Multivariate Data Analysis to Assess Process Evolution and Systematic Root Causes Investigation in Tablet Manufacturing at an Industrial Scale—A Case Study Focused on Improving Tablet Hardness
Abstract
1. Introduction
2. Materials and Methods
2.1. Product Summary
2.2. Process Summary
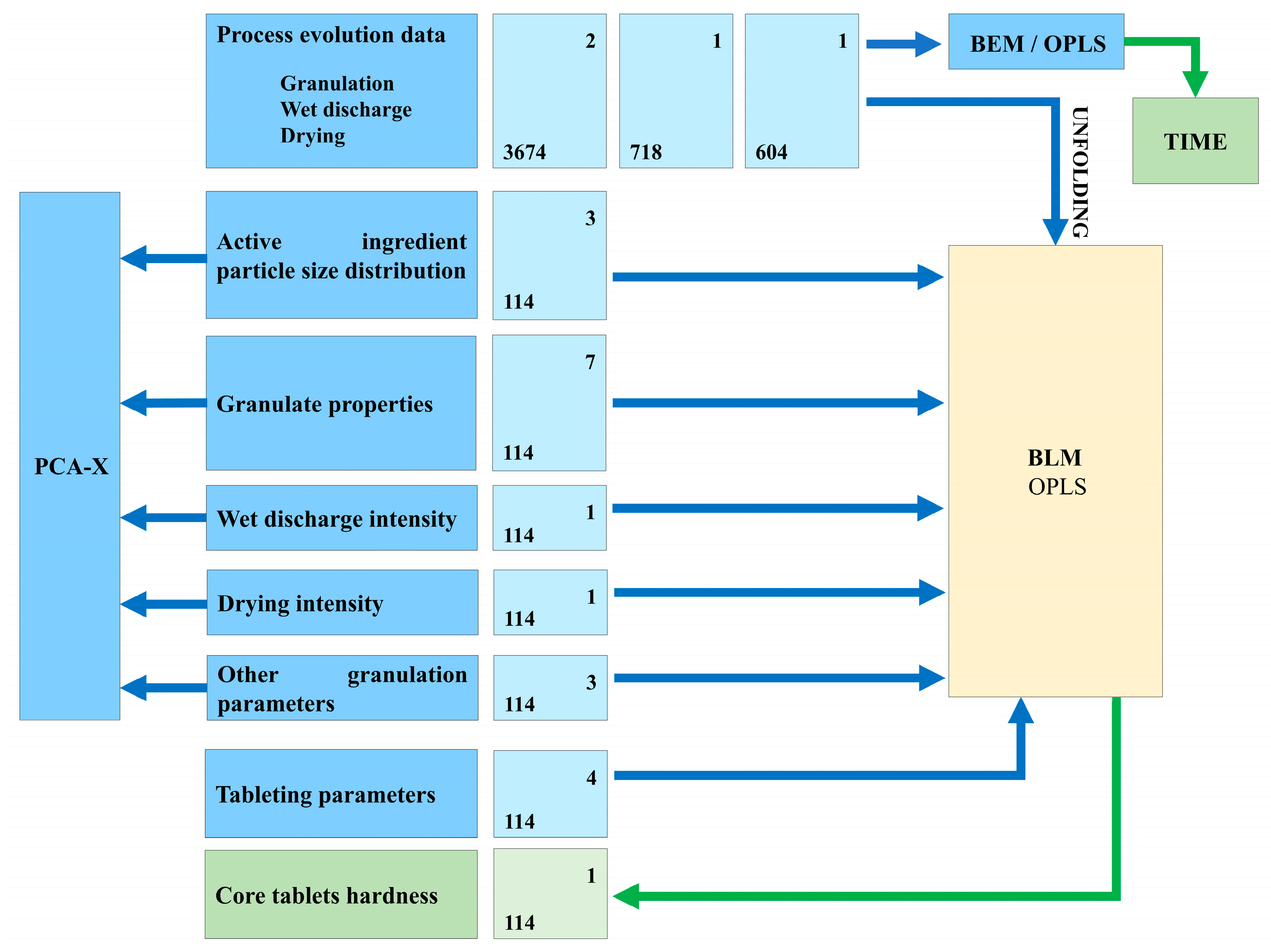
2.3. Data Collection and Organization
- Batch conditions data (one row per batch): active ingredient batch particle size distribution, granulation water quantity, granulation nozzle type, wet discharge intensity, wet discharge time, drying intensity, inlet air humidity, average tableting speed, average feeder speed, and average compression force.
- Batch evolution data (multiple rows per batch): process variables recorded at regular time points during granulation, wet discharge, and drying.
- Product quality data (one row per batch): granule and tablet quality attributes.
2.4. Data Analysis
2.5. Effectiveness Check of Improvement Actions
- 1.
