Abstract
Transmucosal drug products, such as aerosols, films, semisolids, suppositories, and tablets, have been developed for the treatment of various human diseases and conditions. Transmucosal drug absorption is highly influenced by the biological structures of the mucosa and the physiological environment specific to the administration route (e.g., nasal, rectal, and vaginal). Over the last few decades, in vitro permeation testing (IVPT) using animal tissues or in vitro cell cultures have been utilized as a cost-effective and efficient tool for evaluating drug release and permeation behavior, assisting in formulation development and quality control of transmucosal drug delivery systems. This review summarizes the key mucosal permeation barriers associated with representative transmucosal administration routes, as well as considerations for IVPT method development. It highlights various IVPT methods, including vertical diffusion cell, flow-through diffusion cell, Ussing chamber, and transwell systems. Additionally, future perspectives are discussed, such as the use of optical methods to study in vitro drug permeation and the development of in vitro–in vivo correlation (IVIVC) for transmucosal drug development. The potential of IVPT as part of in vitro bioequivalence assessment strategies for locally acting transmucosal drug products is also highlighted.
1. Introduction
Mucosal membranes are commonly targeted for therapeutic delivery, owing to their rich blood supply and lack of a keratinized stratum corneum, which enable enhanced drug permeability and more efficient absorption of therapeutics for both systemic and localized drug actions compared to the skin [1]. Non-oral mucosal delivery routes (e.g., nasal, rectal, vaginal) offer significant advantages, such as bypassing first-pass metabolism and enzymatic degradation associated with oral drug delivery, enabling targeted delivery to the site of action, enhancing bioavailability, and reducing systemic side effects. These mucosal administration routes are particularly valuable for delivering proteins, hormones, and other therapeutics with poor oral bioavailability, as well as for diseases and conditions (e.g., bacterial vaginosis, ulcerative colitis) at the local sites of action [2,3].
The efficiency of transmucosal drug delivery is generally influenced by: (1) the biological structure and mucus composition of the mucosa, which vary across administration routes [4]; (2) the physicochemical properties of the drug, which determine its primary transport pathway (e.g., paracellular vs. transcellular) through the mucosal tissues; and (3) dosage forms or delivery systems utilized, which affect drug release and permeation behavior. Currently, transmucosal drug products approved by the U.S. Food and Drug Administration (FDA) are available in a variety of dosage forms, such as aerosols, films, semisolids, sprays, and tablets. Since these are considered complex drug products, it is critical to understand the impact of critical material and formulation parameters on drug permeation across the mucosa to facilitate the development of both innovator and generic transmucosal drug products.
Over the last few decades, in vitro permeation testing (IVPT) has been widely employed to assist in the selection of new chemical entities, formulation development, and to ensure consistent product performance of transmucosal drug products. IVPT typically involves the utilization of human or animal tissues mounted in diffusion cells, offering a cost-effective and efficient means of evaluating drug permeation behavior across biological membranes. This approach is instrumental in supporting formulation development, bioequivalence assessments, and regulatory review and approvals, particularly for locally acting semisolid drugs. Recent advances in IVPT methodologies also include the use of cell models and 3D-bioprinted tissues.
This review highlights recent progress in IVPT methodologies for evaluating drug permeation across mucosal membranes, particularly for locally acting transmucosal drug products. It provides an overview of the physiological environment and main transmucosal permeation barriers across different transmucosal routes and highlights various in vitro permeation techniques. The review also identifies key challenges and future perspectives for enhancing IVPT approaches to support drug product development.
2. Main Permeation Barriers of Representative Mucosal Membranes
Understanding the physiological environment and permeation barriers of mucosal membranes is essential for developing biorelevant in vitro methods to study drug permeation. Figure 1 provides an overview of the main permeation barriers associated with different mucosal membranes, and Table 1 summarizes their physiological characteristics.
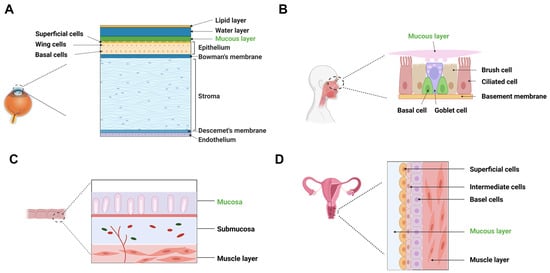
Figure 1.
Main permeation barriers of representative mucosal administration routes. (A) cornea; (B) nasal mucosa (respiratory region); (C) rectal mucosa; (D) vaginal mucosa.

Table 1.
Physiological environment of different mucosa.
2.1. Ocular Mucosa
Topical ocular drug delivery is a well-established approach for treating eye diseases affecting the eye surface and anterior chamber, such as inflammation and glaucoma. Drug absorption following topical ocular administration involves a complex process, including dilution in tear fluid (7–30 μL), diffusion across the mucin layer, penetration through the multilayered cornea, and transfer into the aqueous humor to reach the target sites such as the iris-ciliary body. While some drug absorption may occur through the conjunctiva/sclera, corneal absorption remains the primary route, particularly for diseases affecting the eye surface and anterior chamber [7]. However, short drug retention in the precorneal area due to the nasolacrimal drainage effect and the limited capacity (7–10 μL) of the cul-de-sac [17,18,19] may restrict ocular drug permeation and bioavailability.
The cornea, composed of epithelium, stroma, and endothelium, presents distinct drug permeation barriers: (1) Epithelium is lipophilic and composed of non-keratinized stratified squamous cells. Tight junctions, arranged in a “ribbon-like” structure, restrict paracellular diffusion, allowing better permeability for hydrophobic drugs while limiting hydrophilic drug transport [20,21,22,23]; (2) Stroma composed of a lamellar arrangement of collagen fibrils, accounts for 90% of corneal thickness and is highly hydrated, posing a significant barrier to lipophilic drugs but facilitating hydrophilic drug diffusion; and (3) Endothelium consists of a monolayer of leaky junctions, allowing the passage of macromolecules between the aqueous humor and stroma, which maintaining corneal clarity [24].
The mucin layer on corneal and conjunctival epithelia plays a protective role by restricting the penetration of foreign molecules and maintaining mucosal barrier integrity [24]. For instance, the presence of membrane-bound mucins has been shown to prevent corneal penetration of rose Bengal, an anionic organic dye used clinically to evaluate epithelial damage in conditions such as dry eye syndrome [5]. Although the gel-like mucin layer permits the diffusion of small molecules, its mesh size may restrict the transport of macromolecules and microbes. To prolong precorneal retention and enhance corneal penetration, mucoadhesive formulations are often designed to interact with the mucus layer, improving drug absorption and therapeutic effect [7].
A comprehensive understanding of the barrier properties and drug transport pathways within the cornea is critical for designing biorelevant IVPT methods to evaluate drug permeation. Effective models should incorporate tear fluid dynamics, mucin interactions, and layer-specific permeability to accurately predict in vivo performance and support formulation optimization of ocular drug delivery systems.
2.2. Nasal Mucosa
The nasal mucosa is a promising target for drug delivery due to its high vascularization, large surface area, and non-invasive accessibility. Intranasal drug products are commonly used to treat localized conditions such as nasal allergies, sinusitis, and congestion, providing rapid symptom relief with minimal systemic adverse effects [8]. Additionally, the olfactory region enables drug delivery into the central nervous system (CNS), making it an attractive pathway for neurological therapies [25,26].
The nasal respiratory mucosa consists of epithelium, basement membrane, and lamina propria. The epithelium is primarily composed of pseudostratified columnar epithelial cells, goblet cells, and mucous and serous glands, interconnected by tight junctions that restrict paracellular diffusion of hydrophilic drugs [27,28]. The pseudostratified columnar epithelial cells are further categorized into mucus secreting, ciliated, non-ciliated and basal cells. Ciliated and non-ciliated cells, covered with non-motile microvilli, provide a large surface area for drug absorption, while basal cells serve as progenitors within the collagen-based basement membrane, which overlays the lamina propria [26]. The nasal mucus layer, rich in mucin, may trap large molecular weight (MW) drugs (e.g., peptides and proteins) [29]. Moreover, mucociliary clearance affects drug residence and absorption efficiency [30]. Approximately 20–40 mL of mucus is secreted daily, covering extensive nasal mucosa surface area (160 cm2). This continuous mucus turnover can reduce drug residence time and potentially bioavailability [31]. Interestingly, the olfactory region in humans and primates contains minimal ciliated epithelium, leading to reduced drug clearance and prolonged drug retention [32,33], which is advantages for CNS drug delivery.
In order to develop biorelevant IVPT models for nasal drug delivery, it is essential to accurately mimic the nasal environment. Key factors to consider include mucin-drug interactions, mucus flow dynamics, mucociliary clearance mechanisms, and epithelial barriers along with drug transport pathways.
