Fundamental and Targeted Approaches in Pulmonary Arterial Hypertension Treatment
Abstract
1. Introduction
2. Pathophysiology of PAH
3. Drug Classes and Mechanisms in PAH
3.1. Conventional Approaches for PAH Management
3.1.1. Endothelin Receptor Antagonists
3.1.2. Phosphodiesterase Type 5 Inhibitors
3.1.3. Prostacyclin Pathway-Targeted Drugs
3.1.4. Soluble Guanylate Cyclase Activators
3.2. Current Pharmacological Approaches
4. Targeted Strategies in PAH Treatment
4.1. Endothelin Receptor Antagonists
4.2. PDE5 Inhibitors
4.3. Prostacyclin Pathway-Targeted Drugs
4.4. Other Strategies
5. Pulmonary Targeting of Nanoparticle Systems for Disease Treatment
5.1. Pulmonary Drug Delivery Route
5.1.1. Inhalation
5.1.2. Intratracheal Injection
5.2. Non-Pulmonary Drug Delivery Route
5.2.1. Intravenous Injection
5.2.2. Intraperitoneal Injection
5.2.3. Oral Administration
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C. Pulmonary arterial hypertension. Orphanet J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, K.K. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir. Med. 2010, 104, 9–21. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsubara, H.; Akagi, S.; Sarashina, T.; Ejiri, K.; Kawakita, N.; Yoshida, M.; Miyoshi, T.; Watanabe, A.; Nishii, N.; et al. Nanoparticle-mediated drug delivery system for pulmonary arterial hypertension. J. Clin. Med. 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Sysol, J.; Machado, R. Classification and pathophysiology of pulmonary hypertension. Contin. Cardiol. Educ. 2018, 4, 2–12. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2015, 37, 67–119. [Google Scholar] [CrossRef]
- Gelzinis, T.A. Pulmonary Hypertension in 2021: Part I—Definition, Classification, Pathophysiology, and Presentation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. Jama 2022, 327, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.C.; DeMarco, T.; Givertz, M.M.; Borlaug, B.A.; Lewis, G.D.; Rame, J.E.; Gomberg-Maitland, M.; Murali, S.; Frantz, R.P.; McGlothlin, D.; et al. World Health Organization Pulmonary Hypertension Group 2: Pulmonary hypertension due to left heart disease in the adult—A summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2012, 31, 913–933. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dorfmüller, P.; Shlobin, O.A.; Ventetuolo, C.E. Group 3 Pulmonary Hypertension: From Bench to Bedside. Circ. Res. 2022, 130, 1404–1422. [Google Scholar] [CrossRef]
- Kim, N.H. Group 4 Pulmonary Hypertension: Chronic Thromboembolic Pulmonary Hypertension: Epidemiology, Pathophysiology, and Treatment. Cardiol. Clin. 2016, 34, 435–441. [Google Scholar] [CrossRef]
- Lahm, T.; Chakinala, M.M. World Health Organization group 5 pulmonary hypertension. Clin. Chest. Med. 2013, 34, 753–778. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef]
- Swinnen, K.; Bijnens, E.; Casas, L.; Nawrot, T.S.; Delcroix, M.; Quarck, R.; Belge, C. Health effects of exposure to residential air pollution in patients with pulmonary arterial hypertension: A cohort study in Belgium. Eur. Respir. J. 2022, 60, 2102335. [Google Scholar] [CrossRef]
- Sofianopoulou, E.; Kaptoge, S.; Gräf, S.; Hadinnapola, C.; Treacy, C.M.; Church, C.; Coghlan, G.; Gibbs, J.S.R.; Haimel, M.; Howard, L.S.; et al. Traffic exposures, air pollution and outcomes in pulmonary arterial hypertension: A UK cohort study analysis. Eur. Respir. J. 2019, 53, 1801429. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.E.; Berger, R.; Haworth, S.G.; Tulloh, R.; Mache, C.J.; Morrell, N.W.; Aldred, M.A.; Trembath, R.C. Transforming Growth Factor-β Receptor Mutations and Pulmonary Arterial Hypertension in Childhood. Circulation 2005, 111, 435–441. [Google Scholar] [CrossRef]
- Austin, E.D.; Ma, L.; LeDuc, C.; Berman Rosenzweig, E.; Borczuk, A.; Phillips, J.A.; Palomero, T.; Sumazin, P.; Kim, H.R.; Talati, M.H.; et al. Whole Exome Sequencing to Identify a Novel Gene (Caveolin-1) Associated With Human Pulmonary Arterial Hypertension. Circ. Cardiovasc. Genet. 2012, 5, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.W.; Kucera, E.T.; Tian, L.; Mellor, N.E.; Dvorina, N.; Baldwin, W.W.; Aldred, M.A.; Farver, C.F.; Comhair, S.A.A.; Aytekin, M.; et al. Bone Morphogenic Protein Type 2 Receptor Mutation-Independent Mechanisms of Disrupted Bone Morphogenetic Protein Signaling in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2016, 55, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Gräf, S.; Haimel, M.; Bleda, M.; Hadinnapola, C.; Southgate, L.; Li, W.; Hodgson, J.; Liu, B.; Salmon, R.M.; Southwood, M.; et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat. Commun. 