Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvent System Selection for Spherical Agglomerates Preparation
2.3. Preparation of Spherical Agglomerates
2.4. Characterization of Spherical Agglomerates
2.4.1. FTIR Spectroscopy (FTIR)
2.4.2. Percentage Yield
2.4.3. Drug Content
2.4.4. Pre-Compression Parameters (Micromeritic Properties)
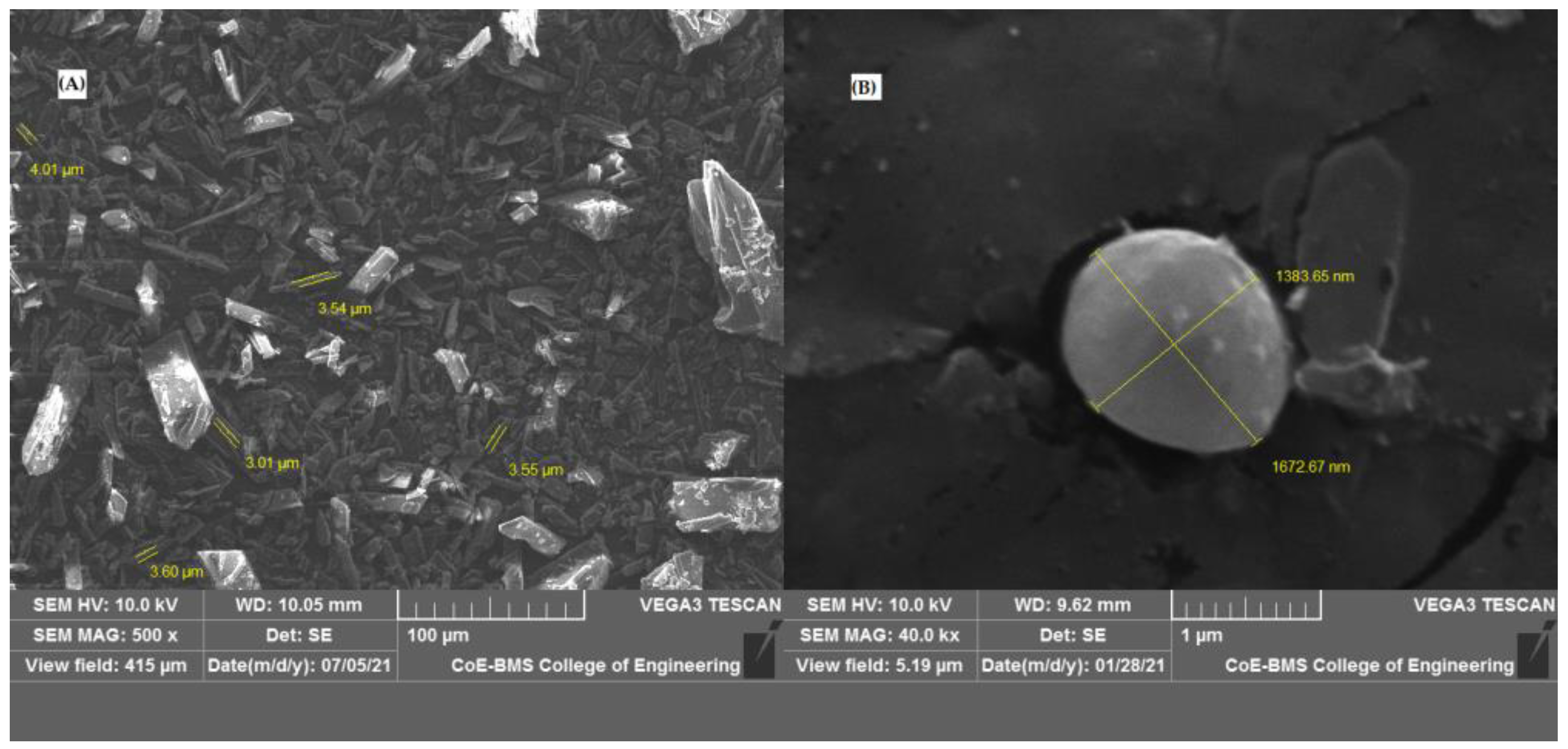
2.4.5. Scanning Electron Microscopy
2.4.6. Particle Size
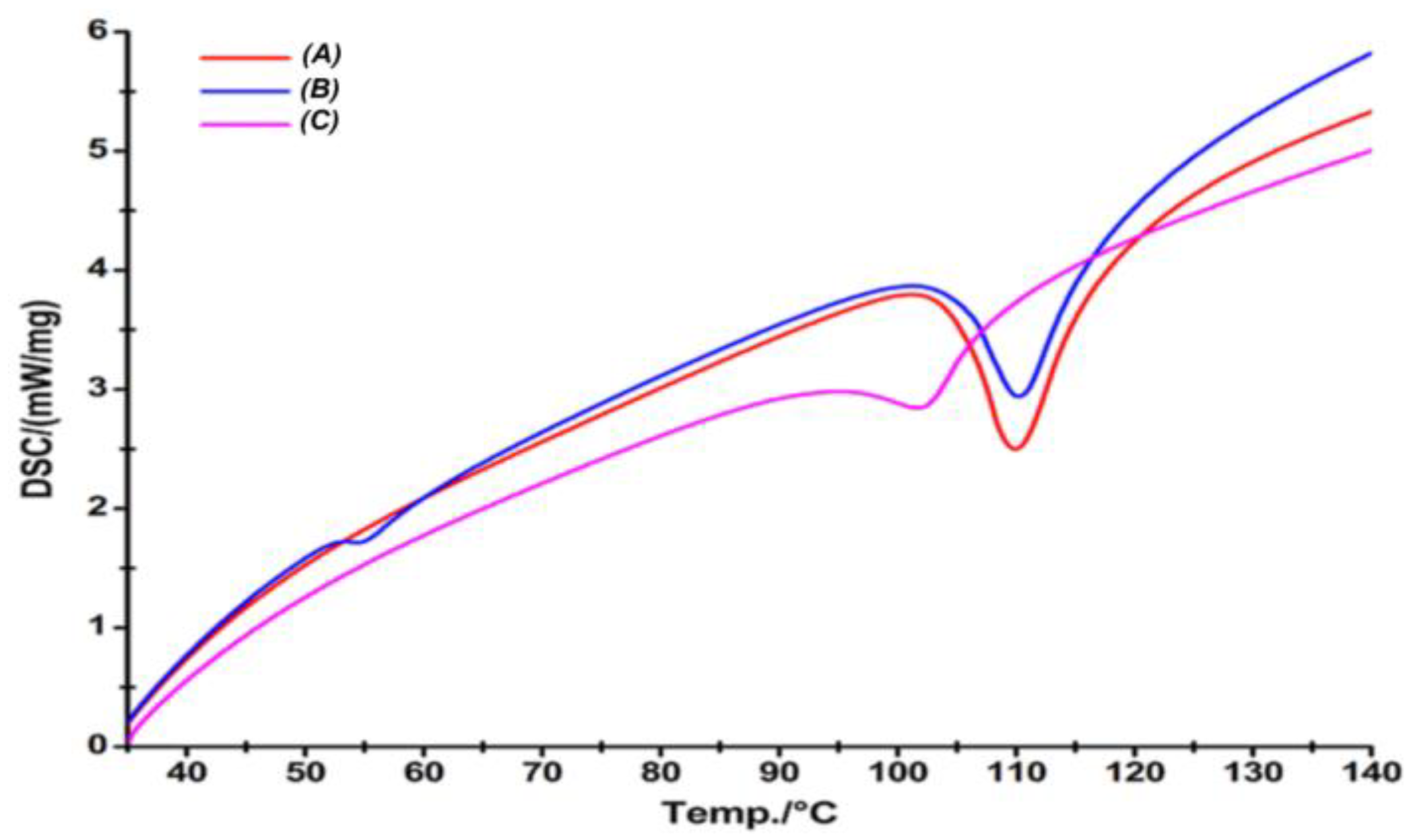
2.4.7. Differential Scanning Calorimetry (DSC)
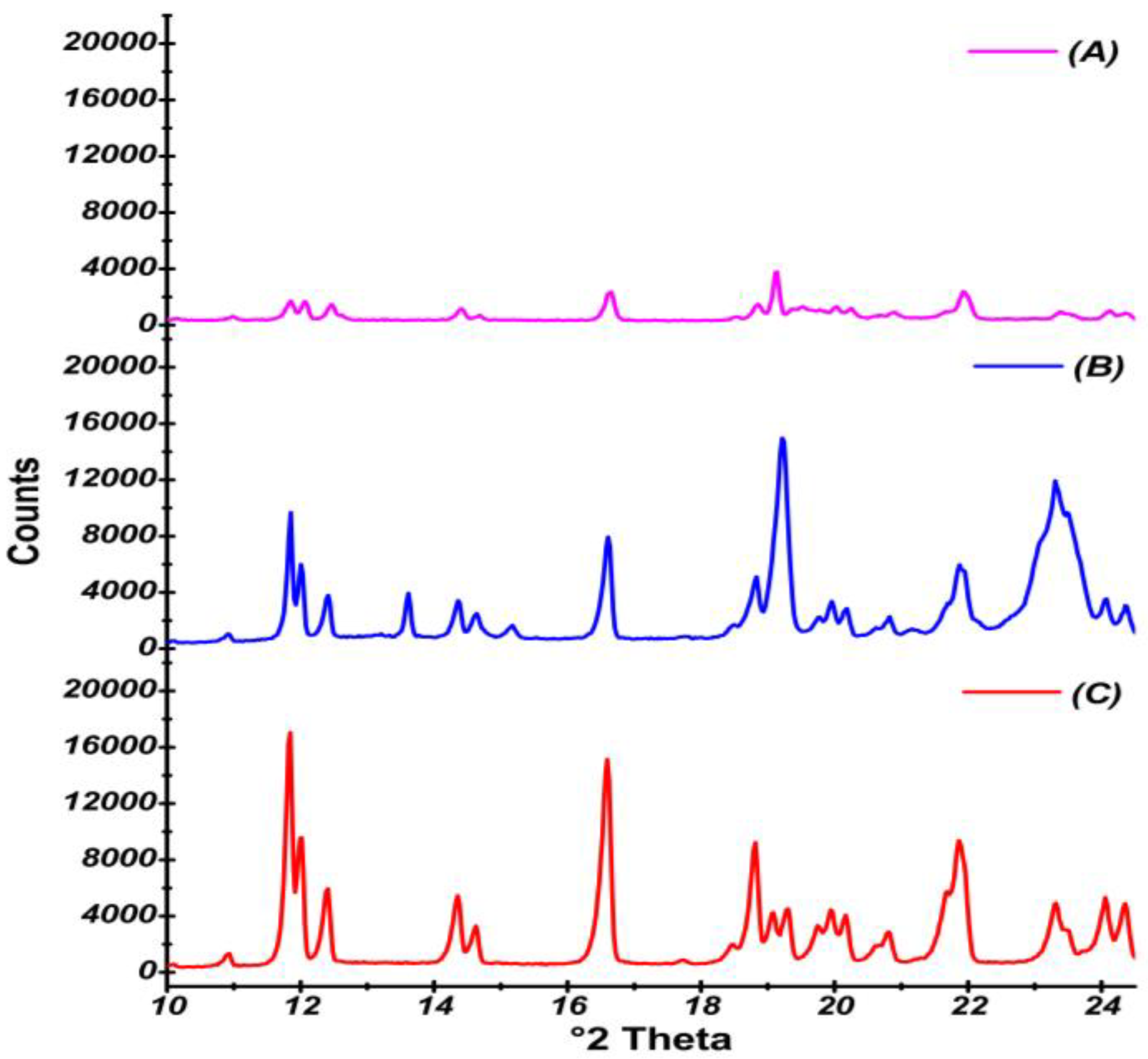
2.4.8. X-Ray Powder Diffraction
2.4.9. In Vitro Release Study of Spherical Agglomerates
2.5. Formulation of Rapid Orodispersible Tablets
2.6. Evaluation of Orodispersible Tablets
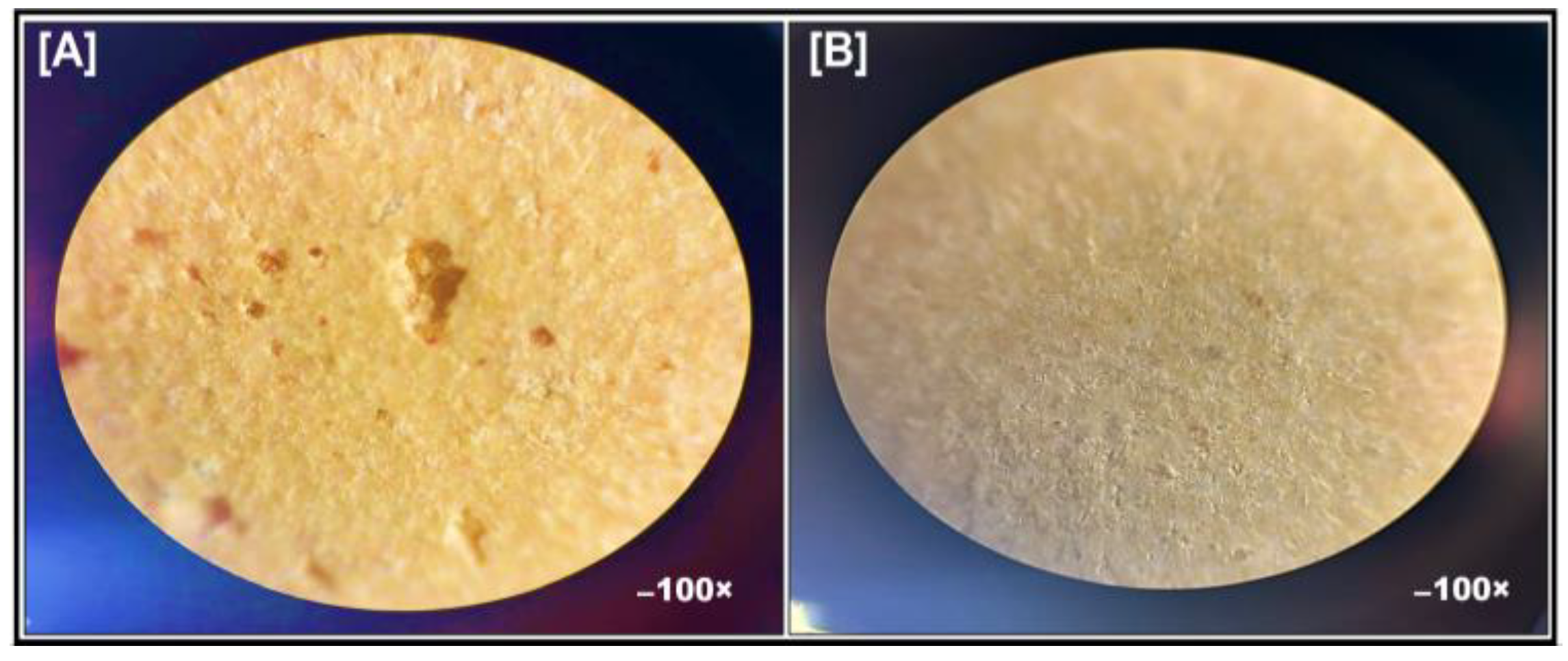
2.6.1. Bright-Field Microscopy
2.6.2. Post-Compression Parameters
Weight Variation
Hardness
Thickness
Friability
Wetting Time
Disintegration Test
Drug Content
2.6.3. In Vitro Dissolution Studies of Prepared Orodispersible Tablets
2.7. Stability Study
2.8. Statistical Analysis
3. Results
3.1. Screening of Solvents for Spherical Agglomerates Preparation
3.2. Evaluation of Prepared Sphere Agglomerates
3.2.1. FTIR Spectroscopy
3.2.2. Percentage Yield
3.2.3. Drug Content
3.2.4. Micromeritic Property
3.2.5. Scanning Electron Microscopy
3.2.6. Particle Size
3.2.7. Differential Scanning Calorimetry
3.2.8. X-Ray Powder Diffraction
3.2.9. In Vitro Release Study of Spherical Agglomerates
3.3. Formulation of Orodispersible Tablets
3.4. Evaluation of Orodispersible Tablets
3.4.1. Bright Field Microscopy
3.4.2. Post-Compression Parameters
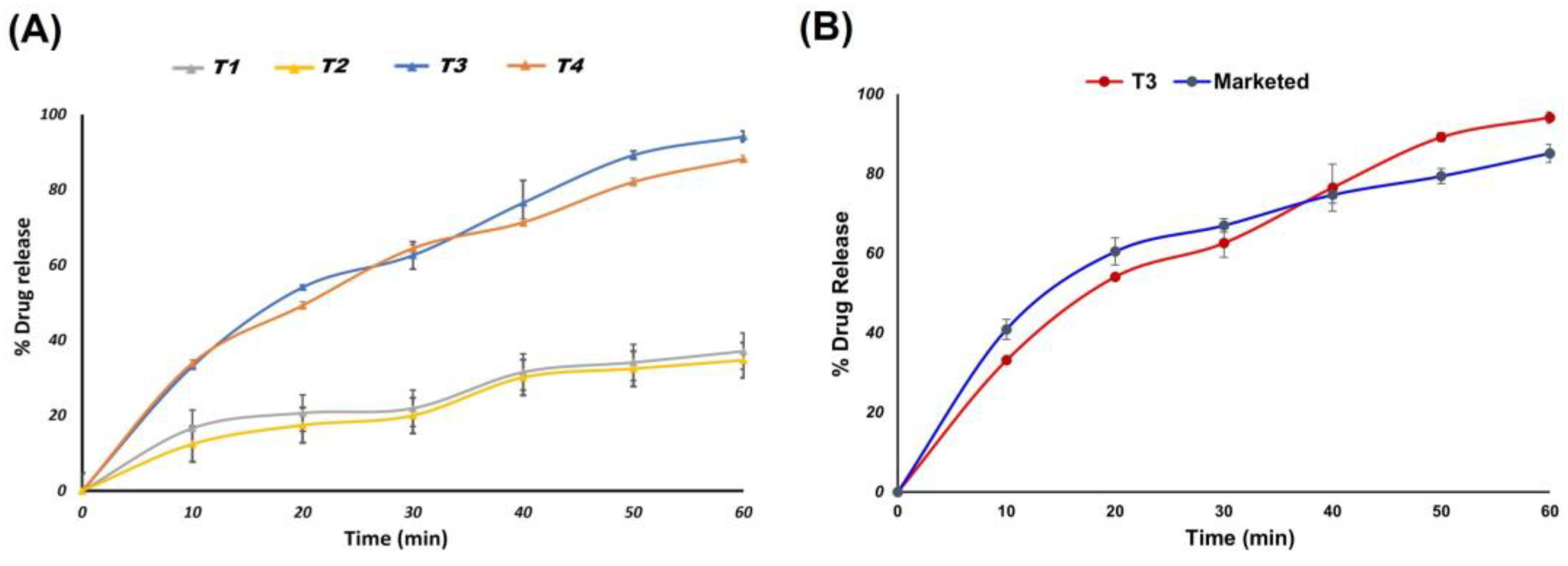
3.4.3. In Vitro Release Study of Prepared Orodispersible Tablets
3.5. Stability Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shankar, R.; Singh, G.P. Prevalence and associated risk factors of hypertension: A cross-sectional study in urban Varanasi. Int. J. Hypertens. 2017, 17, 5491838. [Google Scholar] [CrossRef]
- Mehta, K.K.; Tiwaskar, M.; Kasture, P. Cilnidipine, a Dual L/N-Type Ca2+ Channel Blocker in Hypertension Management: A Review. J. Assoc. Phys. India 2024, 72, 54–58. [Google Scholar]
