Evaluating Manganese-Doped Magnetic Nanoflowers for Biocompatibility and In Vitro Magnetic Hyperthermia Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Determination of Iron Content via Liebig Reaction
2.4. Evaluation of Optical Interference of Mn in the Determination of Iron Content via Liebig Reaction
2.5. Cell Lines
2.6. In Vitro Cytocompatibility Evaluation
2.7. Evaluation of Cellular Uptake Using Prussian Blue Staining and Liebig Reaction
2.8. In Vitro Magnetic Hyperthermia
2.9. Statistics
3. Results and Discussion
3.1. Structural Characterization of NFs
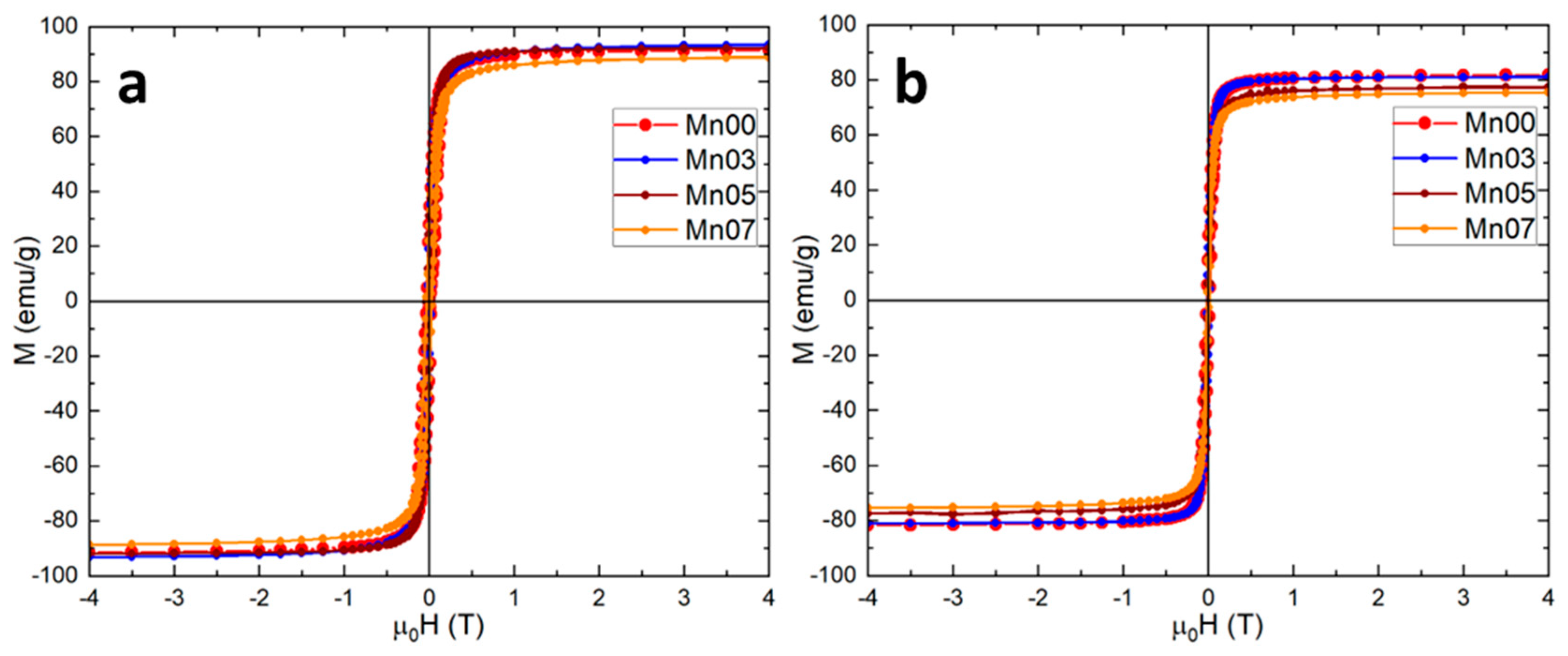
3.2. Magnetic Characterization of NFs
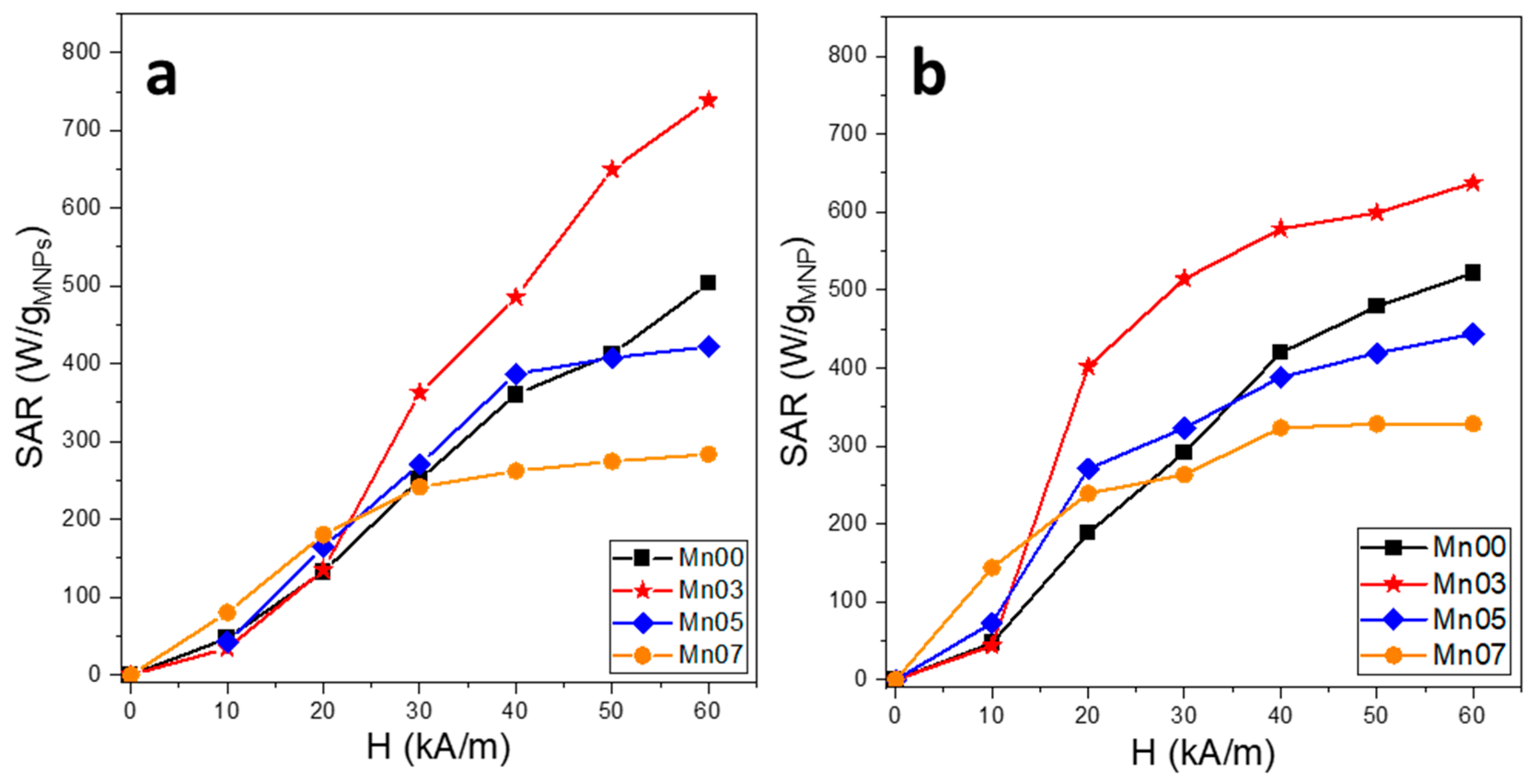
3.3. Magnetic Hyperthermia Properties of NFs
3.4. In Vitro Cytotoxicity Evaluation of NFs
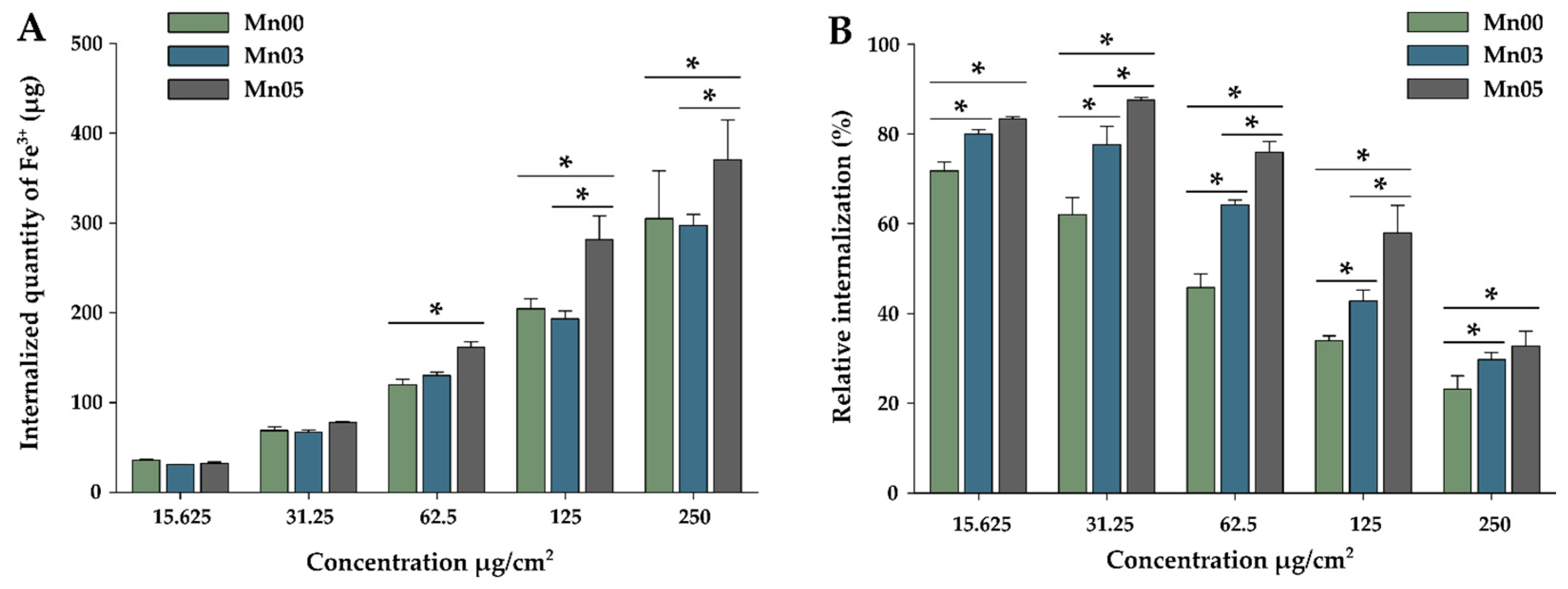
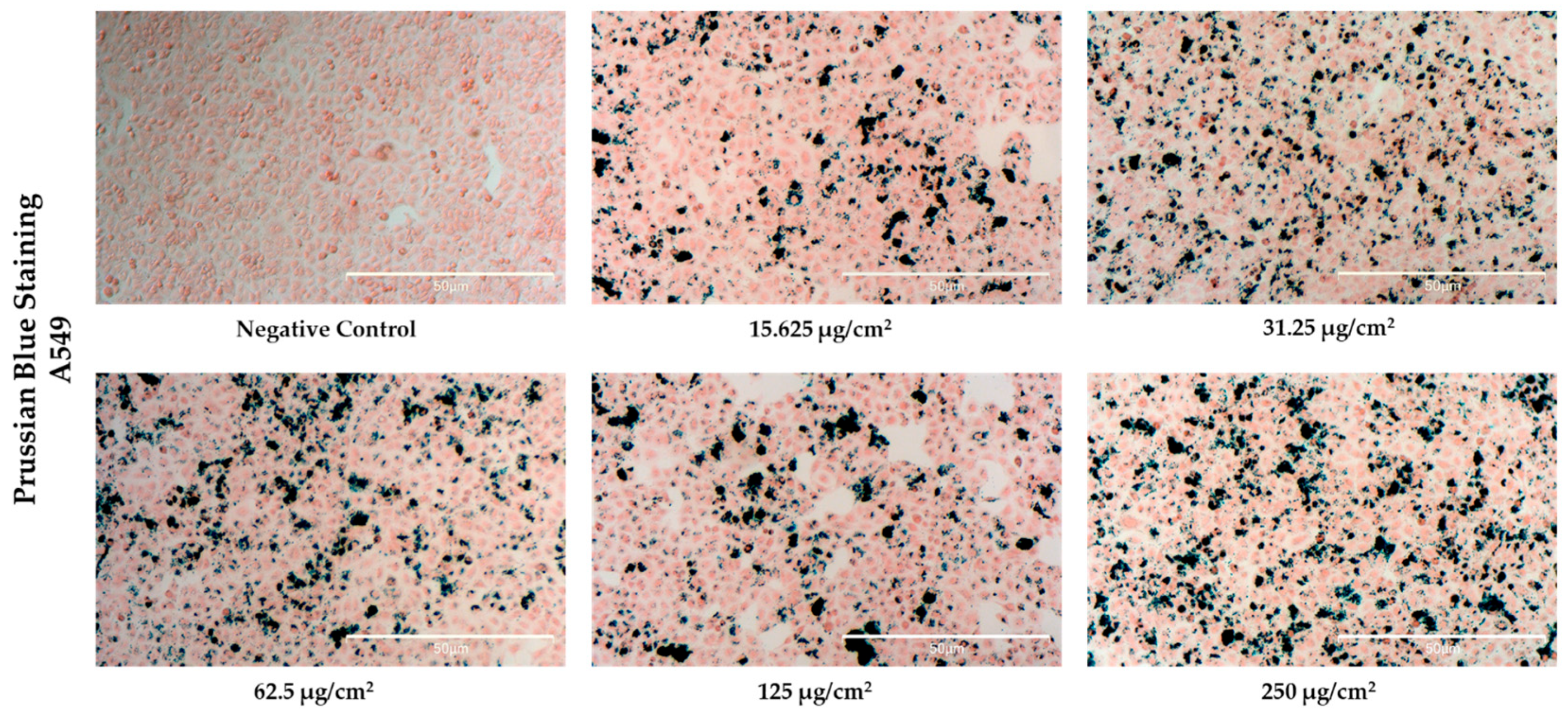
3.5. Cellular Uptake of NFs
3.6. In Vitro Magnetic Hyperthermia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-induced cardiotoxicity: Overview of the roles of oxidative stress. Oxidative Med. Cell. Longev. 2015, 2015, 795602. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; St Clair, D.K. Chemotherapy-induced tissue injury: An insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants 2017, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic Fluid Hyperthermia Based on Magnetic Nanoparticles: Physical Characteristics, Historical Perspective, Clinical Trials, Technological Challenges, and Recent Advances. Adv. Ther. 2020, 3, 2000061. [Google Scholar] [CrossRef]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of an accurate temperature regulation culture system with metallic culture vessel demonstrates different thermal cytotoxicity in cancer and normal cells. Sci. Rep. 2021, 11, 21466. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia-Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.A.; Orue, I.; Phan, M.-H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.-H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Jeun, M.; Lee, S.; Kyeong Kang, J.; Tomitaka, A.; Wook Kang, K.; Il Kim, Y.; Takemura, Y.; Chung, K.W.; Kwak, J.; Bae, S. Physical limits of pure superparamagnetic Fe3O4 nanoparticles for a local hyperthermia agent in nanomedicine. Appl. Phys. Lett. 2022, 100, 092406. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Nedylakova, M.; Medinger, J.; Mirabello, G.; Lattuada, M. Iron oxide magnetic aggregates: Aspects of synthesis, computational approaches and applications. Adv. Colloid Interface Sci. 2024, 323, 103056. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, L.; Colvin, V.L.; Zhang, Q.; Bao, G. Synthesis and Application of Magnetic Nanocrystal Clusters. Ind. Eng. Chem. Res. 2022, 61, 7613–7625. [Google Scholar] [CrossRef]
- Müller, R.; Dutz, S.; Neeb, A.; Cato, A.; Zeisberger, M. Magnetic heating effect of nanoparticles with different sizes and size distributions. J. Magn. Magn. Mater. 2013, 328, 80–85. [Google Scholar] [CrossRef]
- Lévy, M.; Wilhelm, C.; Siaugue, J.-M.; Horner, O.; Bacri, J.-C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 204133. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Park, M.; Do, M.J.; Lee, N.; Ryu, J.H.; Kim, G.W.; Kim, C.; Park, T.G.; Hyeon, T. Chitosan Oligosaccharide-Stabilized Ferrimagnetic Iron Oxide Nanocubes for Magnetically Modulated Cancer Hyperthermia. ACS Nano 2012, 6, 5266–5273. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, W.E.M.; Al-Hazmi, F.S.; Memesh, L.S.; Bronstein, L.M. A novel approach for rapid green synthesis of nearly mono-disperse iron oxide magnetic nanocubes with remarkable surface magnetic anisotropy density for enhancing hyperthermia performance. Colloids Surf. A 2017, 529, 239–245. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Jang, J.; Nah, H.; Lee, J.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical enhancements of MRI contrast and hyperthermic effects by dopant controlled magnetic nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Pathak, S.; Jain, K.; Garg, P.; Pant, M.; Mahapatro, A.K.; Rath, D.; Wang, L.; Kim, S.-K.; et al. A threefold increase in SAR performance for magnetic hyperthermia by compositional tuning in zinc-substituted iron oxide superparamagnetic nanoparticles with superior biocompatibility. J. Alloys Compd. 2023, 968, 171868. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Pathak, S.; Jain, K.; Garg, P.; Pant, M.; Mahapatro, A.K.; Singh, R.K.; Rajput, P.; Kim, S.-K.; et al. Tailored nanoparticles for magnetic hyperthermia: Highly stable aqueous dispersion of Mn-substituted magnetite superparamagnetic nanoparticles by double surfactant coating for improved heating efficiency. J. Alloys Compd. 2024, 976, 172999. [Google Scholar] [CrossRef]
