1. Introduction
Tuberculosis, an ancient and intractable infectious disease caused by Mycobacterium tuberculosis, continues to pose a significant public health threat globally [
1]. According to the latest data from the World Health Organization, there are still a large number of new cases every year, and the emergence of drug-resistant strains has made the treatment situation even more severe [
2]. Macrophages, as a crucial line of defense in the body’s innate immunity [
3], play a central role in the infection process of Mycobacterium tuberculosis [
4]. During the complex interaction between Mycobacterium tuberculosis and macrophages, macrophages exhibit different polarization states, which have a decisive impact on the development of the disease [
5]. M1 macrophages, usually polarized under the stimulation of interferon-γ (IFN-γ) and lipopolysaccharide (LPS), can secrete a variety of pro-inflammatory cytokines such as inducible nitric oxide synthase (iNOS), interleukin (IL-1), and tumor necrosis factor-α (TNF-α), and resist pathogen invasion by releasing antibacterial substances such as reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) [
6]. However, Mycobacterium tuberculosis has evolved a variety of escape mechanisms that can inhibit the M1 polarization of macrophages, thus surviving and multiplying safely within macrophages [
7].
Exosomes, as extracellular vesicles with a diameter of approximately 30–150 nm, are secreted by a variety of cells into the extracellular environment. They encapsulate bioactive substances such as proteins, nucleic acids, and lipids inside and possess the ability to transmit information among cells. In recent years, exosomes have emerged in the field of drug delivery due to their unique advantages such as their natural nanoscale size, good biocompatibility, low immunogenicity, and the ability to cross biological barriers [
8]. Through engineering modifications of exosomes, for example, by modifying specific targeting molecules on their surfaces or loading therapeutic drugs, the targeting ability towards specific cells and the therapeutic effect can be significantly improved.
In recent years, through research on cell surface molecules, many targets that can be used to regulate the targeting ability of exosomes have been discovered. Among them, CD47, as a transmembrane glycoprotein widely expressed on the surfaces of various cells, plays an important role in immune recognition and regulation [
9,
10]. Under normal physiological conditions, CD47 interacts with the signal regulatory protein α (SIRPα) on the surface of macrophages, transmitting a “don’t eat me” signal, thereby inhibiting the phagocytosis of self-cells or foreign substances by macrophages [
11]. This mechanism provides a theoretical basis for the modification of exosomes to target macrophages. By using the anti-CD47 antibody to engineer exosomes, it is possible to change the interaction mode between exosomes and macrophages, enhance the targeting ability of exosomes to macrophages, and then achieve more efficient drug delivery [
12]. Moreover, exosomes have the characteristics of “homing and tropism” to their tissues or cells of origin, so the use of macrophage-derived exosomes in this study for the construction of drug-carrying systems can indirectly further enhance their macrophage-targeting properties.
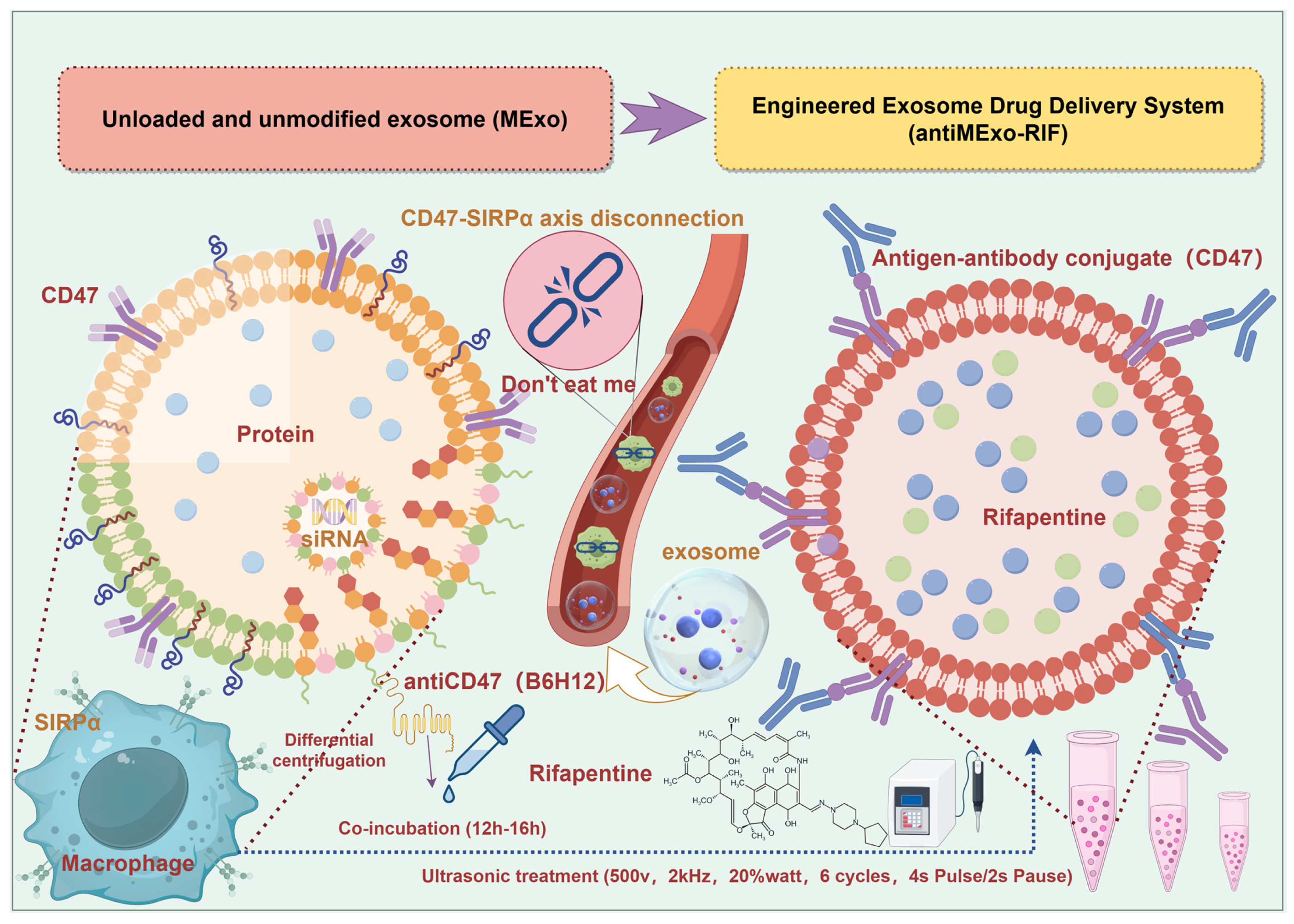
Based on the above background, this study has innovatively constructed an engineered exosome drug delivery system, aiming to take advantage of the excellent properties of exosomes to precisely deliver anti-tuberculosis drugs into macrophages. It is expected that by inducing the polarization of macrophages towards the M1 direction and activating relevant inflammatory pathways, macrophage apoptosis can be ultimately induced, thus breaking the survival shelter of Mycobacterium tuberculosis within macrophages and opening up new avenues for the development of novel and efficient anti-tuberculosis treatment strategies. Through a series of multi-omics analyses and cell function experiments, this study focused on exploring the regulatory mechanism of the engineered exosome drug delivery system on the immune effect of macrophages, providing a brand new theoretical basis and practical guidance for the treatment of tuberculosis.
