Vitamin D Status Determines Metformin Action on Gonadotropin Levels in Postmenopausal Women with Subclinical Hyperthyroidism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Analysis
3. Results
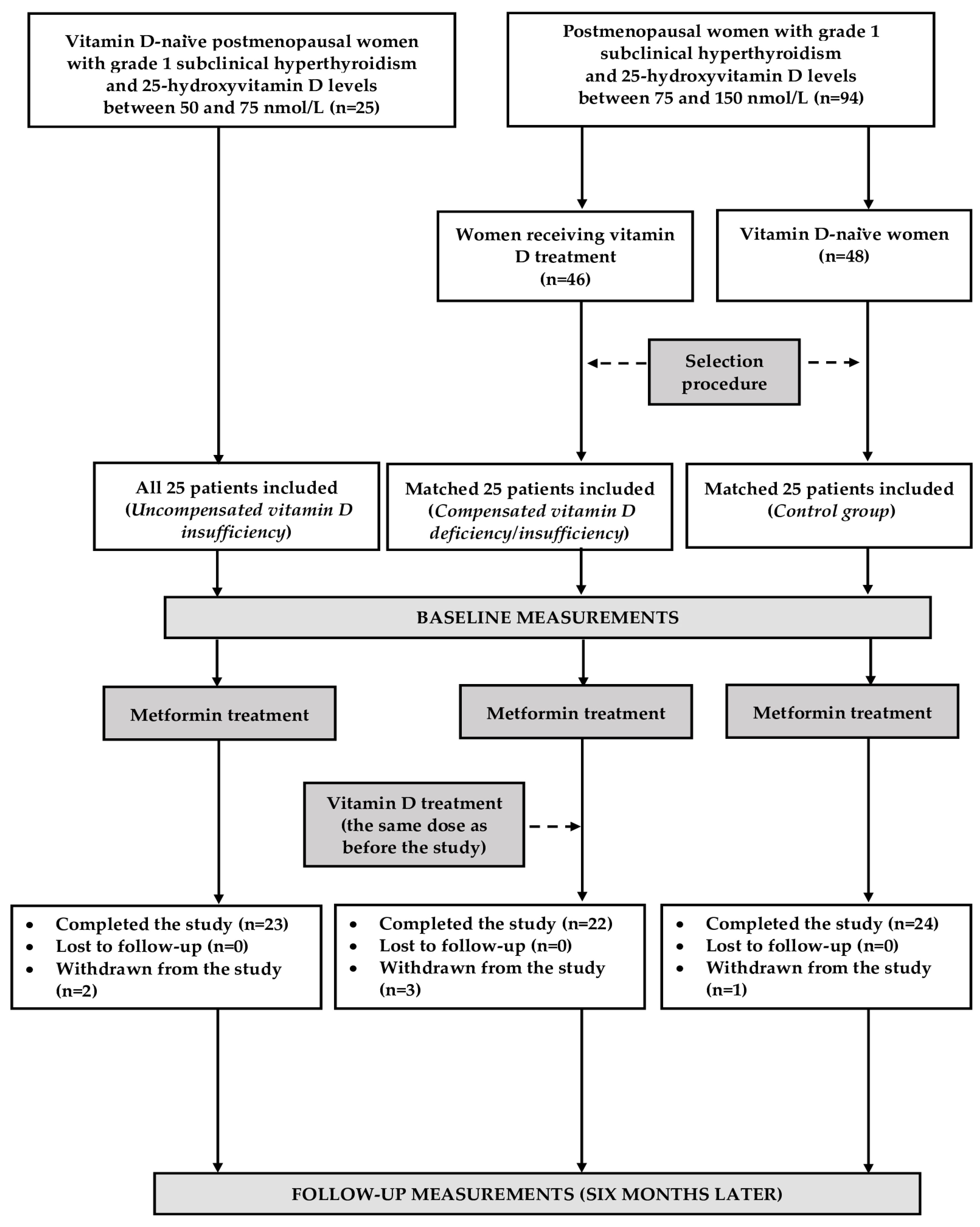
3.1. The Course of This Study
3.2. Baseline Conditions
3.3. The Effect of Metformin on the Investigated Variables
3.4. Between-Group Comparisons
3.5. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haroon, S.M.; Khan, K.; Maqsood, M.; Iqbal, S.; Aleem, M.; Khan, T.U. Exploring the effect of metformin to lower thyroid-stimulating hormone in euthyroid and hypothyroid type-2 diabetic patients. Cureus 2021, 13, e13283. [Google Scholar] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; Aversa, A.; Calogero, A.E.; La Vignera, S. TSH lowering effects of metformin: A possible mechanism of action. J. Endocrinol. Invest. 2021, 44, 1547–1550. [Google Scholar] [CrossRef]
- Bo, Q.J.; Wang, Z.M.; Li, X.B.; Ma, X.; Wang, C.Y.; de Leon, J. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: A systematic review. Psychiatry Res. 2016, 237, 257–263. [Google Scholar] [PubMed]
- Wu, R.R.; Jin, H.; Gao, K.; Twamley, E.W.; Ou, J.J.; Shao, P. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with worst-episode schizophrenia: A double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 2012, 169, 813–821. [Google Scholar] [PubMed]
- Zahra, M.; Shah, M.; Ali, A.; Rahim, R. Effects of metformin on endocrine and metabolic parameters in patients with polycystic ovary syndrome. Horm. Metab. Res. 2017, 49, 103–108. [Google Scholar]
- Velija-Ašimi, Z. Evaluation of endocrine changes in women with the polycystic ovary syndrome during metformin treatment. Bosn. J. Basic Med. Sci. 2013, 13, 180–185. [Google Scholar]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of metformin on serum gonadotropin levels in postmenopausal women with diabetes and prediabetes: A pilot study. Exp. Clin. Endocrinol. Diabetes 2018, 126, 645–650. [Google Scholar]
- Krysiak, R.; Szkróbka, W.; Bednarska-Czerwińska, A.; Okopień, B. Plasma gonadotropin levels in metformin-treated men with prediabetes: A non-randomized, uncontrolled pilot study. Fundam. Clin. Pharmacol. 2021, 35, 466–472. [Google Scholar]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar]
- McNeil, M.A.; Merriam, S.B. Menopause. Ann. Intern. Med. 2021, 174, ITC97–ITC112. [Google Scholar]
- Jiang, S.; Tang, T.; Sheng, Y.; Li, R.; Xu, H. The effects of letrozole and metformin combined with targeted nursing care on ovarian function, LH, and FSH in infertile patients with polycystic ovary syndrome. J. Healthc. Eng. 2022, 2022, 3712166. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Rosuvastatin potentiates gonadotropin-lowering effects of metformin in postmenopausal women: A pilot study. Pharmacology 2023, 108, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, T.; Shojaei-Baghini, A.; Dehbashi, S.; Asadi, N.; Ghaffarpasand, F.; Kazerooni, Y. Effects of metformin plus simvastatin on polycystic ovary syndrome a prospective, randomized, double-blind, placebo-controlled study. Fertil. Steril. 