Exploring the Anticancer Potential of NO-Donor Oxadiazole Assemblies Against Malignant Pleural Mesothelioma
Abstract
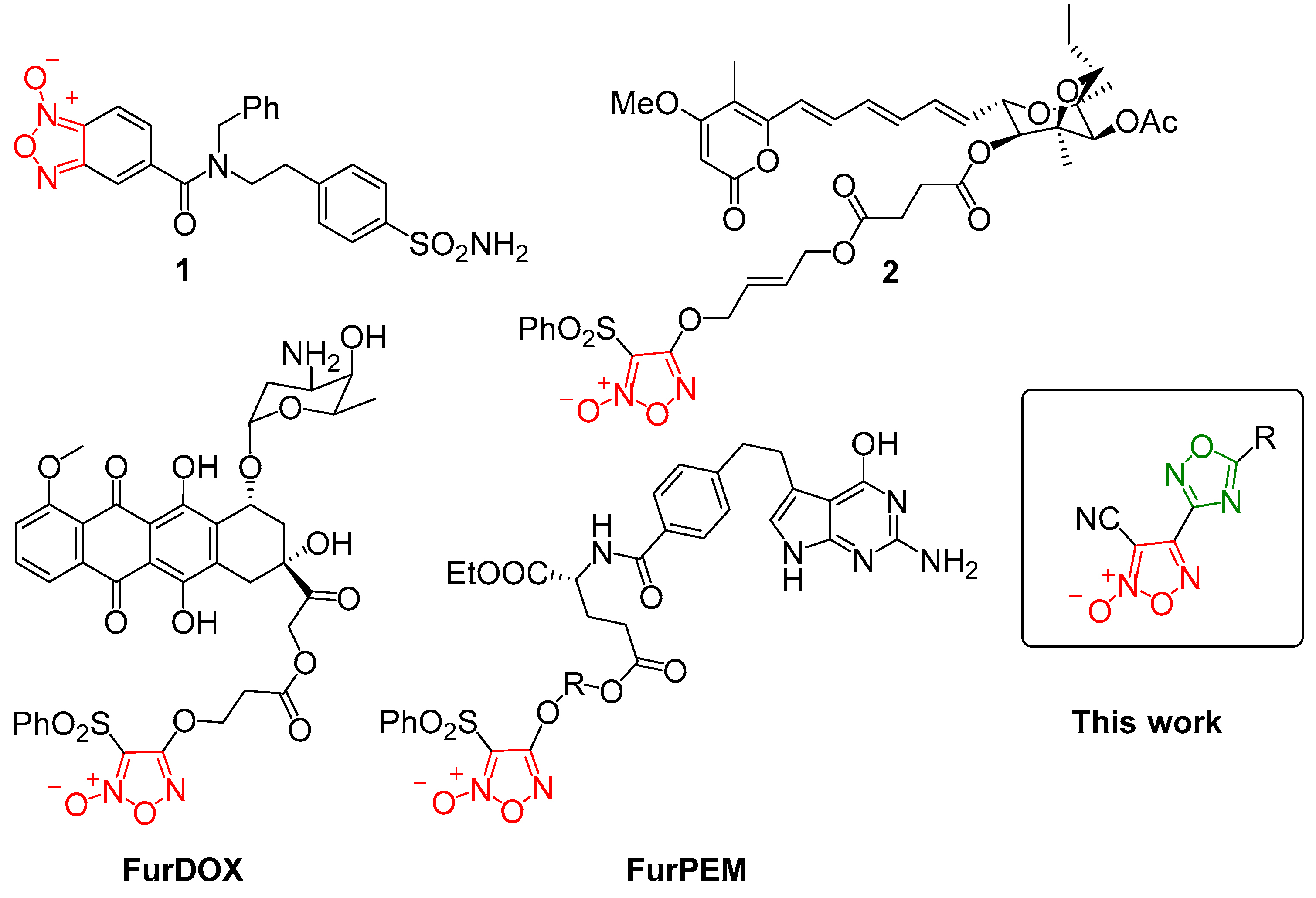
1. Introduction
2. Experimental Section
2.1. General Information
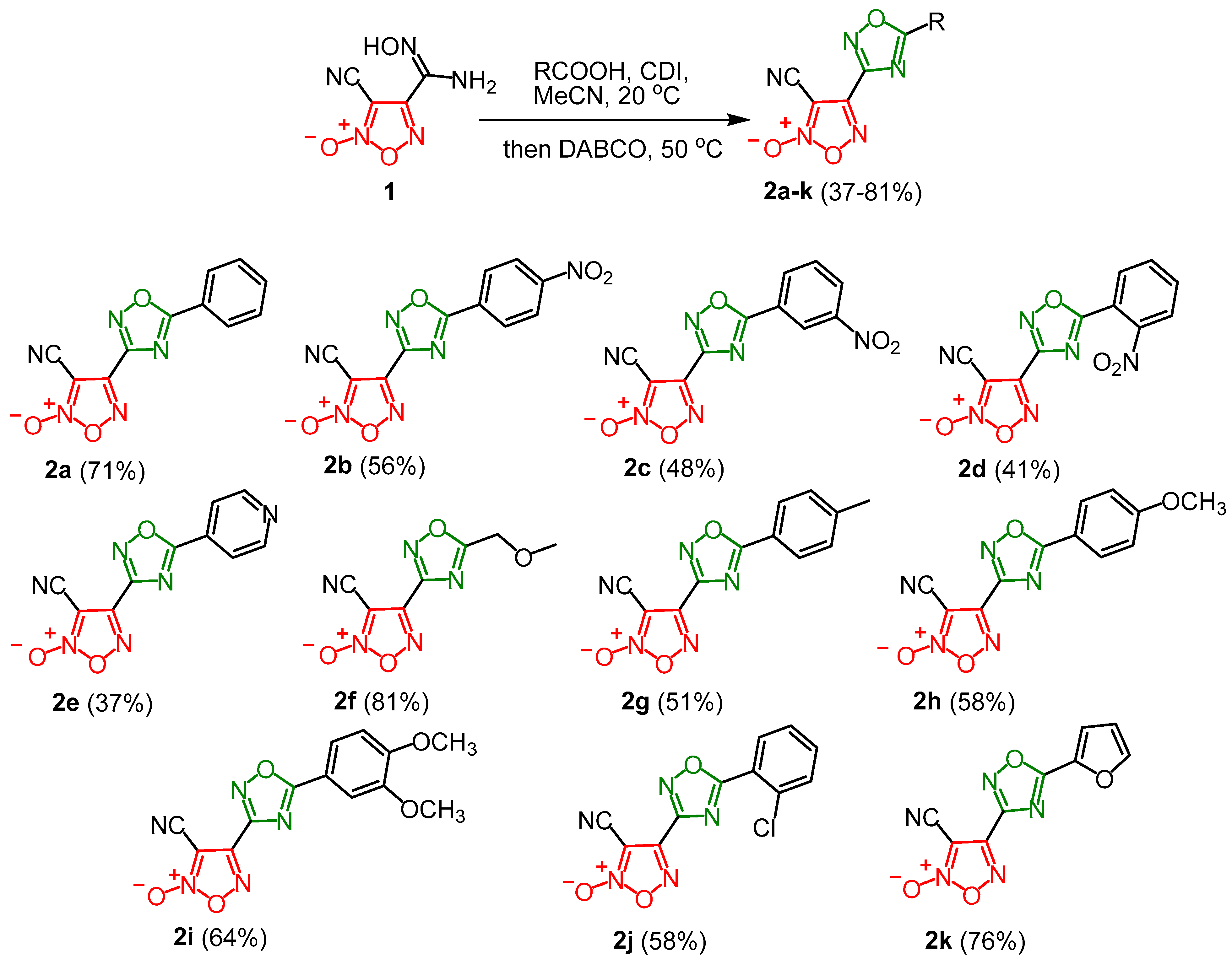
2.2. Synthesis of 3-Cyano-(1,2,4-Oxadiazol-3-yl)Furoxans (General Procedure)
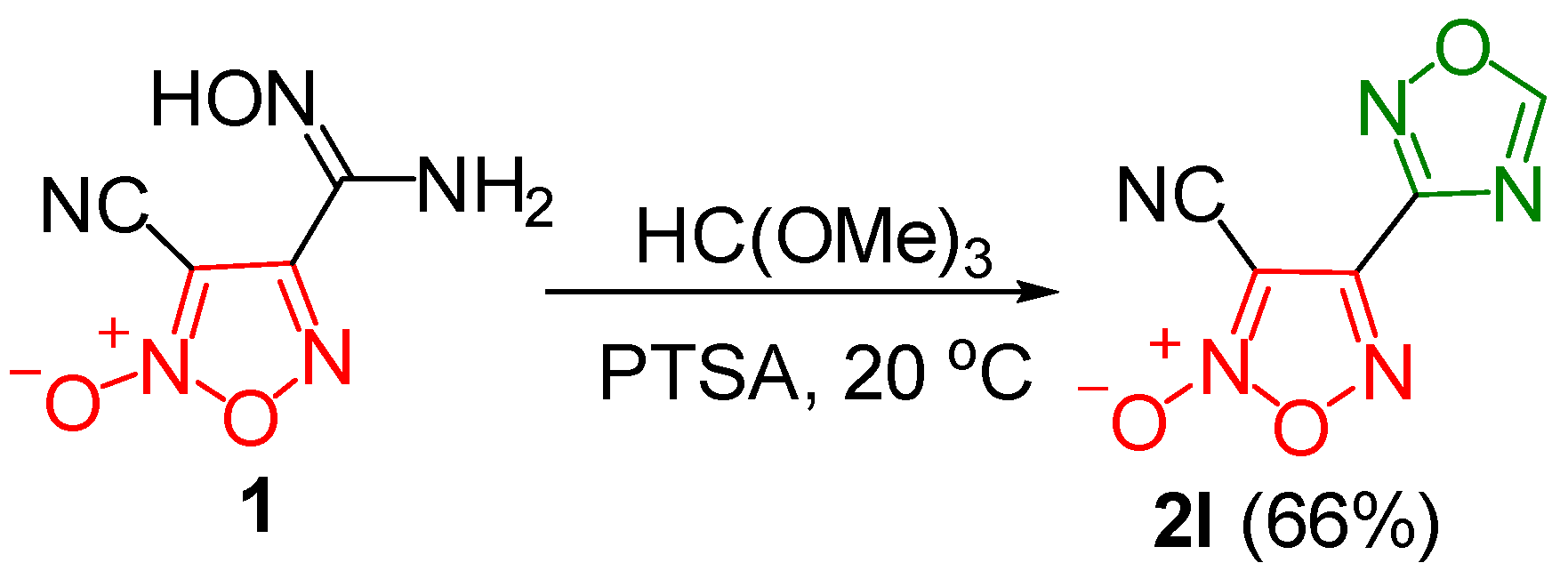
2.3. Synthesis of 3-Cyano-4-(1,2,4-Oxadiazol-3-yl)Furoxan
2.4. NO Release Assay
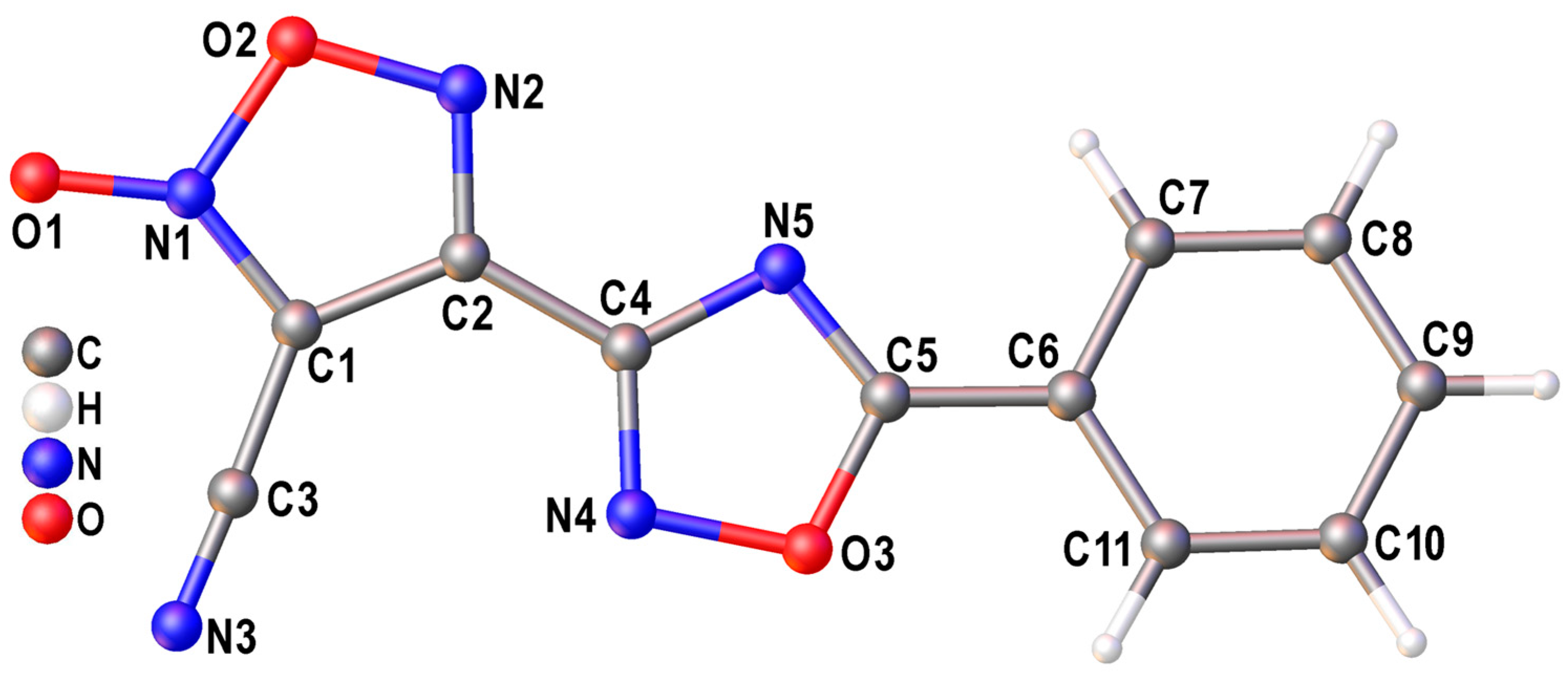
2.5. X-Ray Diffraction Study
2.6. Cell Lines and Culture Conditions
2.7. Evaluation of Anticancer Activity
2.8. Evaluation of NO Release In Vitro
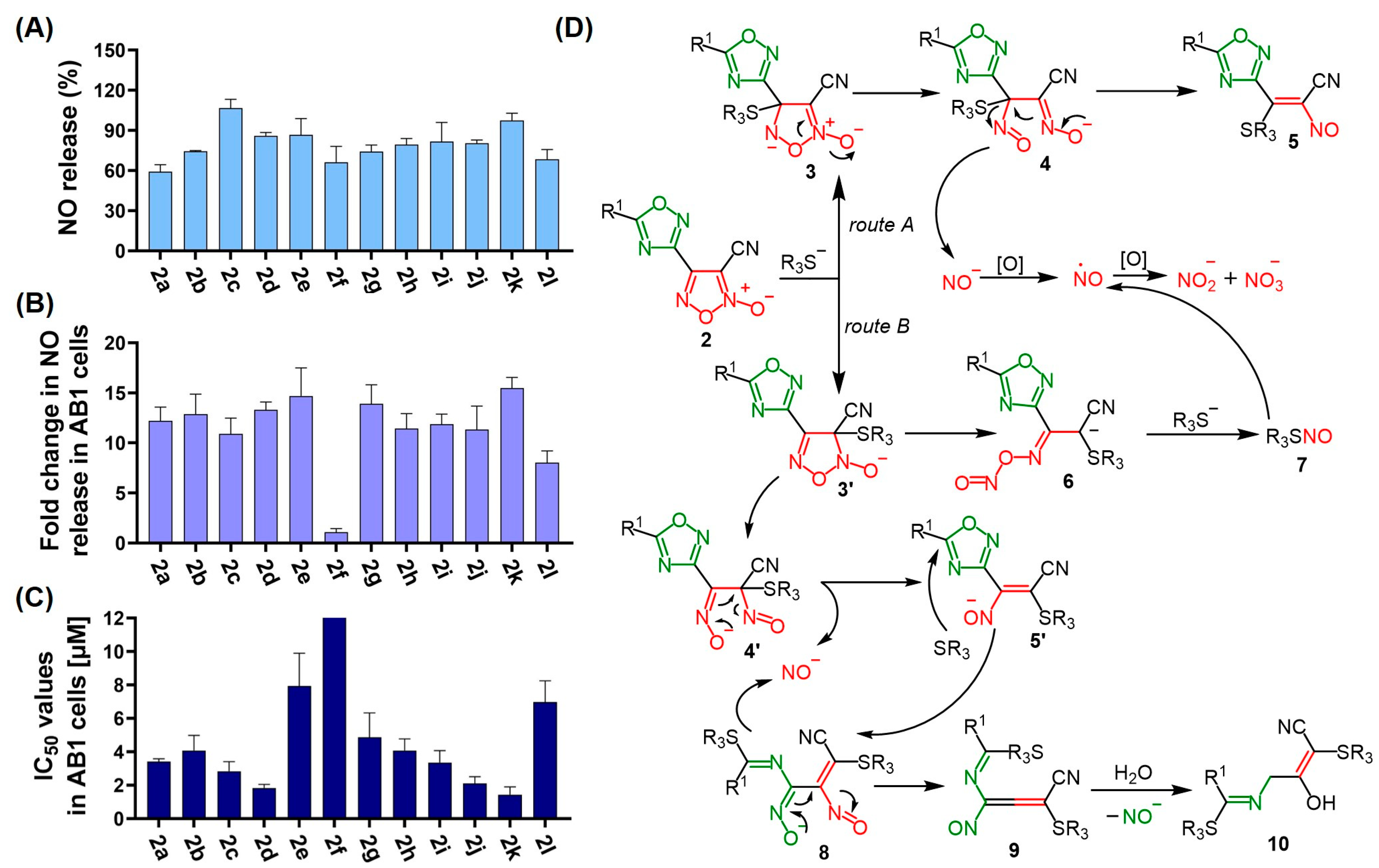
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. X-Ray Crystallography and DFT Calculations
3.3. Anticancer Activity
3.4. NO Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, M.; Crites, M.K.; Rich, P.; Bajantri, B. Malignant Pleural Mesothelioma: A Comprehensive Review. J. Clin. Med. 2024, 13, 5837. [Google Scholar] [CrossRef]
- Lee, A.Y.; Raz, D.J.; He, B.; Jablons, D.M. Update on the molecular biology of malignant mesothelioma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1454–1461. [Google Scholar] [CrossRef]
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant mesothelioma and its non-asbestos causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. [Google Scholar] [CrossRef]
