Utilizing Nanoparticles of Hesperidin Loaded on Layered Double Hydroxide to Reduce Hepatotoxicity Caused by Paracetamol in Rats: Controlling of Biotransformation, Oxidative Stress, Inflammation, and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Agents
2.2. Preparation of Mg AL-LDH and Mg AL-LDH-Hesp
2.3. Characterization
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. Morphology
2.3.3. Particle Size and Zeta Potential Analysis
2.3.4. Entrapment Efficiency (EE) and Drug Loading (DL)
2.3.5. In Vitro Drug Release Studies
2.4. Experimental Design
2.5. Blood Sampling and Serum Analysis
2.6. Liver Sampling and Analysis
2.7. Statistical Analysis
3. Results
3.1. FTIR Analysis
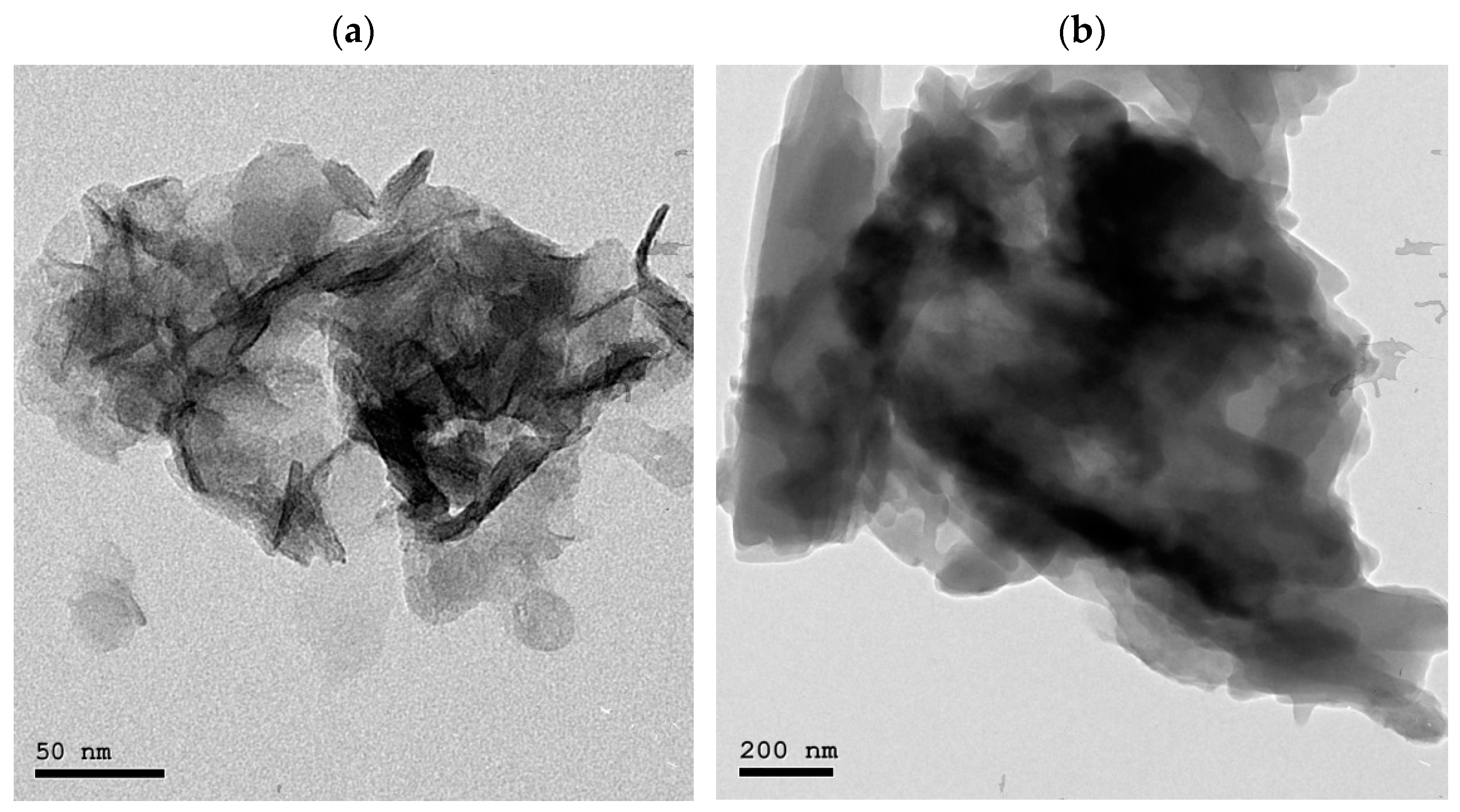
3.2. HRTEM Analysis
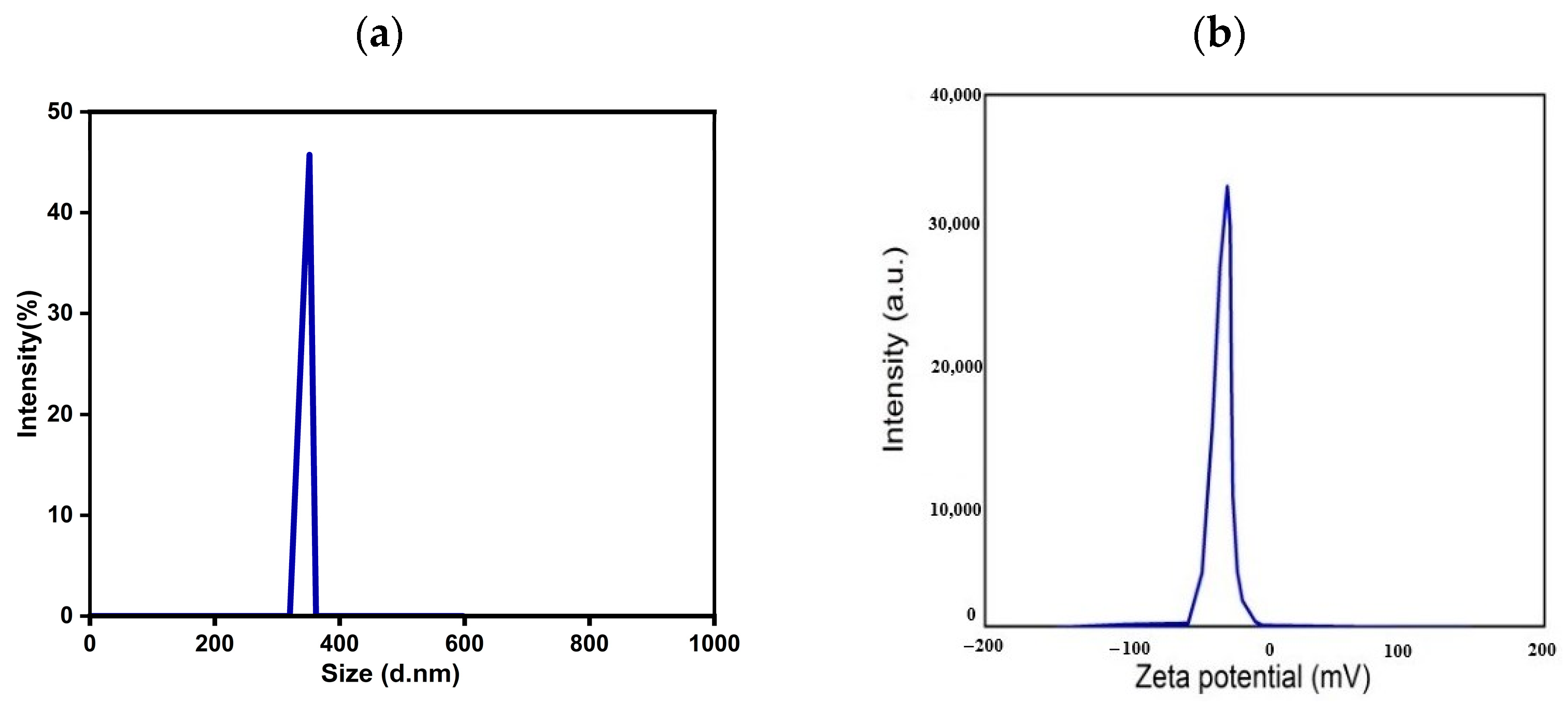
3.3. Particle Size and Zeta Potential Analysis
3.4. Efficiency of Entrapment, Drug Loading, and In Vitro Drug Release Investigation
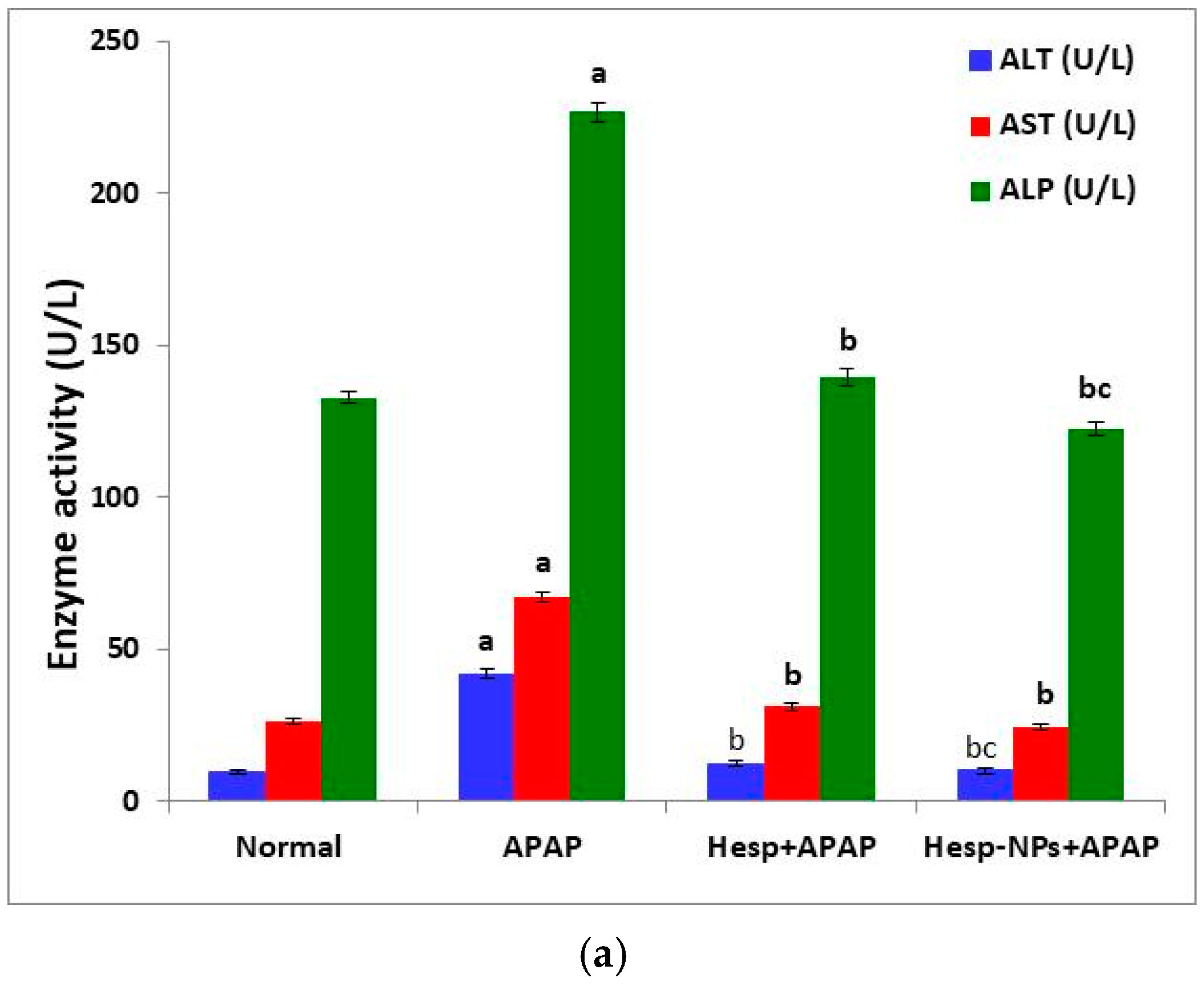
3.5. Effects of Hesp and Hesp-NPs on APAP-Induced Liver Injury
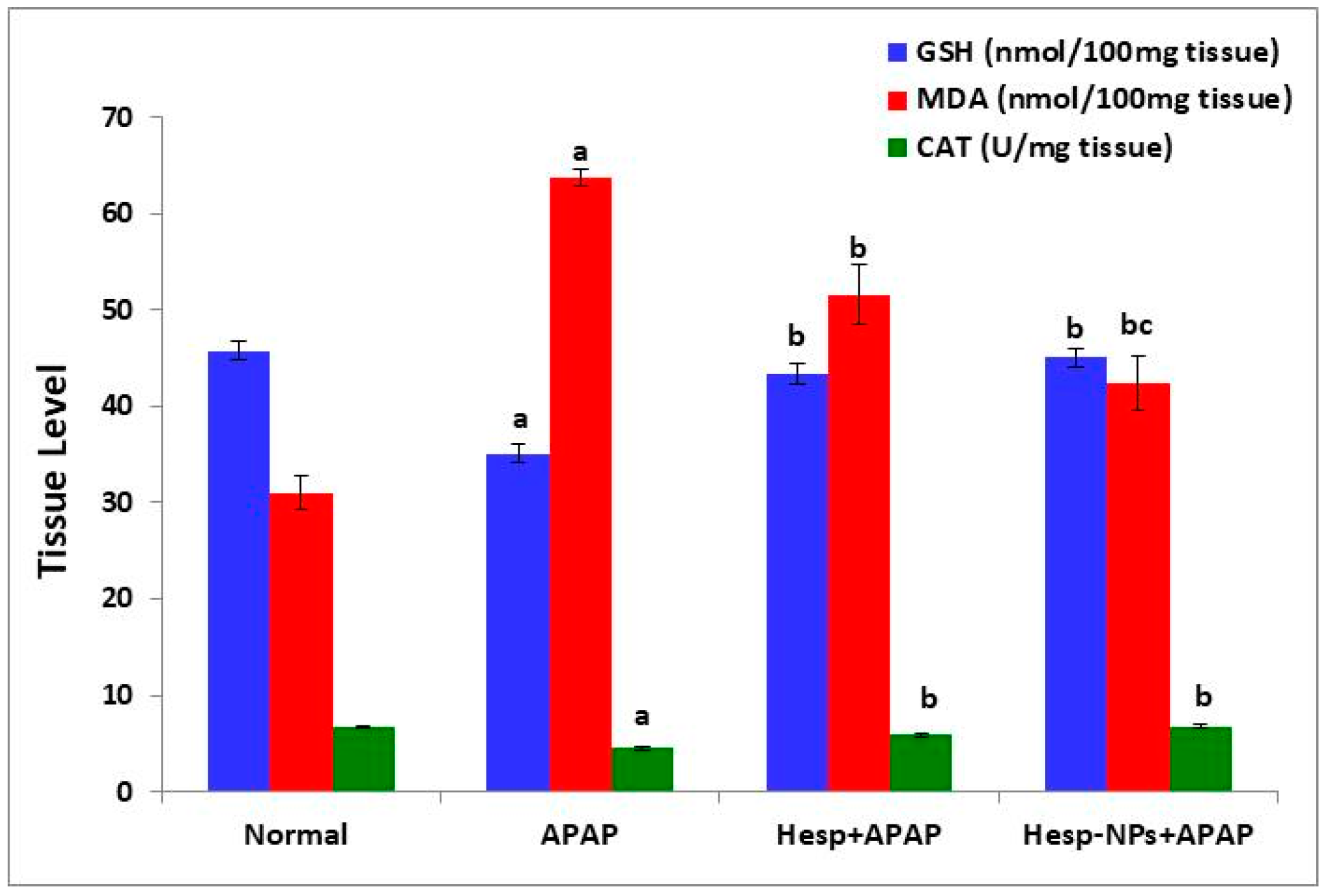
3.6. Effects of Hesp and Hesp-NPs on Liver GSH, MDA, and CAT Levels
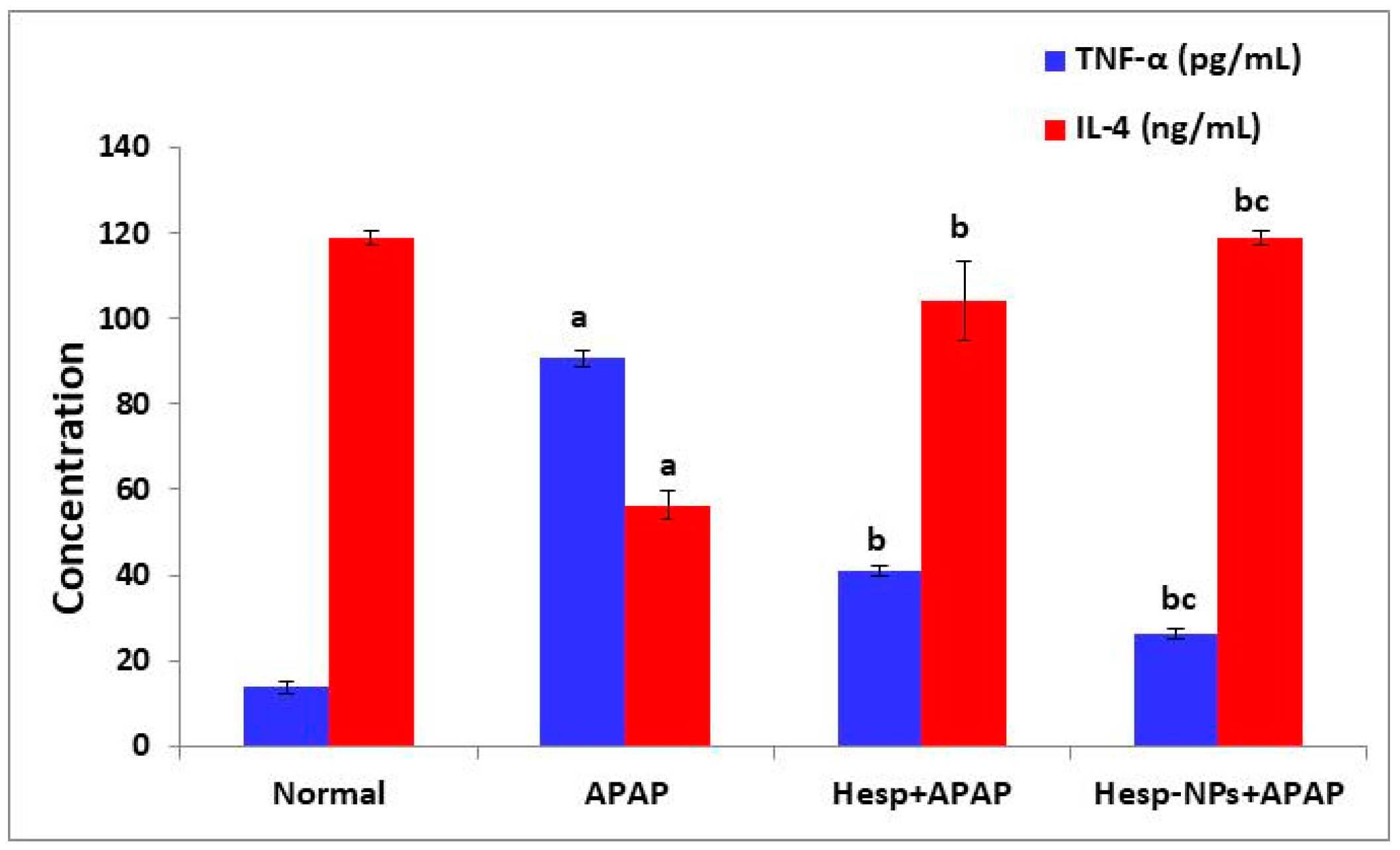
3.7. Effects of Hesp and Hesp-NPs on Serum TNF-α and IL-4 Levels
3.8. Effects of Hesp and Hesp-NPs on mRNA Expression of Various Genes
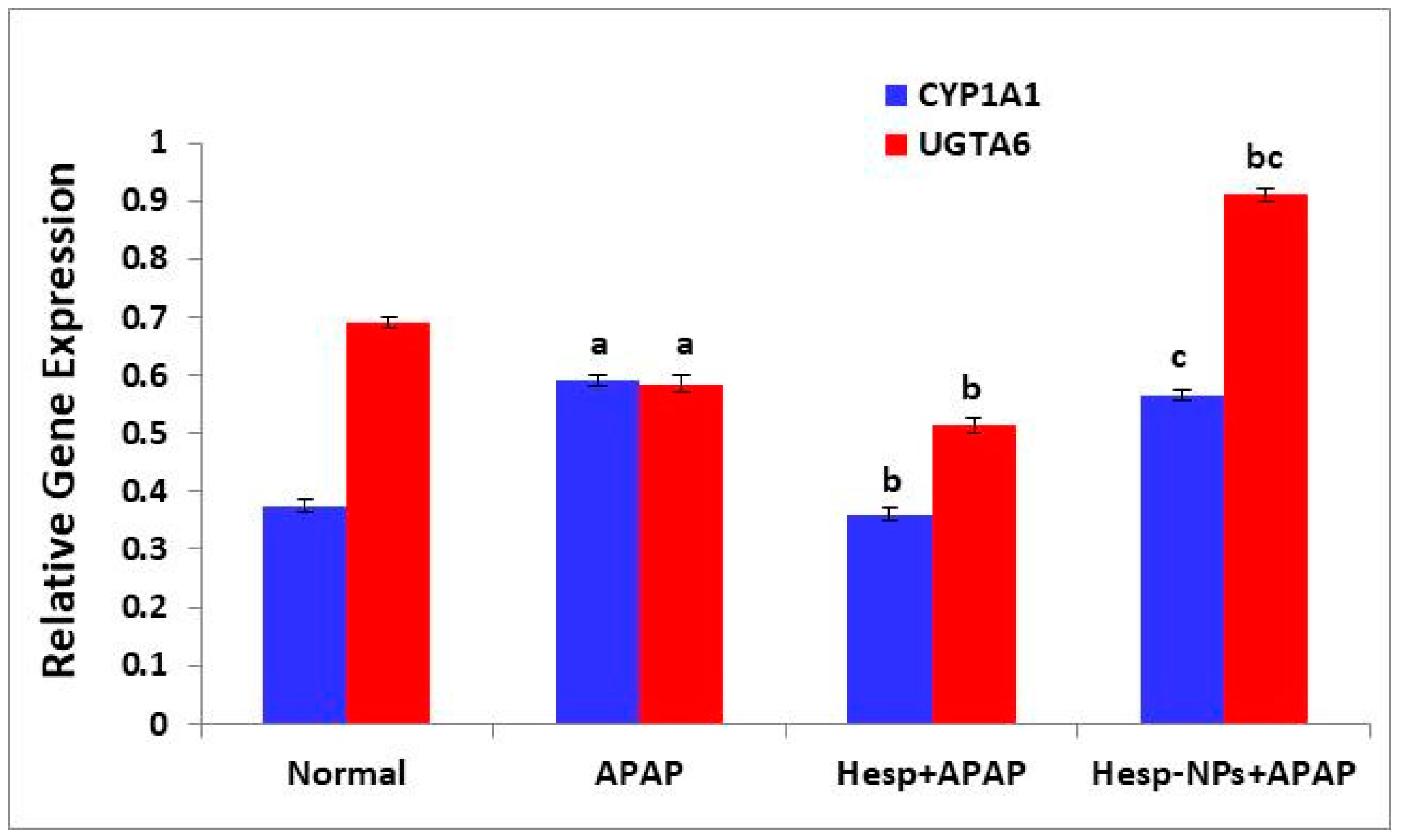
3.8.1. Effects on mRNA Expression of Liver CYP1A1 and UGTA6
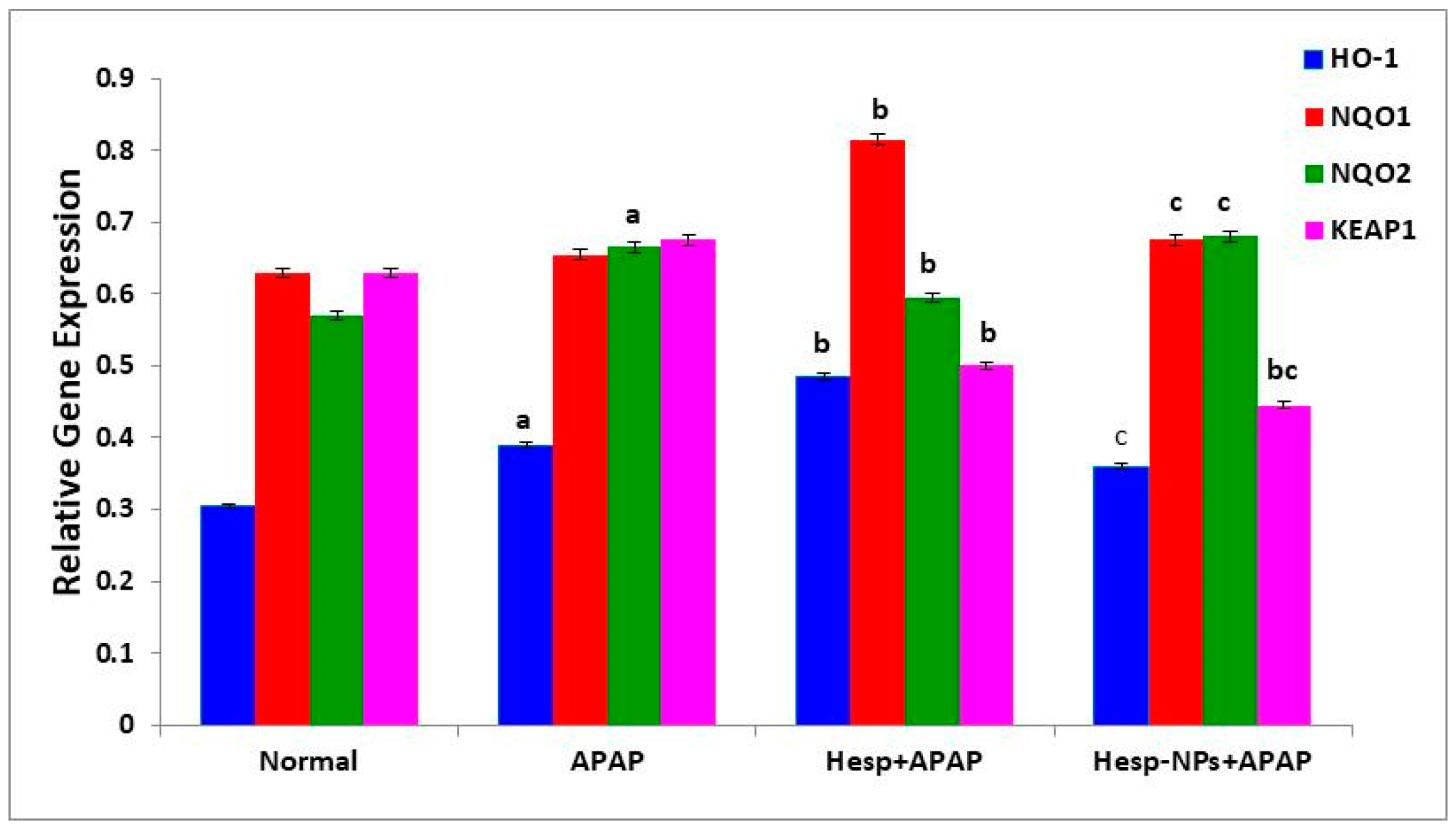
3.8.2. Effects on mRNA Expression of Liver HO-1, NQO1, NQO2, and KEAP1
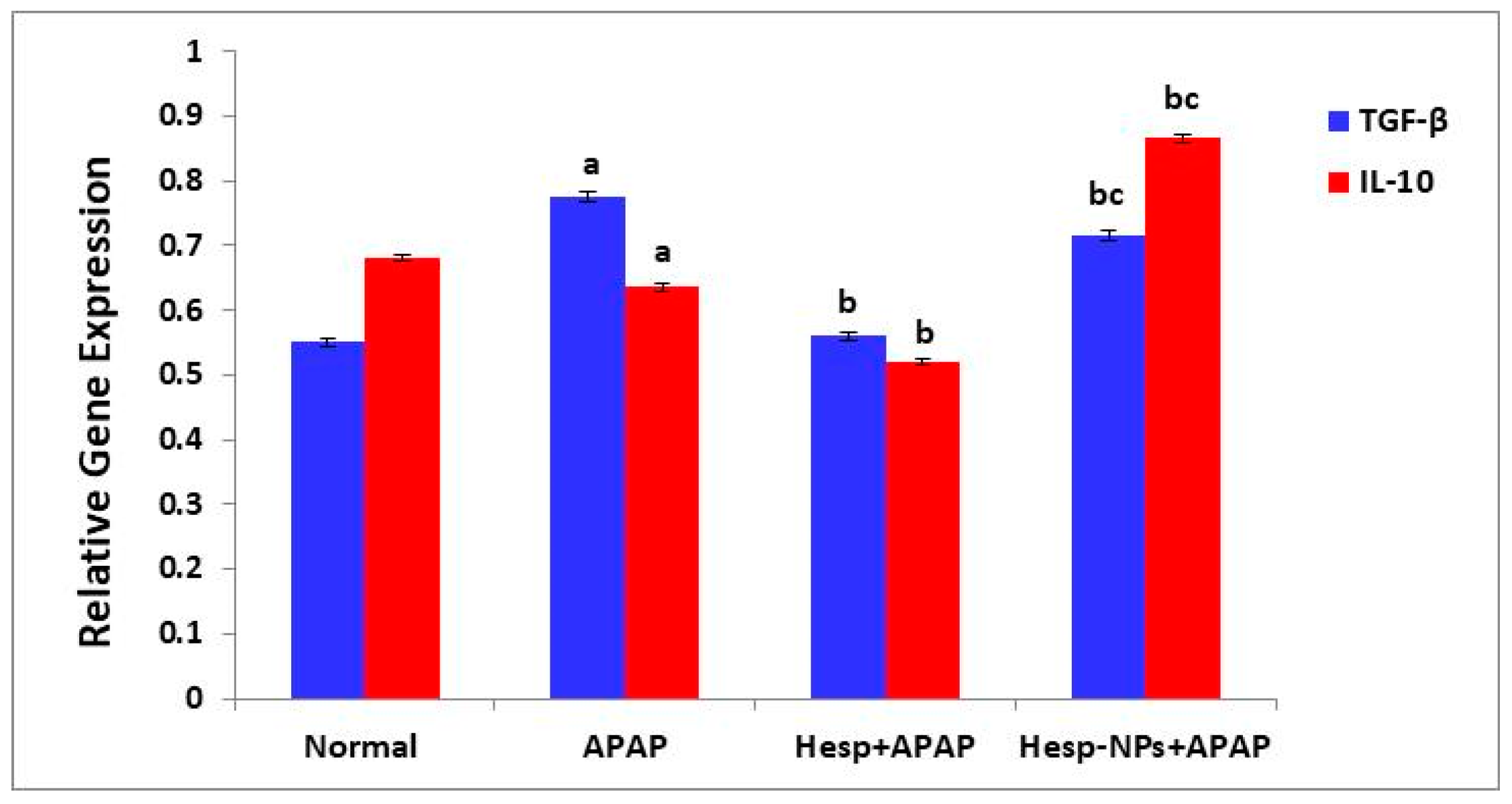
3.8.3. Effects on mRNA Expression of Liver mRNA Expression of TGF-β and IL-10
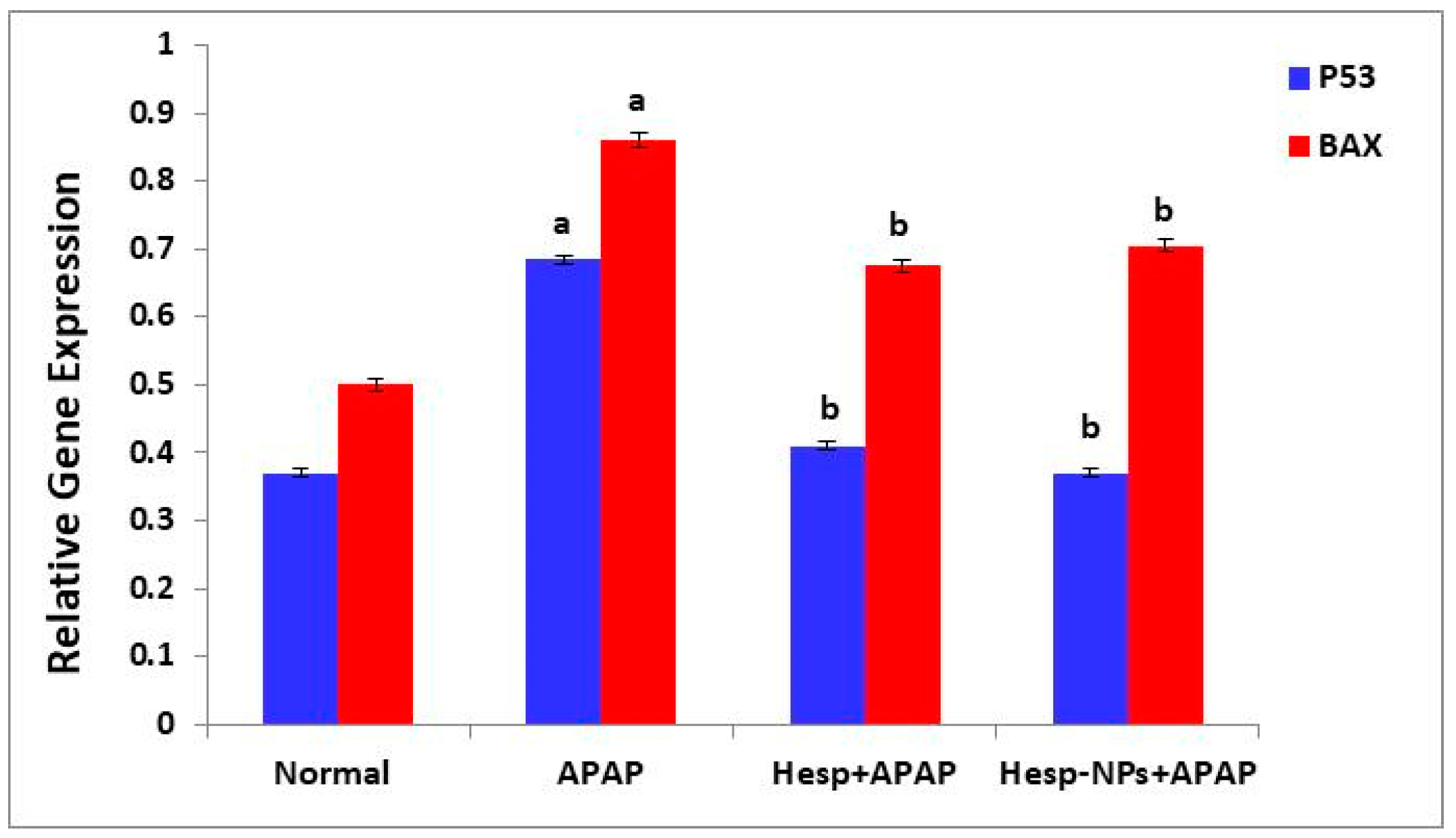
3.8.4. Effects on mRNA Expression of Markers Related to Apoptosis (P53 and BAX) in the Liver
3.9. Effects on Liver Histological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, N.; Mamat, S.; Kamisan, F.; Yahya, F.; Kamarolzaman, M.; Nasir, N.; Mohtarrudin, N.; Tohid, S.; Zakaria, Z. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. Leaves. BioMed Res. Int. 2014, 2014, 695678. [Google Scholar]
- Halim, A.; Nur, N.M.; El-Agamy, E.-S.; Ibrahim, A. Protective effect of hesperidin (HDN) on carbon tetrachloride (CCl4)-induced hepatic toxicity in male albino rats. AIJCR 2017, 8, 20328–20338. [Google Scholar]
- Dart, R.C.; Bailey, E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1219–1230. [Google Scholar]
- Dar, A.; Saxena, R.; Bansal, S. Hepatoprotection: A Hallmark of Citrullus colocynthis L. against Paracetamol Induced Hepatotoxicity in Swiss Albino Rats. Am. J. Plant Sci. 2012, 3, 1022–1027. [Google Scholar]
- Ahmed, O.M. Natural Flavonoids: Chemistry, Therapeutic Potentials, Therapeutic Targets and Mechanisms of Actions. Curr. Pharm. Des. 2021, 27, 455. [Google Scholar]
- Khaled, S.S.; Soliman, H.A.; Abdel-Gabbar, M.; Ahmed, N.A.; Attia, K.A.H.A.; Mahran, H.A.; El-Nahass, E.S.; Ahmed, O.M. The Preventive Effects of Naringin and Naringenin against Paclitaxel-Induced Nephrotoxicity and Cardiotoxicity in Male Wistar Rats. Evid. Based Complement Altern. Med. 2022, 2022, 8739815. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Saleh, A.S.; Ahmed, E.A.; Ghoneim, M.M.; Ebrahim, H.A.; Abdelgawad, M.A.; Abdel-Gabbar, M. Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells and Hesperetin in the Treatment of Streptozotocin-Induced Type 1 Diabetes in Wistar Rats. Pharmaceuticals 2023, 16, 859. [Google Scholar] [CrossRef]