2. Materials and Methods
2.1. Extraction, Modification, Drug Loading and Characterization of Exosomes (Scheme 1)
2.1.1. Exosome Extraction
The culture conditions for RAW264.7 cells are as follows: Dulbecco’s modified Eagle medium (DMEM) high-glucose (4.5 g/L glucose), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, maintained in a humidified incubator at 37 °C with 5% CO2 under saturated humidity. Before collecting the culture supernatant of macrophages, the cells should be cultured in exosome-free medium to eliminate the influence of serum-derived exosomes.
Scheme 1.
Schematic diagram of the preparation steps of the engineered exosome drug delivery system and the targeting principle of macrophages (By Figdraw, ID:ITSWS9f8e8).
Scheme 1.
Schematic diagram of the preparation steps of the engineered exosome drug delivery system and the targeting principle of macrophages (By Figdraw, ID:ITSWS9f8e8).
The collected cell culture supernatant is subjected to low-speed centrifugation, typically at 300× g for 10 min, to remove cells and larger cellular debris. This is followed by medium-speed centrifugation at 2000× g for 10–30 min to eliminate smaller cellular debris and large vesicles. Subsequently, ultracentrifugation is performed at 10,000× g for 30 min to remove medium-sized membrane vesicles and protein aggregates. Finally, ultracentrifugation is carried out at 100,000× g at 4 °C for 70 min to pellet exosomes. The supernatant was carefully discarded, and the precipitate was resuspended with an appropriate amount of phosphate buffers (PBS). Subsequently, ultracentrifugation was carried out again at 4 °C with the same rotation speed and time to further purify the exosomes. Finally, the obtained exosome precipitate was resuspended with a small amount of PBS and stored at −80 °C for later use.
2.1.2. Exosome Engineering Modification
The purified exosomes were incubated with the diluted (1:1000) anti-CD47 antibody (B6H12) at 4 °C for 12–16 h to ensure that the antibody had sufficient time to bind to the exosomes. Meanwhile, a control group was set up, in which the exosomes were only incubated in the same reaction buffer (Phosphate buffers, PBS) without the addition of the antibody.
2.1.3. Exosome Drug Loading
First, rifapentine was dissolved (dimethyl sulfoxide, DMSO) and mixed with exosomes at a ratio of 1:1. After ultrasonic treatment (500 v, 2 kHz, 20% watt, 6 cycles, 4s Pulse/2s Pause) using the ultrasonic method, the mixture was allowed to stand for one hour. Subsequently, the unloaded drugs were separated by centrifugation through an ultrafiltration tube (100 KD). The precipitate was collected and resuspended to obtain the exosome solution loaded with rifapentine.
Macrophage exosomes (MExo) and the engineered exosome delivery system (antiMExo-RIF) were prepared by the above methods.
2.1.4. Characterization of the Engineered Exosome Drug Loading System
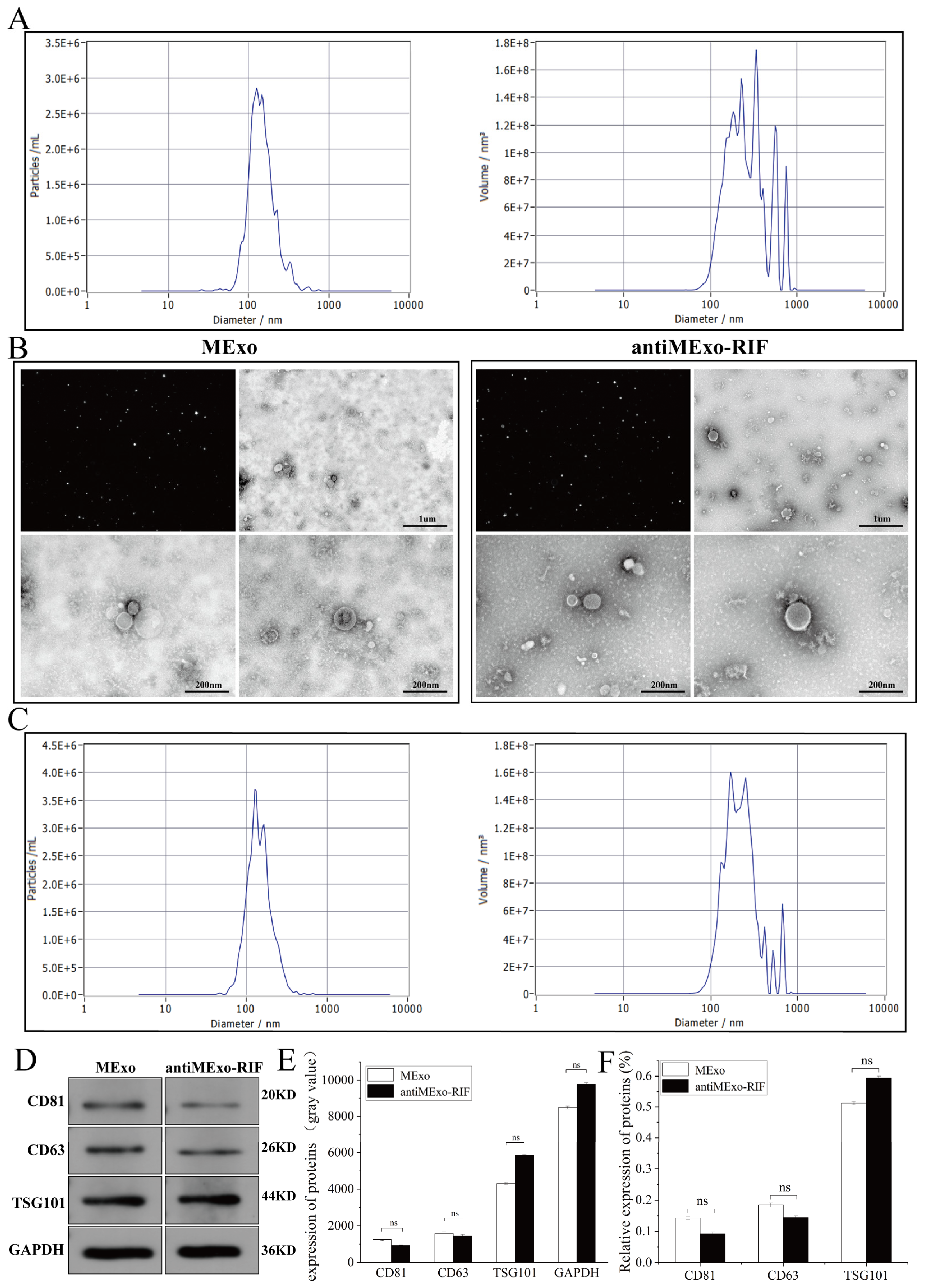
Transmission electron microscopy (TEM) (HITACHI, HT7800/HT7700, Tokyo, Japan): An appropriate amount of the exosome suspension was dropped onto a copper grid and negatively stained with a negative staining solution. Then, the morphology of the exosomes was observed under a transmission electron microscope. Typical exosomes are cup-shaped or round with a double-membrane structure.
Nanoparticle tracking analysis (NTA) (Particle Metrix, ZetaView, Hallbergmoos Germany). Using a nanoparticle tracking analyzer, the exosome suspension was diluted to an appropriate concentration, and then its particle size distribution and concentration were analyzed. According to the NTA results, it was confirmed that the particle size of exosomes was mainly distributed between 30 and 150 nm, and the concentration information of exosomes was obtained at the same time.
