Senotherapeutic Potential of Araliadiol in Senescent Human Dermal Fibroblasts: An In Vitro Study Using Three Senescence Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Culture
2.2. Senescence Cell Models
2.3. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
2.4. Intracellular Reactive Oxygen Species (ROS) Measurement
2.5. Adenosine Triphosphate (ATP) Content Assay
2.6. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blot Analysis
2.8. Immunofluorescence Staining
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
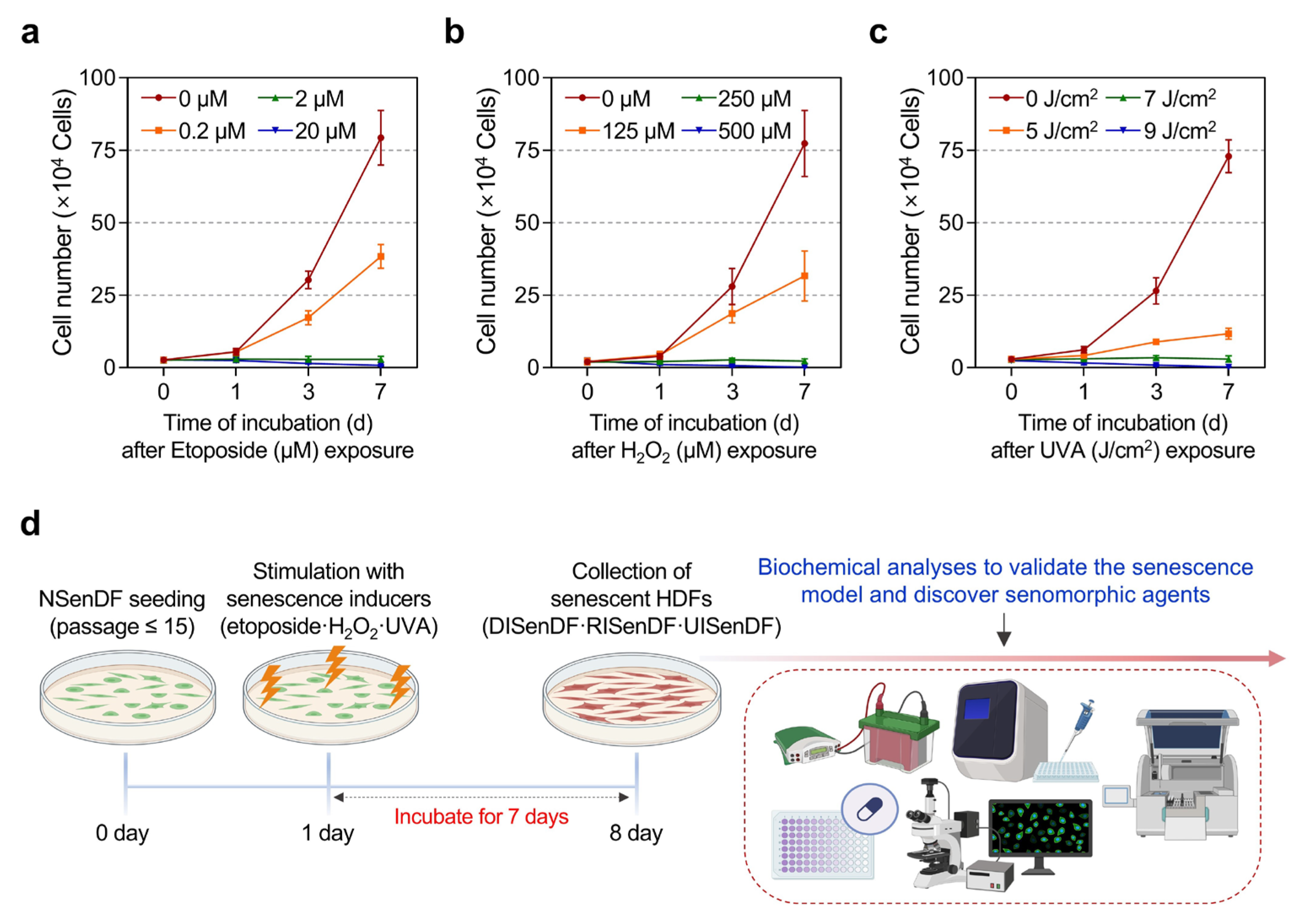
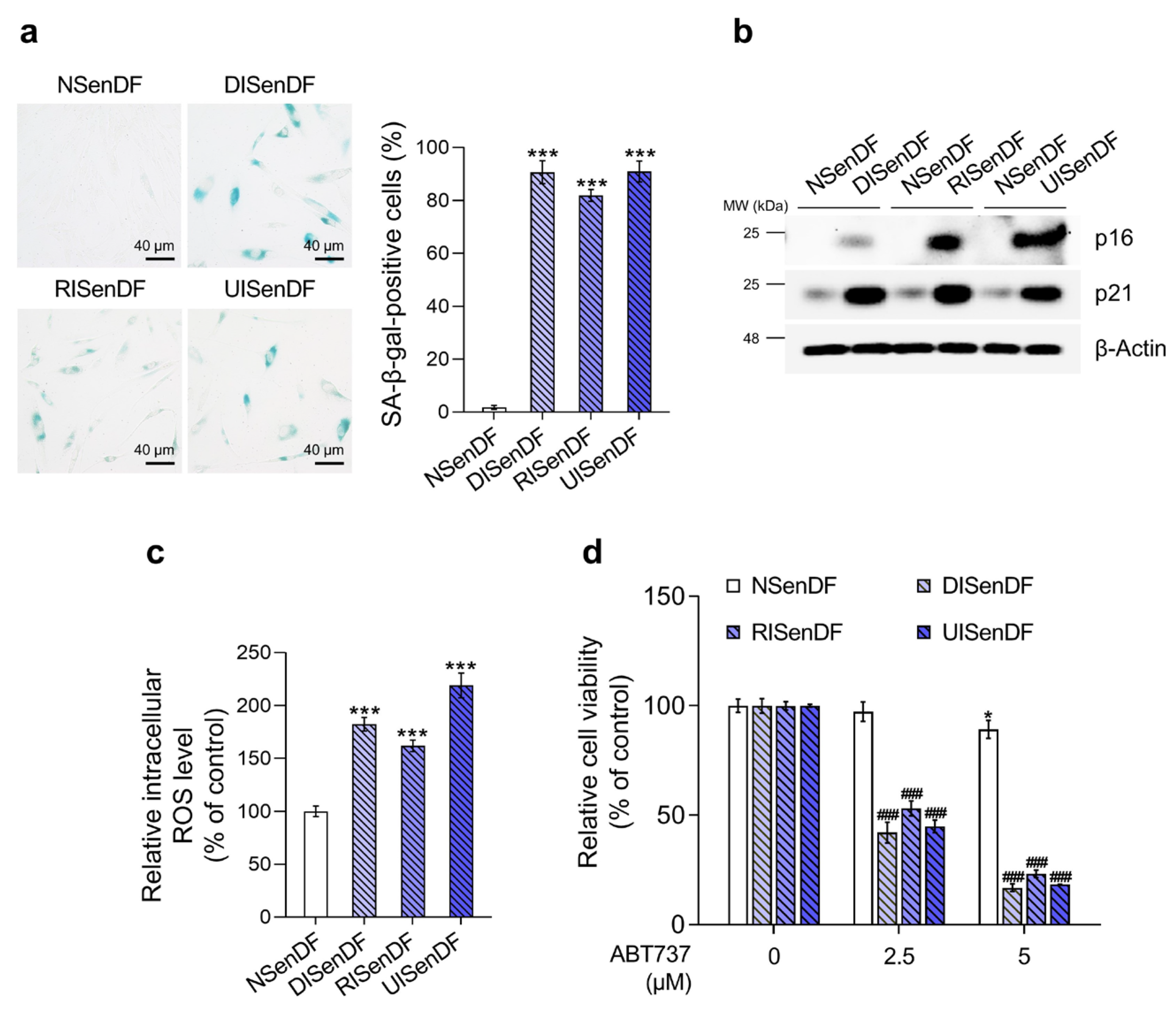
3.1. Establishment of Three Senescence Models in Human Dermal Fibroblasts for Identifying Senotherapeutic Candidates
3.2. Araliadiol Does Not Exhibit Senolytic Activity but Exerts Senomorphic Effects in Senescent Dermal Fibroblasts
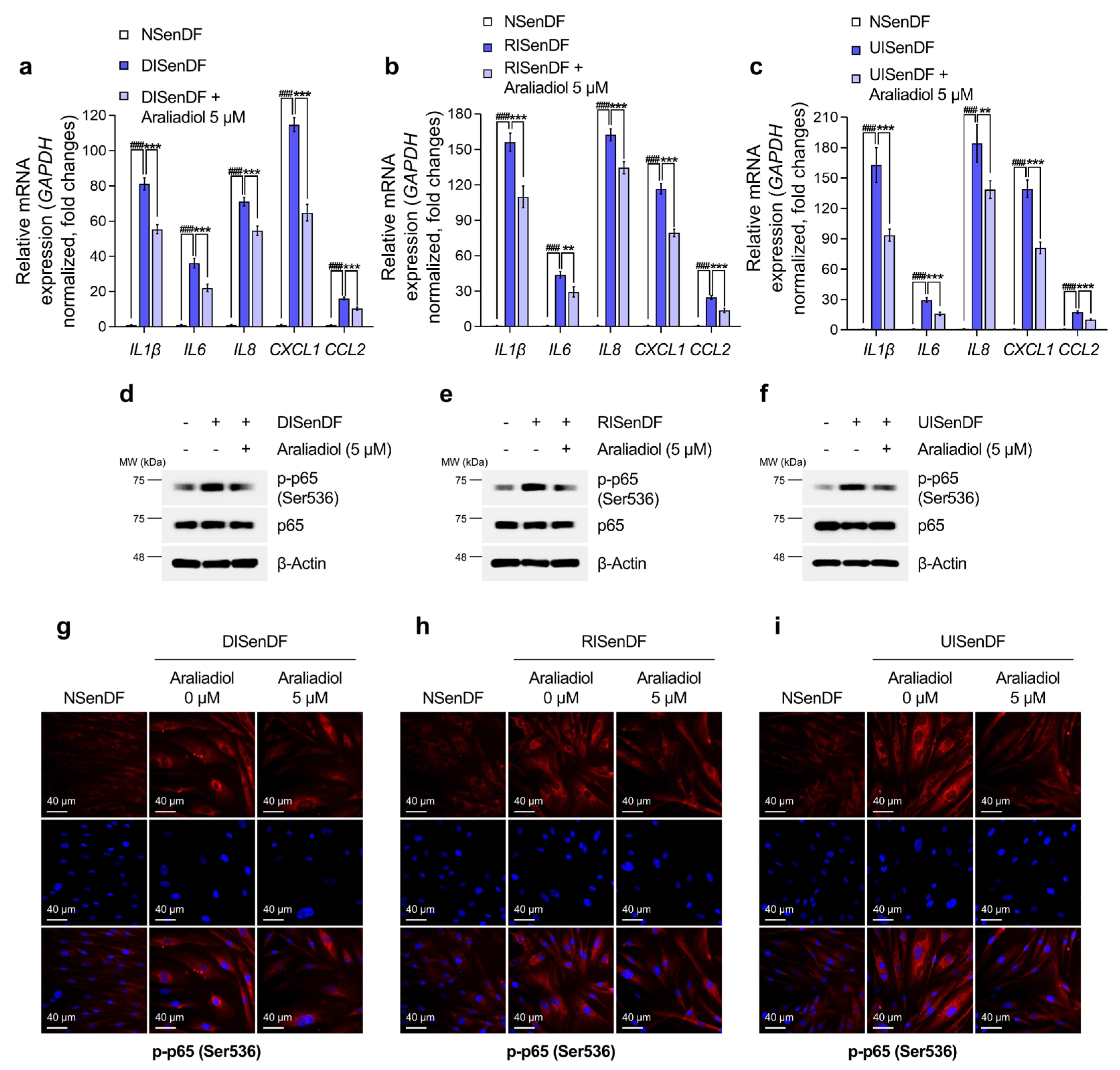
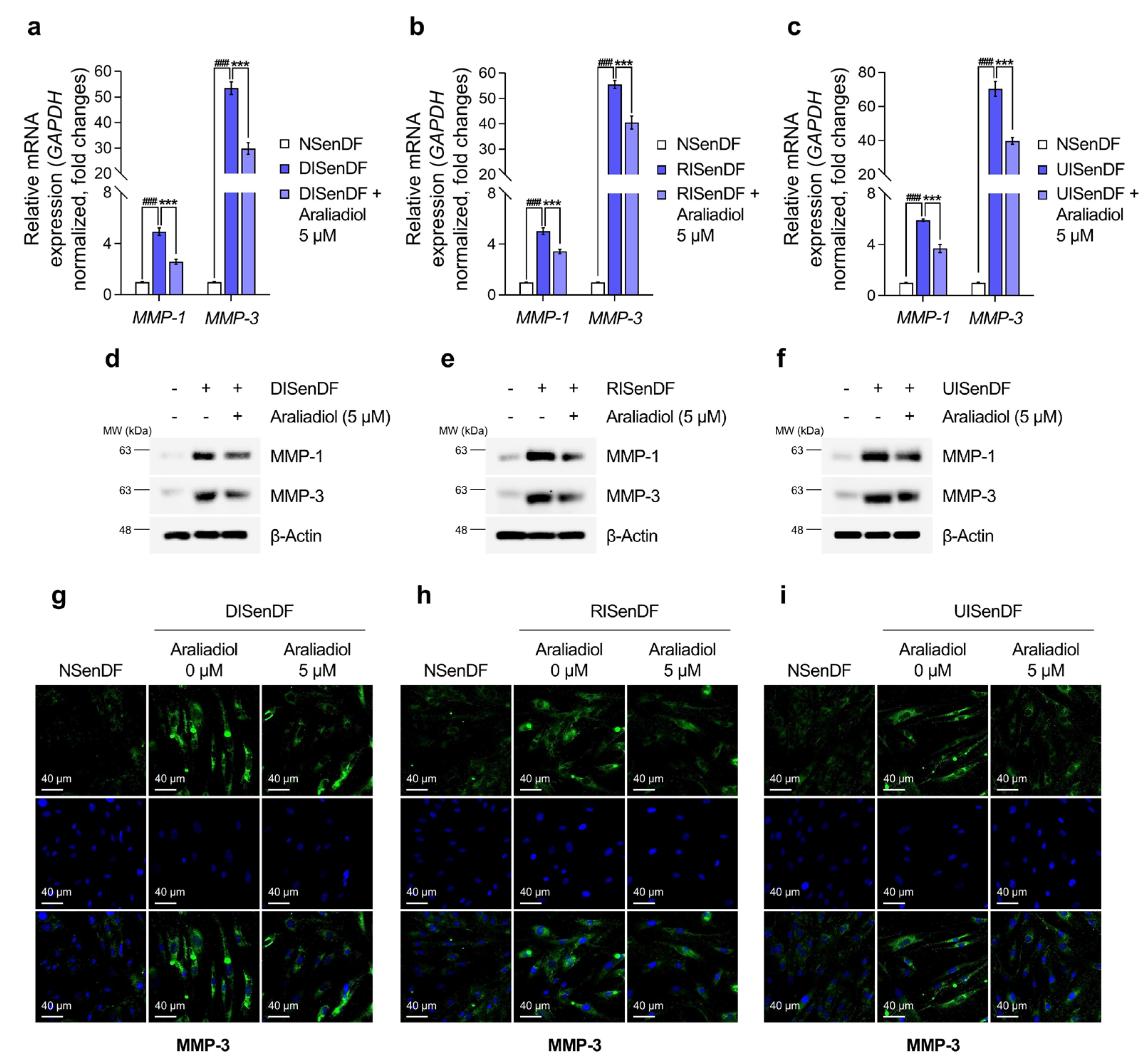
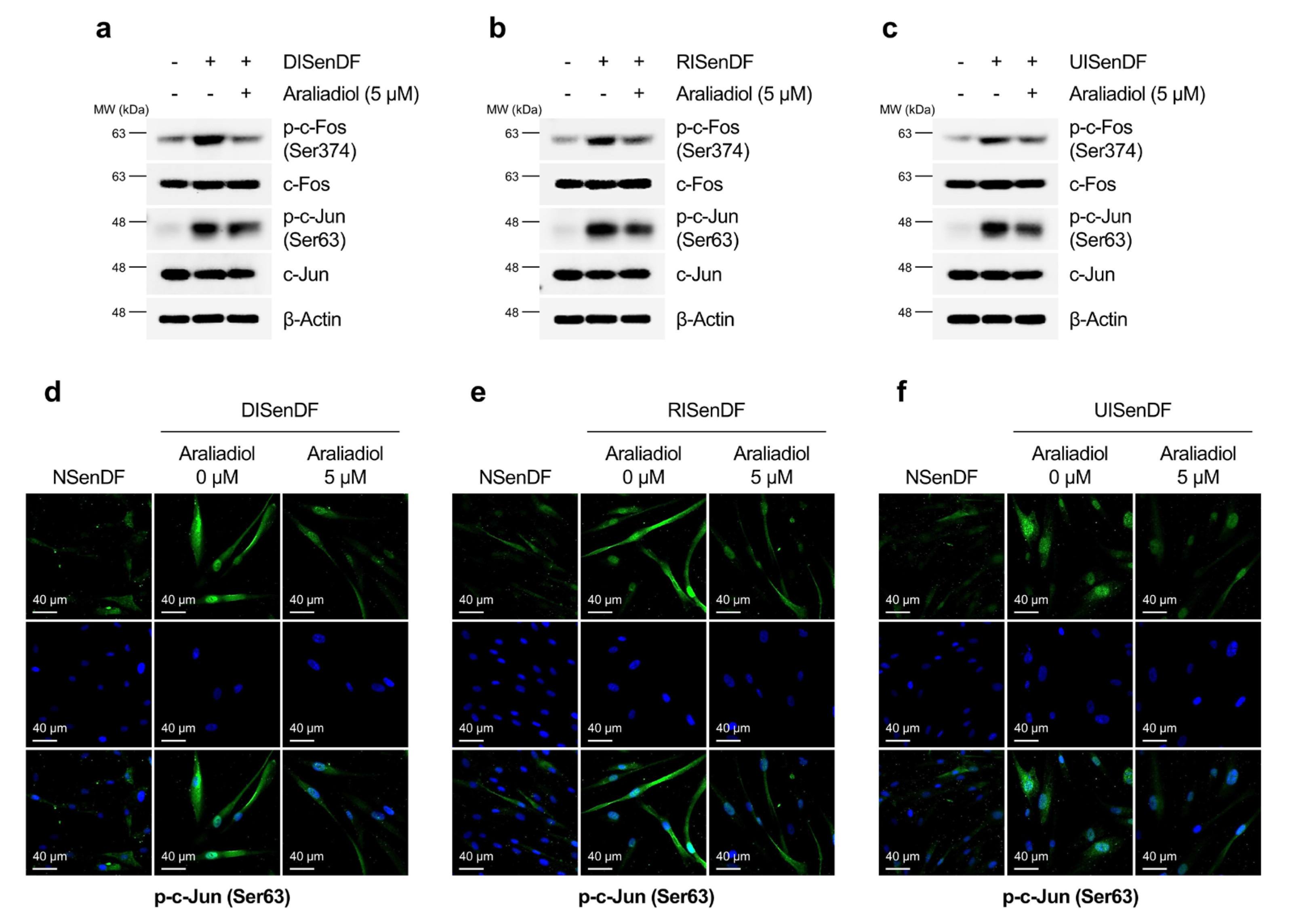
3.3. Araliadiol Suppresses AP-1 Activation and MMP Expression in Senescent Dermal Fibroblasts
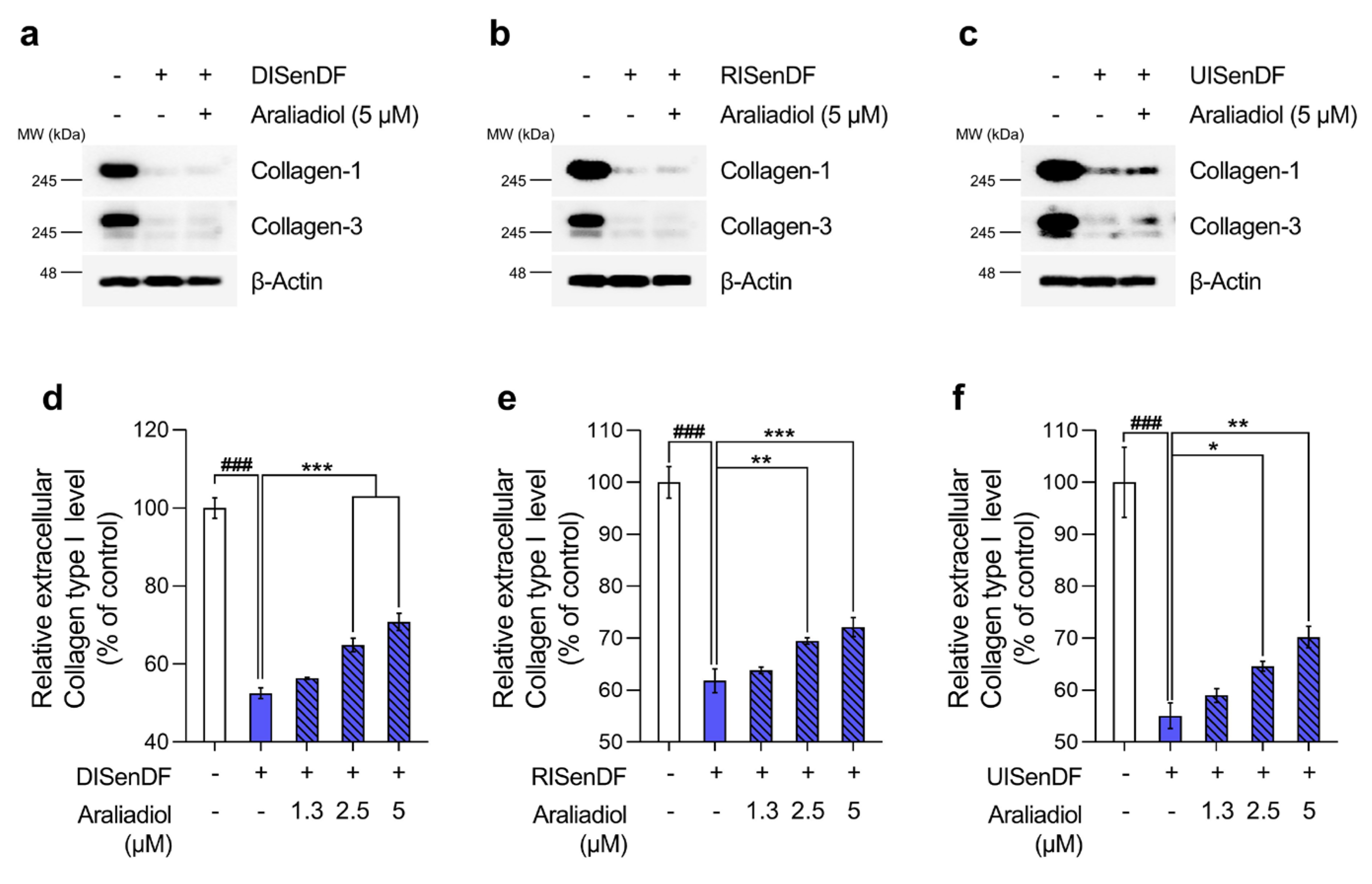
3.4. Araliadiol Increases Extracellular Collagen Content Without Altering Intracellular Collagen Expression in Senescent Dermal Fibroblasts
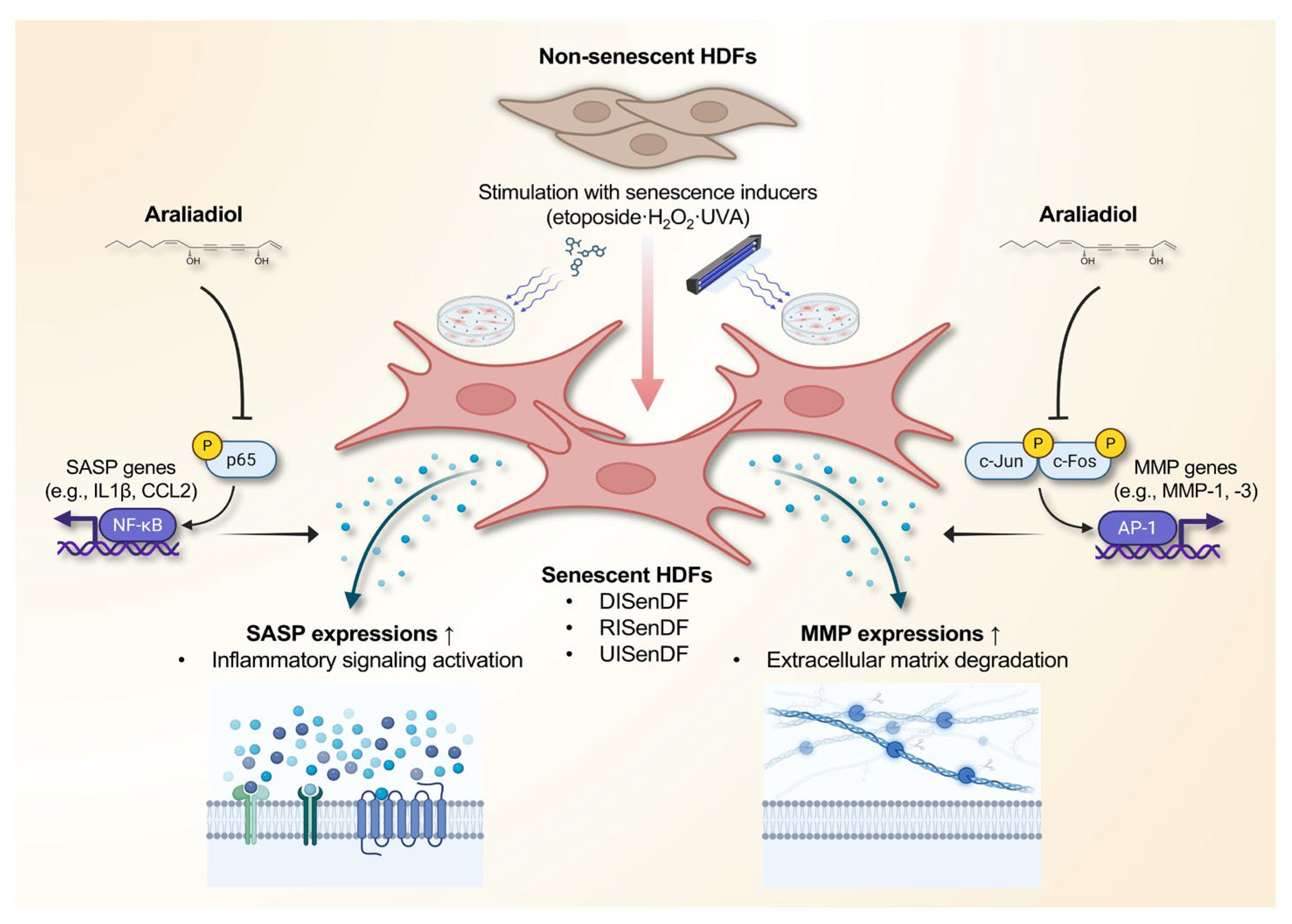
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA-β-gal | Senescence-associated β-galactosidase |
| UV | Ultraviolet |
| SASP | Senescence-associated secretory phenotype |
| MMPs | Matrix metalloproteinases |
| AP-1 | Activator protein-1 |
| QoL | Quality of life |
| ATP | Adenosine triphosphate |
| BCL-2 | B-cell lymphoma-2 |
| HSP90 | Heat shock protein 90 |
| BCA | Bicinchoninic acid |
| FDA | Food and Drug Administration |
| LPS | Lipopolysaccharide |
| H2O2 | Hydrogen peroxide |
| UVA | Ultraviolet A |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HDF | Human dermal fibroblast |
| FBS | Fetal bovine serum |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| TBS-T | Tris-buffered saline with Tween-20 |
| BCL-xL | B-cell lymphoma-extra large |
| cDNA | Complementary DNA |
| CCL2 | C-C motif chemokine ligand 2 |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| qRT-PCR | Quantitative reverse transcriptase–polymerase chain reaction |
| dNTPs | Deoxynucleotide triphosphates |
| DTT | Dithiothreitol |
| ECL | Enhanced chemiluminescence |
| ECM | Extracellular matrix |
