Salicylic Acid-Mediated Silver Nanoparticle Green Synthesis: Characterization, Enhanced Antimicrobial, and Antibiofilm Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of SA-AgNPs
2.3. Study of the Effect of Different Factors on SA-AgNPs Biosynthesis
2.3.1. Effect of Ultrasonic Time
2.3.2. Effect of Volume Ratio
2.3.3. Effect of pH
2.4. Characterization of SA-AgNP
2.5. Assay of Salicylic Acid
2.6. Assay of Silver Ions
2.7. Stability of SA-AgNPs
2.8. Antimicrobial Activity of SA-AgNPs
2.8.1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration (MIC and MBC)
2.8.2. Time-Kill Assay
2.9. Antibiofilm Ability Studies
2.9.1. Inhibition of Biofilm Formation
2.9.2. Live and Dead Bacteria Assay
2.10. Determination of Leakage of DNA, RNA, and Protein Concentrations in Bacterial Cell
2.11. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis of Salicylic Acid-Silver Nanoparticles (SA-AgNPs)
3.2. Study of the Effect of Different Factors on the Biosynthesis of SA-AgNPs
3.2.1. Effect of Ultrasonic Time
3.2.2. Effect of Volume Ratio
3.2.3. Effect of pH
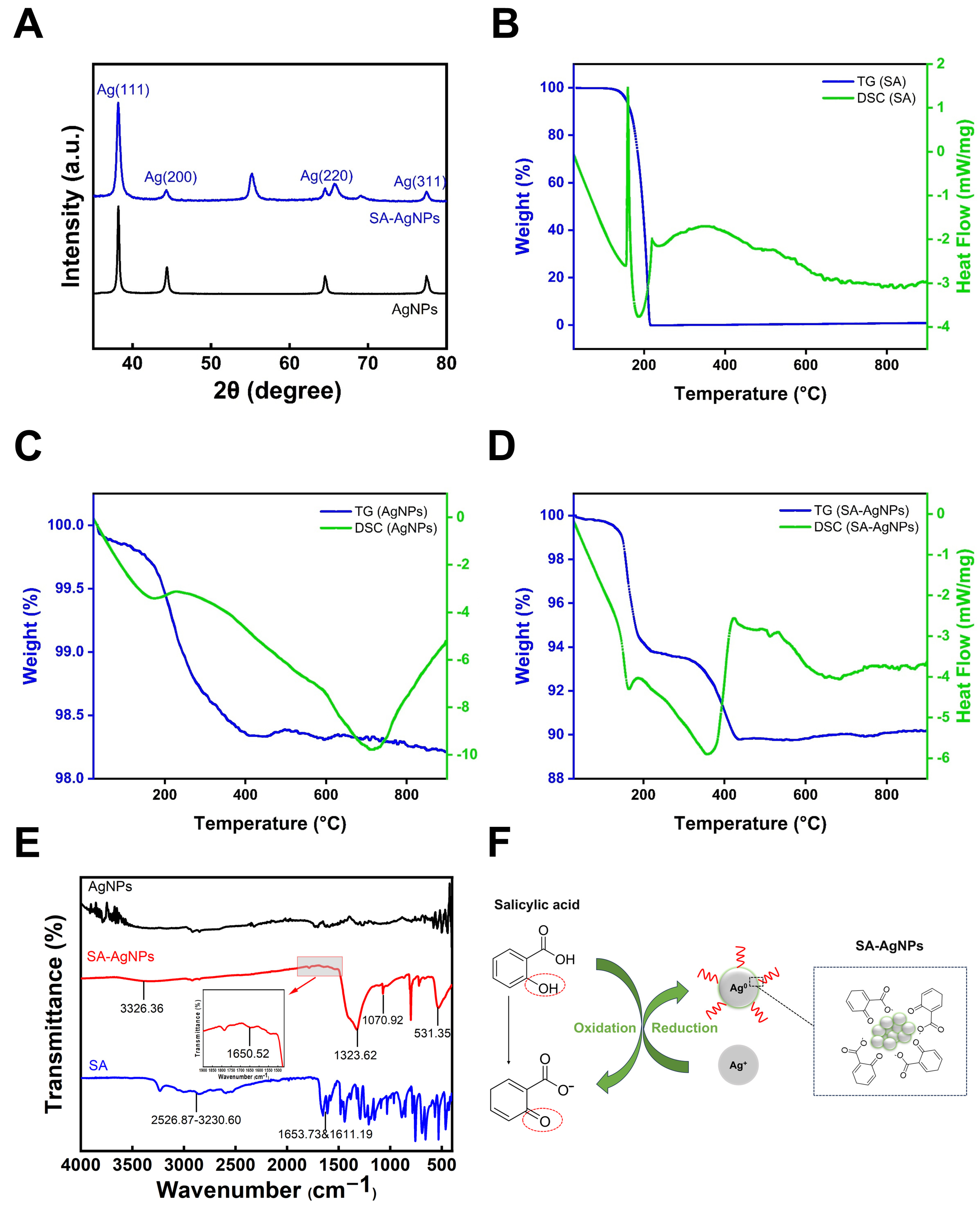
3.3. Characterization of SA-AgNPs
3.3.1. UV-Visible Spectrophotometer Analysis
3.3.2. Hydrodynamic Particle Size and Zeta Potential Measurement
3.3.3. Transmission Electron Microscopy (TEM)
3.3.4. Scanning Electron Microscope-Energy Dispersive X-Ray Spectroscopy (SEM-EDX)
3.3.5. X-Ray Diffraction Analysis
3.3.6. Thermogravimetric-Differential Scanning Calorimetry (TG-DSC)
3.3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Assay of Salicylic Acid
3.5. Assay of Silver Ions
3.6. Stability of SA-AgNPs
3.7. Antibacterial Activity of SA-AgNPs
3.7.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
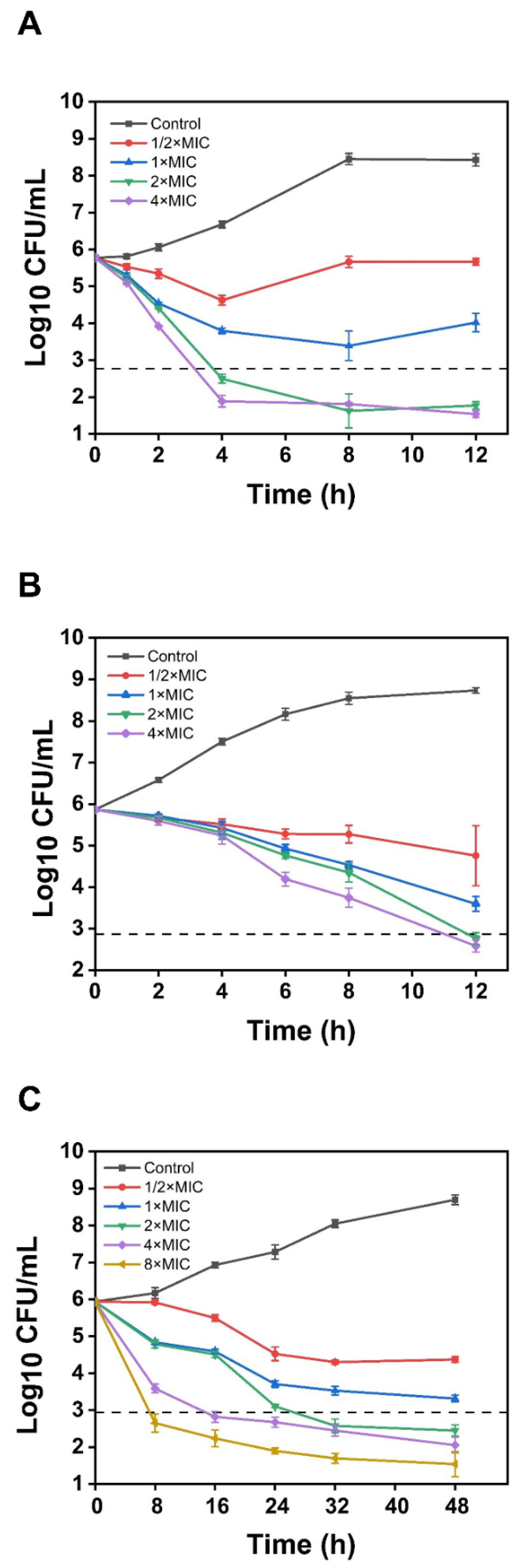
3.7.2. Time-Kill Assay
3.8. Antibiofilm Ability Studies
3.8.1. Biofilm Formation Inhibition
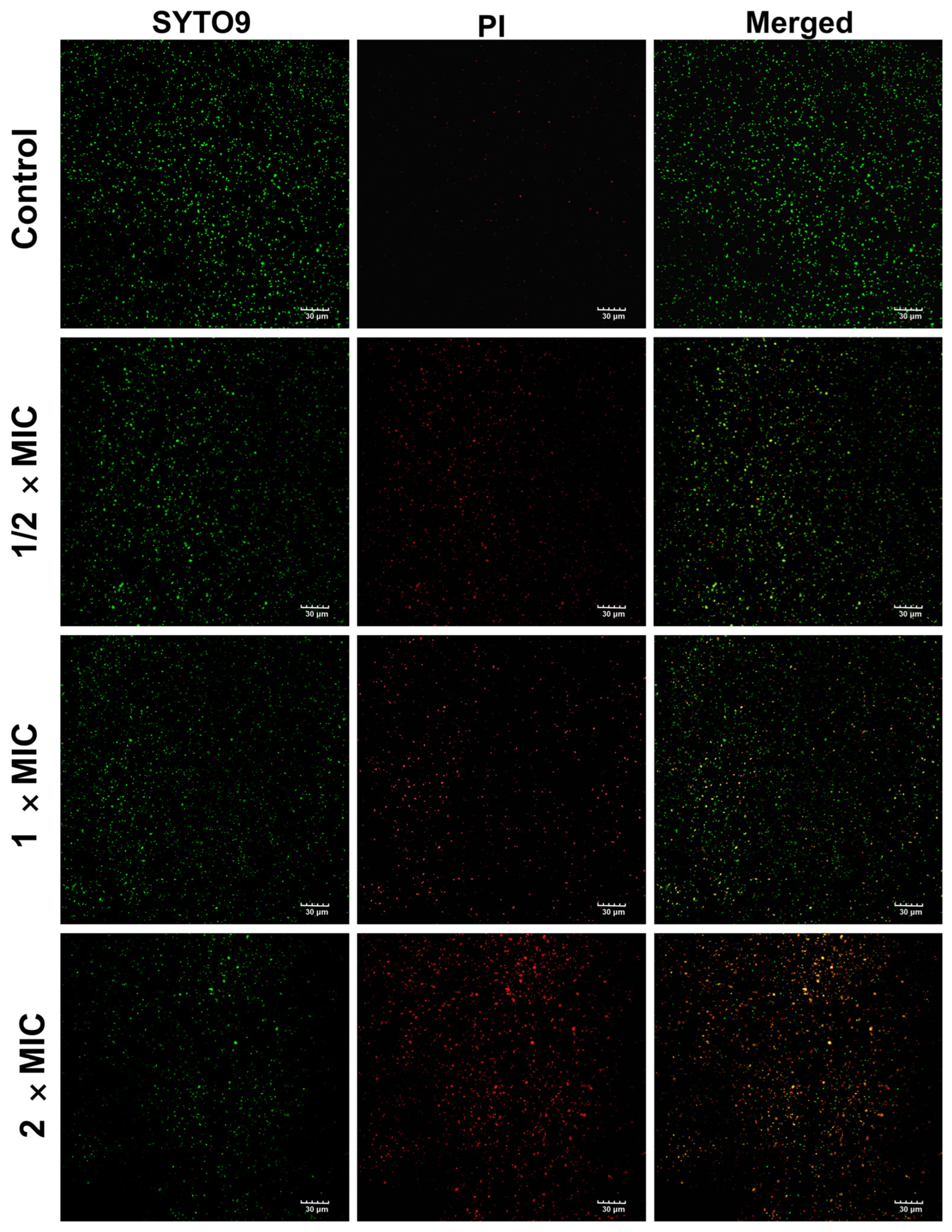
3.8.2. Live and Dead Bacteria Assay
3.9. Effect of SA-AgNPs on the Leakage of DNA, RNA, and Protein in Bacterial Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990-2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S. A parallel and silent emerging pandemic: Antimicrobial resistance (AMR) amid COVID-19 pandemic. J. Infect. Public Health 2023, 16, 611–617. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, C.; Shao, L.Q. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Shi, J.J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Shah, S.Z.A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Ben Khedher, N.; et al. Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: A review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent advancements and unexplored biomedical applications of green synthesized Ag and Au nanoparticles: A review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wei, X.Y.; Zhong, L.; Chan, C.L.; Li, H.Y.; Sun, H.Z. Metal-based approaches for the fight against antimicrobial resistance: Mechanisms, opportunities, and challenges. J. Am. Chem. Soc. 2025, 147, 12361–12380. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, M.J.; Xu, X.H.; Gao, P.; Xu, Z.L.; Zhang, Q.; Li, H.Y.; Yan, A.X.; Kao, R.Y.T.; Sun, H.Z. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Qiao, X.L.; Chen, J.G.; Wang, X.J.; Ding, S.Y. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; del Val, J.; Pou, J. Production of silver nanoparticles by laser ablation in open air. Appl. Surf. Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications-a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mandavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Rónavari, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Biao, L.H.; Tan, S.N.; Zhang, X.W.; Gao, J.; Liu, Z.G.; Fu, Y.J. Synthesis and characterization of proanthocyanidins-functionalized Ag nanoparticles. Colloids Surf. B-Biointerfaces 2018, 169, 438–443. [Google Scholar] [CrossRef]
- Sun, C.C.; Zhi, H.X.; Li, H.; Li, J.C.; Shao, K.; Lin, Y.M.; Fu, Y.J.; Liu, Z.G. Synthesis, characterization and antimicrobial study of cinnamic acid functionalized Ag nanoparticles. Nanocomposites 2022, 8, 95–101. [Google Scholar] [CrossRef]
- Hairil Anuar, A.H.; Abd Ghafar, S.A.; Hanafiah, R.M.; Lim, V.; Mohd Pazli, N.F.A. Critical evaluation of green synthesized silver nanoparticles-kaempferol for antibacterial activity against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2024, 19, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.J.; Yuan, Y.; Liu, Y.W.; Niu, F.L. Green synthesis of gallic acid-coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells. Process Biochem. 2015, 50, 357–366. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Noh, H.J.; Kim, H.S.; Jun, S.H.; Kang, Y.H.; Cho, S.; Park, Y. Biogenic silver nanoparticles with chlorogenic acid as a bioreducing agent. J. Nanosci. Nanotechnol. 2013, 13, 5787–5793. [Google Scholar] [CrossRef]
