Use of Computational Intelligence in Customizing Drug Release from 3D-Printed Products: A Comprehensive Review
Abstract
1. Introduction
2. Limitations of 3D Printing for Drug Delivery Systems Manufacturing
3. Role of Computational Intelligence in Addressing Limitations of 3D Printing
| 3D Printer | Models Used in This Study | Purpose of the CI | Reference |
|---|---|---|---|
| Binder jetting | U-Net | Determine drug distribution in printed tablets | [11] |
| Bioprinter | nnU-Net | Medical image segmentation | [66] |
| DT, MLR | Predict shape recovery ratio | [67] | |
| 3D printing and neural network co-modelling | Model the relationship between electric field imaging and electroanatomical features of cochlear implants | [68] | |
| ANN | Design and optimize pseudo-bone drug delivery scaffold for controlled release of simvastatin | [69] | |
| Regression neural network, classification neural network, Bayesian optimization | Predict cell viability | [70] | |
| Hierarchical machine learning | Optimize and predict print outcomes | [71] | |
| Relative least general generalization algorithm, MLR | Predict printability based on ink composition | [72] | |
| SVM, convolutional neural network (CNN) | Anomaly detection | [73] | |
| RF | Predict printability from rheological data | [74] | |
| Direct ink writing printer | DT, RF, DL | Predict printability of hydrogel formulations | [75] |
| PCA, linear discriminant analysis (LDA), partial least square (PLS), k-means, density-based spatial clustering of applications with noise, hierarchical clustering, t-distributed stochastic neighbor embedding | Verify drug and dose of orodispersible films | [76] | |
| DLP | CNN | Assess print fidelity and uniformity | [77] |
| SVM, multiple regression analysis | Predict drug release | [78] | |
| ANN | Optimize and predict ibuprofen release | [79] | |
| Self-organizing maps, generalized regression neural network | Predict the influence of tablet thickness on release rates | [19] | |
| FDM | MLR, DT, SVM, partial least squares | Predict drug dissolution profiles from rheological data | [80] |
| Evolutionary algorithm | Identify structures for a prescribed drug release profile | [81] | |
| GAs | Optimize capsule geometry for desired release profiles | [82] | |
| GAN, a bag of features | Generate 3D porous structures | [83] | |
| Multivariate linear regression, k-nearest neighbor, SVM, RF, (traditional) neural networks, DL | Predict key fabrication parameters (e.g., temperature, filament characteristics) | [61] | |
| ANN | Geometry classification and surface area-to-volume ratio prediction | [84] | |
| RF, k-nearest neighbor, ANN, SVM, logistic regression | Predict hot melt extrusion temperature, filament properties, printability, dissolution time | [85] | |
| Stochastic gradient descent, DT, Naïve Bayes (NB), multilayer perceptron, SVM, PCA, CNN | Quality control and anomaly detection for fabricated microneedles | [2] | |
| Extreme gradient-boosted trees | Predict personalized insulin dosages for IoT-reconfigurable system | [37] | |
| RF, light gradient-boosting machine, DT, extreme gradient boosted trees, SVR, k-nearest neighbor, kernel ridge regression, multilayer perceptron | Forecast CO2 emissions from pharmaceutical FDM printing | [86] | |
| DT | Relate mechanical properties of filaments to printability | [87] | |
| Self-organizing maps, multilayer perceptron | Predict drug release properties | [88] | |
| GAN | Create new formulations for 3D printing | [89] | |
| ANN, SVM, RF | Predict printability and filament properties | [90] | |
| PCA | Compare the printability of the polymer and identify the contribution of each mechanical property | [91] | |
| Inkjet printing | ANN, SVM, RF | Develop predictive models for printing outcomes | [92] |
| SLA | Machine vision, SVM, k-nearest neighbor, logistic regression, DT, RF, gradient-boosting, multilayer perceptron | Anomaly detection for quality control | [38] |
| Semi-solid extrusion (SSE) | SVM, Gaussian model, DT | Optimizing 3D printing parameters | [93] |
| SLS | PCA, t-distributed stochastic neighbor embedding, RF, logistic regression, SVM, gradient boosting, extreme gradient boosting, DT, multilayer perceptron, k-nearest neighbor, extremely randomized trees | Predict printability of formulations | [94] |
| Deep ensembles, extreme gradient boosting | Predict printability of SLS formulations | [21] | |
| Sprayed Multi Adsorbed Droplet Reposting Technology (SMART) | DT, RF, k-nearest neighbor, light gradient boosting machine, extreme gradient boost | Identify factors and predict drug loading efficiency of microparticles | [95] |
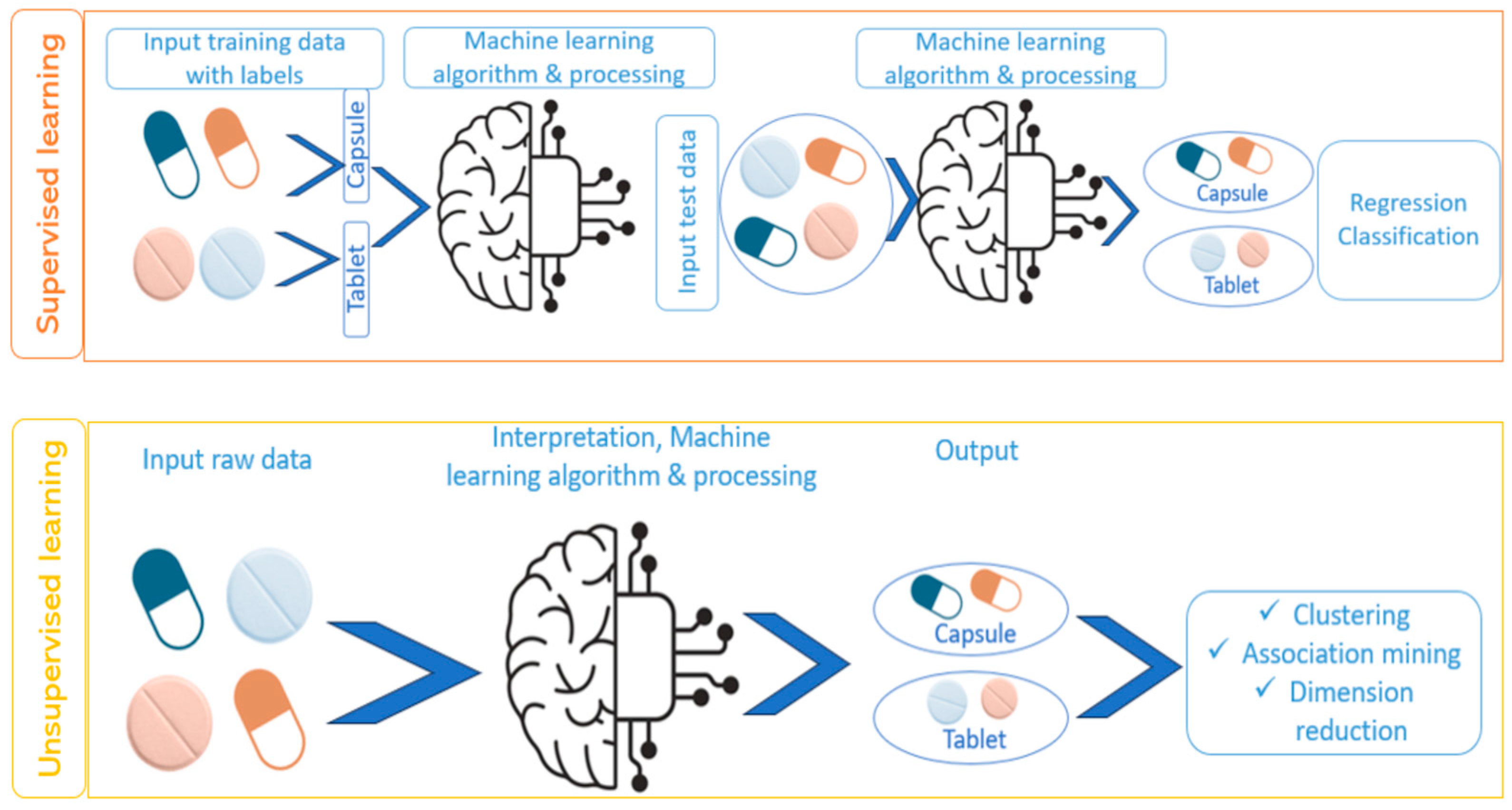
4. Classification of Machine-Learning Techniques
4.1. Implementation of Machine-Learning Techniques
4.2. Evaluation of the Performance of Machine-Learning Models
5. Machine-Learning Techniques for Customizing Drug Release from 3D-Printed Pharmaceuticals
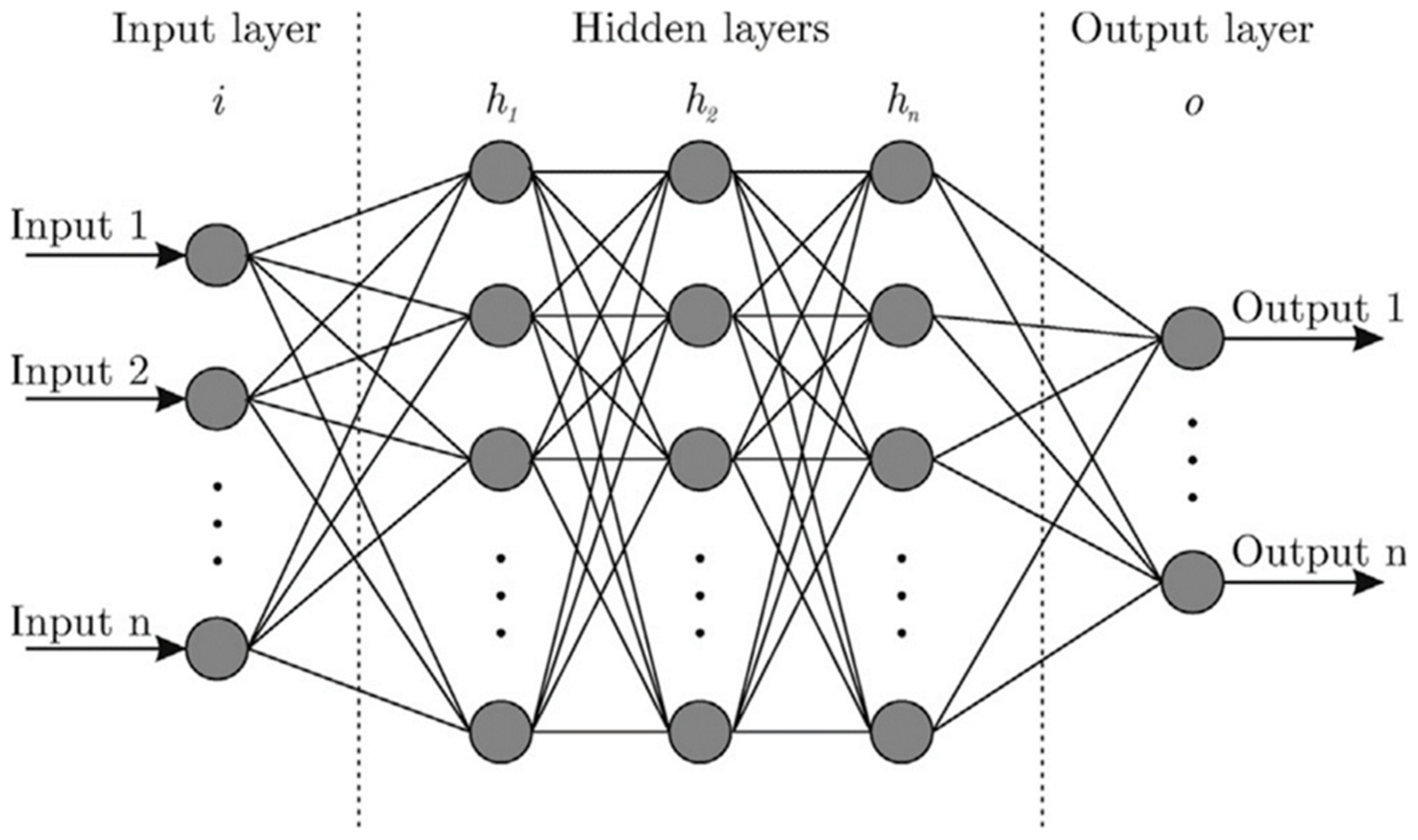
5.1. Artificial Neural Networks for Predicting Drug Release Profiles
5.2. Genetic Algorithms for Optimizing Dosage Form Geometry for Desired Drug Release Profile
5.3. Other Machine-Learning Techniques for Drug Dissolution Prediction
6. Real-World Examples of Computational Intelligence Integration in Pharmaceutical 3D Printing
