Non-Invasive Delivery of CRISPR/Cas9 Ribonucleoproteins (Cas9 RNPs) into Cells via Nanoparticles for Membrane Transport
Abstract
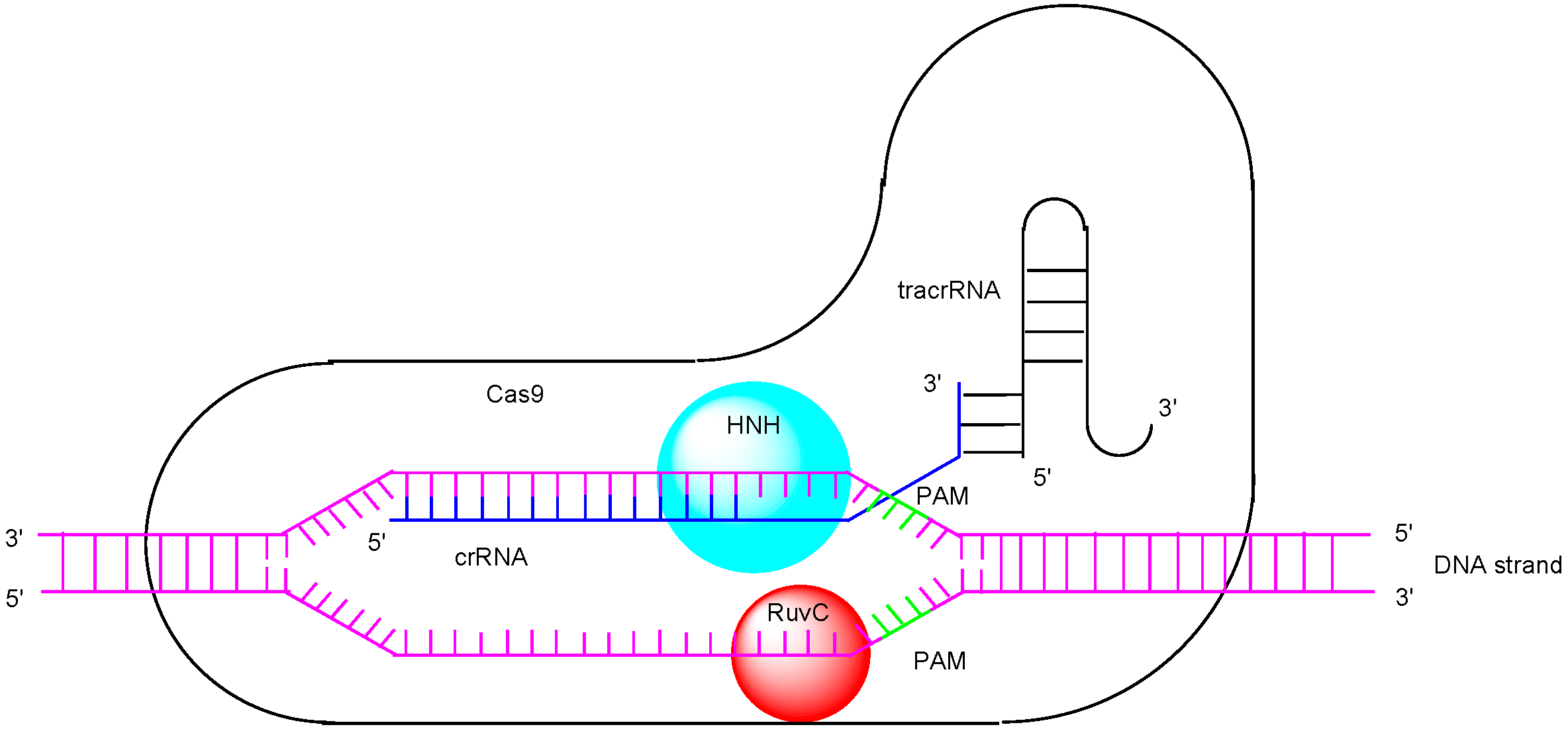
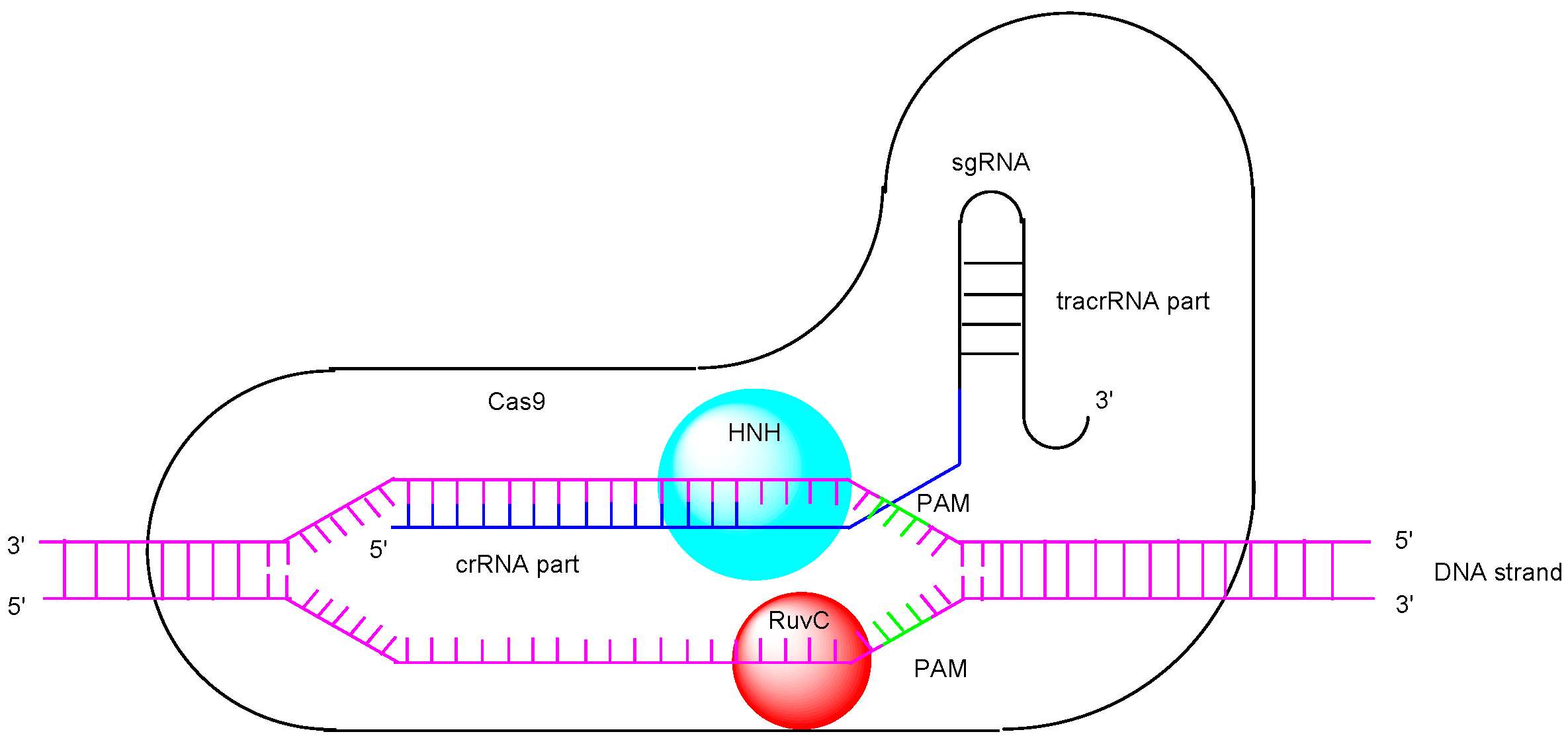
1. Introduction
2. Discussion
2.1. Membrane Impermeability/Permeability of Substances
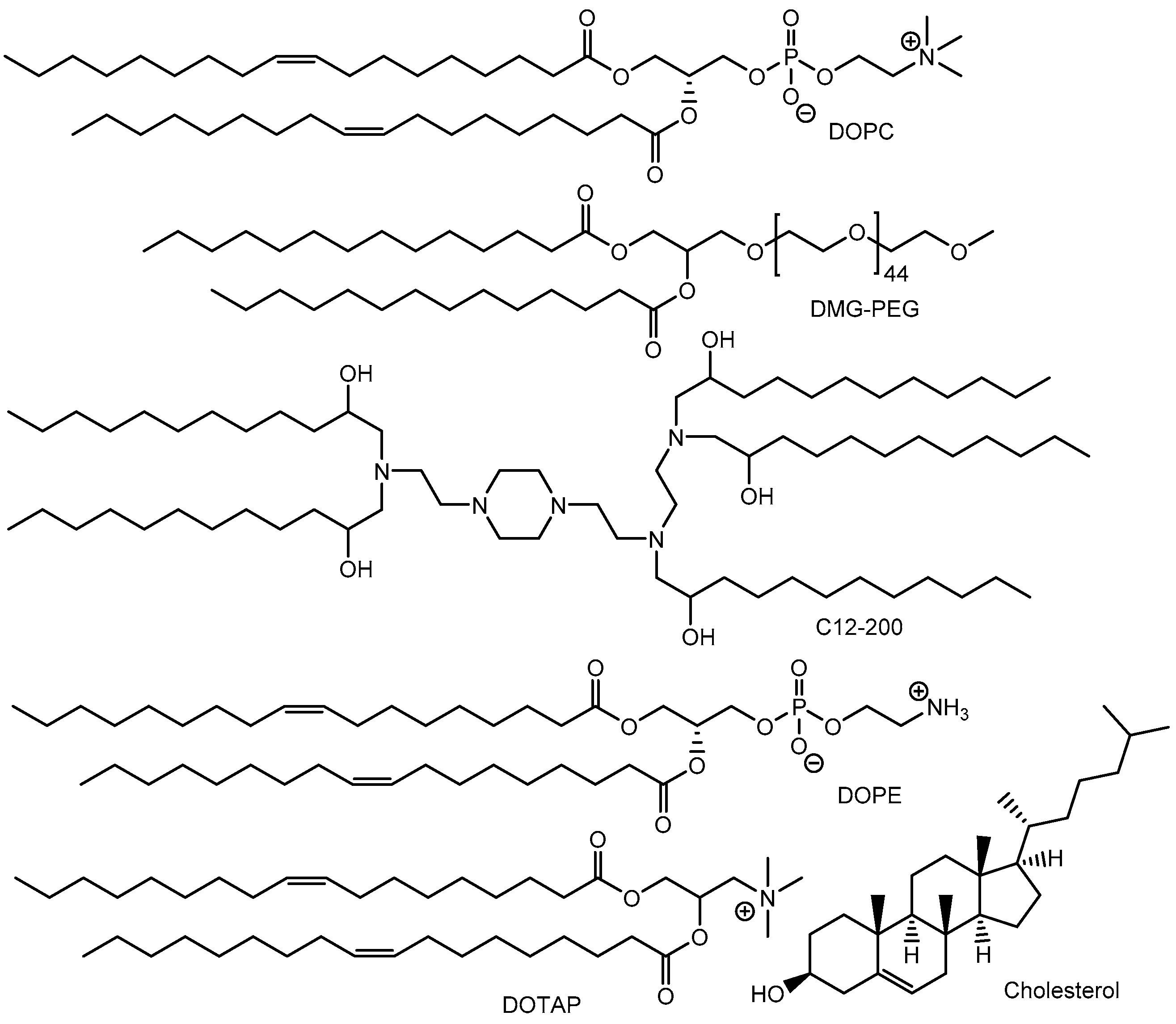
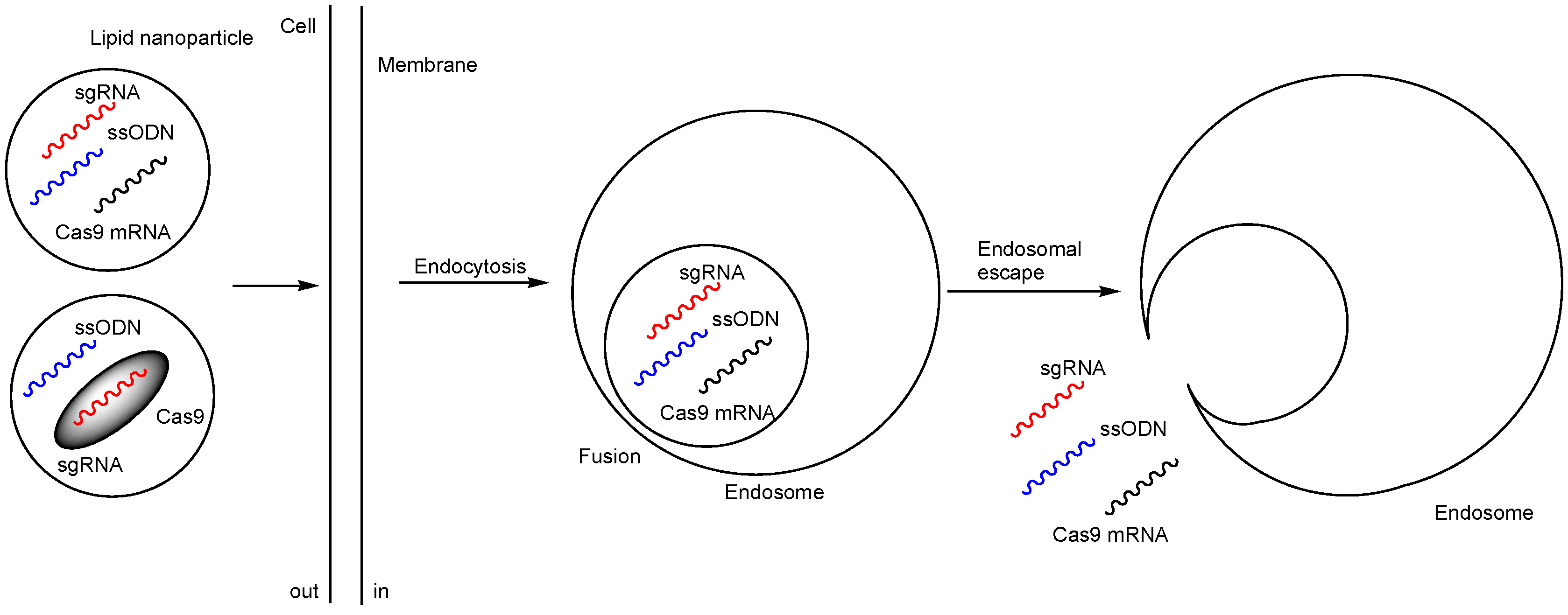
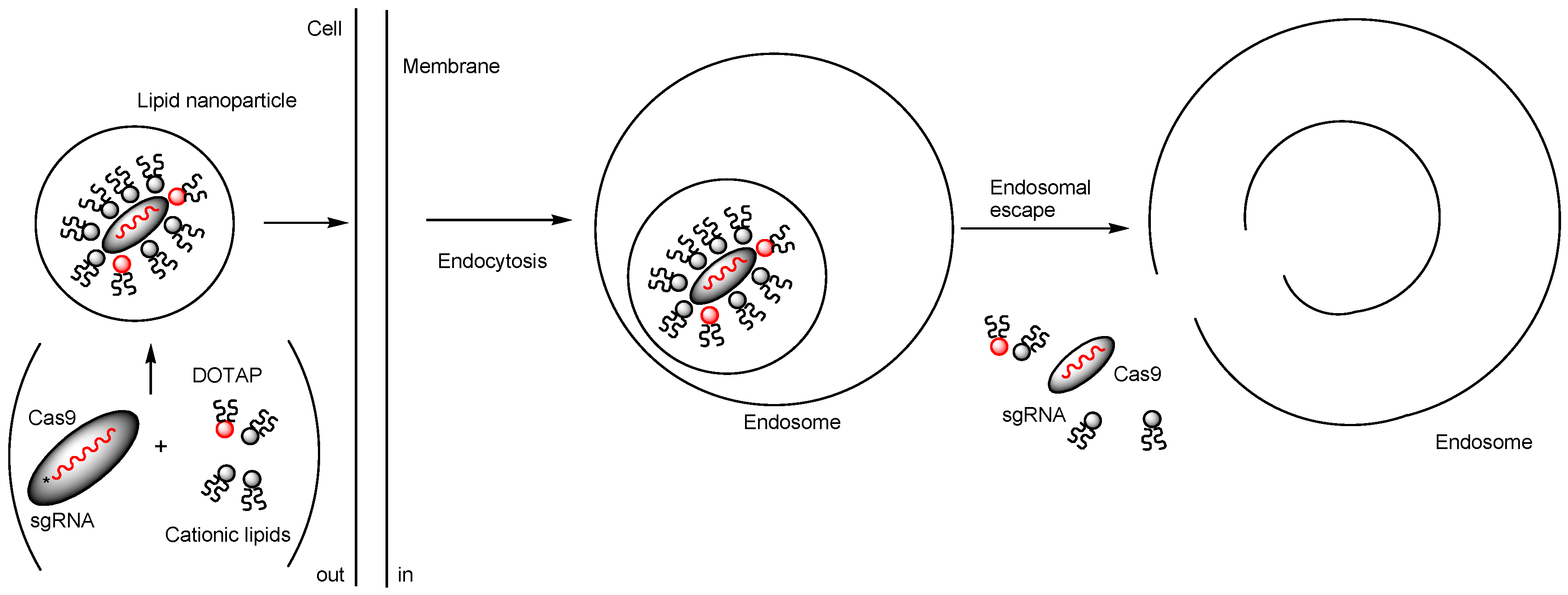
2.2. Nanoparticles Encapsulating Cas9 RNPs
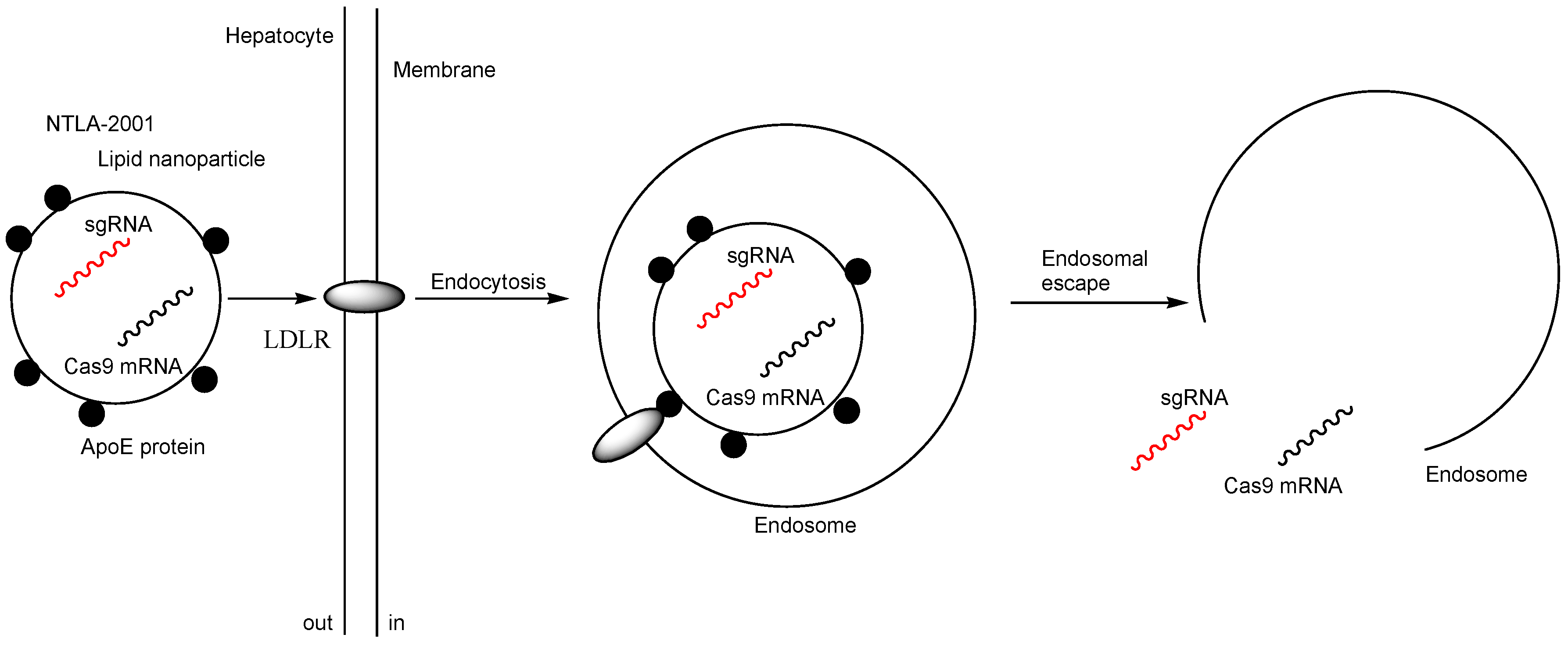
2.2.1. Distribution to Tissues, Such as the Liver, Involved in the Reticuloendothelial System
2.2.2. Restricted Distribution to the Target Sites via Local Injections
2.2.3. Selective and Effective Distribution Through Non-Invasive Methods
| # | Formulations | Administration | Diseases | Targeting Gene | Results | Status | Refs. |
|---|---|---|---|---|---|---|---|
| i | NTLA-2001 (LNPs encapsulating Cas9 mRNA and sgRNA targeting TTR | Intravenous injection | TTR amyloidosis | TTR | Preclinical studies showed that at day 28 the mean reduction in serum TTR protein concentration was 52% in the group that received a dose of 0.1 mg per kilogram and was 87% in the group that received a dose of 0.3 mg per kilogram. | Phase 1 clinical trial (NCT04601051) | [17] |
| ii | LNP-based Cas RNP delivery system using optimally designed ssODNs | Intravenous injection | TTR amyloidosis | TTR | RNP-loaded LNPs with anti-TTR sgRNA (sgTTR-G269) and designed ssODNs with a complementation rate of 50% (calculated Tm: 30 °C) suppressed TTR the highest (34%) as TTR protein levels in serum, quantified 1 week after intravenous administration in mice. | Basic research | [21] |
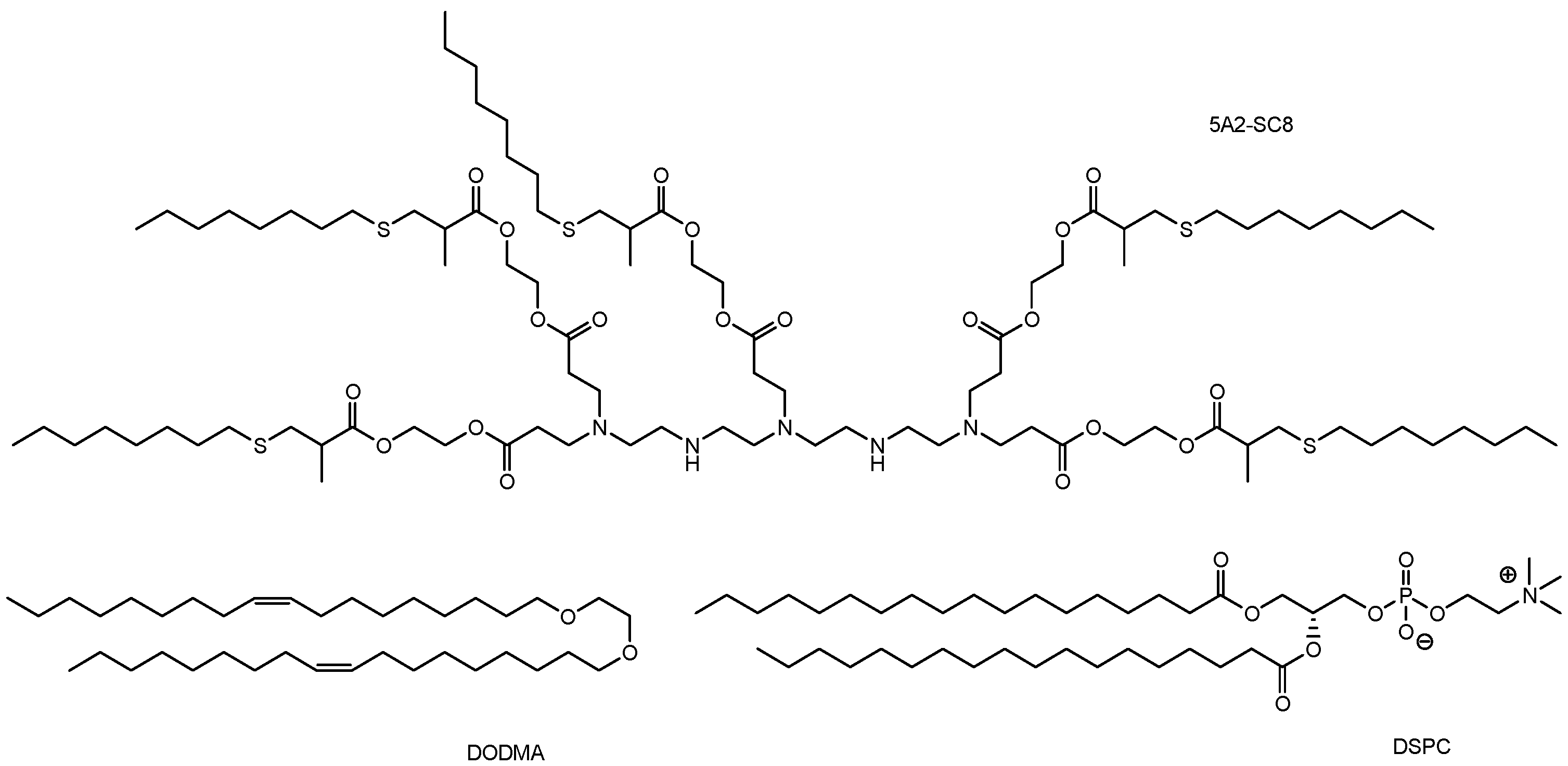
| iii | 5A2-DOT-5 LNPs encapsulating Cas9/sgP53/sgPTEN/sgRB1 RNPs | Intravenous injection | Induction of cancer | P53, PTEN, or RB1 | Generation of visible tumors on the liver in adult C57BL/6 mice. | Basic research | [28] |
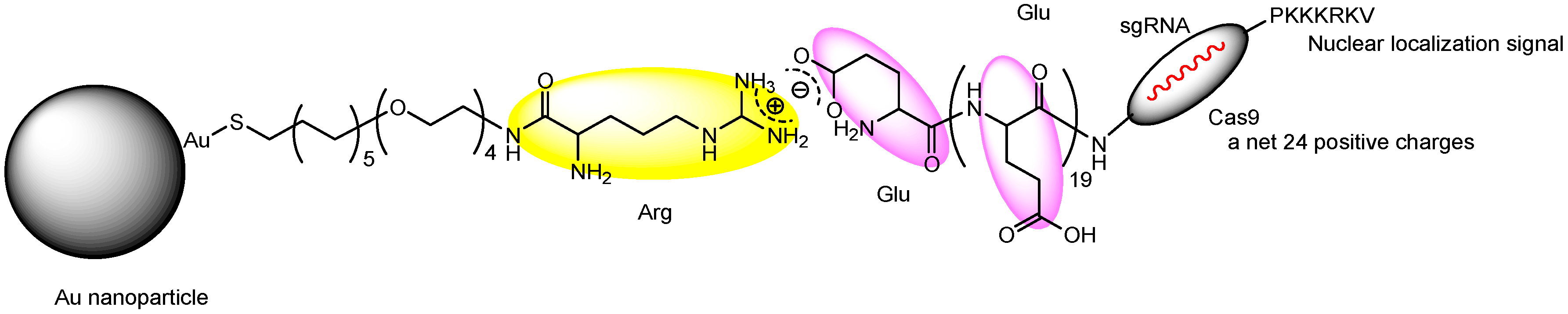
| iv | Nanoassemblies composed of Cas9 RNP with oligo (20) Glu tags (Cas9E20) and Arg-AuNPs electrostatically connected between Glu and Arg | Intravenous injection | - | PTEN | >8% and >4% gene editing efficiency in macrophages of the liver and the spleen, respectively. | Basic research | [31] |
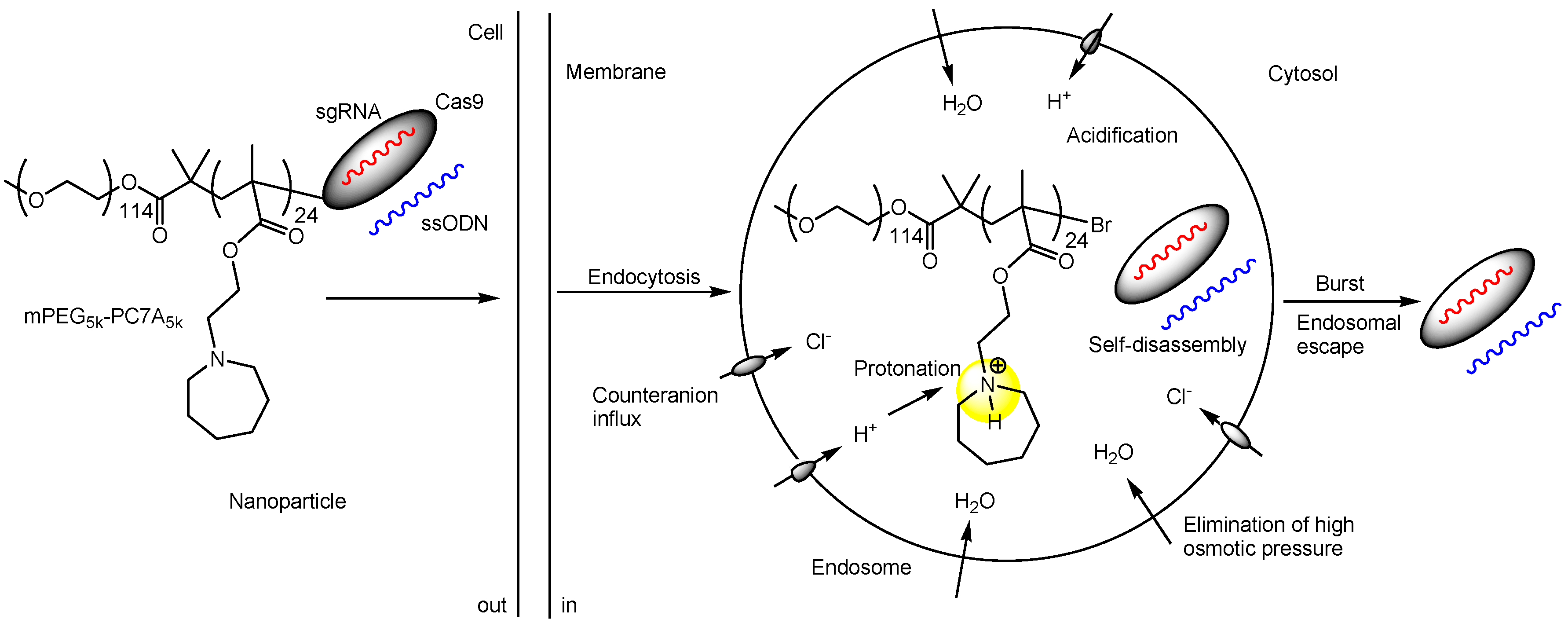
| v | Nanoparticles composed of Cas9 RNPs with ssODNs and mPEG-PC7A | Intravenous, intratracheal, or intramuscular injection | - | A STOP cassette that consists of three SV40 polyA sequences to prevent transcription of the downstream tdTomato | Intravenously, intratracheally, and intramuscularly injected NHEJ-NP (29.4 nm in diameter) induced efficient gene editing in mouse liver, lung, and skeletal muscle, respectively. | Basic research | [33] |
