An Inhaled Nanoemulsion Encapsulating a Herbal Drug for Non-Small Cell Lung Cancer (NSCLC) Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Culture
2.3. Quantification of Cela Through Ultra-Performance Liquid Chromatography (UPLC)
2.4. Preparation of Nanoemulsion (NE) and Construction of Pseudo-Ternary Phase Diagrams
2.5. Physicochemical Characterization
2.5.1. Hydrodynamic Size and Surface Charge Analysis
2.5.2. Transmission Electron Microscopy
2.5.3. Drug Entrapment Efficiency
2.5.4. In Vitro Drug Release
2.5.5. In Vitro Aerosolization
2.6. Stability Studies
2.7. Cell Culture Studies
2.7.1. Cytotoxicity Studies
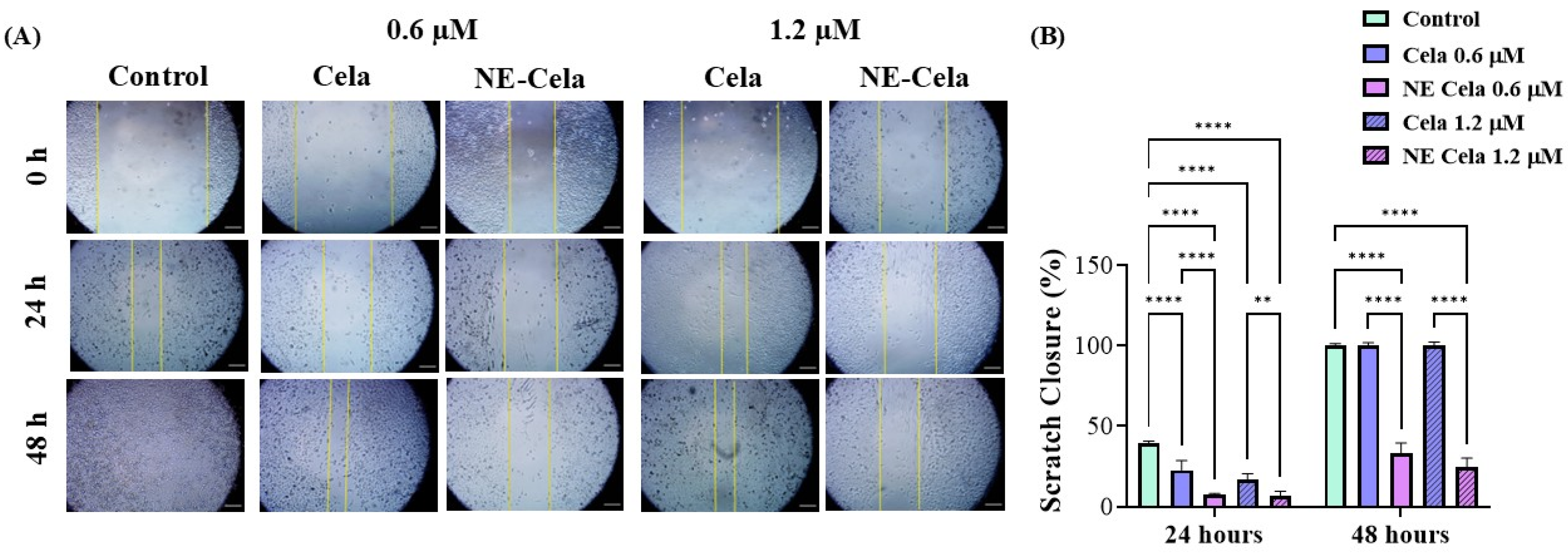
2.7.2. Wound Healing Assay
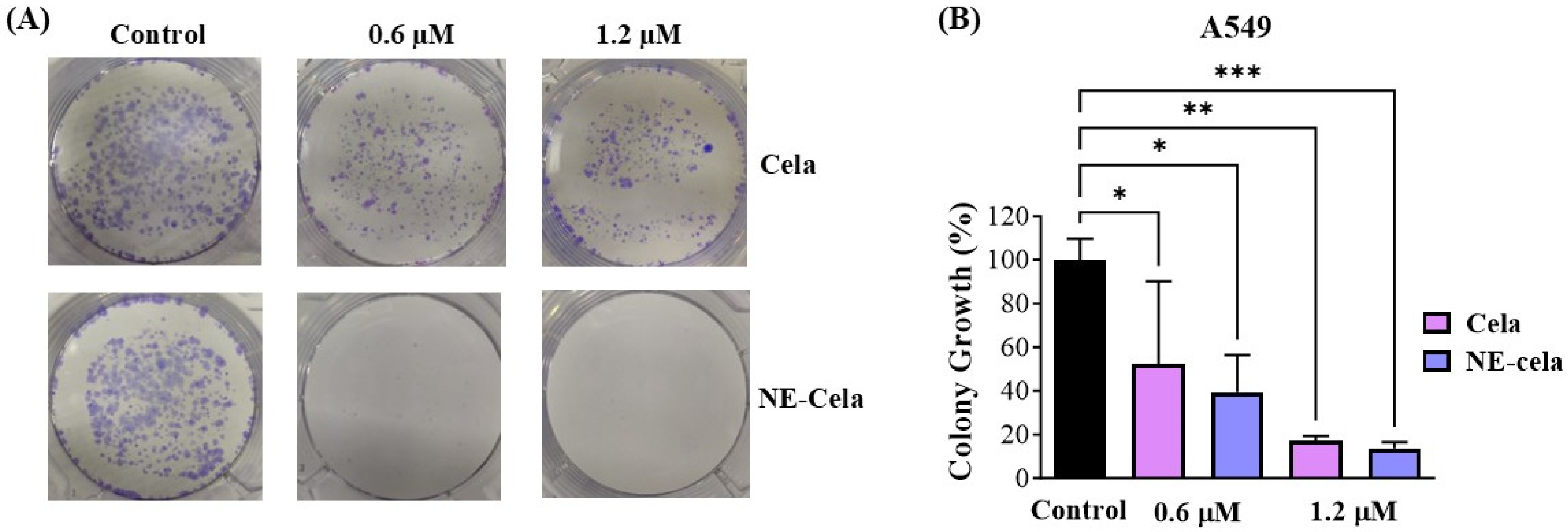
2.7.3. Clonogenic Assay
2.7.4. Spheroid Assay
2.7.5. Live–Dead Assay
2.8. Data Representation and Statistical Analysis
3. Results
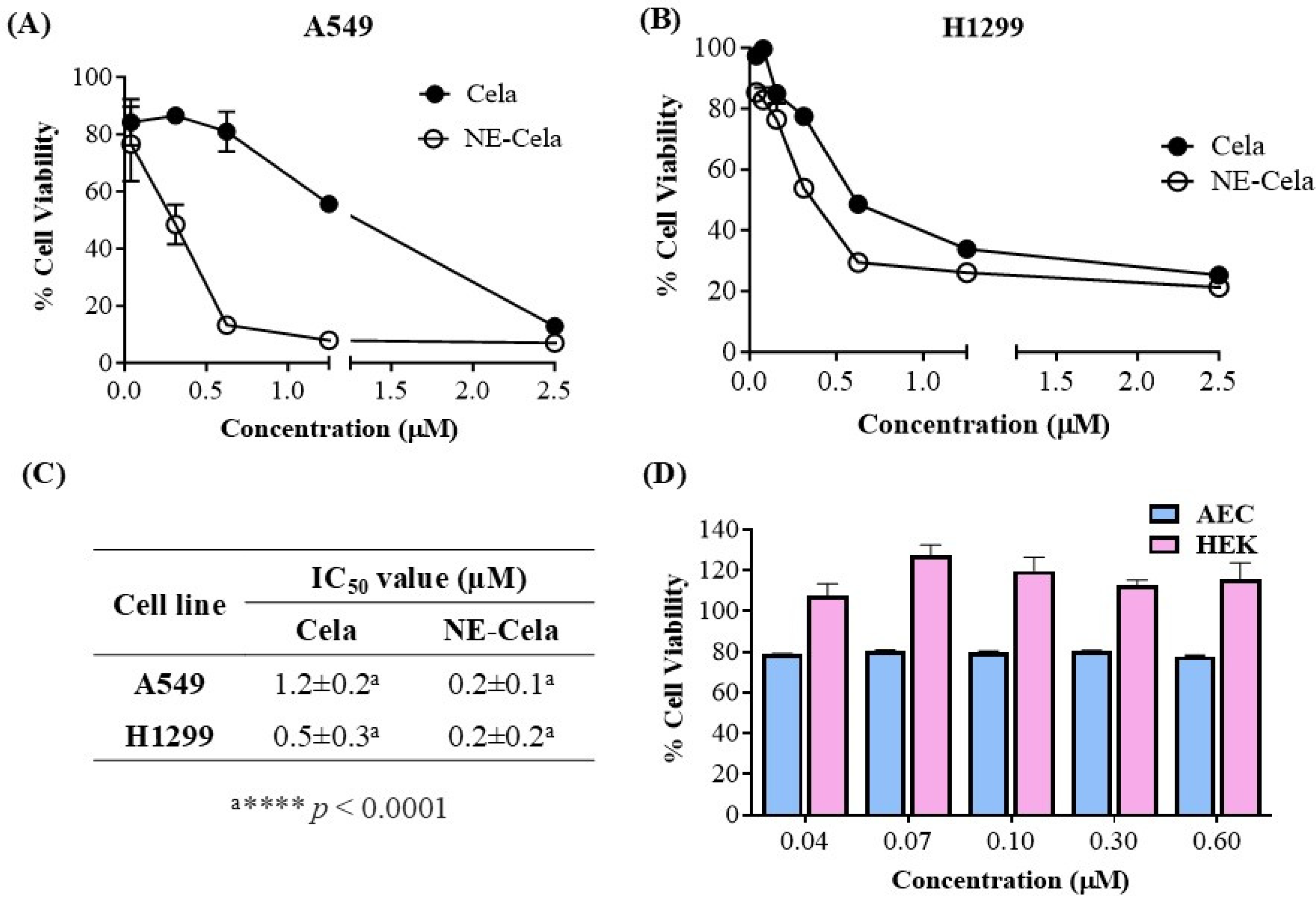
3.1. Quantification of Cela by UPLC
3.2. Selection of NE Components and Construction of Pseudo-Ternary Phase Diagrams
3.3. Physicochemical Characterization
3.3.1. Size and Surface Charge
3.3.2. Morphological Assessment with Transmission Electron Microscopy (TEM)
3.3.3. Drug Encapsulation
3.3.4. In Vitro Drug Release
3.3.5. In Vitro Aerosolization
3.4. Cell Culture Studies
3.4.1. Cytotoxicity Studies
3.4.2. Wound Healing Assay
3.4.3. Clonogenic Assay
3.4.4. 3D Spheroid Assay
Spheroid Volume
Live–Dead Assay