- Sakr, H.I.; Khowailed, A.A.; Bastawy, N.A.; Gaber, S.S.; Ahmed, O.M.; Gaber, A.S. Hesperidin and experimentally-induced arthritis in male rats. J. Med. Clin. Res. 2017, 10, 29567–29585. [Google Scholar]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Wanjari, U.R. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.; Jabir, M.S.; Hameed, A.H. Fabrication of hesperidin nanoparticles loaded by poly lactic co-Glycolic acid for improved therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef]
- Rojas, R.; Palena, M.; Jimenez-Kairuz, A.; Manzo, R.; Giacomelli, C. Modeling drug release from a layered double hydroxide–ibuprofen complex. Appl. Clay Sci. 2012, 62, 15–20. [Google Scholar]
- Adefegha, S.A.; Rosa Leal, D.B.; Olabiyi, A.A.; Oboh, G.; Castilhos, L.G. Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep. 2017, 22, 563–571. [Google Scholar] [PubMed]
- El-Shahawy, A.A.; Abdel-Moneim, A.; Ebeid, A.S.; Eldin, Z.E.; Zanaty, M.I. A novel layered double hydroxide-hesperidin nanoparticles exert antidiabetic, antioxidant and anti-inflammatory effects in rats with diabetes. Mol. Biol. Rep. 2021, 48, 5217–5232. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; El-Shahawy, A.; Yousef, A.I.; Abd El-Twab, S.M.; Elden, Z.E.; Taha, M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int. J. Biol. Macromol. 2020, 154, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Yeh, M.-H.; Kao, S.-T.; Hung, C.-M.; Liu, C.-J.; Huang, Y.-Y.; Yeh, C.-C. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol. Lett. 2010, 194, 42–49. [Google Scholar] [PubMed]
- Çetin, A.; Çiftçi, O.; Otlu, A. Protective effect of hesperidin on oxidative and histological liver damage following carbon tetrachloride administration in Wistar rats. Arch. Med. Sci. 2016, 12, 486–493. [Google Scholar] [CrossRef]
- Arif Hussain, T.; Imad Uddin, M.D.; Irfan Uddin, M.D.; Nadeem, M.D.; Talha, H.; Prashanth, K. Hepato-Protective Studies of Poloxamer—188 by Paracetomol induced liver toxicity in Rats. Res. J. Pharm. Tech. 2019, 12, 574–578. [Google Scholar] [CrossRef]
- Abd-Eltawab Tammam, A.; AKhalaf, A.A.; RZaki, A.; Mansour Khalifa, M.; AIbrahim, M.; MMekkawy, A.; EAbdelrahman, R.; Farghali, A.; ANoshy, P. Hesperidin protects rats’ liver and kidney from oxidative damage and physiological disruption induced by nickel oxide nanoparticles. Front. Physiol. 2022, 13, 912625. [Google Scholar]
- Hollands, M.; Logan, J. An examination of commercial kits for the determination of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) in serum. Can. Med. Assoc. J. 1966, 95, 303. [Google Scholar]
- Bowers, G.N.; McComb, R.B. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin. Chem. 1966, 12, 70–89. [Google Scholar]
- Witt, I.; Trendelenburg, C. Gemeinsame Studie zur Erstellung von Richtwerten für klinisch-chemische Kenngrößen im Kindesalter. J. Clin. Chem. Clin. Biochem. 1982, 20, 235–242. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar]
