PEGylated Terpesome-Loaded 3D-Printed Aripiprazole Ocuserts for the Treatment of Ocular Candidiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PEGylated Terpesomes
2.2.2. Characterization of PEGylated Terpesomes
Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
Entrapment Efficiency (EE%)
Central Composite Design for Formulation Optimization
Selection of the Optimum Formulation
Morphological Characterization by Transmission Electron Microscopy (TEM)
Differential Scanning Calorimetry (DSC)
Storage Stability Evaluation
Ocular Tolerance via pH Evaluation
Ex Vivo Corneal Permeation Studies
Confocal Laser Scanning Microscopy (CLSM) Evaluation
Formulation and 3D Printing of Ocuserts
In Vitro Antifungal Efficacy
In Vivo Ocular Evaluation
Histopathological Examination
3. Results and Discussion
3.1. Optimization of PEG-TERs via Central Composite Design
3.2. Effect of Formulation Variables on EE%
3.3. Effect of Formulation Variables on PS
3.4. PDI Evaluation
3.5. Effect of Formulation Variables on ZP
3.6. Selection of the Optimum AR-Loaded PEG-TERs
3.7. Morphological Evaluation by TEM
3.8. Thermal Evaluation by Differential Scanning Calorimetry (DSC)
3.9. Short-Term Storage Stability
3.10. Ocular Tolerance Testing (pH Evaluation)
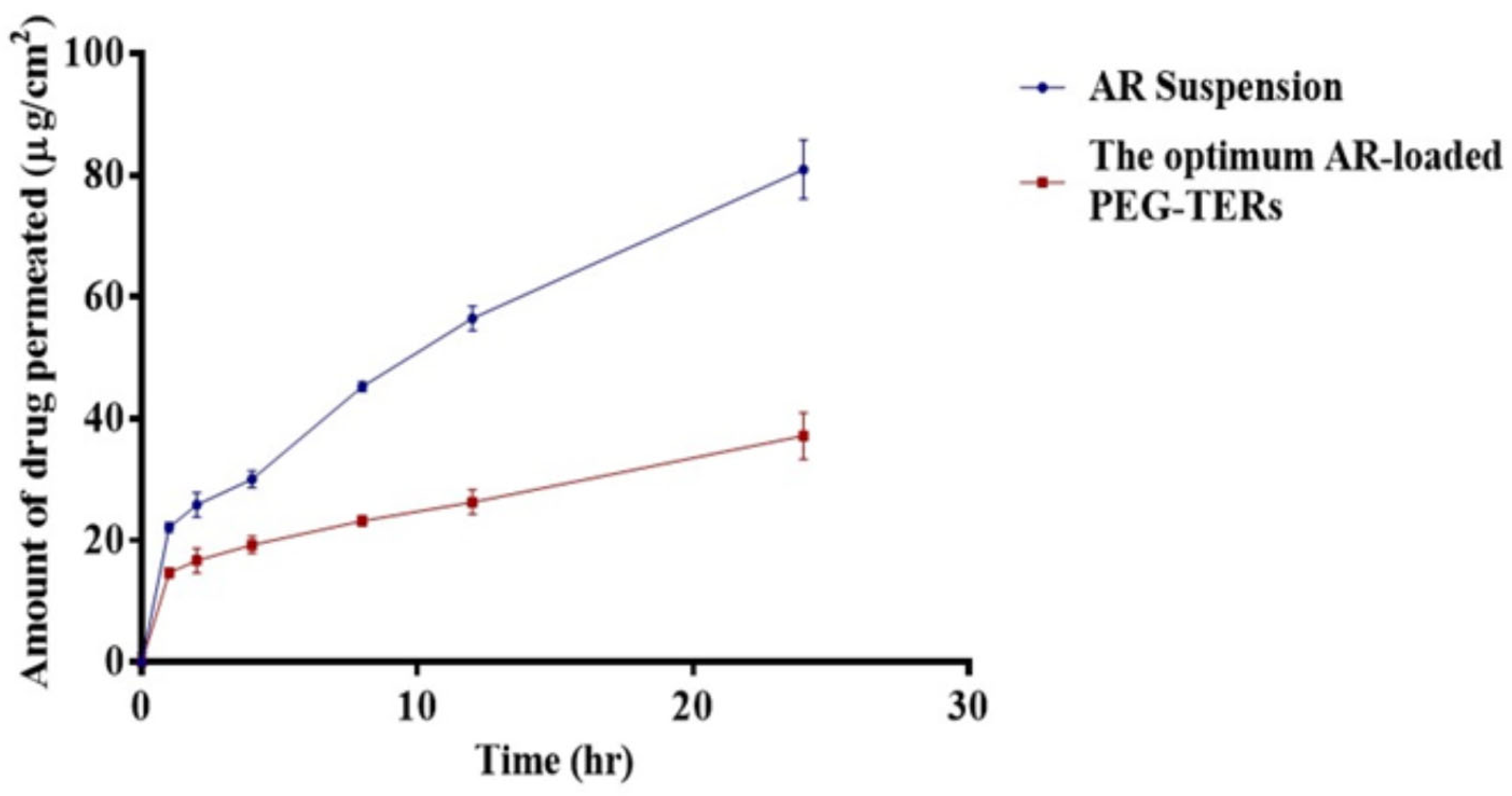
3.11. Ex Vivo Corneal Permeation Evaluation
3.12. Corneal Permeation Evaluation by CLSM
3.13. In Vitro Antifungal Activity
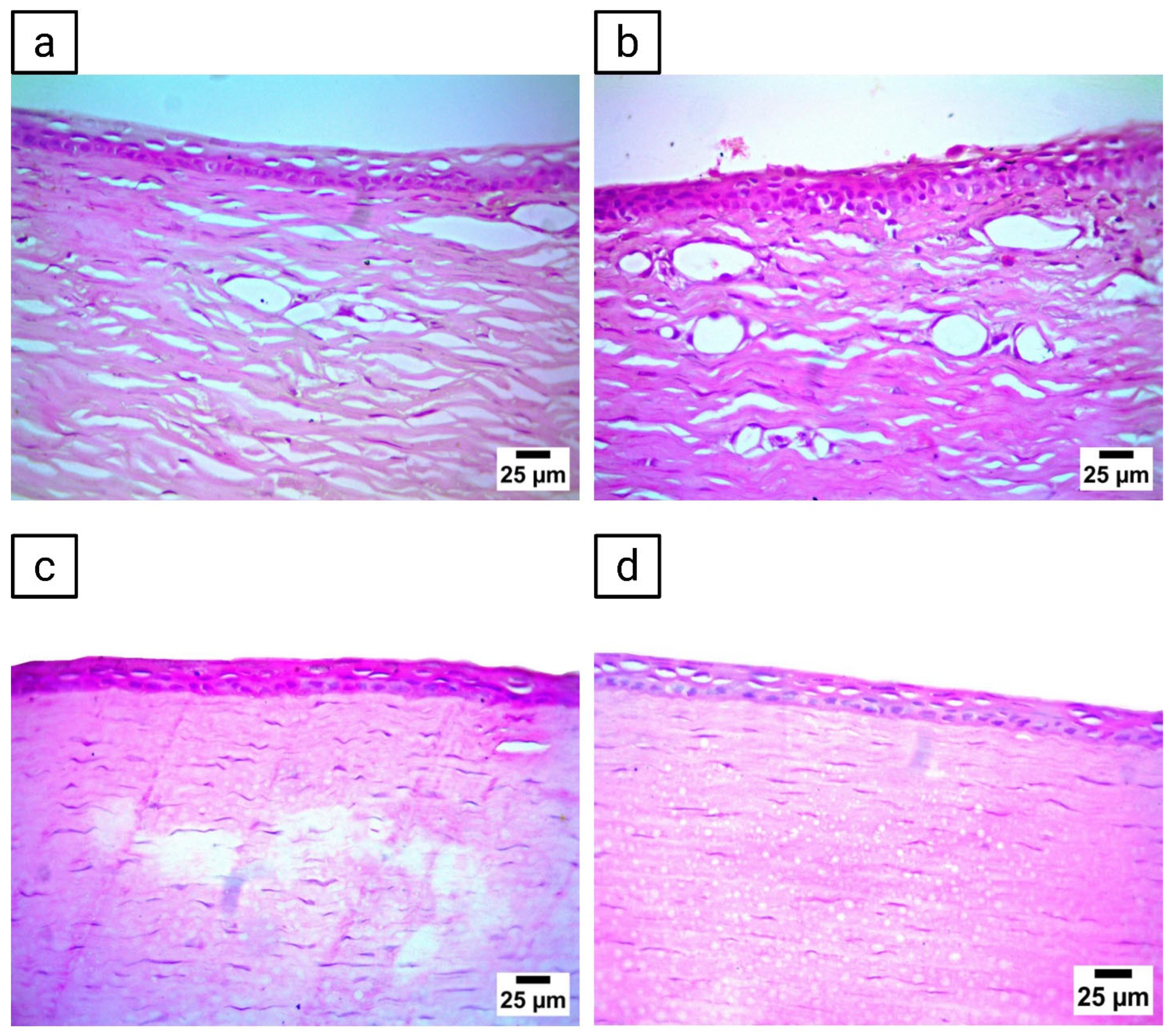
3.14. Histopathological Evaluation
3.15. Future Perspectives and Benefits of the Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrillo, F.; Maione, A.; Spampinato, M.; Massa, L.D.; Guida, M.; Zarrelli, A.; Galdiero, E.; Longobardo, L. Antifungal and Antibiofilm Activities of 2-Aminobenzoic Acid Derivatives Against a Clinical Ocular Candida albicans Isolate for Biomedical Applications. Antibiotics 2025, 14, 432. [Google Scholar] [CrossRef]
- Hetta, H.F.; Melhem, T.; Aljohani, H.M.; Salama, A.; Ahmed, R.; Elfadil, H.; Alanazi, F.E.; Ramadan, Y.N.; Battah, B.; Rottura, M.; et al. Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways. Pharmaceuticals 2025, 18, 364. [Google Scholar] [CrossRef]