3.5. Stability Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corson, T.W.; Crews, C.M. Molecular Understanding and Modern Application of Traditional Medicines: Triumphs and Trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Abdallah, Q.M.; Kazi, M.; Khaleel, M.A.; Al-Deeb, I.; Nasr, A.-R.T.; Phillips, R.M. Utilization of novel self-nanoemulsifying formulations (SNEFs) loaded paclitaxel for the treatment prosperity of bladder cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101514. [Google Scholar] [CrossRef]
- Çapcı, A.; Lorion, M.M.; Wang, H.; Simon, N.; Leidenberger, M.; Borges Silva, M.C.; Moreira, D.R.M.; Zhu, Y.; Meng, Y.; Chen, J.Y.; et al. Artemisinin–(Iso)quinoline Hybrids by C−H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. 2019, 58, 13066–13079. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vieweger, M.; Zhang, K.; Yin, H.; Wang, H.; Li, X.; Li, S.; Hu, S.; Sparreboom, A.; Evers, B.M.; et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat. Commun. 2020, 11, 972. [Google Scholar] [CrossRef]
- Wang, Z.; Little, N.; Chen, J.; Lambesis, K.T.; Le, K.T.; Han, W.; Scott, A.J.; Lu, J. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat. Nanotechnol. 2021, 16, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef]
- Chen, S.-R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium wilfordii Hook F. Front. Pharmacol. 2018, 9, 104. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Al-Jamal, K.T. Recent progress in nanotechnology-based drug carriers for celastrol delivery. Biomater. Sci. 2021, 9, 6355–6380. [Google Scholar] [CrossRef]
