Exploring Manipulated Prescribed Medicines for Novel Leads in 3D Printed Personalized Dosage Forms
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Parameters
2.2. Data Cleaning
2.3. Data Analysis and Endpoints
- Children:
- Neonates, both preterm and term: from the day of birth up to 27 days;
- Infants (or toddlers): from 1 month (28 days) to 23 months;
- Children: from 2 years to 11 years; and
- Adolescents: from 12 years to less than 18 years.
- Adults: 18 until 65 years of age
- Elderly: older than 65 years of age.
3. Results
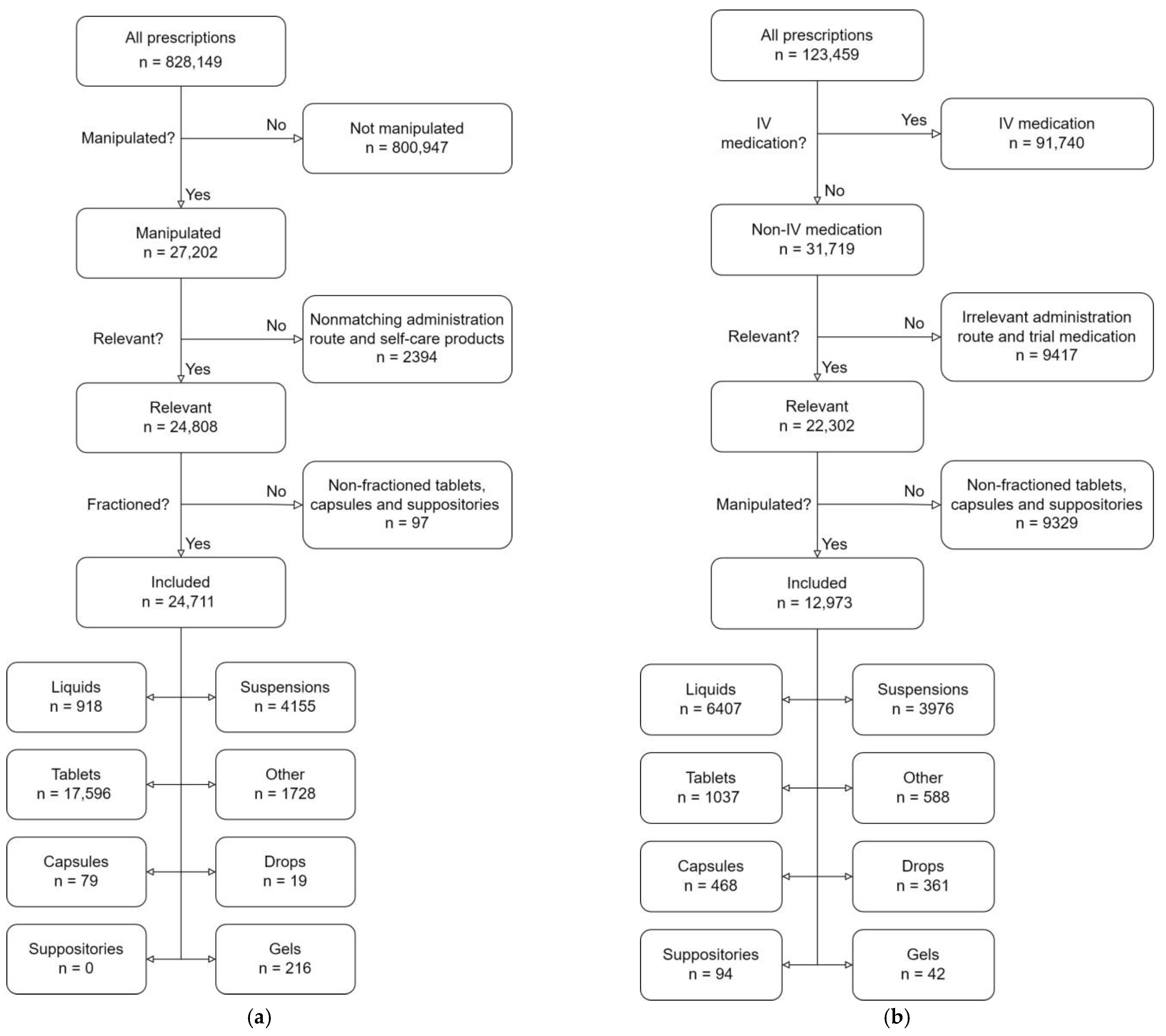
3.1. Data Cleaning
3.2. Data Analysis and Endpoints
Dosage Forms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Manipulations per Drug Dosage Form
| Drug Dosage Form | Manipulation for Dose Accuracy |
|---|---|
| Tablet |
|
| Capsule |
|
| Sachet (powder) |
|
| Oral liquid |
|
| Suppository |
|
| Intravenous injection |
|
Appendix B. Data Cleaning
- Administration routes that are suitable for printed medication included the following: oral, oral/inhalation, oromucosal, rectal, sublingual, and vaginal administrations.
- Anatomical Chemical Therapeutic (ATC) codes (as maintained by the World Health Organization) that are not suitable for printed medication were removed; A01A, A06AG, D., G01, J06, J07, M02, P03, R01A, R03A, R03B, S01, S02, S03, V07, V08, V09, V10, V20, missing ATC value.
- Partial administration, every entry that did not contain any indication of partial administration of a standard unit was removed. These indications include measures of weight, volume, fractions, named fractions, and numerical values.
- To prevent accidental removal based on free text, all entries related to tubes, syringes, and dissolving tablets/capsules in water were stored in a separate dataset.
- Based on the free text, entries were removed, primarily based on free text in the prescription instructions. This included a misspelling of previous removal criteria, anything related to test patients, anything other than prescription medication (bandages, non-prescription medication, sunscreen), internal notes (“discussed with the doctor”, “automatically repeat every three months”, “medication not dispensed”, etc.), medication used in clinical trials.
- Afterward, the dataset containing entries related to tubes, syringes, and dissolving was added to the included dataset. Integer numbers were removed, such as “X pieces” in a text note.
- The dose per unit and the dose per gift were calculated. If this did result in an integer number of units required, the entry was removed.