7. Other Methods for Optimizing Drug Release from 3D-Printed Products
7.1. Design of Experiments
7.2. Finite-Element Analysis
7.3. Mechanism-Based Kinetic Modeling
8. Comparison of Computational Intelligence with Other Methods for Optimizing Drug Release
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Suriyaamporn, P.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. The Artificial Intelligence-Powered New Era in Pharmaceutical Research and Development: A Review. AAPS PharmSciTech 2024, 25, 188. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, M.R.; Alseed, M.M.; Karagoz, A.A.; Tasoğlu, S. Machine Learning-Enabled Prediction of 3D-Printed Microneedle Features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, X.; Ouyang, D.; Williams, R.O., III. Emerging Artificial Intelligence (AI) Technologies Used in the Development of Solid Dosage Forms. Pharmaceutics 2022, 14, 2257. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Lee, J.C.; Lee, K.; Han, H.-K.; Kim, K.H.; Jeong, S.H. Recent trends and perspectives of artificial intelligence-based machine learning from discovery to manufacturing in biopharmaceutical industry. J. Pharm. Investig. 2023, 53, 803–826. [Google Scholar] [CrossRef]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Sultana, A.; Maseera, R.; Rahamanulla, A.; Misiriya, A. Emerging of artificial intelligence and technology in pharmaceuticals: Review. Future J. Pharm. Sci. 2023, 9, 65. [Google Scholar] [CrossRef]
- Ali, K.A.; Mohin, S.K.; Mondal, P.; Goswami, S.; Ghosh, S.; Choudhuri, S. Influence of artificial intelligence in modern pharmaceutical formulation and drug development. Future J. Pharm. Sci. 2024, 10, 53. [Google Scholar] [CrossRef]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in artificial intelligence for drug delivery and development: A comprehensive review. Comput. Biol. Med. 2024, 178, 108702. [Google Scholar] [CrossRef]
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Hase, F.; Aspuru-Guzik, A.; Allen, C. Machine learning directed drug formulation development. Adv. Drug Deliv. Rev. 2021, 175, 113806. [Google Scholar] [CrossRef]
- Bao, Z.; Bufton, J.; Hickman, R.J.; Aspuru-Guzik, A.; Bannigan, P.; Allen, C. Revolutionizing Drug Formulation Development: The Increasing Impact of Machine Learning. Adv. Drug Deliv. Rev. 2023, 202, 115108. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, J.; Jiang, J.; Zhang, Y.; Giri, B.R.; Kulkarni, V.R.; Aghda, N.H.; Wang, J.; Maniruzzaman, M. Novel 3D Printed Modular Tablets Containing Multiple Anti-Viral Drugs: A Case of High Precision Drop-on-Demand Drug Deposition. Pharm. Res. 2022, 39, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rajendran, K.; Yogeshwaran, V.N.; Radhakrishnan, B. The Influence of Artificial Intelligence in Drug Discovery and Development. In Role of Artificial Intelligence, Telehealth, and Telemedicine in Medical Virology; Chatterjee, J.M., Sujatha, R., Saxena, S.K., Eds.; Springer Nature: Singapore, 2024; pp. 53–81. [Google Scholar]
- Khan, O.; Parvez, M.; Kumari, P.; Parvez, S.; Ahmad, S. The future of pharmacy: How AI is revolutionizing the industry. Intell. Pharm. 2023, 1, 32–40. [Google Scholar] [CrossRef]
- Yang, S.; Kar, S. Application of artificial intelligence and machine learning in early detection of adverse drug reactions (ADRs) and drug-induced toxicity. Artif. Intell. Chem. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; McCoubrey, L.E.; Elbadawi, M.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advancing pharmacy and healthcare with virtual digital technologies. Adv. Drug Deliv. Rev. 2022, 182, 114098. [Google Scholar] [CrossRef]
- Mosley-Kellum, K.; Bagde, A.; Spencer, S.; Dev, S.; Singh, M. Development of 3D DLP Printed Sustained Release Ibuprofen Tablets and Their Pharmacokinetic Evaluation in Rats. AAPS PharmSciTech 2023, 24, 88. [Google Scholar] [CrossRef]
- Digkas, T.; Porfire, A.; Van Renterghem, J.; Samaro, A.; Borodi, G.; Vervaet, C.; Crișan, A.G.; Iurian, S.; De Beer, T.; Tomuta, I. Development of Diclofenac Sodium 3D Printed Cylindrical and Tubular-Shaped Tablets through Hot Melt Extrusion and Fused Deposition Modelling Techniques. Pharmaceuticals 2023, 16, 1062. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef]
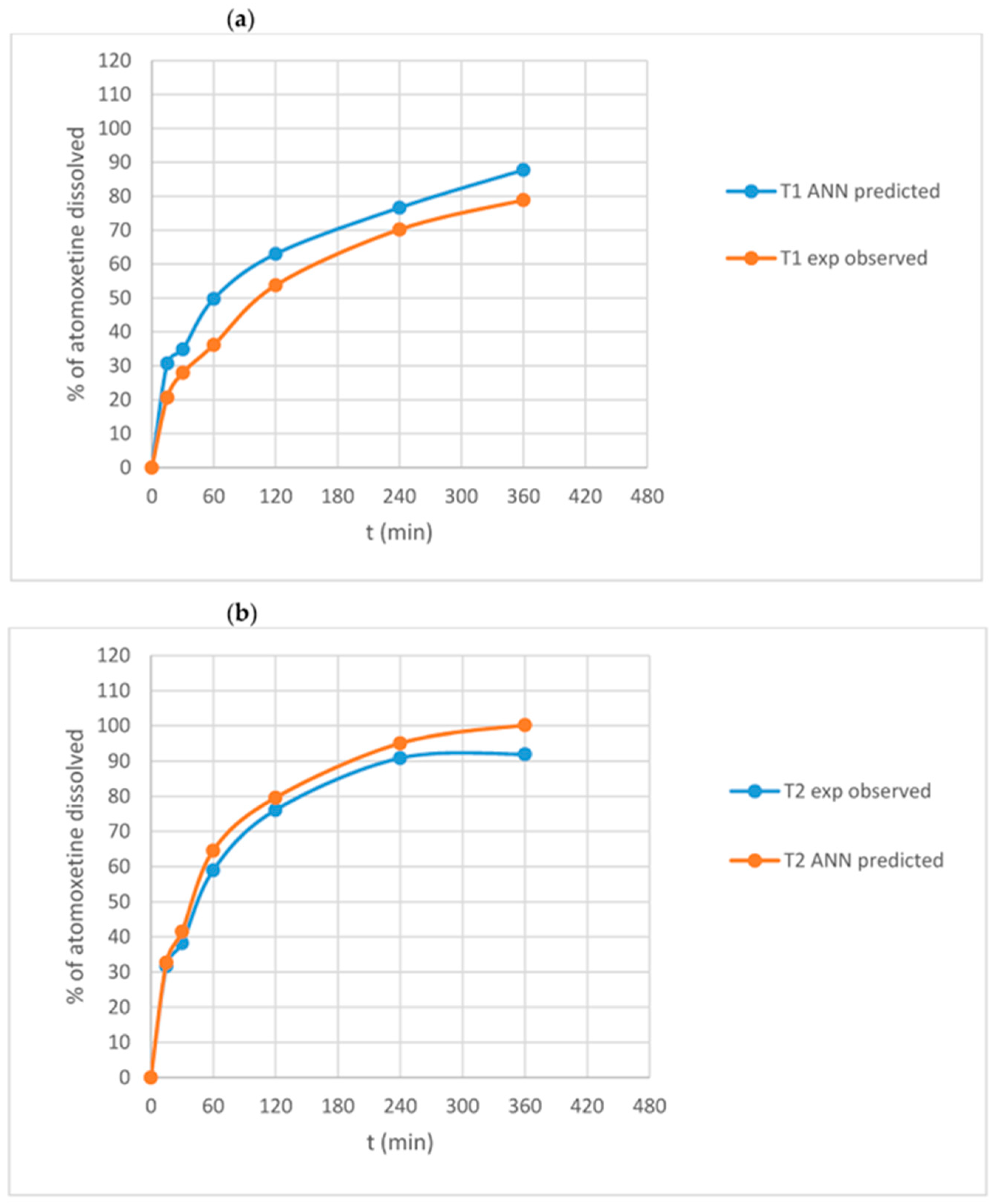
- Stanojevic, G.; Medarevic, D.; Adamov, I.; Pesic, N.; Kovacevic, J.; Ibric, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2021, 26, 11. [Google Scholar] [CrossRef]
- Xu, X.; Seijo-Rabina, A.; Awad, A.; Rial, C.; Gaisford, S.; Basit, A.W.; Goyanes, A. Smartphone-enabled 3D printing of medicines. Int. J. Pharm. 2021, 609, 121199. [Google Scholar] [CrossRef]
- Abdalla, Y.; Ferianc, M.; Awad, A.; Kim, J.; Elbadawi, M.; Basit, A.W.; Orlu, M.; Rodrigues, M. Smart laser Sintering: Deep Learning-Powered powder bed fusion 3D printing in precision medicine. Int. J. Pharm. 2024, 661, 124440. [Google Scholar] [CrossRef]
- Milliken, R.L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. Application of 3D printing in early phase development of pharmaceutical solid dosage forms. Int. J. Pharm. 2024, 653, 123902. [Google Scholar] [CrossRef]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.N.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, X.; Wang, M.; Wang, L. A New Model of a 3D-Printed Shell with Convex Drug Release Profile. Dissolut. Technol. 2018, 25, 24–28. [Google Scholar] [CrossRef]