2018, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Wort, S.J.; Perros, F.; Dorfmüller, P.; Huertas, A.; Montani, D.; Cohen-Kaminsky, S.; Humbert, M. Inflammation in Pulmonary Arterial Hypertension. CHEST 2012, 141, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Savai, R.; Al-Tamari, H.M.; Sedding, D.; Kojonazarov, B.; Muecke, C.; Teske, R.; Capecchi, M.R.; Weissmann, N.; Grimminger, F.; Seeger, W.; et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat. Med. 2014, 20, 1289–1300. [Google Scholar] [CrossRef]
- Shah, A.J.; Beckmann, T.; Vorla, M.; Kalra, D.K. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 5850. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Gaine, S.P. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur. Respir. Rev. 2015, 24, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Nanomedicine-mediated therapeutic approaches for pulmonary arterial hypertension. Drug Discov. Today 2023, 28, 103599. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abou-Saleh, H.; Kameno, Y.; Marei, I.; de Nucci, G.; Ahmetaj-Shala, B.; Shala, F.; Kirkby, N.S.; Jennings, L.; Al-Ansari, D.E.; et al. Studies on metal–organic framework (MOF) nanomedicine preparations of sildenafil for the future treatment of pulmonary arterial hypertension. Sci. Rep. 2021, 11, 4336. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.A.; Basalious, E.B.; ELGazayerly, O.N. Respirable controlled release polymeric colloid (RCRPC) of bosentan for the management of pulmonary hypertension: In vitro aerosolization, histological examination and in vivo pulmonary absorption. Drug Deliv. 2016, 24, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lebron, B.N.; Risbano, M.G. Ambrisentan: A review of its use in pulmonary arterial hypertension. Ther. Adv. Respir. Dis. 2017, 11, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kendre, P.N.; Chaudhari, P.D. Effect of amphiphilic graft co-polymer-carrier on physical stability of bosentan nanocomposite: Assessment of solubility, dissolution and bioavailability. Eur. J. Pharm. Biopharm. 2018, 126, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zancan, L.R.; Bruinsmann, F.A.; Paese, K.; Türck, P.; Bahr, A.; Zimmer, A.; Carraro, C.C.; Schenkel, P.C.; Belló-Klein, A.; Schwertz, C.I.; et al. Oral delivery of ambrisentan-loaded lipid-core nanocapsules as a novel approach for the treatment of pulmonary arterial hypertension. Int. J. Pharm. 2021, 610, 121181. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Patel, B.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Inhaled sildenafil as an alternative to oral sildenafil in the treatment of pulmonary arterial hypertension (PAH). J. Control. Release 2017, 250, 96–106. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Yi, E.-J.; Hwang, K.-M.; Cho, C.-H.; Park, C.-W.; Kim, J.-Y.; Rhee, Y.-S.; Park, E.-S. Formulation and evaluation of carrier-free dry powder inhaler containing sildenafil. Drug Deliv. Transl. Res. 2019, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R. Four-year review of sildenafil citrate. Rev. Urol. 2002, 4 (Suppl. 3), S26–S38. [Google Scholar] [PubMed]
- Bhogal, S.; Khraisha, O.; Al Madani, M.; Treece, J.; Baumrucker, S.J.; Paul, T.K. Sildenafil for Pulmonary Arterial Hypertension. Am. J. Ther. 2019, 26, e520–e526. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, N.; Sharma, R.; Ghadi, R.; Date, T.; Bhargavi, N.; Chaudhari, D.; Katiyar, S.S. Self-nanoemulsifying formulation for oral delivery of sildenafil: Effect on physicochemical attributes and in vivo pharmacokinetics. Drug Deliv. Transl. Res. 2023, 13, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Elbardisy, B.; Boraie, N.; Galal, S. Tadalafil nanoemulsion mists for treatment of pediatric pulmonary hypertension via nebulization. Pharmaceutics 2022, 14, 2717. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, R.; Jiang, X.; Wen, L.; Wu, D.C.; Liu, D.; Yuan, P.; Wang, Y.L.; Jing, Z.C. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc. Res. 2013, 99, 395–403. [Google Scholar] [CrossRef]