- Mutti, L.; Peikert, T.; Robinson, B.W.; Scherpereel, A.; Tsao, A.S.; de Perrot, M.; Woodard, G.A.; Jablons, D.M.; Wiens, J.; Hirsch, F.R. Scientific advances and new frontiers in mesothelioma therapeutics. J. Thorac. Oncol. 2018, 13, 1269–1283. [Google Scholar] [CrossRef]
- Huerta, S. Nitric oxide for cancer therapy. Future Sci. OA 2015, 1, Fso44. [Google Scholar] [CrossRef]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. The role of nitric oxide in pleural disease. Respir. Med. 2021, 179, 106350. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Kahlos, K.; Puhakka, A.; Lakari, E.; Säily, M.; Pääkkö, P.; Kinnula, V. Expression of inducible nitric oxide synthase in healthy pleura and in malignant mesothelioma. Br. J. Cancer 2000, 83, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Puhakka, A.; Kahlos, K.; Säily, M.; Pääkkö, P.; Koistinen, P.; Kinnula, V. Endothelial nitric oxide synthase is strongly expressed in malignant mesothelioma but does not associate with vascular density or the expression of VEGF, FLK1 or FLT1. Histopathology 2001, 39, 179–186. [Google Scholar] [CrossRef]
- Riganti, C.; Orecchia, S.; Silvagno, F.; Pescarmona, G.; Betta, P.G.; Gazzano, E.; Aldieri, E.; Ghigo, D.; Bosia, A. Asbestos induces nitric oxide synthesis in mesothelioma cells via Rho signaling inhibition. Am. J. Respir. Cell Mol. Biol. 2007, 36, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ren, H.; Liu, J.; Wang, Y.; Meng, Z.; He, Z.; Miao, W.; Chen, G.; Li, X. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale 2019, 11, 5474–5488. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.; Chilka, S.; Bonavida, B. Nitric oxide donors: Novel cancer therapeutics. Int. J. Oncol. 2008, 33, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.; Schiefer, I.T. Pharmacokinetics and pharmacodynamics of nitric oxide mimetic agents. Nitric Oxide 2019, 84, 69–78. [Google Scholar] [CrossRef]
- Schönafinger, K. Heterocyclic NO prodrugs. Il Farmaco 1999, 54, 316–320. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Burilov, A.R. Novel structural hybrids on the base of benzofuroxans and furoxans. Mini-review. Curr. Top. Med. Chem. 2017, 17, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Ermondi, G.; Fruttero, R.; Di Stilo, A.; Ferretti, C.; Gasco, A. Furoxans as nitric oxide donors. 4-Phenyl-3-furoxancarbonitrile: Thiol-mediated nitric oxide release and biological evaluation. J. Med. Chem. 1994, 37, 4412–4416. [Google Scholar] [CrossRef]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2013, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Bua, S.; Nocentini, A.; Bonardi, A.; Palma, G.; Ciampi, G.; Giliberti, A.; Iannelli, F.; Bruzzese, F.; Supuran, C.T.; de Nigris, F. Harnessing Nitric Oxide-Donating Benzofuroxans for Targeted Inhibition of Carbonic Anhydrase IX in Cancer. J. Med. Chem. 2024, 67, 15892–15907. [Google Scholar] [CrossRef]