2.3. Rectal Mucosa
The rectum, the terminal portion of the large intestine, offers an alternative administration route for drug delivery with both local and systemic applications. Structurally, a normal adult human rectum is about 12–15 cm long with a surface area of 200–400 cm2 [34,35]. Unlike the small intestine, it lacks villi and microvilli, resulting in a limited absorptive surface area. The rectal epithelium consists of columnar cells and goblet cells that are responsible for secreting mucus, forming a 100 μm-thick layer that acts as a protective barrier and may hinder drug permeation [12,36].
Drug absorption across the rectal epithelium occurs through transcellular and paracellular routes [13,37,38]. However, several physiological factors affect drug permeation: (1) Low fluid volume (1–3 mL) limits drug dissolution, impacting drug absorption [36,39,40]; (2) Neutral pH with low buffering capacity, which may be altered by administrated drugs, potentially causing irritant; and (3) Presence of stool increases the viscosity of the rectal fluid, which may limit drug dissolution and mobility in the viscous rectal fluid [37,39,41]. Other factors such as rectal wall motility and formulation retention ability in the rectum also influence drug permeation and absorption [37,42]. For effective drug permeation and absorption through the rectal route, a drug or conjugate must strike a balance between lipophilicity (to cross the epithelial layer) and hydrophilicity (to ensure dissolution in the rectal fluid) [28]. Most rectal drug candidates are small molecules with MW < 500 g/mol and logP values ranging from 1.20 to 4.88.
To develop biorelevant IVPT models to assist in rectal drug development and quality control, it is essential to replicate the mucosal environment. Incorporating mucosal viscosity, and pH variability in IVPT systems may potentially improve predictive accuracy for in vivo performance, supporting formulation optimization and bioequivalence assessments.
2.4. Vaginal Mucosa
The vagina is a fibromuscular tube extending from the cervix to the vulva, measuring approximately 7–10 cm in length and 4 cm in width [43,44,45]. It is composed of four distinct layers: (1) Non-keratinizing stratified squamous epithelium; (2) Lamina propria (or tunica), which is rich in collagen, elastin, vascular and lymphatic channels; (3) Muscle layer with smooth muscle fibers arranged in both circular and longitudinal directions; and (4) Areolar connective tissue interwoven with a vascular plexus [34].
The vaginal epithelium consists of basal and parabasal layers, held together by tight junctions, desmosomes, and intermediate junctions [46]. Drug absorption involves two key steps: dissolution in the vaginal fluid followed by permeation through the epithelium. Previous studies indicated that most drugs permeated through the vagina via passive diffusion [47]. Vaginal fluid (~0.51 mL, pH 3.5 to 4.5), is composed of cervical mucus, glandular secretions, transudates, and endometrial fluids [16,48,49]. This fluid forms a thin mucus layer that influences drug absorption, bioadhesion and permeation, which could reduce the overall drug efficacy and residence time at the target tissue [48,50]. The mucus barrier provides greater resistance to large or charged molecules, while small, uncharged molecules diffuse more readily [51].
In order to assess the released drug mobility in the vaginal fluid and permeation mechanisms across the vaginal membrane, a biorelevant IVPT model should closely mimic the vaginal environment. Key considerations include the use of simulated vaginal fluid (to replicate the pH and mucosal composition) and biological membrane such as animal vaginal tissues, excised human vaginal tissues, or artificial vaginal membranes, to simulate permeation barriers.
3. Drug Permeation Mechanisms Across Mucosa
Mucoadhesion, drug dissolution, and epithelial penetration are critical steps for effective drug permeation and absorption through mucosal tissues. Drug dissolution and diffusion through the mucosal layer largely depend on the physiological environment (e.g., pH, volume, viscosity) of the mucosal tissues, the physicochemical properties (e.g., partition coefficient, MW) of the drug, and the formulation matrix and release kinetics. Mucus, composed of glycoprotein (mucin, MW: ~1–40 × 106 kDa), lipids, salts, proteins, and carbohydrates, forms a hydrogel-like barrier that protects epithelial cells [4,52]. Mucin, primarily secreted by goblet cells, creates a mesh-like network that traps large molecules and restricts drug transport [53,54]. Effective permeation requires the ability of a drug to diffuse through mucus, equilibrate laterally, and partition into underlying tissues, typically driven by a concentration gradient [55]. Strategies to improve mucus penetration include the use of mucus-penetrating particles [56] and proteolytic enzymes to degrade mucin [57].
Drug penetration through epithelial tissues occurs via two main pathways: (1) Paracellular pathway which relies on passive diffusion through tight junctions and is primarily used by hydrophilic compounds (e.g., peptides). Molecules with radii greater than 15 Å have significant permeation limitations due to tight-junction selectivity. (2) Transcellular pathway is preferred by lipophilic drugs to diffuse across epithelial cell membranes [4]. Transcellular transport mechanisms include passive diffusion, carrier-mediated transport (active or facilitated diffusion) and endocytosis. Drug molecules move through passive diffusion along a concentration or electrochemical gradient without requiring energy. Unlike carrier-mediated transport, this process is not saturable and exhibits low structural selectivity [58]. Overall, drug lipophilicity, MW, ionization state, and dissolution rate strongly influence drug transport pathways and subsequent absorption. For instance, lipophilic molecules with MW less than 1 kDa are absorbed rapidly and efficiently via the transcellular pathway across the nasal mucosa [8]. Similarly, lipid solubility (e.g., carbonic anhydrase inhibitor) correlates directly with ocular permeation rates [59]. It has been reported that the acidic environment (pH 3.5–4.5) facilitates drug dissolution, however, the vaginal mucus may restrict permeation of ionized compounds [60].
4. IVPT Considerations and Systems
IVPT is a critical tool for assessing drug permeation using excised tissues or cell membranes applied in diffusion cells or Transwell systems. Since its first introduction in the mid-1970s, IVPT systems have undergone significant advancements [61]. Commonly used IVPT systems include vertical diffusion cell (VDC) apparatus, flow-through cell (FTC) apparatus, and Ussing chamber systems. Either an infinite or finite dosing strategy is implemented depending upon the study objective, with permeation rates estimated once a steady state is reached. In recent years, Transwell systems using cell layers and 3D tissue models have been developed to evaluate in vitro permeation behavior in vitro under biorelevant conditions. The receptor media used for IVPT of representative mucosae were summarized in Table 2.

Table 2.
The receptor media used in transmucosal IVPT studies.
4.1. Tissue-Based Models
For tissue models, it is critical to validate tissue integrity and thickness before and after IVPT studies to ensure reliable data. The IVPT duration has been reported to be up to 24 h for cornea, vaginal, rectal mucosa and 8 h for nasal mucosa [78,88,89,90]. In addition, tissue surface temperature should be carefully monitored and controlled throughout testing. Maintaining sink conditions is important to prevent saturation effects, particularly for poorly water-soluble drugs. Tissue variability must also be addressed by using tissues from multiple donors and conducting tests with sufficient replicates to ensure statistical robustness. Table 3 summarizes the tissue models commonly used for transmucosal permeation studies.

Table 3.
Tissue models commonly used in transmucosal IVPT studies.
4.1.1. VDC Apparatus
The VDC apparatus, originally developed by Franz [61], is widely used to evaluate in vitro performance, including in vitro release test (IVRT) and IVPT for topical drug products. It consists of two compartments: a donor cell and a receptor cell, with the tissue membrane mounted between them as the permeation barrier (Figure 2A). A water jacket is used to maintain a constant test temperature (e.g., 37 °C). A simulated body fluid or buffered solution is added to the receptor cell [47,83], while a test formulation is applied to the donor cell. Homogeneous mixing and proper hydrodynamics in the receptor cell are obtained using a magnetic stirring bar. The receptor cell features a sampling port for withdrawing aliquots at predefined time intervals, enabling time-course analysis and medium replacement to maintain sink conditions. Key parameters to be considered for IVPT using a VDC apparatus include: dosing amount, effective permeation area, receptor volume, and hydrodynamic conditions in the receptor cell [109]. These parameters must be carefully selected and controlled to minimize IVPT data variability and ensure reproducible and reliable results. While the VDC system is simple to use, a potential limitation is the decreasing of diffusion gradient caused by the accumulation of the permeating drug in the receptor compartment [88]. Strategies to maintain sink conditions such as replacing and replenishing receptor media at each sampling time point, as well as optimizing sampling intervals can help mitigate this issue.

Figure 2.
Schematic diagram of tissue-based (A) vertical diffusion cell (VDC), (B) flow-through vertical cell apparatus (FTC), (C) vertical Ussing chamber apparatus and (D) transwell system.
