Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MNP Functionalization
2.3. Design of Experiment (DoE)
2.4. Folic Acid–Chitosan Conjugation Synthesis
2.5. Quercetin Loading
2.6. Physicochemical Characterization
2.7. In Vitro Drug Release
2.8. Cytotoxicity Assay
2.9. Data Analysis
3. Results and Discussion
3.1. Design of Experiment
3.2. Folic Acid-Chitosan Conjugation
3.3. Optimized Nanoparticles
3.4. Physicochemical Characterization
3.5. In Vitro Drug Release
3.6. Colon Cancer Cytotoxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MNP | Magnetic nanoparticle |
| CS | Chitosan |
| FA-CS | Chitosan folate |
| Q | Quercetin |
| Dh | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| PdI | Polydispersity index |
| PBS | Phosphate buffer solution |
| HCT-116 | Human colon cancer epithelial cell line |
| IC50 | Inhibitory concentration 50% |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
| FT-IR | Fourier-transform infrared spectroscopy |
| FEG-SEM | Field emission gun scanning electron microscope |
References
- Soares, G.A.; Faria, J.V.C.; Pinto, L.A.; Prospero, A.G.; Pereira, G.M.; Stoppa, E.G.; Buranello, L.P.; Bakuzis, A.F.; Baffa, O.; Miranda, J.R.A. Long-Term Clearance and Biodistribution of Magnetic Nanoparticles Assessed by AC Biosusceptometry. Materials 2022, 15, 2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An Overview and Bibliometric Analysis on the Colorectal Cancer Therapy by Magnetic Functionalized Nanoparticles for the Responsive and Targeted Drug Delivery. J. Nanobiotechnol. 2021, 19, 399. [Google Scholar] [CrossRef]
- Lu, Q.; Yao, X.; Zheng, H.; Ou, J.; You, J.; Zhang, Q.; Guo, W.; Xu, J.; Geng, L.; Liu, Q.; et al. SS-31 Modification Alleviates Ferroptosis Induced by Superparamagnetic Iron Oxide Nanoparticles in Hypoxia/Reoxygenation Cardiomyocytes. Heliyon 2024, 10, e38584. [Google Scholar] [CrossRef]