- Anand, K.; Mandal, P.; Karmakar, S.; Bhowmik, R.; Shaharyar, M.A.; Mandal, A.; Sarkar, A.; De, A.; Chakraborty, S.; Ray, S.; et al. Evaluation of Cilnidipine-Loaded Self-Micro-Emulsifying Drug Delivery System (SMEDDS) by Quantification of Comparative Pharmacokinetic Parameters Using Validated LC-ESI-MS/MS Bioanalytical Method and Pharmacodynamic Assessment. Ann. Pharm. Fr. 2024, 82, 1134–1149. [Google Scholar] [CrossRef]
- Ohno, S.; Ishii, A.; Yanagita, M.; Yokoi, H. Calcium channel blocker in patients with chronic kidney disease. Clin. Exp. Nephrol. 2022, 26, 20715. [Google Scholar] [CrossRef]
- Shaikh, F.; Patel, M.; Patel, V.; Patel, A.; Shinde, G.; Shelke, S. Formulation and optimization of cilnidipine loaded nanosuspension for the enhancement of solubility, dissolution and bioavailability. J. Drug Deliv. Sci. Technol. 2022, 69, 103066. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Song, W.; Gu, D.; Hu, Q. Investigation of inclusion complex of cilnidipine with hydroxypro-pyl-β-cyclodextrin. Carbohydr. Polym. 2012, 90, 1719–1724. [Google Scholar] [CrossRef]
- Mohana, M.; Vijayalakshmi, S. Development and characterization of solid dispersion-based orodispersible tablets of cilnidipine. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 83. [Google Scholar] [CrossRef]
- Mhetre, R.L.; Hol, V.B.; Chanshetti, R.R.; Dhole, S.N. Optimisation of cilnidipine nanoparticles using box-behnken de-sign: In-vitro, toxicity and bioavailability assessment. Mater. Technol. 2022, 37, 1796–1807. [Google Scholar] [CrossRef]
- Liu, Q.; Mai, Y.; Gu, X.; Zhao, Y.; Di, X.; Ma, X. A wet-milling method for the preparation of cilnidipinenanosus-pension with enhanced dissolution and oral bioavailability. J. Drug Deliv. Sci. Technol. 2020, 55, 101371. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Ma, Y.; Wu, S.; Gong, J. Optimization of Green Spherical Agglomeration Process Based on Response Surface Methodology for Preparation of High-Performance Spherical Particles. Int. J. Pharm. 2024, 662, 124515. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.K.; Sorathiya, K.R.; Chauhan, N.P.; Patel, J.M.; Parikh, R.K.; Sheth, N.R. Influence of polymers/excipients on de-velopment of agglomerated crystals of secnidazole by crystallo-co-agglomeration technique to improve processability. Drug Dev. Ind. Pharm. 2013, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.A.; Osman, D.A.; El-Fattah, A.; Amany, I. Improvement of Class II Drug Properties by Crystal Agglomeration Design. Azhar Int. J. Pharm. Med. Sci. 2024, 4, 125–140. [Google Scholar] [CrossRef]