Western blot (WB). The concentration of exosomes and protein extracted from cell samples was determined using the BCA assay. Protein samples were loaded into the wells of polyacrylamide gels (PAGE gels). Under an electric field, proteins migrated through the gel based on molecular weight for separation. The separated proteins were then transferred from the gel onto PVDF or nitrocellulose membranes, typically via electrophoretic or semi-dry transfer. The membranes were blocked with blocking buffer (5% skimmed milk) to reduce non-specific antibody binding. Subsequently, the membranes were incubated with primary antibodies specific to target proteins for 12 h, followed by washing to remove unbound primary antibodies. Horseradish peroxidase (HRP)-conjugated secondary antibodies were then applied for 2 h, and signals were detected using a chemiluminescence imager. The details of antibodies used are as follows: GAPDH (SanYing, #60004-1-Ig, 1:10,000, Hangzhou, China), CD63 (Abcam, #Ab216130, 1:1000, Cambridge, UK), CD81 (Abclonal, #A22528, 1:3000, Woburn, MA, USA), TSG101 (Affinity, #DF8427, 1:2000, Cincinnati, OH, USA), P65 (SanYing, #10745-1-AP, 1:3000), CD86 (SanYing, #68674-2-Ig, 1:3000), CD206 (SanYing, #18704-1-AP, 1:3000), iNOS (Affinity, #AF0199, 1:2000).
2.2. Validation of Macrophage Targeting in Engineered Exosome Drug Delivery Systems
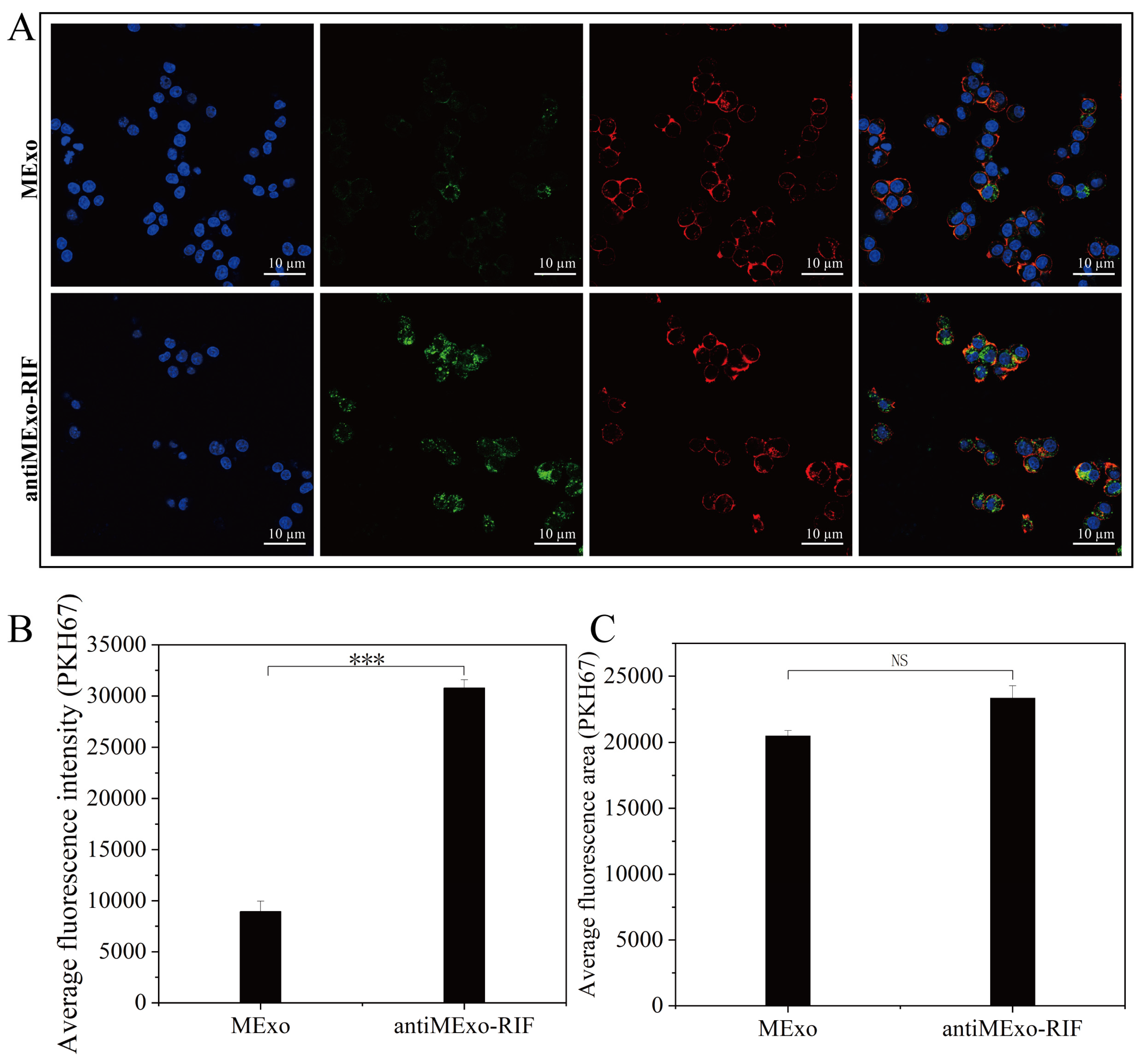
To validate the successful engineering of exosomes, PKH67 (MCE, 257277-27-3) labeled antiMExo-RIF (10 µg) was co-incubated with macrophages overnight (12), with a control group set for comparison. After fixation with 4% paraformaldehyde, nuclear staining (Servicebio, DAPI, G1012-100ML, Wuhan, China) and cytoskeletal staining (Life iLab, Alexa Fluor 555, AC8L022, Beijing, China) were performed. Subsequently, images were captured using confocal microscopy (Germany, Zeiss LSM 880, Oberkochen, Germany), and quantitative analysis of the average fluorescence intensity and fluorescence area was conducted.
2.3. Transcriptome Sequencing and Proteomic Quantitative Analysis
2.3.1. Sample Collection and Processing
After macrophages were altered by the engineered exosome drug delivery system (10 µg, 12), the macrophages were collected. For transcriptomics samples, total RNA was extracted using the TRIzol kit per instructions. Cells were first washed with cold PBS, then lysed with TRIzol reagent. After chloroform treatment and centrifugation, the aqueous phase was separated, and RNA was precipitated with isopropanol and washed with ethanol. The RNA pellet was then dissolved in DEPC-treated water. For proteomics samples, total protein was extracted using cell lysis buffer as instructed. Cells were washed with cold PBS, lysed with protease inhibitor-containing buffer, and sonicated if needed. After centrifugation to remove debris, the supernatant was collected as the total protein extract.
2.3.2. Transcriptome Sequencing and Proteomic Quantitative Analysis
After quality inspection of the RNA samples, transcriptome sequencing was carried out. The RNA libraries were sequenced by OE Biotech, Inc., Shanghai, China. The mass spectrometry analysis of peptides was performed using liquid chromatography–mass spectrometry (Thermo Scientific™, Waltham, MA, USA; Orbitrap™ Waltham, MA, USA; Astral™, Asheville, NC, USA). By comparing the signal intensities of corresponding peptides in different samples, the relative quantification of the proteins corresponding to the peptides was conducted. By comparing the information in the spectral library and integrating it into the corresponding proteins, a quantitative analysis of the relative expression levels of proteins in different samples was achieved.