| vi | Nanocomplexes (200–400 nm in diameter) electrostatically composed of a Cas9 RNP and cationic LAH5 peptides | - | - | CCR5 | Nanocomplexes targeting CCR5 exhibited the gene editing across the membrane in diverse cell lines. | Basic research | [34] |
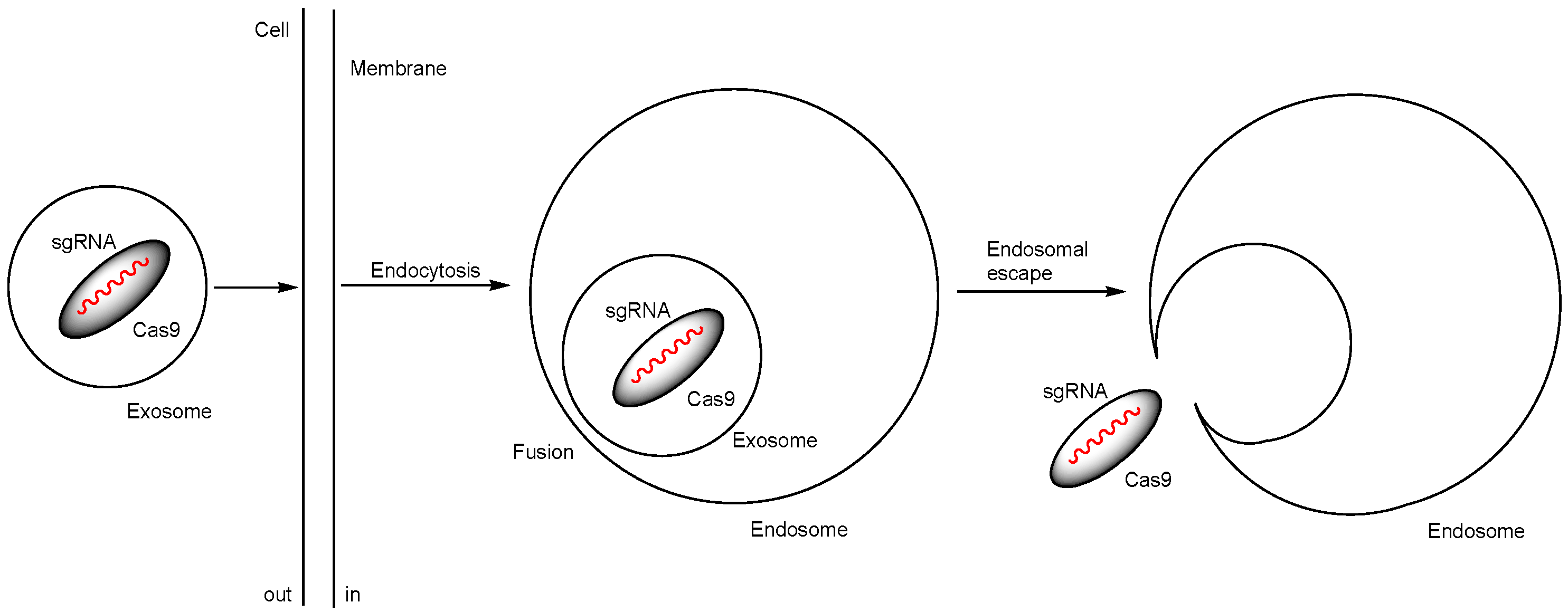
| vii | ExosomeRNP nanocomplexes, prepared by loading Cas9 RNPs into purified exosomes isolated from hepatic stellate cells through electroporation | Intravenous injection | liver injury, chronic liver fibrosis, or hepatocellular carcinoma | PUMA, CcnE1, or KAT5 | ExosomeRNP targeting PUMA ameliorated acute liver injury. ExosomeRNP targeting CcnE1 ameliorated chronic liver fibrosis. ExosomeRNP targeting KAT5 administered by tail vein injections ameliorated orthotopic hepatocellular carcinoma (HCC). | Basic research | [36] |
| viii | LNP-encapsulated tdTomato-targeted CRISPR strategy in the loxP-3xStop-loxP-tdTomato reporter system | Intrastromal injection | corneal diseases | A STOP cassette that consists of three SV40 polyA sequences to prevent transcription of the downstream tdTomato | Transfection not only of the stromal cells but also of the endothelial cells in all wild-type eyes of 3-month-old hybrid B6129F1-loxP-3xStop-loxP-tdTomato wild-type mice. | Basic research | [37] |
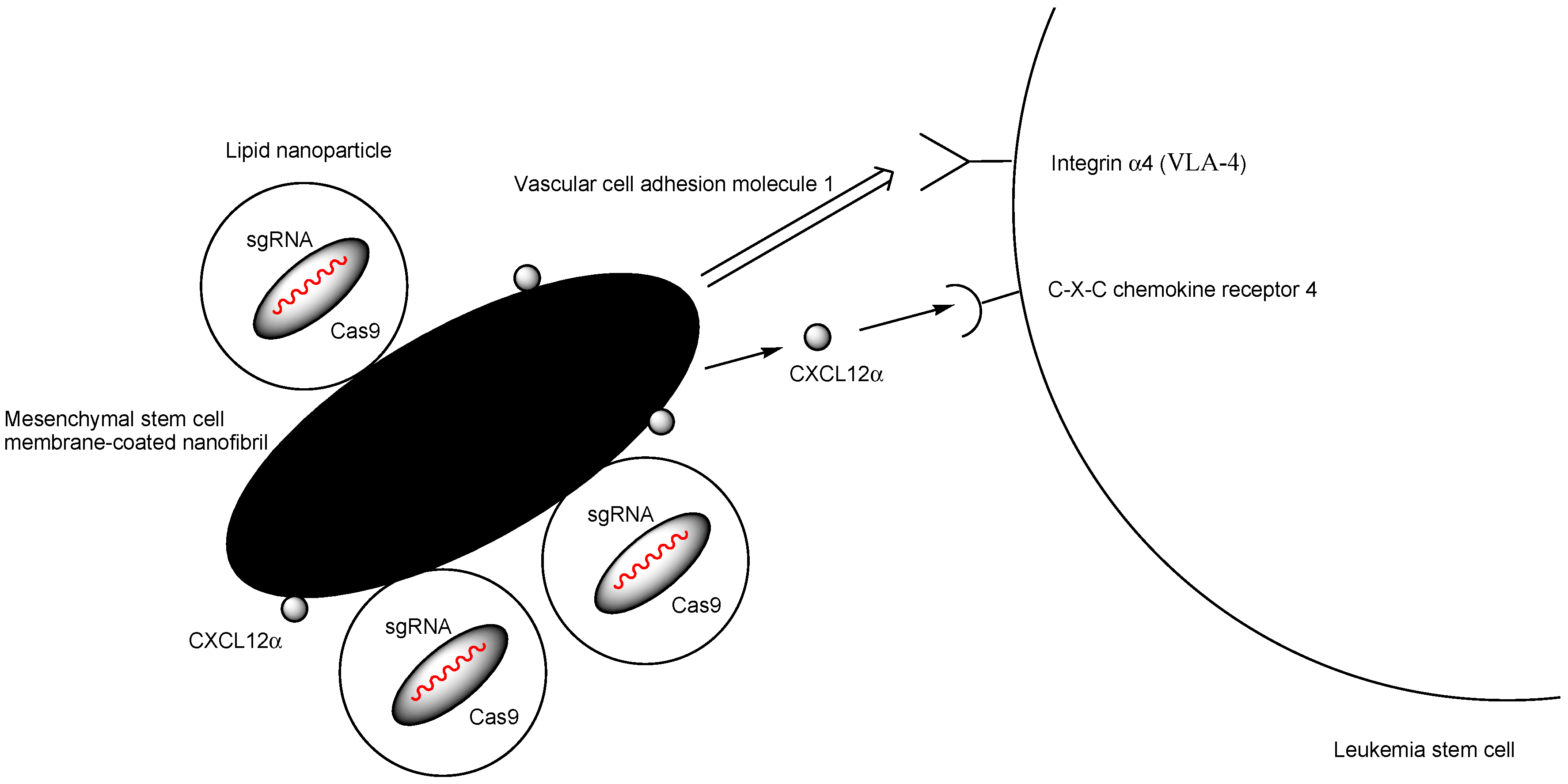
| ix | MSCM-NFs loading (a) bioreducible LNPs encapsulating Cas9 RNP targeting the critical gene IL1RAP in human LSCs and (b) CXCL12α that is a ligand of CXCR4 | Injection into the bone marrow cavity | acute myeloid leukemia | IL1RAP or CXCL12α | IL1RAP knockout reduced LSC colony-forming capacity and leukemic burden. | Basic research | [39] |
| x | PEGylated nanocapsules encapsulating Cas9 RNPs | Intracerebral injection | CNS diseases | A STOP cassette that consists of three SV40 polyA sequences to prevent transcription of the downstream tdTomato | Genome editing of striatal neurons, which was determined by the tdTomato reporter system. | Basic research | [43] |
| xi | CRISPR–gold targeting the mGluR5 gene | Intracranial injection | fragile X syndrome | mGluR5 | Reduction in local mGluR5 protein levels in the striatum by 40–50% after an intracranial injection into the brains of Fmr1 knockout mice and eventual reduction in hyperlocomotor activities such as excessive digging and jumping behavior. | Basic research | [45] |
| xii | Nanocomplexes coated with phenylboronate and lipoic acid and containing Cas9 RNP | Subcutaneous injection | psoriasis | NLRP3 | 33.7% gene disruption at the NLRP3 locus in psoriatic skin tissues. | Basic research | [48] |
