology and Management (ICIPTM), Noida, India, 21–23 February 2024; pp. 1–6. [Google Scholar]
- Zhou, Q.; Lan, L.; Wang, W.; Xu, X. Identifying effective immune biomarkers in alopecia areata diagnosis based on machine learning methods. BMC Med. Inform. Decis. Mak. 2025, 25, 23. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, Y.; Hao, K.; Liu, C.; Yu, R.; Huang, A.; Wu, C.; Yang, Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, 2306360. [Google Scholar] [CrossRef]
- Rezapour Sarabi, M.; Alseed, M.M.; Karagoz, A.A.; Tasoglu, S. Machine Learning-Enabled Prediction of 3D-Printed Microneedle Features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef]
- He, W.; Kong, S.; Lin, R.; Xie, Y.; Zheng, S.; Yin, Z.; Huang, X.; Su, L.; Zhang, X. Machine Learning Assists in the Design and Application of Microneedles. Biomimetics 2024, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.A.; Dhondale, M.R.; Agrawal, A.K.; Serrano, D.R.; Mishra, B.; Kumar, D. Advancements in microneedle fabrication techniques: Artificial intelligence assisted 3D-printing technology. Drug Deliv. Transl. Res. 2024, 14, 1458–1479. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Wu, S.J.; Wu, J.; Kaser, S.J.; Roh, H.; Shiferaw, R.D.; Yuk, H.; Zhao, X. A 3D printable tissue adhesive. Nat. Commun. 2024, 15, 1215. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Gao, Y.; Mathivanan, S.; Kong, L.; Tao, Y.; Dong, Y.; Li, X.; Bhattacharyya, A.; Zhao, X.; et al. 3D bioprinting of human neural tissues with functional connectivity. Cell Stem Cell 2024, 31, 260–274. [Google Scholar] [CrossRef]
- Fang, J.-H.; Liu, C.-H.; Hsu, R.-S.; Chen, Y.-Y.; Chiang, W.-H.; Wang, H.-M.D.; Hu, S.-H. Transdermal Composite Microneedle Composed of Mesoporous Iron Oxide Nanoraspberry and PVA for Androgenetic Alopecia Treatment. Polymers 2020, 12, 1392. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Li, Z.; Jiao, S.; Xiao, T.; Wu, Y.; Chen, C.; Guo, S.; Li, X.; Pan, Z.; Li, J.; et al. Black phosphorus nanosheets encapsulated microneedle for multifunctional therapy for androgenic alopecia. J. Nanobiotechnol. 2025, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Liu, Z.; Guo, T.; Ai, X.; He, Y.; Hou, X.; Feng, N. Synergistic treatment of androgenetic alopecia with follicular co-delivery of minoxidil and cedrol in metal–organic frameworks stabilized by covalently cross-linked cyclodextrins. Int. J. Pharm. 2024, 654, 123948. [Google Scholar] [CrossRef]
- Ananth, P.; Koland, M. Topical Delivery of Fenugreek Seed Extract Loaded Solid Lipid Nanoparticles Based Hydrogels for Alopecia. J. Pharm. Res. Int. 2021, 33, 231–241. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnady, R.E.; Abdon, M.S.; Shaheen, H.R.; Eladawy, R.M.; Azar, Y.O.; Al Raish, S.M. The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus. Pharmaceutics 2025, 17, 584. https://doi.org/10.3390/pharmaceutics17050584
Elnady RE, Abdon MS, Shaheen HR, Eladawy RM, Azar YO, Al Raish SM. The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus. Pharmaceutics. 2025; 17(5):584. https://doi.org/10.3390/pharmaceutics17050584
Chicago/Turabian StyleElnady, Rana E., Manar S. Abdon, Hagar R. Shaheen, Reem M. Eladawy, Yasmena O. Azar, and Seham M. Al Raish. 2025. "The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus" Pharmaceutics 17, no. 5: 584. https://doi.org/10.3390/pharmaceutics17050584
APA StyleElnady, R. E., Abdon, M. S., Shaheen, H. R., Eladawy, R. M., Azar, Y. O., & Al Raish, S. M. (2025). The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus. Pharmaceutics, 17(5), 584. https://doi.org/10.3390/pharmaceutics17050584