2.3.3. Bioinformatics Analysis
Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were used to analyze the enrichment of the differentially expressed genes in aspects such as biological processes, cellular components, and molecular functions, as well as their enrichment in different signaling pathways, so as to reveal the main biological functions and pathways involved in the changes of gene expression in macrophages after the intervention of the engineered exosome drug delivery system.
For the proteomic data, after log transformation, two parameters were used to jointly evaluate the differences in protein expression between groups, namely, Fold change (FC, calculated from log2FC, where log2(Fold change) = mean of the experimental group—mean of the control group) and the p-value obtained through the t-test. The differential screening conditions were as follows: p-value < 0.05, FC ≥ 2.0 or FC ≤ 1/2.0. Subsequently, GO enrichment analysis and KEGG pathway analysis were used to analyze the enrichment of the differentially expressed proteins.
2.4. Verification of Macrophage Polarization and Key Inflammatory Pathways
According to the BCA assay, the exosome concentration was determined to be 1 μg/μL. Subsequently, 10 μg of MExo and antiMExo-RIF were co-cultured with macrophages for 12 h. After incubation, cell samples were collected, and total cellular protein was extracted. The expression levels of the M1 macrophage markers, inducible nitric oxide synthase (iNOS) and CD86, as well as the M2 macrophage marker CD206, were detected by Western blotting technology to evaluate the polarization state of the macrophages. The enzyme-linked immunosorbent assay (ELISA) kits were used to detect the contents of inflammatory factors such as iNOS, IL-1, and tumor necrosis factor-α (TNF-α) in the co-culture supernatant, reflecting the inductive effect of the engineered exosome drug delivery system on the inflammatory response of macrophages. Western blotting was adopted to detect the nuclear translocation of P65, a key protein in the NF-κB pathway, so as to verify whether the engineered exosomes activated the NF-κB pathway.
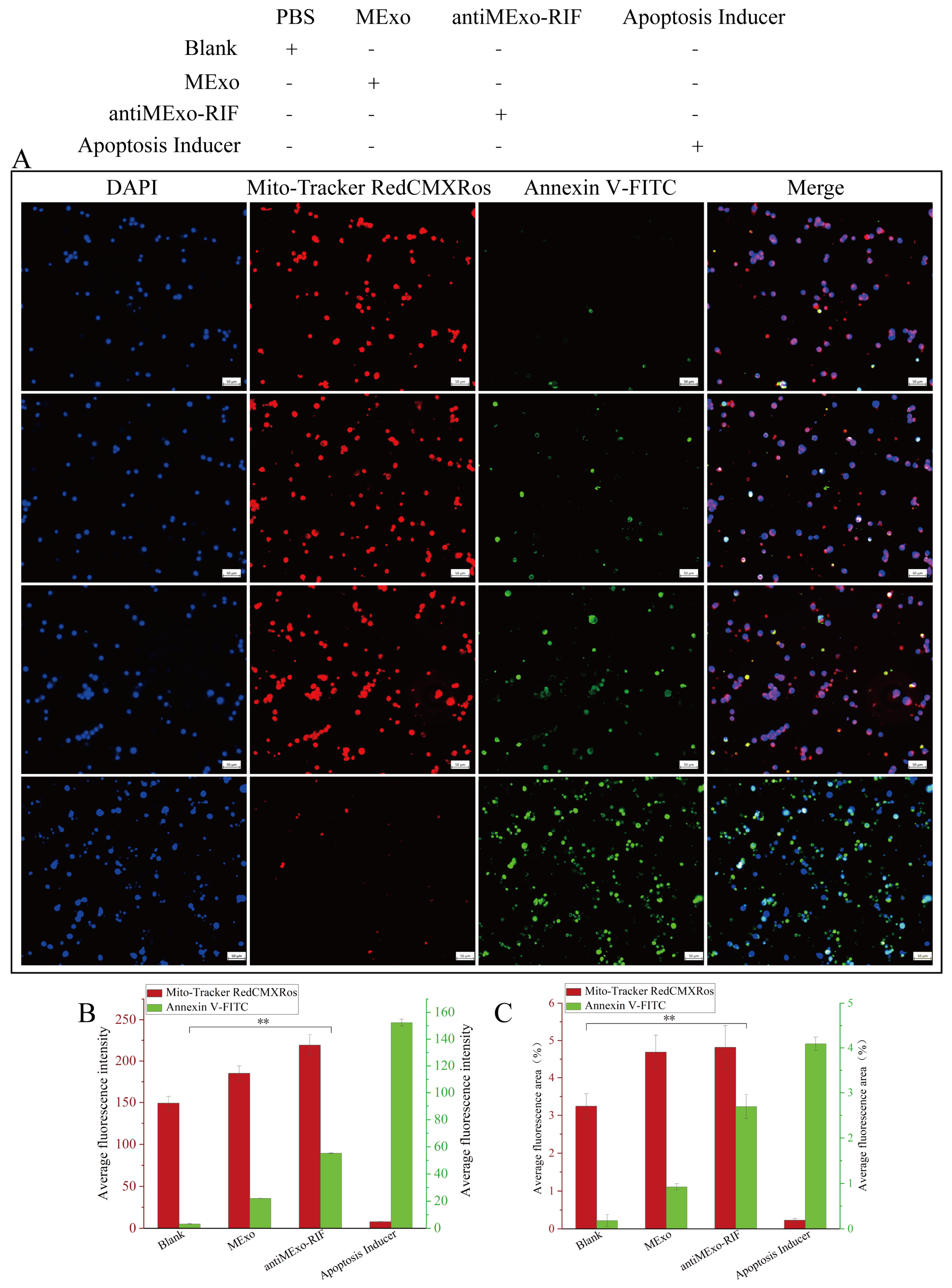
2.5. Macrophage Apoptosis
The Mitochondria Membrane Potential and Apoptosis Detection Kit with Mito-Tracker Red CMXRos and Annexin V-FITC was used to perform apoptosis staining on the macrophages after co-culturing. The live cells with maintained mitochondrial membrane potential were marked by red fluorescence, while the apoptotic cells were marked by green fluorescence.
The experimental data were derived from three independent replicate experiments. Descriptive statistics are presented as mean ± standard deviation. Comparisons between two groups were performed using the independent samples t-test, while comparisons among multiple groups were conducted using one-way analysis of variance (ANOVA).
4. Discussion
In this study, a macrophage-derived engineered exosome drug delivery system was successfully constructed to target macrophages, induce macrophage polarization towards M1, exert its pro-inflammatory and antibacterial effects, activate relevant inflammatory pathways to further promote the expression of inflammatory factors, and ultimately induce macrophage apoptosis, thereby destroying the living environment of Mycobacterium tuberculosis and achieving an antibacterial effect. From a molecular perspective, the mechanisms behind this phenomenon involve multiple aspects.
From the perspective of immune recognition, CD47, as an important “don’t eat me” signal molecule, interacts with SIRPα on the surface of macrophages and inhibits the phagocytic activity of macrophages under normal physiological conditions [
13,
14]. When we engineered the RAW264.7 exosomes with anti-CD47 antibody, the antibody bound to CD47 or related antigens on the surface of exosomes, effectively blocking this inhibitory signal. This is like removing a barrier that prevents macrophages from “eating”, enabling macrophages to recognize and phagocytose exosomes more actively [
15,
16]. The core principle of this engineered exosome-based drug delivery system resembles the Trojan Horse strategy from ancient Greek mythology. It involves encapsulating therapeutic agents (the soldiers) within exosomes (the wooden horse). Through engineered surface modifications, these exosomes can mislead macrophages (the city of Troy) into keeping their cell membrane channels open, thereby enabling entry into the cellular interior and achieving sustained drug release effects.