- Ng, S.W.; Chan, Y.; Chellappan, D.K.; Madheswaran, T.; Zeeshan, F.; Chan, Y.L.; Collet, T.; Gupta, G.; Oliver, B.G.; Wark, P.; et al. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed. Pharmacother. 2019, 109, 1785–1792. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zhou, Z.; Yan, Y.; Fu, W.; Zou, P.; Ni, D. ML385, an Nrf2 Inhibitor, Synergically Enhanced Celastrol Triggered Endoplasmic Reticulum Stress in Lung Cancer Cells. ACS Omega 2024, 9, 43697–43705. [Google Scholar] [CrossRef]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.-K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Gong, Y.; Wang, X.; Zhang, L.; Zhao, H.; Li, J.; Zhu, J.; Huang, X.; Zhao, C.; et al. Celastrol elicits antitumor effects by inhibiting the STAT3 pathway through ROS accumulation in non-small cell lung cancer. J. Transl. Med. 2022, 20, 525. [Google Scholar] [CrossRef]
- Holder, J.E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C.M.; Byrne, L.J.; Bertolo, E.; Wilson, C.M. The use of nanoparticles for targeted drug delivery in non-small cell lung cancer. Front. Oncol. 2023, 13, 1154318. [Google Scholar] [CrossRef]
- What Is Lung Cancer?|Types of Lung Cancer|American Cancer Society. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/what-is.html (accessed on 21 September 2023).
