Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Colon Cancer Stem Cells
2.3. Characterization of Isolated Colon Cancer Stem Cells
2.3.1. Spheroid Formation
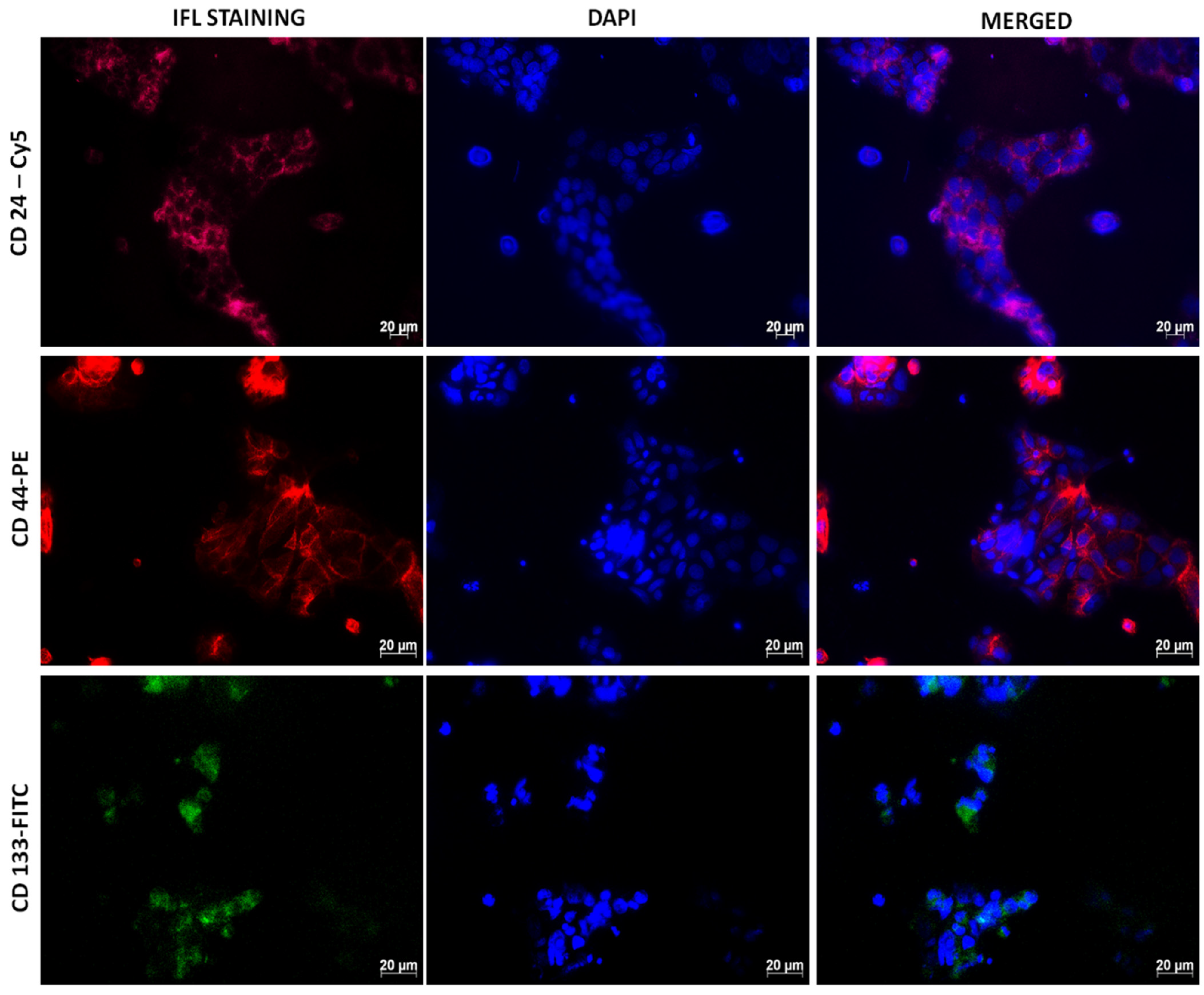
2.3.2. Immunofluorescence (IFL)
2.3.3. Flow Cytometry
2.4. Synthesis of Nanobiocomposite
2.5. Photodynamic Therapy on Isolated CCSCs
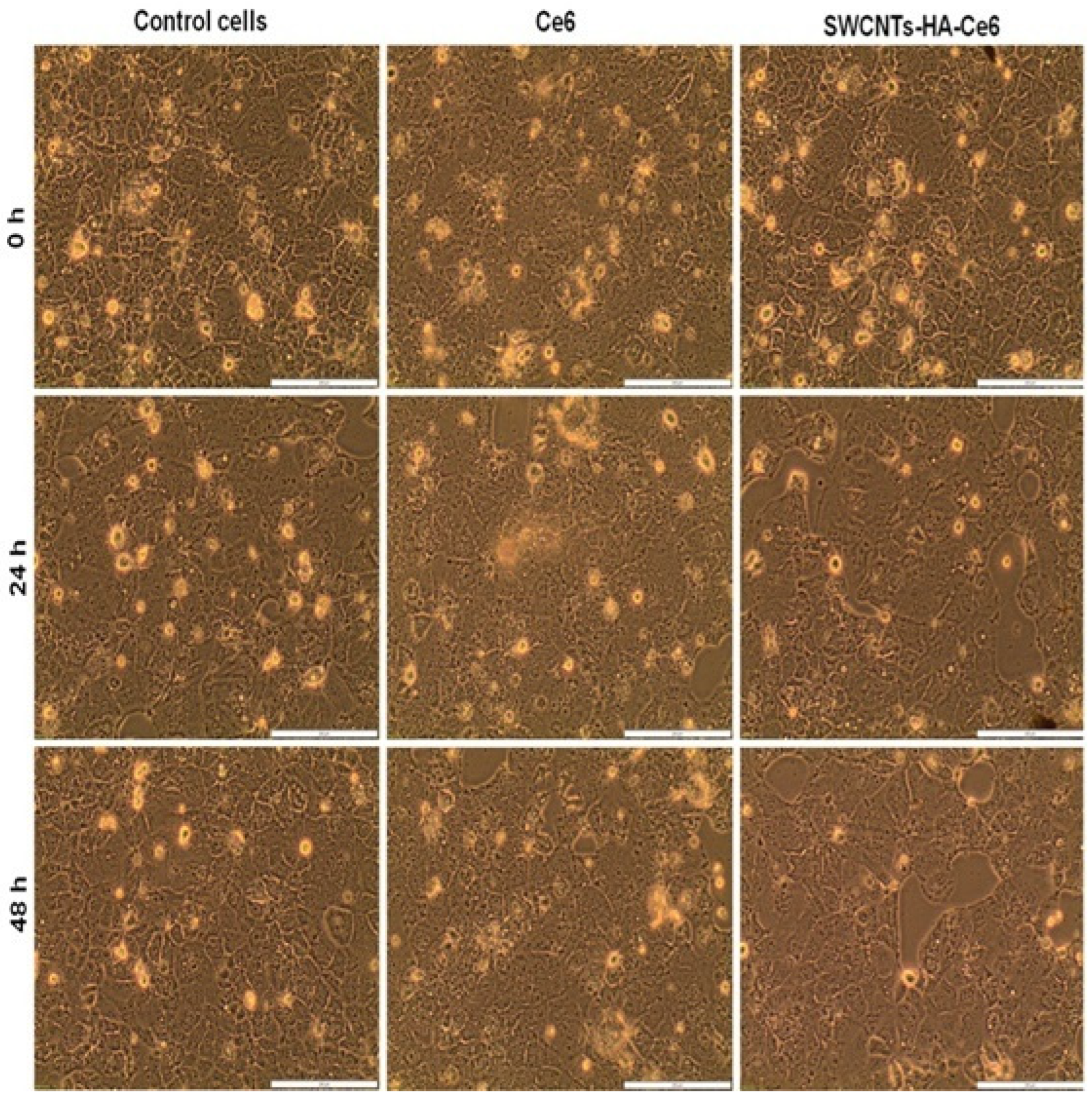
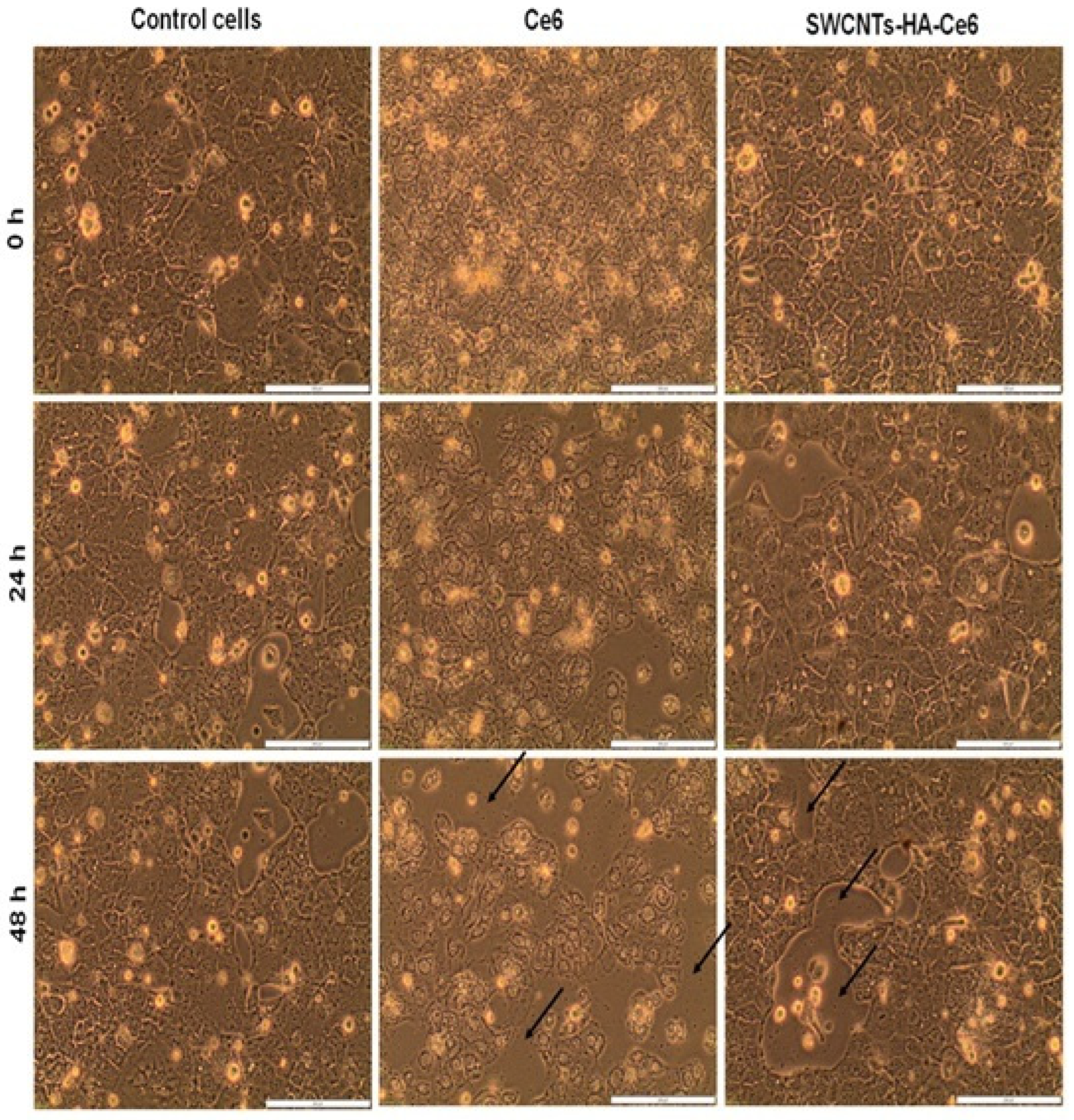
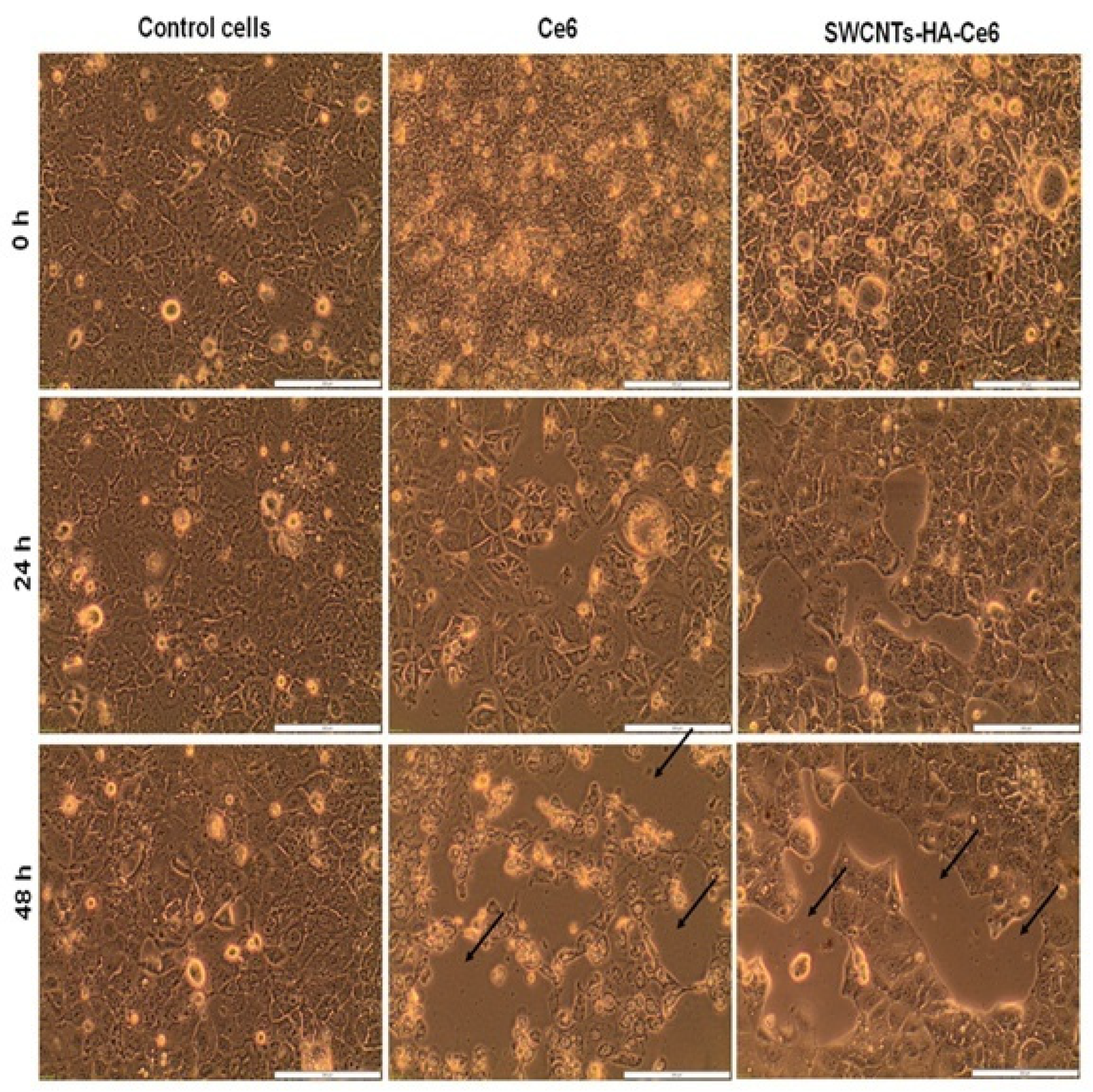
2.5.1. Morphology Studies
2.5.2. Cytotoxicity Assay Using Lactate Dehydrogenase (LDH) Assay
2.5.3. Flow Cytometry Annexin V–Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI)
2.5.4. Intracellular Reactive Oxygen Species Detection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of CCSCs: Morphology, Flow Cytometry, Immunofluorescence
3.1.1. Spheroid Formation of Isolated CCSCs
3.1.2. Flow Cytometry Analysis
3.1.3. Immunofluorescence