| xiii | Nanocapsules composed of nanoassembled, engineered DNAzyme shells encasing Cas9 RNP | Intratumoral injection | cancer | MIR-21 | Tumor growth inhibition of up to 75.94%. | Basic research | [49] |
| xiv | Nanoparticles encapsulating Cas9 RNP targeting Nrf2 gene and antitumor photosensitizer chlorin e6 (Ce6) and covered with iRGD | Intravenous injection | cancer | Nrf2 | The Nrf2 protein is associated with angiogenesis promotion. Hypoxia-inducible factor 1α (HIF1α) and vascular endothelial growth factor-A (VEGF-A) are representative angiogenetic factors. Ce6 and Cas9 RNP-encapsulated NP treatment resulted in 50.0 and 44.6% reduction in HIF1α and VEGF-A levels, respectively. | Basic research | [54] |
| xv | GSH-responsive SNCs conjugated with glucose and RVG peptide and encapsulating Cas9 mRNA and App659 sgRNA or Th sgRNA | Intravenous injection | CNS diseases including Alzheimer’s disease | App659 or Th | Up to 6.1% amyloid precursor protein (App) gene editing efficiency for thalamus/hypothalamus (resulting in 19.1% reduction in the expression level of intact APP) or up to 3.9% tyrosine hydroxylase (Th) gene editing efficiency for thalamus/hypothalamus (resulting in 30.3% reduction in the expression level of Th), respectively. | Basic research | [55] |
| xvi | Disulfide-cross-linked polymeric shell nanocapsules decorated with angiopep-2 peptide, encapsulating Cas9 RNP (approx. 30 nm in diameter) | Intravenous injection | glioblastoma | PLK1 | High PLK1 gene editing efficiency in a brain tumor (up to 38.1%) in orthotopic CSC2-Luc GSC tumor-bearing mice. | Basic research | [56] |
| xvii | CRISPR/Cas9-based nanomedicine by fabricating an angiopep-2 decorated, guanidinium and fluorine functionalized polymeric NPs loading Cas9/gRNA RNP | - | glioblastoma | PLK1 | 32% gene knockout and 67% protein reduction in the proto-oncogene polo-like kinase 1 (PLK1) (in vitro). | Basic research | [57] |
| xviii | LNPs and polymer Pluronic F127-encapsulated CRISPR/Cas plasmid | Oral administration | colitis-associated colorectal cancer | CD98 | Reduction in CD98 expression and demonstration of therapeutic efficacy against CAC. | Basic research | [58] |
| xix | Cas9 RNP-encapsulated NPs covered with anti-TfR and anti-α-7 AchR bispecific mAbs | Intravenous injection | CNS diseases | Certain proteins involved in CNS diseases | Under analysis in Tashima lab. | Basic research | - |
2.2.4. Promising Delivery of Intravenously Administered Cas9 RNP-Encapsulated Nanoparticles to the Brain
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Narula, A. Gene Editing and Crop Improvement Using CRISPR-Cas9 System. Front. Plant Sci. 2017, 8, 1932. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Li, T.; Yang, B.; Spalding, M.H. TALEN-mediated genome editing: Prospects and perspectives. Biochem. J. 2014, 462, 15–24. [Google Scholar] [CrossRef]