| EIA | Enzyme immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| FITC | Fluorescein isothiocyanate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| ICD-11 | International Classification of Diseases, 11th Revision |
| IgG | Immunoglobulin G |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| M-MLV | Moloney murine leukemia virus |
| MMP-1 | Matrix metalloproteinase-1 |
| MMP-3 | Matrix metalloproteinase-3 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NSenDF | Non-senescent dermal fibroblasts |
| DISenDF | DNA damage–induced senescent dermal fibroblasts |
| RISenDF | ROS-induced senescent dermal fibroblasts |
| UISenDF | UVA-induced senescent dermal fibroblasts |
| RLU | Relative luminescence unit |
| SD | Standard deviation |
| WHO | World Health Organization |
References
- Chen, M.; Li, J.; Ding, Y.; Zhang, C. Skin aging research enters a new era in China. J. Investig. Dermatol. 2024, 144, 1921–1922. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Nan, L.; Guo, P.; Hui, W.; Xia, F.; Yi, C. Recent advances in dermal fibroblast senescence and skin aging: Unraveling mechanisms and pioneering therapeutic strategies. Front. Pharmacol. 2025, 16, 1592596. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.; Watson, R.E. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin1. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Ogata, Y.; Yamada, T.; Hasegawa, S.; Sanada, A.; Iwata, Y.; Arima, M.; Nakata, S.; Sugiura, K.; Akamatsu, H. SASP-induced macrophage dysfunction may contribute to accelerated senescent fibroblast accumulation in the dermis. Exp. Dermatol. 2021, 30, 84–91. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and Immunosenescence as Part of Skin Aging—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Xia, W.; He, T.; Calderone, K.; Bou-Gharios, G.; Voorhees, J.J.; Dlugosz, A.A.; Fisher, G.J. Matrix metalloproteinase-1 expression in fibroblasts accelerates dermal aging and promotes papilloma development in mouse skin. J. Investig. Dermatol. 2023, 143, 1700–1707.e1. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.-H.; Lee, D.H.; Lee, S.-H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef]
- Igarashi, T.; Nishino, K.; Nayar, S.K. The appearance of human skin: A survey. Found. Trends® Comput. Graph. Vis. 2007, 3, 1–95. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Psychological and social implications of aging skin: Normal aging and the effects of cutaneous disease. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–14. [Google Scholar]