- Tasca, F.; Antiochia, R. Biocide Activity of Green Quercetin-Mediated Synthesized Silver Nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef]
- Jones, A.W. Early drug discovery and the rise of pharmaceutical chemistry. Drug Test. Anal. 2011, 3, 337–344. [Google Scholar] [CrossRef]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Li, Y.; Si, Y.-F.; Fu, J.; Dong, H.; Sun, S.-S.; Zhang, F.; She, Y.-H.; Zhang, Z.-Q. Synthesis of nanosilver particles mediated by microbial surfactants and its enhancement of crude oil recovery. Energy 2023, 272, 127123. [Google Scholar] [CrossRef]
- Deng, S.P.; Zhang, J.Y.; Ma, Z.W.; Wen, S.W.; Tan, S.Z.; Cai, J.Y. Facile Synthesis of long-term stable silver nanoparticles by kaempferol and their enhanced antibacterial activity against Escherichia coli and Staphylococcus aureus. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2766–2778. [Google Scholar] [CrossRef]
- Kuzma, M.; Nyúl, E.; Mayer, M.; Fischer, E.; Perjési, P. HPLC analysis of in vivo intestinal absorption and oxidative metabolism of salicylic acid in the rat. Biomed. Chromatogr. 2016, 30, 2044–2052. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, X.; Kong, X.; Jiang, Y.; Mo, L.; Li, M.; Jin, Y.; Han, Y.; Li, X.L.; Jin, T.; et al. Monitoring of salicylic acid content in human saliva and its relationship with plasma concentrations. J. Pharm. Biomed. Anal. 2022, 219, 114961. [Google Scholar] [CrossRef] [PubMed]
- Katta, C.; Shaikh, A.S.; Bhale, N.; Jyothi, V.; Kaki, V.R.; Dikundwar, A.G.; Singh, P.K.; Shukla, R.; Mishra, K.; Madan, J. Naringenin-Capped Silver Nanoparticles Amalgamated Gel for the Treatment of Cutaneous Candidiasis. AAPS Pharmscitech 2023, 24, 126. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 204–216. [Google Scholar] [CrossRef]
- M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI: Wayne, PA, USA, 2012.
- M26-A; Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. CLSI: Wayne, PA, USA, 1999.