2010, 94, 2208–2213. [Google Scholar]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Myo-inositol enhances the inhibitory effect of metformin on gonadotropin levels in postmenopausal women. Gynecol. Obstet. Invest. 2022, 87, 373–380. [Google Scholar]
- Krysiak, R.; Basiak, M.; Machnik, G.; Okopień, B. Impaired gonadotropin-lowering effects of metformin in postmenopausal women with autoimmune thyroiditis: A pilot study. Pharmaceuticals 2023, 16, 922. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Vitamin D status determines the impact of metformin on gonadotropin levels in postmenopausal women. J. Clin. Med. 2023, 12, 3715. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Subclinical hyperthyroidism enhances gonadotropin-lowering effects of metformin in postmenopausal women. J. Clin. Pharmacol. 2024, 65, 318–327. [Google Scholar] [CrossRef]
- Taderegew, M.M.; Woldeamanuel, G.G.; Wondie, A.; Getawey, A.; Abegaz, A.N.; Adane, F. Vitamin D deficiency and its associated factors among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. BMJ Open 2023, 13, e075607. [Google Scholar]
- Dominguez, L.J.; Veronese, N.; Marrone, E.; Di Palermo, C.; Iommi, C.; Ruggirello, R.; Caffarelli, C.; Gonnelli, S.; Barbagallo, M. Vitamin D and risk of incident type 2 diabetes in older adults: An updated systematic review and meta-analysis. Nutrients 2024, 16, 1561. [Google Scholar] [CrossRef]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Mirenayat, F.S.; Saneei, P. Serum vitamin D levels in relation to metabolic syndrome: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13224. [Google Scholar]
- Kadiyala, R.; Peter, R.; Okosieme, O.E. Thyroid dysfunction in patients with diabetes: Clinical implications and screening strategies. Int. J. Clin. Pract. 2010, 64, 1130–1139. [Google Scholar] [PubMed]
- Chen, R.H.; Chen, H.Y.; Man, K.M.; Chen, S.J.; Chen, W.; Liu, P.L.; Chen, Y.H.; Chen, W.C. Thyroid diseases increased the risk of type 2 diabetes mellitus: A nation-wide cohort study. Medicine 2019, 98, e15631. [Google Scholar]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [PubMed]
- Pang, B.; Li, L.; Liu, X.; Cao, Z.; Pang, T.; Wang, Q.; Wei, J. Association between serum vitamin D level and Graves’ disease: A systematic review and meta-analysis. Nutr. J. 2024, 23, 60. [Google Scholar] [PubMed]
- Wiersinga, W.M.; Poppe, K.G.; Effraimidis, G. Hyperthyroidism: Aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 2023, 11, 282–298. [Google Scholar]
- Mammen, J.S. Thyroid and aging. Endocrinol. Metab. Clin. N. Am. 2023, 52, 229–243. [Google Scholar]
- Zhao, Y.; Yue, R. Aging adipose tissue, insulin resistance, and type 2 diabetes. Biogerontology 2024, 25, 53–69. [Google Scholar] [CrossRef]