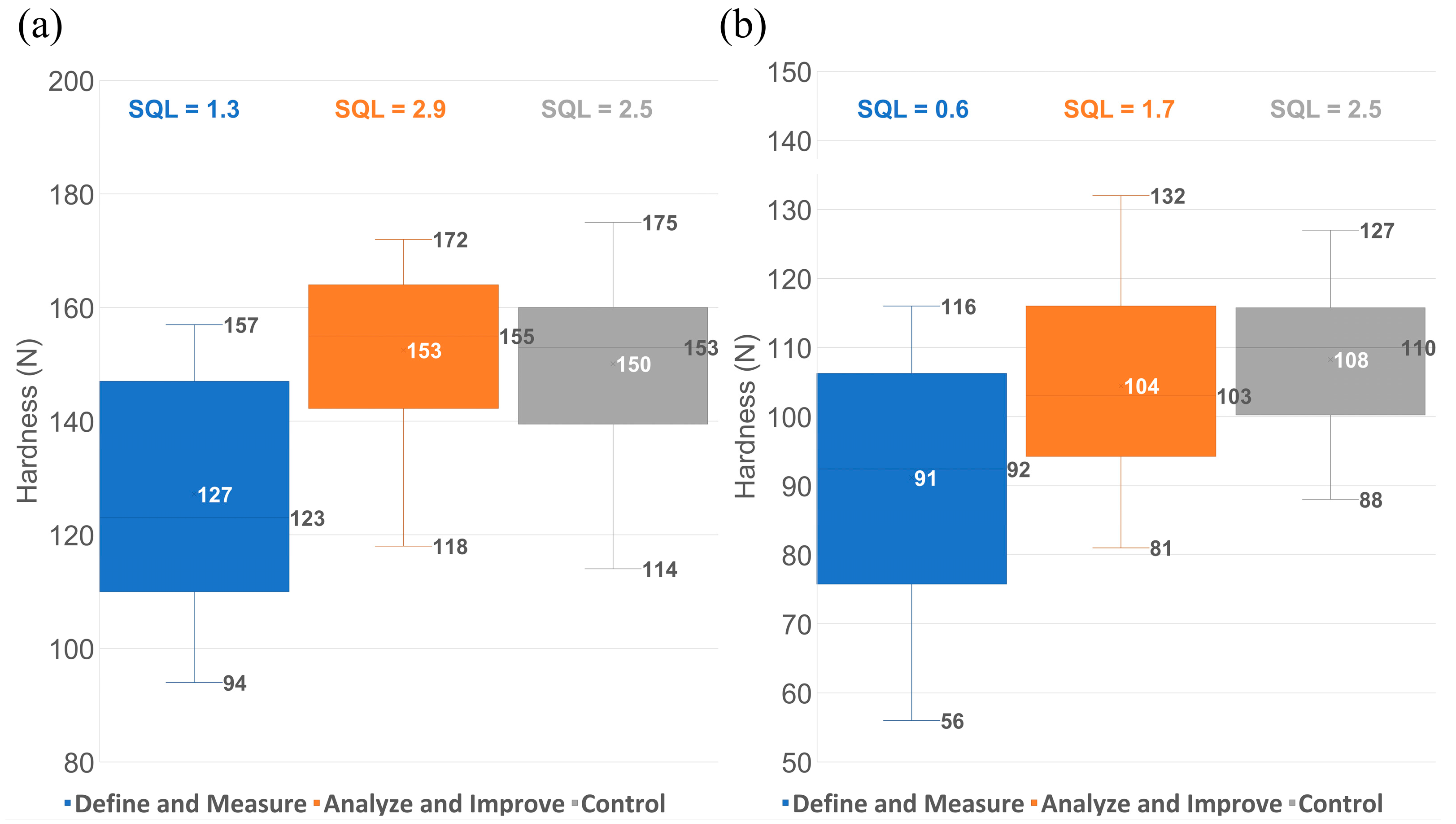
- Define and Measure phases: batches belonging to campaigns from 2 to 5, where the increased hardness variability and tendency for results close to the lower limit and below it was observed; these were the batches that defined the problem statement and triggered the extensive investigation.
- 2.
- Analyze and Improve phases: batches belonging to campaigns from 6 to 14, where improvement actions were gradually implemented based on the outcome of the statistical analysis performed.
- 3.
- Control phase: additional 46 batches not included in the initial models; part of campaigns from 15 to 19 where all improvement actions were in place.