- Albash, R.; Yousry, C.; El Hassab, M.A.; Eldehna, W.M.; Alaa-Eldin, A.A. Investigation of Moxifloxacin-loaded terpenes enriched cationic cerosomes (TECs) as an adjunct pulmonary therapy for COVID-19: In-silico study; D-optimal optimization; aerodynamic simulation assessment and cytotoxic evaluation. J. Drug Deliv. Sci. Technol. 2025, 106, 106683. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Zolotareva, D.; Zazybin, A.; Belyankova, Y.; Bayazit, S.; Dauletbakov, A.; Seilkhanov, T.; Kemelbekov, U.; Aydemir, M. Heterocyclic Antidepressants with Antimicrobial and Fungicide Activity. Molecules 2025, 30, 1102. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J.H.; Lee, J. Aripiprazole repurposed as an inhibitor of biofilm formation and sterol biosynthesis in multidrug-resistant Candida albicans. Int. J. Antimicrob. Agents 2019, 54, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Reitler, P.; Regan, J.; DeJarnette, C.; Srivastava, A.; Carnahan, J.; Tucker, K.M.; Meibohm, B.; Peters, B.M.; Palmer, G.E. The atypical antipsychotic aripiprazole alters the outcome of disseminated Candida albicans infections. Infect. Immun. 2024, 92, e00072-24. [Google Scholar] [CrossRef]
- Sawant, K.; Pandey, A.; Patel, S. Aripiprazole loaded poly(caprolactone) nanoparticles: Optimization and in vivo pharmacokinetics. Mater. Sci. Eng. C 2016, 66, 230–243. [Google Scholar] [CrossRef]
- Alimohammadvand, S.; Zenjanab, M.K.; Pakchin, P.S.; Abdolahinia, E.D.; Barar, J.; Omidi, Y.; Pourseif, M.M.; Fathi, M.; Shayegh, J. Aripiprazole-loaded niosome/chitosan-gold nanoparticles for breast cancer chemo-photo therapy. BMC Biotechnol. 2024, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, H.R.F.; Basri, M.; Samiun, W.S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 10, 6469–6471. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; El-Nabarawi, M.A.; Teaima, M.H.; Hassan, M.; Hamed, M.I.A.; Elrashedy, A.A.; Albash, R. Integration of terpesomes loaded Levocetrizine dihydrochloride gel as a repurposed cure for Methicillin-Resistant Staphylococcus aureus (MRSA)-Induced skin infection; D-optimal optimization, ex-vivo, in-silico, and in-vivo studies. Int. J. Pharm. 2023, 633, 122621. [Google Scholar] [CrossRef]
- Teaima, M.H.; Eltabeeb, M.A.; El-Nabarawi, M.A.; Abdellatif, M.M. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: In-vitro, ex-vivo, and in-vivo evaluation. Drug Deliv. 2022, 29, 2549–2560. [Google Scholar] [CrossRef]