- Aquino, V.R.R.; Aquino, J.C.R.; Coaquire, J.A.H.; Bakuzis, A.F.; Sousa, M.H.; Morais, P.C. New synthesis route for high quality iron oxide-based nanorings: Structural and magnetothermal evaluations. Mater. Des. 2023, 232, 112082. [Google Scholar] [CrossRef]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Levy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Anujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T.K. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: Microwave assisted synthesis and the role of core-to-core interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef]
- Hemery, G.; Keyes, A.C., Jr.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning sizes, morphologies, and magnetic properties of mono- vs. multi-core iron oxide nanoparticles through control of added water in the polyol synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef] [PubMed]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron Oxide Monocrystalline Nanoflowers for Highly Efficient Magnetic Hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Bender, P.; Fock, J.; Frandsen, C.; Hansen, M.F.; Balceris, C.; Ludwig, F.; Posth, O.; Wetterskog, E.; Bogart, L.K.; Southern, P.; et al. Relating Magnetic Properties and High Hyperthermia Performance of Iron Oxide Nanoflowers. J. Phys. Chem. C 2018, 122, 3068–3077. [Google Scholar] [CrossRef]
- Storozhuk, L.; Besenhard, M.O.; Mourdikoudis, S.; LaGrow, A.P.; Lees, M.R.; Tung, L.D.; Gavriilidis, A.; Thanh, N.T.K. Stable Iron Oxide Nanoflowers with Exceptional Magnetic Heating Efficiency: Simple and Fast Polyol Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 45870–45880. [Google Scholar] [CrossRef]
- Bejko, M.; Al Yaman, Y.; Keyes, A.; Bagur, A.; Rosa, P.; Gayot, M.; Weill, F.; Mornet, S.; Sandre, O. Structure-Function Relationship of Iron Oxide Nanoflowers: Optimal Sizes for Magnetic Hyperthermia Depending on Alternating Magnetic Field Conditions. ChemPhysChem 2024, 25, e202400023. [Google Scholar] [CrossRef]