- Mir, A.; Edraki, A.; Lee, J.; Sontheimer, E.J. Type II-C CRISPR-Cas9 biology, mechanism, and application. ACS Chem. Biol. 2018, 13, 357–365. [Google Scholar] [CrossRef]
- Le Rhun, A.; Escalera-Maurer, A.; Bratovič, M.; Charpentier, E. CRISPR-Cas in Streptococcus pyogenes. RNA Biol. 2019, 16, 380–389. [Google Scholar] [CrossRef]
- Gehrke, F.; Schindele, A.; Puchta, H. Nonhomologous end joining as key to CRISPR/Cas-mediated plant chromosome engineering. Plant Physiol. 2022, 188, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ren, S.; Yu, S.; Pan, H.; Li, T.; Ge, S.; Zhang, J.; Xia, N. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int. J. Mol. Sci. 2020, 21, 6461. [Google Scholar] [CrossRef]
- Waddington, S.N.; Privolizzi, R.; Karda, R.; O’Neill, H.C. A broad overview and review of CRISPR-Cas technology and stem cells. Curr. Stem Cell Rep. 2016, 2, 9–20. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Tashima, T. Effective cancer therapy based on selective drug delivery into cells across their membrane using receptor-mediated endocytosis. Bioorg. Med. Chem. Lett. 2018, 28, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Laughlin, C.D.; D’Aquili, E.G. Biogenetic Structuralism; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Leavy, S.A. Biogenetic Structuralism. Yale J. Biol. Med. 1976, 49, 420–421. [Google Scholar]
- Chen, Y.; Cai, Y.; Xu, Q.; Chen, Z.W. Atomic force bio-analytics of polymerization and aggregation of phycoerythrin-conjugated immunoglobulin G molecules. Mol. Immunol. 2004, 41, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Vieira, M.; Saraiva, M.J. Transthyretin: A multifaceted protein. Biomol. Concepts 2014, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Li, B. Nonviral Delivery of CRISPR/Cas Systems in mRNA Format. Adv. Nanobiomed. Res. 2022, 2, 2200082. [Google Scholar] [CrossRef]
- Klösgen, B.; Helfrich, W. Cryo-Transmission Electron Microscopy of a Superstructure of Fluid Dioleoylphosphatidylcholine (DOPC) Membranes. Biophys J. 1997, 73, 3016–3029. [Google Scholar] [CrossRef]
- Walther, J.; Porenta, D.; Wilbie, D.; Seinen, C.; Benne, N.; Yang, Q.; de Jong, O.G.; Lei, Z.; Mastrobattista, E. Comparative analysis of lipid Nanoparticle-Mediated delivery of CRISPR-Cas9 RNP versus mRNA/sgRNA for gene editing in vitro and in vivo. Eur. J. Pharm. Biopharm. 2024, 196, 114207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, T.; Ou, J.; Huang, J.; Liang, P. Homology-based repair induced by CRISPR-Cas nucleases in mammalian embryo genome editing. Protein Cell 2022, 13, 316–335. [Google Scholar] [CrossRef]