- Sun, M.; Bi, J.; Zhao, Y.; Gong, J. Particle Design of Drugs via Spherical Crystallization: A Review from Fundamental Aspects to Technology Development. Cryst. Growth Des. 2024, 24, 2266–2287. [Google Scholar] [CrossRef]
- Diwan, R.; Ravi, P.R.; Pathare, N.S.; Aggarwal, V. Pharmacodynamic, pharmacokinetic and physical characterization of cilnidipine loaded solid lipid nanoparticles for oral delivery optimized using the principles of design of experiments. Colloids Surf. B Biointerfaces 2020, 193, 111073. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Yu, C.; Yao, M.; Xu, S.; Tang, W. Strategy of selecting solvent systems for spherical agglomeration by the Lifshitz-van der Waals acid-base approach. Chem. Eng. Sci. 2020, 220, 115613. [Google Scholar] [CrossRef]
- Jadhav, N.; Pawar, A.; Paradkar, A. Design and evaluation of deformable talc agglomerates prepared by crystal-lo-co-agglomeration technique for generating heterogenous matrix. AAPS PharmaSciTech 2007, 8, E61–E67. [Google Scholar] [CrossRef]
- Fadke, J.; Desai, J.; Thakkar, H. Formulation Development of Spherical Crystal Agglomerates of Itraconazole for Preparation of Directly Compressible Tablets with Enhanced Bioavailability. AAPS PharmSciTech 2015, 16, 1434–1444. [Google Scholar] [CrossRef][Green Version]
- Alhamhoom, Y.; Said, A.K.; Kumar, A.; Nanjappa, S.H.; Wali, D.; Rahamathulla, M.; Farhana, S.A.; Ahmed, M.M.; Shivanandappa, T.B. Sublingual Fast-Dissolving Thin Films of Loratadine: Characterization, In Vitro and Ex Vivo Evaluation. Polymers 2024, 16, 2919. [Google Scholar] [CrossRef]
- Varia, U.; Patel, A.; Katariya, H. Formulation and optimization of polymeric agglomerates of Bosentan monohydrate by crystallo-co-agglomeration technique. Bull. Natl. Res. Cent. 2022, 46, 156. [Google Scholar] [CrossRef]