- Nejad, T.M.; Mohammadi, F.; Abdollahi, F.; Gorgulu, O.; Motalebi, S.A. Predictors of health-related quality of life among older patients with skin diseases. J. Pak. Assoc. Dermatol. 2024, 34, 151–158. [Google Scholar]
- Gupta, M.A.; Gilchrest, B.A. Psychosocial aspects of aging skin. Dermatol. Clin. 2005, 23, 643–648. [Google Scholar] [CrossRef]
- Hess, U.; Huppertz, D.; Mauersberger, H.; Kastendieck, T. Wrinkles are neither beautiful nor nice: The effect of facial wrinkles on person perception and interpersonal closeness. Acta Psychol. 2023, 241, 104077. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Chen, F.; Zeng, R.; Tong, R. Targeted delivery strategy: A beneficial partner for emerging senotherapy. Biomed. Pharmacother. 2022, 155, 113737. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.; Kim, G.; Park, C.H.; Lee, D.; Lee, S.H.; Chung, J. Attenuation of intrinsic ageing of the skin via elimination of senescent dermal fibroblasts with senolytic drugs. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1125–1135. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef]
- Masilamani, A.P.; Dettmer-Monaco, V.; Monaco, G.; Cathomen, T.; Kuckuck, I.; Schultze-Seemann, S.; Huber, N.; Wolf, P. An Anti-PSMA Immunotoxin Reduces Mcl-1 and Bcl2A1 and specifically induces in combination with the bad-like BH3 Mimetic ABT-737 apoptosis in prostate cancer cells. Cancers 2020, 12, 1648. [Google Scholar] [CrossRef]
- Zarbock, A. The shady side of dasatinib. Blood 2012, 119, 4817–4818. [Google Scholar] [CrossRef] [PubMed]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Maitray, A.; Rishi, P. Methoxsalen-induced macular toxicity. Indian J. Ophthalmol. 2017, 65, 1243–1245. [Google Scholar] [CrossRef]
- Farhadi, M.; Fattahi, E.; Kouchesfahani, H.M.; Shockravi, A.; Parivar, K. The adverse effects of methoxsalen on the oogenesis of Balb/C mice. Cell J. 2013, 15, 348. [Google Scholar]
- Gonzales, M.M.; Garbarino, V.; Zilli, E.M.; Petersen, R.; Kirkland, J.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): A pilot clinical trial. J. Prev. Alzheimer’s Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Rapamycin for the aging skin. Aging 2019, 11, 12822. [Google Scholar] [CrossRef]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. Geroscience 2019, 41, 861–869. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Yang, Y.; Zhang, S.; Wang, J.; Zhang, D.; Yu, H. Metformin attenuates UVA-induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int. J. Mol. Sci. 2022, 23, 6960. [Google Scholar] [CrossRef]