- Caracciolo, G.; Caminiti, R. Efficient Escape from Endosomes Determines the Superior Efficiency of Multicomponent Lipoplexes. J. Phys. Chem. B 2009, 113, 4995–4997. [Google Scholar] [CrossRef] [PubMed]
- Hafez, I.M.; Maurer, N.; Cullis, P.R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001, 8, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ito, S.; Yoshino, F.; Suzuki, Y.; Zhao, L.; Komatsu, N. Polyglycerol Grafting Shields Nanoparticles from Protein Corona Formation to Avoid Macrophage Uptake. ACS Nano. 2020, 14, 7216–7226. [Google Scholar] [CrossRef]
- Torrice, M. Does nanomedicine have a delivery problem? ACS Cent. Sci. 2016, 2, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Cheng, Q.; Min, Y.L.; Olson, E.N.; Siegwart, D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Watanabe, S.; Goel, I.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B.; Teramura, Y. Promotion of cell membrane fusion by cell-cell attachment through cell surface modification with functional peptide-PEG-lipids. Biomaterials 2020, 253, 120113. [Google Scholar] [CrossRef]
- Lee, Y.W.; Mout, R.; Luther, D.C.; Liu, Y.; Castellanos-García, L.; Burnside, A.S.; Ray, M.; Tonga, G.Y.; Hardie, J.; Nagaraj, H.; et al. In Vivo Editing of Macrophages through Systemic Delivery of CRISPR-Cas9-Ribonucleoprotein-Nanoparticle Nanoassemblies. Adv. Ther. 2019, 2, 1900041. [Google Scholar] [CrossRef]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.-W.; Tay, T.; Sasaki, K.; Rotello, V.M. Efficient Gene Editing through Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, X.; Wang, Y.; Ye, M.; Zhao, Y.; Yandell, B.S.; Gong, S. pH-Responsive Polymer Nanoparticles for Efficient Delivery of Cas9 Ribonucleoprotein With or Without Donor DNA. Adv. Mater. 2022, 34, e2110618. [Google Scholar] [CrossRef]
- Öktem, M.; Mastrobattista, E.; de Jong, O.G. Amphipathic Cell-Penetrating Peptide-Aided Delivery of Cas9 RNP for In Vitro Gene Editing and Correction. Pharmaceutics 2023, 15, 2500. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, C.R.; Bai, M.J.; Zhu, D.; Chen, J.W.; Wang, H.F.; Li, M.A.; Wu, C.; Li, Z.R.; Huang, M.S. Exosome-mediated miRNA delivery promotes liver cancer EMT and metastasis. Am. J. Transl. Res. 2020, 12, 1080–1095. [Google Scholar]
- Wan, T.; Zhong, J.; Pan, Q.; Zhou, T.; Ping, Y.; Liu, X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 2022, 8, eabp9435. [Google Scholar] [CrossRef] [PubMed]