- Fonner, D.E.; Banker, G.S.; Swarbrick, J. Micromeritics of granular pharmaceutical solids I. J. Pharm. Sci. 1966, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Park, C.; Kang, T.; Park, Y.J.; Oh, E.; Lee, B.J. Micromeritic properties and instrumental analysis of physical mixtures and solid dispersions with adsorbent containing losartan: Comparison of dissolution-differentiating factors. Powder Technol. 2015, 272, 269–275. [Google Scholar] [CrossRef]
- Müller, D.; Fimbinger, E.; Brand, C. Algorithm for the determination of the angle of repose in bulk material analysis. Powder Technol. 2021, 383, 598–605. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, A. Scanning electron microscope. In Characterization of Polymers and Fibres; Elsevier: Amsterdam, The Netherlands, 2022; pp. 387–419. [Google Scholar]
- Choi, M.-J.; Woo, M.R.; Choi, H.-G.; Jin, S.G. Effects of Polymers on the Drug Solubility and Dissolution Enhancement of Poorly Water-Soluble Rivaroxaban. Int. J. Mol. Sci. 2022, 23, 9491. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.; Pastorin, G.; Ng, W.K.; Tan, R.B.H. Spherical agglomerates of pure drug nanoparticles for improved pulmonary delivery in dry powder inhalers. J. Nanopart. Res. 2013, 15, 1560. [Google Scholar] [CrossRef]
- Avlani, D.; Kumar, A.; Shivakumar, H.N. Development of dispersible vaginal tablets of tenofovir loaded mucoadhesive chitosan microparticles for anti-HIV pre-exposure prophylaxis. Mol. Pharm. 2023, 20, 5006–5018. [Google Scholar] [CrossRef]
- Gowrav, M.P.; Siree, K.G.; Amulya, T.M.; Bharathi, M.B.; Ghazwani, M.; Alamri, A.; Alalkami, A.Y.; Kumar, T.M.P.; Ahmed, M.M.; Rahamathulla, M. Novel Rhinological Application of Polylactic Acid—An In Vitro Study. Polymers 2023, 15, 2521. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Tajalli, B.A.S. Evaluation of physicomechanical properties of drug-excipients agglomerates obtained by crystallization. Pharm. Dev. Technol. 2010, 16, 243–249. [Google Scholar] [CrossRef]
- Zaki, R.M.; El Sayeh Abou El Ela, A.; Almurshedi, A.S.; Aldosari, B.N.; Aldossari, A.A.; Ibrahim, M.A. Fabrication and Assessment of Orodispersible Tablets Loaded with Cubosomes for the Improved Anticancer Activity of Simvastatin against the MDA-MB-231 Breast Cancer Cell Line. Polymers 2023, 15, 1774. [Google Scholar] [CrossRef]
- El, M.G.M.; Sergany, R.N. Fast disintegrating tablets of nisoldipine for intra-oral administration. Pharm. Dev. Technol. 2014, 19, 641–650. [Google Scholar] [CrossRef]