3. Results and Discussions
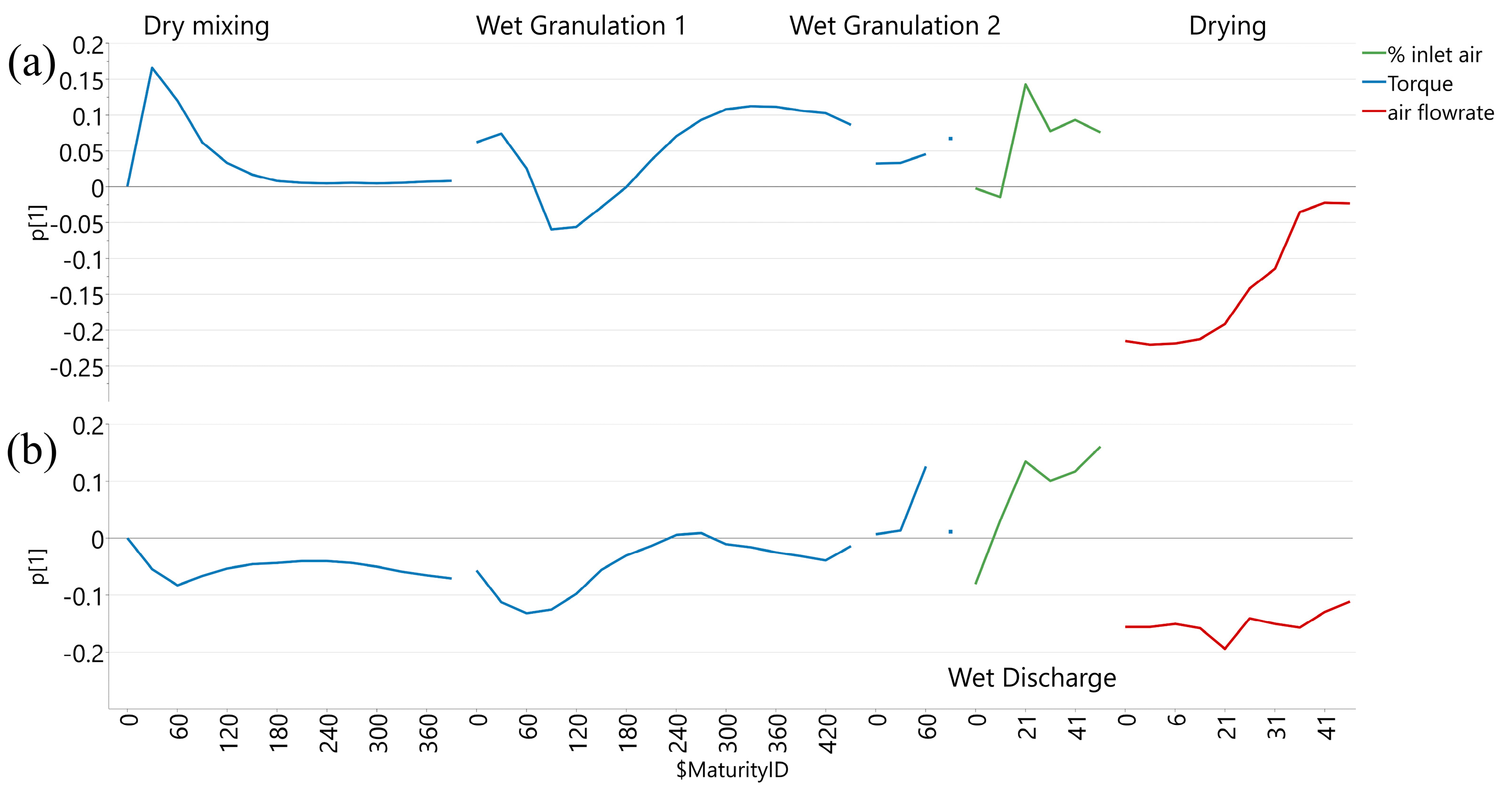
3.1. Influence of Process Evolution on Core Tablet Hardness
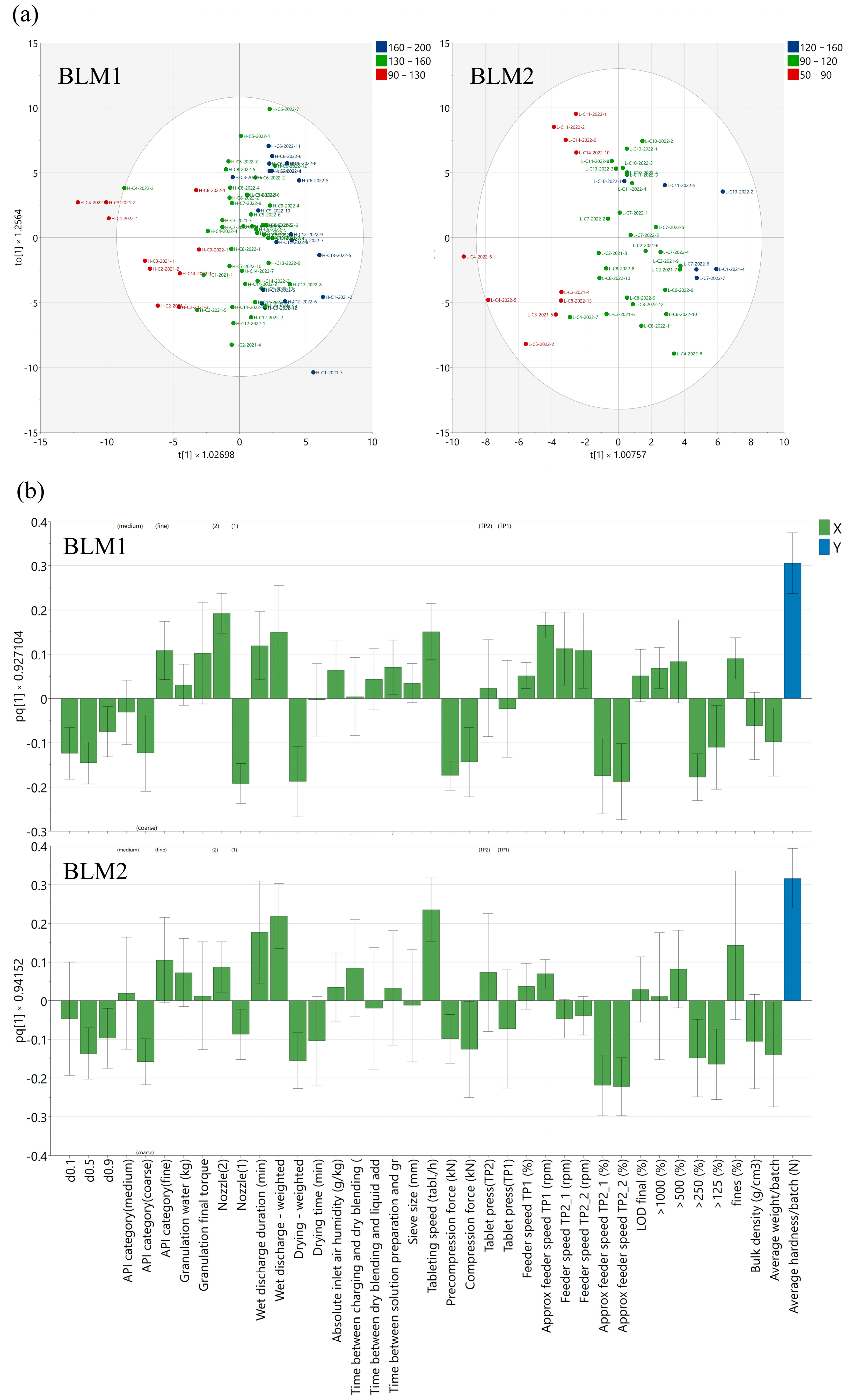
3.2. Influences of Active Ingredient, Granulation Conditions, and Intermediate Product Properties on Core Tablet Hardness
3.3. Influences of Tableting Process on Core Tablet Hardness
3.4. In-Depth Analysis of Batch Groups Based on Most Significant Process Conditions
3.5. Long-Term Effectiveness of the Proposed Control Strategy
- Waste reduction:
- ○
- Fewer product losses during the prolonged set-up needed for lower hardness batches.