- Winnard, P.T., Jr.; Kluth, J.B.; Raman, V. Noninvasive Optical Tracking of Red Fluorescent Protein–Expressing Cancer Cells in a Model of Metastatic Breast Cancer. Neoplasia 2006, 8, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili Mohanna, S.Z.; Djaksigulova, D.; Hill, A.M.; Wagner, P.K.; Simpson, E.M.; Leavitt, B.R. LNP-mediated delivery of CRISPR RNP for wide-spread in vivo genome editing in mouse cornea. J. Control Release 2022, 350, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Kim, H.S.; Chen, Y.; Li, Y.; LaMere, M.W.; Chen, C.; Wang, H.; Gong, J.; Palumbo, C.D.; Ashton, J.M.; et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci. Adv. 2021, 7, eabg3217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bandara, S.R.; Tan, Z.; Leal, C. Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc. Natl. Acad. Sci. USA 2023, 120, e2301067120. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Ray, M.; Lee, Y.W.; Scaletti, F.; Rotello, V.M. In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges. Bioconjug. Chem. 2017, 28, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.C.; Sabo, J.K.; Kang, M.H.; Allen, R.; Applegate, E.; Kim, S.E.; Kwon, Y.; Seth, A.; Lemus, N.; Salinas-Rios, V.; et al. Genome editing in the mouse brain with minimally immunogenic Cas9 RNPs. Mol. Ther. 2023, 31, 2422–2438. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.M.; Wang, Y.; Neuman, S.S.; Snow, K.J.; Murray, S.A.; Lutz, C.M.; Bondarenko, V.; Felton, J.; Gimse, K.; Xie, R.; et al. Efficient in vivo neuronal genome editing in the mouse brain using nanocapsules containing CRISPR-Cas9 ribonucleoproteins. Biomaterials 2023, 293, 121959. [Google Scholar] [CrossRef]
- Sajjanar, B.; Saxena, S.; Bisht, D.; Singh, A.K.; Manjunatha Reddy, G.B.; Singh, R.; Singh, R.P.; Kumar, S. Effect of nicotinic acetylcholine receptor alpha 1 (nAChRα1) peptides on rabies virus infection in neuronal cells. Neuropeptides 2016, 57, 59–64. [Google Scholar] [CrossRef]
- Lee, B.; Lee, K.; Panda, S.; Gonzales-Rojas, R.; Chong, A.; Bugay, V.; Park, H.M.; Brenner, R.; Murthy, N.; Lee, H.Y. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat. Biomed. Eng. 2018, 2, 497–507. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.S.; Gomez-Mancilla, B.; Neri, G.; Willemsen, R.; Gasparini, F. Fragile X syndrome: A preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology 2014, 231, 1217–1226. [Google Scholar] [CrossRef]
- Tan, E.; Wan, T.; Pan, Q.; Duan, J.; Zhang, S.; Wang, R.; Gao, P.; Lv, J.; Wang, H.; Li, D.; et al. Dual-responsive nanocarriers for efficient cytosolic protein delivery and CRISPR-Cas9 gene therapy of inflammatory skin disorders. Sci. Adv. 2024, 10, eadl4336. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, Y.; Wang, B.; Luo, D.; Wang, J.; He, Y.; Feng, L.; Xu, Y.; Xie, S.; Chen, M.; et al. Autonomous Feedback-Driven Engineered DNAzyme-Coated Trojan Horse-like Nanocapsules for On-Demand CRISPR/Cas9 Delivery. ACS Nano 2024, 18, 13950–13965. [Google Scholar] [CrossRef] [PubMed]