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Barabadi, H.; Mohammadzadeh, A.; Vahidi, H.; Rashedi, M.; Saravanan, M.; Talank, N.; Alizadeh, A. Penicillium chrysogenum-derived silver nanoparticles: Exploration of their antibacterial and biofilm inhibitory activity against the standard and pathogenic Acinetobacter baumannii compared to tetracycline. J. Clust. Sci. 2022, 33, 1929–1942. [Google Scholar] [CrossRef]
- Sonbol, H.; Mohammed, A.E.; Korany, S.M. Soil fungi as biomediator in silver nanoparticles formation and antimicrobial efficacy. Int. J. Nanomed. 2022, 17, 2843–2863. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Ullah, R.; Ahmad, M.; Ali, A.; Ullah, Z.; Ali, M.; Al-Joufi, F.A.; Zahoor, M.; Sher, H. Green synthesis of silver nanoparticles using Euphorbia wallichii leaf extract: Its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules 2022, 27, 3525. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, J.W.; Shin, U.C.; Lee, M.W.; Kim, D.J.; Kim, S.W. Inhibitory activity of silver nanoparticles synthesized using Lycopersicon Esculentum against biofilm formation in Candida species. Nanomaterials 2019, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.R.; Hannan, F.A.; Henari, F.; Akhtar, S. Effects of different parts of the okra plant (Abelmoschus esculentus) on the Phytosynthesis of silver nanoparticles: Evaluation of synthesis conditions, nonlinear optical and antibacterial properties. Nanomaterials 2022, 12, 4174. [Google Scholar] [CrossRef]
- Zuñiga-Miranda, J.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Heredia-Moya, J.; Vizuete, K.; Debut, A.; Barba-Ostria, C.; Coyago-Cruz, E.; et al. Phytosynthesis of silver nanoparticles using Mansoa alliacea (Lam.) A.H. gentry (Bignoniaceae) leaf extract: Characterization and their biological activities. Pharmaceutics 2024, 16, 1247. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Vieira, Í.R.S.; Souza, L.M.d.S.; Florêncio, I.; Silva, I.G.M.d.; Tavares Junior, A.G.; Machado, Y.A.A.; Santos, L.C.d.; Taube, P.S.; Nakazato, G.; et al. Green synthesis of silver nanoparticles using Paullinia cupana Kunth leaf extract collected in different seasons: Biological studies and catalytic properties. Pharmaceutics 2025, 17, 356. [Google Scholar] [CrossRef]