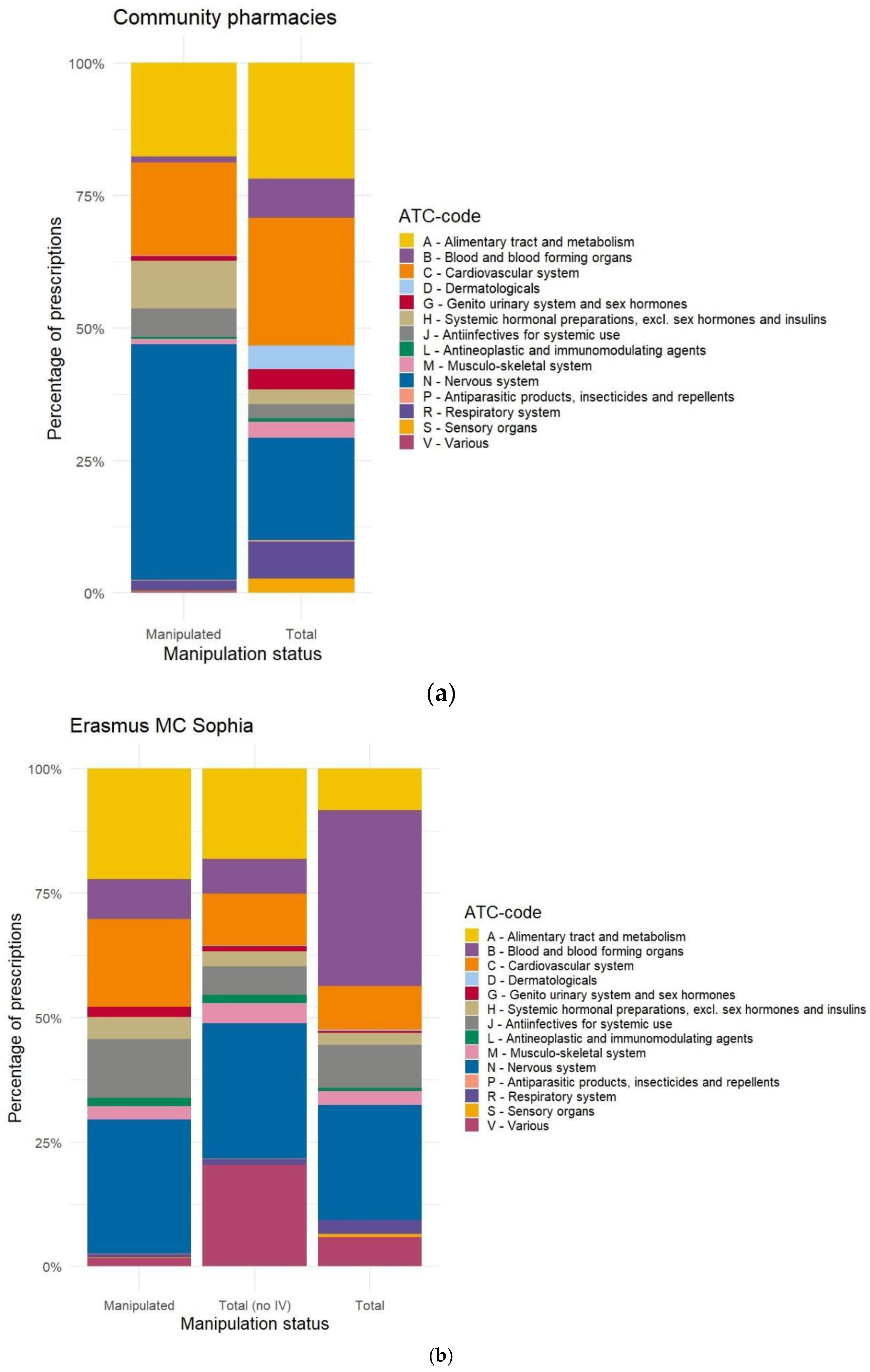
Appendix C. Distribution of Manipulated Prescriptions Versus Source Data, Based on ATC-Code
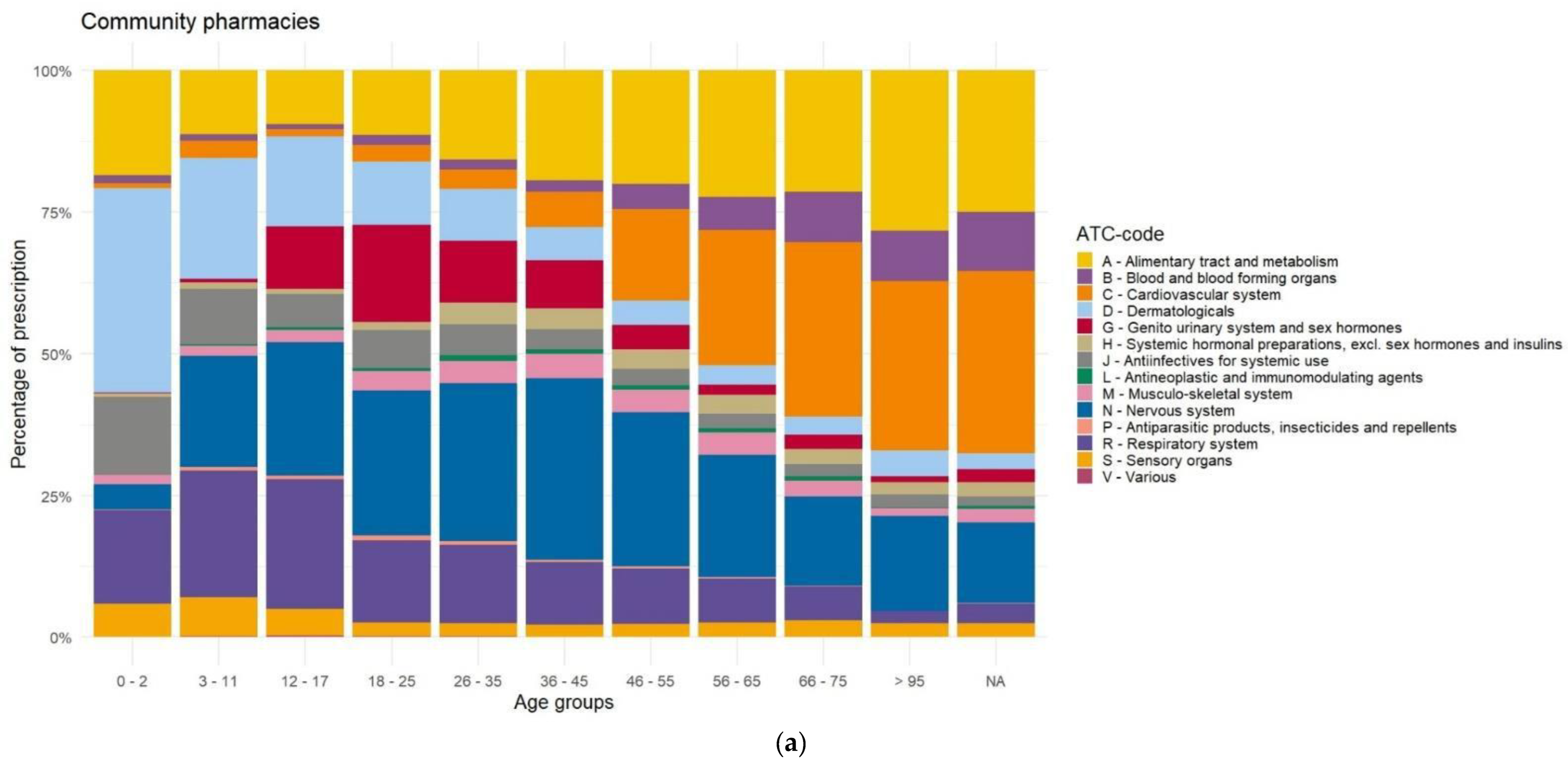
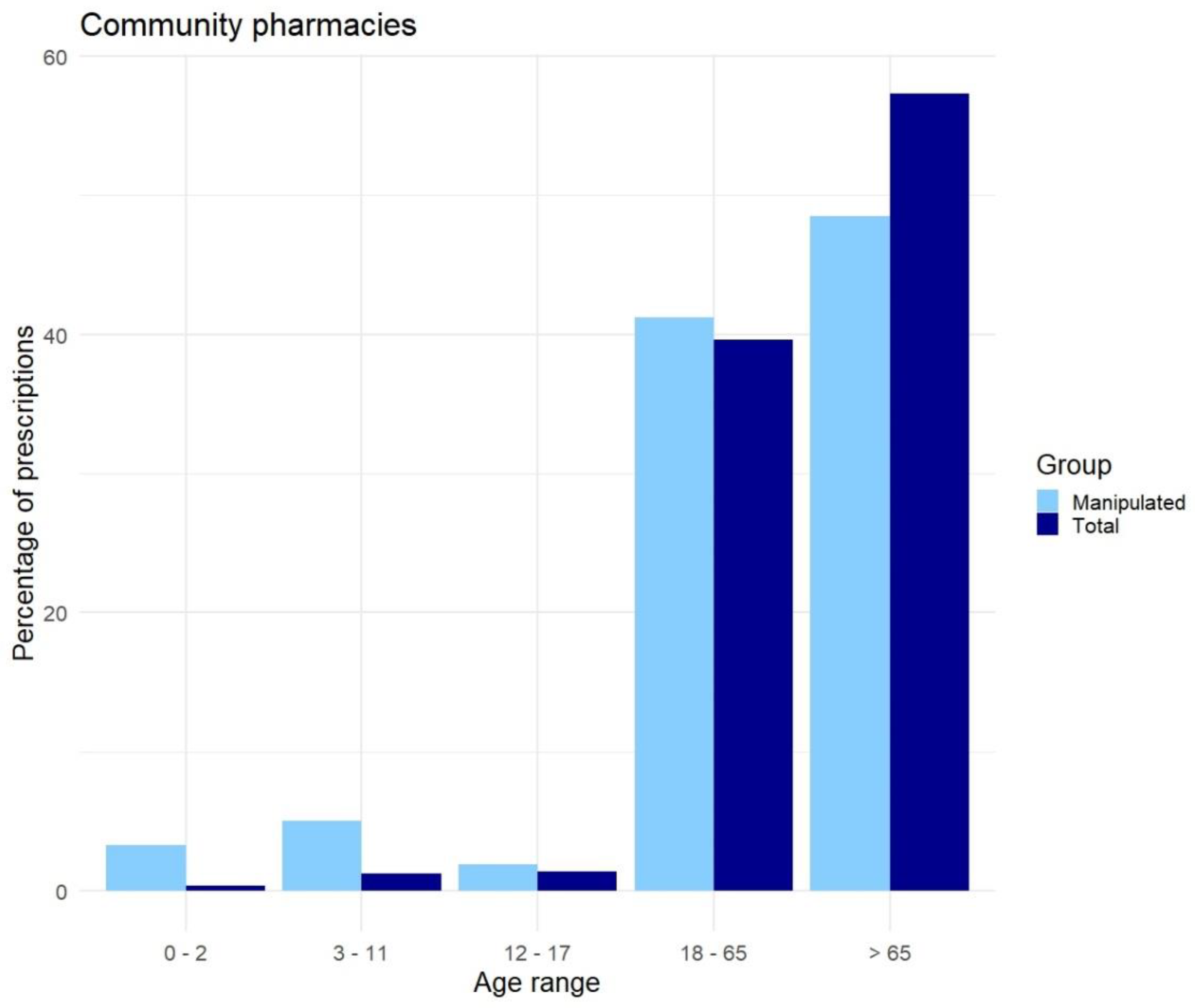
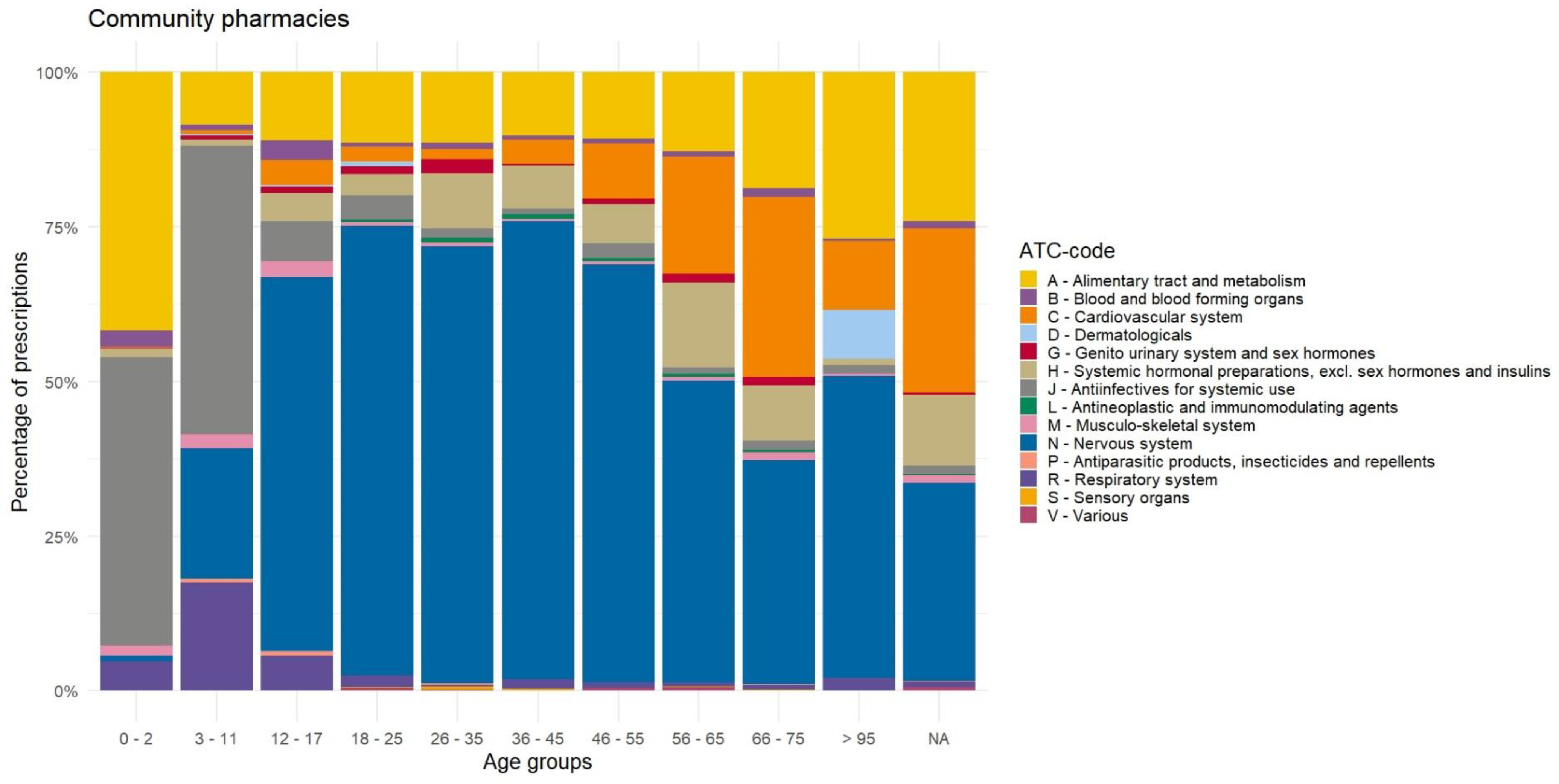
Appendix D. Distribution of Prescriptions Based on ATC-Code Ranked by Age, Before Data Cleaning

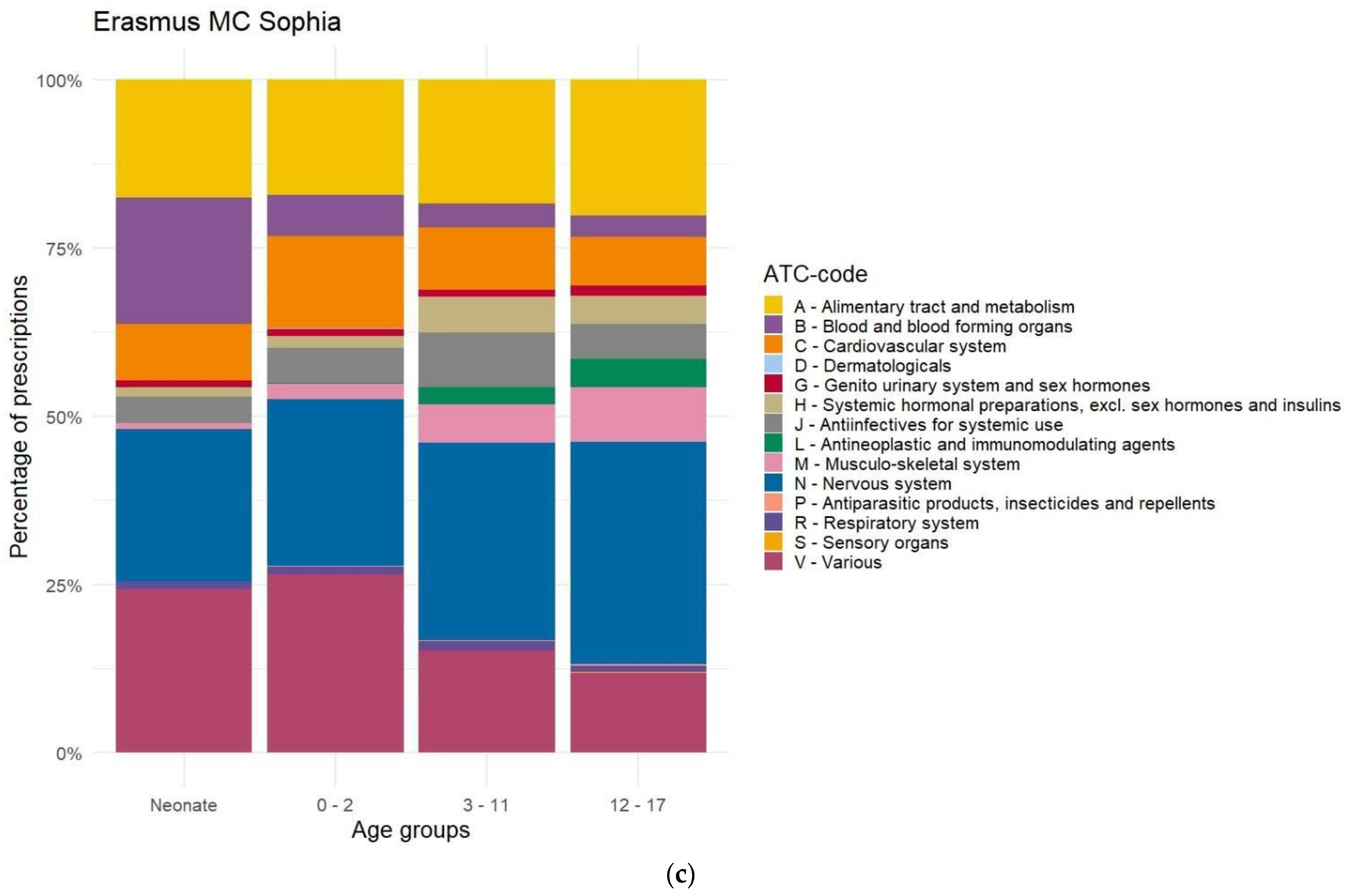
Appendix E. Distribution of Prescriptions-Based ATC-Code Ranked by Age, After Data Cleaning

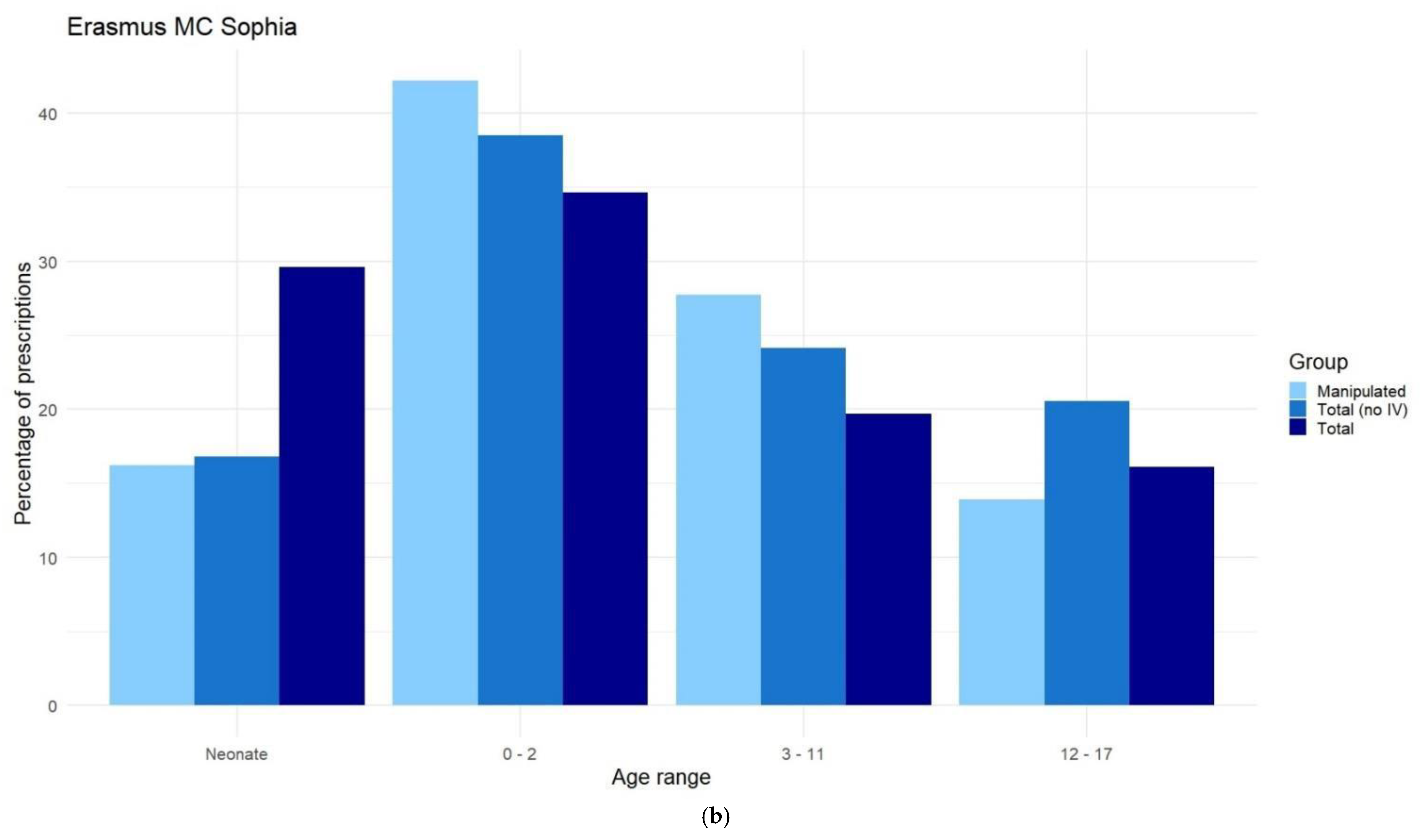
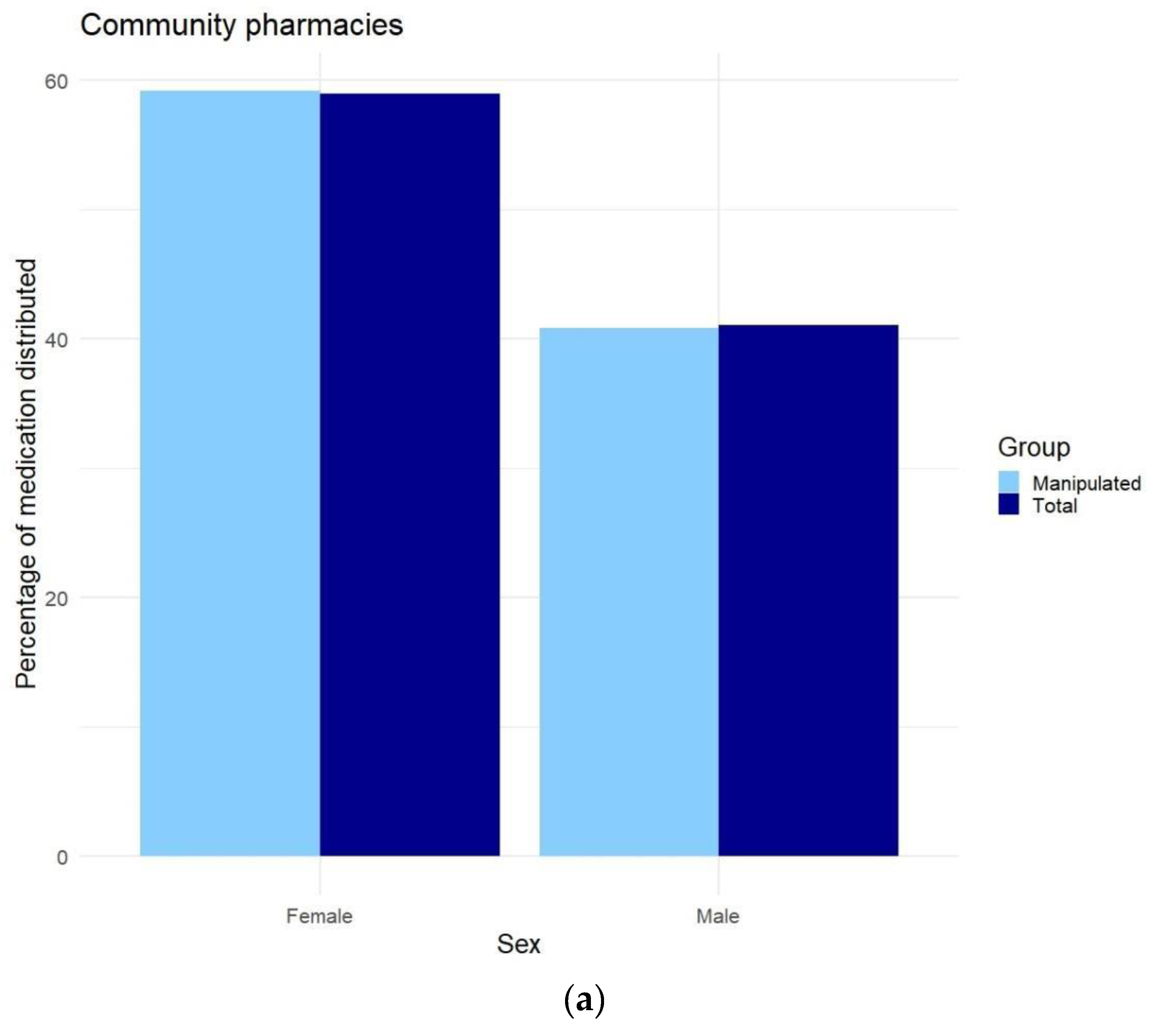
Appendix F. Differences in Prescriptions Ranked by Gender
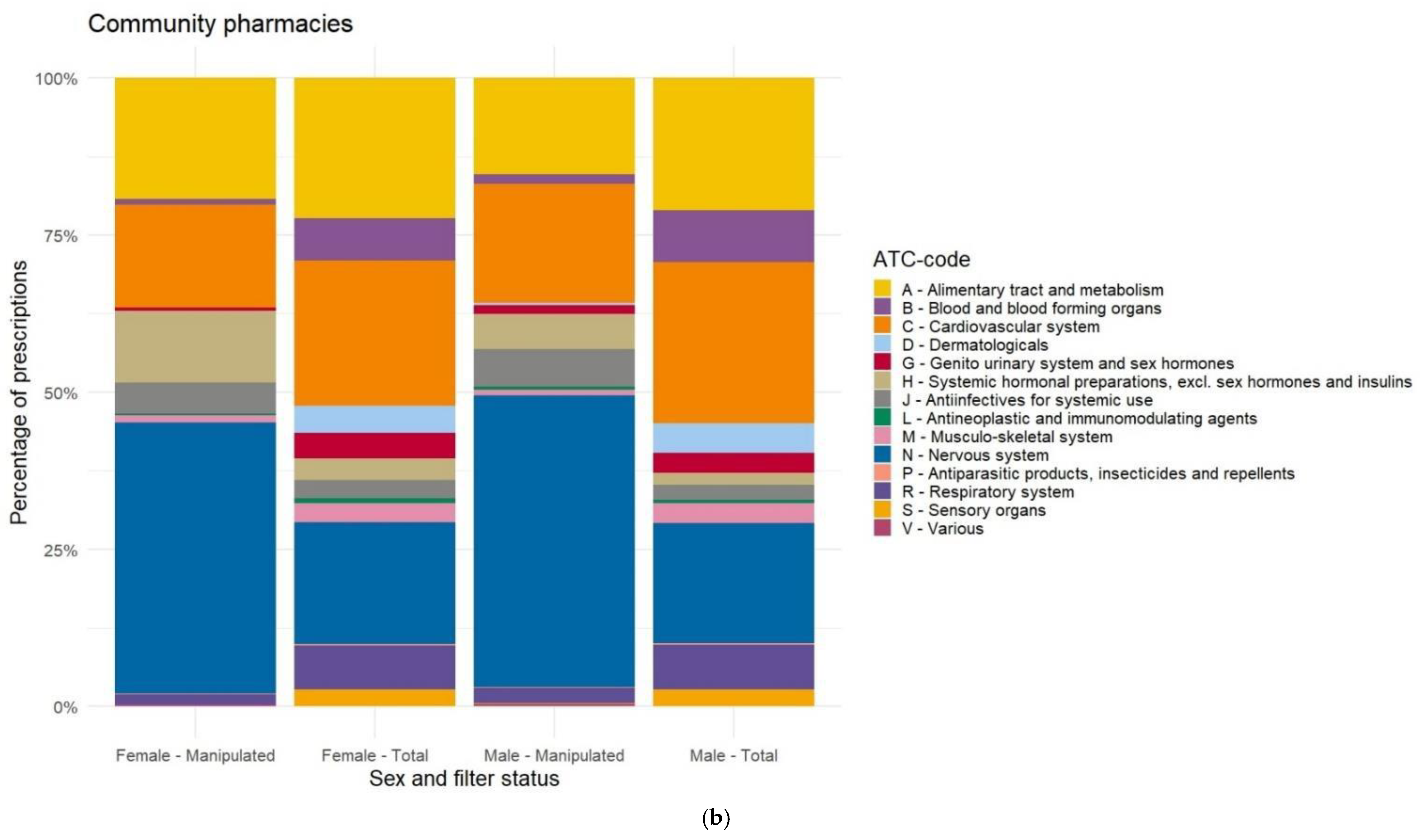
References
- Lind, J.; Kalvemark Sporrong, S.; Kaae, S.; Rantanen, J.; Genina, N. Social aspects in additive manufacturing of pharmaceutical products. Expert Opin. Drug Deliv. 