4.1.2. FTC Apparatus
The FTC system features a vertical arrangement similar to the VDC apparatus (Figure 2B), but includes input and output ports in the receptor cell to enable continuous fluid flow. This design better simulates in vivo conditions compared to the static VDC apparatus, as it effectively maintains sink conditions and mimics blood flow beneath biological membranes [110]. Flow rate is a critical parameter that influences drug permeation rates in the FTC apparatus. Depending upon drug solubility, low flow rates may lead to drug saturation in the receptor fluid, thus violating sink conditions and reducing permeation rates for highly permeable drugs. Conversely, high flow rates may cause excessive dilution, potentially underestimating permeation rates for low-permeability drugs [111]. The FTC system offers numerous advantages, including auto-sampling, easy maintenance of sink conditions, and simulation of physiological conditions such as blood flow beneath biological membranes [110]. However, the pumped volume must significantly exceed the receptor’s volume to thoroughly wash the permeating substance. Therefore, the receptor chamber volume should be kept small to manage the effluent volume effectively [88].
4.1.3. Ussing Chamber System
The Ussing chamber system, introduced by Hans Ussing in the early 1950s [112], allows precise control of temperature and oxygenation while simultaneously measuring electric parameters to monitor tissue viability during IVPT studies [113]. The Ussing chamber system is classified into two main types: circulating chambers and continuously perfused chambers. The Ussing chamber system typically consists of two main functional components: a chamber unit that houses the biological tissue and maintains hydrostatic pressure to prevent tissue damage caused by bending [114]; and an electrical circuit that measures current (I) and voltage (V) to calculate resistance (R) and more complex parameters such as impedance and capacitance [115]. The circulating chamber features a U-shaped tubing system filled with carbogen gas (95% of O2 and 5% of CO2) and N2 to maintain constant stirring and ensure mucosal viability [88]. Ussing chambers are available in vertical (Figure 2C) and horizontal configurations, tailored for specific experiments. Key parameters to be considered for the Ussing chamber system include temperature, gassing, electrical interferences (noise), and offset voltages [115]. While the Ussing chamber system can support both native human or animal tissue and cell models [113], the viscosity of semisolids or dissolved solutes may impair gaseous and nutrient exchange, affecting cell viability and data accuracy [88].
4.2. Cell-Based Models
Cell-based models utilizing immortalized human cells or primary cell cultures assess drug permeation behavior and biocompatibility [116]. These cell-based models can reduce the reliance on animal tissues and address translational challenges while enabling reproducible and ethically accepted methods in early drug development. Transepithelial electrical resistance (TEER) measurements are used to monitor the integrity of tight junctions and mucosal barriers [81]. However, simplified cell models may not fully replicate the complex tissue environment, which limits their capacity for formulation evaluation [117].
4.2.1. Transwell System
The transwell system (Figure 2D), equipped with polycarbonate or polyester membrane inserts cultured with cells, has been used for drug permeation studies [38,81]. Cell monolayers, acting as permeation barriers, typically take several days to form after cell seeding, and TEER measurements ensure monolayer integrity. Inserts with intact cell monolayer layers are transferred to the receiving wells containing a receptor medium (e.g., simulated vaginal fluid) and a test formulation is added to the insert/donor chamber. Samples are collected from receiving wells at predetermined time points and replaced with fresh media. It is critical to monitor TEER during IVPT to ensure cell monolayer integrity throughout the study [81]. Table 4 provides an overview of cell models reported for transmucosal IVPT using transwells. While transwell models reduce animal use and support mechanistic studies, experimental variability can arise due to culture conditions and poorly characterized lipid compositions [118].

Table 4.
Representative cell models reported for transmucosal IVPT using a transwell.
4.2.2. Corneal Cell Models
Primary human corneal epithelium (HCE) cells are not ideal for large-scale permeation studies, since primary cells cease to grow after a few passages in culture. As a result, immortalized human corneal epithelium cell lines such as HCE-S and SV 40 HCE-T are typically preferred for such studies, as shown in Table 4 [121,134]. HCE-S cells [119,135] can be readily cultured in basic DMEM and serum-based media, offering a distinct advantage over existing corneal epithelial cell lines. Although SV 40 HCE-T has been widely applied for in vitro models of HCE cells, the genomic content of HCE-T cells appeared to be different from a normal healthy genome, and they also showed a significant number of heterogeneous cell populations based on an array-based comparative genomic hybridization analysis [135]. On the other hand, tetracycline-responsive HPV 16-E6/E7-transduced HCE cell clones [121] exhibited tightly controlled inducible proliferation, relatively normal differentiation of the corneal epithelium, and characteristic HCE morphology. More recently, a human organotypic cornea with typical corneal structures was developed, which contains a single layer of endothelial cells and a multilayered epithelium with flat superficial cells. However, the barrier functions of the human organotypic cornea still need to be thoroughly evaluated.
4.2.3. Nasal Cell Models
Immortalized nasal cell lines have been shown to have high proliferation rates, greater reproducibility, and lower costs, and are easier to maintain in culture compared to primary cell cultures [123,136]. RPMI 2650 is the only cell line derived from human nasal tissue; however, it has several limitations: (1) it lacks tight-junction expression; (2) it does not differentiate into goblet or ciliated cells; and (3) it fails to reach confluence [137,138]. Although RPMI 2650 cells have been used in some permeation studies, results were highly dependent on culture conditions. It was reported that the air–liquid interface culture of RPMI2650 cells could be suitable for in vitro permeation studies across nasal mucosa [71].
4.2.4. Rectal Cell Model
The Caco-2 monolayer model has been the main model for studying drug permeation through the rectal membrane. For example, rectal delivery of polymeric nanoparticles containing an anti-HIV drug, dapivirine, was evaluated using Caco-2 monolayers. The retention of the drug within the Caco-2 cell monolayers and pig mucosa showed a similar trend for different nanoparticle formulations, indicating in vivo relevance [81].
4.2.5. Vaginal Cell Models
Ectocervical cells appear to be the most interesting in vitro model for studying vaginal drug permeability histologically. Human ectocervical epithelial (hECE) cells have been shown to maintain most of the phenotypic and biological features of the human cervicovaginal epithelium in vivo, including intercellular tight junctions and polarization of apical and basolateral surfaces. However, a significant lack of human ectocervical explants limits this model’s application. In contrast, several continuous human cervicovaginal cell lines, which are easier to maintain than primary cells, are more readily accessible. Cultured cervical cell lines such as CaSki, ECE16-1, and HT3 have been shown to have leaky epithelia based on both TEER and pyranine permeability studies, resembling the epithelia derived from human explants [126,139]. The commercially available EpiVaginal® model composed of untransformed human vaginal ectocervical (VEC) epithelial cells and nonepithelial elements such as dendritic cells and fibroblasts, presenting unique organotypic characteristics, has been utilized for IVPT of various drugs [123]. However, this model lacks a mucosal component and is expensive, which may limit its applicability. HVE® is composed of A431 cells derived from a vulval epidermoid carcinoma cultivated on an inert polycarbonate filter at the air–liquid interface in a chemically defined medium. This model has been shown to be histologically similar to the in vivo vaginal mucosa and is used to screen and assess active ingredients or formulations for vaginal administration [88].
It is critical to note that research involving human and animal tissues requires review and approval or exemption from an institutional review board (IRB). In the United States, IRB exemption is generally granted for research using unidentifiable human tissue samples from cadavers. However, this may differ across countries. Nevertheless, researchers must carefully review tissue sources and material transfer details with their IRB and complete the appropriate human research ethics training according to institutional and national requirements. Additionally, research involving cells must be reviewed and approval by an institutional biosafety committee (IBC) to ensure compliance with relevant biosafety guidelines and to protect public health and the environment. Adherence to both biosafety and human research ethics guidelines is essential for conducting responsible and ethical research.
4.3. Data Analysis
Transmucosal diffusion is generally a passive process driven by a concentration gradient. Initially, drug molecules must be released from the formulation vehicle to reach the mucosal interface, after which they partition into the upper layers of the mucosal tissue. A key parameter influencing the permeation rate is the drug’s lipid–water partition coefficient.
For tissue-based models, the concentration gradient across the mucosa may not be linear at the start of IVPT. Over time, however, a steady state is achieved, at which point Fick’s first law of diffusion can be applied to describe drug transport across the mucosal barrier. Fick’s first law relates to the amount of solute (dosed drug, Q), membrane area (A), time period (T) regarding the concentration gradient (ΔCs), the diffusion coefficient of the mucosal membrane (D), and the transport path length (h) (Equation (1)). A steady-state flux, Jss, can then be defined (Equation (2)). Higuchi suggested that flux is more appropriately expressed in terms of thermodynamic activity, rather than relying on a concentration approximation [140]. The constant maximum flux occurs when the solute (drug) solubility reaches the maximum value (Ss) in the tissue (Equation (3)). The thermodynamic activity of the solute (dosed drug) is generally defined as the fractional solubility of the solute (drug) in the tissue (Cs/Ss), which can also be expressed in terms of the solute (drug) concentration in the vehicle (formulation, Cv) and the partition coefficient K (K = Cs/Ss, Equation (4)) [141].