- Kurra, H.; Velidandi, A.; Sarvepalli, M.; Pabbathi, N.P.P.; Godishala, V. Aqueous cymbopogon citratus extract mediated silver nanoparticles: Part I. influence of synthesis parameters, characterization, and biomedical studies. Nanomaterials 2025, 15, 328. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle?: A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Smith, S.L.; Nissamudeen, K.M.; Philip, D.; Gopchandran, K.G. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2008, 71, 186–190. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B-Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dhaliwal, A.S. Novel green synthesis and characterization of the antioxidant activity of silver nanoparticles prepared from Nepeta leucophylla root extract. Anal. Lett. 2019, 52, 213–230. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Abdel-Rahman, R.M.; Fouda, M.M.G.; Vojtova, L.; Uhrova, L.; Hassan, A.F.; Al-Deyab, S.S.; El-Shamy, I.E.; Jancar, J. Preparation, characterization and cytotoxicity of schizophyllan/silver nanoparticle composite. Carbohydr. Polym. 2014, 102, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ahani, M.; Khatibzadeh, M. Green synthesis of silver nanoparticles using gallic acid as reducing and capping agent: Effect of pH and gallic acid concentration on average particle size and stability. Inorg. Nano-Met. Chem. 2022, 52, 234–240. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.H.; Yadav, D.; Mitra, S.; Mukhopadhyay, K. Biosynthesis of silver nanoparticles using culture supernatant of Shewanella sp. ARY1 and their antibacterial activity. Int. J. Nanomed. 2020, 15, 8295–8310. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Adil, S.F.; Tahir, M.N.; Tremel, W.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomed. 2013, 8, 1507–1516. [Google Scholar] [CrossRef]
- Mat Yusuf, S.N.A.; Che Mood, C.N.A.; Ahmad, N.H.; Sandai, D.; Lee, C.K.; Lim, V. Optimization of biogenic synthesis of silver nanoparticles from flavonoid-rich Clinacanthus nutans leaf and stem aqueous extracts. R. Soc. Open Sci. 2020, 7, 200065. [Google Scholar] [CrossRef]
- Mandal, D.; Dash, S.K.; Das, B.; Chattopadhyay, S.; Ghosh, T.; Das, D.; Roy, S. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed. Pharmacother. 2016, 83, 548–558. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, S.L.; Li, K.; Chen, J.; Bae, B.; Hwang, I.; Ahn, E.Y.; Park, Y.; Chun, K.H.; Lee, J. Ecofriendly and enhanced biogenic synthesis of silver nanoparticles using deep eutectic solvent-based green tea extracts. J. Clean. Prod. 2022, 379, 134655. [Google Scholar] [CrossRef]