- Lo, S.F.; Kytzia, H.-J.; Schumann, G.; Swartzentruber, M.; Vader, H.L.; Weber, F.; Doumas, B.T. Interlaboratory comparison of the Doumas bilirubin reference method. Clin. Biochem. 2009, 42, 1328–1330. [Google Scholar] [PubMed]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA, 1982. [Google Scholar]
- Griesmacher, A.; Kindhauser, M.; Andert, S.E.; Schreiner, W.; Toma, C.; Knoebl, P.; Pietschmann, P.; Prager, R.; Schnack, C.; Schemthaner, G. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am. J. Med. 1995, 98, 469–475. [Google Scholar] [PubMed]
- Claiborne, A. Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Beutler, E.; Mary, K.Y. Erythrocyte glutathione reductase. Blood 1963, 21, 573–585. [Google Scholar]
- Northoff, H.; Weinstock, C.; Berg, A. The cytokine response to strenuous exercise. Int. J. Sports Med. 1994, 15, S167–S171. [Google Scholar]
- Adinolfi, A.; Adinolfi, M. Alpha-feto-protein during development and in disease. J. Med. Genet. 1975, 12, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Hu-Li, J.; Shevach, E.; Mizuguchi, J.; Ohara, J.; Mosmann, T.; Paul, W. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J. Exp. Med. 1987, 165, 157–172. [Google Scholar]
- Abd-Elbaset, M.; Mansour, A.M.; Ahmed, O.M.; Abo-Youssef, A.M. The potential chemotherapeutic effect of β-ionone and/or sorafenib against hepatocellular carcinoma via its antioxidant effect, PPAR-γ, FOXO-1, Ki-67, Bax, and Bcl-2 signaling pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1611–1624. [Google Scholar]
- Shaker, O.J.; Sourour, D.A. Protective effects of pomegranate seed extract on streptozotocin-induced β-cell damage in Rats: Inhibition of pancreatic nuclear factor kappa beta, transforming growth factor beta and matrix metalloproteinase-2 genes expression. Int. J. Adv. Res. 2013, 1, 88–102. [Google Scholar]
- Ashok, I.; Sheeladevi, R. Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biol. 2014, 2, 820–831. [Google Scholar] [PubMed]
- Asiri, Y.A. Probucol attenuates cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid. Med. Cell Longev. 2010, 3, 308–316. [Google Scholar] [PubMed]
- Yamashita, Y.; Ueyama, T.; Nishi, T.; Yamamoto, Y.; Kawakoshi, A.; Sunami, S.; Iguchi, M.; Tamai, H.; Ueda, K.; Ito, T.; et al. Nrf2-Inducing Anti-Oxidation Stress Response in the Rat Liver—New Beneficial Effect of Lansoprazole. PLoS ONE 2014, 9, e97419. [Google Scholar]
- Yan, X.; Cheng, X.; Zhou, L.; He, X.; Zheng, W.; Chen, H. Dexmedetomidine alleviates lipopolysaccharide-induced lung injury in Wistar rats. Oncotarget 2017, 8, 44410–44417. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Moore, M.P.; Moore, A.N.; Healy, J.C.; Roberts, M.D.; Rector, R.S.; Martin, J.S. Curcumin supplementation mitigates NASH development and progression in female Wistar rats. Physiol. Rep. 2018, 6, e13789. [Google Scholar] [PubMed]
- Wang, L.; Li, H. Effects of resveratrol on the Nrf2 and HO-1 expression in diabetic vascular endothelial cells. Int. J. Clin. Exp. Med. 2017, 10, 684–691. [Google Scholar]
- O’Bryan, M.K.; Gerdprasert, O.; Nikolic-Paterson, D.J.; Meinhardt, A.; Muir, J.A.; Foulds, L.M.; Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1744–R1755. [Google Scholar]