The in vitro permeation rate is typically calculated by plotting the cumulative amount of drug permeated per unit of surface area against the square root of time. The slope of the regression line (derived from the linear portion of the curve) represents the permeation rate [38].
where, Cn is the drug concentration at the respective time point, Cn−1 is the drug concentration at the previous time point, V is the medium volume in the receptor cell, Vr is the sampling volume, and A is the permeation area. ΔQt is the difference in the drug amount permeated between two time points (Δt), and C0 is the initial drug concentration in the donor chamber.
Beyond the steady state, a transient model can be used to describe time-dependent drug permeation. Fick’s second law is used as a basic model for this, assuming that the tissue membrane is a pseudo-homogeneous membrane and that both drug partition coefficient and diffusion coefficient remain constant over time (Equation (5)). Various modeling approaches, including compartmental and complex models, have been developed to account for these dynamics [141,142].
where, C is the concentration of the permeating solute (drug) at time t and depth x within the tissue membrane.
For cell-based models, the apparent permeability coefficient (Papp) is calculated using the following equation:
where Q is the total amount of drug permeated into the receptor compartment (μg), A is the diffusion area (cm2), C is the initial drug concentration in the donor compartment (μg/cm3), and t is the total duration of IVPT [38].
5. Future Perspectives
IVPT has long been instrumental in studying transmucosal permeation mechanisms and supporting drug product development. With significant advances in biomedical sciences and biotechnology, innovative techniques have emerged to enhance our understanding of transmucosal drug permeation. Among these, non-destructive optical methods, particularly confocal laser scanning microscopy (CLSM) and confocal Raman spectroscopy and near-infrared (NIR) spectroscopy, have shown great promise in studying the permeation of topical drug products. These optical methods can provide more detailed information on functional groups of drug compounds. For instance, CLSM in combination with other techniques such as transmission electron microscopy and scanning electron microscopy has been utilized to explore the corneal penetration pathways of labeled peptides and to examine changes in cytoskeletal structure and tight-junction permeability when exposed to penetration-enhancing agents [143,144,145]. CLSM has also been employed to identify the transcellular routes through which various nanocapsules penetrate the cornea, both ex vivo and in vivo [146]. In terms of nasal drug permeation, CLSM has provided valuable insights into the permeation and uptake mechanisms of therapeutic proteins, such as human calcitonin and insulin, through ex vivo nasal epithelia [147,148]. CLSM has also been utilized in elucidating the impact of size and polyethylene glycol (PEG) coating density on the transport of poly(lactic acid)–poly(ethylene glycol) (PLA–PEG) nanoparticles and microparticles across the nasal mucosa in rats [149]. Oranat et.al used confocal Raman spectroscopy to measure the local concentrations of the topical vaginal anti-HIV microbicide (tenofovir) in fluids, drug delivery gels, and tissues. Their findings demonstrated a strong correlation between tenofovir tissue permeation obtained using confocal Raman spectroscopy and that obtained using LC-MS/MS [150]. Furthermore, the combination of confocal Raman spectroscopy and optical coherence tomography (OCT) has enabled temporally and spatially specific measurements of tenofovir concentration across the full depth of excised porcine rectal mucosa [151]. Despite its potential as a minimally invasive and label-free analytical tool for determining drug concentrations during the permeation process, confocal Raman spectroscopy has certain limitations: (1) high variability in tissue composition and structure, which can complicate Raman spectral analysis [152]; and (2) ongoing diffusion and changes in tissue hydration during IVPT, which can alter Raman spectra. As a result, confocal Raman spectroscopy is still considered a semi-quantitative technique.
As drug delivery systems become more complex, IVPT is expected to evolve to include more sophisticated models, such as organ-on-a-chip systems, to replicate human physiological conditions more effectively. Recent efforts have focused on building models, such as a nasal epithelial mucosa, to mimic nasal tissue physiology in vitro [153,154] for drug transport studies of aerosols [155]. Advanced tools such as the dynamic micro tissue engineering system (DynaMiTES) [156] were used to build a 3D hemi-cornea construct composed of human keratocytes (HCKeCa) and epithelial cells (HCE-T) [157]. Although this ocular tissue-on-a-chip model was developed to study the disease progression of macular degeneration, it could potentially be useful for studying drug permeability [158,159].
In vitro–in vivo correlation (IVIVC) is defined by the U.S. FDA as a predictive mathematical model describing the relationship between the in vitro properties of a dosage form and its relevant in vivo response. While IVIVC has been extensively developed for extended-release oral drug products, regulatory agencies currently do not permit the use of IVIVC as a surrogate for bioequivalence (BE) studies of locally acting transmucosal drug products. Recent advancements have demonstrated the feasibility of developing an IVIVC for dermatological products such as transdermal patches [160]. For most percutaneous drug products, the rate-limiting step in absorption is drug transport across the stratum corneum (SC), rather than its release from a transdermal formulation. Similarly, the epithelium of mucosae may serve as the primary barrier to drug absorption in the mucosal membrane. This suggests that IVPT studies using exercised tissues with good integrity could potentially correlate well with in vivo absorption of transmucosal drugs. For systemically acting drug products, BE, required for generic drug approval, is evaluated based on plasma pharmacokinetic approaches, which can provide accurate and direct evidence through area under the plasma concentration curve (AUC) and maximum concentration (Cmax). However, BE assessment for locally acting topical drug products often relies on costly comparative in vivo clinical endpoint studies, which can be challenging [161]. Recognizing these challenges, the U.S. FDA has made significant efforts in recent years to provide guidance documents supporting in vitro characterization-based BE approaches to demonstrate BE for topical dermatological drug products [162,163,164,165,166]. IVPT is a key component of the in vitro characterization-based BE approaches recommended in several product-specific guidance (PSG) for topical drug products to compare prospective generic products and reference drug products [167,168,169,170,171]. The endpoints of IVPT are based on parameters that characterize the rate (flux, J) and extent (total cumulative amount, AMT) of drug permeation. The maximum flux (Jmax) at the peak of the drug flux profile and the AMT should both be compared for locally acting test topical products and reference standards (RSs). This is somewhat analogous to the comparison of the Cmax and AUC for systemically acting test products and RSs [172]. Similar in vitro characterization-based approaches are currently under investigation for locally acting semisolid dosage forms administrated via mucosal routes, such as vaginal and rectal. Early evidence supports the feasibility of in vitro characterization-based BE approaches for locally acting transmucosal drug products [173,174,175].
In silico prediction of mucosal drug permeation may be an alternative to experimental permeation studies in the future. Various in silico models, such as the Potts–Guy model, have been developed to correlate transdermal permeability coefficients and drug molecule characteristics (such as lipophilicity and MW) [121]. Although there is currently no simple model to predict mucosal drug permeation, it is expected that this will be addressed in the near future with advances in physiologically based pharmacokinetic (PBPK) modeling.
6. Summary and Outlook
IVPT has emerged as a pivotal tool in evaluating the performance of transmucosal drug products, offering insights into their absorption mechanisms and predicting in vivo drug behavior. The integration of IVPT with IVIVC for locally acting drug products, including those administered through the mucosal routes, holds significant potential for advancing regulatory science and streamlining drug approval processes. However, challenges remain, including variability in mucosal tissue properties and the need for standardized IVPT protocols. Continued refinement of more biorelevant and robust IVPT methodologies, coupled with advanced analytical tools and deeper insights into drug permeation mechanisms, is expected to drive the development of safer and more effective transmucosal drug products. As these methods become more standardized and validated, they are likely to play a crucial role in the FDA’s regulatory strategies, facilitating the development and approval of both innovator and generic transmucosal drug products, which will ultimately improve public access to medications.