- Devanesan, S.; Ponmurugan, K.; AlSalhi, M.S.; Al-Dhabi, N.A. Cytotoxic and Antimicrobial efficacy of silver nanoparticles synthesized using a traditional phytoproduct, Asafoetida Gum. Int. J. Nanomed. 2020, 15, 4351–4362. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.S.; Cheng, W.J.; Ma, Y.F.; Zhang, Y.M.; Li, M.S.; Zheng, Y.G.; Zhang, D.S.; Wu, L.F. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Zingiber officinale and its application in synthesis of silver nanoparticles. Front. Nutr. 2022, 9, 917094. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.; Ramesh, B.; Kumaran, S.; Radhakrishnan, M.; Saravanan, D.; Saravanan, P.; Pugazhvendan, S.R.; Nalinasundari, M.S. Development of nanobiomaterial for wound healing based on silver nanoparticles loaded on chitosan hydrogel. 3 Biotech 2021, 11, 490. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.Y.; Zhang, J.C.; Zhang, L.G.; Wang, W.; Zhan, J.B.; Liao, Y.; Wu, C.Y.; Yu, W.; Zhang, J.H. A matrine-based supramolecular ionic salt that enhances the water solubility, transdermal delivery, and bioactivity of salicylic acid. Chem. Eng. J. 2023, 468, 143480. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Goto, M. Characterization and cytotoxicity evaluation of biocompatible amino acid esters used to convert salicylic acid into ionic liquids. Int. J. Pharm. 2018, 546, 31–38. [Google Scholar] [CrossRef]
- Begum, I.; Ameen, F.; Soomro, Z.; Shamim, S.; AlNadhari, S.; Almansob, A.; Al-Sabri, A.; Arif, A. Facile fabrication of malonic acid capped silver nanoparticles and their antibacterial activity. J. King Saud Univ.-Sci. 2021, 33, 101231. [Google Scholar] [CrossRef]
- Setyorini, D.A.; Noviandri, I.; Amran, M.B.; Rizki, W.O.S.; Serunting, M.A. A green-micro-synthesis of curcumin functionalized silver nanoparticles for bacteria inhibition and glucose sensor electrode modifier. Mater. Today Sustain. 2024, 25, 100648. [Google Scholar] [CrossRef]
- Al Baloushi, K.S.Y.; Senthilkumar, A.; Kandhan, K.; Subramanian, R.; Kizhakkayil, J.; Ramachandran, T.; Shehab, S.; Kurup, S.S.; Alyafei, M.A.M.; Dhaheri, A.S.A.; et al. Green synthesis and characterization of silver nanoparticles using moringa peregrina and their toxicity on MCF-7 and Caco-2 human cancer cells. Int. J. Nanomed. 2024, 19, 3891–3905. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Ji, Z.J.; Ra, M.; Yu, B.; Shen, Y.Q.; Cong, H.L. Nature-inspired biogenic synthesis of silver nanoparticles for antibacterial applications. Mater. Today Chem. 2023, 27, 101339. [Google Scholar] [CrossRef]