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.B.; et al. Synthesis of Ferromagnetic Fe0.6Mn0.4O3 Nanoflowers as a New Class of Magnetic Theranostic Platform for In Vivo T1-T2 Dual-Mode Magnetic Resonance Imaging and Magnetic Hyperthermia Therapy. Adv. Healthc. Mater. 2016, 5, 2092–2104. [Google Scholar] [CrossRef]
- Bender, P.; Honecker, D.; Fernandez Barquin, L. Supraferromagnetic correlations in clusters of magnetic nanoflowers. Appl. Phys. Lett. 2019, 115, 132406. [Google Scholar] [CrossRef]
- Neuman, S.; Kuger, L.; Carsten-Rene, A.; Franzreb, M.; Rafaja, D. Influence of the hierarchical architecture of multi-core iron oxide nanoflowers on their magnetic properties. Sci. Rep. 2023, 13, 5673. [Google Scholar] [CrossRef]
- Moya, C.; Escoda-Torroella, M.; Rodríguez-Álvarez, J.; Figueroa, A.I.; García, Í.; Ferrer-Vidal, I.B.; Gallo-Cordova, A.; Morales, M.P.; Aballe, L.; Rodríguez, A.F.; et al. Unveiling the crystal and magnetic texture of iron oxide nanoflowers. Nanoscale 2024, 16, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Lobaz, V.; Klupp Taylor, R.N.; Peukert, W. Highly magnetizable superparamagnetic colloidal aggregates with narrowed size distribution from ferrofluid emulsion. J. Colloid Interface Sci. 2012, 374, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yan, Y.; Roberts, C.; Liu, Z.; Chen, Y. The role of dipole interactions in hyperthermia heating colloidal clusters of densely-packed superparamagnetic nanoparticles. Sci. Rep. 2018, 8, 4704. [Google Scholar] [CrossRef] [PubMed]
- Krasia-Christoforou, T.; Socoliuc, V.; Knudsen, K.D.; Tombácz, E.; Turcu, R.; Vékás, L. From Single-Core Nanoparticles in Ferrofluids to Multi-Core Magnetic Nanocomposites: Assembly Strategies, Structure, and Magnetic Behavior. Nanomaterials 2020, 10, 2178. [Google Scholar] [CrossRef]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.F.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Puerto Morales, M.; et al. Formation Mechanism of Maghemite Nanoflowers Synthesized by a Polyol-Mediated Process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef]