- Jing, Z.C.; Yu, Z.X.; Shen, J.Y.; Wu, B.X.; Xu, K.F.; Zhu, X.Y.; Pan, L.; Zhang, Z.L.; Liu, X.Q.; Zhang, Y.S.; et al. Vardenafil in pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 183, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Melian, E.B.; Goa, K.L. Beraprost. Drugs 2002, 62, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef]
- Clapp, L.H.; Finney, P.; Turcato, S.; Tran, S.; Rubin, L.J.; Tinker, A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am. J. Respir. Cell. Mol. Biol. 2002, 26, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Hayashi, E.; Yamamoto, S.; Kobayashi, C.; Tamura, Y.; Sawazaki, R.; Tamura, F.; Tahara, K.; Kasahara, T.; Ishihara, T.; et al. Encapsulation of beraprost sodium in nanoparticles: Analysis of sustained release properties, targeting abilities and pharmacological activities in animal models of pulmonary arterial hypertension. J. Control. Release 2015, 197, 97–104. [Google Scholar] [CrossRef]
- Jain, P.P.; Leber, R.; Nagaraj, C.; Leitinger, G.; Lehofer, B.; Olschewski, H.; Olschewski, A.; Prassl, R.; Marsh, L.M. Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries. Int. J. Nanomed. 2014, 9, 3249–3261. [Google Scholar] [CrossRef]
- Plaunt, A.J.; Islam, S.; Macaluso, T.; Gauani, H.; Baker, T.; Chun, D.; Viramontes, V.; Chang, C.; Corboz, M.R.; Chapman, R.W.; et al. Development and Characterization of Treprostinil Palmitil Inhalation Aerosol for the Investigational Treatment of Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Nossaman, B.; Pankey, E.; Kadowitz, P. Stimulators and activators of soluble guanylate cyclase: Review and potential therapeutic indications. Crit. Care Res. Pract. 2012, 2012, 290805. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Gupta, N.; Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Cocktail of Superoxide Dismutase and Fasudil Encapsulated in Targeted Liposomes Slows PAH Progression at a Reduced Dosing Frequency. Mol. Pharm. 2017, 14, 830–841. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; McMurtry, I.F.; Oka, M.; Nozik-Grayck, E.; Komatsu, M.; Ahsan, F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control. Release 2013, 167, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Renard, S.; Provencher, S.; Bonnet, S. Anti-inflammatory and immunosuppressive agents in PAH. Handb. Exp. Pharmacol. 2013, 218, 437–476. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Mohammed, N.; Gopalan, R.C.; Ayoub, Y.A.; Nasim, M.T.; Assi, K.H. Development and Optimisation of Inhalable EGCG Nano-Liposomes as a Potential Treatment for Pulmonary Arterial Hypertension by Implementation of the Design of Experiments Approach. Pharmaceutics 2023, 15, 539. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, X.; Cao, G.; Liu, Y.; Liu, C.; Sun, H.; Pang, X. Intratracheal instillation of ethyl pyruvate nanoparticles prevents the development of shunt-flow-induced pulmonary arterial hypertension in a rat model. Int. J. Nanomed. 2016, 11, 2587–2599. [Google Scholar] [CrossRef]
- Kuang, T.; Wang, J.; Pang, B.; Huang, X.; Burg, E.D.; Yuan, J.X.; Wang, C. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm. Pharmacol. Ther. 2010, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Usta, D.Y.; Timur, B.; Teksin, Z.S. Formulation development, optimization by Box-Behnken design, characterization, in vitro, ex-vivo, and in vivo evaluation of bosentan-loaded self-nanoemulsifying drug delivery system: A novel alternative dosage form for pulmonary arterial hypertension treatment. Eur. J. Pharm. Sci. 2022, 174, 106159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, W.; Ye, L.; Zhu, Y.; Tian, Y.; Chen, L.; Yang, J.; Miao, M.; Shi, Y.; Azevedo, H.S.; et al. Targeted Delivery of Sildenafil for Inhibiting Pulmonary Vascular Remodeling. Hypertension 2019, 73, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Hefnawy, A.; Almotairy, A.; Alobaida, A.; Alyahya, M.; Althobaiti, A.; Adel Ali Youssef, A.; Elkanayati, R.M.; Ashour, E.A.; Smyth, H.D.C.; et al. Formulation and evaluation of inhaled Sildenafil-loaded PLGA microparticles for treatment of pulmonary arterial hypertension (PAH): A novel high drug loaded formulation and scalable process via hot melt extrusion technology (Part I). Int. J. Pharm. 2024, 655, 124044. [Google Scholar] [CrossRef]
- Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Inhaled combination of sildenafil and rosiglitazone improves pulmonary hemodynamics, cardiac function, and arterial remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L119–L130. [Google Scholar] [CrossRef]