- Ferri, E.; Casati, M.; Cesari, M.; Vitale, G.; Arosio, B. Vitamin D in physiological and pathological aging: Lesson from centenarians. Rev. Endocr. Metab. Disord. 2019, 20, 273–282. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes: Standards of care in diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef]
- Biondi, B.; Bartalena, L.; Cooper, D.S.; Hegedüs, L.; Laurberg, P.; Kahaly, G.J. The 2015 European Thyroid Association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur. Thyroid J. 2015, 4, 149–163. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2019, 163, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhai, T.; Zhang, J.A. Metformin reduces autoimmune antibody levels in patients with Hashimoto’s thyroiditis: A systematic review and meta-analysis. Autoimmunity 2020, 53, 353–361. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, C.; Chen, M.; Feng, P. Subclinical hyperthyroidism and the risk of dementia: A meta-analysis. Brain Behav. 2024, 14, 70037. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.; Xu, Y.; Li, L.; Chen, J.; Mai, X.; Xu, J.; Zhang, Z.; Yang, R.; Sun, J.; et al. Effect of subclinical hyperthyroidism on osteoporosis: A meta-analysis of cohort studies. Endocrine 2020, 69, 39–48. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Chen, X.L.; Guo, L.Y.; Jiang, P.R.; Lu, H.; Pan, K.; Guo, L.; Hu, Y.T.; Bao, A.M. Downregulation of peripheral luteinizing hormone rescues ovariectomy-associated cognitive deficits in APP/PS1 mice. Neurobiol. Aging 2024, 135, 60–69. [Google Scholar] [CrossRef]
- Villanueva, M.T. Hooking FSH as a potential target for Alzheimer disease. Nat. Rev. Drug. Discov. 2022, 21, 259. [Google Scholar] [CrossRef]
- Kim, S.M.; Sultana, F.; Korkmaz, F.; Rojekar, S.; Pallapati, A.; Ryu, V.; Lizneva, D.; Yuen, T.; Rosen, C.J.; Zaidi, M. Neuroendocrinology of bone. Pituitary 2024, 27, 761–777. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, S.; Fonseca, V.A.; Shi, L. Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open 2019, 9, e024954. [Google Scholar] [CrossRef]
- Cai, Y.; Jun, G.; Zhuang, X. Metformin treatment reduces the incidence of osteoporosis: A two-sample Mendelian randomized study. Osteoporos. Int. 2024, 35, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr. Med. Chem. 2007, 14, 1199–1206. [Google Scholar]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [PubMed]
- Tosca, L.; Froment, P.; Rame, C.; McNeilly, J.R.; McNeilly, A.S.; Maillard, V.; Dupont, J. Metformin decreases GnRH- and activin-induced gonadotropin secretion in rat pituitary cells: Potential involvement of adenosine 5’ monophosphate-activated protein kinase (PRKA). Biol. Reprod. 2011, 84, 351–362. [Google Scholar] [PubMed]
- Chang, E.; Kim, Y. Vitamin D insufficiency exacerbates adipose tissue macrophage infiltration and decreases AMPK/SIRT1 activity in obese rats. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef]