- Bertuit, E.; Neveu, S.; Abou-Hassan, A. High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device. Nanomaterials 2022, 12, 119. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Corrales-Pérez, B.; Cabrero, P.; Force, C.; Veintemillas-Verdaguer, S.; Ovejero, J.G.; Morales, M.D.P. Magnetic Harvesting and Degradation of Microplastics using Iron Oxide Nanoflowers prepared by a Scaled-up Procedure. Chem. Eng. J. 2024, 490, 151725. [Google Scholar] [CrossRef]
- Iacovita, C.; Fizeșan, I.; Pop, A.; Scorus, L.; Dudric, R.; Stiufiuc, G.; Vedeanu, N.; Tetean, R.; Loghin, F.; Stiufiuc, R.; et al. In Vitro Intracellular Hyperthermia of Iron Oxide Magnetic Nanoparticles, Synthesized at High Temperature by a Polyol Process. Pharmaceutics 2020, 12, 424. [Google Scholar] [CrossRef]
- Iacovita, C.; Dudric, R.; Vomir, M.; Ersen, O.; Donnio, B.; Gallani, J.L.; Rastei, M.V. Imaging large iron-oxide nanoparticles clusters by field-dependent magnetic force microscopy. J. Phys. Chem. C 2021, 125, 24001–24010. [Google Scholar] [CrossRef]
- Del Sol-Fernández, S.; Portilla-Tundidor, Y.; Gutiérrez, L.; Odio, O.F.; Reguera, E.; Barber, D.F.; Morales, M.P. Flower-like Mn-Doped Magnetic Nanoparticles Functionalized with αvβ3-Integrin-Ligand to Efficiently Induce Intracellular Heat after Alternating Magnetic Field Exposition, Triggering Glioma Cell Death. ACS Appl. Mater. Interfaces 2019, 11, 26648–26663. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Manganese-Doped Magnetic Nanoclusters for Hyperthermia and Photothermal Glioblastoma Therapy. ACS Appl. Nano Mater. 2020, 3, 2026–2037. [Google Scholar] [CrossRef]
- Shaw, S.; Kailashiya, J.; Gupta, S.K.; Prajapat, C.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N. MnFe2O4 nano-flower: A prospective material for bimodal hyperthermia. J. Alloys Compd. 2022, 899, 163192. [Google Scholar] [CrossRef]
- Yan, Z.; Chaluvadi, A.; FitzGerald, S.; Spence, S.; Bleyer, C.; Zhu, J.; Crawford, T.M.; Getman, R.B.; Watt, J.; Huber, D.L.; et al. Effect of manganese substitution of ferrite nanoparticles on particle grain structure. Nanoscale Adv. 2022, 4, 3957–3965. [Google Scholar] [CrossRef]
- Phalake, S.S.; Lad, M.S.; Kadam, K.V.; Tofail, S.A.M.; Thorat, N.D.; Khot, V.M. Application of MnxFe1–xFe2O4 (x = 0–1) Nanoparticles in Magnetic Fluid Hyperthermia: Correlation with Cation Distribution and Magnetostructural Properties. ACS Omega 2022, 7, 44187–44198. [Google Scholar] [CrossRef]