- Ragelle, H.; Rahimian, S.; Guzzi, E.A.; Westenskow, P.D.; Tibbitt, M.W.; Schwach, G.; Langer, R. Additive manufacturing in drug delivery: Innovative drug product design and opportunities for industrial application. Adv. Drug Deliv. Rev. 2021, 178, 113990. [Google Scholar] [CrossRef]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication 2024, 16, 32001. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pombo, L.; Carou-Senra, P.; Rodríguez-Martínez, E.; Januskaite, P.; Rial, C.; Félix, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Customizable orodispersible films: Inkjet printing and data matrix encoding for personalized hydrocortisone dosing. Int. J. Pharm. 2024, 655, 124005. [Google Scholar] [CrossRef]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed Oral Dosage Forms: Mechanical Properties, Computational Approaches and Applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Paccione, N.; Guarnizo-Herrero, V.; Ramalingam, M.; Larrarte, E.; Pedraz, J.L. Application of 3D printing on the design and development of pharmaceutical oral dosage forms. J. Control. Release 2024, 373, 463–480. [Google Scholar] [CrossRef]
- Awad, A.; Goyanes, A.; Basit, A.W.; Zidan, A.S.; Xu, C.; Li, W.; Narayan, R.J.; Chen, R.K. A Review of State-of-the-Art on Enabling Additive Manufacturing Processes for Precision Medicine. J. Manuf. Sci. Eng. 2022, 145, 010802. [Google Scholar] [CrossRef]
- Sultana, N.; Ali, A.; Waheed, A.; Aqil, M. 3D Printing in pharmaceutical manufacturing: Current status and future prospects. Mater. Today Commun. 2024, 38, 107987. [Google Scholar] [CrossRef]
- Poudel, I.; Mita, N.; Babu, R.J. 3D printed dosage forms, where are we headed? Expert Opin. Drug Deliv. 2024, 21, 1595–1614. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Isreb, M.; Forbes, R.T.; Oga, E.F.; Alhnan, M.A. Additive Manufacturing of a Point-of-Care “Polypill”: Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease. Adv. Healthc. Mater. 2020, 9, e2000236. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar] [CrossRef]
- Fawzi, F.; Sedky, M.; Abohammar, Y.; Sharara, H.; Serry, M. Open Source AI-Enhanced 3D-Printed Insulin Pump. In Proceedings of the 2023 IEEE Applied Sensing Conference (APSCON) 2023, Bengaluru, India, 23–25 January 2023; pp. 1–4. [Google Scholar]
- Sun, S.; Alkahtani, M.E.; Gaisford, S.; Basit, A.W.; Elbadawi, M.; Orlu, M. Virtually Possible: Enhancing Quality Control of 3D-Printed Medicines with Machine Vision Trained on Photorealistic Images. Pharmaceutics 2023, 15, 2630. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, Á.; Gaisford, S.; Basit, A.W. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, Á.; Gaisford, S.; Basit, A.W. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol. Sci. 2021, 42, 745–757. [Google Scholar] [CrossRef]
- Goh, G.D.; Sing, S.L.; Yeong, W.Y. A review on machine learning in 3D printing: Applications, potential, and challenges. Artif. Intell. Rev. 2021, 54, 63–94. [Google Scholar] [CrossRef]
- Biswas, A.A.; Dhondale, M.R.; Agrawal, A.K.; Serrano, D.R.; Mishra, B.; Kumar, D. Advancements in microneedle fabrication techniques: Artificial intelligence assisted 3D-printing technology. Drug Deliv. Transl. Res. 2024, 15, 1433. [Google Scholar] [CrossRef]
- Muhindo, D.; Elkanayati, R.; Srinivasan, P.; Repka, M.A.; Ashour, E.A. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 57. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [Google Scholar] [CrossRef]
- Arora, N.; Dua, S.; Singh, V.K.; Singh, S.K.; Senthilkumar, T. A comprehensive review on fillers and mechanical properties of 3D printed polymer composites. Mater. Today Commun. 2024, 40, 109617. [Google Scholar] [CrossRef]
- Parulski, C.; Jennotte, O.; Lechanteur, A.; Evrard, B. Challenges of fused deposition modeling 3D printing in pharmaceutical applications: Where are we now? Adv. Drug Deliv. Rev. 2021, 175, 113810. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Ramasamy, S.K.; Venkatesan, G.; Lee, J.; Barathi, S.; Kandasamy, S.; Sarangi, P.K. The comprehensive review on 3D printing- pharmaceutical drug delivery and personalized food and nutrition. Food Chem. 2024, 459, 1470348. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, S.; Shah, N.; Shah, S.; Momin, I.; Shah, S. 3D printing chronicles in medical devices and pharmaceuticals: Tracing the evolution and historical milestones. J. Biomater. Sci. 2024, 35, 2723–2766. [Google Scholar] [CrossRef]
- Jørgensen, A.K.; Ong, J.J.; Parhizkar, M.; Goyanes, Á.; Basit, A.W. Advancing non-destructive analysis of 3D printed medicines. Trends Pharmacol. Sci. 2023, 44, 379–393. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Lu, A.; Williams, R.O., III; Maniruzzaman, M. 3D printing of biologics-what has been accomplished to date? Drug Discov. Today 2024, 29, 103823. [Google Scholar] [CrossRef]