- Xu, Z.P.; Stevenson, G.; Lu, C.-Q.; Lu, G.Q. Dispersion and size control of layered double hydroxide nanoparticles in aqueous solutions. J. Phys. Chem. B 2006, 110, 16923–16929. [Google Scholar]
- Bourdi, M.; Eiras, D.P.; Holt, M.P.; Webster, M.R.; Reilly, T.P.; Welch, K.D.; Pohl, L.R. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem. Res. Toxicol. 2007, 20, 208–216. [Google Scholar]
- Nathiya, S.; Rajaram, S.; Abraham, P. Hesperidin alleviates antitubercular drug induced oxidative stress, inflammation and apoptosis in rat liver. Int. J. Biomed. Res. 2016, 7, 439–446. [Google Scholar]
- Mihajlovic, M.; Vinken, M. Mitochondria as the Target of Hepatotoxicity and Drug-Induced Liver Injury: Molecular Mechanisms and Detection Methods. Int. J. Mol. Sci. 2022, 23, 3315. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.J. Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction. Cell Mol. Life Sci. 2024, 81, 34. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Powell, E.E.; Irvine, K.M.; Martin, J.H. Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br. J. Clin. Pharmacol. 2016, 81, 210–222. [Google Scholar] [CrossRef]
- Ashry, S.K.; Ahmed, S.A.; Salem, H.E.; Wahdan, M.M. Alpha fetoprotein; a prognostic marker for early detection of liver regeneration in acute paracetamol toxicity. QJM Int. J. Med. 2018, 111 (Suppl. 1), hcy200.056. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Abdul-Hamid, M.M.; El-Bakry, A.M.; Mohamed, H.M.; Rahman, F.E.-Z.S.A. Camellia sinensis and epicatechin abate doxorubicin-induced hepatotoxicity in male Wistar rats via their modulatory effects on oxidative stress, inflammation, and apoptosis. J. Appl. Pharm. Sci. 2019, 9, 30–44. [Google Scholar]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef]
- Prescott, L. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 1980, 10, 291–298. [Google Scholar] [CrossRef]
- Ben-Shachar, R.; Chen, Y.; Luo S Hartman, C.; Reed, M.; Nijhout, H.F. The biochemistry of acetaminophen hepatotoxicity and rescue: A mathematical model. Theor. Biol. Med. Model. 2012, 9, 1–22. [Google Scholar] [CrossRef]
- Liao, J.; Lu, Q.; Li, Z.; Li, J.; Zhao, Q.; Li, J. Acetaminophen-induced liver injury: Molecular mechanism and treatments from natural products. Front. Pharmacol. 2023, 14, 1122632. [Google Scholar] [CrossRef]
- Abdallah, A.A.M.; Bafail, R.; Zaman, A.Y.; Aldhafiri, A.J.; Alalawi, A.; Omran, F.M.; Baghdadi, H.H.; Abdellah, W.A.; Alsharif, A.M.; Al Thagfan, S.S.; et al. Acute paracetamol toxicity-induced inflammatory and oxidative effects are relieved by Aleppo galls: A novel experimental study. Int. J. Physiol. Pathophysiol. Pharmacol. 2023, 15, 1–11. [Google Scholar] [PubMed]
- Ahmed, H.M.; Shehata, H.H.; El-Saeed, G.S.M.; Abou Gabal, H.H.; El-Daly, S.M. Paracetamol Overdose Induces Acute Liver Injury accompanied by oxidative stress and inflammation. Egypt. J. Chem. 2023, 66, 399–408. [Google Scholar]
- Iwalokun, B.; Efedede, B.; Alabi-Sofunde, J.; Oduala, T.; Magbagbeola, O.; Akinwande, A. Hepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage in mice. J. Med. Food 2006, 9, 524–530. [Google Scholar]
- Yanpallewar, S.; Sen, S.; Tapas, S.; Kumar, M.; Raju, S.; Acharya, S. Effect of Azadirachta indica on paracetamol-induced hepatic damage in albino rats. Phytomedicine 2003, 10, 391–396. [Google Scholar]