- Marcano, L.; Orue, I.; García-Prieto, A.; Abrudan, R.M.; Alonso, J.; Barquín, L.F.; Valencia, S.; Muela, A.; Fdez-Gubieda, M.L. Controlled Magnetic Anisotropy in Single Domain Mn-doped Biosynthesized Nanoparticles. J. Phys. Chem. C 2020, 124, 22827–22838. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Lucaciu, C.M.; Nitica, S.; Fizesan, I.; Filip, L.; Bilteanu, L.; Iacovita, C. Enhanced Magnetic Hyperthermia Performance of Zinc Ferrite Nanoparticles under a Parallel and a Transverse Bias DC Magnetic Field. Nanomaterials 2022, 12, 3578. [Google Scholar] [CrossRef]
- Morales, I.; Costo, R.; Mille, N.; Carrey, J.; Hernando, A.; de la Presa, P. Time-dependent AC magnetometry and chain formation in magnetite: The influence of particle size, initial temperature and the shortening of the relaxation time by the applied field. Nanoscale Adv. 2021, 3, 5801–5812. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, 2003712. [Google Scholar] [CrossRef]
- Cabrera, D.; Coene, A.; Leliaert, J.; Artés-Ibáñez, E.J.; Dupré, L.; Telling, N.D.; Teran, F.J. Dynamical Magnetic Response of Iron Oxide Nanoparticles Inside Live Cells. ACS Nano 2018, 12, 2741–2752. [Google Scholar] [CrossRef]
- Mejías, R.; Hernández Flores, P.; Talelli, M.; Tajada-Herráiz, J.L.; Brollo, M.E.F.; Portilla, Y.; Morales, M.P.; Barber, D.F. Cell-Promoted Nanoparticle Aggregation Decreases Nanoparticle-Induced Hyperthermia under an Alternating Magnetic Field Independently of Nanoparticle Coating, Core Size, and Subcellular Localization. ACS Appl. Mater. Interfaces 2019, 11, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; Cabreira-Gomes, R.; Torres, T.E.; Valdés, D.P.; Lima, E., Jr.; De Biasi, E.; Zysler, R.D.; Ibarra, M.R.; Goya, G.F. Low-Dimensional Assemblies of Magnetic MnFe2O4 Nanoparticles and Direct In Vitro Measurements of Enhanced Heating Driven by Dipolar Interactions: Implications for Magnetic Hyperthermia. ACS Appl. Nano Mater. 2020, 3, 8719–8731. [Google Scholar] [CrossRef]
- Kameda, R.; Suzuki, T.; Kato, I.; Suga, K.; Watanabe, K.; Nagino, K.; Hayashi, K.; Arai, S.; Nagao, D. Intracellular Magnetic Control over the Reversible Assembly of Silica-Based Magnetic Composite Particles for Photothermal Therapy. ACS Appl. Nano Mater. 2023, 6, 3873–3883. [Google Scholar] [CrossRef]