- Shahbazi, R.; Behbahani, F.K. Synthesis, Modifications, and Applications of Iron-Based Nanoparticles. Mol. Divers. 2024, 28, 4515–4552. [Google Scholar] [CrossRef]
- Ansari, S.R.; Hempel, N.-J.; Asad, S.; Svedlindh, P.; Bergström, C.A.S.; Löbmann, K.; Teleki, A. Hyperthermia-Induced In Situ Drug Amorphization by Superparamagnetic Nanoparticles in Oral Dosage Forms. ACS Appl. Mater. Interfaces 2022, 14, 21978–21988. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Muhammad, A.; Tian, L.; Liu, Y.; Chen, L.; Yang, P. Biopolymer Coating for Particle Surface Engineering and Their Biomedical Applications. Mater. Today Bio 2022, 16, 100407. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Naeimi, H.; Goliaei, B.; Bigdeli, B.; Sadighi, A.; Dehghani, S.; Lotfabadi, A.; Hosseini, M.; Nezamtaheri, M.S.; Amanlou, M.; et al. Magnetic Bio-Metal–Organic Framework Nanocomposites Decorated with Folic Acid Conjugated Chitosan as a Promising Biocompatible Targeted Theranostic System for Cancer Treatment. Mater. Sci. Eng. C 2019, 99, 805–815. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Almalki, F. Design and Synthesis of Multi-Functional Superparamagnetic Core-Gold Shell Nanoparticles Coated with Chitosan and Folate for Targeted Antitumor Therapy. Nanomaterials 2020, 11, 32. [Google Scholar] [CrossRef]
- Dabaghi, M.; Quaas, R.; Hilger, I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers 2020, 12, 2562. [Google Scholar] [CrossRef]
- Cintra, E.R.; Hayasaki, T.G.; Sousa-Junior, A.A.; Silva, A.C.G.; Valadares, M.C.; Bakuzis, A.F.; Mendanha, S.A.; Lima, E.M. Folate-Targeted PEGylated Magnetoliposomes for Hyperthermia-Mediated Controlled Release of Doxorubicin. Front. Pharmacol. 2022, 13, 854430. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.-A.; Maghiar, T.-A.; Alaya, A.; Olah, N.