- Tabeshpour, J.; Hosseinzadeh, H.; Hashemzaei, M.; Karimi, G. A review of the hepatoprotective effects of hesperidin, a flavanon glycoside in citrus fruits, against natural and chemical toxicities. DARU J. Pharm. Sci. 2020, 28, 305–317. [Google Scholar]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [PubMed]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Pharmacological significance of hesperidin and hesperetin, two citrus flavonoids, as promising antiviral compounds for prophylaxis against and combating COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X211042540. [Google Scholar]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An Up-to-Date Review on Citrus Flavonoids: Chemistry and Benefits in Health and Diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar]
- Chen, X.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar]
- Aggarwal, V.; Tuli, H.S.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.A. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar]
- Ahmad, S.T.; Arjumand, W.; Nafees, S.; Seth, A.; Ali, N.; Rashid, S.; Sultana, S. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol. Lett. 2012, 208, 149–161. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, Z.; Ren, S.; Leng, J.; Hu, J.-N.; Liu, Z.; Chen, C.; Li, W. Improved protective effects of American ginseng berry against acetaminophen-induced liver toxicity through TNF-α-mediated caspase-3/-8/-9 signaling pathways. Phytomedicine 2018, 51, 128–138. [Google Scholar] [PubMed]
- Emam, H.T.; Madboly, A.G. Ameliorative effects of hesperidin and melatonin against acetaminophen-induced nephrotoxicity in adult albino rats. Egypt J. Forensic Sci. Appli. Toxicol. 2021, 21, 31–46. [Google Scholar]
- Talebi, S.F.; Kooshki, A.; Zarein, M.; Seify, M.; Dolatshahi, B.; Shoorei, H.; Bhandari, R.K. Protective effect of hesperidin on malathion-induced ovarian toxicity in mice: The role of miRNAs, inflammation, and apoptosis. Toxicol. Rep. 2024, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kazak, F.; Yarim, G.F.; Anadol, E.; Salt, A. Hesperidin alleviates inflammation in the metabolic syndrome model. Vet. Arhiv. 2024, 94, 67–76. [Google Scholar] [CrossRef]
- Hassan, R.A.; Hozayen, W.G.; Abo Sree, H.T.; Al-Muzafar, H.M.; Amin, K.A.; Ahmed, O.M. Naringin and Hesperidin Counteract Diclofenac-Induced Hepatotoxicity in Male Wistar Rats via Their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Oxid. Med. Cell. Longev. 2021, 2021, 9990091. [Google Scholar]
- Ali, Y.A.; Soliman, H.A.; Abdel-Gabbar, M.; Ahmed, N.A.; Attia, K.A.A.; Shalaby, F.M.; El-Nahass, E.; Ahmed, O.M. Rutin and Hesperidin Revoke the Hepatotoxicity Induced by Paclitaxel in Male Wistar Rats via Their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Evid.-Based Complement. Altern. Med. 2023, 2023, 2738351. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar]