- Le, W.; Chen, B.; Cui, Z.; Shi, D. Detection of cancer cells based on glycolytic-regulated surface electrical charges. Biophys. Rep. 2019, 5, 10–18. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Cao, S.; Liang, Y.; Xu, Y. Warburg Effects in Cancer and Normal Proliferating Cells: Two Tales of the Same Name. Genom. Proteom. Bioinform. 2019, 17, 273–286. [Google Scholar] [CrossRef]
- Preta, G. New Insights Into Targeting Membrane Lipids for Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 571237. [Google Scholar] [CrossRef] [PubMed]
- Cressman, S.; Sun, Y.; Maxwell, E.J.; Fang, N.; Chen, D.D.Y.; Cullis, P.R. Binding and Uptake of RGD-Containing Ligands to Cellular avβ3 Integrins. Int. J. Pept. Res. Ther. 2009, 15, 49–59. [Google Scholar] [CrossRef]
- Deng, S.; Li, X.; Liu, S.; Chen, J.; Li, M.; Chew, S.Y.; Leong, K.W.; Cheng, D. Codelivery of CRISPR-Cas9 and chlorin e6 for spatially controlled tumor-specific gene editing with synergistic drug effects. Sci. Adv. 2020, 6, eabb4005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Xie, R.; Burger, J.C.; Tong, Y.; Gong, S. Overcoming the Blood–Brain Barrier for Gene Therapy via Systemic Administration of GSH-Responsive Silica Nanocapsules. Adv. Mater. 2023, 35, e2208018. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-targeted CRISPR/Cas9 nanomedicine for effective glioblastoma therapy. J. Control Release 2022, 351, 739–751. [Google Scholar] [CrossRef]
- Ma, L.; Ma, Y.; Gao, Q.; Liu, S.; Zhu, Z.; Shi, X.; Dai, F.; Reis, R.L.; Kundu, S.C.; Cai, K.; et al. Mulberry Leaf Lipid Nanoparticles: A Naturally Targeted CRISPR/Cas9 Oral Delivery Platform for Alleviation of Colon Diseases. Small 2024, 20, 2307247. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Aguet, F.; Danuser, G.; Schmid, S.L. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol. 2010, 191, 1381–1393. [Google Scholar] [CrossRef]
- Cureton, D.K.; Harbison, C.E.; Cocucci, E.; Parrish, C.R.; Kirchhausen, T. Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures. J. Virol. 2012, 86, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Kibbey, R.G.; Rizo, J.; Gierasch, L.M.; Anderson, R.G. The LDL Receptor Clustering Motif Interacts with the Clathrin Terminal Domain in a Reverse Turn Conformation. J. Cell Biol. 1998, 142, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kida, S.; Kinoshita, M.; Tanaka, S.; Okumura, M.; Koshimura, Y.; Morimoto, H. Non-clinical evaluation of a blood-brain barrier-penetrating enzyme for the treatment of mucopolysaccharidosis type I. Mol Genet Metab. 2019, 126, S83–S84. [Google Scholar] [CrossRef]