3.2. Targeted Delivery of SWCNTs-HA-Ce6 on CCSCs
3.3. Photodynamic Therapy Effect on Isolated CCSCs Using SWCNTs-HA-Ce6
3.3.1. Cell Morphology Study
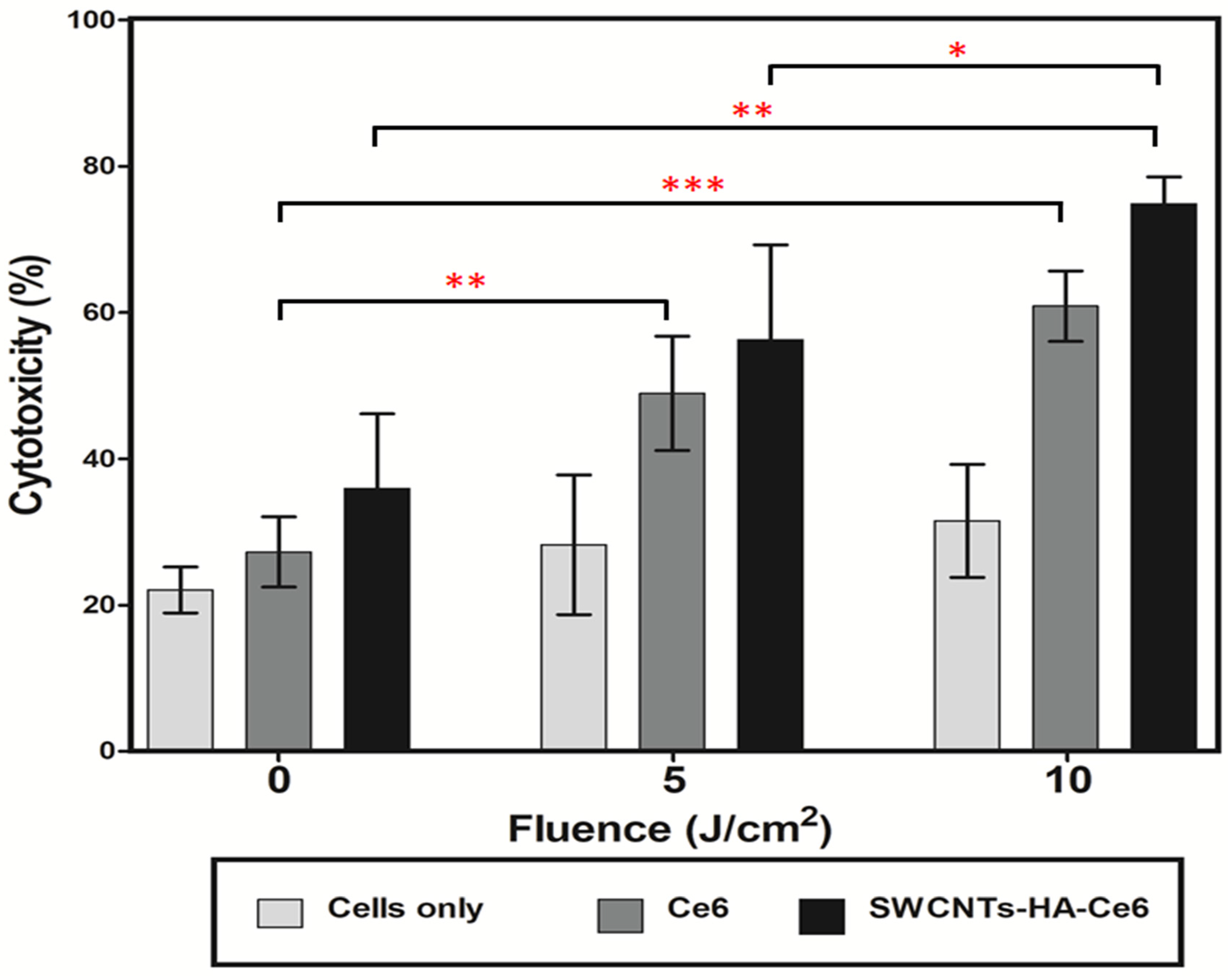
3.3.2. LDH Cytotoxicity Assay
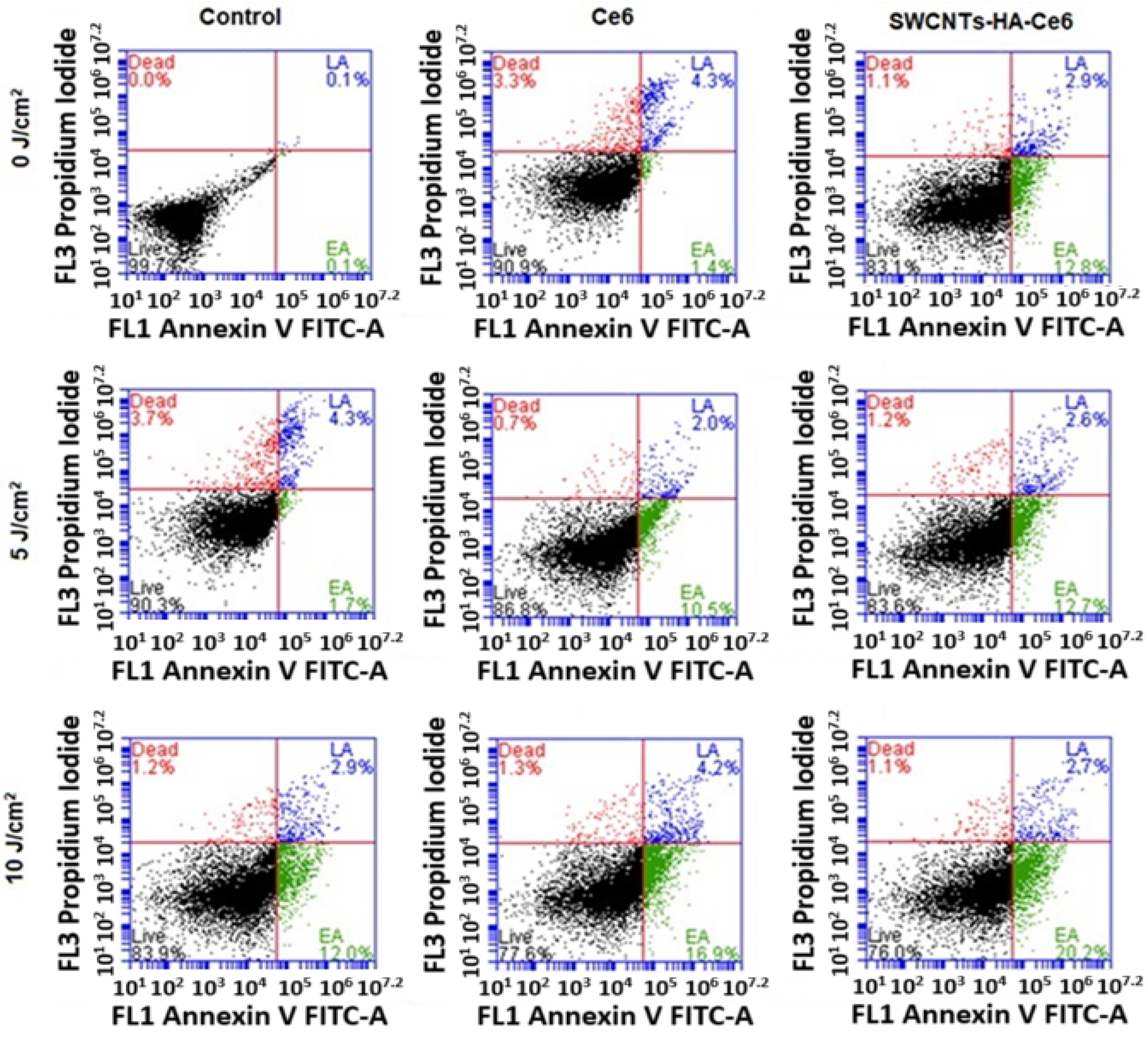
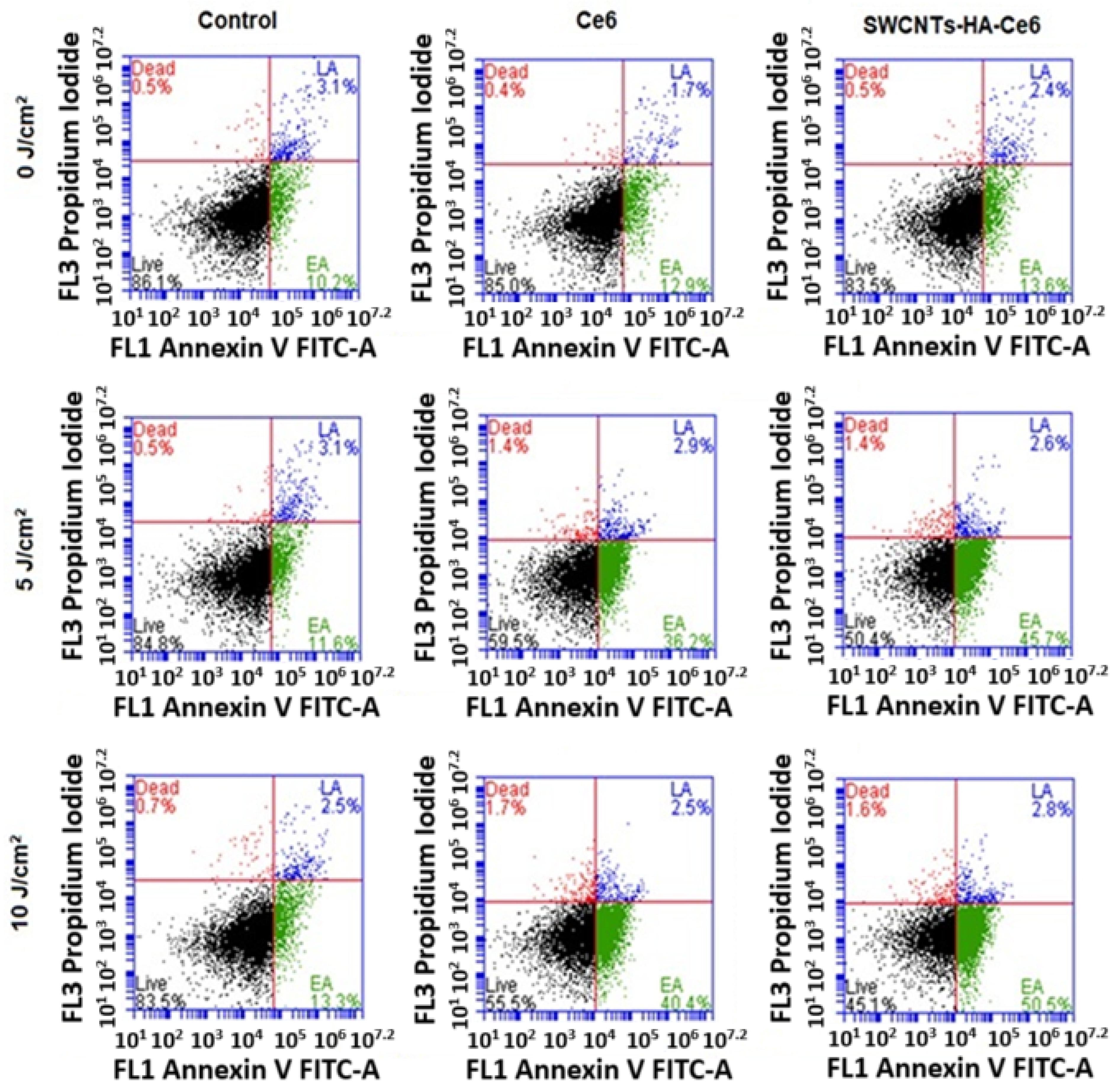
3.3.3. Annexin V–Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) Assay Using Flow Cytometry
3.3.4. Oxidative Stress Visualization Immediately Following Laser Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current targeted therapy for metastatic colorectal cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Bayat, M.R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar] [PubMed]
- Kim, T.E.; Chang, J.E. Recent studies in photodynamic therapy for cancer treatment: From basic research to clinical trials. Pharmaceutics 2023, 15, 2257. [Google Scholar] [CrossRef]