2017, 14, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Li, W.; Tian, W.; Zheng, M.; Shen, L.; Hong, Y. A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022. Pharmaceuticals 2023, 16, 1521. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maciñeiras, X.; Bendicho-Lavilla, C.; Rial, C.; Garba-Mohammed, K.; Worsley, A.; Díaz-Torres, E.; Orive-Martínez, C.; Orive-Mayor, A.; Basit, A.W.; Alvarez-Lorenzo, C.; et al. Advancing medication compounding: Use of a pharmaceutical 3D printer to auto-fill minoxidil capsules for dispensing to patients in a community pharmacy. Int. J. Pharm. 2025, 671, 125251, ISSN 0378-5173. [Google Scholar] [CrossRef] [PubMed]
- Beer, N.; Hegger, I.; Kaae, S.; De Bruin, M.L.; Genina, N.; Alves, T.L.; Hoebert, J.; Kalvemark Sporrong, S. Scenarios for 3D printing of personalized medicines—A case study. Explor. Res. Clin. Soc. Pharm. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; Phang, F.J.F.; Yeo, L.S.; Ngu, L.H.; How, B.S. Future era of techno-economic analysis: Insights from review. Front. Sustain. 2022, 3, 924047. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- FabRx. Available online: https://www.fabrx.co.uk/ (accessed on 5 February 2025).
- Triastek. Available online: https://www.triastek.com/ (accessed on 5 February 2025).
- Aprecia. Available online: https://www.aprecia.com/ (accessed on 5 February 2025).
- DiHeSys. Available online: https://www.digital-health-systems.com/ (accessed on 5 February 2025).
- Dosermedical. Available online: https://dosermedical.com/ (accessed on 5 February 2025).