Author Contributions
Conceptualization, J.S. (Juan Song), L.X. and J.S. (Jie Shen); writing—original draft preparation, J.S. (Juan Song), L.X., and Z.X.; writing—review and editing, J.S. (Jie Shen), Z.X., J.S. (Juan Song) and L.X.; supervision and funding acquisition, J.S. (Jie Shen). All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a grant (1U01FD007656). The contents are those of the author(s) and do not necessarily represent the official views of or endorsement by FDA/HHS or the U.S. government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank biorender.com for providing a platform for creating figures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| BE | bioequivalence |
| FDA | Food and Drug Administration |
| FTC | flow-through cell |
| IVIVC | in vitro–in vivo correlation |
| IVRT | in vitro release testing |
| IVPT | in vitro permeation testing |
| PSG | product-specific guidance |
| TEER | transepithelial electrical resistance |
| VDC | vertical diffusion cell |
References
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Brunaugh, A.D.; Smyth, H.D.C.; Williams Iii, R.O. Rectal and Vaginal Drug Delivery. In Essential Pharmaceutics; Brunaugh, A.D., Smyth, H.D.C., Williams Iii, R.O., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 149–161. [Google Scholar]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Chastain, J. General Considerations in Ocular Drug Delivery. In Ophthalmic Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2003; pp. 59–107. [Google Scholar]
- Xu, X.; Al-Ghabeish, M.; Rahman, Z.; Krishnaiah, Y.S.; Yerlikaya, F.; Yang, Y.; Manda, P.; Hunt, R.L.; Khan, M.A. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int. J. Pharm. 2015, 493, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K. Ophthalmic Drug Delivery Systems, Second Edition, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef]
- Eichner, H.; Behbehani, A.A.; Hochstrasser, K. Diagnostic value of nasal secretions, current state: Normal values. 1. [Nasensekretdiagnostik, aktueller Stand: Normalwerte. Teil 1]. Laryngol Laryngol. Rhinol. Otol. 1983, 62, 561–565. [Google Scholar] [CrossRef]
- Hua, S. Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Front. Pharmacol. 2019, 10, 1196. [Google Scholar] [CrossRef]
- Goldwyn, R.M. Gray’s anatomy. Plast. Reconstr. Surg. 1985, 76, 147–148. [Google Scholar] [CrossRef]
- Batchelor, H. Rectal Drug Delivery. In Pediatric Formulations: A Roadmap; Bar-Shalom, D., Rose, K., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014; Volume 11, pp. 303–310. [Google Scholar] [CrossRef]
- Anderson, D.J.; Marathe, J.; Pudney, J. The structure of the human vaginal stratum corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Paul, K.; Agnew, K.; Gaussman, R.; Coombs, R.W.; Hitti, J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J. Clin. Microbiol. 2011, 49, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. The dynamics and drainage of tears. Int. Ophthalmol. Clin. 1973, 13, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Gasset, A.; Klyce, S.D., Jr.; Baum, J.L. Determination of Tear Volume and Tear Flow. Investig. Ophthalmol. Vis. Sci. 1966, 5, 264–276. [Google Scholar]
- Ehlers, N. The precorneal film. Biomicroscopical, histological and chemical investigations. Acta Ophthalmol. Suppl. 1965, 81, 81–134. [Google Scholar]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef]
- Huang, H.S.; Schoenwald, R.D.; Lach, J.L. Corneal penetration behavior of beta-blocking agents III: In vitro-in vivo correlations. J. Pharm. Sci. 1983, 72, 1279–1281. [Google Scholar] [CrossRef]
- Klyce, S.D.; Crosson, C.E. Transport processes across the rabbit corneal epithelium: A review. Curr. Eye Res. 1985, 4, 323–331. [Google Scholar] [CrossRef]
- McLaughlin, B.J.; Caldwell, R.B.; Sasaki, Y.; Wood, T.O. Freeze-fracture quantitative comparison of rabbit corneal epithelial and endothelial membranes. Curr. Eye Res. 1985, 4, 951–961. [Google Scholar] [CrossRef]
- Sunkara, G.; Kompella, U. Membrane Transport Processes in the Eye. In Ophthalmic Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2003; pp. 13–58. [Google Scholar]
- Veronesi, M.C.; Alhamami, M.; Miedema, S.B.; Yun, Y.; Ruiz-Cardozo, M.; Vannier, M.W. Imaging of intranasal drug delivery to the brain. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 1–31. [Google Scholar]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.L.; Darekar, A.; Saudagar, R. A recent review on nasal microemulsion for treatment of cns disorder. Int. J. Curr. Pharm. Res. 2017, 9, 5. [Google Scholar] [CrossRef]
- Bedse, A.; Sadique, S.M.S.; Kunde, V.; Jaiswal, S. Drug Delivery on Rectal Absorption: Suppositories. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 70–76. [Google Scholar]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef]
- Merkus, F.W.; Verhoef, J.C.; Schipper, N.G.; Marttin, E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Mygind, N. Structure and ultrastructure of the nose. Nasal Allergy 1979, 2, 3–38. [Google Scholar]
- Kumar, T.A.; David, G.; Puri, V. Ovulation in rhesus monkeys suppressed by intranasal administration of progesterone and norethisterone. Nature 1977, 270, 532–534. [Google Scholar] [CrossRef]
- Herbst, A.L. Comprehensive Gynecology; Mosby Elsevier Health Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st Century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef]
- van Hoogdalem, E.; de Boer, A.G.; Breimer, D.D. Pharmacokinetics of rectal drug administration, Part I. General considerations and clinical applications of centrally acting drugs. Clin. Pharmacokinet. 1991, 21, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sibinovska, N.; Božič, D.; Bošković Ribarski, M.; Kristan, K. Prediction of pharmacokinetic studies outcome for locally acting nasal sprays by using different in vitro methods. Int. J. Pharm. 2021, 601, 120569. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Sarmento, B.; das Neves, J. Formulation and delivery of anti-HIV rectal microbicides: Advances and challenges. J. Control Release 2014, 194, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef]
- de Boer, A.G.; Moolenaar, F.; de Leede, L.G.; Breimer, D.D. Rectal drug administration: Clinical pharmacokinetic considerations. Clin. Pharmacokinet. 1982, 7, 285–311. [Google Scholar] [CrossRef]
- van Hoogdalem, E.J.; de Boer, A.G.; Breimer, D.D. Pharmacokinetics of rectal drug administration, Part II. Clinical applications of peripherally acting drugs, and conclusions. Clin. Pharmacokinet. 1991, 21, 110–128. [Google Scholar] [CrossRef]
- Woolfson, A.D.; Malcolm, R.K.; Gallagher, R. Drug delivery by the intravaginal route. Crit. Rev. Ther. Drug Carrier Syst. 2000, 17, 509–555. [Google Scholar] [CrossRef]
- Funt, M.I.; Thompson, J.D.; Birch, H. Normal vaginal axis. South. Med. J. 1978, 71, 1552, 1534–1535. [Google Scholar] [CrossRef]
- Van De Graaff, K.M. Human Anatomy; McGraw-Hill Higher Education: New York, NY, USA, 2001. [Google Scholar]
- Schuchner, E.B.; Foix, A.; Borenstein, C.A.; Marchese, C. Electron microscopy of human vaginal epithelium under normal and experimental conditions. J. Reprod. Fertil. 1974, 36, 231–233. [Google Scholar] [CrossRef]
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016. [Google Scholar] [CrossRef]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J. Physiology and ecology of the vagina. Scand. J. Infect. Dis. Suppl. 1983, 40, 31–35. [Google Scholar] [PubMed]
- Brannon-Peppas, L. Novel vaginal drug release applications. Adv. Drug Deliv. Rev. 1993, 11, 169–177. [Google Scholar] [CrossRef]
- Khanvilkar, K.; Donovan, M.D.; Flanagan, D.R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001, 48, 173–193. [Google Scholar] [CrossRef]
- Schoonen, A.; Moolenaar, F.; Huizinga, T. Release of drugs from fatty suppository bases I. The release mechanism. Int. J. Pharm. 1979, 4, 141–152. [Google Scholar] [CrossRef]
- Lawson, L.B.; Norton, E.B.; Clements, J.D. Defending the mucosa: Adjuvant and carrier formulations for mucosal immunity. Curr. Opin. Immunol. 2011, 23, 414–420. [Google Scholar] [CrossRef]
- Shakya, P.; Madhav, N.S.; Shakya, A.K.; Singh, K. Palatal mucosa as a route for systemic drug delivery: A review. J. Control. Release 2011, 151, 2–9. [Google Scholar] [CrossRef]