- Orlic, D.; Hill, J.M.; Arai, A.E. Stem cells for myocardial regeneration. Circ. Res. 2002, 91, 1092–1102. [Google Scholar] [CrossRef]
- Adams, J.M.; Strasser, A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008, 68, 4018–4021. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Sun, H.R.; Wang, S.; Yan, S.C.; Zhang, Y.; Nelson, P.J.; Jia, H.L.; Qin, L.X.; Dong, Q.Z. Therapeutic strategies targeting cancer stem cells and their microenvironment. Front. Oncol. 2019, 9, 1104. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Von Roemeling, C.A.; Wang, Y.; Qie, Y.; Yuan, H.; Zhao, H.; Liu, X.; Yang, Z.; Yang, M.; Deng, W.; Bruno, K.A.; et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.A.; Han, S.; Kim, C.S.; Wen, Y.; Sampaio, D.R.T.; Harris, J.T.; Homan, K.A.; Swain, J.L.; Emelianov, S.Y.; Sood, A.K.; et al. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat. Commun. 2021, 12, 5410. [Google Scholar] [CrossRef]
- Nelson, A.L. Antibody fragments: Hope and hype. MAbs 2010, 2, 77–83. [Google Scholar] [CrossRef]
- Kawelah, M.R.; Han, S.; Dincer, C.A.; Jeon, J.; Brisola, J.; Hussain, A.F.; Jeevarathinam, A.S.; Bouchard, R.; Marras, A.E.; Truskett, T.M.; et al. Antibody-Conjugated Polymersomes with Encapsulated Indocyanine Green J-Aggregates and High Near-Infrared Absorption for Molecular Photoacoustic Cancer Imaging. ACS Appl. Mater. Interfaces 2024, 16, 5598–5612. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes--the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Kostarelos, K. Pharmacology of carbon nanotubes: Toxicokinetics, excretion and tissue accumulation. Adv. Drug Deliv. Rev. 2013, 65, 2111–2119. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon nanotubes: Current perspectives on diverse applications in targeted drug delivery and therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective photodynamic