- Nakamura, K.; Akagi, S.; Ejiri, K.; Yoshida, M.; Miyoshi, T.; Toh, N.; Nakagawa, K.; Takaya, Y.; Matsubara, H.; Ito, H. Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 5885. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Nakamura, K.; Matsubara, H.; Kondo, M.; Miura, D.; Matoba, T.; Egashira, K.; Ito, H. Intratracheal Administration of Prostacyclin Analogue-incorporated Nanoparticles Ameliorates the Development of Monocrotaline and Sugen-Hypoxia-induced Pulmonary Arterial Hypertension. J. Cardiovasc. Pharmacol. 2016, 67, 290–298. [Google Scholar] [CrossRef]
- Liu, A.; Li, B.; Yang, M.; Shi, Y.; Su, J. Targeted treprostinil delivery inhibits pulmonary arterial remodeling. Eur. J. Pharmacol. 2022, 923, 174700. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Alobaida, A.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. CAR, a homing peptide, prolongs pulmonary preferential vasodilation by increasing pulmonary retention and reducing systemic absorption of liposomal fasudil. Mol. Pharm. 2019, 16, 3414–3429. [Google Scholar] [CrossRef]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD packaged in peptide-studded-liposomes: Properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Patel, B.; Nahar, K.; Ahsan, F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur. J. Pharm. Biopharm. 2014, 88, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Drug Delivery to the lungs: Challenges and Opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Yıldız-Peköz, A.; Ehrhardt, C. Advances in Pulmonary Drug Delivery. Pharmaceutics 2020, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Patwa, A.; Shah, A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J. Anaesth. 2015, 59, 533–541. [Google Scholar] [CrossRef]
- Scheuch, G.; Kohlhaeufl, M.J.; Brand, P.; Siekmeier, R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv. Drug Deliv. Rev. 2006, 58, 996–1008. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, R.; Zhou, J.; Mao, S. Recent advances in controlled pulmonary drug delivery. Drug Discov. Today 2015, 20, 380–389. [Google Scholar] [CrossRef]
- Bisserier, M.; Mathiyalagan, P.; Zhang, S.; Elmastour, F.; Dorfmüller, P.; Humbert, M.; David, G.; Tarzami, S.; Weber, T.; Perros, F. Regulation of the methylation and expression levels of the BMPR2 gene by SIN3a as a novel therapeutic mechanism in pulmonary arterial hypertension. Circulation 2021, 144, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tai, Y.-Y.; Tang, Y.; Zhao, J.; Negi, V.; Culley, M.K.; Pilli, J.; Sun, W.; Brugger, K.; Mayr, J. BOLA (BolA Family Member 3) deficiency controls endothelial metabolism and glycine homeostasis in pulmonary hypertension. Circulation 2019, 139, 2238–2255. [Google Scholar] [CrossRef]
- Eid, H.M.; Turkia, T.H.; Ali, A.A.; Aboud, H.M. A Novel Chitosan-coated Leciplex Loaded with Ambrisentan as a Possible Pulmonary Nanosystem: Optimization, Characterization, and Pharmacokinetics Assessments. J. Pharm. Sci. 2024, 113, 2320–2330. [Google Scholar] [CrossRef]
- Ferguson, S.K.; Pak, D.I.; Hopkins, J.L.; Harral, J.W.; Redinius, K.M.; Loomis, Z.; Stenmark, K.R.; Borden, M.A.; Schroeder, T.; Irwin, D.C. Pre-clinical assessment of a water-in-fluorocarbon emulsion for the treatment of pulmonary vascular diseases. Drug Deliv. 2019, 26, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Rashid, J.; Absar, S.; Al-Saikhan, F.I.; Ahsan, F. Liposomal aerosols of nitric oxide (NO) donor as a long-acting substitute for the ultra-short-acting inhaled NO in the treatment of PAH. Pharm. Res. 2016, 33, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Muenster, U.; Zhao, J.; Zhang, L.; Becker-Pelster, E.-M.; Rosenbruch, M.; Mao, S. Exploring polyvinylpyrrolidone in the engineering of large porous PLGA microparticles via single emulsion method with tunable sustained release in the lung: In vitro and in vivo characterization. J. Control. Release 2017, 249, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Pai, S.B.; Bellamkonda, R.V.; Thompson, D.H.; Singh, J. Cerivastatin nanoliposome as a potential disease modifying approach for the treatment of pulmonary arterial hypertension. J. Pharmacol. Exp. Ther. 2018, 366, 66–74. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, L.-Z.; Pan, J.-A.; Gao, Q.; Zhang, J.-F.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z.; Zhang, H.-L. Microfluidic fabrication of inhalable large porous microspheres loaded with H2S-releasing aspirin derivative for pulmonary arterial hypertension therapy. J. Control. Release 2021, 329, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Ntokou, A.; Dave, J.M.; Kauffman, A.C.; Sauler, M.; Ryu, C.; Hwa, J.; Herzog, E.L.; Singh, I.; Saltzman, W.M.; Greif, D.M. Macrophage-derived PDGF-B induces muscularization in murine and human pulmonary hypertension. JCI Insight 2021, 6, e139067. [Google Scholar] [CrossRef]