- Albash, R.; Abdellatif, M.M.; Hassan, M.; Badawi, N.M. Tailoring terpesomes and leciplex for the effective ocular conveyance of moxifloxacin hydrochloride (Comparative assessment): In-vitro, ex-vivo, and in-vivo evaluation. Int. J. Nanomed. 2021, 16, 5247–5263. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality considerations on the pharmaceutical applications of fused deposition modeling 3D printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef]
- Frohn-Sörensen, P.; Geueke, M.; Engel, B.; Löffler, B.; Bickendorf, P.; Asimi, A.; Bergweiler, G.; Schuh, G. Design for 3D Printed Tools: Mechanical Material Properties for Direct Polymer Additive Tooling. Polymers 2022, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef]
- Naguib, M.J.; Hassan, Y.R.; Abd-Elsalam, W.H. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int. J. Pharm. 2021, 607, 121010. [Google Scholar] [CrossRef]
- Das, S.; D’Souza, S.; Gorimanipalli, B.; Shetty, R.; Ghosh, A.; Deshpande, V. Ocular surface infection mediated molecular stress responses: A review. Int. J. Mol. Sci. 2022, 23, 3111. [Google Scholar] [CrossRef] [PubMed]
- Pal Kaur, I.; Kanwar, M. Ocular preparations: The formulation approach. Drug Dev. Ind. Pharm. 2002, 28, 473–493. [Google Scholar] [CrossRef]
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’Emanuele, A.; Dinarvand, R. Recent developments of nanostructures for the ocular delivery of natural compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef]
- Tsung, T.H.; Tsai, Y.C.; Lee, H.P.; Chen, Y.H.; Lu, D.W. Biodegradable polymer-based drug-delivery systems for ocular diseases. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef]
- Ahmed, S.; Attia, H.; Saher, O.; Fahmy, A.M. Augmented glycerosomes as a promising approach against fungal ear infection: Optimization and microbiological, ex vivo and in vivo assessments. Int. J. Pharm. X 2024, 8, 100295. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbari, M.A.; Elbesh, R.M.; Khaleel, E.F.; Badi, R.M.; Eldehna, W.M.; Elkaeed, E.B.; El Hassab, M.A.; Ahmed, S.M.; Mosallam, S. Sonophoresis mediated diffusion of caffeine loaded Transcutol® enriched cerosomes for topical management of cellulite. Eur. J. Pharm. Sci. 2024, 201, 106875. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, K.; Induri, M.; Sudhakar, M. Validated spectrophotometric quantification of aripiprazole in pharmaceutical formulations by using multivariate technique. Adv. Pharm. Bull. 2013, 3, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Fahmy, A.M.; Shamsel-Din, H.A.; Ibrahim, A.B.; Bogari, H.A.; Malatani, R.T.; Abdelbari, M.A.; Mosallam, S. Intranasal propranolol hydrochloride-loaded PLGA-lipid hybrid nanoparticles for brain targeting: Optimization and biodistribution study by radiobiological evaluation. Eur. J. Pharm. Sci. 2025, 208, 107061. [Google Scholar] [CrossRef]
- Albash, R.; Ali, S.K.; Abdelmonem, R.; Agiba, A.M.; Aldhahri, R.; Saleh, A.; Kassem, A.B.; Abdellatif, M.M. Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies. Pharmaceuticals 2025, 18, 633. [Google Scholar] [CrossRef]
- Eltabeeb, M.A.; Hamed, R.R.; El-Nabarawi, M.A.; Teaima, M.H.; Hamed, M.I.A.; Darwish, K.M.; Hassan, M.; Abdellatif, M.M. Nanocomposite alginate hydrogel loaded with propranolol hydrochloride kolliphor® based cerosomes as a repurposed platform for Methicillin-Resistant Staphylococcus aureus-(MRSA)-induced skin infection; in-vitro, ex-vivo, in-silico, and in-vivo evaluation. Drug Deliv. Transl. Res. 2024, 15, 556–576. [Google Scholar] [CrossRef]
- Djordjević Filijović, N.; Pavlović, A.; Nikolić, K.; Agbaba, D. Validation of an HPLC method for determination of aripiprazole and its impurities in pharmaceuticals. Acta Chromatogr. 2014, 26, 13–28. [Google Scholar] [CrossRef]
- Ahmed, S.; Farag, M.M.; Attia, H.; Balkhi, B.; Adel, I.M.; Nemr, A.A. Exploring the potential of antifungal-loaded proniosomes to consolidate corneal permeation in fungal keratitis: A comprehensive investigation from laboratory characterization to microbiological evaluation. Int. J. Pharm. X 2025, 9, 100322. [Google Scholar] [CrossRef]
- Ahmed, S.; Farag, M.M.; Attia, H.; Balkhi, B.; Adel, I.M.; Nemr, A.A. Terconazole loaded edge-activated hybrid elastosome for revamped corneal permeation in ocular mycosis: In-vitro characterization, statistical optimization, microbiological assessment, and in-vivo evaluation. Int. J. Pharm. X 2025, 9, 100333. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; Elsayed, I.; Aboelwafa, A.A.; Elshafeey, A.H.; Hassan, M. Ocular Mucoadhesive and Biodegradable Sponge-Like Inserts for the Sustained and Controlled Delivery of Voriconazole; Preparation, D-optimal Factorial Optimization and in-vivo Evaluation. J. Pharm. Sci. 2024, 113, 961–973. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-Mahallawi, A.M. Statistical optimization of hyaluronic acid enriched ultradeformable elastosomes for ocular delivery of voriconazole via Box-Behnken design: In vitro characterization and in vivo evaluation. Drug Deliv. 2021, 28, 77–86. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120–132. [Google Scholar] [CrossRef]
- Al-Zheery, W.H.; Kassab, H.J. Effect of Different Types of Terpenes on Disulfiram Loaded Transdermal Invasomes Preparation and in-vitro Characterization. Iraqi J. Pharm. Sci. 2025, 34, 78–89. [Google Scholar] [CrossRef]
- Eltabeeb, M.A.; Abdellatif, M.M.; El-Nabarawi, M.A.; Teaima, M.H.; Hamed, M.I.A.; Darwish, K.M.; Hassan, M.; Hamdan, A.M.; Hamed, R.R. Chitosan decorated oleosomes loaded propranolol hydrochloride hydrogel repurposed for Candida albicans-vaginal infection. Nanomedicine 2024, 19, 1369–1388. [Google Scholar] [CrossRef]
- Salama, A.H.; Aburahma, M.H. Ufasomes nano-vesicles-based lyophilized platforms for intranasal delivery of cinnarizine: Preparation, optimization, ex-vivo histopathological safety assessment and mucosal confocal imaging. Pharm. Dev. Technol. 2016, 21, 706–715. [Google Scholar] [CrossRef]
- Agiba, A.M.; Huerta, L.G.R.; Ulloa-Castillo, N.A.; Sierra-Valdez, F.J.; Beigi-Boroujeni, S.; Lozano, O.; Aguirre-Soto, A. Fusion of polymer-coated liposomes and centrifugally spun microfibers as hybrid materials to enhance sustained release. Nanoscale Adv. 2024, 7, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Pilato, S.; Carradori, S.; Melfi, F.; Di Giacomo, S.; Ciavarella, S.; Ciulla, M.; Fontana, A.; Di Profio, P.; Aschi, M.; Moffa, S.; et al. Phenolic Terpenes in Liposomal Bilayers: Unraveling Physicochemical Interactions and Membrane Perturbation via Biophysical and Computational Approach-Es. J. Colloid Interface Sci. 2025, 700, 138358. [Google Scholar] [CrossRef] [PubMed]