- Alhamhoom, Y.; Kumaraswamy, T.; Kumar, A.; Nanjappa, S.H.; Prakash, S.S.; Rahamathulla, M.; Thajudeen, K.Y.; Ahmed, M.M.; Shivanandappa, T.B. Formulation and Evaluation of pH-Modulated Amorphous Solid Dispersion-Based Orodispersible Tablets of Cefdinir. Pharmaceutics 2024, 16, 866. [Google Scholar] [CrossRef] [PubMed]
- Dalvadi, H.; Parmar, K.; Yadav, S. Spherical agglomeration to improve dissolution and micromeritic properties of an anticancer drug, Bicalutamide. Drug Dev. Ind. Pharm. 2019, 45, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.; Lasher, J.; Alexander, K.S.; Baki, G. A new modified wetting test and an alternative disintegration test for orally disintegrating tablets. J. Pharm. Biomed. Anal. 2016, 120, 391–396. [Google Scholar] [CrossRef]
- Brniak, W.; Jachowicz, R.; Pelka, P. The practical approach to the evaluation of methods used to determine the dis-integration time of orally disintegrating tablets (ODTs). Saudi Pharm. J. 2015, 23, 437–443. [Google Scholar] [CrossRef]
- Hellberg, E.; Westberg, A.; Appelblad, P.; Mattsson, S. Evaluation of dissolution techniques for orally disintegrating mini-tablets. J. Drug Deliv. Sci. Technol. 2021, 61, 102191. [Google Scholar] [CrossRef]
- Karemore, M.N.; Bali, N.R. Gellan gum based gastroretentive tablets for bioavailability enhancement of cilnidipine in human volunteers. Int. J. Biol. Macromol. 2021, 174, 424–439. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S.Y.; Park, S.J.; Lee, J.; Cho, K.H.; Jee, J.P.; Kim, H.C.; Maeng, H.J.; Jang, D.J. Development and evaluation of a reconstitutable dry suspension to improve the dissolution and oral absorption of poorly water-soluble celecoxib. Pharmaceutics 2018, 10, 140. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; El Nabarawi, M.A.; Shalaby, S.H.; El-Bary, A.A. Sertraline-cyclodextrin complex orodispersible sublingual tablet: Optimization, stability, and pharmacokinetics. J. Pharm. Innov. 2021, 16, 53–66. [Google Scholar] [CrossRef]
- Rahate, N.B.; Bodhankar, M.M.; Dhoke, P.N. Crystallo-co-agglomeration: A novel technique to improve flow and compressibility. J. Drug Deliv. Ther. 2013, 3, 178–183. [Google Scholar] [CrossRef][Green Version]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef]