- Ma, L.-F.; Xu, L.L.; Yuan, L.-J.; Yang, X.; Wu, R.; Bao, S.-M.; Chen, Y.-L.; Duan, H.-L.; Fang, L.; Zhao, H.-J.; et al. Discovery of NO Donor-Aurovertin Hybrids as Dual Ferroptosis and Apoptosis Inducers for Treating Triple Negative Breast Cancer. J. Med. Chem. 2024, 67, 13089–13105. [Google Scholar] [CrossRef] [PubMed]
- Vlastos, F.; Hillas, G.; Vidal, P.; Lacomme, S.; Galateau-Sallé, F.; Vollmer, E.; Guzman-Costabel, J.; Vignaud, J.M.; Martinet, N. Survey and biological insights of pemetrexed-related therapeutic improvement in mesothelioma: The Nancy Centre of Biological Resources’ Mesothelioma Cohort. J. Thorac. Oncol. 2009, 4, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Yi, B.; Zhang, P.; Liu, J.; Zhang, C.; Zhou, H. Novel furoxan NO-donor pemetrexed derivatives: Design, synthesis, and preliminary biological evaluation. Med. Chem. Res. 2009, 18, 495–510. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenium microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Efficient assembly of mono- and bis(1,2,4-oxadiazol-3-yl)furoxan scaffolds via tandem reactions of furoxanylamidoximes. RSC Adv. 2015, 5, 47248–47260. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.B.; Frank, H.; Palshof, T. Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma. Br. J. Cancer 2008, 99, 44–50. [Google Scholar] [CrossRef][Green Version]