-K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, J.; Ghorbani Haghjo, A.; Mesgari Abbasi, M.; Dastranj Tabrizi, A.; Yaqoubi, S.; Sanajou, D.; Ashrafi Jigheh, Z.; Namvaran, A.; Mohammadi, A.; Mohammadi Khoshraj, J.; et al. Antitumor Effect of Quercetin Loaded Chitosan Nanoparticles on Induced Colon Cancer in Wistar Rats. Adv. Pharm. Bull. 2019, 9, 409–415. [Google Scholar] [CrossRef]
- Mandić, L.; Sadžak, A.; Erceg, I.; Baranović, G.; Šegota, S. The Fine-Tuned Release of Antioxidant from Superparamagnetic Nanocarriers under the Combination of Stationary and Alternating Magnetic Fields. Antioxidants 2021, 10, 1212. [Google Scholar] [CrossRef]
- De Castro, C.H.; Nunes, A.; Ramalho, L.; Colugnati, D.; Mendes, E.; Sousa, M.; Bakuzis, A.; Souza, A.; Zufelato, N. Manganese Ferrite-Based Nanoparticles Induce Ex Vivo, but Not in Vivo, Cardiovascular Effects. Int. J. Nanomed. 2014, 9, 3299–3312. [Google Scholar] [CrossRef]
- Profirio, D.M.; Pessine, F.B.T. Formulation of Functionalized PLGA Nanoparticles with Folic Acid-Conjugated Chitosan for Carboplatin Encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Shah, S.A.; Majeed, A.; Rashid, K.; Awan, S.-U. PEG-Coated Folic Acid-Modified Superparamagnetic MnFe2O4 Nanoparticles for Hyperthermia Therapy and Drug Delivery. Mater. Chem. Phys. 2013, 138, 703–708. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell Uptake and in Vitro Toxicity of Magnetic Nanoparticles Suitable for Drug Delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Bio-Inspired Encapsulation and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Belanova, A.A.; Gavalas, N.; Makarenko, Y.M.; Belousova, M.M.; Soldatov, A.V.; Zolotukhin, P.V. Physicochemical Properties of Magnetic Nanoparticles: Implications for Biomedical Applications In Vitro and In Vivo. Oncol. Res. Treat 2018, 41, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (Epr) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Das, R.; Gaur, P. Preparation of Chitosan Nanoparticles and Their In-Vitro Characterization. Int. J. Life Sci. Sci. Res. 2018, 4, 1713–1720. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Zarrabi, A.; Rahgozar, S. The Antitoxic Effects of Quercetin and Quercetin-Conjugated Iron Oxide Nanoparticles (QNPs) against Induced Toxicity in PC12 Cells. Int. J. Nanomed. 2019, 14, 6813–6830. [Google Scholar] [CrossRef]
- Asif, A.; Desu, P.; Alavala, R.; Rao, G.; Sreeharsha, N.; Meravanige, G. Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach. Pharmaceutics 2022, 14, 584. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Zeng, F.; Chen, L.; Peng, Z.; Kong, L.X. Synthesis and Characterization of Folate Conjugated Chitosan and Cellular Uptake of Its Nanoparticles in HT-29 Cells. Carbohydr. Res. 2011, 346, 801–806. [Google Scholar] [CrossRef]
- Nahar, A.; Hanium Maria, K.; Liba, S.I.; Anwaruzzaman, M.; Khan, M.N.I.; Islam, A.; Choudhury, S.; Hoque, S.M. Surface-Modified CoFe2O4 Nanoparticles Using Folate-Chitosan for Cytotoxicity Studies, Hyperthermia Applications and Positive/Negative Contrast of MRI. J. Magn. Magn. Mater. 2022, 554, 169282. [Google Scholar] [CrossRef]
- Viswanadh, M.K.; Mehata, A.K.; Sharma, V.; Priya, V.; Varshney, N.; Mahto, S.K.; Muthu, M.S. Bioadhesive Chitosan Nanoparticles: Dual Targeting and Pharmacokinetic Aspects for Advanced Lung Cancer Treatment. Carbohydr. Polym. 2021, 274, 118617. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, P.; Rashid, T.U.; Khan, M.N.; Mallik, A.K.; Khan, M.N.I.; Khan, M.; Rahman, M.M. Core–Shell Drug Carrier from Folate Conjugated Chitosan Obtained from Prawn Shell for Targeted Doxorubicin Delivery. J. Mater. Sci. Mater. Med. 2017, 28, 55. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lin, F.-H.; Tsai, K.-C.; Wei, M.-F.; Tsai, H.-M.; Wong, J.-M.; Shieh, M.