- Wiley, L.; Cheek, M.; LaFar, E.; Ma, X.; Sekowski, J.; Tanguturi, N.; Iltis, A. The Ethics of Human Embryo Editing via CRISPR-Cas9 Technology: A Systematic Review of Ethical Arguments, Reasons, and Concerns. HEC Forum, 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Singh, A.; Irfan, H.; Fatima, E.; Nazir, Z.; Verma, A.; Akilimali, A. Revolutionary breakthrough: FDA approves CASGEVY, the first CRISPR/Cas9 gene therapy for sickle cell disease. Ann. Med. Surg. 2024, 86, 4555–4559. [Google Scholar] [CrossRef]
- The U.S. Food and Drug Administration, Human Gene Therapy Products Incorporating Human Genome Editing, Guidance for Industry, January 2024. Available online: https://www.fda.gov/media/156894/download (accessed on 1 January 2025).
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 886. [Google Scholar] [CrossRef]
- Shen, Y.; Gwak, H.; Han, B. Advanced manufacturing of nanoparticle formulations of drugs and biologics using microfluidics. Analyst 2024, 49, 614–637. [Google Scholar] [CrossRef]
- Hussain, M.; Binici, B.; O’Connor, L.; Perrie, Y. Production of mRNA lipid nanoparticles using advanced crossflow micromixing. J. Pharm. Pharmacol. 2024, 76, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Saffie-Siebert, S.; Torabi-Pour, N.; Gibson, A.; Sutera, F.M.; Dehsorkhi, A.; Baran-Rachwalska, P.; Quinn, S. Toward a large-batch manufacturing process for silicon-stabilized lipid nanoparticles: A highly customizable RNA delivery platform. Mol. Ther. Methods Clin. Dev. 2024, 32, 101299. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals and Medical Devices Agency (PMDA), White-Paper for Quality and Safety for Gene Therapy Products Using Gene Editing Technology. Available online: https://www.pmda.go.jp/files/000237636.pdf (accessed on 1 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashima, T. Non-Invasive Delivery of CRISPR/Cas9 Ribonucleoproteins (Cas9 RNPs) into Cells via Nanoparticles for Membrane Transport. Pharmaceutics 2025, 17, 201. https://doi.org/10.3390/pharmaceutics17020201
Tashima T. Non-Invasive Delivery of CRISPR/Cas9 Ribonucleoproteins (Cas9 RNPs) into Cells via Nanoparticles for Membrane Transport. Pharmaceutics. 2025; 17(2):201. https://doi.org/10.3390/pharmaceutics17020201
Chicago/Turabian StyleTashima, Toshihiko. 2025. "Non-Invasive Delivery of CRISPR/Cas9 Ribonucleoproteins (Cas9 RNPs) into Cells via Nanoparticles for Membrane Transport" Pharmaceutics 17, no. 2: 201. https://doi.org/10.3390/pharmaceutics17020201
APA StyleTashima, T. (2025). Non-Invasive Delivery of CRISPR/Cas9 Ribonucleoproteins (Cas9 RNPs) into Cells via Nanoparticles for Membrane Transport. Pharmaceutics, 17(2), 201. https://doi.org/10.3390/pharmaceutics17020201