- Roark, K.M.; Iffland, P.H. Rapamycin for longevity: The pros, the cons, and future perspectives. Front. Aging 2025, 6, 1628187. [Google Scholar] [CrossRef]
- Mazumder, A.; Singh, A.; Jha, S. A Review on Metformin: Clinical Significance and Side Effects. Int. J. Pharm. Res. 2021, 13, 60–69. [Google Scholar] [CrossRef]
- Sidiropoulou, P.; Katsarou, M.; Sifaki, M.; Papasavva, M.; Drakoulis, N. Topical calcineurin and mammalian target of rapamycin inhibitors in inflammatory dermatoses: Current challenges and nanotechnology-based prospects. Int. J. Mol. Med. 2024, 54, 85. [Google Scholar] [CrossRef]
- Pantazopoulos, D.; Papachristou, S.; Gouveri, E.; Papi, M.; Papazoglou, D.; Papanas, N. Metformin: Old Drug, New Therapeutic Potential in the Skin? A Brief Narrative Review. Adv. Ther. 2025, 42, 3606–3620. [Google Scholar] [CrossRef]
- Zhang, J.; Shimozaki, K.; Hattori, S.; Pastukh, V.; Maloney, D.; Hogan, M.V.; Wang, J.H. Metformin lotion promotes scarless skin tissue formation through AMPK activation, TGF-β1 inhibition, and reduced myofibroblast numbers. PLoS ONE 2024, 19, e0311147. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar] [PubMed]
- Meier, B.P.; Lappas, C.M. The influence of safety, efficacy, and medical condition severity on natural versus synthetic drug preference. Med. Decis. Mak. 2016, 36, 1011–1019. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M. The dark side of beauty: An in-depth analysis of the health hazards and toxicological impact of synthetic cosmetics and personal care products. Front. Public Health 2024, 12, 1439027. [Google Scholar] [CrossRef]
- Sahu, G.; Goswami, P.; Taj, T.; Pal, R.; Ashique, S.; Bhowmick, M.; Bhowmick, P.; Farid, A. Medicinal and Nutritional Importance of Centella asiatica in Human Health. In Medicinal Plants and Their Bioactive Compounds in Human Health: Volume 1; Springer: Berlin/Heidelberg, Germany, 2024; pp. 221–249. [Google Scholar]
- Cheng, W.-L.; Lin, T.-Y.; Tseng, Y.-H.; Chu, F.-H.; Chueh, P.-J.; Kuo, Y.-H.; Wang, S.-Y. Inhibitory effect of human breast cancer cell proliferation via p21-mediated G1 cell cycle arrest by araliadiol isolated from Aralia cordata Thunb. Planta Medica 2011, 77, 164–168. [Google Scholar] [CrossRef]