therapy for colon cancer cells using chlorin e6 coated hyaluronic acid-based carbon nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P. The Use of Biopolymer Functionalized Single-Walled Carbon Nanotubes for Effective Targeted Photodynamic Therapy of Colon Cancer Stem Cells. Ph.D. Dissertation, University of Johannesburg, Johannesburg, South Africa, 2021. [Google Scholar]
- Yao, H.J.; Zhang, Y.G.; Sun, L.; Liu, Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.H.; Pai, S.; Rao, R.; Sripathi, H.; Prabhu, S. Techniques of immunofluorescence and their significance. Indian. J. Dermatol. VE 2008, 74, 415–419. [Google Scholar] [CrossRef]
- Ong, C.W.; Kim, L.G.; Kong, H.H.; Low, L.Y.; Iacopetta, B.; Soong, R.; Salto-Tellez, M. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod. Pathol. 2010, 23, 450–457. [Google Scholar] [CrossRef]
- Coco, C.; Zannoni, G.F.; Caredda, E.; Sioletic, S.; Boninsegna, A.; Migaldi, M.; Rizzo, G.; Bonetti, L.R.; Genovese, G.; Stigliano, E.; et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 71. [Google Scholar] [CrossRef]
- Slomiany, M.G.; Dai, L.; Tolliver, L.B.; Grass, G.D.; Zeng, Y.; Toole, B.P. Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin. Cancer Res. 2009, 15, 7593–7601. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Lee, H.J.; Choe, G.; Jheon, S.; Sung, S.W.; Lee, C.T.; Chung, J.H. CD24, a novel cancer biomarker, predicting disease-free survival of non-small cell lung carcinomas: A retrospective study of prognostic factor analysis from the viewpoint of forthcoming (seventh) new TNM classification. J. Thorac. Oncol. 2010, 5, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Napier, S.L.; Healy, Z.R.; Schnaar, R.L.; Konstantopoulos, K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: The roles of CD44v and alternative sialofucosylated selectin ligands. J. Biol. Chem. 2007, 282, 3433–3441. [Google Scholar] [CrossRef]