- Kimura, S.; Egashira, K.; Chen, L.; Nakano, K.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Hara, K.; Morishita, R.; Sueishi, K. Nanoparticle-mediated delivery of nuclear factor κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009, 53, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Vinjamuri, B.P.; Mahmoud, A.A.; Shamma, R.N.; Mansour, S.M.; Ammar, H.O.; Ghorab, M.M.; Chougule, M.B.; Chablani, L. Design and evaluation of novel inhalable sildenafil citrate spray-dried microparticles for pulmonary arterial hypertension. J. Control. Release 2019, 302, 126–139. [Google Scholar] [CrossRef]
- Shahin, H.; Vinjamuri, B.P.; Mahmoud, A.A.; Mansour, S.M.; Chougule, M.B.; Chablani, L. Formulation and optimization of sildenafil citrate-loaded PLGA large porous microparticles using spray freeze-drying technique: A factorial design and in-vivo pharmacokinetic study. Int. J. Pharm. 2021, 597, 120320. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ji, H.; Li, Z.; Qiao, W.; Wang, C.; Tang, J. In vivo pharmacokinetics and in vitro release of imatinib mesylate-loaded liposomes for pulmonary delivery. Int. J. Nanomed. 2021, 16, 1221–1229. [Google Scholar] [CrossRef]
- Rashid, J.; Alobaida, A.; Al-Hilal, T.A.; Hammouda, S.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. Repurposing rosiglitazone, a PPAR-γ agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH. J. Control. Release 2018, 280, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rawat, A.; Ahsan, F. Feasibility study of aerosolized prostaglandin E1 microspheres as a noninvasive therapy for pulmonary arterial hypertension. J. Pharm. Sci. 2010, 99, 1774–1789. [Google Scholar] [CrossRef]
- Gupta, V.; Davis, M.; Hope-Weeks, L.J.; Ahsan, F. PLGA microparticles encapsulating prostaglandin E 1-hydroxypropyl-β-cyclodextrin (PGE 1-HPβCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm. Res. 2011, 28, 1733–1749. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, Y.; Yan, F. Exosomes enriched by miR-429-3p derived from ITGB1 modified Telocytes alleviates hypoxia-induced pulmonary arterial hypertension through regulating Rac1 expression. Cell Biol. Toxicol. 2024, 40, 32. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ibrahim, H.M.; Ahsan, F. Peptide–micelle hybrids containing fasudil for targeted delivery to the pulmonary arteries and arterioles to treat pulmonary arterial hypertension. J. Pharm. Sci. 2014, 103, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Patel, B.; Ahsan, F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: Preparation and characterization. Pharm. Res. 2014, 31, 1553–1565. [Google Scholar] [CrossRef]
- Nahar, K.; Absar, S.; Patel, B.; Ahsan, F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int. J. Pharm. 2014, 464, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Absar, S.; Gupta, N.; Kotamraju, V.R.; McMurtry, I.F.; Oka, M.; Komatsu, M.; Nozik-Grayck, E.; Ahsan, F. Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol. Pharm. 2014, 11, 4374–4384. [Google Scholar] [CrossRef]
- Rashid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiao, W.; Wang, C.; Wang, H.; Ma, M.; Han, X.; Tang, J. DPPC-coated lipid nanoparticles as an inhalable carrier for accumulation of resveratrol in the pulmonary vasculature, a new strategy for pulmonary arterial hypertension treatment. Drug Deliv. 2020, 27, 736–744. [Google Scholar] [CrossRef]
- Sun, C.-K.; Zhen, Y.-Y.; Lu, H.-I.; Sung, P.-H.; Chang, L.-T.; Tsai, T.-H.; Sheu, J.-J.; Chen, Y.-L.; Chua, S.; Chang, H.-W. Reducing TRPC1 Expression through Liposome-Mediated siRNA Delivery Markedly Attenuates Hypoxia-Induced Pulmonary Arterial Hypertension in a Murine Model. Stem Cells Int. 2014, 2014, 316214. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.A.; Wertheim, B.M.; Xiao, W.; Loscalzo, J.; Zhang, Y.Y. Nanoparticle delivery of VEGF and SDF-1α as an approach for treatment of pulmonary arterial hypertension. Pulm. Circ. 2024, 14, e12412. [Google Scholar] [CrossRef] [PubMed]