- Fujimori, H.; Ohba, T.; Mikami, M.; Nakamura, S.; Ito, K.; Kojima, H.; Takahashi, T.; Iddamalgoda, A.; Shimazawa, M.; Hara, H. The protective effect of Centella asiatica and its constituent, araliadiol on neuronal cell damage and cognitive impairment. J. Pharmacol. Sci. 2022, 148, 162–171. [Google Scholar] [CrossRef]
- Park, S.; Park, H.W.; Seo, D.B.; Yoo, D.S.; Bae, S. In vitro hair growth-promoting effects of araliadiol via the p38/PPAR-γ signaling pathway in human hair follicle stem cells and dermal papilla cells. Front. Pharmacol. 2024, 15, 1482898. [Google Scholar] [CrossRef]
- Jo, Y.H.; Yeon, S.W.; Ahn, J.H.; Turk, A.; Liu, Q.; Kim, M.-O.; Hwang, B.Y.; Park, S.-Y.; Lee, M.K. Polyacetylenes from the adventitious roots of Centella asiatica with glucose uptake stimulatory activity. J. Biotechnol. 2023, 368, 53–59. [Google Scholar] [CrossRef]
- Park, S.; Cho, S.; Shin, H.-J.; Baek, S.; Gwon, H.-I.; Lee, J.; Yoo, D.S.; Park, H.W.; Seo, D.B.; Bae, S. Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells. Biomedicines 2025, 13, 1408. [Google Scholar] [CrossRef]
- Stair-Nawy, S.; Csóka, A.B.; Stern, R. Hyaluronidase expression in human skin fibroblasts. Biochem. Biophys. Res. Commun. 1999, 266, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; LeGrand, C.F.; Kinnear, B.F.; Sobota, R.M.; Ramalingam, R.; Dye, D.E.; Raghunath, M.; Lane, E.B.; Coombe, D.R. In vitro expansion of keratinocytes on human dermal fibroblast-derived matrix retains their stem-like characteristics. Sci. Rep. 2019, 9, 18561. [Google Scholar] [CrossRef]
- Ashrafi, E.; Sauvageau, D.; Elliott, J.A. Effects of different cryopreservation parameters on the differences between trypan blue and fluorescent SYTO 13/GelRed assays. Cryobiology 2024, 116, 104883. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell cycle arrest is not senescence. Aging 2011, 3, 94. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.J.; Kim, H.S.; Shin, H.-J.; Kim, J.-S.; Lee, J.N.; Lee, J.H.; Bae, S. Phloroglucinol enhances anagen signaling and alleviates H2O2-induced oxidative stress in human dermal papilla cells. J. Microbiol. Biotechnol. 2024, 34, 812–827. [Google Scholar] [CrossRef]