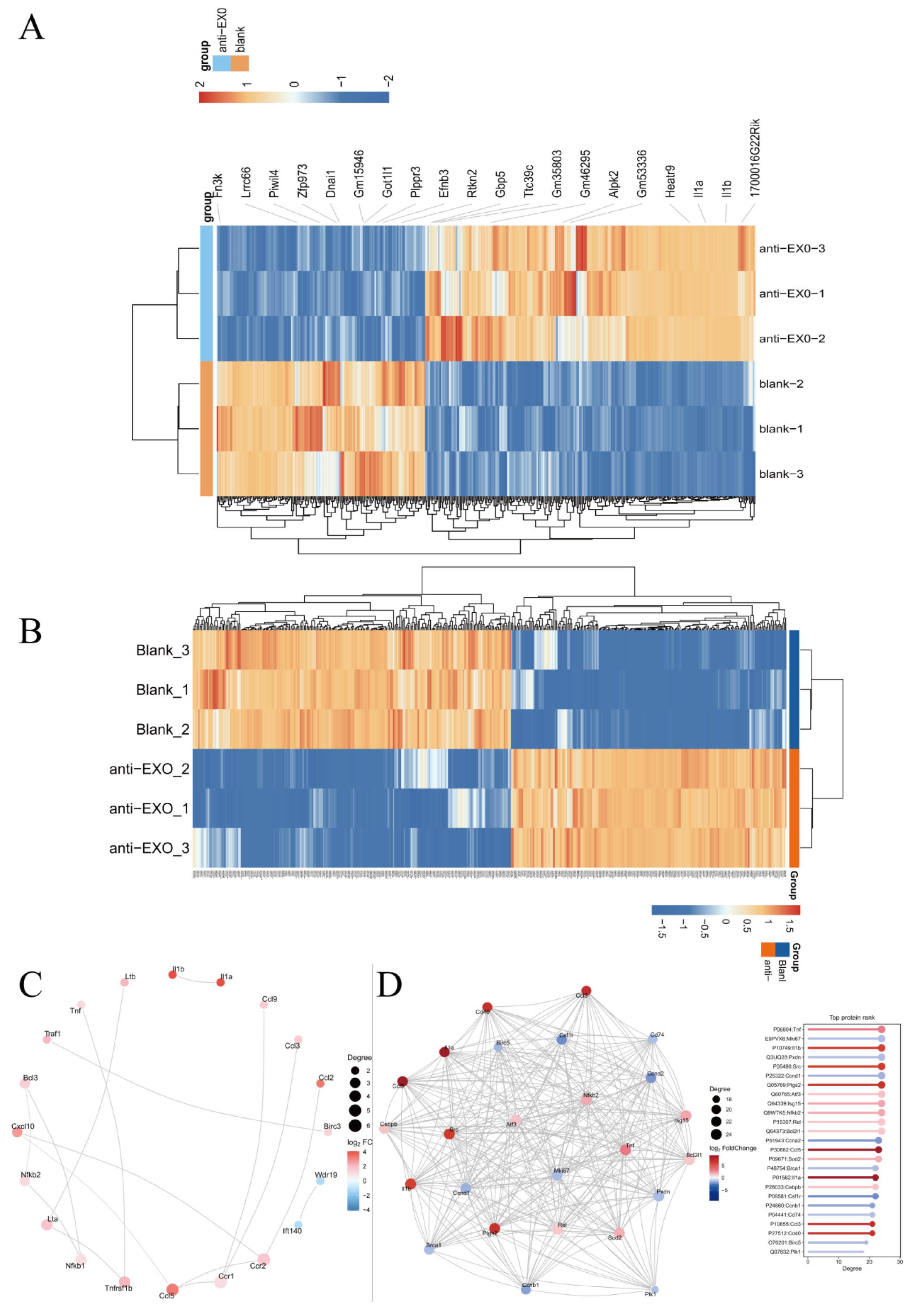
The highly differentially expressed genes and proteins screened by transcriptomics and proteomics were consistent and mutually verified. In particular, IL-1α and IL-1β, as important pro-inflammatory cytokines, play a central role in the immune activation of macrophages. They can induce the activation of multiple immune cells and promote the release of inflammatory mediators, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-1) [
17], which was also proven by the ELISA results. After the engineered exosome drug delivery system entered macrophages, the differential expressions of IL-1α and IL-1β indicated that they initiated a series of subsequent inflammatory cascade reactions in macrophages, including M1 polarization of macrophages and the activation of inflammatory pathways. Since IL-1α and IL-1β can regulate the phagocytic ability, antigen presentation function, and cytokine secretion pattern of macrophages [
18], they can enhance the phagocytosis of Mycobacterium tuberculosis by macrophages, promote antigen processing and presentation, and thus activate the adaptive immune response.
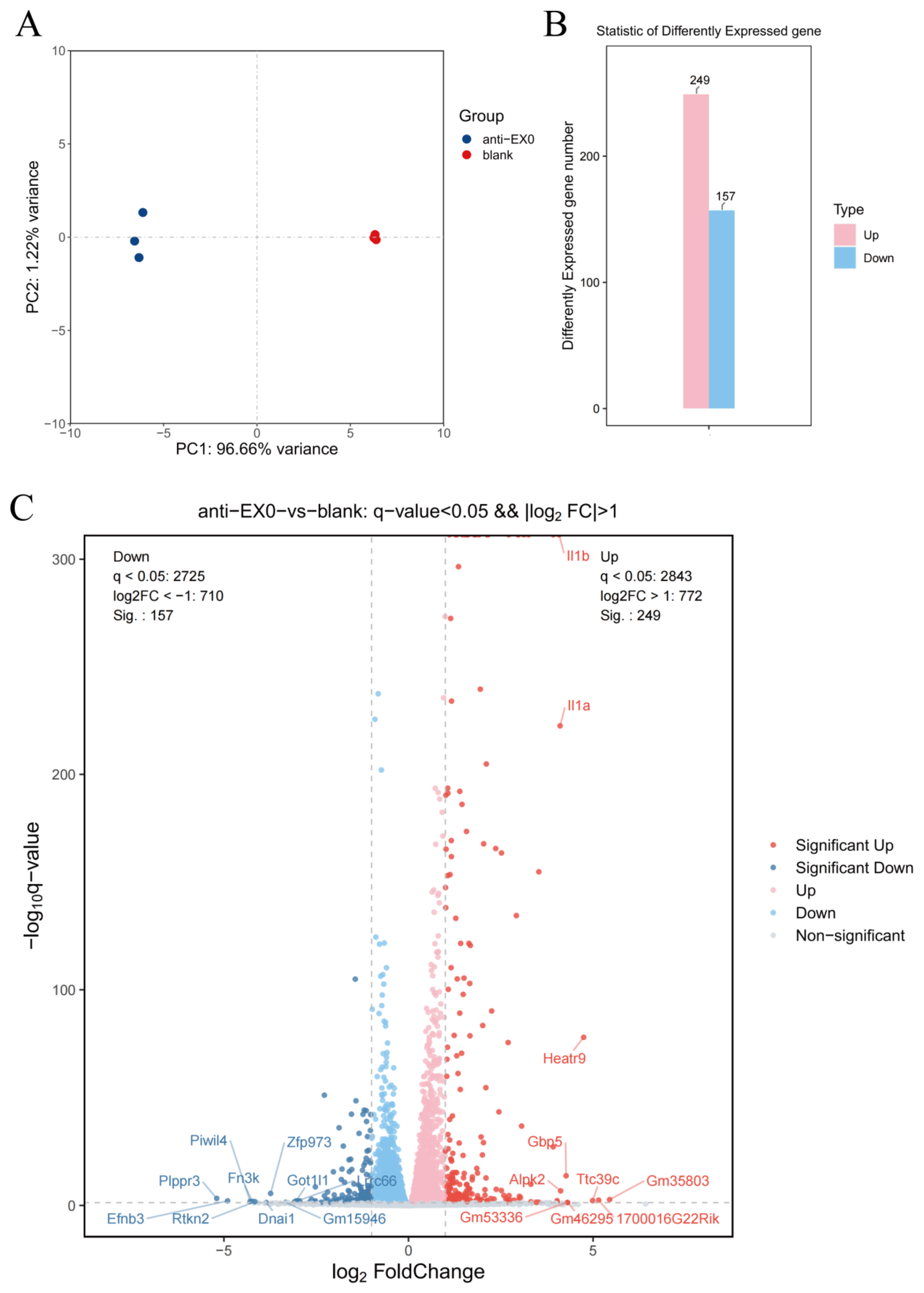
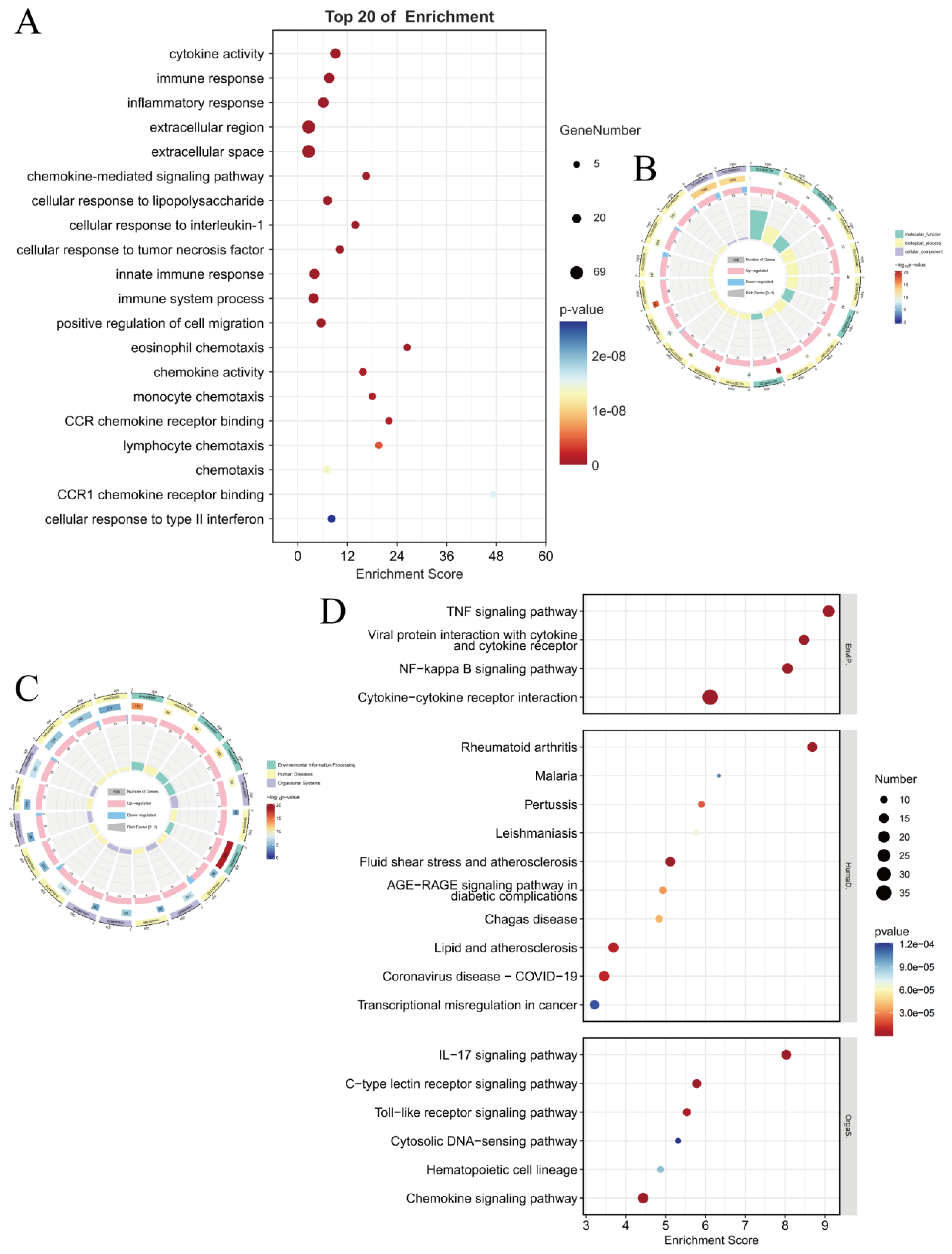
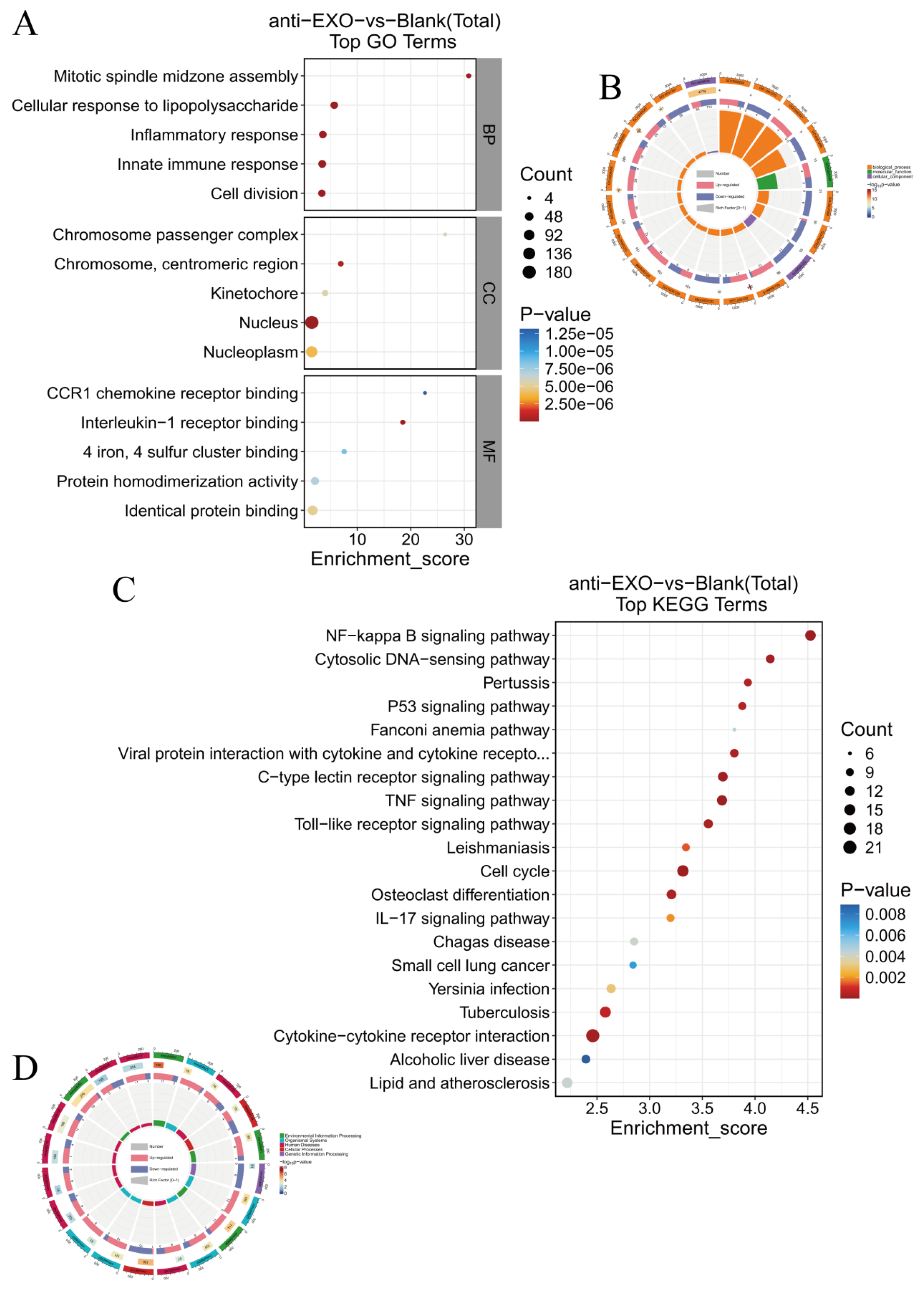
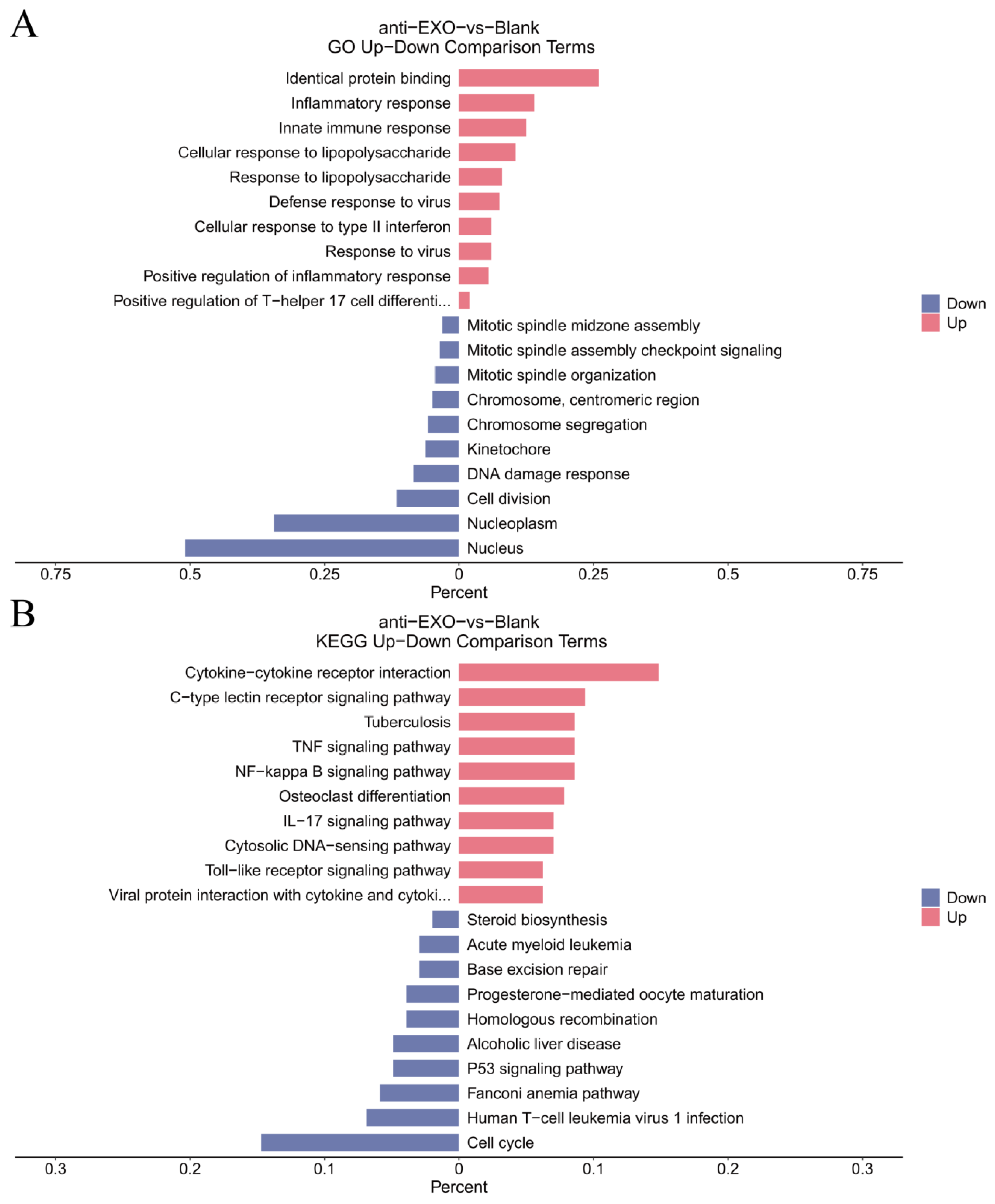
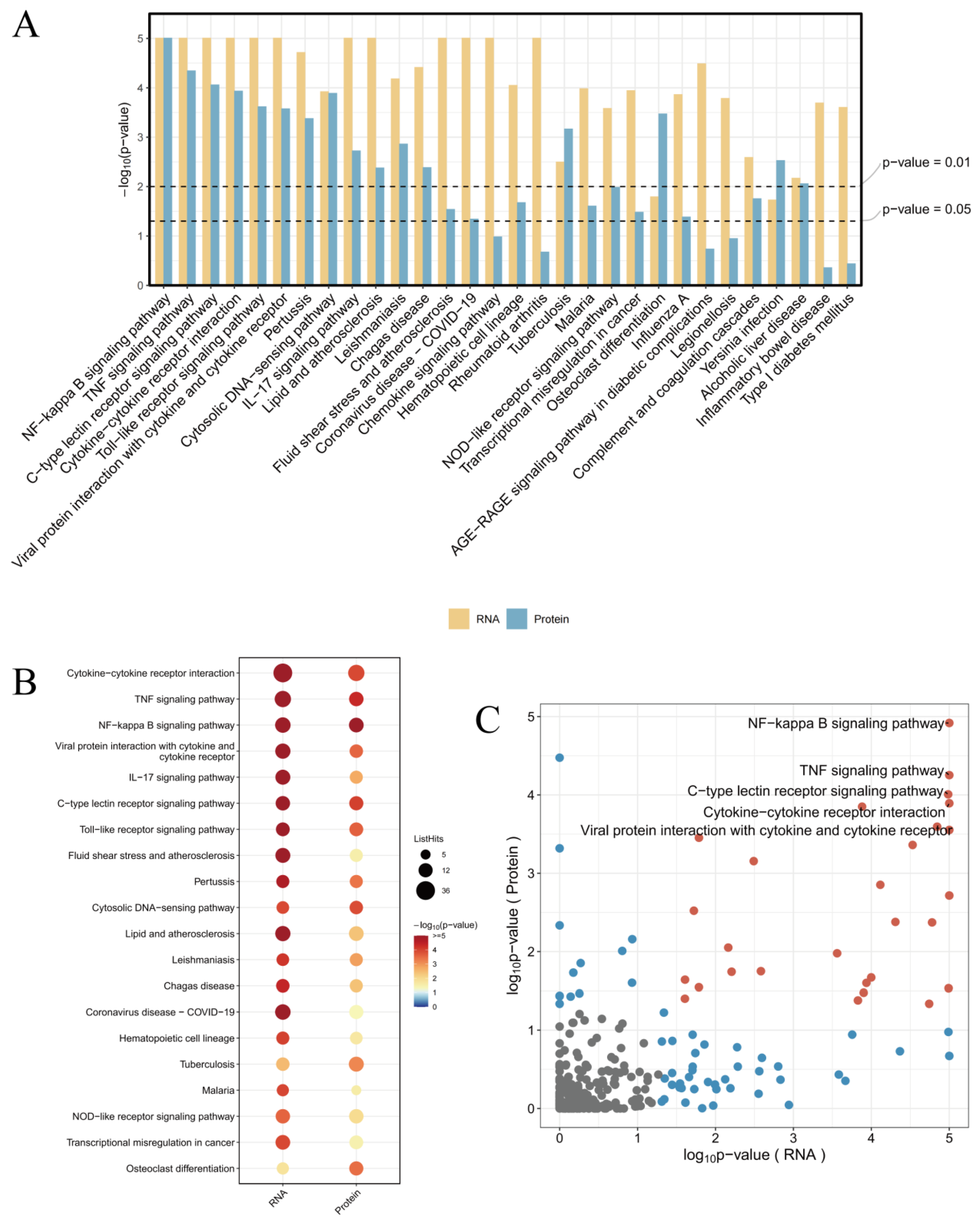
The combined analysis of transcriptomics and proteomics can comprehensively reveal the panorama of gene expression. By leveraging the complementary nature of transcriptomics and proteomics, more complete gene expression information can be obtained, enhancing the accuracy and reliability of the research. The mutual validation of multi-omics data helps reduce false-positive results. The study found that the enriched pathways showed consistency between transcriptomics and proteomics, particularly in the NF-κB signaling pathway, TNF signaling pathway, and Cytokine–cytokine receptor interaction, which were the most significant pathways (
Figure 9A). This indicates that these pathways are regulated by antiMExo-RIF at both the gene and protein expression levels. The bubble chart (