- Garala, K.C.; Patel, J.M.; Dhingani, A.P.; Dharamsi, A.T. Quality by design (QbD) approach for developing agglomer-ates containing racecadotril and loperamide hydrochloride by crystallo-co-agglomeration. Powder Technol. 2013, 247, 128–146. [Google Scholar] [CrossRef]
- Chen, C.; Xie, X.; Li, Y.; Zhou, C.; Song, Y.; Yan, Z.; Yang, X. Influence of different polymers on crystallization tendency and dissolution behavior of cilnidipine in solid dispersions. Drug Dev. Ind. Pharm. 2014, 40, 441–451. [Google Scholar] [CrossRef]
- Umaredkar, A.A.; Dangre, P.V.; Mahapatra, D.K.; Dhabarde, D.M. Fabrication of chitosan-alginate polyelectrolyte complexed hydrogel for controlled release of cilnidipine: A statistical design approach. Mater. Technol. 2020, 35, 697–707. [Google Scholar] [CrossRef]
- Nitsure, A.; Patel, D.; Wairkar, S. Improved processability of ethambutol hydrochloride by spherical agglomeration. Pharm. Dev. Technol. 2020, 25, 376–384. [Google Scholar] [CrossRef]
- Chatzizaharia, K.A.; Hatziavramid, S.S.; Patel, R.; Soniwala, M.; Chavda, J. Development and optimization of press coated tablets of release engineered valsartan for pulsatile delivery. Drug Dev. Ind. Pharm. 2015, 41, 1835–1846. [Google Scholar] [CrossRef]
- Roy, S.K.; Das, P.; Mondal, A.; Mandal, A.; Kuotsu, K. Design, formulation and evaluation of multiparticulate time programmed system of ramipril for pulsed release: An approach in the management of early morning surge in blood pressure. J. Drug Deliv. Sci. Technol. 2021, 62, 102344. [Google Scholar] [CrossRef]
- Gallo, L.; Veronica, R.R.M.; Bucala, V. Development of porous spray-dried inhalable particles using an organic solvent-free technique. Powder Technol. 2019, 342, 642–652. [Google Scholar] [CrossRef]
- Song, H.; Moon, C.; Lee, B.J.; Oh, E. MesoporousProvastatin solid dispersion granules incorporable into Orally Disintegrating Tablets. J. Pharm. Sci. 2018, 107, 1186–1195. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Desai, B.G.; Murthy, S.; Sharma, A. The disintegration and dissolution of Nabumetone Dispersible Tablets. Pharm. Technol. 2007, 31, 60–69. [Google Scholar]
- Indian Pharmacopoeia; Controller of Publications; Government of India: Delhi, India, 2018; Volume II, pp. 1143–1144.
