Hesperetin Nanoparticle Powder as a Potential Antioxidant Nutraceutical Ingredient: Fabrication, Characterization, and Comparative Dissolution in Vegetarian and Non-Vegetarian Capsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hesperetin Loaded HPBCD and PVPK30 Nanoparticle Powder (HHPNP)
2.3. Establishment of a Calibration Curve for Hesperetin
2.4. Determination of Yield and Encapsulation Efficiency of HHPNP
2.5. Water Solubility Analysis
2.6. Particle Size Analysis
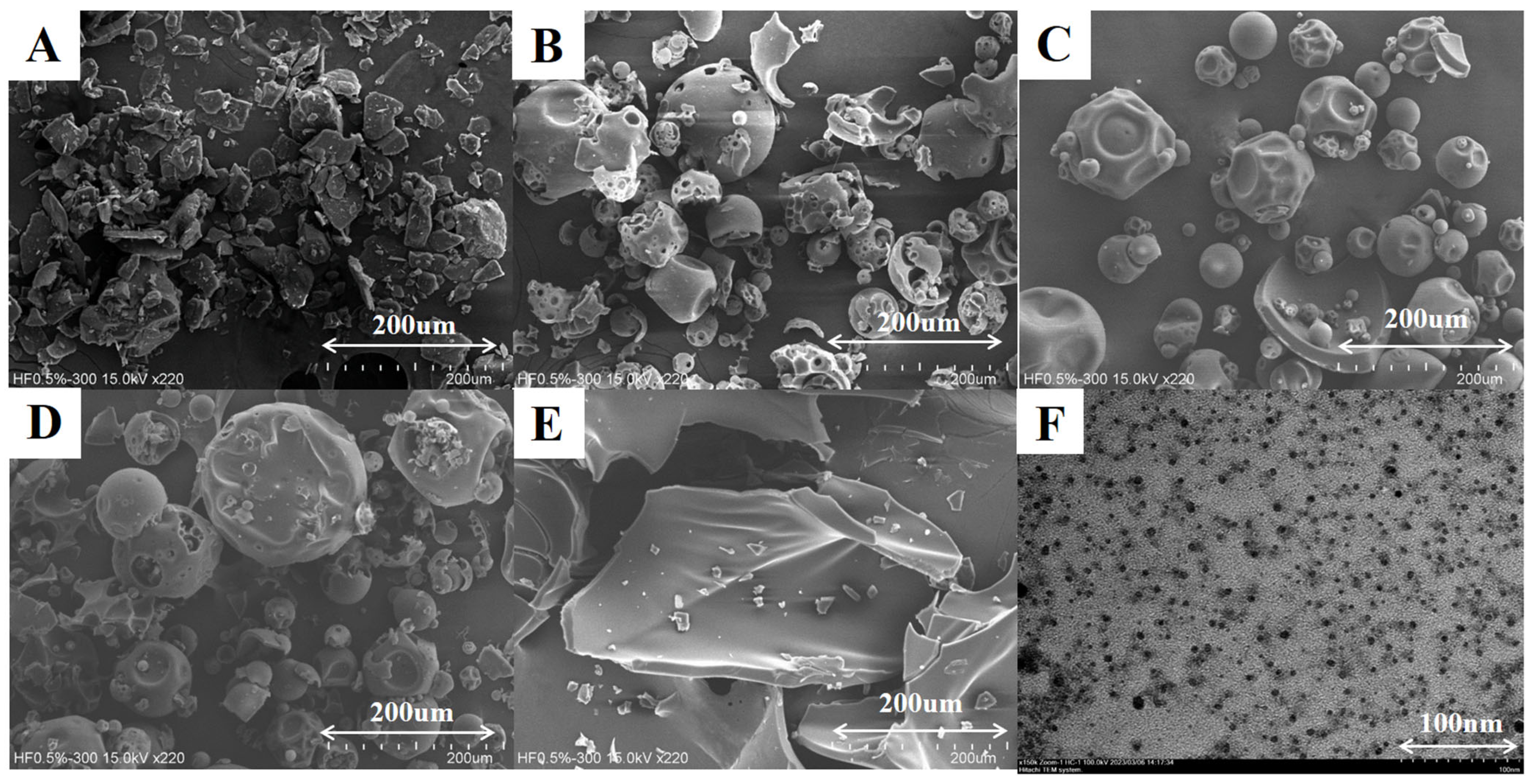
2.7. Scanning/Transmission Electron Microscopy (SEM/TEM)
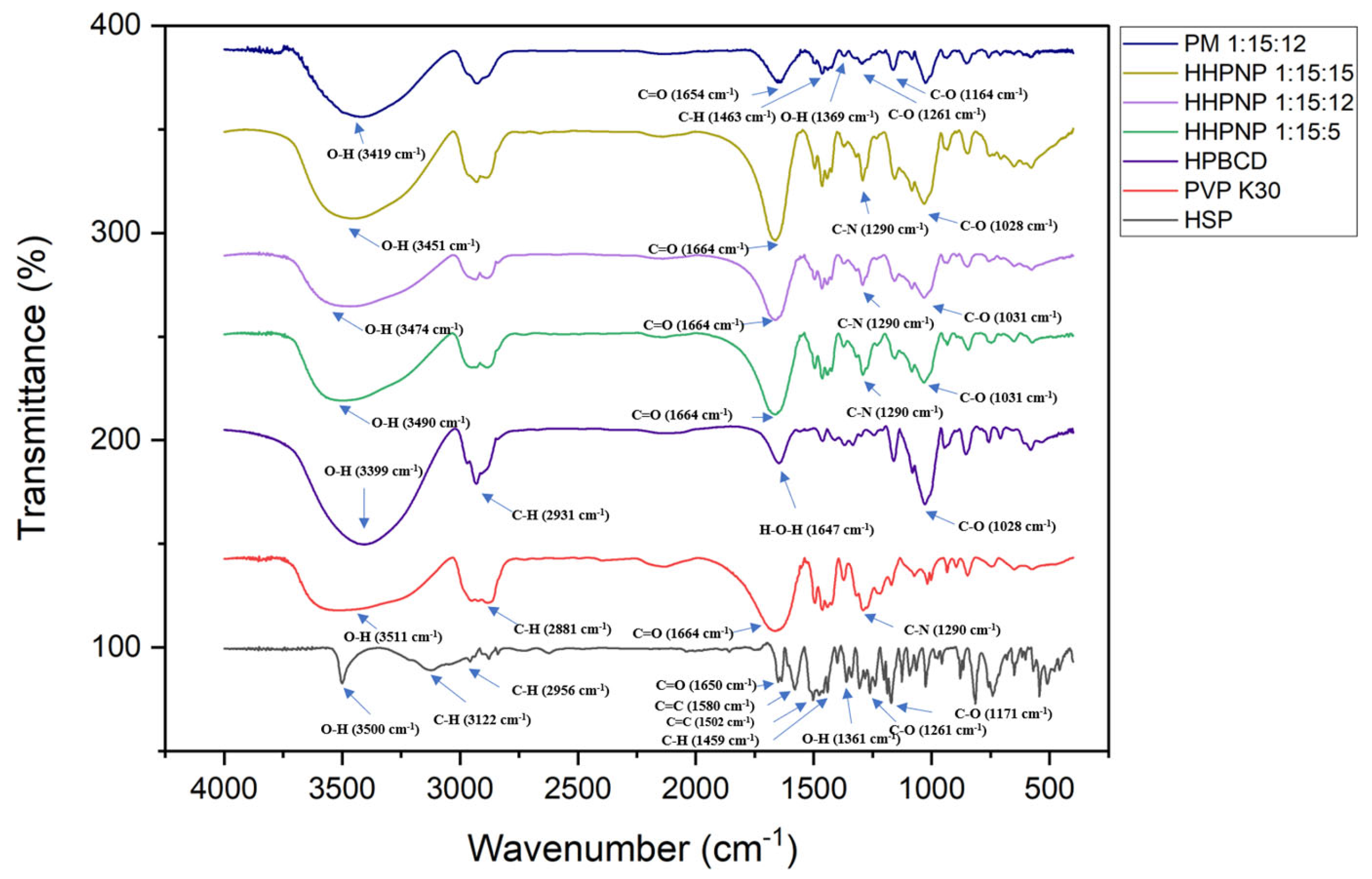