- Ng, N.S.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc 2021, 11, e3877. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Park, S.H.; Woo, J.E.; Park, B.J. Correlation Between External and Internal Skin Aging Markers by Skin Depth. J. Cosmet. Dermatol. 2025, 24, e70354. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The molecular mechanism of polyphenols with anti-aging activity in aged human dermal fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; Van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Thomas, C.; Erni, R.; Wu, J.Y.; Fischer, F.; Lamers, G.; Grigolon, G.; Mitchell, S.J.; Zarse, K.; Carreira, E.M.; Ristow, M. A naturally occurring polyacetylene isolated from carrots promotes health and delays signatures of aging. Nat. Commun. 2023, 14, 8142. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix metalloproteinases on skin photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, T.H.; Park, S.-Y.; Yi, T.H.; Kim, S.Y. Enzyme-modified Panax ginseng inhibits UVB-induced skin aging through the regulation of procollagen type I and MMP-1 expression. Food Funct. 2014, 5, 265–274. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.E.; Choi, S.I.; Lee, H.R.; JU LEE, Y.; Jang, M.J.; Son, H.J.; Lee, H.S.; HUN OH, C.; Kim, B.H. UV radiation-induced skin aging in hairless mice is effectively prevented by oral intake of sea buckthorn (Hippophae rhamnoides L.) fruit blend for 6 weeks through MMP suppression and increase of SOD activity. Int. J. Mol. Med. 2012, 30, 392–400. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.-H.; Tu, X.-L.; Zhang, C.; Zhao, M.; Zhang, Q.-W.; Gao, F.-H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Riekki, R.; Jukkola, A.; Sassi, M.L.; Höyhtyä, M.; Kallioinen, M.; Risteli, J.; Oikarinen, A. Modulation of skin collagen metabolism by irradiation: Collagen synthesis is increased in irradiated human skin. Br. J. Dermatol. 2000, 142, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Rabheru, K.; Byles, J.E.; Kalache, A. How “old age” was withdrawn as a diagnosis from ICD-11. Lancet Healthy Longev. 2022, 3, e457–e459. [Google Scholar] [CrossRef]
- Stambler, I.; Alekseev, A.; Matveyev, Y.; Khaltourina, D. Advanced pathological ageing should be represented in the ICD. Lancet Healthy Longev. 2022, 3, e11. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11: International Classification of Diseases 11th Revision: The Global Standard for Diagnostic Health Information; World Health Organization: Geneva, Switzerland, 2025.