- Elkhodairy, K.A.; Hassan, M.A.; Afifi, S.A. Formulation and optimization of orodispersible tablets of flutamide. Saudi Pharm. J. 2014, 22, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moqbel, H.A.; Meshad, A.N.; Nabarawi, M.A. A pharmaceutical study on chlorzoxazoneorodispersible tablets: Formulation, in-vitro and in-vivo evaluation. Drug Deliv. 2016, 23, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Yassin, S.; Goodwin, D.J.; Anderson, A.; Sibik, J.; Ian, W.D.; Gladden, L.F. The disintegration process in microcrystalline cellulose based tablets, part 1: Influence of temperature, porosity and superdisintegrants. J. Pharm. Sci. 2015, 104, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Alsenaidy, M.A.; Almalki, Z.S.; Fayed, M.H. Development and Optimization of Sildenafil Orodispersible Mini-Tablets (ODMTs) for Treatment of Pediatric Pulmonary Hypertension Using Response Surface Methodology. Pharmaceutics 2023, 15, 923. [Google Scholar] [CrossRef]
- El-Rasoul, S.; Shazly, G.A. PropafenoneHCl fast dissolving tablets containing subliming agent prepared by direct compression. Saudi Pharm. J. 2017, 25, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zaborenko, N.; Shi, Z.; Corredor, C.C.; Smith, G.B.M.; Zhang, L.; Hermans, A. First-principles and empirical approaches to predicting in vitro dissolution for pharmaceutical formulation and process development and for product release testing. AAPS J. 2019, 21, 32. [Google Scholar] [CrossRef]





| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| Cilnidipine | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| GMS | 100 | - | - | - | 50 | 50 | - | 50 |
| PVP K-30 | - | 100 | - | - | 50 | - | 50 | - |
| HPMC E50 | - | - | 100 | - | - | 50 | - | - |
| PEG 6000 | - | - | - | 100 | - | - | 50 | 50 |
| Talc | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Ingredients | T1 * | T2 ^ | T3 * | T4 ^ |
|---|---|---|---|---|
| CIL | 10 | 10 | - | - |
| Spherical agglomerates (~10 mg CIL) | - | - | 15 | 15 |
| Directly compressible mannitol | 19 | 24 | 19 | 24 |
| Microcrystalline cellulose (Avicel -PH 102) | 20 | 25 | 20 | 25 |
| Croscarmellose sodium | 4 | 4 | 4 | 4 |
| Cros-povidone | 4 | 4 | 4 | 4 |
| Ammonium bicarbonate | 15 | - | 15 | - |
| Magnesium stearate | 2 | 2 | 2 | 2 |
| Talc | 1 | 1 | 1 | 1 |
| Total tablet weight | 75 | 70 | 80 | 80 |
| Peak Position (cm−1) | Functional Group | Peak Position in CIL (cm−1) | Peak Position in PM (cm−1) | Peak Position in SAs (cm−1) |
|---|---|---|---|---|
| 3000–2800 | N-H (Stretch) | 3289.0 | 3288.63 | 3280.12 |
| 1725–1705 | C=O (Stretch) | 1697.18 | 1698.16 | 1689.13 |
| 1662–1626 | C=C (Stretch) | 1648.22 | 1646.31 | 1638.34 |
| 1550–1475 | NO2 (Stretch) | 1523.49 | 1522.12 | 1539.30 |
| Formulations | Percentage Yield | % Drug Content |
|---|---|---|
| F1 | 66.82 ± 0.08 | 83.65 ± 0.42 |
| F2 | 82.02 ± 0.06 | 95.18 ± 0.23 |
| F3 | 76.12 ± 0.01 | 94.18 ± 0.12 |
| F4 | 84.02 ± 0.04 | 97.49 ±0.29 |
| F5 | 72.09 ± 0.02 | 91.26 ± 0.16 |
| F6 | 73.18 ± 0.06 | 91.83 ± 0.18 |
| F7 | 80.08 ± 0.03 | 94.23 ± 0.02 |
| F8 | 76.12 ± 0.01 | 94.03 ± 0.06 |
| Formulations | Carr’s Index % | Hausner’s Ratio | Angle of Repose (°) |
|---|---|---|---|
| CIL | 33.6 ± 0.08 | 1.52 ± 0.06 | 31.82 ± 0.02 |
| F1 | 14.11 ± 0.01 | 1.16 ± 0.06 | 23.60 ± 0.02 |
| F2 | 13.50 ± 0.06 | 1.17 ± 0.03 | 22.40 ± 0.03 |
| F3 | 14.90 ± 0.08 | 1.18 ± 0.05 | 23.20 ± 0.08 |
| F4 | 11.66 ± 0.01 | 1.11 ± 0.03 | 23.01 ± 0.03 |
| F5 | 13.82 ± 0.02 | 1.16 ± 0.02 | 24.20 ± 0.07 |
| F6 | 14.73 ± 0.03 | 1.17 ± 0.08 | 23.08 ± 0.02 |
| F7 | 14.22 ± 0.02 | 1.16 ± 0.01 | 23.30 ± 0.03 |