- ○
- Significantly reduced risk of eroded tablets after film-coating, leading to visual sorting for the removal of defective tablets
- ○
- Significantly reduced risk of broken tablets during packaging caused by the low hardness
- Reduction in inter-batch variability through defining more standardized processing conditions.
- Process efficiency improvement through increased tableting throughput, as batches with medium and high hardness can be processed at a higher tableting speed.
- Improved product quality at core tablets in-process control level through a reduction in the deviation rate for hardness values below the acceptable limit.
- Cost reduction through a reduction in overall processing and batch release times.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, B.; Wang, J.; Zeng, J.; Zhao, L.; Wang, Y.; Feng, Y.; Du, R. A review of high shear wet granulation for better process understanding, control and product development. Powder Technol. 2021, 381, 204–223. [Google Scholar] [CrossRef]
- Wang, B.; Sun, X.; Xiang, J.; Guo, X.; Cheng, Z.; Liu, W.; Tan, S. A critical review on granulation of pharmaceuticals and excipients: Principle, analysis and typical applications. Powder Technol. 2022, 401, 117329. [Google Scholar] [CrossRef]
- De Simone, V.; Caccavo, D.; Lamberti, G.; D’Amore, M.; Barba, A.A. Wet-granulation process: Phenomenological analysis and process parameters optimization. Powder Technol. 2018, 340, 411–419. [Google Scholar] [CrossRef]
- Sarkar, S.; Chaudhuri, B. DEM modeling of high shear wet granulation of a simple system. Asian J. Pharm. Sci. 2018, 13, 220–228. [Google Scholar] [CrossRef]
- Casian, T.; Iurian, S.; Gâvan, A.; Porfire, A.; Pop, A.L.; Crișan, S.; Pușcaș, A.M.; Tomuță, I. In-Depth Understanding of Granule Compression Behavior under Variable Raw Material and Processing Conditions. Pharmaceutics 2022, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Toshev, K.; Endekovska, I.; Ackovska, M.T.; Stojanovska, N.A. Optimization Study of Aqueous Film-Coating Process in the Industrial Scale Using Design of Experiments. Farmacia 2023, 71, 29–37. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Morton, D.A.V.; Hapgood, K.P. Predicting tablet strength from the wet granulation conditions via the unified compaction curve. Procedia Eng. 2015, 102, 517–526. [Google Scholar] [CrossRef]
- Gabbott, I.P.; Al Husban, F.; Reynolds, G.K. The combined effect of wet granulation process parameters and dried granule moisture content on tablet quality attributes. Eur. J. Pharm. Biopharm. 2016, 106, 70–78. [Google Scholar] [CrossRef]
- Veronica, N.; Goh, H.P.; Kang, C.Y.X.; Liew, C.V.; Heng, P.W.S. Influence of spray nozzle aperture during high shear wet granulation on granule properties and its compression attributes. Int. J. Pharm. 2018, 553, 474–482. [Google Scholar] [CrossRef]
- Domokos, A.; Pusztai, É.; Madarász, L.; Nagy, B.; Gyürkés, M.; Farkas, A.; Fülöp, G.; Casian, T.; Szilágyi, B.; Nagy, Z.K. Combination of PAT and mechanistic modeling tools in a fully continuous powder to granule line: Rapid and deep process understanding. Powder Technol. 2021, 388, 70–81. [Google Scholar] [CrossRef]