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. Perioperative Nivolumab in Resectable Lung Cancer. N. Engl. J. Med. 2024, 390, 1756–1769. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Spira, A.; Angevin, E.; Su, W.-C.; Hong, D.S.; et al. Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). Int. J. Oncol. 2021, 59, 90. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, B.S.; McNeil, M.J.; Pham, L.T.D.; Chen, Y.; Rivera, J.; Acuna, C.; Sniderman, L.; Sakaan, F.M.; Aceituno, A.M.; Villegas, C.A.; et al. Treatment-related mortality in children with cancer in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Oncol. 2023, 24, 967–977. [Google Scholar] [CrossRef]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert. Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef]
- Dilalla, V.; Chaput, G.; Williams, T.; Sultanem, K. Radiotherapy side effects: Integrating a survivorship clinical lens to better serve patients. Curr. Oncol. 2020, 27, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Dabas, P.; Danda, A. Revolutionizing cancer treatment: A comprehensive review of CAR-T cell therapy. Med. Oncol. 2023, 40, 275. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Hong, L.; Zhang, J.; Le, X. MET Amplification as a Resistance Driver to TKI Therapies in Lung Cancer: Clinical Challenges and Opportunities. Cancers 2023, 15, 612. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef]
- Yusri, M.A.A.; Sekar, M.; Wong, L.S.; Gan, S.H.; Ravi, S.; Subramaniyan, V.; Rani, N.N.I.M.; Chidambaram, K.; Begum, M.Y.; Ramar, M.; et al. Celastrol: A Potential Natural Lead Molecule for New Drug Design, Development and Therapy for Memory Impairment. Drug Des. Dev. Ther. 2023, 17, 1079–1096. [Google Scholar] [CrossRef]
- Qi, X.; Qin, J.; Ma, N.; Chou, X.; Wu, Z. Solid self-microemulsifying dispersible tablets of celastrol: Formulation development, charaterization and bioavailability evaluation. Int. J. Pharm. 2014, 472, 40–47. [Google Scholar] [CrossRef]
- Xu, S.; Fan, R.; Wang, L.; He, W.; Ge, H.; Chen, H.; Xu, W.; Zhang, J.; Xu, W.; Feng, Y.; et al. Synthesis and biological evaluation of celastrol derivatives as potent antitumor agents with STAT3 inhibition. J. Enzym. Inhib. Med. Chem. 2021, 37, 236–251. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Molecular Targets of Celastrol in Cancer: Recent Trends and Advancements—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1040842817304006 (accessed on 21 September 2023).
- Lai, X.; Zhao, Y.; Shi, Z.; Xing, L.; Li, X.; Jia, L.; Lin, K. Plant-derived paclitaxel-loaded ultra-small Fe3O4 nanoparticles for MR imaging-mediated antitumor therapy. Ind. Crops Prod. 2025, 228, 120902. [Google Scholar] [CrossRef]