- Miklosz, A.; Chabowski, A.; Zendzian-Piotrowska, M.; Gorski, J. Effects of hyperthyroidism on lipid content and composition in oxidative and glycolytic muscles in rats. J. Physiol. Pharmacol. 2012, 63, 403–410. [Google Scholar]
- Chang, E. Effects of vitamin D supplementation on adipose tissue inflammation and NF-κB/AMPK activation in obese mice fed a high-fat diet. Int. J. Mol. Sci. 2022, 23, 10915. [Google Scholar] [CrossRef]
- Takano, A.P.; Diniz, G.P.; Barreto-Chaves, M.L. AMPK signaling pathway is rapidly activated by T3 and regulates the cardiomyocyte growth. Mol. Cell. Endocrinol. 2013, 37, 43–50. [Google Scholar]
- Li, H.X.; Gao, J.M.; Liang, J.Q.; Xi, J.M.; Fu, M.; Wu, Y.J. Vitamin D3 potentiates the growth inhibitory effects of metformin in DU145 human prostate cancer cells mediated by AMPK/mTOR signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 711–717. [Google Scholar]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Davis, S.; Jones, M. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer 1999, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Baig, M.S.; Helmy, T.; Gersh, F.L. Controversies regarding postmenopausal hormone replacement therapy for primary cardiovascular disease prevention in women. Cardiol. Rev. 2021, 29, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Mezzullo, M.; Gambineri, A.; Di Dalmazi, G.; Fazzini, A.; Magagnoli, M.; Baccini, M.; Vicennati, V.; Pelusi, C.; Pagotto, U.; Fanelli, F. Steroid reference intervals in women: Influence of menopause, age and metabolism. Eur. J. Endocrinol. 2021, 184, 395–407. [Google Scholar] [CrossRef]
- Iglesias, P.; Díez, J.J. Hyperthyroidism and cardiovascular risk factors: A nationwide study. Endocrinol. Diabetes Nutr. 2023, 70, 255–261. [Google Scholar] [CrossRef]
- Trofimiuk-Müldner, M.; Konopka, J.; Sokołowski, G.; Dubiel, A.; Kieć-Klimczak, M.; Kluczyński, Ł.; Motyka, M.; Rzepka, E.; Walczyk, J.; Sokołowska, M.; et al. Current iodine nutrition status in Poland (2017): Is the Polish model of obligatory iodine prophylaxis able to eliminate iodine deficiency in the population? Public Health Nutr. 2020, 23, 2467–2477. [Google Scholar]
- Kłapcińska, B.; Poprzecki, S.; Danch, A.; Sobczak, A.; Kempa, K. Selenium levels in blood of Upper Silesian population: Evidence of suboptimal selenium status in a significant percentage of the population. Biol. Trace Elem. Res. 2005, 108, 1–15. [Google Scholar] [CrossRef]


| Variable | Uncompensated Vitamin D Insufficiency | Compensated Vitamin D Deficiency/Insufficiency | Control Group |
|---|---|---|---|
| Number (n) | 23 | 22 | 24 |
| Age (years) | 65 ± 8 | 64 ± 9 | 65 ± 7 |
| Multinodular goiter/solitary adenomas (%) | 74/26 | 73/23 | 75/25 |
| Smokers (%)/number of cigarettes a day (n)/duration of smoking (years) | 43/9 ± 6/30 ± 11 | 45/9 ± 5/31 ± 14 | 38/10 ± 6/32 ± 12 |