- Mohamed, E.E.; Ahmed, O.M.; Abdel-Moneim, A.; Zoheir, K.M.A.; Elesawy, B.H.; Al Askary, A.; Hassaballa, A.; El-Shahawy, A.A.G. Protective Effects of Naringin-Dextrin Nanoformula against Chemically Induced Hepatocellular Carcinoma in Wistar Rats: Roles of Oxidative Stress, Inflammation, Cell Apoptosis, and Proliferation. Pharmaceuticals 2022, 15, 1558. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Ahmed, O.M.; Zoheir, K.M.A.; El-Shahawy, A.A.G.; Tamur, S.; Shams, A.; Burcher, J.T.; Bishayee, A.; Abdel-Moneim, A. Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation. Cancers 2023, 15, 5102. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Al-Muzafar, H.M.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.S.; Abdelazeem, W.H. The Preventive Effects and the Mechanisms of Action of Navel Orange Peel Hydroethanolic Extract, Naringin, and Naringenin in N-Acetyl-p-aminophenol-Induced Liver Injury in Wistar Rats. Oxid. Med. Cell Longev. 2019, 2019, 2745352. [Google Scholar] [PubMed]
- Chen, J.; Fan, X.; Chen, J.; Luo, X.; Huang, X.; Zhou, Z.; He, Y.; Feng, S.; Jiao, Y.; Wang, R.; et al. Effects of hesperidin on the histological structure, oxidative stress, and apoptosis in the liver and kidney induced by NiCl2. Front. Vet. Sci. 2024, 11, 1424711. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; Abdelazem, A.Z.; Hashem, K.S.; Attia, Y.A. Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1405–1417. [Google Scholar]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Hassan, M.I.; Habib, S.; Islam, S. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Berens, H.M.; Tyler, K.L. The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J. Virol. 2011, 85, 3858–3871. [Google Scholar]
- Spampanato, C.; De Maria, S.; Sarnataro, M.; Giordano, E.; Zanfardino, M.; Baiano, S.; Cartenì, M.; Morelli, F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 2012, 40, 935–941. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, A.A.; Fahim, H.I.; Zaky, M.Y. Quercetin and naringenin abate diethylnitrosamine/acetylaminofluorene-induced hepatocarcinogenesis in Wistar rats: The roles of oxidative stress, inflammation and cell apoptosis. Drug Chem. Toxicol. 2022, 45, 262–273. [Google Scholar]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [PubMed]
- Li, C.; Cheng, L.; Wu, H.; He, P.; Zhang, Y.; Yang, Y.; Chen, J.; Chen, M. Activation of the KEAP1 NRF2 ARE signaling pathway reduces oxidative stress in Hep2 cells. Molecular. Med. Rep. 2018, 18, 2541–2550. [Google Scholar]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, M.; Feng, J.; Wufur, R.; Liu, K.; Hu, S.; Zhang, Y. Different inhibition of Nrf2 by two Keap1 isoforms α and β to shape malignant behaviour of human hepatocellular carcinoma. Int. J. Mol. Sci. 2022, 23, 10342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-P.; Deng, J.-S.; Huang, S.-S.; Wu, S.-H.; Chen, C.-C.; Liao, J.-C.; Chen, H.-Y.; Lin, H.-Y.; Huang, G.-J. Sanghuangporus sanghuang mycelium prevents paracetamol-induced hepatotoxicity through regulating the MAPK/NF-κB, Keap1/Nrf2/HO-1, TLR4/PI3K/Akt, and CaMKKβ/LKB1/AMPK pathways and suppressing oxidative stress and inflammation. Antioxidants 2021, 10, 897. [Google Scholar] [CrossRef]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Subramanya, S.B.; Venkataraman, B.; Meeran, M.F.N.; Goyal, S.N.; Patil, C.R.; Ojha, S. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int. J. Mol. Sci. 2018, 19, 3776. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Barua, C.C.; Karnam, K.C.; Dahiya, V.; Ellutla, M. Antiproliferative and antioxidant potential of hesperetin against benzo (a) pyrene-induced lung carcinogenesis in Swiss albino mice. Chem.-Biol. Interact. 2015, 242, 345–352. [Google Scholar] [CrossRef]