- Simon, M.C.; Laios, K.; Nikolakakis, I.; Papaioannou, T.G. Three-Dimensional Printing Technology in Drug Design and Development: Feasibility, Challenges, and Potential Applications. J. Pers. Med. 2024, 14, 1080. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Xu, X.; Ong, J.J.; Teyeb, A.; Gaisford, S.; Campos-Álvarez, A.; Stulz, A.; Marcuta, C.; Kraschew, L.; Mohr, W.; et al. A case study on decentralized manufacturing of 3D printed medicines. Int. J. Pharm. X 2023, 5, 100184. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharm. 2020, 577, 119066. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Goyanes, A.; Telford, R.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. Int. J. Pharm. 2018, 549, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Januskaite, P.; Goyanes, A.; Wilson, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Prediction of Solid-State Form of SLS 3D Printed Medicines Using NIR and Raman Spectroscopy. Pharmaceutics 2022, 14, 589. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Xu, X.; Goyanes, A.; Rowland, M.; Wilsdon, D.; Gaisford, S.; Basit, A.W. Releasing fast and slow: Non-destructive prediction of density and drug release from SLS 3D printed tablets using NIR spectroscopy. Int. J. Pharm. X 2023, 5, 100148. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Rodríguez-Pombo, L.; Ong, J.J.; Basit, A.W.; Santoveña-Estévez, A.; Fariña, J.B.; Alvarez-Lorenzo, C.; Goyanes, A. Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing. Int. J. Pharm. X 2022, 4, 100133. [Google Scholar] [CrossRef] [PubMed]
- Mora-Castaño, G.; Rodríguez-Pombo, L.; Carou-Senra, P.; Januskaite, P.; Rial, C.; Bendicho-Lavilla, C.; Couce, M.L.; Millán-Jiménez, M.; Caraballo, I.; Basit, A.W.; et al. Optimising 3D printed medications for rare diseases: In-line mass uniformity testing in direct powder extrusion 3D printing. Int. J. Pharm. 2024, 668, 124964. [Google Scholar] [CrossRef]
- Elbadawi, M.; Castro, B.M.; Gavins, F.K.H.; Ong, J.J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. M3diseen a novel machine learning approach for predicting the 3d printability of medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef]
- McCoubrey, L.E.; Elbadawi, M.; Pasqua, O.D.; Orlu, M.; Gaisford, S.; Basit, A.W. Harnessing machine learning for development of microbiome therapeutics. Gut. Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Dedeloudi, A.; Weaver, E.; Lamprou, D.A. Machine learning in additive manufacturing & Microfluidics for smarter and safer drug delivery systems. Int. J. Pharm. 2023, 636, 122818. [Google Scholar] [CrossRef]
- Han, R.; Xiong, H.; Ye, Z.; Yang, Y.; Huang, T.; Jing, Q.; Lu, J.; Pan, H.; Ren, F.; Ouyang, D. Predicting physical stability of solid dispersions by machine learning techniques. J. Control. Release 2019, 311–312, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, E.; Chen, X.; Liu, Z. Machine Learning Enables Process Optimization of Aerosol Jet 3D Printing Based on the Droplet Morphology. ACS Appl. Mater. Interfaces 2023, 15, 14532–14545. [Google Scholar] [CrossRef]
- Sokmen, S.; Cakmak, S.; Oksuz, I. 3D printing of an artificial intelligence-generated patient-specific coronary artery segmentation in a support bath. Biomed. Mater. 2024, 19, 035038. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, P.; Wang, J.; Duggal, I.; Maniruzzaman, M.; Banerjee, S. Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling. Pharmaceutics 2023, 15, 1266. [Google Scholar] [CrossRef]
- Lei, I.M.; Jiang, C.; Lei, C.L.; Rijk SRd Tam, Y.C.; Swords, C.; Sutcliffe, M.P.F.; Malliaras, G.G.; Bance, M.; Huang, Y.Y.S. 3D printed biomimetic cochleae and machine learning co-modelling provides clinical informatics for cochlear implant patients. Nat. Commun. 2021, 12, 6260. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Podina, L.; Silva, J.D.; Kohandel, M. Cell viability prediction and optimization in extrusion-based bioprinting via neural network-based Bayesian optimization models. Biofabrication 2024, 16, 025016. [Google Scholar] [CrossRef]
- Bone, J.M.; Childs, C.M.; Menon, A.; Poczos, B.; Feinberg, A.W.; LeDuc, P.R.; Washburn, N.R. Hierarchical Machine Learning for High-Fidelity 3D Printed Biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 7021–7031. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.; An, S.H.; Kim, W.-D.; Kim, S.-H. Machine learning-based design strategy for 3D printable bioink: Elastic modulus and yield stress determine printability. Biofabrication 2020, 12, 035018. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Shao, X.; Gu, G.X. Monitoring Anomalies in 3D Bioprinting with Deep Neural Networks. ACS Biomater. Sci. Eng. 2023, 9, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Nadernezhad, A.; Groll, J. Machine Learning Reveals a General Understanding of Printability in Formulations Based on Rheology Additives. Adv. Sci. 2022, 9, e2202638. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Balabani, S.; Hirayama, R.; Huang, J. Machine Learning in Predicting Printable Biomaterial Formulations for Direct Ink Writing. Research 2023, 6, 0197. [Google Scholar] [CrossRef]
- O’Reilly, C.S.; Elbadawi, M.; Desai, N.; Gaisford, S.; Basit, A.W.; Orlu, M. Machine Learning and Machine Vision Accelerate 3D Printed Orodispersible Film Development. Pharmaceutics 2021, 13, 2187. [Google Scholar] [CrossRef]