- Bardina, E.E.; Matnurov, E.M.; Bakaev, I.V.; Rakhmanova, M.I.; Davydova, M.P.; Artem’ev, A.V.; Babak, M.V.; Gushchin, A.L. Dinuclear gold (I) complexes with pyrimidine-and m-carborane-based bisphosphine ligands: Synthesis, structure, photoluminescence and cytotoxicity against malignant pleural mesothelioma. New J. Chem. 2024, 48, 11918–11924. [Google Scholar] [CrossRef]
- Han, C.; Huang, Z.; Zheng, C.; Wan, L.; Zhang, L.; Peng, S.; Zhang, Y. Novel hybrids of (phenylsulfonyl) furoxan and anilinopyrimidine as potent and selective epidermal growth factor receptor inhibitors for intervention of non-small-cell lung cancer. J. Med. Chem. 2013, 56, 4738–4748. [Google Scholar] [CrossRef]
- Liu, M.M.; Chen, X.Y.; Huang, Y.Q.; Feng, P.; Guo, Y.L.; Yang, G.; Chen, Y. Hybrids of phenylsulfonylfuroxan and coumarin as potent antitumor agents. J. Med. Chem. 2014, 57, 9343–9356. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Bohn, H.; Brendel, J.; Martorana, P.A.; Schönafinger, K. Cardiovascular actions of the furoxan CAS 1609, a novel nitric oxide donor. Br. J. Pharmacol. 1995, 114, 1605. [Google Scholar] [CrossRef]