- Nagy, B.; Galata, D.L.; Farkas, A.; Nagy, Z.K. Application of Artificial Neural Networks in the Process Analytical Technology of Pharmaceutical Manufacturing—A Review. AAPS J. 2022, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Casian, T.; Nagy, B.; Kovács, B.; Galata, D.L.; Hirsch, E.; Farkas, A. Challenges and Opportunities of Implementing Data Fusion in Process Analytical Technology—A Review. Molecules 2022, 27, 4846. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Zhou, Q.; Zhao, L.; Wang, Y.; Shen, L.; Feng, Y.; Du, R. High shear wet granulation: Improved understanding of the effects of process variables on granule and tablet properties of a high-dose, high-hydrophobicity API based on quality by design and multivariate analysis approaches. Adv. Powder Technol. 2022, 33, 103369. [Google Scholar] [CrossRef]
- Crisan, S.; Pop, A.L.; Hentes, P.; Lacatusu, I.; Badea, N.; Zetu, C.; Ciobanu, A.M.; Penes, O.N.; Valentin, V.; Ozon, E.A.; et al. Pre-Formulation, Formulation and Pilot Scale-Up Studies To Establish the Qualitative and Quantitative Composition of an Innovative Nanoform Dietary Supplement for Menopausal Therapy. Farmacia 2024, 72, 369–380. [Google Scholar] [CrossRef]
- Grangeia, H.B.; Silva, C.; Simões, S.P.; Reis, M.S. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Mathe, R.; Casian, T.; Tomuţă, I. Multivariate feed forward process control and optimization of an industrial, granulation based tablet manufacturing line using historical data. Int. J. Pharm. 2020, 591, 119988. [Google Scholar] [CrossRef]
- Daniyan, I.; Adeodu, A.; Mpofu, K.; Maladzhi, R.; Kana-Kana Katumba, M.G. Application of lean Six Sigma methodology using DMAIC approach for the improvement of bogie assembly process in the railcar industry. Heliyon 2022, 8, e09043. [Google Scholar] [CrossRef]
- Destro, F.; Barolo, M. A review on the modernization of pharmaceutical development and manufacturing—Trends, perspectives, and the role of mathematical modeling. Int. J. Pharm. 2022, 620, 121715. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Amelia, R.; Zaman, M.B.; Merrett, B.K.; Leslie, P. Improving liquid distribution by reducing dimensionless spray flux in wet granulation-A pharmaceutical manufacturing case study. Chem. Eng. J. 2010, 164, 340–349. [Google Scholar] [CrossRef]
- Lourenço, V.; Lochmann, D.; Reich, G.; Menezes, J.C.; Herdling, T.; Schewitz, J. A quality by design study applied to an industrial pharmaceutical fluid bed granulation. Eur. J. Pharm. Biopharm. 2012, 81, 438–447. [Google Scholar] [CrossRef]
- Nagy, B.; Szabados-Nacsa, Á.; Fülöp, G.; Turák Nagyné, A.; Galata, D.L.; Farkas, A.; Mészáros, L.A.; Nagy, Z.K.; Marosi, G. Interpretable artificial neural networks for retrospective QbD of pharmaceutical tablet manufacturing based on a pilot-scale developmental dataset. Int. J. Pharm. 2023, 633, 122620. [Google Scholar] [CrossRef] [PubMed]
- Muteki, K.; Swaminathan, V.; Sekulic, S.S.; Reid, G.L. Feed-forward process control strategy for pharmaceutical tablet manufacture using latent variable modeling and optimization technologies. In Proceedings of the IFAC Proceedings Volumes (IFAC-PapersOnline), Sofia, Bulgaria, 12–14 September 2012. [Google Scholar]
- Mathe, R.; Casian, T.; Marina, A.; Marusca, D.D.; Tomuta, I. Multivariate Data Analysis for Tableting Performance Improvement at Industrial Scale. A Case Study Focused on Understanding and Avoiding the Occurrence of Capping and Lamination. Farmacia 2023, 71, 1–12. [Google Scholar] [CrossRef]
- Sultan, T.; Xu, X.; Hasan Rozin, E.; Sorjonen, J.; Ketolainen, J.; Wikström, H.; Martin de Juan, L.; Tajarobi, P.; Cetinkaya, C. Effect of shape on the physical properties of pharmaceutical tablets. Int. J. Pharm. 2022, 624, 121993. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guideline on Process Validation for Finished Products—Information and Data to be Provided in Regulatory Submissions; European Medicines Agency (EMA): London, UK, 2016; pp. 1–15. [Google Scholar]