- Gadhave, D.; Quadros, M.; Ugale, A.R.; Goyal, M.; Gupta, V. A Nanoemulgel for Nose-to-Brain delivery of Quetiapine—QbD-Enabled formulation development & in-vitro characterization. Int. J. Pharm. 2023, 648, 123566. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Chauhan, G.; Wang, X.; Quadros, M.; Vats, M.; Gupta, V. Chitosan/bovine serum albumin layer-by-layer assembled particles for non-invasive inhaled drug delivery to the lungs. Int. J. Biol. Macromol. 2024, 271, 132526. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Zhu, J.; Zhu, J.; Shen, J.; Liu, Z.; Yang, Y.; Chen, Q. Nanoparticle-Mediated Delivery of Inhaled Immunotherapeutics for Treating Lung Metastasis. Adv. Mater. 2021, 33, e2007557. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Y.; Liu, Y.; Li, R.; Huang, D.-B.; Pan, Y.-Y. Nanomedicine in lung cancer: Current states of overcoming drug resistance and improving cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1654. [Google Scholar] [CrossRef]
- Chauhan, G.; Wang, X.; Yousry, C.; Gupta, V. Scalable Production and In Vitro Efficacy of Inhaled Erlotinib Nanoemulsion for Enhanced Efficacy in Non-Small Cell Lung Cancer (NSCLC). Pharmaceutics 2023, 15, 996. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gadhave, D.; Chauhan, G.; Gupta, V. Development and characterization of inhaled nintedanib-loaded PLGA nanoparticles using scalable high-pressure homogenization technique. J. Drug Deliv. Sci. Technol. 2024, 91, 105233. [Google Scholar] [CrossRef]
- Wang, X.; Chauhan, G.; Tacderas, A.R.L.; Muth, A.; Gupta, V. Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2023, 24, 5204. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Parvathaneni, V.; Shukla, S.K.; Barasa, L.; Perron, J.C.; Yoganathan, S.; Muth, A.; Gupta, V. Tyrosine kinase inhibitor conjugated quantum dots for non-small cell lung cancer (NSCLC) treatment. Eur. J. Pharm. Sci. 2019, 133, 145–159. [Google Scholar] [CrossRef]
- Wang, X.; Choudhary, S.M.; Chauhan, G.; Muth, A.; Gupta, V. Transferrin-conjugated polymeric nanoparticles for receptor-mediated delivery of resveratrol-cyclodextrin complex in non-small cell lung cancer (NSCLC) cells. J. Drug Deliv. Sci. Technol. 2025, 105, 106588. [Google Scholar] [CrossRef]
- Goyal, M.; Tulsyan, G.; Kanabar, D.D.; Chavan, T.; Muth, A.; Gupta, V. Poly vinyl pyrrolidone (PVP) based inhaled delivery carriers for olaparib for non-small cell lung cancer (NSCLC) treatment. J. Drug Deliv. Sci. Technol. 2023, 87, 104767. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K.; Kolluru, S.; Huen, M.; Mulla, N.; Mehra, N.; Kanabar, D.; Palakurthi, S.; Ayehunie, S.; Muth, A.; et al. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Aldawsari, M.F.; Iqbal, M.; Almutairy, B.K.; Soliman, G.A.; Aboudzadeh, M.A. Diosmin-Loaded Nanoemulsion-Based Gel Formulation: Development, Optimization, Wound Healing and Anti-Inflammatory Studies. Gels 2023, 9, 95. [Google Scholar] [CrossRef]
- Siddiqui, A.; Jain, P.; Alex, T.S.; Ali, M.A.; Hassan, N.; Haneef, J.; Naseef, P.P.; Kuruniyan, M.S.; Mirza, M.A.; Iqbal, Z. Investigation of a Minocycline-Loaded Nanoemulgel for the Treatment of Acne Rosacea. Pharmaceutics 2022, 14, 2322. [Google Scholar] [CrossRef]