- Baruah, K.; Haque, M.; Langbang, L.; Das, S.; Aguan, K.; Roy, A.S. Ocimum sanctum mediated green synthesis of silver nanoparticles: A biophysical study towards lysozyme binding and anti-bacterial activity. J. Mol. Liq. 2021, 337, 116422. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.F.; Huang, C.Z. Detection of ferulic acid based on the plasmon resonance light scattering of silver nanoparticles. Talanta 2007, 72, 1698–1703. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Swami, A.; Srisathiyanarayanan, D.; Shirude, P.S.; Pasricha, R.; Mandale, A.B.; Sastry, M. Synthesis of Aqueous Au Core–Ag shell nanoparticles using tyrosine as a pH-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air–water interface. Langmuir 2004, 20, 7825–7836. [Google Scholar] [CrossRef]
- Bhutto, A.A.; Kalay, Ş.; Sherazi, S.T.H.; Culha, M. Quantitative structure–activity relationship between antioxidant capacity of phenolic compounds and the plasmonic properties of silver nanoparticles. Talanta 2018, 189, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M.; Maczka, E. Zeta potential and particle size in dispersions of alumina in 50-50 w/w ethylene glycol-water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130168. [Google Scholar] [CrossRef]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef]
- Yan, Y.; Li, G.; Su, M.; Liang, H. Scutellaria baicalensis polysaccharide-mediated green synthesis of smaller silver nanoparticles with enhanced antimicrobial and antibiofilm activity. ACS Appl. Mater. Interfaces 2024, 16, 45289–45306. [Google Scholar] [CrossRef]
- Shao, Y.; Luan, Y.; Hao, C.; Song, J.; Li, L.; Song, F. Antimicrobial protection of two controlled release silver nanoparticles on simulated silk cultural relic. J. Colloid Interface Sci. 2023, 652, 901–911. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; MacGregor-Ramiasa, M.; Zilm, P.; Majewski, P.; Vasilev, K. ‘Chocolate’ silver nanoparticles: Synthesis, antibacterial activity and cytotoxicity. J. Colloid Interface Sci. 2016, 482, 151–158. [Google Scholar] [CrossRef]