- European Medicines Agency (EMA). Guideline on the Investigation of Bioequivalence; European Medicines Agency (EMA): London, UK, 2010; pp. 1–27. [Google Scholar]
- European Medicines Agency (EMA). ICH Topic Q 1 A (R2) Stability Testing of new Drug Substances and Products; European Medicines Agency (EMA): London, UK, 2003; pp. 1–20. [Google Scholar]
- European Medicines Agency (EMA). Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use; Chapter 5: Production; European Medicines Agency (EMA): London, UK, 2014; pp. 1–12. [Google Scholar]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis Basic Principles and Applications; Umetrics Academy, Ed.; MKS Umetrics: Umea, Sweden, 2013; ISBN 9789197373050. [Google Scholar]
- Hadian, H.; Rahimifard, A. Multivariate statistical control chart and process capability indices for simultaneous monitoring of project duration and cost. Comput. Ind. Eng. 2019, 130, 788–797. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, H.C.; Hsu, W.Y.; Huang, A.N.; Kuo, H.P. Stage-wise characterization of the high shear granulation process by impeller torque changing rate. Adv. Powder Technol. 2019, 30, 1513–1521. [Google Scholar] [CrossRef]
- Benali, M.; Gerbaud, V.; Hemati, M. Effect of operating conditions and physico–chemical properties on the wet granulation kinetics in high shear mixer. Powder Technol. 2009, 190, 160–169. [Google Scholar] [CrossRef]
- Boonkanokwong, V.; Frank, R.P.; Valliappan, P.; Remy, B.; Khinast, J.G.; Glasser, B.J. Flow of granular materials in a bladed mixer: Effect of particle properties and process parameters on impeller torque and power consumption. Adv. Powder Technol. 2018, 29, 2733–2752. [Google Scholar] [CrossRef]
- Shah, U.V.; Karde, V.; Ghoroi, C.; Heng, J.Y.Y. Influence of particle properties on powder bulk behaviour and processability. Int. J. Pharm. 2017, 518, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Kleinebudde, P. Impact of functionalized particle structure on roll compaction/dry granulation and tableting of calcium carbonate. Int. J. Pharm. 2018, 544, 235–241. [Google Scholar] [CrossRef]
- Tan, B.M.J.; Loh, Z.H.; Soh, J.L.P.; Liew, C.V.; Heng, P.W.S. Distribution of a viscous binder during high shear granulation—Sensitivity to the method of delivery and its impact on product properties. Int. J. Pharm. 2014, 460, 255–263. [Google Scholar] [CrossRef]
- Pohl, S.; Kleinebudde, P. A review of regime maps for granulation. Int. J. Pharm. 2020, 587, 119660. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D.; York, P.; Rowe, R.C. Binder-substrate interactions in wet granulation. 3: The effect of excipient source variation. Int. J. Pharm. 1992, 80, 179–190. [Google Scholar] [CrossRef]
- De Leersnyder, F.; Vanhoorne, V.; Bekaert, H.; Vercruysse, J.; Ghijs, M.; Bostijn, N.; Verstraeten, M.; Cappuyns, P.; Van Assche, I.; Vander Heyden, Y.; et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: Influence of process parameters and wet granule transfer. Eur. J. Pharm. Sci. 2018, 115, 223–232. [Google Scholar] [CrossRef]