- Walia, N.; Zhang, S.; Wismer, W.; Chen, L. A low energy approach to develop nanoemulsion by combining pea protein and Tween 80 and its application for vitamin D delivery. Food Hydrocoll. Health 2022, 2, 100078. [Google Scholar] [CrossRef]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, F.; Zhang, Z.; Zhu, F.; Xue, J.; Tan, X.; Zhang, L.; Jia, X. Formulation and Evaluation of Celastrol-Loaded Liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Li, Y.; An, G.-S.; Ni, J.-H.; Jia, H.-T.; Li, S.-Y. DNA Damage-Response Pathway Heterogeneity of Human Lung Cancer A549 and H1299 Cells Determines Sensitivity to 8-Chloro-Adenosine. Int. J. Mol. Sci. 2018, 19, 1587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Bao, X.; Chen, M.; Lin, R.; Zhuyan, J.; Zhen, T.; Xing, K.; Zhou, W.; Zhu, S. Mechanisms and Future of Non-Small Cell Lung Cancer Metastasis. Front. Oncol. 2020, 10, 585284. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-loaded chitosomes for treatment of malignant pleural mesothelioma—A rare thoracic cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Ahmad, G.; El Sadda, R.; Botchkina, G.; Ojima, I.; Egan, J.; Amiji, M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017, 406, 71–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Wang, M.; Zhang, X.; Zhang, H.; Tong, X.; Xiao, Y. Competitive profiling of celastrol targets in human cervical cancer HeLa cells via quantitative chemical proteomics. Mol. BioSyst. 2016, 13, 83–91. [Google Scholar] [CrossRef]
- Jang, S.Y.; Jang, S.-W.; Ko, J. Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptor α. Cancer Lett. 2011, 300, 57–65. [Google Scholar] [CrossRef]
- Moreira, H.; Szyjka, A.; Gąsiorowski, K. Chemopreventive activity of celastrol in drug–resistant human colon carcinoma cell cultures. Oncotarget 2018, 9, 21211–21223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, D.; Hang, T.; Wu, Z.; Liu, J.; Xu, Q.; Xie, X.; Zuo, J.; Wang, Z.; Zhou, Y. Preparation, characterization, and assessment of the antiglioma effects of liposomal celastrol. Anti-Cancer Drugs 2012, 23, 515. [Google Scholar] [CrossRef] [PubMed]
- Boridy, S.; Soliman, G.M.; Maysinger, D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine 2012, 7, 1149–1165. [Google Scholar] [CrossRef]
- Peng, X.; Wang, J.; Song, H.; Cui, D.; Li, L.; Li, J.; Lin, L.; Zhou, J.; Liu, Y. Optimized Preparation of Celastrol-Loaded Polymeric Nanomicelles Using Rotatable Central Composite Design and Response Surface Methodology. J. Biomed. Nanotechnol. 2012, 8, 491–499. [Google Scholar] [CrossRef]
- Lee, W.D.; Liang, Y.J.; Chen, B.H. Effects of tanshinone nanoemulsion and extract on inhibition of lung cancer cells A549. Nanotechnology 2016, 27, 495101. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, S.A.; Min, K.A.; Jee, J.-P.; Jin, S.G.; Cho, K.H. Development of Alectinib-Suspended SNEDDS for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1694. [Google Scholar] [CrossRef]
- Sharifi, F.; Jahangiri, M.; Nazir, I.; Asim, M.H.; Ebrahimnejad, P.; Hupfauf, A.; Gust, R.; Bernkop-Schnürch, A. Zeta potential changing nanoemulsions based on a simple zwitterion. J. Colloid. Interface Sci. 2021, 585, 126–137. [Google Scholar] [CrossRef]
- Bunchongprasert, K.; Shao, J. Cytotoxicity and permeability enhancement of Capmul®MCM in nanoemulsion formulation. Int. J. Pharm. 2019, 561, 289–295. [Google Scholar] [CrossRef]
- Gomes Daré, R.; Beatriz Chieco Costa, A.; Silva Martins, T.; Lopes, L.B. Simvastatin and adenosine-co-loaded nanostructured lipid carriers for wound healing: Development, characterization and cell-based investigation. Eur. J. Pharm. Biopharm. 2024, 205, 114533. [Google Scholar] [CrossRef]