- Bagde, A.; Dev, S.; KSriram, L.M.; Spencer, S.D.; Kalvala, A.; Nathani, A.; Salau, O.; Mosley-Kellum, K.; Dalvaigari, H.; Rajaraman, S.; et al. Biphasic burst and sustained transdermal delivery in vivo using an AI-optimized 3D-printed MN patch. Int. J. Pharm. 2023, 636, 122647. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Morimura, C.; Ozeki, T. Effective and simple prediction model of drug release from “ghost tablets” fabricated using a digital light projection-type 3D printer. Int. J. Pharm. 2021, 604, 120721. [Google Scholar] [CrossRef]
- Madžarević, M.; Medarević, D.; Vulović, A.; Šušteršič, T.; Djuriš, J.; Filipović, N.; Ibric, S. Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef]
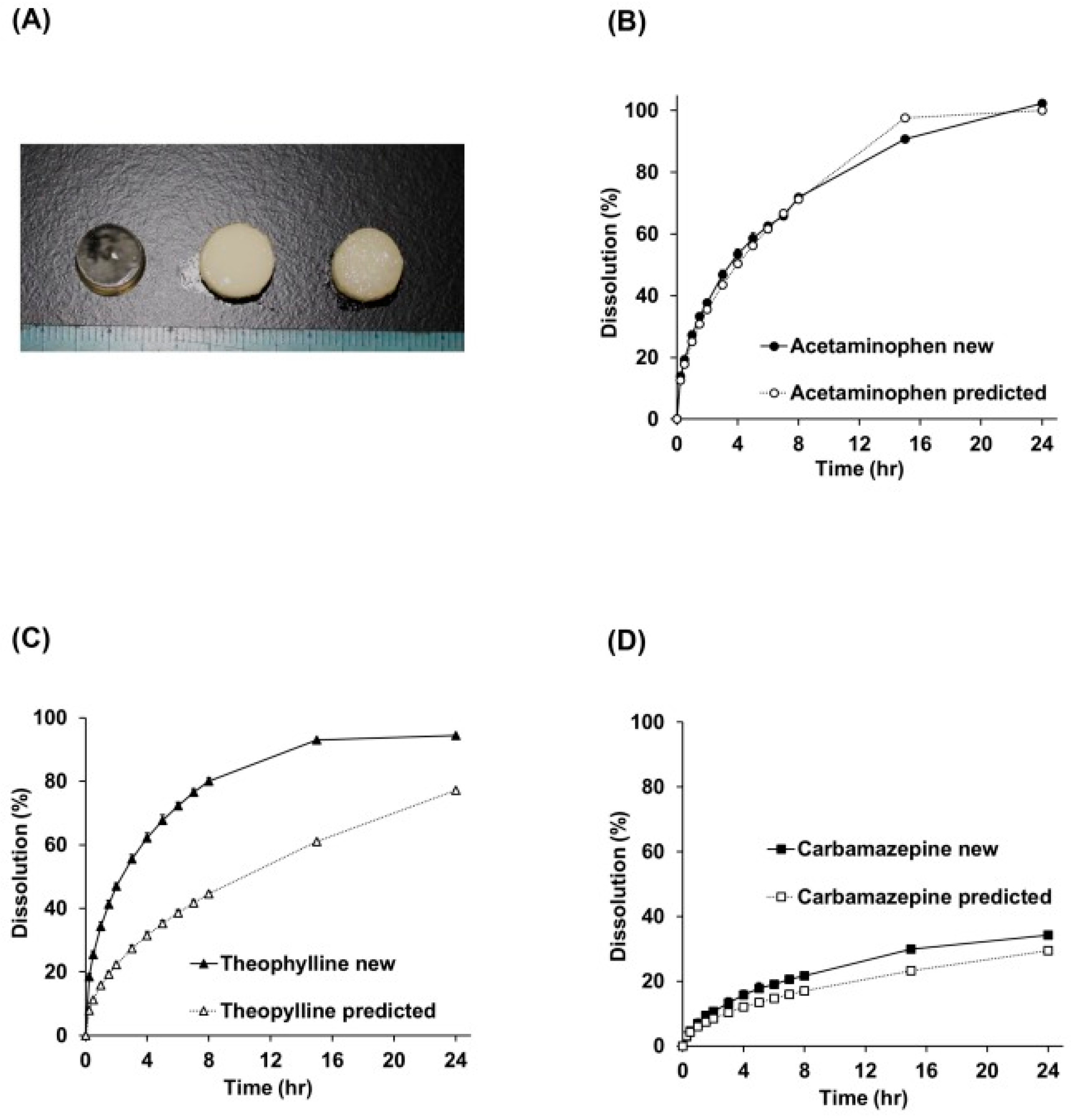
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef]
- Grof, Z.; Štěpánek, F. Artificial intelligence based design of 3D-printed tablets for personalised medicine. Comput. Chem. Eng. 2021, 154, 107492. [Google Scholar] [CrossRef]
- Hu, J.; Wan, J.; Xi, J.; Shi, W.; Qian, H. AI-driven design of customized 3D-printed multi-layer capsules with controlled drug release profiles for personalized medicine. Int. J. Pharm. 2024, 656, 124114. [Google Scholar] [CrossRef]
- Siegkas, P. Generating 3D porous structures using machine learning and additive manufacturing. Mater. Des. 2022, 220, 110858. [Google Scholar] [CrossRef]
- Mazur, H.; Erbrich, L.; Quodbach, J. Investigations into the use of machine learning to predict drug dosage form design to obtain desired release profiles for 3D printed oral medicines. Pharm. Dev. Technol. 2023, 28, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Elbadawi, M.; Ong, J.J.; Pollard, T.D.; Song, Z.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Alkahtani, M.E.; Basit, A.W.; Elbadawi, M.; Gaisford, S. Optimizing environmental sustainability in pharmaceutical 3D printing through machine learning. Int. J. Pharm. 2023, 648, 123561. [Google Scholar] [CrossRef]
- Đuranoví, M.; Obeid, S.; Madžarevíc, M.; Cvijić, S.; Ibrić, S. Paracetamol extended release FDM 3D printlets: Evaluation of formulation variables on printability and drug release. Int. J. Pharm. 2021, 592, 120053. [Google Scholar] [CrossRef]
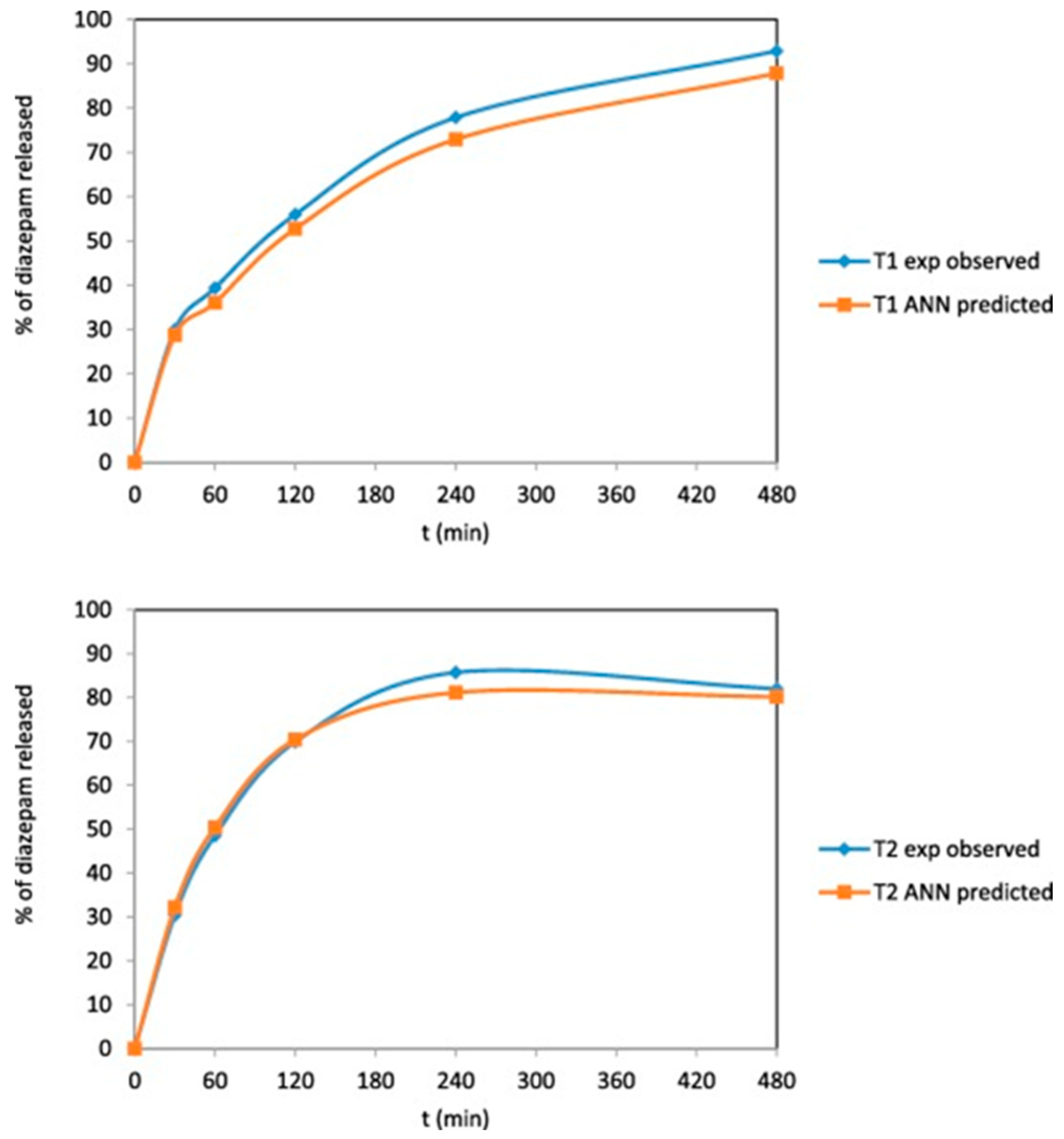
- Obeid, S.; Madzarevic, M.; Krkobabic, M.; Ibric, S. Predicting drug release from diazepam FDM printed tablets using deep learning approach: Influence of process parameters and tablet surface/volume ratio. Int. J. Pharm. 2021, 601, 120507. [Google Scholar] [CrossRef]
- Elbadawi, M.; Li, H.; Sun, S.; Alkahtani, M.E.; Basit, A.W.; Gaisford, S. Artificial intelligence generates novel 3D printing formulations. Appl. Mater. Today 2024, 36, 102061. [Google Scholar] [CrossRef]
- Ong, J.J.; Castro, B.M.; Gaisford, S.; Cabalar, P.; Basit, A.W.; Pérez, G.; Goyanes, A. Accelerating 3D printing of pharmaceutical products using machine learning. Int. J. Pharm. X 2022, 4, 100120. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Scoutaris, N.; Gong, Y.; Hui, H.-W.; Kumar, S.; Douroumis, D. Investigation on hot melt extrusion and prediction on 3D printability of pharmaceutical grade polymers. Int. J. Pharm. 2021, 604, 120755. [Google Scholar] [CrossRef]
- Carou-Senra, P.; Ong, J.J.; Castro, B.M.; Seoane-Vianõ, I.; Rodríguez-Pombo, L.; Cabalar, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Pérez, G.; Goyanes, A. Predicting pharmaceutical inkjet printing outcomes using machine learning. Int. J. Pharm. 2023, X5, 12. [Google Scholar] [CrossRef]
- Bozorg, N.M.; Leclercq, M.; Lescot, T.; Bazin, M.; Gaudreault, N.; Dikpati, A.; Fortin, M.-A.; Droit, A.; Bertrand, N. Design of experiment and machine learning inform on the 3D printing of for biomedical. Biomater. Adv. 2023, 153, 213533. [Google Scholar] [CrossRef]
- Abdalla, Y.; Elbadawi, M.; Ji, M.; ME, A.; Awad, A.; Orlu, M.; Gaisford, S.; Basit, A.W. Machine learning using multi-modal data predicts the production of selective laser sintered 3D printed drug products. Int. J. Pharm. 2023, 633, 122628. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Aghda, N.H.; Jiang, J.; Habib, A.M.; Ouyang, D.; Maniruzzaman, M. 3D bioprinted microparticles: Optimizing loading efficiency using advanced DoE technique and machine learning modeling. Int. J. Pharm. 2022, 628, 122302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Moon, S.K.; Ngo, T.H. Hybrid Machine Learning Method to Determine the Optimal Operating Process Window in Aerosol Jet 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 17994–18003. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Ma, L.; Yu, S.; Xu, X.; Amadi, S.M.; Zhang, J.; Wang, Z. Application of artificial intelligence in 3D printing physical organ models. Mater. Today Bio. 2023, 23, 100792. [Google Scholar] [CrossRef]
- Ramesh, S.; Deep, A.; Tamayol, A.; Kamaraj, A.; Mahajan, C.; Madihally, S. Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting 2024, 38, e00331. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Liu, Z.; Zhang, H. Machine learning enables electrical resistivity modeling of printed lines in aerosol jet 3D printing. Sci. Rep. 2024, 14, 14614. [Google Scholar] [CrossRef]
- Furxhi, I.; Murphy, F.; Mullins, M.; Arvanitis, A.; Poland, C.A. Practices and Trends of Machine Learning Application in Nanotoxicology. Nanomaterials 2020, 10, 116. [Google Scholar] [CrossRef]
- Vieira, S.; Pinaya, W.H.L.; Mechelli, A. Chapter 2-Main concepts in machine learning. In Machine Learning; Mechelli, A., Vieira, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–44. [Google Scholar]