- Mao, Z. Frontiers in Skin Rejuvenation: Recent Advances in Anti-Aging Skincare Technologies Based on Proteins, Peptides, and Peptide Derivatives. Mod. Health Sci. 2025, 8, 69. [Google Scholar] [CrossRef]
- Bose, S.; Malik, J.; Mandal, S.C. Application of phytochemicals in pharmaceuticals. In Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 55–68. [Google Scholar]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Egbuna, C.; Kumar, S.; Ifemeje, J.C.; Ezzat, S.M.; Kaliyaperumal, S. Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gurley, B. Emerging technologies for improving phytochemical bioavailability: Benefits and risks. Clin. Pharmacol. Ther. 2011, 89, 915–919. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of phytochemicals with conventional anticancer drugs in form of nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Kim, H.S.; Park, S.C.; Park, J.T.; Kim, H.S.; Oh, W.K.; Cho, K.A. Identification of a novel senomorphic agent, avenanthramide C, via the suppression of the senescence-associated secretory phenotype. Mech. Ageing Dev. 2020, 192, 111355. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Xu, Q.; Jiang, Z.; He, R.; Fu, Q.; Sun, Y. Pyrroloquinoline Quinone Is an Effective Senomorphic Agent to Target the Pro-Inflammatory Phenotype of Senescent Cells. Aging Cell 2025, e70138. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, E.Y.; Kim, Y.H.; Cha, S.Y.; Kim, H.Y.; Nam, Y.K.; Park, J.S.; Kim, S.Y.; Lee, Y.J.; Yoon, J.H. Epigallocatechin gallate in Camellia sinensis ameliorates skin aging by reducing mitochondrial ROS production. Pharmaceuticals 2025, 18, 612. [Google Scholar] [CrossRef]
- Iswarya, B.; John, C.M. Modulating senescence-associated secretory phenotype–driven paracrine effects to overcome therapy-induced senescence: Senolytic effects of hesperidin and quercetin in A549 lung adenocarcinoma cells. Mol. Biol. Rep. 2025, 52, 795. [Google Scholar] [CrossRef]
- Lee, J.-W.; Ryu, H.W.; Lee, S.U.; Son, T.H.; Park, H.A.; Kim, M.O.; Yuk, H.J.; Ahn, K.-S.; Oh, S.-R. Protective effect of polyacetylene from Dendropanax morbifera Leveille leaves on pulmonary inflammation induced by cigarette smoke and lipopolysaccharide. J. Funct. Foods 2017, 32, 358–366. [Google Scholar] [CrossRef]
- Best, B.P. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 2009, 12, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Wilson, D.M. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef]
- Farage, M.; Miller, K.; Elsner, P.; Maibach, H. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the senescence-associated phenotype in human skin fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuchi, H.; Sun, L.; Zhou, X.; Lin, J. Human amnion-derived mesenchymal stem cells protect against UVA irradiation-induced human dermal fibroblast senescence, in vitro. Mol. Med. Rep. 2017, 16, 2016–2022. [Google Scholar] [CrossRef]
- Vo, P.H.; Do, N.M.; Nguyen, S.T. Primary evaluation the effects of Boesenbergia pandurata ethanol extract on etoposide-induced senescence in fibroblasts. Biomed. Res. Ther. 2024, 11, 6869–6882. [Google Scholar] [CrossRef]
- Petrocelli, J.J.; McKenzie, A.I.; de Hart, N.M.; Reidy, P.T.; Mahmassani, Z.S.; Keeble, A.R.; Kaput, K.L.; Wahl, M.P.; Rondina, M.T.; Marcus, R.L. Disuse-induced muscle fibrosis, cellular senescence, and senescence-associated secretory phenotype in older adults are alleviated during re-ambulation with metformin pre-treatment. Aging Cell 2023, 22, e13936. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases—Past and future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef]
- Bogdanowicz, P.; Bensadoun, P.; Noizet, M.; Béganton, B.; Philippe, A.; Alvarez-Georges, S.; Doat, G.; Tourette, A.; Bessou-Touya, S.; Lemaitre, J.-M. Senomorphic activity of a combination of niacinamide and hyaluronic acid: Correlation with clinical improvement of skin aging. Sci. Rep. 2024, 14, 16321. [Google Scholar] [CrossRef]
- He, M.; Zhou, P.; Chen, H.; Zhang, J.; Zhang, Y.; Zheng, X.; Zhu, W.; Han, L. (-)-α-Bisabolol inhibits D-Gal-induced HSF cellular senescence in vitro and prevents skin aging in vivo by reducing SASP. Iran. J. Basic Med. Sci. 2024, 27, 1105. [Google Scholar] [PubMed]
- Zonari, A.; Brace, L.E.; Al-Katib, K.; Porto, W.F.; Foyt, D.; Guiang, M.; Cruz, E.A.O.; Marshall, B.; Gentz, M.; Guimarães, G.R. Senotherapeutic peptide treatment reduces biological age and senescence burden in human skin models. Npj Aging 2023, 9, 10. [Google Scholar] [CrossRef]
- Loo, Y.C.; Hu, H.-C.; Yu, S.-Y.; Tsai, Y.-H.; Korinek, M.; Wu, Y.-C.; Chang, F.-R.; Chen, Y.-J. Development on potential skin anti-aging agents of Cosmos caudatus Kunth via inhibition of collagenase, MMP-1 and MMP-3 activities. Phytomedicine 2023, 110, 154643. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, F.; Ceriotti, L.; Meloni, M. Extra cellular matrix deposition and assembly in dermis spheroids. Clin. Cosmet. Investig. Dermatol. 2021, 14, 935–943. [Google Scholar] [CrossRef]
- Pu, S.-Y.; Huang, Y.-L.; Pu, C.-M.; Kang, Y.-N.; Hoang, K.D.; Chen, K.-H.; Chen, C. Effects of oral collagen for skin anti-aging: A systematic review and meta-analysis. Nutrients 2023, 15, 2080. [Google Scholar] [CrossRef]
- Bar, O.; Valiukevičienė, S. Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects. Cosmetics 2025, 12, 129. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Lim, S.S.; Choi, J.-S.; Kang, Y.-H. Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation-induced human dermal fibroblasts. Nutr. Res. Pract. 2007, 1, 279–284. [Google Scholar] [CrossRef]