- Mashru, R.; Sutariya, V.; Sankalia, M.; Sankalia, J. Transbuccal delivery of lamotrigine across porcine buccal mucosa: In vitro determination of routes of buccal transport. J. Pharm. Pharm. Sci. 2005, 8, 54–62. [Google Scholar]
- Ensign, L.M.; Tang, B.C.; Wang, Y.-Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4, 138ra79. [Google Scholar] [CrossRef]
- Müller, C.; Leithner, K.; Hauptstein, S.; Hintzen, F.; Salvenmoser, W.; Bernkop-Schnürch, A. Preparation and characterization of mucus-penetrating papain/poly (acrylic acid) nanoparticles for oral drug delivery applications. J. Nanopart. Res. 2013, 15, 1353. [Google Scholar] [CrossRef]
- Shargel, L. Applied Biopharmaceutics & Pharmacokinetics, 4th ed.; Appleton & Lange: Stamford, CT, USA, 1999. [Google Scholar]
- Maren, T.H.; Jankowska, L.; Sanyal, G.; Edelhauser, H.F. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp. Eye Res. 1983, 36, 457–479. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Saettone, M.F.; Chetoni, P.; Cerbai, R.; Mazzanti, G.; Braghiroli, L. Evaluation of ocular permeation enhancers: In vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int. J. Pharm. 1996, 142, 103–113. [Google Scholar] [CrossRef]
- Liu, J.; Fu, S.; Wei, N.; Hou, Y.; Zhang, X.; Cui, H. The effects of combined menthol and borneol on fluconazole permeation through the cornea ex vivo. Eur. J. Pharmacol. 2012, 688, 1–5. [Google Scholar] [CrossRef]
- Ranta, V.P.; Laavola, M.; Toropainen, E.; Vellonen, K.S.; Talvitie, A.; Urtti, A. Ocular pharmacokinetic modeling using corneal absorption and desorption rates from in vitro permeation experiments with cultured corneal epithelial cells. Pharm. Res. 2003, 20, 1409–1416. [Google Scholar] [CrossRef]
- Reichl, S.; Döhring, S.; Bednarz, J.; Müller-Goymann, C.C. Human cornea construct HCC-an alternative for in vitro permeation studies? A comparison with human donor corneas. Eur. J. Pharm. Biopharm. 2005, 60, 305–308. [Google Scholar] [CrossRef]
- Al-Ghabeish, M.; Xu, X.; Krishnaiah, Y.S.; Rahman, Z.; Yang, Y.; Khan, M.A. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int. J. Pharm. 2015, 495, 783–791. [Google Scholar] [CrossRef]
- Tegtmeyer, S.; Papantoniou, I.; Müller-Goymann, C.C. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur. J. Pharm. Biopharm. 2001, 51, 119–125. [Google Scholar] [CrossRef]
- Richter, T.; Keipert, S. In vitro permeation studies comparing bovine nasal mucosa, porcine cornea and artificial membrane: Androstenedione in microemulsions and their components. Eur. J. Pharm. Biopharm. 2004, 58, 137–143. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Vellonen, K.S.; Palmgrén, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hämäläinen, K.M.; Auriola, S.; Urtti, A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Quantitative estimation of drug permeation through nasal mucosa using in vitro membrane permeability across Calu-3 cell layers for predicting in vivo bioavailability after intranasal administration to rats. Eur. J. Pharm. Biopharm. 2020, 149, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef]
- Wadell, C.; Björk, E.; Camber, O. Permeability of porcine nasal mucosa correlated with human nasal absorption. Eur. J. Pharm. Sci. 2003, 18, 47–53. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Simmen, D.; Hilbe, M.; Boderke, P.; Ditzinger, G.; Sandow, J.; Lang, S.; Rubas, W.; Merkle, H.P. Validation of excised bovine nasal mucosa as in vitro model to study drug transport and metabolic pathways in nasal epithelium. J. Pharm. Sci. 2000, 89, 396–407. [Google Scholar] [CrossRef]
- Pund, S.; Rasve, G.; Borade, G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef]
- Henriques, P.; Bicker, J.; Silva, S.; Doktorovová, S.; Fortuna, A. Nasal-PAMPA: A novel non-cell-based high throughput screening assay for prediction of nasal drug permeability. Int. J. Pharm. 2023, 643, 123252. [Google Scholar] [CrossRef]
- Srivatsa Palakurthi, S.; Bharat Charbe, N.; Recalde Phillips, S.Y.; Alge, D.L.; Lu, D.; Palakurthi, S. Development of optimal in vitro release and permeation testing method for rectal suppositories. Int. J. Pharm. 2023, 640, 123042. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Rabea, Y.K.; El-Ela, F.I.A.; Khallaf, R.A. Glycerosomal thermosensitive in situ gel of duloxetine HCl as a novel nanoplatform for rectal delivery: In vitro optimization and in vivo appraisal. Drug Deliv. Transl. Res. 2022, 12, 3083–3103. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Trapani, A.; Franco, C.D.; Mandracchia, D.; Trapani, G.; Franchini, C.; Corbo, F.; Tripodo, G.; Kolev, I.N.; Stoyanov, G.S.; et al. In vitro and ex vivo studies on diltiazem hydrochloride-loaded microsponges in rectal gels for chronic anal fissures treatment. Int. J. Pharm. 2019, 557, 53–65. [Google Scholar] [CrossRef]
- Eissa, E.M.; El Sisi, A.M.; Bekhet, M.A.; El-Ela, F.I.A.; Kharshoum, R.M.; Ali, A.A.; Alrobaian, M.; Ali, A.M.A. pH-Sensitive In Situ Gel of Mirtazapine Invasomes for Rectal Drug Delivery: Protruded Bioavailability and Anti-Depressant Efficacy. Pharmaceuticals 2024, 17, 978. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef] [PubMed]
- das Neves, J.; Araújo, F.; Andrade, F.; Michiels, J.; Ariën, K.K.; Vanham, G.; Amiji, M.; Bahia, M.F.; Sarmento, B. In vitro and ex vivo evaluation of polymeric nanoparticles for vaginal and rectal delivery of the anti-HIV drug dapivirine. Mol. Pharm. 2013, 10, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Bhargava, H.N. Comparison of in vitro dissolution and permeation of fluconazole from different suppository bases. Drug Dev. Ind. Pharm. 1999, 25, 691–694. [Google Scholar] [CrossRef]
- Ravani, L.; Esposito, E.; Bories, C.; Moal, V.L.; Loiseau, P.M.; Djabourov, M.; Cortesi, R.; Bouchemal, K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013, 454, 695–702. [Google Scholar] [CrossRef]
- van Eyk, A.D.; van der Bijl, P. Porcine vaginal mucosa as an in vitro permeability model for human vaginal mucosa. Int. J. Pharm. 2005, 305, 105–111. [Google Scholar] [CrossRef]
- McCarron, P.A.; Donnelly, R.F.; Gilmore, B.F.; Woolfson, A.D.; McClelland, R.; Zawislak, A.; Price, J.H. Phototoxicity of 5-Aminolevulinic Acid in the HeLa Cell Line as an Indicative Measure of Photodynamic Effect After Topical Administration to Gynecological Lesions of Intraepithelial Form. Pharm. Res. 2004, 21, 1871–1879. [Google Scholar] [CrossRef]
- Berginc, K.; Skalko-Basnet, N.; Basnet, P.; Kristl, A. Development and evaluation of an in vitro vaginal model for assessment of drug’s biopharmaceutical properties: Curcumin. AAPS PharmSciTech 2012, 13, 1045–1053. [Google Scholar] [CrossRef]
- Sulistiawati; Enggi, C.K.; Isa, H.T.; Wijaya, S.; Ardika, K.A.R.; Asri, R.M.; Donnelly, R.F.; Permana, A.D. Validation of spectrophotometric method to quantify cabotegravir in simulated vaginal fluid and porcine vaginal tissue in ex vivo permeation and retention studies from thermosensitive and mucoadhesive gels. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120600. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Zeng, W.; Ge, S.; Lu, H.; Wu, C.; Ge, L.; Liang, D.; Xu, Y. Proniosome-derived niosomes for tacrolimus topical ocular delivery: In vitro cornea permeation, ocular irritation, and in vivo anti-allograft rejection. Eur. J. Pharm. Sci. 2014, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Karasulu, E.; Yavasoğlu, A.; Evrensanal, Z.; Uyanikgil, Y.; Karasulu, H.Y. Permeation studies and histological examination of sheep nasal mucosa following administration of different nasal formulations with or without absorption enhancers. Drug Deliv. 2008, 15, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Aqil, M.; Sultana, Y. A grafted copolymer-based nanomicelles for topical ocular delivery of everolimus: Formulation, characterization, ex-vivo permeation, in-vitro ocular toxicity, and stability study. Eur. J. Pharm. Sci. 2021, 159, 105735. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef]
- Resende, A.P.; Silva, B.; Braz, B.S.; Nunes, T.; Gonçalves, L.; Delgado, E. Ex vivo permeation of erythropoietin through porcine conjunctiva, cornea, and sclera. Drug Deliv. Transl. Res. 2017, 7, 625–631. [Google Scholar] [CrossRef]
- de Fraissinette, A.; Kolopp, M.; Schiller, I.; Fricker, G.; Gammert, C.; Pospischil, A.; Vonderscher, J.; Richter, F. In vitro tolerability of human nasal mucosa: Histopathological and scanning electron-microscopic evaluation of nasal forms containing Sandostatin®. Cell Biol. Toxicol. 1995, 11, 295–301. [Google Scholar] [CrossRef]