- Liu, P.; Yue, C.; Sheng, Z.; Gao, G.; Li, M.; Yi, H.; Zheng, C.; Wang, B.; Cai, L. Photosensitizer-conjugated redox-responsive dextran theranostic nanoparticles for near-infrared cancer imaging and photodynamic therapy. Polym. Chem. 2014, 5, 874–881. [Google Scholar] [CrossRef]
- Yoo, J.O.; Ha, K.S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, F. Photodynamic Therapy (PDT): Principles, Mechanisms and Applications; Nova Science Publishers: New York, NY, USA, 2017. [Google Scholar]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef] [PubMed]






| Parameters | |
|---|---|
| Laser type | Semiconductor diode |
| Wavelength (nm) | 660 |
| Emission | Continuous wave |
| Power output | 104 mW |
| Power density | 11.45 mW/cm2 |
| Fluences (J/cm2) | 5 and 10 |
| Irradiation time | 7 min 16 s for 5 J/cm2 14 min 32 s for 10 J/cm2 |
| Photosensitizer | Chlorin e6 |
| Height of laser | 8 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaram, P.; Dhilip Kumar, S.S.; Abrahamse, H. Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy. Pharmaceutics 2025, 17, 469. https://doi.org/10.3390/pharmaceutics17040469
Sundaram P, Dhilip Kumar SS, Abrahamse H. Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy. Pharmaceutics. 2025; 17(4):469. https://doi.org/10.3390/pharmaceutics17040469
Chicago/Turabian StyleSundaram, Prabhavathi, Sathish Sundar Dhilip Kumar, and Heidi Abrahamse. 2025. "Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy" Pharmaceutics 17, no. 4: 469. https://doi.org/10.3390/pharmaceutics17040469
APA StyleSundaram, P., Dhilip Kumar, S. S., & Abrahamse, H. (2025). Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy. Pharmaceutics, 17(4), 469. https://doi.org/10.3390/pharmaceutics17040469