- Silki; Sinha, V.R. Enhancement of In Vivo Efficacy and Oral Bioavailability of Aripiprazole with Solid Lipid Nanoparticles. AAPS PharmSciTech 2018, 19, 1264–1273. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Gazar, A.A.; Abdelbary, A.A.; Elshafeey, A.H.; Mosallam, S. Brij® integrated bilosomes for improving the transdermal delivery of niflumic acid for effective treatment of osteoarthritis: In vitro characterization, ex vivo permeability assessment, and in vivo study. Int. J. Pharm. 2023, 640, 123024. [Google Scholar] [CrossRef]
- Teshima, M.; Kawakami, S.; Nishida, K.; Nakamura, J.; Sakaeda, T.; Terazono, H.; Kitahara, T.; Nakashima, M.; Sasaki, H. Prednisolone retention in integrated liposomes by chemical approach and pharmaceutical approach. J. Control. Release 2004, 97, 211–218. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and In Vivo Skin Deposition Assay. AAPS PharmSciTech 2018, 19, 2174–2184. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed]






| Factors (Independent ariables) | Factor type | Levels | ||
| (−1) | (+1) | |||
| X1: Amount of lipid (mg) | Numeric | 100 | 150 | |
| X2: Amount of terpenes (mg) | Numeric | 10 | 30 | |
| X3: Brij® HLB value | Numeric | 5 | 15 | |
| Responses (Dependent variables) | Constraints | |||
| Y1: EE% | Maximize | |||
| Y2: PS (nm) | Minimize | |||
| Y3: ZP (mV) | Maximize (Absolute value) | |||
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors |
|---|---|---|---|---|---|
| EE% | 0.89 | 0.87 | 0.83 | 19.80 | X1, X2, X3 |
| PS (nm) | 0.90 | 0.88 | 0.87 | 29.93 | X1, X2, X3 |
| ZP (mV) | 0.91 | 0.88 | 0.81 | 16.64 | X1, X2, X3 |
| Formula Code | Lipid Amount (mg) | Terpene Amount (mg) | HLB of Brij® | EE (%) | PS (nm) | PDI | ZP (mV) |
|---|---|---|---|---|---|---|---|
| F1 | 100 | 10 | 5 | 94.80 ± 0.15 | 308.23 ± 2.00 | 0.730 ± 0.012 | −30.92 ± 0.50 |
| F2 | 100 | 30 | 5 | 66.27 ± 0.07 | 355.18 ± 4.50 | 0.727 ± 0.025 | −30.80 ± 0.50 |
| F3 | 100 | 20 | 10 | 88.54 ± 5.02 | 304.44 ± 3.50 | 0.529 ± 0.025 | −34.53 ± 1.50 |
| F4 | 100 | 10 | 15 | 84.82 ± 0.11 | 228.14 ± 3.00 | 0.825 ± 0.02 | −33.70 ± 0.30 |
| F5 | 100 | 30 | 15 | 89.34 ± 0.45 | 302.96 ± 2.00 | 0.431 ± 0.025 | −36.75 ± 1.85 |
| F6 | 125 | 20 | 5 | 86.73 ± 1.41 | 535.50 ± 2.50 | 0.874 ± 0.005 | −36.2 ± 1.00 |
| F7 | 125 | 10 | 10 | 92.28 ± 0.06 | 404.02 ± 2.21 | 0.627 ± 0.025 | −34.26 ± 1.00 |