- Fernández-Afonso, Y.; Ruta, S.; Páez-Rodríguez, A.; van Zanten, T.S.; Gleadhall, S.; Fratila, R.M.; Moros, M.; Morales, M.d.P.; Satoh, A.; Chantrell, R.W.; et al. Reversible Alignment of Nanoparticles and Intracellular Vesicles During Magnetic Hyperthermia Experiments. Adv. Funct. Mater. 2024, 34, 2405334. [Google Scholar] [CrossRef]
- Fizesan, I.; Iacovita, C.; Pop, A.; Kiss, B.; Dudric, R.; Stiufiuc, R.; Lucaciu, C.M.; Loghin, F. The Effect of Zn-Substitution on the Morphological, Magnetic, Cytotoxic, and In Vitro Hyperthermia Properties of Polyhedral Ferrite Magnetic Nanoparticles. Pharmaceutics 2021, 13, 2148. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Mesquita, M.S.; Garcia, L.M.; dos Santos, P.R.; de Marangoni de Viveiros, C.C.; da Fonseca, R.D.; Báo, S.N. Radiofrequency Driving Antitumor Effect of Graphene Oxide-Based Nanocomposites: A Hill Model Analysis. Nanomedicine 2023, 19, 397–412. [Google Scholar] [CrossRef]
- Abbas, F.; Jan, T.; Iqbal, J.; Naqvi, M.S.; Ahmad, I. Inhibition of Neuroblastoma cancer cells viability by ferromagnetic Mn doped CeO2 monodisperse nanoparticles mediated through reactive oxygen species. Mater. Chem. Phys. 2016, 173, 146–151. [Google Scholar] [CrossRef]
- Valdés-Solís, T.; Valle-Vigón, P.; Álvarez, S.; Marbán, G.; Fuertes, A.B. Manganese ferrite nanoparticles synthesized through a nanocasting route as a highly active Fenton catalyst. Catal Commun. 2007, 8, 2037–2042. [Google Scholar] [CrossRef]
- Kalaiselvan, C.R.; Laha, S.S.; Somvanshi, S.B.; Tabish, T.A.; Thorat, N.D.; Sahu, N.K. Manganese ferrite (MnFe2O4) nanostructures for cancer theranostics. Coord. Chem. Rev. 2022, 473, 214809. [Google Scholar] [CrossRef]
- Tasso, M.; Ghilini, F.; Cathcarth, M.; Picco, A.S. Toxicity Assessment of Nanoferrites. In Spinel Nanoferrites. Topics in Mining, Metallurgy and Materials Engineering; Sharma, S.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 233–314. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Nitica, S.; Fizesan, I.; Dudric, R.; Barbu-Tudoran, L.; Pop, A.; Loghin, F.; Vedeanu, N.; Lucaciu, C.M.; Iacovita, C. A Fast, Reliable Oil-In-Water Microemulsion Procedure for Silica Coating of Ferromagnetic Zn Ferrite Nanoparticles Capable of Inducing Cancer Cell Death In Vitro. Biomedicines 2022, 10, 1647. [Google Scholar] [CrossRef] [PubMed]