- Ispikoudi, M.; Amvrazis, M.; Kontogiorgis, C.; Koumbis, A.E.; Litinas, K.E.; Hadjipavlou-Litina, D.; Fylaktakidou, K.C. Convenient synthesis and biological profile of 5-amino-substituted 1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.M.; Mohamed, M.F.A.; Al-Sanea, M.M.; Moustafa, A.H.; Abdelhamid, A.A.; Gomaa, H.A.M. Novel aryl carboximidamides and 3-aryl-1,2,4-oxadiazoles analogues of naproxen as dual selective COX-2/15-LOX inhibitors: Design, synthesis and Docking studies. Bioorganic Chem. 2019, 85, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.S.; Larin, A.A.; Fershtat, L.L.; Anikina, L.V.; Pukhov, S.A.; Klochkov, S.G.; Makhova, N.N. Synthesis, structural characterization and cytotoxic activity of heterocyclic compounds containing the furoxan ring. Arkivoc 2017, 3, 250–268. [Google Scholar] [CrossRef]
- Hasegawa, U.; Wang, T.; Chen, J.J.; Uyama, H.; van der Vlies, A.J. Furoxan-Bearing Micelles for Nitric Oxide Delivery. Macromol. Biosci. 2016, 16, 1009–1018. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef] [PubMed]

 | |||||
|---|---|---|---|---|---|
| Entry | Activation Reagent | Base | Time, h | T, °C | Yield of 2a, a % |
| 1 | SOCl2 | Cs2CO3 | 10 | 20 | 20 |
| 2 | SOCl2 | Cs2CO3 | 6 | 20 | 14 |
| 3 | SOCl2 | Cs2CO3 | 12 | 20 | 48 |
| 4 | SOCl2 | Cs2CO3 | 5 | 50 | 21 |
| 5 | SOCl2 | Cs2CO3 | 48 | 20 | 23 |
| 6 | SOCl2 | TEA | 7 | 20 | 33 |
| 7 | CDI | DBU | 12 | 20 | 37 |
| 8 | CDI | TEA | 7 | 20 | 52 |
| 9 | CDI | DABCO | 3 | 20 | 56 |
| 10 | CDI | DABCO | 3 | 50 | 71 |
| Compound | IC50, μM a | SFJU77 b | ||
|---|---|---|---|---|
| AB1 | JU77 | MRC-5 | ||