- Lakio, S.; Siiriä, S.; Räikkönen, H.; Airaksinen, S.; Närvänen, T.; Antikainen, O.; Yliruusi, J. New insights into segregation during tabletting. Int. J. Pharm. 2010, 397, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Hilden, J.; Litster, J.D. Novel use of monodisperse granules to deconvolute impacts of granule size versus granule solid fraction on tablet tensile strength. Adv. Powder Technol. 2015, 26, 553–562. [Google Scholar] [CrossRef]
- Macho, O.; Gabrišová, Ľ.; Brokešová, J.; Svačinová, P.; Mužíková, J.; Galbavá, P.; Blaško, J.; Šklubalová, Z. Systematic study of paracetamol powder mixtures and granules tabletability: Key role of rheological properties and dynamic image analysis. Int. J. Pharm. 2021, 608, 121110. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, I.; Finke, J.H.; John, E.; Juhnke, M.; Kwade, A. The influence of particle size on the application of compression and compaction models for tableting. Int. J. Pharm. 2021, 599, 120424. [Google Scholar] [CrossRef] [PubMed]
- Sochon, R.P.J.; Zomer, S.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. The variability of pharmaceutical granulation. Chem. Eng. J. 2010, 164, 285–291. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Zhang, Z.; Yu, J.; Wang, F.; Shi, X.; Qiao, Y. A compression behavior classification system of pharmaceutical powders for accelerating direct compression tablet formulation design. Int. J. Pharm. 2019, 572, 118742. [Google Scholar] [CrossRef]
- Peeters, E.; Silva, A.F.T.; Fonteyne, M.; De Beer, T.; Vervaet, C.; Remon, J.P. Influence of extended dwell time during pre- and main compression on the properties of ibuprofen tablets. Eur. J. Pharm. Biopharm. 2018, 128, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Llusa, M.; Faqih, A.; Levin, M.; Muzzio, F.J. Influence of shear intensity and total shear on properties of blends and tablets of lactose and cellulose lubricated with magnesium stearate. Int. J. Pharm. 2007, 336, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Blahova Prudilova, B.; Detobel, F.; Remon, J.P.; De Beer, T.; Vervaet, C. Optimizing feed frame design and tableting process parameters to increase die-filling uniformity on a high-speed rotary tablet press. Int. J. Pharm. 2018, 548, 54–61. [Google Scholar] [CrossRef]
- Mendez, R.; Muzzio, F.; Velazquez, C. Study of the effects of feed frames on powder blend properties during the filling of tablet press dies. Powder Technol. 2010, 200, 105–116. [Google Scholar] [CrossRef]
- Staniforth, J.N.; Baichwal, A.R.; Hart, J.P.; Heng, P.W.S. Effect of addition of water on the rheological and mechanical properties of microcrystalline celluloses. Int. J. Pharm. 1988, 41, 231–236. [Google Scholar] [CrossRef]
- Lura, A.; Tardy, G.; Kleinebudde, P.; Breitkreutz, J. Tableting of mini-tablets in comparison with conventionally sized tablets: A comparison of tableting properties and tablet dimensions. Int. J. Pharm. X 2020, 2, 100061. [Google Scholar] [CrossRef]
- Eiliazadeh, B.; Pitt, K.; Briscoe, B. Effects of punch geometry on powder movement during pharmaceutical tabletting processes. Int. J. Solids Struct. 2004, 41, 5967–5977. [Google Scholar] [CrossRef]
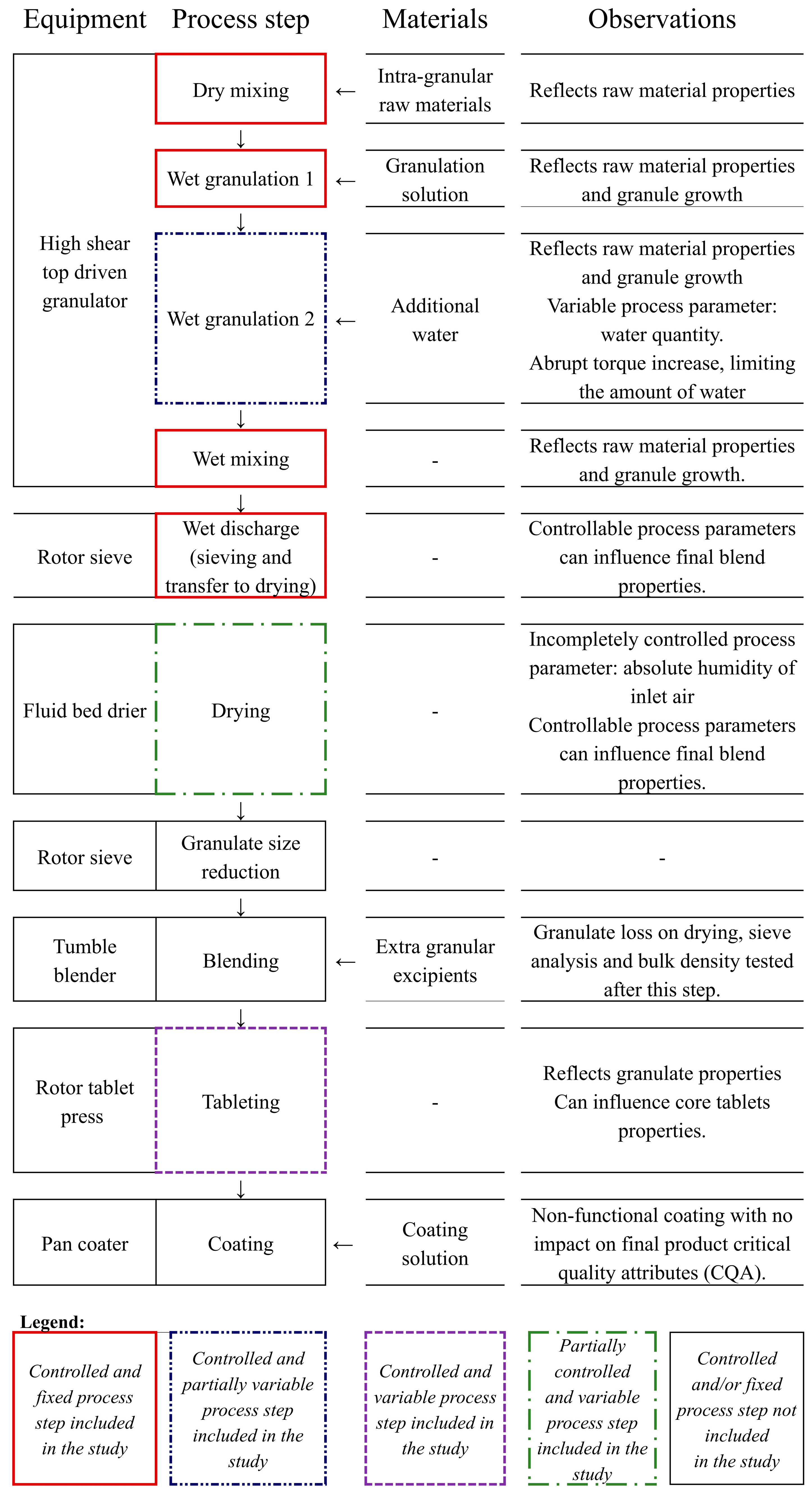


| Variable Type | Attribute (Unit of Measure) | Data Source | |
|---|---|---|---|
| Raw material | API | Particle size distribution (PSD) by laser diffraction (d0.1; d0.5; d0.9) (μm) | Certificate of analysis; one value/batch |
| Process parameters | Wet granulation | Mixer speed (rpm) | Equipment report; Datapoints at 30 s interval |
| Torque value (Nm) | |||
| Quantity of granulation water (kg) | Batch records | ||
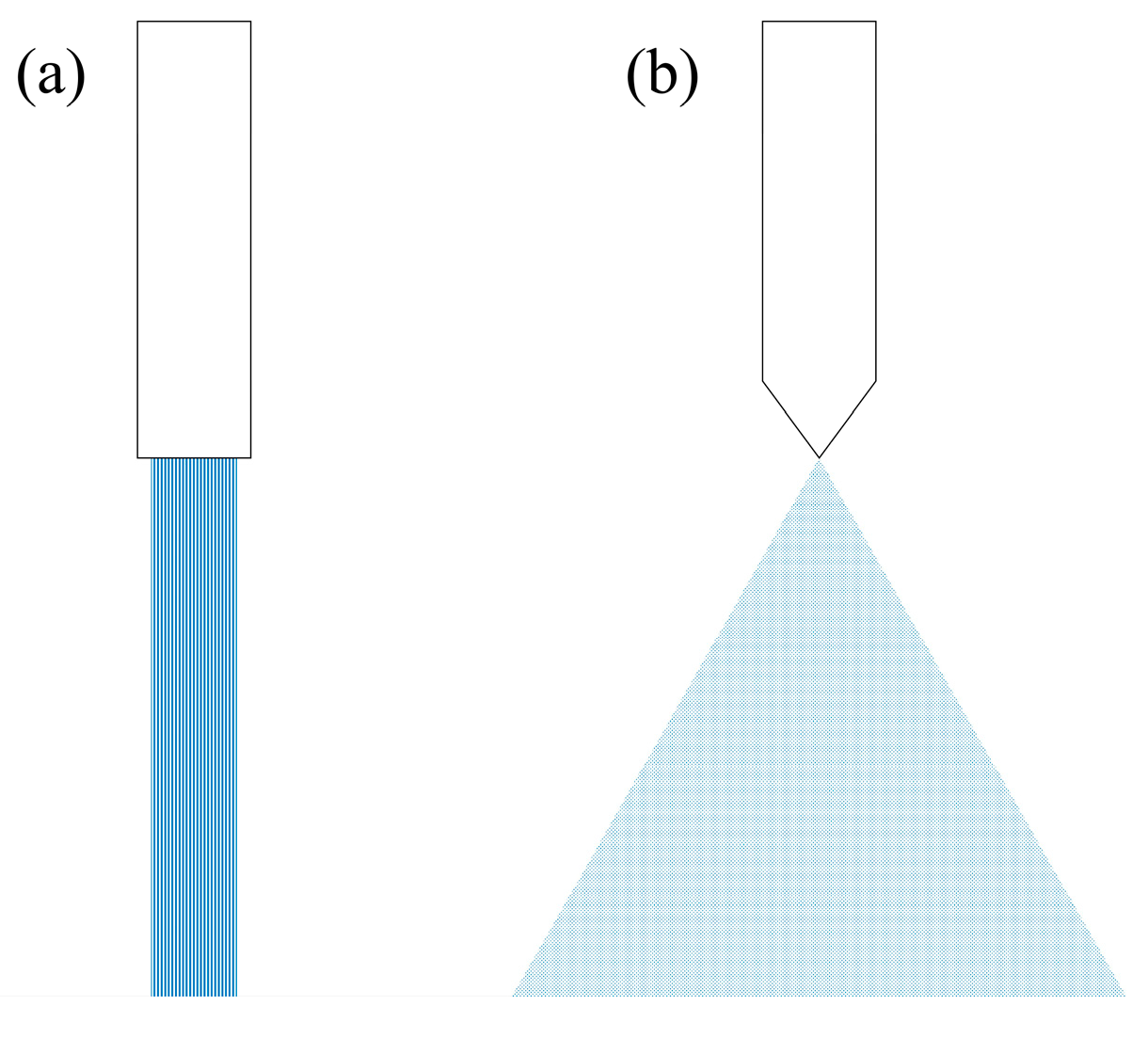