- Thomas, S.; Palahnuk, H.; Amini, H.; Akseli, I. Data-smart machine learning methods for predicting composition-dependent Young’s modulus of pharmaceutical compacts. Int. J. Pharm. 2021, 592, 120049. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, W.; Yang, Y.; Ouyang, D. Interpretable machine learning methods for in vitro pharmaceutical formulation development. Food Front. 2021, 2, 195–207. [Google Scholar] [CrossRef]
- Shahiwala, A.F.; Qawoogha, S.S.; Faruqui, N. Designing Optimum Drug Delivery Systems Using Machine Learning Approaches: A Prototype Study of Niosomes. AAPS PharmSciTech 2023, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ye, Z.; Su, Y.; Ouyang, D. Predicting complexation performance between cyclodextrins and guest molecules by integrated machine learning and molecular modeling techniques. Acta Pharm. Sin. B 2019, 9, 1241–1252. [Google Scholar] [CrossRef]
- Vougas, K.; Sakellaropoulos, T.; Kotsinas, A.; Foukas, G.-R.P.; Ntargaras, A.; Koinis, F.; Polyzos, A.; Myrianthopoulos, V.; Zhou, H.; Narang, S.; et al. Machine learning and data mining frameworks for predicting drug response in cancer: An overview and a novel in silico screening process based on association rule mining. Pharmacol. Ther. 2019, 203, 107395. [Google Scholar] [CrossRef] [PubMed]
- de Vargas, V.W.; Schneider Aranda, J.A.; dos Santos Costa, R.; da Silva Pereira, P.R.; Victória Barbosa, J.L. Imbalanced data preprocessing techniques for machine learning: A systematic mapping study. Knowl. Inf. Syst. 2023, 65, 31–57. [Google Scholar] [CrossRef]
- Sun, Y.; Soh, S. Printing Tablets with Fully Customizable Release Profiles for Personalized Medicine. Adv. Mater. 2015, 27, 7847–7853. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. VIEW 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Butler, C.T.; Rodgers, A.M.; Curtis, A.M.; Donnelly, R.F. Chrono-tailored drug delivery systems: Recent advances and future directions. Drug Deliv. Transl. Res. 2024, 14, 1756–1775. [Google Scholar] [CrossRef]
- Tan, Y.J.N.; Yong, W.P.; Low, H.R.; Kochhar, J.S.; Khanolkar, J.; Lim, T.S.E.; Sun, Y.; Wong, J.Z.E.; Soh, S. Customizable drug tablets with constant release profiles via 3D printing technology. Int. J. Pharm. 2021, 598, 120370. [Google Scholar] [CrossRef]
- Manini, G.; Benali, S.; Raquez, J.-M.; Goole, J. Proof of concept of a predictive model of drug release from long-acting implants obtained by fused-deposition modeling. Int. J. Pharm. 2022, 618, 121663. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Ravi, P.; Shiakolas, P.S. Effects of slicing parameters on measured fill density for 3D printing of precision cylindrical constructs using Slic3r. SN Appl. Sci. 2021, 3, 390. [Google Scholar] [CrossRef]
- Domsta, V.; Hänsch, C.; Lenz, S.; Gao, Z.; Matin-Mann, F.; Scheper, V.; Lenarz, T.; Seidlitz, A. The Influence of Shape Parameters on Unidirectional Drug Release from 3D Printed Implants and Prediction of Release from Implants with Individualized Shapes. Pharmaceutics 2023, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting Drug Release from 3D Printed Oral Medicines Based on the Surface Area to Volume Ratio of Tablet Geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef]
- Hattori, Y.; Kubota, S.; Otsuka, M. Pharmaceutical evaluation of matrix tablets prepared using a fused deposition modelling type three-dimensional printer-Effect of geometrical internal microstructural factors on drug release from enteric-polymer tablets containing rebamipide. J. Pharm. Pharmacol. 2020, 72, 787–797. [Google Scholar] [CrossRef]
- Thanawuth, K.; Limmatvapirat, S.; Rojviriya, C.; Sriamornsak, P. Controlled Release of Felodipine from 3D-Printed Tablets with Constant Surface Area: Influence of Surface Geometry. Pharmaceutics 2023, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Crisan, A.G.; Iurian, S.; Porfire, A.; Rus, L.M.; Bogdan, C.; Casian, T.; Lucacel, R.C.; Turza, A.; Porav, S.; Tomuță, I. QbD guided development of immediate release FDM-3D printed tablets with customizable API doses. Int. J. Pharm. 2022, 613, 121411. [Google Scholar] [CrossRef]
- Than, Y.M.; Titapiwatanakun, V. Statistical design of experiment-based formulation development and optimization of 3D printed oral controlled release drug delivery with multi target product profile. J. Pharm. Investig. 2021, 51, 715–734. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Visan, A.I.; Ristoscu, C.; Mihailescu, I.N. Artificial Neural Network Algorithms for 3D Printing. Materials 2020, 14, 163. [Google Scholar] [CrossRef]
- Katoch, S.; Chauhan, S.S.; Kumar, V. A review on genetic algorithm: Past, present, and future. Multimed. Tools Appl. 2021, 80, 8091–8126. [Google Scholar] [CrossRef]
- Devi, R.V.; Sathya, S.S.; Coumar, M.S. Evolutionary algorithms for de novo drug design—A survey. Appl. Soft Comput. 2015, 27, 543–552. [Google Scholar] [CrossRef]
- Kanyilmaz, A.; NavarroTichell, P.R.; Loiacono, D. A genetic algorithm tool for conceptual structural design with cost and embodied carbon optimization. Eng. Appl. Artif. Intell. 2022, 112, 104711. [Google Scholar] [CrossRef]
- FABRX: M3DIMAKER STUDIO. Available online: https://fabrx-ai.com/m3dimakerStudio (accessed on 18 February 2025).