| 2a | 3.4 ± 0.2 | 3.7 ± 1.0 | 3.7 ± 0 | 1.0 |
| 2b | 4.1 ± 0.9 | 2.7 ± 0.2 | 4.4 ± 0.6 | 1.6 |
| 2c | 2.8 ± 0.5 | 1.2 ± 0.2 | 4.3 ± 0.3 | 3.6 |
| 2d | 1.8 ± 0.2 | 0.87 ± 0.07 | 2.2 ± 0.2 | 2.5 |
| 2e | 7.9 ± 2.0 | 4.2 ± 0.8 | 4.7 ± 0.3 | 1.1 |
| 2f | >150 | >150 | >150 | - |
| 2g | 4.9 ± 1.4 | 3.1 ± 0.2 | 4.8 ± 0.3 | 1.5 |
| 2h | 4.1 ± 0.7 | 2.9 ± 0.3 | 4.2 ± 0.7 | 1.4 |
| 2i | 3.3 ± 0.7 | 1.6 ± 0.4 | 4 ± 0.6 | 2.5 |
| 2j | 2.1 ± 0.3 | 1.4 ± 0.2 | 3.3 ± 0.3 | 2.4 |
| 2k | 1.4 ± 0.4 | 0.99 ± 0.13 | 1.5 ± 0.3 | 1.5 |
| 2l | 7.0 ± 1.3 | 5.4 ± 1.3 | 4.6 ± 1 | 0.9 |
| Cisplatin c | 5.8 ± 1 | 4.2 ± 0.5 | 3.1 ± 1.2 | 0.7 |
| Compound | NO Released (%) a | Fold Change | ||||
|---|---|---|---|---|---|---|
| 1 h | 24 h | 48 h | 24 h/1 h | 48 h/1 h | 48 h/24 h | |
| 1 | 70 ± 5 | 81 ± 4 | 84 ± 10 | 1.2 | 1.2 | 1.0 |
| 2a | 50 ± 8 | 59 ± 5 | 89 ± 6 | 1.2 | 1.8 | 1.5 |
| 2b | 51 ± 11 | 74 ± 1 | 80 ± 4 | 1.5 | 1.6 | 1.1 |
| 2c | 61 ± 8 | 107 ± 8 | 107 ± 10 | 1.8 | 1.8 | 1.0 |
| 2d | 69 ± 1 | 86 ± 3 | 89 ± 3 | 1.2 | 1.3 | 1.0 |
| 2e | 53 ± 7 | 87 ± 13 | 87 ± 11 | 1.6 | 1.6 | 1.0 |
| 2f | 50 ± 7 | 66 ± 11 | 71 ± 11 | 1.3 | 1.4 | 1.1 |
| 2g | 59 ± 1 | 74 ± 6 | 81 ± 10 | 1.3 | 1.4 | 1.1 |
| 2h | 54 ± 6 | 79 ± 5 | 86 ± 4 | 1.5 | 1.6 | 1.1 |
| 2i | 54 ± 14 | 82 ± 14 | 94 ±14 | 1.5 | 1.7 | 1.1 |
| 2j | 53 ± 4 | 80 ± 3 | 84 ± 2 | 1.5 | 1.6 | 1.1 |
| 2k | 73 ± 7 | 97 ± 6 | 104 ± 12 | 1.3 | 1.4 | 1.1 |