| F8 | 12.52 ± 0.03 | 1.14 ± 0.06 | 23.60 ± 0.06 |
| Formulations | Dissolution Efficiency (%) |
|---|---|
| CIL | 26.27 ± 0.06 |
| F1 | 37.57 ± 0.08 |
| F2 | 56.81 ± 0.03 |
| F3 | 45.89 ± 0.03 |
| F4 | 57.01 ± 0.01 |
| F5 | 36.98 ± 0.05 |
| F6 | 34.05 ± 0.06 |
| F7 | 41.37 ± 0.03 |
| F8 | 40.34 ± 0.01 |
| Parameters | ODTs of CIL | ODTs of SAs | ||
|---|---|---|---|---|
| T1 * | T2 ^ | T3 * | T4 ^ | |
| Weight variation (mg) | 66.12 ± 0.22 | 68.16 ± 0.26 | 73.94 ± 0.38 | 72.35 ± 0.44 |
| Hardness (Kg) | 3.2 ± 0.22 | 3.1 ± 0.23 | 3.5 ± 0.21 | 3.4 ± 0.12 |
| Thickness (mm) | 2.52 ± 0.07 | 2.48 ± 0.03 | 2.70± 0.06 | 2.58 ± 0.04 |
| % Friability | 0.23 ± 0.08 | 0.26 ± 0.06 | 0.28 ± 0.03 | 0.27 ± 0.06 |
| Drug content (%) | 93.92 ± 0.18 | 91.06 ± 0.23 | 93.12 ±0.13 | 96.39 ±0.26 |
| Wetting time (sec) | 19.82 ± 0.04 | 21.68 ± 0.08 | 6.22 ± 0.03 | 7.61 ± 0.06 |
| Disintegration time (sec) | 21.12 ± 0.01 | 25.12 ± 0.06 | 6.26 ± 0.29 | 8.26 ± 0.21 |
| Dissolution (in 60 min.) | 37.10 ± 0.37 | 34.70 ± 0.27 | 94.16 ± 1.41 | 84.75 ± 1.69 |
| PARAMETERS | 0 Months | 1 Months | 2 Months | 3 Months | ||||
|---|---|---|---|---|---|---|---|---|
| Controlled | Accelerated | Controlled | Accelerated | Controlled | Accelerated | Controlled | Accelerated | |
| Weight variation (mg) | 73.96 ± 0.34 | 73.96 ± 0.34 | 73.95 ± 0.30 | 73.93 ± 0.21 | 73.95 ± 0.18 | 73.93 ± 0.23 | 73.91 ± 0.17 | 73.89 ± 0.21 |
| Hardness (Kg) | 3.5 ± 0.23 | 3.5 ± 0.23 | 3.5 ± 0.18 | 3.5 ± 0.13 | 3.5 ± 0.14 | 3.5 ± 0.19 | 3.5 ± 0.12 | 3.4 ± 0.13 |
| Thickness (mm) | 2.70 ± 0.03 | 2.70 ± 0.03 | 2.70 ± 0.04 | 2.70 ± 0.06 | 2.70 ± 0.03 | 2.70 ± 0.06 | 2.70 ± 0.08 | 2.68 ± 0.08 |
| % Friability | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.28 ± 0.023 | 0.28 ± 0.025 | 0.28 ± 0.72 | 0.28 ± 0.98 | 0.28 ± 0.84 | 0.29 ± 0.95 |
| Drug content (%) | 95.12 ± 0.13 | 95.12 ± 0.13 | 95.11 ± 0.07 | 95.07 ± 0.11 | 94.93 ± 0.11 | 94.99 ± 0.12 | 94.90 ± 0.11 | 93.93 ± 0.13 |
| Wetting time (sec) | 6.21 ± 0.04 | 6.21 ± 0.04 | 6.22 ± 0.02 | 6.24 ± 0.01 | 6.26 ± 0.03 | 6.29 ± 0.04 | 6.27 ± 0.02 | 6.36 ± 0.04 |
| Disintegration time (sec) | 6.26 ± 0.24 | 6.26 ± 0.24 | 6.27 ± 0.21 | 6.28 ± 0.11 | 6.28 ± 0.19 | 6.29 ± 0.21 | 6.32 ± 0.14 | 6.85 ± 0.23 |
| Dissolution (60 min) | 94.16 ± 1.34 | 94.16 ± 1.34 | 94.14 ± 1.12 | 94.08 ± 1.30 | 94.01 ± 1.18 | 94.03 ± 1.28 | 93.82 ± 1.12 | 93.58 ± 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamhoom, Y.; Prakash, S.S.; Kumar, A.; Nanjappa, S.H.; Rahamathulla, M.; Kamath, M.S.; Farhana, S.A.; Ahmed, M.M.; Boreddy-Shivanandappa, T. Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine. Pharmaceutics 2025, 17, 170. https://doi.org/10.3390/pharmaceutics17020170
Alhamhoom Y, Prakash SS, Kumar A, Nanjappa SH, Rahamathulla M, Kamath MS, Farhana SA, Ahmed MM, Boreddy-Shivanandappa T. Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine. Pharmaceutics. 2025; 17(2):170. https://doi.org/10.3390/pharmaceutics17020170
Chicago/Turabian StyleAlhamhoom, Yahya, Sanjana S. Prakash, Avichal Kumar, Shivakumar Hagalavadi Nanjappa, Mohamed Rahamathulla, Megha S. Kamath, Syeda Ayesha Farhana, Mohammed Muqtader Ahmed, and Thippeswamy Boreddy-Shivanandappa. 2025. "Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine" Pharmaceutics 17, no. 2: 170. https://doi.org/10.3390/pharmaceutics17020170
APA StyleAlhamhoom, Y., Prakash, S. S., Kumar, A., Nanjappa, S. H., Rahamathulla, M., Kamath, M. S., Farhana, S. A., Ahmed, M. M., & Boreddy-Shivanandappa, T. (2025). Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine. Pharmaceutics, 17(2), 170. https://doi.org/10.3390/pharmaceutics17020170