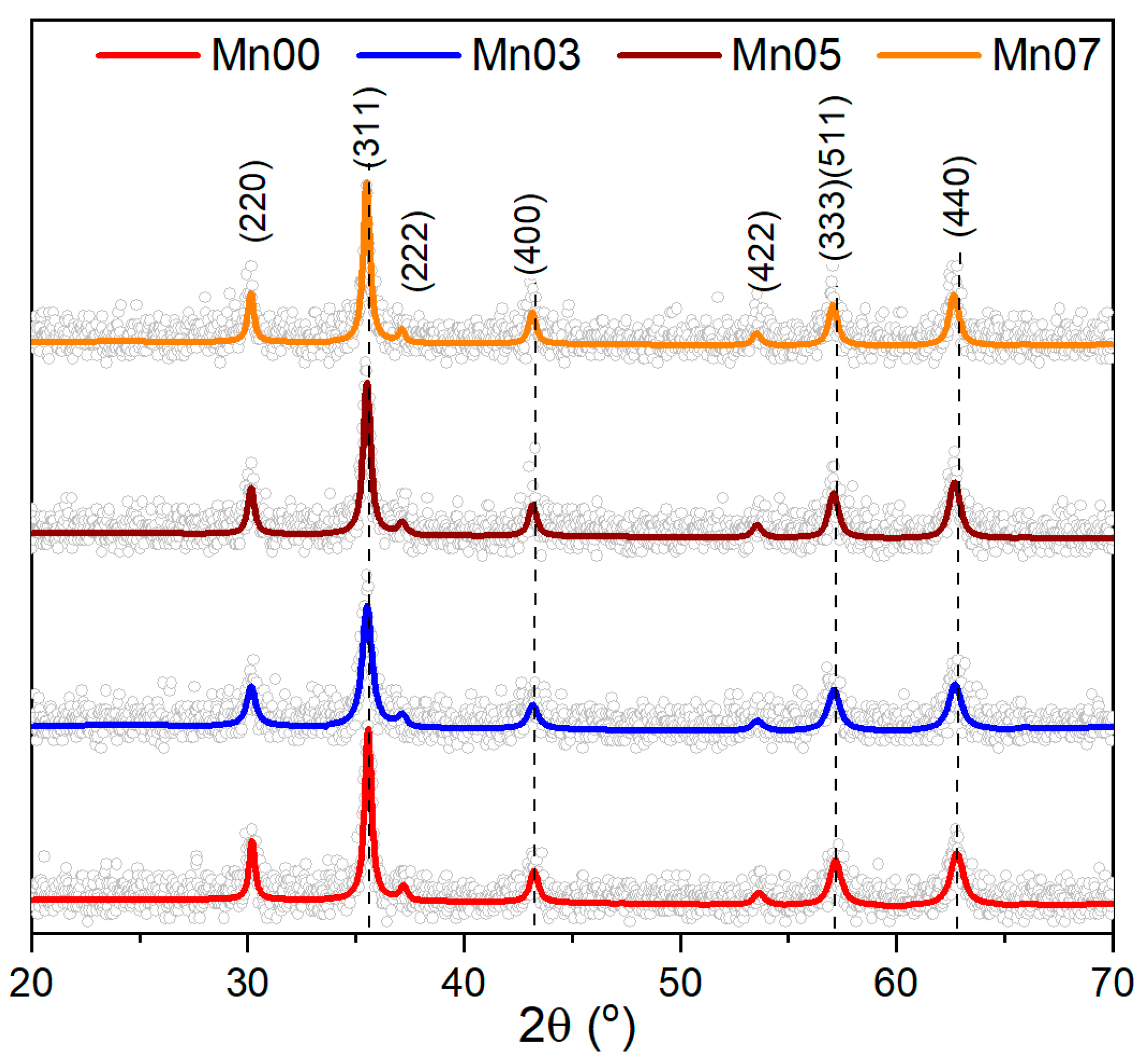


| Sample | TEM Diameter (nm) | Log Standard Deviation (nm) | XRD Diameter (nm) | Lattice Parameter (Å) |
|---|---|---|---|---|
| Mn00 | 222 ± 3 | 49 ± 4 | 33 ± 5 | 8.379 |
| Mn03 | 120 ± 1 | 23 ± 2 | 21 ± 1 | 8.384 |
| Mn05 | 118 ± 1 | 13 ± 1 | 23 ± 1 | 8.397 |
| Mn07 | 112 ± 1 | 18 ± 2 | 24 ± 2 | 8.424 |
| Sample | 4 K | 300 K | ||||
|---|---|---|---|---|---|---|
| Ms (emu/g) | Hc (mT) | Mr (emu/g) | Ms (emu/g) | Hc (mT) | Mr (emu/g) | |
| Mn00 | 91.5 | 31.8 | 31.22 | 81.5 | 17.1 | 19.51 |
| Mn03 | 93.1 | 29.5 | 28.67 | 81.0 | 15.8 | 15.44 |
| Mn05 | 92.0 | 24.4 | 28.45 | 77.3 | 14.7 | 15.42 |
| Mn07 | 88.5 | 23.5 | 27.62 | 75.3 | 12.6 | 14.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petru, A.-E.; Iacovita, C.; Fizeșan, I.; Dudric, R.; Crestin, I.-V.; Lucaciu, C.M.; Loghin, F.; Kiss, B. Evaluating Manganese-Doped Magnetic Nanoflowers for Biocompatibility and In Vitro Magnetic Hyperthermia Efficacy. Pharmaceutics 2025, 17, 384. https://doi.org/10.3390/pharmaceutics17030384
Petru A-E, Iacovita C, Fizeșan I, Dudric R, Crestin I-V, Lucaciu CM, Loghin F, Kiss B. Evaluating Manganese-Doped Magnetic Nanoflowers for Biocompatibility and In Vitro Magnetic Hyperthermia Efficacy. Pharmaceutics. 2025; 17(3):384. https://doi.org/10.3390/pharmaceutics17030384
Chicago/Turabian StylePetru, Andreea-Elena, Cristian Iacovita, Ionel Fizeșan, Roxana Dudric, Ionut-Valentin Crestin, Constantin Mihai Lucaciu, Felicia Loghin, and Bela Kiss. 2025. "Evaluating Manganese-Doped Magnetic Nanoflowers for Biocompatibility and In Vitro Magnetic Hyperthermia Efficacy" Pharmaceutics 17, no. 3: 384. https://doi.org/10.3390/pharmaceutics17030384
APA StylePetru, A.-E., Iacovita, C., Fizeșan, I., Dudric, R., Crestin, I.-V., Lucaciu, C. M., Loghin, F., & Kiss, B. (2025). Evaluating Manganese-Doped Magnetic Nanoflowers for Biocompatibility and In Vitro Magnetic Hyperthermia Efficacy. Pharmaceutics, 17(3), 384. https://doi.org/10.3390/pharmaceutics17030384