- Sorino, C.; Negri, S.; Spanevello, A.; Visca, D.; Scichilone, N. Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur. J. Intern Med. 2020, 75, 15–18. [Google Scholar] [CrossRef]
- Mehta, P.P.; Ghoshal, D.; Pawar, A.P.; Kadam, S.S.; Dhapte-Pawar, V.S. Recent advances in inhalable liposomes for treatment of pulmonary diseases: Concept to clinical stance. J. Drug Deliv. Sci. Technol. 2020, 56, 101509. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Young, P.M. Liposomes for Inhalation. J. Aerosol. Med. Pulm. Drug Deliv. 2024, 37, 100–110. [Google Scholar] [CrossRef]
- Zangiabadian, M.; Malekshahian, D.; Arabpour, E.; Abadi, S.S.D.; Yazarlou, F.; Bostanghadiri, N.; Centis, R.; Aghababa, A.A.; Farahbakhsh, M.; Nasiri, M.J.; et al. Amikacin liposome and Mycobacterium avium complex: A systematic review. PLoS ONE 2022, 17, e0279714. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Basurto, D.; Eedara, B.B.; Mansour, H.M. Biocompatible biodegradable polymeric nanocarriers in dry powder inhalers (DPIs) for pulmonary inhalation delivery. J. Pharm. Investig. 2024, 54, 145–160. [Google Scholar] [CrossRef]
- Zendehdel Baher, S.; Yaqoubi, S.; Asare-Addo, K.; Hamishehkar, H.; Nokhodchi, A. Dry Powder Formulation of Simvastatin Nanoparticles for Potential Application in Pulmonary Arterial Hypertension. Pharmaceutics 2022, 14, 895. [Google Scholar] [CrossRef]
- Paranjpe, M.; Finke, J.H.; Richter, C.; Gothsch, T.; Kwade, A.; Büttgenbach, S.; Müller-Goymann, C.C. Physicochemical characterization of sildenafil-loaded solid lipid nanoparticle dispersions (SLN) for pulmonary application. Int. J. Pharm. 2014, 476, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Liu, J.; Shi, L.; Zhang, T.; Liu, J.; Qiu, S.; Ruiz, M.; Dupuis, J.; Zhu, L.; Wang, L.; et al. Au-modified ceria nanozyme prevents and treats hypoxia-induced pulmonary hypertension with greatly improved enzymatic activity and safety. J. Nanobiotechnol. 2024, 22, 492. [Google Scholar] [CrossRef]
- Wang, Z.; Cuddigan, J.L.; Gupta, S.K.; Meenach, S.A. Nanocomposite microparticles (nCmP) for the delivery of tacrolimus in the treatment of pulmonary arterial hypertension. Int. J. Pharm. 2016, 512, 305–313. [Google Scholar] [CrossRef]
- Teymouri Rad, R.; Dadashzadeh, S.; Vatanara, A.; Alavi, S.; Ghasemian, E.; Mortazavi, S.A. Tadalafil nanocomposites as a dry powder formulation for inhalation, a new strategy for pulmonary arterial hypertension treatment. Eur. J. Pharm. Sci. 2019, 133, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Honmane, S.; Hajare, A.; More, H.; Osmani, R.A.M.; Salunkhe, S. Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy. J. Liposome Res. 2019, 29, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Leal, J.; Soto, M.R.; Smyth, H.D.C.; Ghosh, D. Aerosolizable Lipid Nanoparticles for Pulmonary Delivery of mRNA through Design of Experiments. Pharmaceutics 2020, 12, 1042. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, L. Passive lung-targeted drug delivery systems via intravenous administration. Pharm. Dev. Technol. 2014, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, X.; Yang, Z.; Zhao, J.; Wang, X.; Zhang, Q.; Yuan, M.; Xie, L.; Liu, H.; He, Q. The Potential Efficacy of R8-Modified Paclitaxel-Loaded Liposomes on Pulmonary Arterial Hypertension. Pharm. Res. 2013, 30, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, X.; Lou, H.; Wang, Z.; Xiang, S.; Qiu, Y.; Hu, F.; Yu, F.; Yuan, H. Active Anchoring Stimuli-Responsive Nano-Craft to Relieve Pulmonary Vasoconstriction by Targeting Smooth Muscle Cell for Hypoxic Pulmonary Hypertension Treatment. Adv. Healthc. Mater. 2024, 13, 2400113. [Google Scholar] [CrossRef]
- Dunn, A.W.; Kalinichenko, V.V.; Shi, D. Highly Efficient In Vivo Targeting of the Pulmonary Endothelium Using Novel Modifications of Polyethylenimine: An Importance of Charge. Adv. Healthc. Mater. 2018, 7, 1800876. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-J.; Hsu, H.-H.; Chang, G.-J.; Lin, S.-H.; Chen, W.-J.; Huang, C.-C.; Pang, J.-H.S. Prostaglandin E1 Attenuates Pulmonary Artery Remodeling by Activating Phosphorylation of CREB and the PTEN Signaling Pathway. Sci. Rep. 2017, 7, 9974. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef]