Figure 9B) shows that these pathways exhibit higher significance at the RNA level and involve a larger number of genes, while at the protein level, the significance is lower and fewer proteins are involved. This discrepancy may reflect the complex relationship between transcriptional regulation and translational regulation, as well as potential additional regulatory mechanisms at the protein level. However, the ranking of the number of genes and proteins involved in these pathways remains consistent. From the volcano plot (
Figure 9C), it can be observed that most signaling pathways show some correlation in significance between the RNA and protein levels, but some pathways deviate significantly from the diagonal. For example, the NF-κB signaling pathway, TNF signaling pathway, and Cytokine–cytokine receptor interaction exhibit high significance at both RNA and protein levels, and are located in the upper right quadrant of the scatter plot, indicating that they are tightly regulated at both levels.
The NF-κB signaling pathway is one of the key pathways that regulate immune and inflammatory responses [
19]. It can be activated by a variety of stimuli in macrophages, including pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and cytokines, etc. [
20]. In this study, the activation of the NF-κB signaling pathway has been verified. This may be caused by the differential expression of cytokines such as IL-1α and IL-1β after the engineered exosome drug delivery system enters macrophages. The activation of the NF-κB signaling pathway can lead to the expression of a series of downstream genes, further amplifying the inflammatory response [
21]. The engineered exosome drug delivery system may regulate the functions of macrophages by affecting the upstream regulatory factors or downstream effector molecules of the NF-κB signaling pathway. For example, specific components in exosomes may bind to receptors on the surface of macrophages to activate or inhibit the NF-κB signaling pathway. The specific mechanism still requires further in-depth research. Studying the mechanism of the NF-κB signaling pathway in the response of macrophages to the exosome drug delivery system can provide a basis for designing more effective treatment strategies. By regulating the activity of the NF-κB signaling pathway, the degree of the inflammatory response can be controlled and the therapeutic effect of the exosome drug delivery system can be improved.