| 2l | 42 ± 5 | 68 ± 8 | 72 ± 8 | 1.6 | 1.7 | 1.1 |
| CAS-1609 | 27 ± 3 | 68 ± 6 | 73 ± 6 | 2.5 | 2.7 | 1.1 |
| CHF-2363 | 26 ± 10 | 71 ± 4 | 77 ± 9 | 2.7 | 3.0 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stebletsova, I.A.; Larin, A.A.; Matnurov, E.M.; Ananyev, I.V.; Babak, M.V.; Fershtat, L.L. Exploring the Anticancer Potential of NO-Donor Oxadiazole Assemblies Against Malignant Pleural Mesothelioma. Pharmaceutics 2025, 17, 230. https://doi.org/10.3390/pharmaceutics17020230
Stebletsova IA, Larin AA, Matnurov EM, Ananyev IV, Babak MV, Fershtat LL. Exploring the Anticancer Potential of NO-Donor Oxadiazole Assemblies Against Malignant Pleural Mesothelioma. Pharmaceutics. 2025; 17(2):230. https://doi.org/10.3390/pharmaceutics17020230
Chicago/Turabian StyleStebletsova, Irina A., Alexander A. Larin, Egor M. Matnurov, Ivan V. Ananyev, Maria V. Babak, and Leonid L. Fershtat. 2025. "Exploring the Anticancer Potential of NO-Donor Oxadiazole Assemblies Against Malignant Pleural Mesothelioma" Pharmaceutics 17, no. 2: 230. https://doi.org/10.3390/pharmaceutics17020230
APA StyleStebletsova, I. A., Larin, A. A., Matnurov, E. M., Ananyev, I. V., Babak, M. V., & Fershtat, L. L. (2025). Exploring the Anticancer Potential of NO-Donor Oxadiazole Assemblies Against Malignant Pleural Mesothelioma. Pharmaceutics, 17(2), 230. https://doi.org/10.3390/pharmaceutics17020230