- Elbardisy, B.; Boraie, N.; Galal, S. Tadalafil Nanoemulsion Mists for Treatment of Pediatric Pulmonary Hypertension via Nebulization. Pharmaceutics 2022, 14, 2717. [Google Scholar] [CrossRef]
- Classification of Surface-Active Agents by “HLB”|CiNii Research. Available online: https://cir.nii.ac.jp/crid/1574231874427670016 (accessed on 24 September 2023).
- Gad, E.A.M.; Khairou, K.S. QSPR for HLB of Nonionic Surfactants Based on Polyoxyethylene Group. J. Dispers. Sci. Technol. 2008, 29, 940–947. [Google Scholar] [CrossRef]
- Amani, A.; York, P.; Chrystyn, H.; Clark, B.J. Evaluation of a Nanoemulsion-Based Formulation for Respiratory Delivery of Budesonide by Nebulizers. AAPS PharmSciTech 2010, 11, 1147–1151. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Sun, J.; Zou, H.; Sun, Y.; Luo, J.; Xie, Q.; Rong, A.; Wang, H.; Li, X.; et al. An Osimertinib-Perfluorocarbon Nanoemulsion with Excellent Targeted Therapeutic Efficacy in Non-small Cell Lung Cancer: Achieving Intratracheal and Intravenous Administration. ACS Nano 2022, 16, 12590–12605. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharmacother. 2020, 129, 110369. [Google Scholar] [CrossRef]
- Said-Elbahr, R.; Nasr, M.; Alhnan, M.A.; Taha, I.; Sammour, O. Simultaneous pulmonary administration of celecoxib and naringin using a nebulization-friendly nanoemulsion: A device-targeted delivery for treatment of lung cancer. Expert. Opin. Drug Deliv. 2022, 19, 611–622. [Google Scholar] [CrossRef]
- Yong, J.; Shu, H.; Zhang, X.; Yang, K.; Luo, G.; Yu, L.; Li, J.; Huang, H. Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases. Int. J. Nanomed. 2024, 19, 1723–1748. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Abdulmalek, E.; Abdul Rahman, M.B. Modeling the Effect of Composition on Formation of Aerosolized Nanoemulsion System Encapsulating Docetaxel and Curcumin Using D-Optimal Mixture Experimental Design. Int. J. Mol. Sci. 2020, 21, 4357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Chen, H.; You, J.; Peng, B.; Cao, F.; Zhang, X.; Chen, Q.; Uzan, G.; Xu, L.; et al. Celastrol improves the therapeutic efficacy of EGFR-TKIs for non-small-cell lung cancer by overcoming EGFR T790M drug resistance. Anti-Cancer Drugs 2018, 29, 748–755. [Google Scholar] [CrossRef]
- Jun, H.Y.; Kim, T.-H.; Choi, J.W.; Lee, Y.H.; Lee, K.K.; Yoon, K.-H. Evaluation of connectivity map-discovered celastrol as a radiosensitizing agent in a murine lung carcinoma model: Feasibility study of diffusion-weighted magnetic resonance imaging. PLoS ONE 2017, 12, e0178204. [Google Scholar] [CrossRef]
- Shah, B. Microemulsion as a promising carrier for nose to brain delivery: Journey since last decade. J. Pharm. Investig. 2021, 51, 611–634. [Google Scholar] [CrossRef]