-J. Folic Acid-Conjugated Chitosan Nanoparticles Enhanced Protoporphyrin IX Accumulation in Colorectal Cancer Cells. Bioconjug. Chem. 2010, 21, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Chen, W.; Jiang, F.; Mo, M.; Bi, Y.; Kong, F. Synthesis, Characterization and in Vitro Digestion of Folate Conjugated Chitosan-Loaded Proanthocyanidins Nanoparticles. Food Res. Int. 2023, 163, 112141. [Google Scholar] [CrossRef] [PubMed]
- Naik, T.R.R.; Shivashankar, S.A.; Bindu, P.J. In-Vitro Antioxidant and Anticancer Activities of MnFe2O4 Nanoparticles Synthesized Using Spinach Leaves Extract. Appl. Nanomed. 2022, 2, 330–337. [Google Scholar]
- Barreto, A.C.H.; Santiago, V.R.; Mazzetto, S.E.; Denardin, J.C.; Lavín, R.; Mele, G.; Ribeiro, M.E.N.P.; Vieira, I.G.P.; Gonçalves, T.; Ricardo, N.M.P.S.; et al. Magnetic Nanoparticles for a New Drug Delivery System to Control Quercetin Releasing for Cancer Chemotherapy. J. Nanopart. Res. 2011, 13, 6545–6553. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.; Altawabeyeh, R.; Darweesh, R.S.; Alnabulsi, S. Coating Methotrexate-PLGA Nanoparticles with Folic Acid-Chitosan Conjugate for Cancer Targeting. Pharmacia 2024, 71, 1–9. [Google Scholar] [CrossRef]
- Zhou, J.-F.; Zheng, G.-D.; Wang, W.-J.; Yin, Z.-P.; Chen, J.-G.; Li, J.-E.; Zhang, Q.-F. Physicochemical Properties and Bioavailability Comparison of Two Quercetin Loading Zein Nanoparticles with Outer Shell of Caseinate and Chitosan. Food Hydrocoll. 2021, 120, 106959. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, S.; Ullah, I.; Weera, W. Synthesis and Characterization of Manganese Ferrite Nano-Particles from Low-Grade Manganese Ore and Ferric Oxide Powder via Pyrometallurgy. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Villegas-Peralta, Y.; López-Cervantes, J.; Madera Santana, T.J.; Sánchez-Duarte, R.G.; Sánchez-Machado, D.I.; Martínez-Macías, M.D.R.; Correa-Murrieta, M.A. Impact of the Molecular Weight on the Size of Chitosan Nanoparticles: Characterization and Its Solid-State Application. Polym. Bull. 2021, 78, 813–832. [Google Scholar] [CrossRef]