- Kakehi, M.; Ikenaka, Y.; Nakayama, S.M.; Kawai, Y.K.; Watanabe, K.P.; Mizukawa, H.; Nomiyama, K.; Tanabe, S.; Ishizuka, M. Uridine diphosphate-glucuronosyltransferase (UGT) xenobiotic metabolizing activity and genetic evolution in pinniped species. Toxicol. Sci. 2015, 147, 360–369. [Google Scholar] [CrossRef]
- Stingl, J.C.; Bartels, H.; Viviani, R.; Lehmann, M.; Brockmöller, J. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: A quantitative systematic review. Pharmacol. Ther. 2014, 141, 92–116. [Google Scholar] [CrossRef]
- Rashid, M.H.; Babu, D.; Siraki, A.G. Interactions of the antioxidant enzymes NAD (P) H: Quinone oxidoreductase 1 (NQO1) and NRH: Quinone oxidoreductase 2 (NQO2) with pharmacological agents, endogenous biochemicals and environmental contaminants. Chem.-Biol. Interact. 2021, 345, 109574. [Google Scholar] [CrossRef] [PubMed]
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The modulation of phase II drug-metabolizing enzymes in proliferating and differentiated CaCo-2 cells by hop-derived prenylflavonoids. Nutrients 2020, 12, 2138. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. NQO1 in protection against oxidative stress. Curr. Opin. Toxicol. 2018, 7, 67–72. [Google Scholar] [CrossRef]
- Miettinen, T.P.; Björklund, M. NQO2 is a reactive oxygen species generating off-target for acetaminophen. Mol. Pharm. 2014, 11, 4395–4404. [Google Scholar] [PubMed]
- Nishikawa, S.; Inoue, Y.; Hori, Y.; Miyajima, C.; Morishita, D.; Ohoka, N.; Hida, S.; Makino, T.; Hayashi, H. Anti-inflammatory activity of kurarinone involves induction of HO-1 via the KEAP1/Nrf2 pathway. Antioxidants 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gu, H.; Ye, Y.; Lin, B.; Sun, L.; Deng, W.; Zhang, J.; Liu, J. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem. Toxicol. 2010, 48, 2980–2987. [Google Scholar] [PubMed]
- Wang, X.L.; Yang, M.; Wang, Y. Roles of transforming growth factor-β signaling in liver disease. World J. Hepatol. 2024, 16, 973–979. [Google Scholar] [CrossRef]
- Nakatsukasa, H.; Nagy, P.; Evarts, R.P.; Hsia, C.-C.; Marsden, E.; Thorgeirsson, S.S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Investig. 1990, 85, 1833–1843. [Google Scholar]
- Pérez-Vargas, J.E.; Zarco, N.; Shibayama, M.; Segovia, J.; Tsutsumi, V.; Muriel, P. Hesperidin prevents liver fibrosis in rats by decreasing the expression of nuclear factor-κB, transforming growth factor-β and connective tissue growth factor. Pharmacology 2014, 94, 80–89. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Honarmand, H.; Abdollahi, M.; Ahmadi, A.; Javadi, M.R.; Khoshayand, M.R.; Tabeefar, H.; Mousavi, S.; Mahmoudi, L.; Radfar, M.; Najafi, A. Randomized trial of the effect of intravenous paracetamol on inflammatory biomarkers and outcome in febrile critically ill adults. DARU J. Pharm. Sci. 2012, 20, 12. [Google Scholar]





| Genes | Forward Sequence (5′-3′) | Reserve Sequence (5′-3′) | Ref. |
|---|---|---|---|
| β-ACTIN | TCACCCTGAAGTACCCCATGGAG | TTGGCCTTGGGGTTCAGGGGG | [32] |