| Body mass index (kg/m2) | 24.6 ± 4.7 | 23.8 ± 4.9 | 23.6 ± 5.1 |
| Systolic blood pressure (mmHg) | 130 ± 17 | 128 ± 16 | 127 ± 15 |
| Diastolic blood pressure (mmHg) | 83 ± 6 | 81 ± 5 | 81 ± 6 |
| Daily calciferol intake with food 1 (µg) | 10.0 ± 5.5 | 9.5 ± 5.0 | 11.3 ± 6.0 |
| 25-hydroxyvitamin D (nmol/L) | 62 ± 7 * | 112 ± 18 | 114 ± 17 |
| Glucose (mg/dL) | 110 ± 10 | 112 ± 10 | 108 ± 11 |
| HOMA1-IR | 3.7 ± 1.0 | 3.5 ± 1.1 | 3.9 ± 1.2 |
| HbA1c (%) | 6.0 ± 0.3 | 6.1 ± 0.2 | 6.0 ± 0.3 |
| FSH (U/L) | 78 ± 28 | 69 ± 20 | 75 ± 26 |
| LH (U/L) | 48 ± 18 | 44 ± 20 | 50 ± 23 |
| Estradiol (pmol/L) | 62 ± 25 | 65 ± 28 | 72 ± 23 |
| Progesterone (nmol/L) | 1.0 ± 0.3 | 1.1 ± 0.4 | 1.0± 0.4 |
| TSH (mU/L) | 0.26 ± 0.07 | 0.23 ±0.10 | 0.25 ± 0.10 |
| Free thyroxine (pmol/L) | 18.0 ± 1.9 | 17.8 ± 2.4 | 18.3 ± 2.0 |
| Free triiodothyronine (pmol/L) | 4.8 ± 0.7 | 4.6 ± 1.0 | 4.7 ± 0.9 |
| Prolactin (ng/mL) | 12 ± 10 | 13 ± 8 | 11 ± 6 |
| ACTH (pg/mL) | 37 ± 13 | 34 ± 15 | 32 ± 11 |
| IGF-1 (ng/mL) | 98 ± 40 | 106 ± 38 | 110 ± 50 |
| Variable | Uncompensated Vitamin D Insufficiency | Compensated Vitamin D Deficiency/Insufficiency | Control Group |
|---|---|---|---|
| Δ25-hydroxyvitamin D | 5 ± 15 | 3 ± 11 | −2 ± 14 |
| ΔGlucose | −7 ± 8 * | −16 ± 10 | −15 ± 9 |
| ΔHOMA1-IR | −22 ± 25 * | −46 ± 31 | −49 ± 32 |
| ΔHbA1c | −7 ± 8 * | −13 ± 10 | −13 ± 9 |
| ΔFSH | −26 ± 20 * | −41 ± 22 | −43 ± 25 |
| ΔLH | −17 ± 25 * | −46 ± 26 | −38 ± 21 |
| ΔEstradiol | 10 ± 29 | 8 ± 32 | −4 ± 29 |
| ΔProgesterone | 10 ± 50 | 0 ± 35 | 10 ± 42 |
| ΔTSH | 8 ± 15 | 13 ± 19 | 4 ± 20 |
| ΔFree thyroxine | −3 ± 8 | −4 ± 6 | −3 ± 7 |
| ΔFree triiodothyronine | −4 ± 10 | −4 ± 8 | −6 ± 7 |
| ΔProlactin | −17 ± 30 | −8 ± 28 | 0 ± 35 |
| ΔACTH | 11 ± 20 | 18 ± 23 | 19 ± 25 |
| ΔIGF-1 | 12 ± 15 | 6 ± 19 | 5 ± 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Vitamin D Status Determines Metformin Action on Gonadotropin Levels in Postmenopausal Women with Subclinical Hyperthyroidism. Pharmaceutics 2025, 17, 442. https://doi.org/10.3390/pharmaceutics17040442
Krysiak R, Kowalcze K, Szkróbka W, Okopień B. Vitamin D Status Determines Metformin Action on Gonadotropin Levels in Postmenopausal Women with Subclinical Hyperthyroidism. Pharmaceutics. 2025; 17(4):442. https://doi.org/10.3390/pharmaceutics17040442
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, Witold Szkróbka, and Bogusław Okopień. 2025. "Vitamin D Status Determines Metformin Action on Gonadotropin Levels in Postmenopausal Women with Subclinical Hyperthyroidism" Pharmaceutics 17, no. 4: 442. https://doi.org/10.3390/pharmaceutics17040442
APA StyleKrysiak, R., Kowalcze, K., Szkróbka, W., & Okopień, B. (2025). Vitamin D Status Determines Metformin Action on Gonadotropin Levels in Postmenopausal Women with Subclinical Hyperthyroidism. Pharmaceutics, 17(4), 442. https://doi.org/10.3390/pharmaceutics17040442