- van der Vossen, A.C.; Al-Hassany, L.; Buljac, S.; Brugma, J.D.; Vulto, A.G.; Hanff, L.M. Manipulation of oral medication for children by parents and nurses occurs frequently and is often not supported by instructions. Acta Paediatr. 2019, 108, 1475–1481. [Google Scholar] [CrossRef]
- Richey, R.H.; Hughes, C.; Craig, J.V.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.J.; Turner, M.A. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int. J. Pharm. 2017, 518, 155–166. [Google Scholar] [CrossRef]
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef]
- Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric oral biopharmaceutics: Key considerations and current challenges. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- van Kampen, E.E.M.; Willemsteijn, L.; Ruijgrok, E.J. 3D printing of drugs: Expanding the options for child-tailored pharmacotherapy. Arch. Dis. Child. 2022, 107, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jackson, S.H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef]
- Hilmer, S.N.; McLachlan, A.J.; Le Couteur, D.G. Clinical pharmacology in the geriatric patient. Fundam. Clin. Pharmacol. 2007, 21, 217–230. [Google Scholar] [CrossRef]
- Darwich, A.S.; Polasek, T.M.; Aronson, J.K.; Ogungbenro, K.; Wright, D.F.B.; Achour, B.; Reny, J.L.; Daali, Y.; Eiermann, B.; Cook, J.; et al. Model-Informed Precision Dosing: Background, Requirements, Validation, Implementation, and Forward Trajectory of Individualizing Drug Therapy. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 225–245. [Google Scholar] [CrossRef]
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef]
- WHO. Anatomical Therapeutic Chemical Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 5 February 2025).