- Beitelshees, M.; Hill, A.; Jones, C.H.; Pfeifer, B.A. Phenotypic variation during biofilm formation: Implications for anti-biofilm therapeutic design. Materials 2018, 11, 1086. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Ajlouni, A.W.; Hamdan, E.H.; Alshalawi, R.A.E.; Shaik, M.R.; Khan, M.; Kuniyil, M.; Alwarthan, A.; Ansari, M.A.; Khan, M.; Alkhathlan, H.Z.; et al. Green synthesis of silver nanoparticles using aerial part extract of the Anthemis pseudocotula boiss. Plant and their biological activity. Molecules 2023, 28, 246. [Google Scholar] [CrossRef] [PubMed]





| 0 d | 2 d | 5 d | 7 d | 14 d | 28 d | 35 d | 49 d | 65 d | |
|---|---|---|---|---|---|---|---|---|---|
| λmax (nm) | 411 | 415 | 406 | 405 | 407 | 406 | 408 | 407 | 409 |
| Absorbance | 0.894 | 0.883 | 0.878 | 0.883 | 0.865 | 0.842 | 0.837 | 0.82 | 0.789 |
| Compound | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| C. acnes | E. coli | MRSA | MRSE | S. aureus | |
| SA-AgNPs | 9 | 6 | 8 | 4 | 8 |
| Salicylic Acid | 1800 | 800 | 800 | 800 | 800 |
| Commercial AgNPs | 300 | 200 | 256 | 64 | 256 |
| Ampicillin | 0.5 | - | >512 | 128 | 1 |
| Compound | MBC (µg/mL) | ||||
|---|---|---|---|---|---|
| C. acnes | E. coli | MRSA | MRSE | S. aureus | |
| SA-AgNPs | 18 | 12 | 16 | 8 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, Y.; Xu, Y.; Zhao, Z.; Xu, X. Salicylic Acid-Mediated Silver Nanoparticle Green Synthesis: Characterization, Enhanced Antimicrobial, and Antibiofilm Efficacy. Pharmaceutics 2025, 17, 532. https://doi.org/10.3390/pharmaceutics17040532
Zhang J, Chen Y, Xu Y, Zhao Z, Xu X. Salicylic Acid-Mediated Silver Nanoparticle Green Synthesis: Characterization, Enhanced Antimicrobial, and Antibiofilm Efficacy. Pharmaceutics. 2025; 17(4):532. https://doi.org/10.3390/pharmaceutics17040532
Chicago/Turabian StyleZhang, Jingqing, Yuxu Chen, Yuanyu Xu, Zhimin Zhao, and Xinjun Xu. 2025. "Salicylic Acid-Mediated Silver Nanoparticle Green Synthesis: Characterization, Enhanced Antimicrobial, and Antibiofilm Efficacy" Pharmaceutics 17, no. 4: 532. https://doi.org/10.3390/pharmaceutics17040532
APA StyleZhang, J., Chen, Y., Xu, Y., Zhao, Z., & Xu, X. (2025). Salicylic Acid-Mediated Silver Nanoparticle Green Synthesis: Characterization, Enhanced Antimicrobial, and Antibiofilm Efficacy. Pharmaceutics, 17(4), 532. https://doi.org/10.3390/pharmaceutics17040532