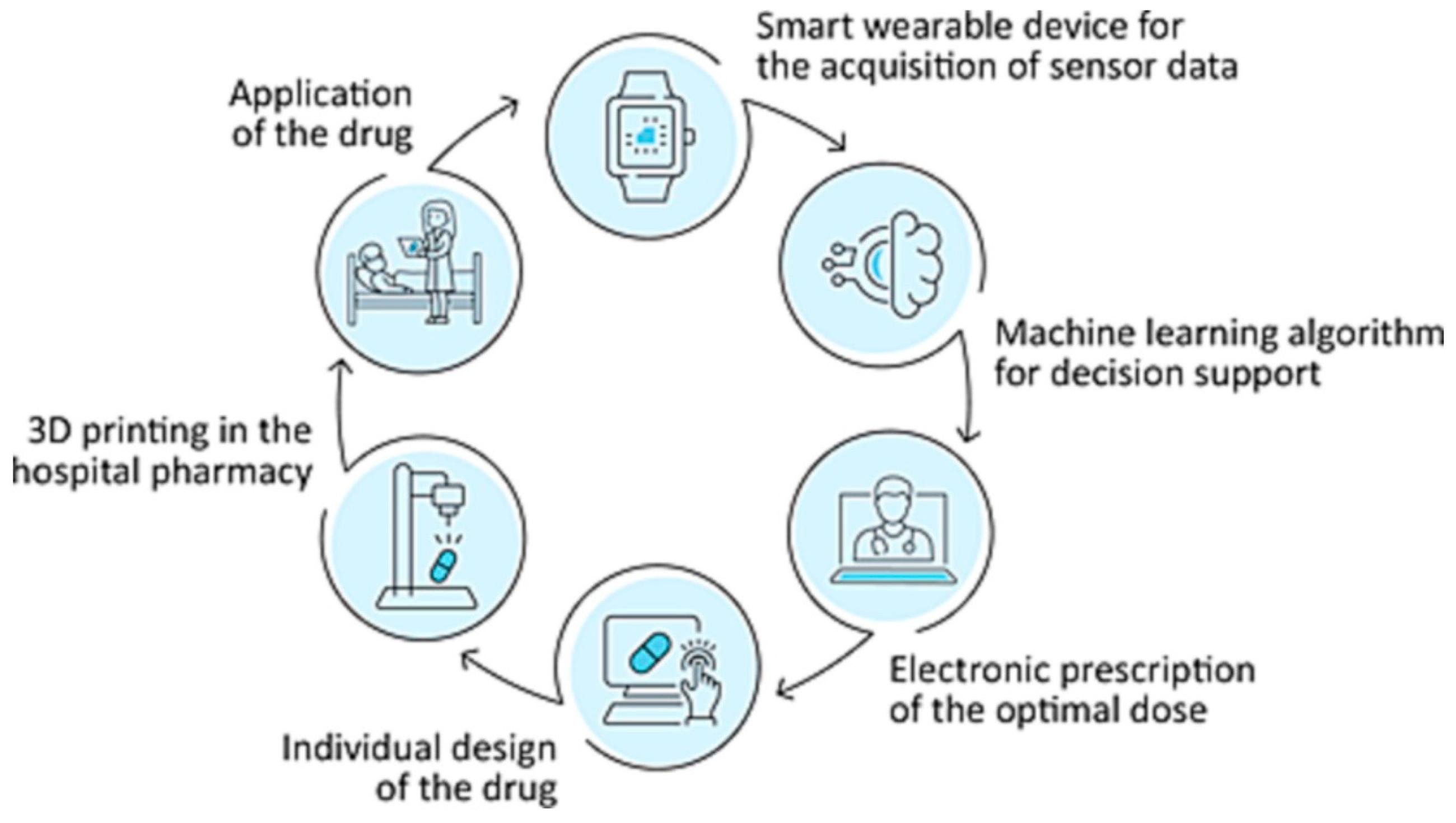
- Langebrake, C.; Gottfried, K.; Dadkhah, A.; Eggert, J.; Gutowski, T.; Rosch, M.; Schönbeck, N.; Gundler, C.; Nürnberg, S.; Ückert, F.; et al. Patient-individual 3D-printing of drugs within a machine-learning-assisted closed-loop medication management–Design and first results of a feasibility study. Clin. eHealth 2023, 6, 3–9. [Google Scholar] [CrossRef]
- Profitiliotis, T.; Tzetzis, D. Chapter 4-Design optimization and finite element analysis of 3D printed drug delivery systems. In Fundamentals and Future Trends of 3D Printing in Drug Delivery; Lamprou, D.A., Fatouros, D.G., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 77–99. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS. J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Singh, B.; Dahiya, M.; Saharan, V.; Ahuja, N. Optimizing drug delivery systems using systematic “design of experiments.” Part II: Retrospect and prospects. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 215–294. [Google Scholar] [CrossRef]
- Alzahrani, A.; Youssef, A.A.A.; Nyavanandi, D.; Tripathi, S.; Bandari, S.; Majumdar, S.; Repka, M.A. Design and optimization of ciprofloxacin hydrochloride biodegradable 3D printed ocular inserts: Full factorial design and in-vitro and ex-vivo evaluations: Part II. Int. J. Pharm. 2023, 631, 122533. [Google Scholar] [CrossRef]
- Jeong, H.M.; Weon, K.-Y.; Shin, B.S.; Shin, S. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules 2020, 25, 2330. [Google Scholar] [CrossRef]
- Partheniadis, I.; Terzi, V.; Nikolakakis, I. Finite Element Analysis and Modeling in Pharmaceutical Tableting. Pharmaceutics 2022, 14, 673. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, P.; Aravena, M.; Ponce, S.; Montelongo, J.H. A Finite Element Method for Modeling Diffusion and Drug Release from Nanocellulose/Nanoporous Silicon Composites. Pharmaceutics 2025, 17, 120. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.Y. Finite element analysis of diffusional drug release from complex matrix systems. I.: Complex geometries and composite structures. J. Control. Release 1997, 49, 277–288. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tzimtzimis, M.; Tsongas, K.; Andreadis, D.; Demiri, E.; Tzetzis, D.; Fatouros, D.G. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro. Eur. J. Pharm. Sci. 2019, 137, 104976. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Shi, J.; Zhou, Z.; Ge, D. Design of three-section microneedle towards low insertion force and high drug delivery amount using the finite element method. Comput. Methods Biomech. Biomed. Eng. 2024, 27, 156–166. [Google Scholar] [CrossRef]
- Angili, S.N.; Morovvati, M.R.; Kardan-Halvaei, M.; Saber-Samandari, S.; Razmjooee, K.; Abed, A.M.; Toghraie, D.; Khandan, A. Fabrication and finite element simulation of antibacterial 3D printed Poly L-lactic acid scaffolds coated with alginate/magnesium oxide for bone tissue regeneration. Int. J. Biol. Macromol. 2023, 224, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Heidari, A.; Nourbakhsh, S.M.; Mohammadi, R.; Semiromi, D. Design and fabrication of elastic two-component polymer-metal disks using a 3D printer under different loads for the lumbar spine. Polym. Test. 2022, 112, 107633. [Google Scholar] [CrossRef]
- Vila Pouca, M.C.P.; Cerqueira, M.R.G.; Ferreira, J.P.S.; Darabi, R.; Ramião, N.A.G.; Sobreiro-Almeida, R.; Castro, A.P.G.; Fernandes, P.R.; Mano, J.F.; Jorge, R.M.N.; et al. Simulating 3D printing on hydrogel inks: A finite element framework for predicting mechanical properties and scaffold deformation. Finite Elem. Anal. Des. 2024, 230, 104098. [Google Scholar] [CrossRef]
- Isaakidou, A.; Ganjian, M.; van Hoften, R.; Saldivar, M.C.; Leeflang, M.; Groetsch, A.; Wątroba, M.; Schwiedrzik, J.; Mirzaali, M.J.; Apachitei, I.; et al. Multi-scale in silico and ex silico mechanics of 3D printed cochlear implants for local drug delivery. Front. Bioeng. Biotechnol. 2024, 11, 1289299. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D.; Bouropoulos, N.; Barmpalexis, P.; Eleftheriadis, G.K.; Fatouros, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 102292. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, K.; Wu, H.; Peng, L.; Chen, Z. Thumb-sized 3D-Printed cymbal microneedle array (CyMA) for enhanced transdermal drug delivery. Eur. J. Pharm. Biopharm. 2025, 207, 114629. [Google Scholar] [CrossRef]
- Haring, A.P.; Tong, Y.; Halper, J.; Johnson, B.N. Programming of Multicomponent Temporal Release Profiles in 3D Printed Polypills via Core–Shell, Multilayer, and Gradient Concentration Profiles. Adv. Healthc. Mater. 2018, 7, 1800213. [Google Scholar] [CrossRef]