| BAX | GACACCTGAGCTGACCTTGG | GAGGAAGTCCAGTGTCCAGC | [33] |
| P53 | CAGCGTGATGATGGTAAGGA | GCGTTGCTCTGATGGTGA | [34] |
| KEAP-1 | GGACGGCAACACTGATTC | TCGTCTCGATCTGGCTCATA | [35] |
| CYP1A1 | GTGGCCTGTATTTTGCTTATG | AGCTCAGGTACGTTTTTCCTA | [35] |
| NQO1 | GGAGACTGTCTGGGAGGAGT | GCTTTGATCTGGTTGTCGGC | [36] |
| NQO2 | TCCAGGAGGCAGAGACTGTT | AGAGGGCACCAGTAACATCG | [36] |
| UGTA1 | ACTCAAAGTATGAGATCCTTGC | TCAAATTCCTGAGACAGGTTC | [35] |
| TGF-β | TGAGTGGCTGTCTTTTGACG | TGGGACTGATCCCATTGATT | [37] |
| HO-1 | CAGAGTTTCTTCGCCAGAGG | TGAGTGTGAGGACCCATCG | [38] |
| IL-10 | GCAGGACTTTAAGGGTTACTTGG | GGGGAGAAATCGATGACAGC | [39] |
| Histopathological Changes | Score | Normal | APAP | Hesp + APAP | Hesp-NPs + APAP |
|---|---|---|---|---|---|
| Hydropic and vacuolar degeneration | 0 | 6 (100%) | - | - | 1 (16.7%) |
| I | - | - | 2 (33.3%) | 4 (66.7%) | |
| II | - | - | 4 (66.7%) | 1 (16.7%) | |
| III | - | 6 (100%) | - | - | |
| Inflammation (inflammatory cell infiltration) | 0 | 6 (100%) | - | 1 (16.7%) | 3 (50.0%) |
| I | - | 1 (16.7%) | 3 (50.0%) | 2 (33.3%) | |
| II | - | 3 (50.0%) | 1 (16.7%) | 1 (16.7%) | |
| III | 2 (33.3%) | 1 (16.7%) | - | ||
| Fibroblast proliferation | 0 | 6 (100%) | - | 1 (16.7%) | 4 (66.7%) |
| I | - | 3 (50.0%) | 5 (83.3%) | 2 (33.3%) | |
| II | - | 2 (33.3%) | - | - | |
| III | 1 (16.7%) | - | |||
| Congested blood vessels and sinusoids | 0 | 6 (100%) | - | 3 (50.0%) | 4 (66.7%) |
| I | - | 2 (33.3%) | 2 (33.3%) | 1 (16.7%) | |
| II | - | 2 (33.3%) | 1 (16.7%) | 1 (16.7%) | |
| III | 2 (33.3%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, D.A.; El-Shahawy, A.A.G.; Zanaty, M.I.; Eldin, Z.E.; Abd-Elbaset, M.; Shams, A.; Tamur, S.; Ahmed, O.M. Utilizing Nanoparticles of Hesperidin Loaded on Layered Double Hydroxide to Reduce Hepatotoxicity Caused by Paracetamol in Rats: Controlling of Biotransformation, Oxidative Stress, Inflammation, and Apoptosis. Pharmaceutics 2025, 17, 429. https://doi.org/10.3390/pharmaceutics17040429
Shaban DA, El-Shahawy AAG, Zanaty MI, Eldin ZE, Abd-Elbaset M, Shams A, Tamur S, Ahmed OM. Utilizing Nanoparticles of Hesperidin Loaded on Layered Double Hydroxide to Reduce Hepatotoxicity Caused by Paracetamol in Rats: Controlling of Biotransformation, Oxidative Stress, Inflammation, and Apoptosis. Pharmaceutics. 2025; 17(4):429. https://doi.org/10.3390/pharmaceutics17040429
Chicago/Turabian StyleShaban, Deyaa A., Ahmed A. G. El-Shahawy, Mohamed I. Zanaty, Zienab E. Eldin, Mohamed Abd-Elbaset, Anwar Shams, Shadi Tamur, and Osama M. Ahmed. 2025. "Utilizing Nanoparticles of Hesperidin Loaded on Layered Double Hydroxide to Reduce Hepatotoxicity Caused by Paracetamol in Rats: Controlling of Biotransformation, Oxidative Stress, Inflammation, and Apoptosis" Pharmaceutics 17, no. 4: 429. https://doi.org/10.3390/pharmaceutics17040429
APA StyleShaban, D. A., El-Shahawy, A. A. G., Zanaty, M. I., Eldin, Z. E., Abd-Elbaset, M., Shams, A., Tamur, S., & Ahmed, O. M. (2025). Utilizing Nanoparticles of Hesperidin Loaded on Layered Double Hydroxide to Reduce Hepatotoxicity Caused by Paracetamol in Rats: Controlling of Biotransformation, Oxidative Stress, Inflammation, and Apoptosis. Pharmaceutics, 17(4), 429. https://doi.org/10.3390/pharmaceutics17040429