- Lindenberg, F.; Sichel, F.; Lechevrel, M.; Respaud, R.; Saint-Lorant, G. Evaluation of Lung Cell Toxicity of Surfactants for Inhalation Route. J. Toxicol. Risk Assess. 2019, 5, 22. [Google Scholar] [CrossRef]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Jang, K.S.B.; Choi, H.J.; Jo, A.; Cheong, J.-H.; Chun, K.-H. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 2014, 47, 697–702. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S.; Pang, L.; Yang, J.; Li, H.J.; Huo, X.; Qian, S.Y. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer. Free Radic. Res. 2019, 53, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-Z.; Wang, S.-C.; Hsi, Y.-T.; Lo, Y.-S.; Lin, C.-C.; Chuang, Y.-C.; Lin, S.-H.; Hsieh, M.-J.; Chen, M.-K. Celastrol induces vincristine multidrug resistance oral cancer cell apoptosis by targeting JNK1/2 signaling pathway. Phytomedicine 2019, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, X.; Li, J.; Zhang, Y.; Wang, X.; Zhang, R.; Qin, X.; Chen, X.; Wang, J.; Liao, W.; et al. Celastrol inhibits lung cancer growth by triggering histone acetylation and acting synergically with HDAC inhibitors. Pharmacol. Res. 2022, 185, 106487. [Google Scholar] [CrossRef]
- Gupta, V.P. Vivek Evaluation of 3D Cell Culture Models for Efficacy Determination of Anticancer Nanotherapeutics. In Drug Delivery with Targeted Nanoparticles; Jenny Stanford Publishing: Singapore, 2021; ISBN 978-1-00-316473-9. [Google Scholar]
- Parvathaneni, V.; Shukla, S.K.; Gupta, V. Development and Characterization of Folic Acid-Conjugated Amodiaquine-Loaded Nanoparticles–Efficacy in Cancer Treatment. Pharmaceutics 2023, 15, 1001. [Google Scholar] [CrossRef]



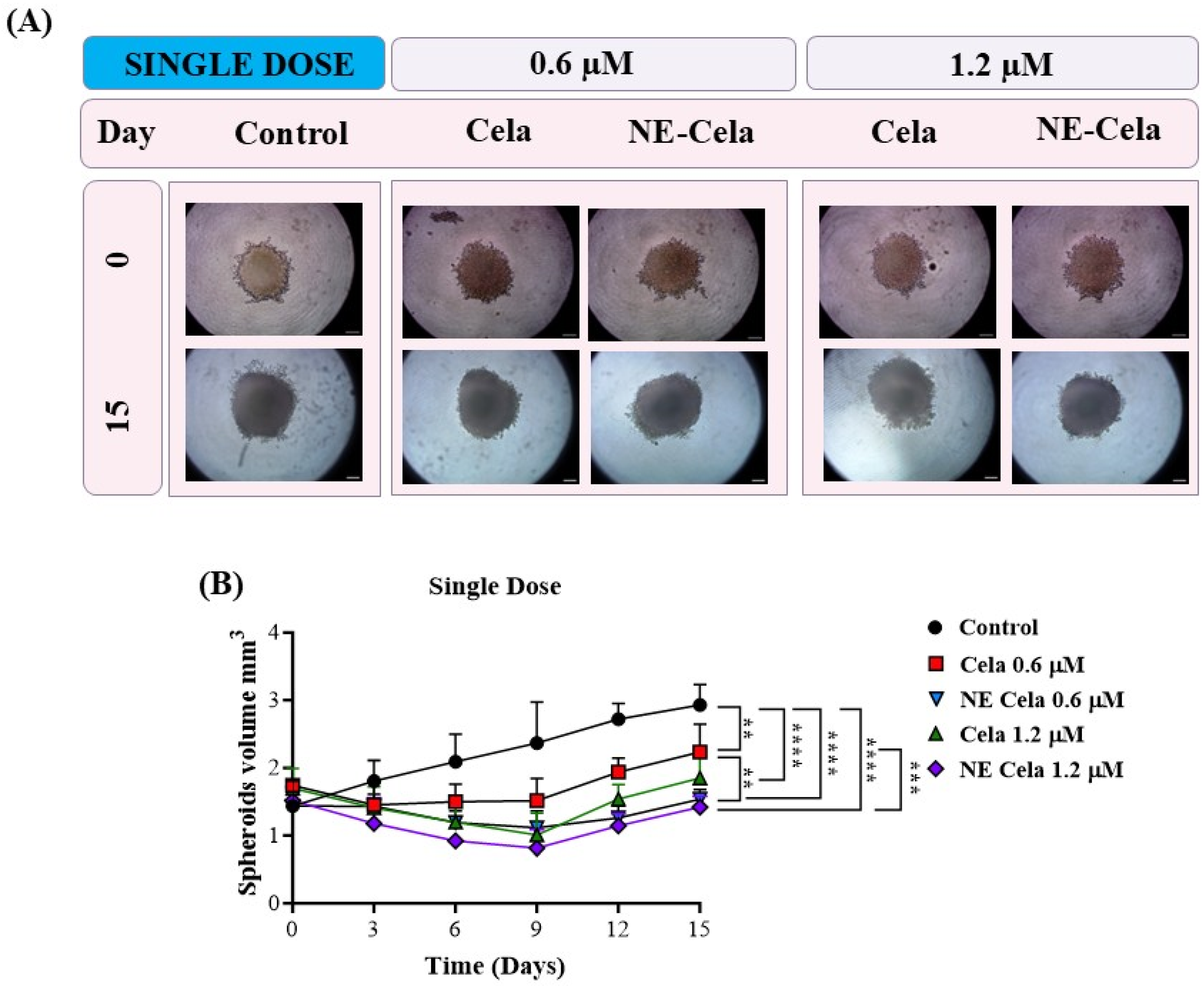

| Spheroid Volume (mm3) | 0.6 μM | 1.2 μM | |||
| Single Dose | Control | Cela | NE-Cela | Cela | NE-Cela |
| Day 0 | 1.4 ± 0.3 | 1.7 ± 0.1 | 1.4 ± 0.3 | 1.7 ± 0.1 | 1.5 ± 0.2 |
| Day 15 | 2.9 ± 0.9 | 2.2 ± 0.4 | 1.5 ± 0.1 | 1.8 ± 0.3 | 1.4 ± 0.1 |
| % Volume change | 107.1 ± 40.4 | 29.4 ± 5.6 | 7.1 ± 1.6 | 5.9 ±1.0 | −6.7 ± 1.01 |
| Multiple Dose | Control | Cela | NE-Cela | Cela | NE-Cela |
| Day 0 | 1.6 ± 0.3 | 1.5 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.2 |
| Day 15 | 2.7 ± 0.4 | 1.1 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | - |
| % Volume change | 68.7 ± 16.4 | −26.7 ± 7.2 | −52.9 ± 11.5 | −37.5 ± 6.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quadros, M.; Goyal, M.; Chauhan, G.; Gadhave, D.; Gupta, V. An Inhaled Nanoemulsion Encapsulating a Herbal Drug for Non-Small Cell Lung Cancer (NSCLC) Treatment. Pharmaceutics 2025, 17, 540. https://doi.org/10.3390/pharmaceutics17050540
Quadros M, Goyal M, Chauhan G, Gadhave D, Gupta V. An Inhaled Nanoemulsion Encapsulating a Herbal Drug for Non-Small Cell Lung Cancer (NSCLC) Treatment. Pharmaceutics. 2025; 17(5):540. https://doi.org/10.3390/pharmaceutics17050540
Chicago/Turabian StyleQuadros, Mural, Mimansa Goyal, Gautam Chauhan, Dnyandev Gadhave, and Vivek Gupta. 2025. "An Inhaled Nanoemulsion Encapsulating a Herbal Drug for Non-Small Cell Lung Cancer (NSCLC) Treatment" Pharmaceutics 17, no. 5: 540. https://doi.org/10.3390/pharmaceutics17050540
APA StyleQuadros, M., Goyal, M., Chauhan, G., Gadhave, D., & Gupta, V. (2025). An Inhaled Nanoemulsion Encapsulating a Herbal Drug for Non-Small Cell Lung Cancer (NSCLC) Treatment. Pharmaceutics, 17(5), 540. https://doi.org/10.3390/pharmaceutics17050540