- Patel, S.K.; Khoder, M.; Peak, M.; Alhnan, M.A. Controlling drug release with additive manufacturing-based solutions. Adv. Drug Deliv. Rev. 2021, 174, 369–386. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Nikita, S.; Thakur, G.; Mishra, S. Artificial intelligence and machine learning applications in biopharmaceutical manufacturing. Trends Biotechnol. 2023, 41, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Mittermaier, M.; Raza, M.M.; Kvedar, J.C. Bias in AI-based models for medical applications: Challenges and mitigation strategies. NPJ Digit. Med. 2023, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Kharate, N.; Anerao, P.; Kulkarni, A.; Abdullah, M. Explainable AI Techniques for Comprehensive Analysis of the Relationship between Process Parameters and Material Properties in FDM-Based 3D-Printed Biocomposites. J. Manuf. Mater. Process. 2024, 8, 171. [Google Scholar] [CrossRef]
- Ukwaththa, J.; Herath, S.; Meddage, D.P.P. A review of machine learning (ML) and explainable artificial intelligence (XAI) methods in additive manufacturing (3D Printing). Mater. Today Commun. 2024, 41, 110294. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Pan, L.; Cao, D.; Jiang, H.; Ding, X. Artificial intelligence facilitates drug design in the big data era. Chemom. Intell. Lab. Syst. 2019, 194, 103850. [Google Scholar] [CrossRef]


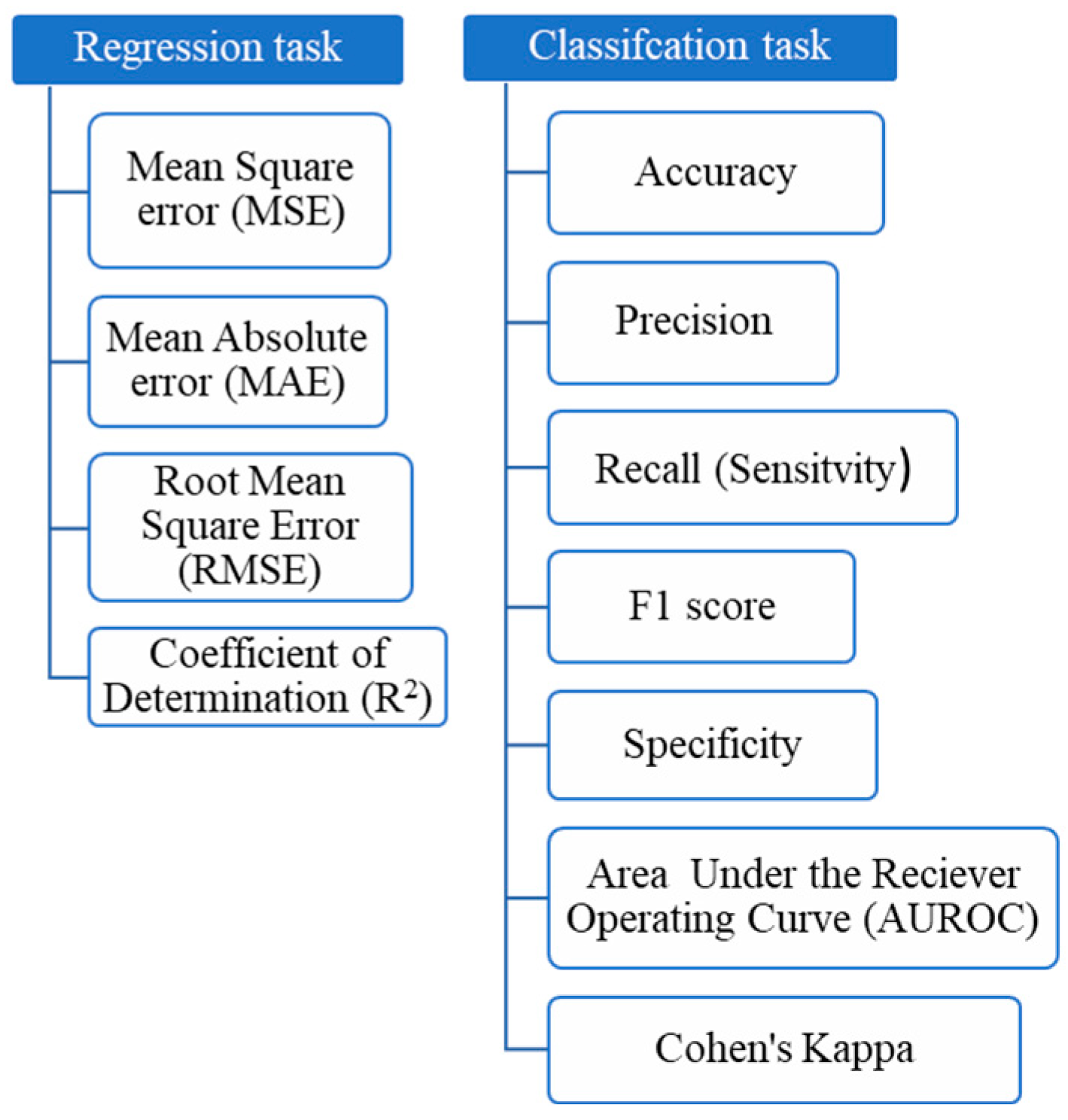

| Metric | Formula |
|---|---|
| MAE | |
| MSE | |
| RMSE | |
| R2 | |
| Accuracy | |
| Precision | |
| Recall (Sensitivity) | |
| F1 Score | |
| Specificity | |
| Cohen’s Kappa |
| Aspect | DoE | FEA | Mechanism-Based Models | CI |
|---|---|---|---|---|
| Main purpose | Identifies key factors affecting drug release through structured experiments | Simulates solid material behavior, such as drug diffusion and polymer degradation | Uses mathematical equations to describe drug release kinetics based on physical and chemical principles | Uses ML to predict and optimize drug release |
| Approach | Statistical method using structured experimental plans | Solves partial differential equations to model drug release from solid matrices | Uses established kinetic models (e.g., Higuchi, Korsmeyer–Peppas) to describe drug release profiles | Learns from experimental and simulation data to make predictions and optimize formulations |
| Data requirement | Requires experimental data but minimizes the number of tests needed | Requires material properties and boundary conditions for accurate simulations | Requires drug release data to fit parameters of kinetic models | Requires large datasets for training predictive models |
| Strength | - Efficient in identifying key formulation and process parameters - Reduces trial-and-error in experiments | - Provides spatial insights into drug diffusion and polymer degradation - Suitable for complex solid structures | - Simple and widely used in pharmaceutical sciences - Provides interpretable and mechanistic insights into drug release | - Can simultaneously integrate multiple factors - Fast optimization and prediction of drug release based on experimental or simulated data |
| Limitations | - Lacks mechanistic insights - Limited to the factors included in the experiment | - Requires high computational power - Needs precise material property data | - Oversimplifies complex drug release mechanisms - May not account for dynamic or multiscale processes | - Requires large datasets for accuracy - “Black-box” nature makes interpretation difficult |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassa, F.M.; Youssef, S.H.; Song, Y.; Garg, S. Use of Computational Intelligence in Customizing Drug Release from 3D-Printed Products: A Comprehensive Review. Pharmaceutics 2025, 17, 551. https://doi.org/10.3390/pharmaceutics17050551
Kassa FM, Youssef SH, Song Y, Garg S. Use of Computational Intelligence in Customizing Drug Release from 3D-Printed Products: A Comprehensive Review. Pharmaceutics. 2025; 17(5):551. https://doi.org/10.3390/pharmaceutics17050551
Chicago/Turabian StyleKassa, Fantahun Molla, Souha H. Youssef, Yunmei Song, and Sanjay Garg. 2025. "Use of Computational Intelligence in Customizing Drug Release from 3D-Printed Products: A Comprehensive Review" Pharmaceutics 17, no. 5: 551. https://doi.org/10.3390/pharmaceutics17050551
APA StyleKassa, F. M., Youssef, S. H., Song, Y., & Garg, S. (2025). Use of Computational Intelligence in Customizing Drug Release from 3D-Printed Products: A Comprehensive Review. Pharmaceutics, 17(5), 551. https://doi.org/10.3390/pharmaceutics17050551