- Alehosseini, E.; Shahiri Tabarestani, H.; Kharazmi, M.S.; Jafari, S.M. Physicochemical, Thermal, and Morphological Properties of Chitosan Nanoparticles Produced by Ionic Gelation. Foods 2022, 11, 3841. [Google Scholar] [CrossRef]
- Bhaskaran, N.A.; Jitta, S.R.; Salwa; Kumar, L.; Sharma, P.; Kulkarni, O.P.; Hari, G.; Gourishetti, K.; Verma, R.; Birangal, S.R.; et al. Folic Acid-Chitosan Functionalized Polymeric Nanocarriers to Treat Colon Cancer. Int. J. Biol. Macromol. 2023, 253, 127142. [Google Scholar] [CrossRef]
- Valencia, M.S.; Franco da Silva Júnior, M.; Xavier Júnior, F.H.; de Oliveira Veras, B.; Fernanda de Oliveira Borba, E.; Gonçalves da Silva, T.; Xavier, V.L.; Pessoa de Souza, M.; Carneiro-da-Cunha, M. das G. Bioactivity and Cytotoxicity of Quercetin-Loaded, Lecithin-Chitosan Nanoparticles. Biocatal. Agric. Biotechnol. 2021, 31, 101879. [Google Scholar] [CrossRef]
- dos Camargo, G.A.; Ferreira, L.; Schebelski, D.J.; Lyra, A.M.; Barboza, F.M.; Carletto, B.; Koga, A.Y.; Semianko, B.C.; Dias, D.T.; Lipinski, L.C.; et al. Characterization and In Vitro and In Vivo Evaluation of Tacrolimus-Loaded Poly(ε-caprolactone) Nanocapsules for the Management of Atopic Dermatitis. Pharmaceutics 2021, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, N.; Romano, M.P.; Zappavigna, S.; Abate, M.; Del Vecchio, V.; Romano, D.; Germinario, C.; Grifa, C.; Filosa, R.; Pappalardo, D. Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers 2023, 15, 1179. [Google Scholar] [CrossRef]
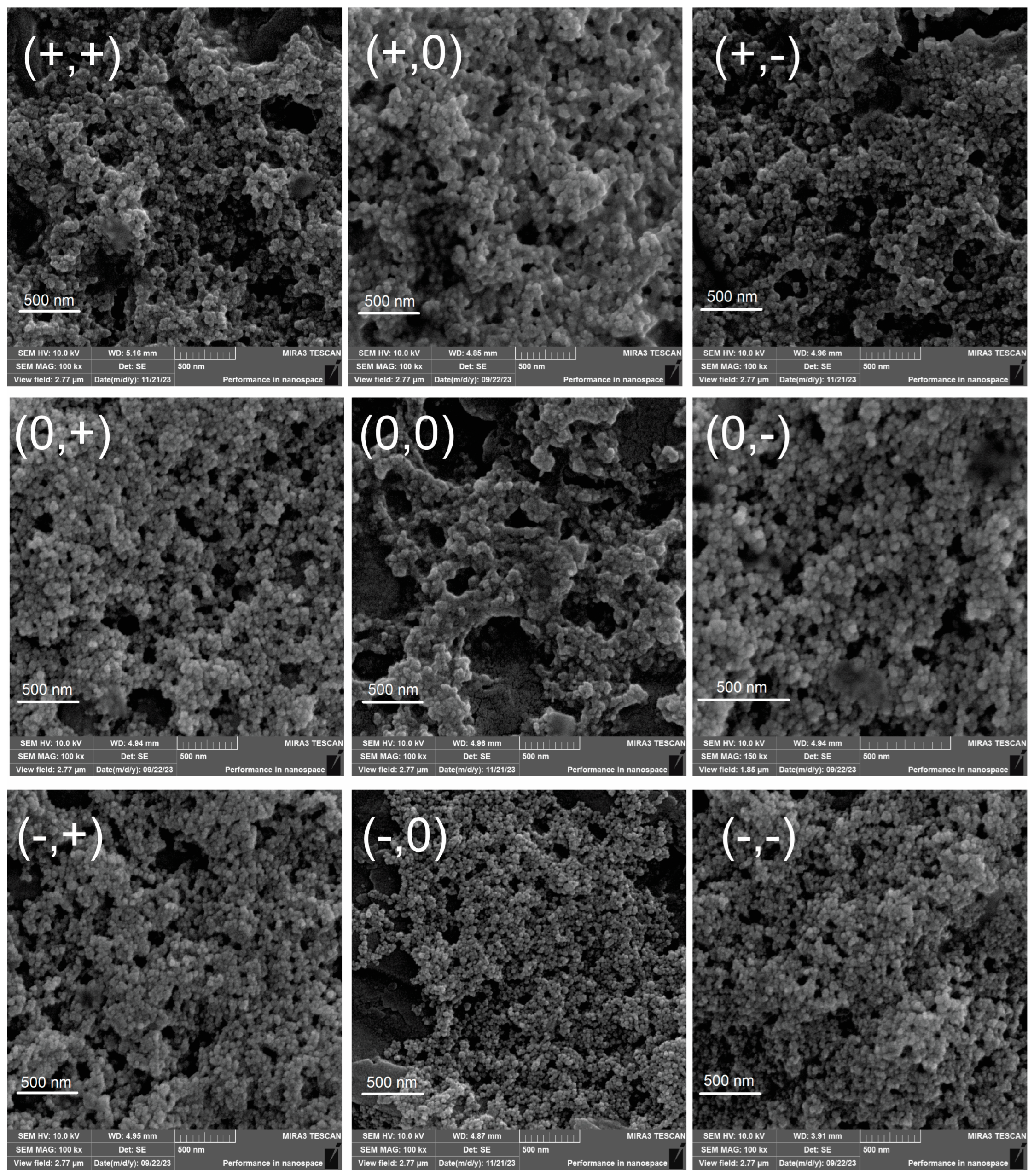
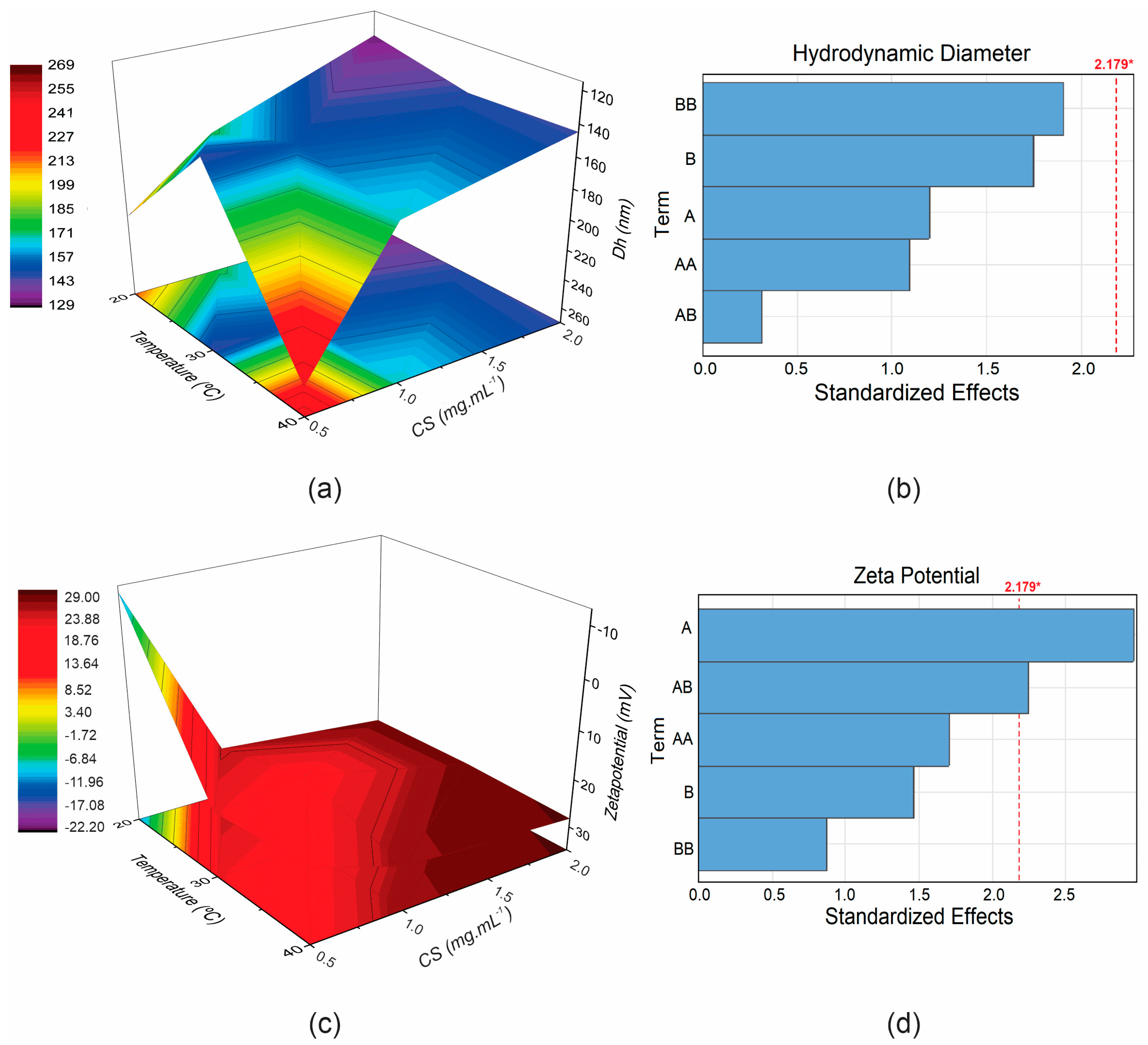




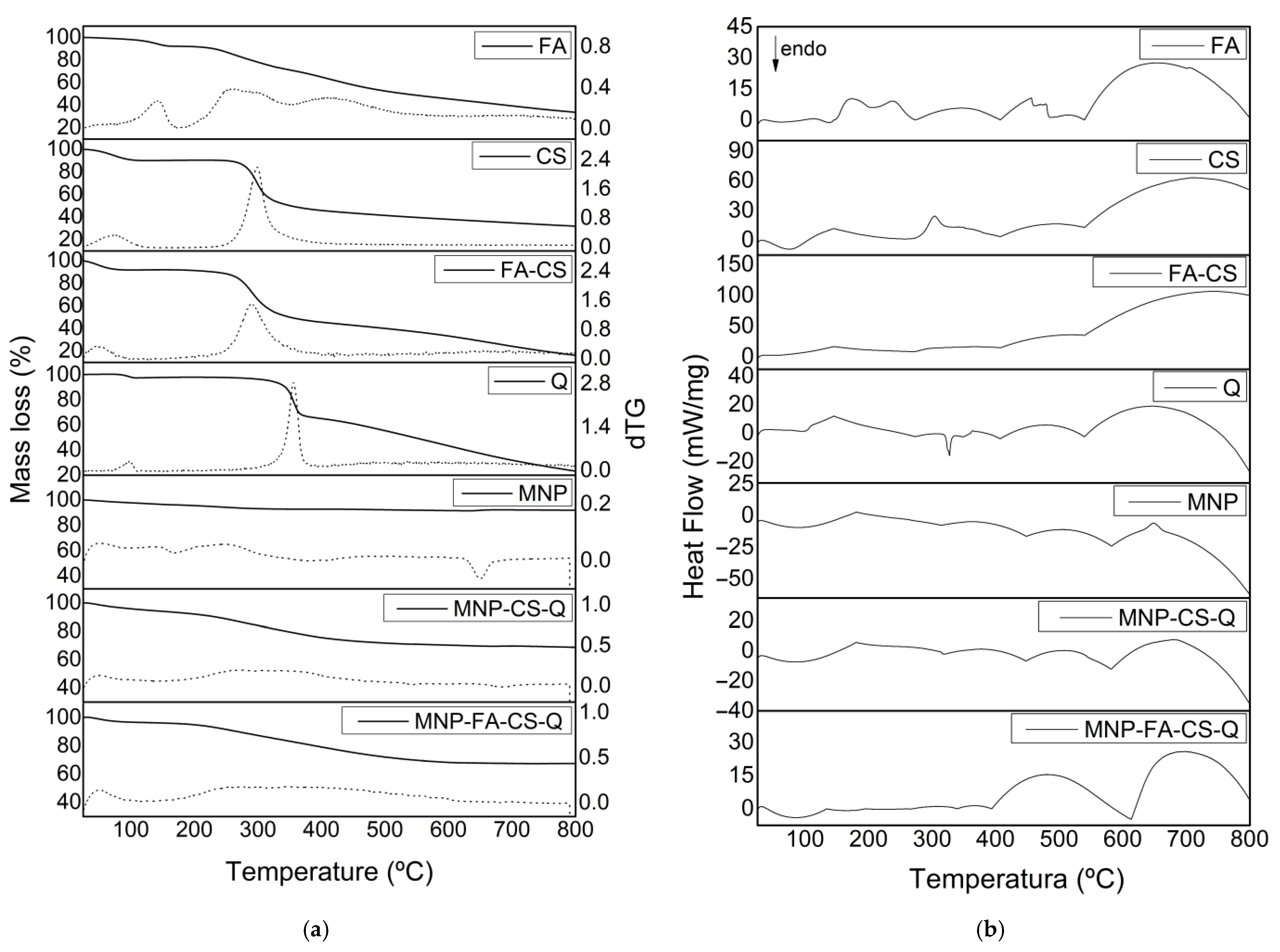

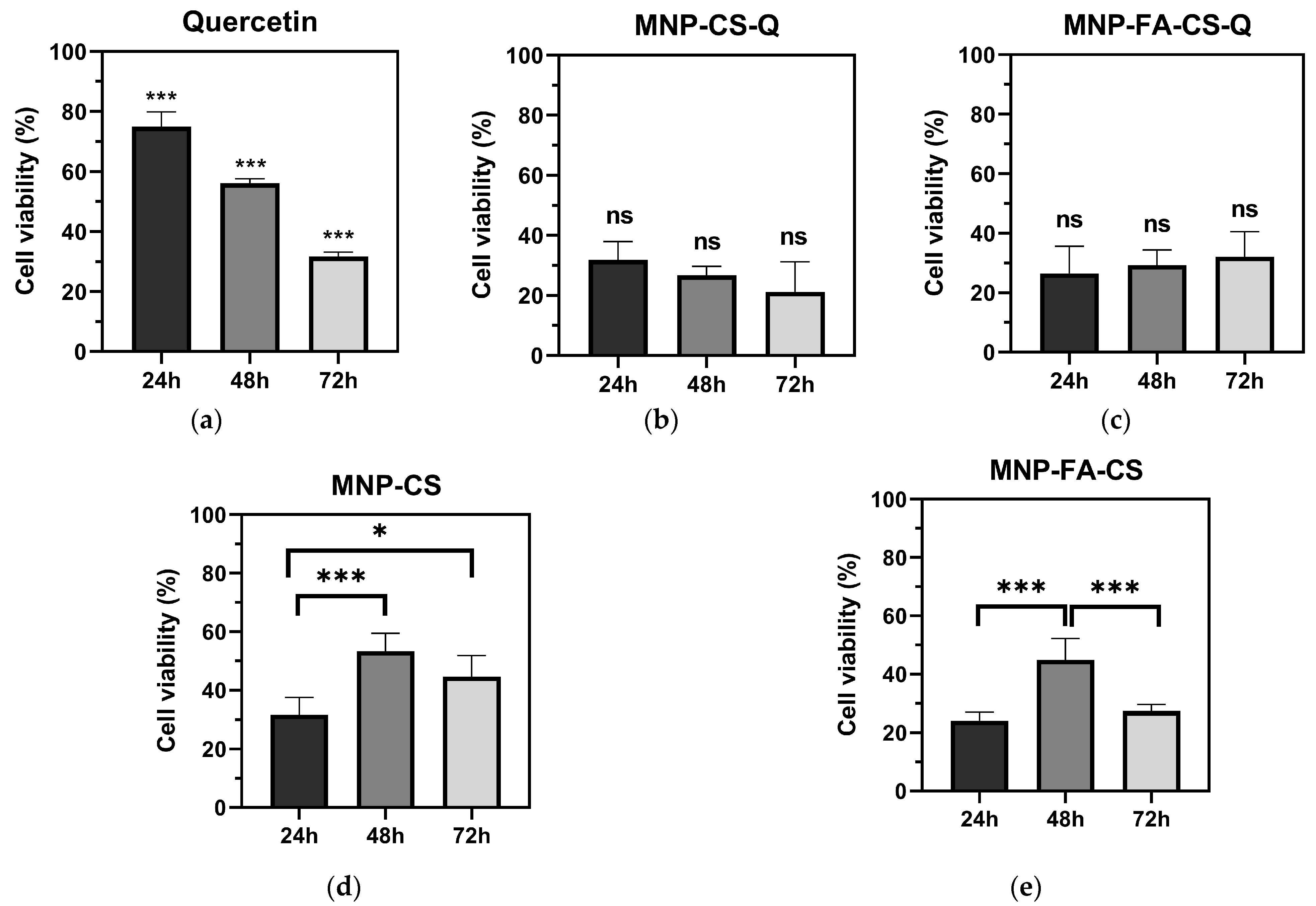
| CS (mg·mL−1) | °C | Dh (nm) | PdI * | ζ (mV) | FEG-SEM (nm) * |