- Griens, A.M.G.F.; Kroon, J.D.L.; Lukaart, J.S.; Postma, D.J.; Verkroost, M.J.S. SFK Data en Feiten 2020 Het Jaar 2019 in Cijfers; Stichting Farmaceutische Kengetallen: The Hague, The Netherlands, 2020. [Google Scholar]
- Bruyndonckx, R.; Adriaenssens, N.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; the ESAC-Net study group. Consumption of antibiotics in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii7–ii13. [Google Scholar] [CrossRef]
- Moor, J.H. Why we need better ethics for emerging technologies. Ethics Inf. Technol. 2005, 7, 111–119. [Google Scholar] [CrossRef]
- Hansson, S.O. How extreme is the precautionary principle? NanoEthics 2020, 14, 245–257. [Google Scholar] [CrossRef]
- KNMP. Aantal Geneesmiddelentekorten over 2023 Hoger Dan Ooit. Available online: https://www.knmp.nl/actueel/nieuws/aantal-geneesmiddelentekorten-over-2023-hoger-dan-ooit (accessed on 5 February 2025).
- Rijksoverheid. Leidraad Verantwoord Wisselen Medicijnen. Available online: https://open.overheid.nl/documenten/ronl-9783b4f97590d758f888567b76ad8aaaf029582d/pdf (accessed on 5 February 2025).
- KNMP. KNMP-Handleiding Geneesmiddelensubstitutie. Available online: https://www.knmp.nl/sites/default/files/2022-02/Handleiding%20substitutie%202018.pdf (accessed on 5 February 2025).
- Richey, R.H.; Craig, J.V.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.J.; Turner, M.A. The manipulation of drugs to obtain the required dose: Systematic review. J. Adv. Nurs. 2012, 68, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.S.; Lee, Y.J. Analysis of liquid medication dose errors made by patients and caregivers using alternative measuring devices. J. Manag. Care Pharm. 2012, 18, 439–445. [Google Scholar] [CrossRef] [PubMed]
- NKFK. Kinderformularium. Available online: https://www.kinderformularium.nl/ (accessed on 5 February 2025).
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Gutsmiedl, K.; Bighelli, I.; Schneider-Thoma, J.; Chaimani, A.; Leucht, S. Efficacy and tolerability of pharmacological and non-pharmacological interventions in older patients with major depressive disorder: A systematic review, pairwise and network meta-analysis. Eur. Neuropsychopharmacol. 2019, 29, 1003–1022. [Google Scholar] [CrossRef]
- NHG. NHG-Richtlijn Slaapproblemen. Available online: https://richtlijnen.nhg.org/standaarden/slaapproblemen (accessed on 5 February 2025).
- Bakker, M.H.; Hugtenburg, J.G.; van Straten, A.; van der Horst, H.E.; Slottje, P. Effectiveness of low-dose amitriptyline and mirtazapine for insomnia disorder: Study protocol of a randomised, double-blind, placebo-controlled trial in general practice (the DREAMING study). BMJ Open 2021, 11, e047142. [Google Scholar] [CrossRef]
- Harvey, B.H.; Slabbert, F.N. New insights on the antidepressant discontinuation syndrome. Hum. Psychopharmacol. 2014, 29, 503–516. [Google Scholar] [CrossRef]
- van Geffen, E.C.; Hugtenburg, J.G.; Heerdink, E.R.; van Hulten, R.P.; Egberts, A.C. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: Tapering versus abrupt discontinuation. Eur. J. Clin. Pharmacol. 2005, 61, 303–307. [Google Scholar] [CrossRef]
- O’Brien, C.P. Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 2005, 66 (Suppl. 2), 28–33. [Google Scholar]
- Darker, C.D.; Sweeney, B.P.; Barry, J.M.; Farrell, M.F.; Donnelly-Swift, E. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst. Rev. 2015, 2015, CD009652. [Google Scholar] [CrossRef]
- Van Leeuwen, E.; van Driel, M.L.; Horowitz, M.A.; Kendrick, T.; Donald, M.; De Sutter, A.I.; Robertson, L.; Christiaens, T. Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults. Cochrane Database Syst. Rev. 2021, 4, CD013495. [Google Scholar] [CrossRef]
- Ibrahim, K.; Cox, N.J.; Stevenson, J.M.; Lim, S.; Fraser, S.D.S.; Roberts, H.C. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, J.A.; Trujillo Rivera, E.A.; Zeng-Treitler, Q.; Faruqe, F.; Morizono, H.; Bost, J.E.; Pollack, M.M.; Patel, A.K. Medications for Children Receiving Intensive Care: A National Sample. Pediatr. Crit. Care Med. 2020, 21, e679–e685. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Hassali, M.A.A.; Mak, V.S.L. Two decades of off-label prescribing in children: A literature review. World J. Pediatr. 2018, 14, 528–540. [Google Scholar] [CrossRef]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 179, 839–847. [Google Scholar] [CrossRef]
- Glaeske, G.; Gerdau-Heitmann, C.; Hofel, F.; Schicktanz, C. “Gender-specific drug prescription in Germany” results from prescriptions analyses. In Sex and Gender Differences in Pharmacology; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 149–167. [Google Scholar] [CrossRef]
- Orlando, V.; Mucherino, S.; Guarino, I.; Guerriero, F.; Trama, U.; Menditto, E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. Int. J. Environ. Res. Public Health 2020, 17, 3926. [Google Scholar] [CrossRef]
- Fernandez-Liz, E.; Modamio, P.; Catalan, A.; Lastra, C.F.; Rodriguez, T.; Marino, E.L. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br. J. Clin. Pharmacol. 2008, 65, 407–417. [Google Scholar] [CrossRef]
- Roe, C.M.; McNamara, A.M.; Motheral, B.R. Gender- and age-related prescription drug use patterns. Ann. Pharmacother. 2002, 36, 30–39. [Google Scholar] [CrossRef]
- CBS. Vrouwen Hebben Vaker Chronische Aandoeningen dan Mannen. Available online: https://www.cbs.nl/nl-nl/achtergrond/2011/10/vrouwen-hebben-vaker-chronische-aandoeningen-dan-mannen (accessed on 5 February 2025).
- CBS. Meer Vrouwen dan Mannen met Psychische Klachten. Available online: https://www.cbs.nl/nl-nl/nieuws/2022/50/meer-vrouwen-dan-mannen-met-psychische-klachten (accessed on 5 February 2025).