- Shewale, H.; Kanugo, A. Formulation Optimization and Evaluation of Patented Solid Lipid Nanoparticles of Ambrisentan for Pulmonary Arterial Hypertension. Recent Pat. Nanotechnol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Jing, Z.C. The limits of oral therapy in pulmonary arterial hypertension management. Ther. Clin. Risk Manag. 2015, 11, 1731–1741. [Google Scholar] [CrossRef]
- Abrahim-Vieira, B.A.; Souza, A.M.T.; Barros, R.C.; Carmo, F.A.D.; Abreu, L.C.L.; Moreira, R.S.S.; HonÓrio, T.S.; Rodrigues, C.R.; Sousa, V.P.; Cabral, L.M. In Silico studies of novel Sildenafil self-emulsifying drug delivery system absorption improvement for pulmonary arterial hypertension. An. Acad. Bras. Ciências 2020, 92, e20191445. [Google Scholar] [CrossRef] [PubMed]
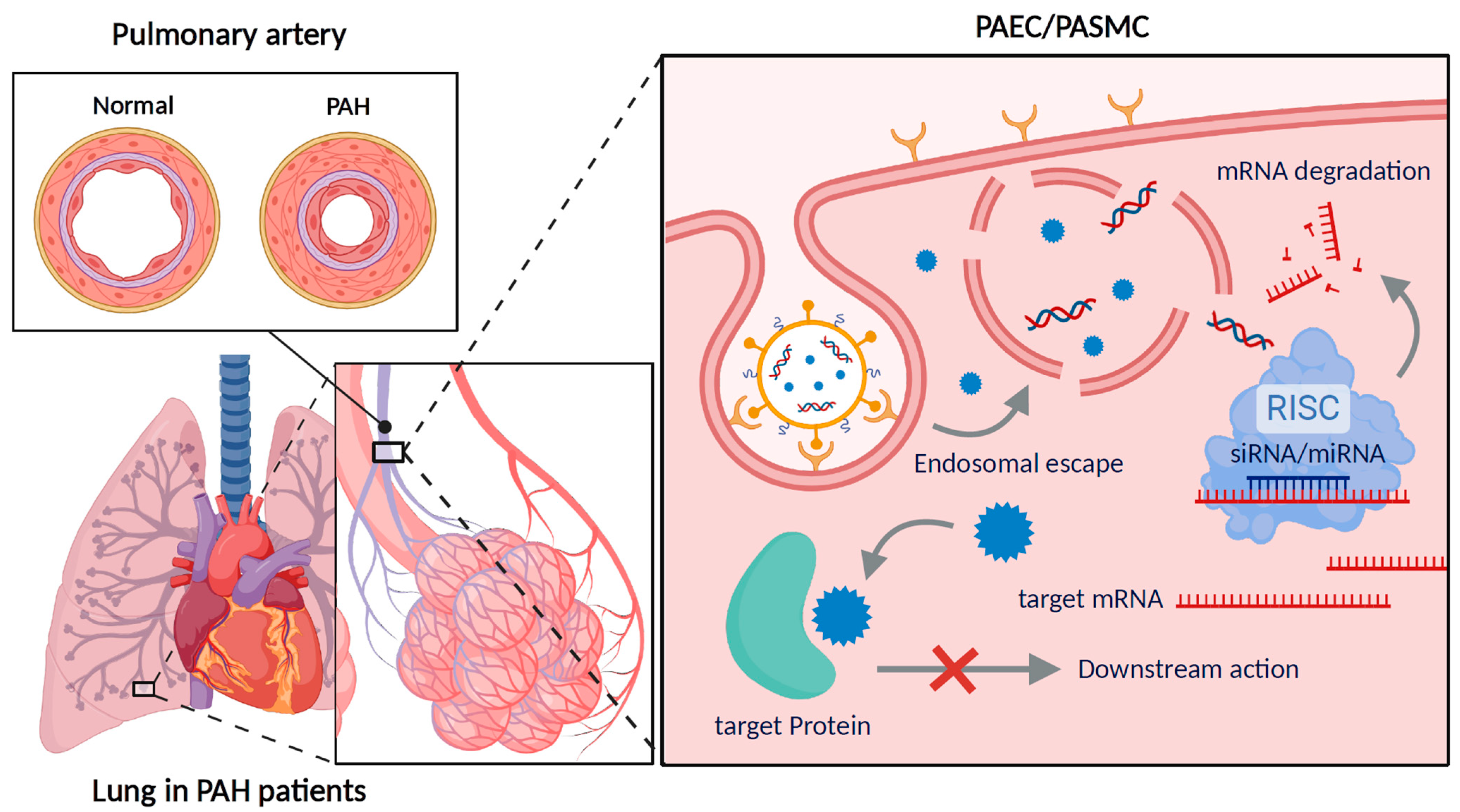
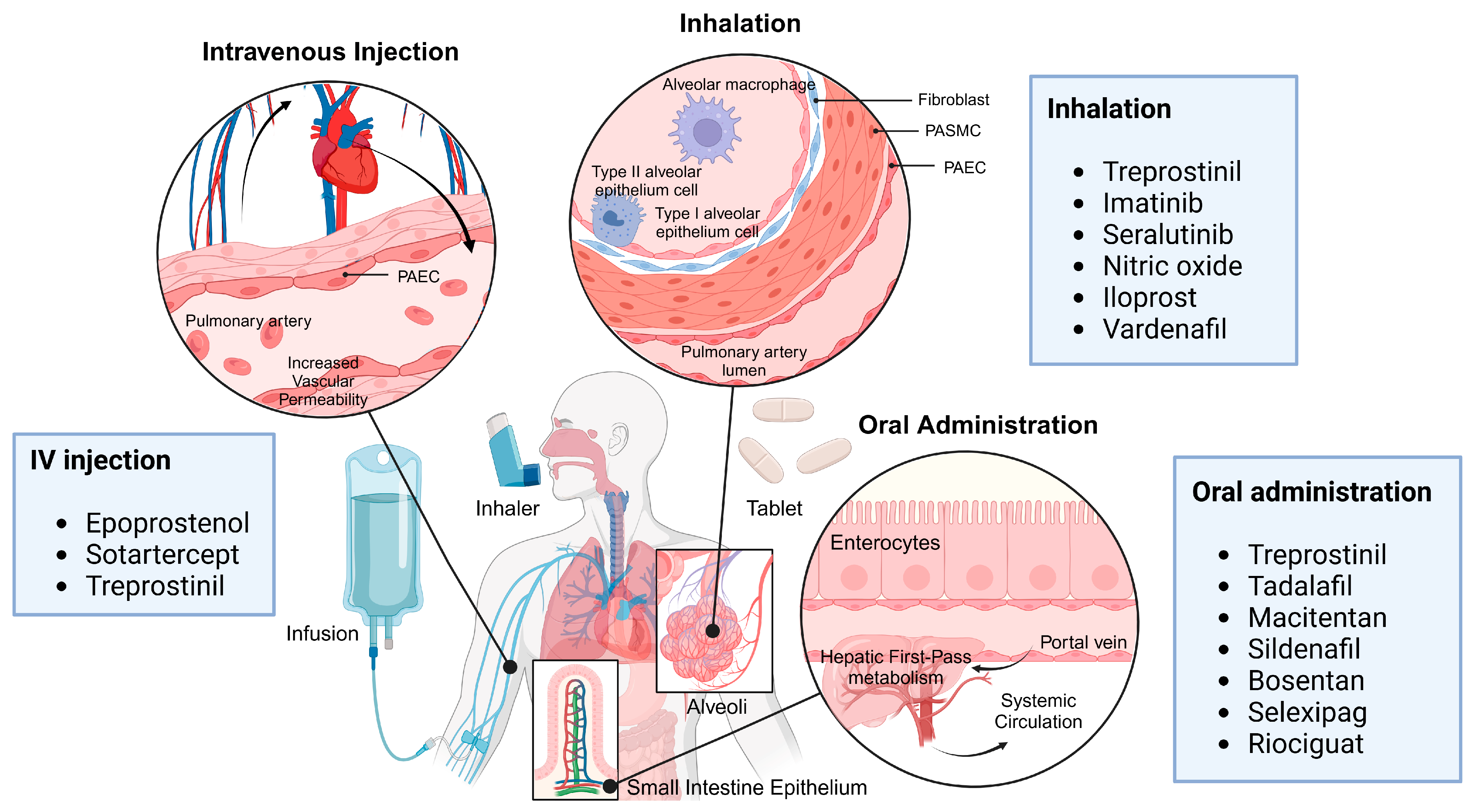
| Drug Class | Drug | Mode of Action |
|---|---|---|
| Endothelin receptor antagonists | Bosentan monohydrate | Reduces the pathogenic effects of elevated endothelin-1 in PAH |
| Macitentan | Reduces vasoconstriction and smooth muscle cell proliferation in pulmonary arteries | |
| Ambrisentan | Inhibits vasoconstriction and cell proliferation while preserving ETB receptor-mediated vasodilation and ET-1 clearance | |
| PDE5 inhibitor | Sildenafil citrate | Increases cGMP levels in pulmonary vascular smooth muscle cells, inducing vasodilation of the pulmonary vascular bed |
| Tadalafil | Increases cGMP levels to enhance smooth muscle relaxation, reducing pulmonary arterial pressure and vascular remodeling in PAH | |
| Vardenafil hydrochloride | Increases cGMP levels to promote smooth muscle relaxation and vasodilation in the pulmonary vasculature in PAH | |
| Prostacyclin analog | Epoprostenol sodium | Activates G protein-coupled receptors to increase cAMP, inhibiting platelet aggregation and inducing vasodilation |
| Iloprost tromethamine | Mimics prostacyclin to induce vasodilation, reduce oxidative stress, and protect endothelial and mitochondrial function in PAH | |
| Treprostinil | Activates prostacyclin receptors to increase cAMP, inducing vasodilation, inhibiting platelet aggregation, and reducing inflammation in PAH | |
| Selective IP receptor agonist | Selexipag | Promotes vasodilation, inhibits vascular smooth muscle proliferation, and reduces inflammation in PAH |
| Soluble guanylate cyclase stimulator | Riociguat | Increases cGMP generation to enhance vasodilation and reduce vascular remodeling in PAH |
| Activin receptor type IIA-Fc fusion protein | Sotatercept | Acts as a ligand trap for activin-class ligands to restore BMP signaling and reduce pulmonary vascular remodeling in PAH |
| Target | Therapeutic Cargo | Therapeutic Strategy | Core Materials for Carriers | Route | Animal Model | Ref. |
|---|---|---|---|---|---|---|
| BMPR2 | SIN3a gene | Increase BMPR2 expression in the epigenetic pathway to overcome BMPR2 silencing caused by methylation | Adeno-associated virus serotype1 (AAV1) | Intratracheal (aerosol inhalation) | Sprague–Dawley rat | [69] |
| BOLA3 | BOLA3 gene | Induce the expression of BOLA3, which plays a key role in PH pathogenesis | Lentiviral vector | Orotracheal instillation | C57BL/6 mice, Sprague–Dawley rat | [70] |
| Endothelin receptor | Ambrisentan | Prevent or reverse histological change caused by elevated levels of ET1, treatment of PAH | Chitosan | Intratracheal | n.a. | [71] |
| Ambrisentan, NaNO2 | Block the vasoconstrictive response in blood vessels, act as a pulmonary vasodilator | Perfluorooctyl bromide emulsion | Intratracheal | Sprague–Dawley rat | [72] | |
| Bosentan | Prevent or reverse histological change caused by elevated levels of ET1, treatment of PAH | PLGA | Intratracheal | Wistar Albino rat | [27] | |
| Guanylate cyclase | Diethylenetriamine NONOate | Activate guanylate cyclase to produces vasodilation and reduce smooth muscle cell proliferation | Liposome (PEGylated) | Intratracheal | Rat | [73] |
| Cinaciguat | Activate soluble guanylate cyclase (increase vasodilation of pulmonary arteries), decrease pulmonary arterial blood pressure | PLGA, polyvinyl pyrrolidone (PVP) | Intratracheal (dry powder insufflator) | Sprague–Dawley rat, Mini-Pig | [74] | |
| HMG-CoA reductase | Cerivastatin | Inhibit smooth muscle cell proliferation, improve endothelial function, reduce inflammation and oxidative stress | Liposomes | Inhalation | Sprague–Dawley rat | [75] |
| IκB | H2S-releasing asprin derivative (ACS14) | Inhibit the EndMT process by suppressing IκB degradation and NF-κB activation | PLGA | Intratracheal | Sprague–Dawley rat | [76] |