| F8 | 125 | 20 | 10 | 87.79 ± 0.14 | 417.25 ± 4.49 | 0.779 ± 0.04 | −34.72 ± 1.24 |
| F9 | 125 | 30 | 10 | 79.77 ± 0.04 | 428.59 ± 5.00 | 0.545 ± 0.03 | −37.43 ± 1.00 |
| F10 | 125 | 20 | 15 | 93.98 ± 4.80 | 285.00 ± 5.00 | 0.551 ± 0.045 | −34.10 ± 0.40 |
| F11 | 150 | 10 | 5 | 96.34 ± 0.58 | 510.57 ± 1.00 | 0.746 ± 0.04 | −41.28 ± 0.50 |
| F12 | 150 | 30 | 5 | 74.53 ± 0.18 | 565.94 ± 5.00 | 0.532 ± 0.089 | −40.20 ± 1.00 |
| F13 | 150 | 20 | 10 | 92.54 ± 0.078 | 513.91 ± 5.00 | 0.615 ± 0.010 | −39.60 ± 0.50 |
| F14 | 150 | 10 | 15 | 99.05 ± 0.05 | 447.15 ± 5.00 | 0.742 ± 0.008 | −34.70 ± 0.30 |
| F15 | 150 | 30 | 15 | 93.84 ± 0.04 | 535.83 ± 3.50 | 0.751 ± 0.045 | −38.25 ± 0.55 |
| Formula | Amount Permeated (µg/cm2) | Flux, Jss (µg/cm2/24 h) | Permeation Coefficient, KP (cm/24 h) |
|---|---|---|---|
| AR | 80.94 ± 4.80 | 11.82 | 0.002 |
| The optimum AR-loaded PEG-TERs | 37.16 ± 3.91 | 5.04 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albash, R.; Hassan, M.; Agiba, A.M.; Abd-Elsalam, W.H.; Aziz, D.; Hassan, Y.R.; Kassem, A.B.; Saleh, A.; Eltabeeb, M.A. PEGylated Terpesome-Loaded 3D-Printed Aripiprazole Ocuserts for the Treatment of Ocular Candidiasis. Pharmaceutics 2025, 17, 1616. https://doi.org/10.3390/pharmaceutics17121616
Albash R, Hassan M, Agiba AM, Abd-Elsalam WH, Aziz D, Hassan YR, Kassem AB, Saleh A, Eltabeeb MA. PEGylated Terpesome-Loaded 3D-Printed Aripiprazole Ocuserts for the Treatment of Ocular Candidiasis. Pharmaceutics. 2025; 17(12):1616. https://doi.org/10.3390/pharmaceutics17121616
Chicago/Turabian StyleAlbash, Rofida, Mariam Hassan, Ahmed M. Agiba, Wessam H. Abd-Elsalam, Diana Aziz, Youssef R. Hassan, Amira B. Kassem, Asmaa Saleh, and Moaz A. Eltabeeb. 2025. "PEGylated Terpesome-Loaded 3D-Printed Aripiprazole Ocuserts for the Treatment of Ocular Candidiasis" Pharmaceutics 17, no. 12: 1616. https://doi.org/10.3390/pharmaceutics17121616
APA StyleAlbash, R., Hassan, M., Agiba, A. M., Abd-Elsalam, W. H., Aziz, D., Hassan, Y. R., Kassem, A. B., Saleh, A., & Eltabeeb, M. A. (2025). PEGylated Terpesome-Loaded 3D-Printed Aripiprazole Ocuserts for the Treatment of Ocular Candidiasis. Pharmaceutics, 17(12), 1616. https://doi.org/10.3390/pharmaceutics17121616