- Lang, S.; Langguth, P.; Oschmann, R.; Traving, B.; Merkle, H.P. Transport and metabolic pathway of thymocartin (TP4) in excised bovine nasal mucosa. J. Pharm. Pharmacol. 1996, 48, 1190–1196. [Google Scholar] [CrossRef]
- Tas, C.; Ozkan, C.K.; Savaser, A.; Ozkan, Y.; Tasdemir, U.; Altunay, H. Nasal administration of metoclopramide from different dosage forms: In vitro, ex vivo, and in vivo evaluation. Drug Deliv. 2009, 16, 167–175. [Google Scholar] [CrossRef]
- Greimel, A.; Bernkop-Schnürch, A.; Del Curto, M.D.; D’Antonio, M. Transport characteristics of a beta sheet breaker peptide across excised bovine nasal mucosa. Drug Dev. Ind. Pharm. 2007, 33, 71–77. [Google Scholar] [CrossRef]
- Osth, K.; Gråsjö, J.; Björk, E. A new method for drug transport studies on pig nasal mucosa using a horizontal Ussing chamber. J. Pharm. Sci. 2002, 91, 1259–1273. [Google Scholar] [CrossRef]
- de Araújo, J.S.M.; Augusto, G.G.X.; Pestana, A.M.; Groppo, F.C.; Rodrigues, F.S.M.; Novaes, P.D.; Franz-Montan, M. Impact of Storage on In Vitro Permeation and Mucoadhesion Setup Experiments Using Swine Nasal Mucosa. AAPS PharmSciTech 2024, 26, 7. [Google Scholar] [CrossRef] [PubMed]
- Reardon, P.M.; Gochoco, C.H.; Audus, K.L.; Wilson, G.; Smith, P.L. In vitro nasal transport across ovine mucosa: Effects of ammonium glycyrrhizinate on electrical properties and permeability of growth hormone releasing peptide, mannitol, and lucifer yellow. Pharm. Res. 1993, 10, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jain, K.; Gowthamarajan, K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Biointerfaces 2014, 113, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Gao, Y.; Nie, S.; Pan, W. The permeation of nalmefene hydrochloride across different regions of ovine nasal mucosa. Chem. Pharm. Bull. 2006, 54, 1722–1724. [Google Scholar] [CrossRef]
- Russo, P.; Sacchetti, C.; Pasquali, I.; Bettini, R.; Massimo, G.; Colombo, P.; Rossi, A. Primary microparticles and agglomerates of morphine for nasal insufflation. J. Pharm. Sci. 2006, 95, 2553–2561. [Google Scholar] [CrossRef]
- Bortolotti, F.; Balducci, A.G.; Sonvico, F.; Russo, P.; Colombo, G. In vitro permeation of desmopressin across rabbit nasal mucosa from liquid nasal sprays: The enhancing effect of potassium sorbate. Eur. J. Pharm. Sci. 2009, 37, 36–42. [Google Scholar] [CrossRef]
- Kamel, R.; Basha, M.; Abd El-Alim, S.H. Development of a novel vesicular system using a binary mixture of sorbitan monostearate and polyethylene glycol fatty acid esters for rectal delivery of rutin. J. Liposome Res. 2013, 23, 28–36. [Google Scholar] [CrossRef]
- Mehta, S.; Verstraelen, H.; Peremans, K.; Villeirs, G.; Vermeire, S.; De Vos, F.; Mehuys, E.; Remon, J.P.; Vervaet, C. Vaginal distribution and retention of a multiparticulate drug delivery system, assessed by gamma scintigraphy and magnetic resonance imaging. Int. J. Pharm. 2012, 426, 44–53. [Google Scholar] [CrossRef]
- Moss, J.A.; Malone, A.M.; Smith, T.J.; Kennedy, S.; Kopin, E.; Nguyen, C.; Gilman, J.; Butkyavichene, I.; Vincent, K.L.; Motamedi, M.; et al. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob. Agents Chemother. 2012, 56, 875–882. [Google Scholar] [CrossRef]
- Vincent, K.L.; Bourne, N.; Bell, B.A.; Vargas, G.; Tan, A.; Cowan, D.; Stanberry, L.R.; Rosenthal, S.L.; Motamedi, M. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: A promising model for testing safety of candidate microbicides. Sex. Transm. Dis. 2009, 36, 312–318. [Google Scholar] [CrossRef]
- Lane, M.E. In vitro permeation testing for the evaluation of drug delivery to the skin. Eur. J. Pharm. Sci. 2024, 201, 106873. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.; Nicolazzo, J.A.; Finnin, B.C. A novel flow through diffusion cell for assessing drug transport across the buccal mucosa in vitro. J. Pharm. Sci. 2009, 98, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Oh, L.; Yi, S.; Zhang, D.; Shin, S.H.; Bashaw, E. In Vitro Skin Permeation Methodology for Over-The-Counter Topical Dermatologic Products. Ther. Innov. Regul. Sci. 2020, 54, 693–700. [Google Scholar] [CrossRef]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3 (Suppl. 2), 123–126. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Modeling the oral cavity: In vitro and in vivo evaluations of buccal drug delivery systems. J. Control Release 2012, 161, 746–756. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores Open Access 2016, 5, 94–108. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Cabrera-Pérez, M.Á.; Bermejo-Sanz, M.; González-Álvarez, M.; González-Álvarez, I.; Vera Lara, V.; Chou Kam, W.-H. Chapter 2—Importance and applications of cell- and tissue-based in vitro models for drug permeability screening in early stages of drug development. In Concepts and Models for Drug Permeability Studies, 2nd ed.; Sarmento, B., Leite Pereira, C., Neves, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2024; pp. 5–41. [Google Scholar] [CrossRef]
- Notara, M.; Daniels, J.T. Characterisation and functional features of a spontaneously immortalised human corneal epithelial cell line with progenitor-like characteristics. Brain Res. Bull. 2010, 81, 279–286. [Google Scholar] [CrossRef]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Mohan, R.R.; Possin, D.E.; Mohan, R.R.; Sinha, S.; Wilson, S.E. Development of genetically engineered tet HPV16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Exp. Eye Res. 2003, 77, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Reichl, S.; Bednarz, J.; Müller-Goymann, C.C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br. J. Ophthalmol. 2004, 88, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Andrade, F.; da Silva, S.B.; Rodrigues, F.; das Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef]
- Cabrera-Pérez, M.Á.; Sanz, M.B.; Sanjuan, V.M.; González-Álvarez, M.; Álvarez, I.G. Importance and applications of cell- and tissue-based in vitro models for drug permeability screening in early stages of drug development. In Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–29. [Google Scholar] [CrossRef]
- Gorodeski, G.I.; Eckert, R.L.; Utian, W.H.; Rorke, E.A. Maintenance of in vivo-like keratin expression, sex steroid responsiveness, and estrogen receptor expression in cultured human ectocervical epithelial cells. Endocrinology 1990, 126, 399–406. [Google Scholar] [CrossRef]
- Gorodeski, G.I.; Romero, M.F.; Hopfer, U.; Rorke, E.; Utian, W.H.; Eckert, R.L. Human uterine cervical epithelial cells grown on permeable support—A new model for the study of differentiation. Differentiation 1994, 56, 107–118. [Google Scholar] [CrossRef]
- Kuramoto, H.; Tamura, S.; Notake, Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am. J. Obstet. Gynecol. 1972, 114, 1012–1019. [Google Scholar] [CrossRef]
- Kaushal, G.; Trombetta, L.; Ochs, R.S.; Shao, J. Delivery of TEM beta-lactamase by gene-transformed Lactococcus lactis subsp. lactis through cervical cell monolayer. Int. J. Pharm. 2006, 313, 29–35. [Google Scholar] [CrossRef]
- Gali, Y.; Ariën, K.K.; Praet, M.; Van den Bergh, R.; Temmerman, M.; Delezay, O.; Vanham, G. Development of an in vitro dual-chamber model of the female genital tract as a screening tool for epithelial toxicity. J. Virol. Methods 2010, 165, 186–197. [Google Scholar] [CrossRef]
- Clark, M.R.; McCormick, T.J.; Doncel, G.F.; Friend, D.R. Preclinical evaluation of UC781 microbicide vaginal drug delivery. Drug Deliv. Transl. Res. 2011, 1, 175–182. [Google Scholar] [CrossRef]
- Schaller, M.; Korting, H.C.; Borelli, C.; Hamm, G.; Hube, B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect. Immun. 2005, 73, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Bein, M.; Korting, H.C.; Baur, S.; Hamm, G.; Monod, M.; Beinhauer, S.; Hube, B. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect. Immun. 2003, 71, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Uckun, F.M. Mucosal safety of PHI-443 and stampidine as a combination microbicide to prevent genital transmission of HIV-1. Fertil. Steril. 2007, 88, 1197–1206. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar]