The TNF signaling pathway is also an important immune and inflammatory regulatory pathway. TNF-α is a key molecule in this pathway, which can be produced by immune cells such as macrophages and plays an important role in the initiation and maintenance of the inflammatory response [
22]. The ELISA results also show that its expression level was significantly increased. The activation of the TNF signaling pathway may be correlated with the differential expression of IL-1α and IL-1β as well as the activation of the NF-κB signaling pathway [
23]. It can further activate other immune cells, amplify the inflammatory response, and also regulate the functions of macrophages, including multiple biological effects such as macrophage activation, cytokine secretion, and cell death [
24,
25]. Subsequent experiments verified that macrophages polarized towards M1 and induced the secretion of multiple cytokines (such as IL-1, iNOS) to participate in the pro-inflammatory process, ultimately inducing macrophage apoptosis.
Cytokine–cytokine receptor interaction exerts its biological functions by binding cytokines to receptors on the cell surface, and is one of the key aspects of immune regulation. It can regulate the activation, proliferation, differentiation, and functions of immune cells [
26,
27]. In this study, the increased secretion of differentially expressed cytokines (such as IL-1α, IL-1β, IL-6, iNOS, and TNF-α, etc.) led to an increase in specific binding with their receptors, which could trigger a cascade amplification of signal transduction. For example, after IL-1α and IL-1β bind to IL-1R1/IL-1RAcP, they activate the nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, and then induce the production of more cytokines. This cascade amplification effect can rapidly amplify the immune response, but it may also lead to excessive inflammatory responses [
28,
29,