| Pdgfb | Pdgfb siRNA | Prevent hypoxia-induced distal pulmonary arteriole muscularization, PH, and RVH | PPMS | Orotracheal instillation | C57BL/6 mice | [77] |
| Nuclear factor κB | Decoy oligonucleotide | Attenuate inflammation, proliferation, development of PAH and pulmonary arterial remodeling | PEG-PLGA | Intratracheal instillation | Rat | [78] |
| PDE5 | Tadalafil | Increase level of cGMP and nitric oxide in pulmonary vasculature to reduce pulmonary arterial pressure | Nanoemulsion | Orotracheal instillation | Sprague–Dawley rat | [36] |
| Sildenafil | PLGA | Intratracheal | Sprague–Dawley rat | [31] | ||
| Carboxymethyl cellulose/sodium alginate hydrogel microparticle | Intratracheal | Albino rat | [79] | |||
| PLGA | Intratracheal | Albino rat | [80] | |||
| PDE5, PPAR-γ | Sildenafil, Rosiglitazone | Increase level of cGMP and nitric oxide in pulmonary vasculature to reduce pulmonary arterial pressure. inhibit PASMC proliferation by modulating cell growth and apoptosis | PLGA | Intratracheal | Sprague–Dawley rat | [56] |
| PDGF-receptor tyrosine kinase | Imatinib | Reverse pulmonary vascular remodeling, anti-proliferative and pro-apoptotic effects | Liposomes | Intratracheal | Sprague–Dawley rat | [81] |
| PPAR-γ | Rosiglitazone | Inhibit PASMC proliferation by modulating cell growth and apoptosis | PLGA | Intratracheal | Sprague–Dawley rat | [82] |
| Prostaglandin E receptors | Prostaglandin E1 | Vasodilatory, anti-inflammatory, anti-aggregatory, and anti-proliferative properties | PLGA | Intratracheal (aerosol inhalation) | Sprague–Dawley rat | [83] |
| PLGA | Intratracheal | Sprague–Dawley rat | [84] | |||
| Rac1 | miR-429-3p | Inhibit proliferation and migration of PASMCs, vascular remodeling of pulmonary arterial walls observed in PAH | Exosomes | Intratracheal | C57BL/6 mice | [85] |
| Rho-kinase | Fasudil | Dilate pulmonary arteries and arterioles, reduces arterial remodeling, prolonged pulmonary preferential vasodilation | Liposomes | Intratracheal | Sprague–Dawley rat | [48] |
| Peptide–micelle hybrid particle | Intratracheal | Rat | [86] | |||
| Nanoerythrosomes | Intratracheal | Sprague–Dawley rat | [87] | |||
| Starch-coated magnetic liposomes (PEGylated) | Intratracheal | Sprague–Dawley rat | [88] | |||
| Nanoerythrosomes | Intratracheal | Sprague–Dawley rat | [62] | |||
| Liposome (PEGylated) | Intratracheal | Sprague–Dawley rat | [89] | |||
| CAR-liposomes (PEGylated) | Intratracheal | Sprague–Dawley rat | [90] | |||
| Liposome (PEGylated) | Intratracheal, intravenous | Sprague–Dawley rat | [60] | |||
| Fasudil, SOD | Liposome (PEGylated) | Intratracheal | Sprague–Dawley rat | [61] | ||
| ROS, HMGB1 | Ethyl pyruvate | Decreased levels of HMGB1, IL-6, TNFα, reactive oxygen species, and ET1 in lung improve pulmonary arterial remodeling | PEG-PLGA | Intratracheal | Sprague–Dawley rat | [51] |
| SIRT1 | Resveratrol | Increased PASMC apoptosis to attenuate pulmonary arterial remodeling and the alleviation of PAH | Lipid nanoparticles | Intratracheal | Sprague–Dawley rat | [91] |
| TRPC1 | TRPC1 siRNA | Attenuate PAH-associated RV and pulmonary arteriolar remodeling | Liposomes | Intratracheal | C57BL/6 mice | [92] |
| VEGFR2 | VEGF, SDF | Block VEGF signaling to facilitate utilizing extra-pulmonary progenitor cells for pulmonary endothelial repair and delayed the thickening of distal pulmonary vessels | Chitosan | Intratracheal (aerosol inhalation) | Athymic nude rat, Sprague–Dawley rat | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Choi, Y.H.; Min, J.-Y.; Lee, J.; Shim, G. Fundamental and Targeted Approaches in Pulmonary Arterial Hypertension Treatment. Pharmaceutics 2025, 17, 224. https://doi.org/10.3390/pharmaceutics17020224
Park JS, Choi YH, Min J-Y, Lee J, Shim G. Fundamental and Targeted Approaches in Pulmonary Arterial Hypertension Treatment. Pharmaceutics. 2025; 17(2):224. https://doi.org/10.3390/pharmaceutics17020224
Chicago/Turabian StylePark, Ji Su, Yong Hwan Choi, Ji-Young Min, Jaeseong Lee, and Gayong Shim. 2025. "Fundamental and Targeted Approaches in Pulmonary Arterial Hypertension Treatment" Pharmaceutics 17, no. 2: 224. https://doi.org/10.3390/pharmaceutics17020224
APA StylePark, J. S., Choi, Y. H., Min, J.-Y., Lee, J., & Shim, G. (2025). Fundamental and Targeted Approaches in Pulmonary Arterial Hypertension Treatment. Pharmaceutics, 17(2), 224. https://doi.org/10.3390/pharmaceutics17020224