- Yamasaki, K.; Kawasaki, S.; Young, R.D.; Fukuoka, H.; Tanioka, H.; Nakatsukasa, M.; Quantock, A.J.; Kinoshita, S. Genomic aberrations and cellular heterogeneity in SV40-immortalized human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 604–613. [Google Scholar] [CrossRef][Green Version]
- Dimova, S.; Brewster, M.E.; Noppe, M.; Jorissen, M.; Augustijns, P. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol. In Vitro 2005, 19, 107–122. [Google Scholar] [CrossRef]
- De Fraissinette, A.; Brun, R.; Felix, H.; Vonderscher, J.; Rummelt, A. Evaluation of the human cell line RPMI 2650 as an in vitro nasal model. Rhinology 1995, 33, 194–198. [Google Scholar]
- Werner, U.; Kissel, T. In-vitro cell culture models of the nasal epithelium: A comparative histochemical investigation of their suitability for drug transport studies. Pharm. Res. 1996, 13, 978–988. [Google Scholar] [CrossRef]
- Gorodeski, G.I.; Merlin, D.; De Santis, B.J.; Frieden, K.A.; Hopfer, U.; Eckert, R.L.; Utian, W.H.; Romero, M.F. Characterization of paracellular permeability in cultured human cervical epithelium: Regulation by extracellular adenosine triphosphate. J. Soc. Gynecol. Investig. 1994, 1, 225–233. [Google Scholar] [CrossRef]
- Higuchi, T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Anissimov, Y.G. Mathematical models in percutaneous absorption. In Percutaneous Absorption; CRC Press: Boca Raton, FL, USA, 2005; pp. 37–80. [Google Scholar]
- Rojanasakul, Y.; Paddock, S.W.; Robinson, J.R. Confocal laser scanning microscopic examination of transport pathways and barriers of some peptides across the cornea. Int. J. Pharm. 1990, 61, 163–172. [Google Scholar] [CrossRef]
- Rojanasakul, Y.; Liaw, J.; Robinson, J.R. Mechanisms of action of some penetration enhancers in the cornea: Laser scanning confocal microscopic and electrophysiology studies. Int. J. Pharm. 1990, 66, 131–142. [Google Scholar] [CrossRef]
- Rojanasakul, Y.; Robinson, J.R. The cytoskeleton of the cornea and its role in tight junction permeability. Int. J. Pharm. 1991, 68, 135–149. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Gref, R.; Calvo, P.; Alonso, M.J. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur. J. Pharm. Sci. 2003, 20, 73–81. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Rothen-Rutishauser, B.; Rist, B.; Beck-Sickinger, A.; Wunderli-Allenspach, H.; Rubas, W.; Sadée, W.; Merkle, H.P. Translocation of human calcitonin in respiratory nasal epithelium is associated with self-assembly in lipid membrane. Biochemistry 1998, 37, 16582–16590. [Google Scholar] [CrossRef]
- Lang, S.; Rothen-Rutishauser, B.; Perriard, J.C.; Schmidt, M.C.; Merkle, H.P. Permeation and pathways of human calcitonin (hCT) across excised bovine nasal mucosa. Peptides 1998, 19, 599–607. [Google Scholar] [CrossRef]
- Vila, A.; Gill, H.; McCallion, O.; Alonso, M.J. Transport of PLA-PEG particles across the nasal mucosa: Effect of particle size and PEG coating density. J. Control Release 2004, 98, 231–244. [Google Scholar] [CrossRef]
- Chuchuen, O.; Henderson, M.H.; Sykes, C.; Kim, M.S.; Kashuba, A.D.; Katz, D.F. Quantitative analysis of microbicide concentrations in fluids, gels and tissues using confocal Raman spectroscopy. PLoS ONE 2013, 8, e85124. [Google Scholar] [CrossRef][Green Version]
- Presnell, A.L.; Chuchuen, O.; Simons, M.G.; Maher, J.R.; Katz, D.F. Full depth measurement of tenofovir transport in rectal mucosa using confocal Raman spectroscopy and optical coherence tomography. Drug Deliv. Transl. Res. 2018, 8, 843–852. [Google Scholar] [CrossRef]
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, M.; Shin, H.-W.; Chung, S. In vitro nasal mucosa gland-like structure formation on a chip. Lab Chip 2017, 17, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, Y.; Li, C.W.; Xia, H.M.; Chao, S.S.; Wang, D.Y.; Wang, Z.P. Live human nasal epithelial cells (hNECs) on chip for in vitro testing of gaseous formaldehyde toxicity via airway delivery. Lab Chip 2014, 14, 677–680. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Cheng, S.; Kourmatzis, A.; Traini, D.; Young, P.; Sheikh, Z.; Ong, H.X. In vitro interactions of aerosol formulations with human nasal epithelium using real-time monitoring of drug transport in a nasal mucosa-on-a-chip. Biosens. Bioelectron. 2023, 223, 115010. [Google Scholar] [CrossRef]
- Mattern, K.; Beißner, N.; Reichl, S.; Dietzel, A. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 1—Engineering of microfluidic system and technical simulations. Eur. J. Pharm. Biopharm. 2018, 126, 159–165. [Google Scholar] [CrossRef]
- Beiβner, N.; Mattern, K.; Dietzel, A.; Reichl, S. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 2—Ocular DynaMiTES for drug absorption studies of the anterior eye. Eur. J. Pharm. Biopharm. 2018, 126, 166–176. [Google Scholar] [CrossRef]
- Chen, L.-J.; Ito, S.; Kai, H.; Nagamine, K.; Nagai, N.; Nishizawa, M.; Abe, T.; Kaji, H. Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Sci. Rep. 2017, 7, 3538. [Google Scholar] [CrossRef]
- Chung, M.; Lee, S.; Lee, B.J.; Son, K.; Jeon, N.L.; Kim, J.H. Wet-AMD on a Chip: Modeling Outer Blood-Retinal Barrier In Vitro. Adv. Healthc. Mater. 2018, 7, 1700028. [Google Scholar] [CrossRef]
- Kondamudi, P.K.; Tirumalasetty, P.P.; Malayandi, R.; Mutalik, S.; Pillai, R. Lidocaine Transdermal Patch: Pharmacokinetic Modeling and In Vitro-In Vivo Correlation (IVIVC). AAPS PharmSciTech 2016, 17, 588–596. [Google Scholar] [CrossRef]
- Baynes, R.; Riviere, J.; Franz, T.; Monteiro-Riviere, N.; Lehman, P.; Peyrou, M.; Toutain, P.L. Challenges obtaining a biowaiver for topical veterinary dosage forms. J. Vet. Pharmacol. Ther. 2012, 35 (Suppl. 1), 103–114. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Draft Product Specific Guidance on Acyclovir Ointment. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_018604.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Product Specific Guidance on Acyclovir Cream. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021478.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Penciclovir. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_020629.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Tacrolimus. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_050777.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Oxymetazoline Hydrochloride. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_208552.pdf (accessed on 30 December 2024).
- Santos, L.L.; Swofford, N.J.; Santiago, B.G. In Vitro Permeation Test (IVPT) for Pharmacokinetic Assessment of Topical Dermatological Formulations. Curr. Protoc. Pharmacol. 2020, 91, e79. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Draft Guidance on Clascoterone. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_213433.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Clindamycin Phosphate. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Clindamycin_Phosphate_crm_50680_RC03-12.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Halobetasol Propionate. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Halobetasol%20propionate_topical%20cream_NDA%2019967_RC04-16.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Draft Guidance on Tazarotene. Guidance Index. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021184-Cre-0.1P.pdf (accessed on 30 December 2024).
- U.S. Food and Drug Administration. In Vitro Permeation Test Studies for Topical Drug Prodcuts Submitted in ANDAs. Guidance Index. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-permeation-test-studies-topical-drug-products-submitted-andas (accessed on 30 December 2024).
- Yue, W.; Megan Kelchen, L.X.; Ghosh, P.; Niu, M.; Raney, S.G.; Shen, J. Impact of fatty acids on in vitro performance of clindamycin phosphate vaginal creams. In Proceedings of the Controlled Release Society Annual Meeting and Exposition #CRS2023, Las Vegas, NV, USA, 24–28 July 2023. [Google Scholar]
- Xie, L.; Megan Kelchen, W.Y.; Ghosh, P.; Niu, M.; Raney, S.G.; Shen, J. Impact of material attributes on in vitro performance of clindamycin phosphate vaginal creams. In Proceedings of the PHARMSCI 360, American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Boston, MA, USA, 16–19 October 2022. [Google Scholar]
- U.S. Food and Drug Administration. FY 2023 GDUFA Science and Research Report. 2023. Available online: https://www.fda.gov/drugs/generic-drugs/fy-2023-gdufa-science-and-research-report (accessed on 30 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).