30]. In-depth research into the role of Cytokine–cytokine receptor interaction in the response of macrophages to the exosome drug delivery system can provide clues for designing more effective treatment strategies [
31]. Combinations of multiple cytokines and receptors form a complex network. Different cytokines can bind to receptors simultaneously or sequentially, influencing each other’s signal transduction. This complex interaction determines the functional state of macrophages and the direction of the immune response.
The interaction between cytokines (TNF itself is also a cytokine) and their receptors can initiate or regulate the TNF signaling pathway [
32]. Meanwhile, the downstream signals activated by cytokine–receptor interactions can converge into the NF-κB signaling pathway. By regulating the activity of NF-κB, they participate in multiple physiological and pathological processes such as immune responses and inflammatory responses. These three pathways are intertwined and jointly play extremely important roles in numerous crucial cellular activities, such as cell signal transduction, immune responses, and inflammatory responses [
33].
Although this study has achieved a series of meaningful results, there are still some limitations. Although transcriptome sequencing and proteomic quantitative analyses have revealed changes in the expression of a large number of genes and proteins, the functional verification of some key molecules is not yet in-depth enough. Although we have found that many genes and proteins have undergone significant changes during the process of engineered exosomes interfering with macrophages, their specific action mechanisms in the entire immune effect process, such as whether there are direct interactions and whether there are upstream and downstream regulatory relationships, still need to be further studied in detail through molecular biology experiments (such as gene knockout, overexpression experiments, and protein–protein interaction experiments). Only by understanding the functions of these key molecules more deeply can we comprehensively elaborate the molecular mechanism of the interaction between engineered exosomes and macrophages and provide a more solid theoretical basis for their application in drug delivery systems.