- Ballering, A.V.; Muijres, D.; Uijen, A.A.; Rosmalen, J.G.M.; Olde Hartman, T.C. Sex differences in the trajectories to diagnosis of patients presenting with common somatic symptoms in primary care: An observational cohort study. J. Psychosom. Res. 2021, 149, 110589. [Google Scholar] [CrossRef]
- SCP. Vrouwen Leven Langer, Maar Zijn ze ook Gezonder? Available online: https://digitaal.scp.nl/emancipatiemonitor2020/vrouwen-leven-langer-maar-zijn-ze-ook-gezonder/ (accessed on 5 February 2025).
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
| Community Pharmacy (All Ages) | Community Pharmacy (Elderly) | Community Pharmacy (Pediatrics) | Elderly Care Home | Sophia Children’s Hospital | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lactulose | A | 1933 | Lactulose | A | 1644 | Amoxicillin | J | 558 | Oxazepam | N | 30 | Macrogol | A | 1055 |
| 2 | Oxazepam | N | 1286 | Oxazepam | N | 818 | Desloratadine | R | 183 | Haloperidol | N | 26 | Caffeine | N | 961 |
| 3 | Pipamperone | N | 1254 | Levothyroxine | H | 811 | Methyl-phenidate | N | 144 | Prednisolone | H | 24 | Furosemide | C | 779 |
| 4 | Levothyroxine | H | 991 | Prednisolone | H | 519 | Nystatin | A | 121 | Lorazepam | N | 19 | Esomeprazole | A | 650 |
| 5 | Haloperidol | N | 716 | Metoprolol tartrate | C | 386 | Amoxicillin/ Clavulanic acid | J | 110 | Risperidone | N | 12 | Lorazepam | N | 575 |
| 6 | Prednisolone | H | 667 | Lorazepam | N | 380 | Omeprazole | A | 109 | Levothyroxine | H | 11 | Ferrous fumarate | B | 480 |
| 7 | Lorazepam | N | 662 | Enalapril | C | 379 | Azithromycin | J | 65 | Macrogol | A | 10 | Paracetamol | N | 452 |
| 8 | Mirtazapine | N | 648 | Metoprolol succinate | C | 365 | Lactulose | A | 64 | Morphine | N | 9 | Cotrimoxazole | J | 439 |
| 9 | Amoxicillin | J | 555 | Bumetanide | C | 341 | Cotrimoxazole | J | 62 | Mirtazapine | N | 9 | Amoxicillin/ Clavulanic acid | J | 369 |
| 10 | Valproic acid | N | 493 | Haloperidol | N | 328 | Nitrofurantoin | J | 62 | Metoprolol tartrate | C | 9 | Hydrochlorothiazide | C | 354 |
| 11 | Metoprolol tartrate | C | 470 | Mirtazapine | N | 305 | Risperidone | N | 59 | Tetrabenazine | N | 8 | Phytonadione | B | 320 |
| 12 | Sertraline | N | 452 | Amlodipine | C | 248 | Ranitidine | A | 54 | Valproic acid | N | 7 | Spironolactone | C | 295 |
| 13 | Enalapril | C | 406 | Clonazepam | N | 225 | Clarithromycin | J | 41 | Trazodone | N | 5 | Prednisolone | H | 276 |
| 14 | Bumetanide | C | 358 | Ivabradine | C | 200 | Cetirizine | R | 33 | Spironolactone | C | 5 | Ibuprofen | M | 230 |
| 15 | Metoprolol succinate | C | 335 | Zolpidem | N | 158 | Ferrous fumarate | B | 32 | Baclofen | M | 5 | Propranolol | C | 202 |
| 16 | Nystatin | A | 316 | Diazepam | N | 155 | Ibuprofen | M | 30 | Carbidopa/ Levodopa | N | 5 | Tramadol | N | 197 |
| 17 | Carbamazepine | N | 306 | Sertraline | N | 131 | Macrogol | A | 28 | Metoprolol succinate | C | 4 | Levetiracetam | N | 196 |
| 18 | Methylphenidate | N | 280 | Nystatin | A | 129 | Aripiprazole | N | 27 | Furosemide | C | 4 | Oxybutynin | G | 187 |
| 19 | Amlodipine | C | 262 | Temazepam | N | 128 | Levetiracetam | N | 24 | Clonidine | C | 184 | |||
| 20 | Risperidone | N | 260 | Risperidone | N | 123 | Ondansetron | A | 23 | Amphotericin B | J | 177 | |||
| 21 | Diazepam | N | 254 | Spironolactone | C | 116 | Miconazole | A | 17 | Dexamethasone | H | 175 | |||
| 22 | Omeprazole | A | 237 | Cholecalciferol | A | 112 | Flucloxacillin | J | 17 | Nystatin | A | 153 | |||
| 23 | Zolpidem | N | 237 | Lisinopril | C | 111 | Levocetirizine | R | 17 | Azithromycin | J | 148 | |||
| 24 | Ivabradine | C | 233 | Sotalol | C | 110 | Pheneticillin | J | 14 | ORS | A | 148 | |||
| 25 | Paroxetine | N | 221 | Omeprazole | A | 109 | Dexamethasone | H | 14 | Midazolam | N | 138 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannekoek, W.; van Kampen, E.E.M.; van Tienen, F.; van der Kuy, P.H.M.; Ruijgrok, E.J. Exploring Manipulated Prescribed Medicines for Novel Leads in 3D Printed Personalized Dosage Forms. Pharmaceutics 2025, 17, 271. https://doi.org/10.3390/pharmaceutics17020271
Pannekoek W, van Kampen EEM, van Tienen F, van der Kuy PHM, Ruijgrok EJ. Exploring Manipulated Prescribed Medicines for Novel Leads in 3D Printed Personalized Dosage Forms. Pharmaceutics. 2025; 17(2):271. https://doi.org/10.3390/pharmaceutics17020271
Chicago/Turabian StylePannekoek, Wouter, Eveline E. M. van Kampen, Frank van Tienen, P. Hugo M. van der Kuy, and Elisabeth J. Ruijgrok. 2025. "Exploring Manipulated Prescribed Medicines for Novel Leads in 3D Printed Personalized Dosage Forms" Pharmaceutics 17, no. 2: 271. https://doi.org/10.3390/pharmaceutics17020271
APA StylePannekoek, W., van Kampen, E. E. M., van Tienen, F., van der Kuy, P. H. M., & Ruijgrok, E. J. (2025). Exploring Manipulated Prescribed Medicines for Novel Leads in 3D Printed Personalized Dosage Forms. Pharmaceutics, 17(2), 271. https://doi.org/10.3390/pharmaceutics17020271