| Nozzle used for liquid addition (type 1 and type 2—Figure 1) | Batch records | ||
| Wet granulate discharge | Inlet air flap position (%) | Equipment report; set point per time interval | |
| Wet discharge intensity index (%; WDi) (low values represent high intensity) | Computed from above variable as weighted average of the various the set-points during a batch with their corresponding durations | ||
| Wet discharge time (min) | Equipment report | ||
| Process parameters | Granule drying | Inlet air flowrate (m3/h) | Equipment report; rounded actual value per time interval |
| Drying intensity index (m3/h; Di) (low values represent low intensity) | Computed from above variable as weighted average of the various the set-points during a batch with their corresponding durations | ||
| Inlet air humidity (g/kg) | Equipment report | ||
| Drying time (min) | Equipment report | ||
| Tableting | Tablet press model (TP1/TP2) | Batch records | |
| Speed (tablets/hour) | Batch records; data points at 1 h interval | ||
| Feeder speed (rpm or %) | |||
| Pre-compression force (kN) | |||
| Compression force (kN) | |||
| Granule properties | Final blend | Particle size analysis by sieving (% retained on 1000; 500; 250; 125 microns sieves and % of fines below 125 microns) | Batch records |
| Bulk density (g/mL) | |||
| Loss on drying (LOD) (%) | |||
| Core tablet properties | Average hardness/batch (N) | ||
| Nr. | Y | X | R2X (cum) | R2Y (cum) | Q2 (cum) | RMSECV |
|---|---|---|---|---|---|---|
| BLM1 | Tablet hardness (tablet H) | API particle size distribution Granulation pp Water quantity Wet discharge pp Drying pp Granule properties Tableting pp | 0.379 | 0.706 | 0.646 | 11.162 |
| BLM2 | Tablet hardness (tablet L) | 0.593 | 0.807 | 0.665 | 9.827 | |
| BLM3 TP1-H | Tablet hardness | Granulation conditions Granulation final torque Water quantity Absolute inlet air humidity (drying) Sieve size Granule properties Tablet press type Tableting feeder speeds (%) | 0.644 | 0.835 | 0.635 | 10.203 |
| BLM4 TP2-H | 0.446 | 0.765 | 0.678 | 10.572 | ||
| BLM5 TP1-L | 0.945 | 0.999 | 0.841 | 4.996 | ||
| BLM6 TP2-L | 0.448 | 0.814 | 0.682 | 9.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathe, R.; Casian, T.; Tomuta, I. Multivariate Data Analysis to Assess Process Evolution and Systematic Root Causes Investigation in Tablet Manufacturing at an Industrial Scale—A Case Study Focused on Improving Tablet Hardness. Pharmaceutics 2025, 17, 213. https://doi.org/10.3390/pharmaceutics17020213
Mathe R, Casian T, Tomuta I. Multivariate Data Analysis to Assess Process Evolution and Systematic Root Causes Investigation in Tablet Manufacturing at an Industrial Scale—A Case Study Focused on Improving Tablet Hardness. Pharmaceutics. 2025; 17(2):213. https://doi.org/10.3390/pharmaceutics17020213
Chicago/Turabian StyleMathe, Rita, Tibor Casian, and Ioan Tomuta. 2025. "Multivariate Data Analysis to Assess Process Evolution and Systematic Root Causes Investigation in Tablet Manufacturing at an Industrial Scale—A Case Study Focused on Improving Tablet Hardness" Pharmaceutics 17, no. 2: 213. https://doi.org/10.3390/pharmaceutics17020213
APA StyleMathe, R., Casian, T., & Tomuta, I. (2025). Multivariate Data Analysis to Assess Process Evolution and Systematic Root Causes Investigation in Tablet Manufacturing at an Industrial Scale—A Case Study Focused on Improving Tablet Hardness. Pharmaceutics, 17(2), 213. https://doi.org/10.3390/pharmaceutics17020213