|---|---|---|---|---|---|
| 0.5 (−) | 20 (−) | 161.43 ± 8.28 | 0.38 ± 0.03 | −1.44 ± 9.75 | 37.54 ± 8.36 |
| 1 (0) | 20 (−) | 169.10 ± 4.95 | 0.35 ± 0.01 | 25.60 ± 0.26 | 46.29 ± 9.66 |
| 2 (+) | 20 (−) | 133.20 ± 2.01 | 0.31 ± 0.02 | 26.63 ± 0.74 | 31.45 ± 8.78 |
| 0.5 (−) | 30 (0) | 150.13 ± 2.11 | 0.28 ± 0.05 | 20.87 ± 1.08 | 24.46 ± 9.60 |
| 1 (0) | 30 (0) | 157.67 ± 1.78 | 0.28 ± 0.01 | 17.43 ± 0.51 | 34.21 ± 11.52 |
| 2 (+) | 30 (0) | 138.50 ± 2.10 | 0.38 ± 0.03 | 26.57 ± 0.55 | 30.09 ± 9.73 |
| 0.5 (−) | 40 (+) | 235.40 ± 6.22 | 0.40 ± 0.04 | 21.93 ± 0.06 | 32.57 ± 6.53 |
| 1 (0) | 40 (+) | 165.97 ± 3.10 | 0.38 ± 0.01 | 27.53 ± 0.76 | 38.75 ± 21.74 |
| 2 (+) | 40 (+) | 142.13 ± 1.36 | 0.35 ± 0.01 | 28.07 ± 1.27 | 36.15 ± 10.82 |
| 0.5 (−) | 20 (−) | 268.70 ± 91.75 | 0.39 ± 0.05 | −22.17 ± 4.72 | - |
| 1 (0) | 20 (−) | 176.87 ± 4.90 | 0.32 ± 0.01 | 23.40 ± 0.62 | - |
| 2 (+) | 20 (−) | 129.40 ± 2.29 | 0.36 ± 0.01 | 28.97 ± 1.16 | - |
| 0.5 (−) | 30 (0) | 141.77 ± 0.46 | 0.26 ± 0.01 | 22.13 ± 0.85 | - |
| 1 (0) | 30 (0) | 146.33 ± 1.46 | 0.27 ± 0.01 | 21.47 ± 0.80 | - |
| 2 (+) | 30 (0) | 154.07 ± 1.96 | 0.39 ± 0.01 | 28.53 ± 0.23 | - |
| 0.5 (−) | 40 (+) | 265.77 ± 6.37 | 0.27 ± 0.05 | 17.43 ± 0.42 | - |
| 1 (0) | 40 (+) | 168.53 ± 3.65 | 0.39 ± 0.05 | 24.40 ± 1.23 | - |
| 2 (+) | 40 (+) | 147.23 ± 3.16 | 0.40 ± 0.04 | 28.33 ± 0.15 | - |
| Terms | β1 | β2 | β12 | β11 | β22 | β0 | R2 |
|---|---|---|---|---|---|---|---|
| Dh | −121.000 ns | −18,000 ns | −0.500 ns | 37.500 ns | 0.322 ns | 492.000 * | 0.556 ns |
| ζ * | 76.400 * | 3870 ns | −0.896 * | −0.038 ns | −14.980 ns | −109.600 * | 0.676 * |
| Samples | ζ (mV) | Dh (nm) | PdI | LE (%) | DL (%) |
|---|---|---|---|---|---|
| MNP | −23.93 ± 1.45 C | 61.38 ± 7.0 D | 0.27 ± 0.06 C | - | - |
| MNP-CS | 30.78 ± 0.80 B | 122.32 ± 8.56 B | 0.46 ± 0.05 B | - | - |