- Lee, H.-R.; Ryu, H.G.; Lee, Y.; Park, J.A.; Kim, S.; Lee, C.E.; Jung, S.; Lee, K.-H. Effect of aronia extract on collagen synthesis in human skin cell and dermal equivalent. Oxidative Med. Cell. Longev. 2022, 2022, 4392256. [Google Scholar] [CrossRef]
- Shim, J.-S.; Choi, E.-J.; Lee, C.-W.; Kim, H.-S.; Hwang, J.-K. Matrix metalloproteinase-1 inhibitory activity of kaempferia pandurata roxb. J. Med. Food 2009, 12, 601–607. [Google Scholar] [CrossRef]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Xia, W.; Hammerberg, C.; Li, Y.; He, T.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell 2013, 12, 661–671. [Google Scholar] [CrossRef]
- Ågren, M.S.; Schnabel, R.; Christensen, L.H.; Mirastschijski, U. Tumor necrosis factor-α-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo. Eur. J. Cell Biol. 2015, 94, 12–21. [Google Scholar] [CrossRef]
- Zigrino, P.; Brinckmann, J.; Niehoff, A.; Lu, Y.; Giebeler, N.; Eckes, B.; Kadler, K.E.; Mauch, C. Fibroblast-derived MMP-14 regulates collagen homeostasis in adult skin. J. Investig. Dermatol. 2016, 136, 1575–1583. [Google Scholar] [CrossRef]
- de Meijer, V.E.; Sverdlov, D.Y.; Popov, Y.; Le, H.D.; Meisel, J.A.; Nosé, V.; Schuppan, D.; Puder, M. Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. PLoS ONE 2010, 5, e11256. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Ooe, M.; Fujii, K.; Matsunaka, H.; Yoshida, M.; Ichihashi, M. Mechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivo. Biofactors 2008, 32, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose Response 2008, 6, 239–251. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef]
- Stefanson, A.; Bakovic, M. Dietary polyacetylene falcarinol upregulated intestinal heme oxygenase-1 and modified plasma cytokine profile in late phase lipopolysaccharide-induced acute inflammation in CB57BL/6 mice. Nutr. Res. 2020, 80, 89–105. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactivity of polyacetylenes in food plants. In Bioactive Foods in Promoting Health; Elsevier: Amsterdam, The Netherlands, 2010; pp. 285–306. [Google Scholar]
- Jakobsen, U.; Kobæk-Larsen, M.; Kjøller, K.D.; Antonsen, S.; Baatrup, G.; Trelle, M.B. Quantification of the anti-neoplastic polyacetylene falcarinol from carrots in human serum by LC-MS/MS. J. Chromatogr. B 2022, 1210, 123440. [Google Scholar] [CrossRef]
| Target mRNA | Sequences of Primer | Amplicons (bp) |
|---|---|---|
| GAPDH | F: 5′-TCCAAAATCAAGTGGGGCGATGC-3′ | 390 |
| R: 5′-GCCAGTAGAGGCAGGGATGATGT-3′ | ||
| IL1β | F: 5′-TTCCCTGCCCACAGACCTTCC-3′ | 116 |
| R: 5′-TGCATCGTGCACATAAGCCTCG-3′ | ||
| IL6 | F: 5′-GTAGCCGCCCCACACAGA-3′ | 101 |
| R: 5′-CATGTCTCCTTTCTCAGGGCTG-3′ | ||
| IL8 | F: 5′-TCTCTTGGCAGCCTTCCTGA-3′ | 172 |
| R: 5′-TTCTGTGTTGGCGCAGTGTG-3′ | ||
| CCL2 | F: 5′-GAGAGGCTGAGACTAACCCAGA-3′ | 259 |
| R: 5′-ATCACAGCTTCTTTGGGACACT-3′ | ||
| CXCL1 | F: 5′-AGGCCACCTGGATTGTGCCTAA-3′ | 281 |
| R: 5′-GCATGTTGCAGGCTCCTCAGAA-3′ | ||
| MMP-1 | F: 5′-GGGCTTGAAGCTGCTTACGA-3′ | 74 |
| R: 5′-ACAGCCCAGTACTTATTCCCTTTG-3′ | ||
| MMP-3 | F: 5′-AGCAAGGACCTCGTTTTCATT-3′ | 261 |
| R: 5′-GTCAATCCCTGGAAAGTCTTCA-3′ |
| Antigen | Host | Clonality (Species Reactivity) | Dilution | Manufacturer (Cat. Number) |
|---|---|---|---|---|
| p16 | Rabbit | Monoclonal (Human) | 1:1000 | Abcam (#108349) |
| p21 | Rabbit | Polyclonal (Human, Monkey) | 1:1000 | CST (#2947) |
| β-Actin | Mouse | Monoclonal (Human, Mouse, Rat···) | 1:1000 | Santa Cruz (#sc-47778) |
| p-p65 (Ser536) | Rabbit | Monoclonal (Human, Mouse, Rat) | 1:1000 | CST (#3033) |
| p65 | Rabbit | Monoclonal (Human, Mouse, Rat···) | 1:1000 | CST (#8242) |
| Collagen-1 | Rabbit | Monoclonal (Human) | 1:1000 | Abcam (#138492) |
| Collagen-3 | Rabbit | Polyclonal (Human, Rat) | 1:1000 | Abcam (#7778) |
| MMP-1 | Mouse | Monoclonal (Human) | 1:1000 | R&D systems (#MAB901) |
| MMP-3 | Rabbit | Polyclonal (Human, Mouse, Rat) | 1:1000 | Invitrogen (#PA5-119639) |
| p-c-Fos (Ser374) | Mouse | Monoclonal (Human, canine) | 1:200 | Santa Cruz (#sc-81485) |
| c-Fos | Rabbit | Monoclonal (Human, Mouse, Rat) | 1:1000 | CST (#2250) |
| p-c-Jun (Ser63) | Mouse | Monoclonal (Human, Mouse, Rat) | 1:200 | Santa Cruz (#sc-822) |
| c-Jun | Rabbit | Polyclonal (Human) | 1:200 | Santa Cruz (#sc-1694) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Baek, S.; Shin, H.-J.; Hwang, J.Y.; Yoo, D.S.; Seo, D.B.; Bae, S. Senotherapeutic Potential of Araliadiol in Senescent Human Dermal Fibroblasts: An In Vitro Study Using Three Senescence Models. Pharmaceutics 2025, 17, 1560. https://doi.org/10.3390/pharmaceutics17121560
Park S, Baek S, Shin H-J, Hwang JY, Yoo DS, Seo DB, Bae S. Senotherapeutic Potential of Araliadiol in Senescent Human Dermal Fibroblasts: An In Vitro Study Using Three Senescence Models. Pharmaceutics. 2025; 17(12):1560. https://doi.org/10.3390/pharmaceutics17121560
Chicago/Turabian StylePark, Seokmuk, Seyeol Baek, Hee-Jae Shin, Jeong Yi Hwang, Dae Sung Yoo, Dae Bang Seo, and Seunghee Bae. 2025. "Senotherapeutic Potential of Araliadiol in Senescent Human Dermal Fibroblasts: An In Vitro Study Using Three Senescence Models" Pharmaceutics 17, no. 12: 1560. https://doi.org/10.3390/pharmaceutics17121560
APA StylePark, S., Baek, S., Shin, H.-J., Hwang, J. Y., Yoo, D. S., Seo, D. B., & Bae, S. (2025). Senotherapeutic Potential of Araliadiol in Senescent Human Dermal Fibroblasts: An In Vitro Study Using Three Senescence Models. Pharmaceutics, 17(12), 1560. https://doi.org/10.3390/pharmaceutics17121560