| MNP-CS-Q | −11.45 ± 1.10 D | 388.45 ± 14.50 A | 0.69 ± 0.04 A | 80.45 ± 4.10 A | 24.30 ± 0.93 A |
| MNP-FA-CS | 48.38 ± 4.10 A | 93.50 ± 3.98 C | 0.23 ± 0.04 D | - | - |
| MNP-FA-CS-Q | −25.20 ± 1.90 E | 365.10 ± 29.01 A | 0.38 ± 0.05 B | 54.40 ± 5.13 B | 17.85 ± 1.40 B |
| Model | Equation | MNP-CS | MNP-FA-CS | Drug Transport Mechanism | ||
|---|---|---|---|---|---|---|
| Linear Equation | R | Linear Equation | R | |||
| Zero-order | Q = Q0 + K0t | y = 0.1779x + 2.3376 | 0.9320 | y = 0.1327x + 4.8285 | 0.7519 | - |
| First-order | dC/dT = −Kt | y = −0.0008x + 1.9898 | 0.9356 | y = −0.0006x + 1.9785 | 0.7572 | - |
| Higuchi | Q = KH t1/2 | y = 0.8817x − 0.8562 | 0.9896 | y = 0.9963x − 3.4131 | 0.8710 | - |
| Korsmeyer–Peppas | Mt/M∞ = Ktn | y = 0.3657x + 2.0074 | 0.9989 | y = 0.1865x + 4.3682 | 0.9924 | Fickian diffusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, J.B.d.; Bueno, J.N.S.; Kanunfre, C.C.; Miranda, J.R.d.A.; Bakuzis, A.F.; Ferrari, P.C. Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment. Pharmaceutics 2025, 17, 467. https://doi.org/10.3390/pharmaceutics17040467
Macedo JBd, Bueno JNS, Kanunfre CC, Miranda JRdA, Bakuzis AF, Ferrari PC. Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment. Pharmaceutics. 2025; 17(4):467. https://doi.org/10.3390/pharmaceutics17040467
Chicago/Turabian StyleMacedo, Júlia Borges de, Julia Narayana Schoroeder Bueno, Carla Cristine Kanunfre, José Ricardo de Arruda Miranda, Andris Figueiroa Bakuzis, and Priscileila Colerato Ferrari. 2025. "Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment" Pharmaceutics 17, no. 4: 467. https://doi.org/10.3390/pharmaceutics17040467
APA StyleMacedo, J. B. d., Bueno, J. N. S., Kanunfre, C. C., Miranda, J. R. d. A., Bakuzis, A. F., & Ferrari, P. C. (2025). Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment. Pharmaceutics, 17(4), 467. https://doi.org/10.3390/pharmaceutics17040467