2.8. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
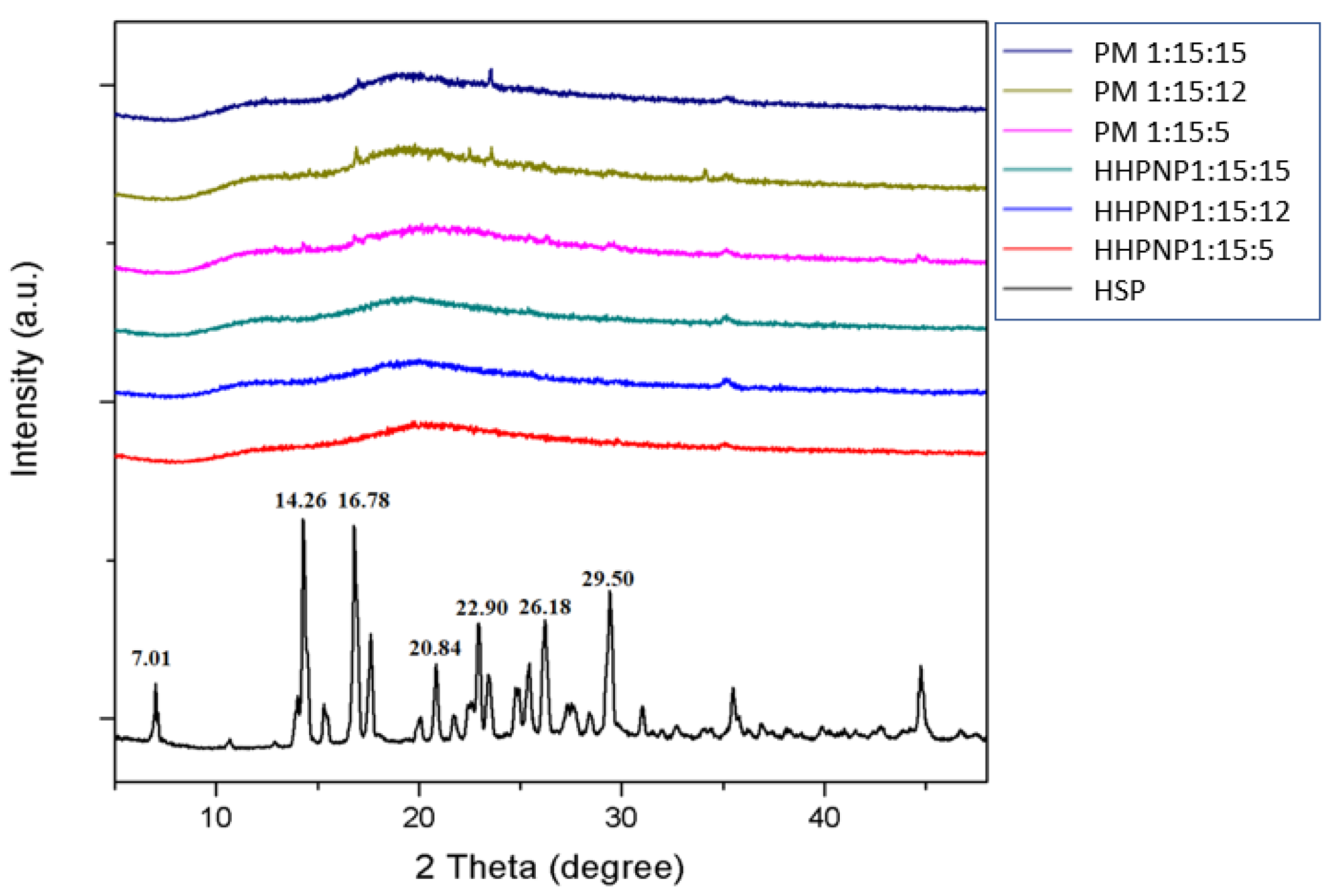
2.9. X-Ray Diffraction (XRD) Analysis
2.10. In Vitro Antioxidant Activities of HHPNP
2.11. Dissolution Studies
2.12. Statistical Analysis
3. Results and Discussion
3.1. Mechanism of Nanoparticle Formation of HHPNP
3.2. Encapsulation Mechanism of Hesperetin in Nanoparticle Delivery Systems
3.3. Effect of Particle Size Reduction and Amorphous Transformation on HSP Water Solubility
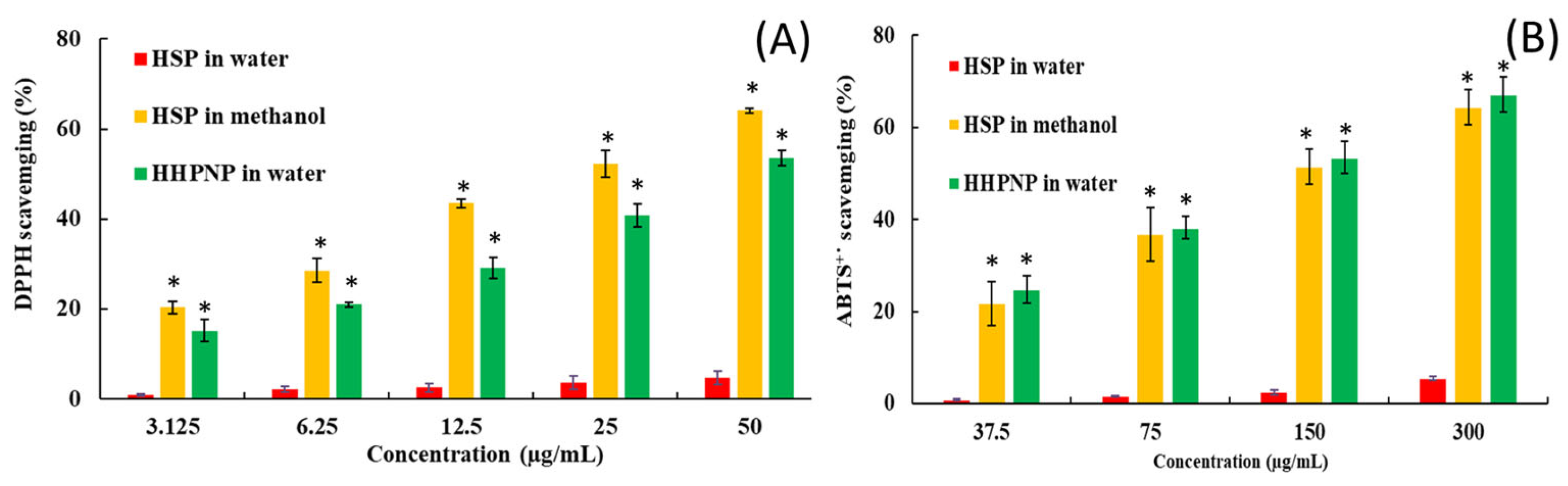
3.4. Effect of Nanoparticle Formulation on Antioxidant Activity
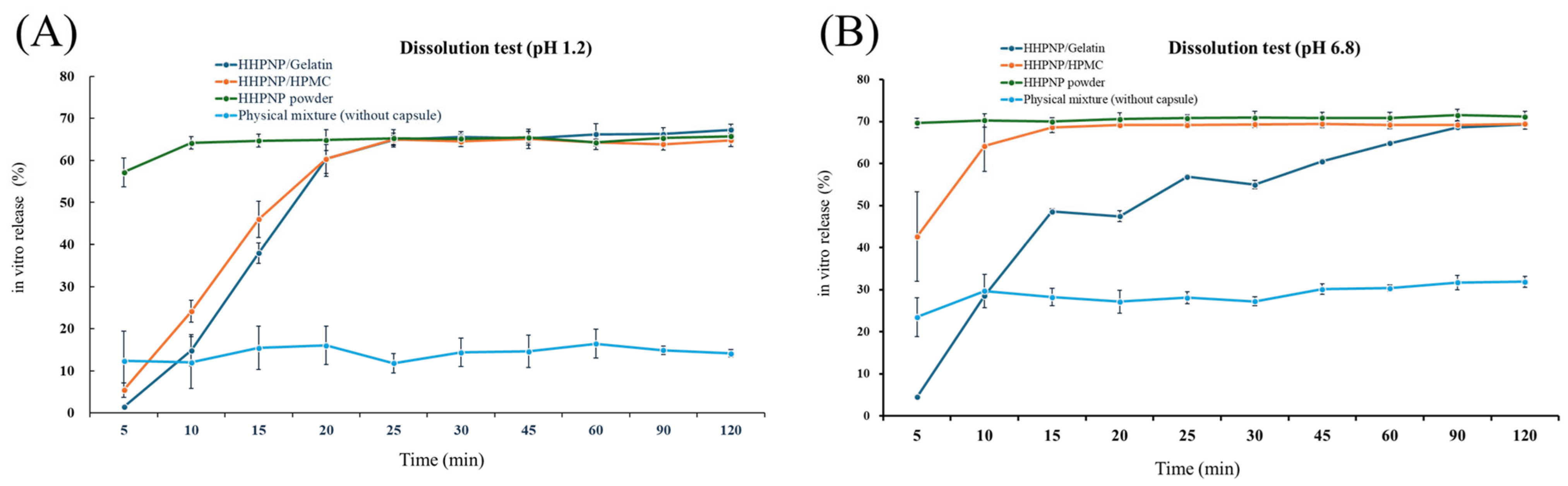
3.5. Dissolution Profiles of HSP Nanoparticle Powders in Gelatin and HPMC Capsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Market.us. Global Nutraceuticals Market by Type (Dietary Supplements, Functional Beverages, and Functional Food) by Form (Capsules, Liquid and Gummies, and Other Forms), by Sales Channel, by Region and Companies—Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023–2032; Report ID 31458; Market.us: New York, NY, USA, 2023. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Song, B.; Hao, M.; Zhang, S.; Niu, W.; Li, Y.; Chen, Q.; Li, S.; Tong, C. Comprehensive review of hesperetin: Advancements in pharmacokinetics, pharmacological effects, and novel formulations. Fitoterapia 2024, 179, 106206. [Google Scholar] [CrossRef] [PubMed]
- Market.us. Global Hesperetin Market Size by Source (Natural Sources, Synthetic Sources), by Formulation (Pharmaceutical Formulations, Dietary Supplements), by Application (Health Supplements, Pharmaceuticals), by End-User (Healthcare Providers, Retail Sector), by Distribution Channel (Online Retail, Offline Retail), by Geographic Scope and Forecast; Verified Market Reports; Market.us: New York, NY, USA, 2025. [Google Scholar]
- Gu, S.F.; Wang, L.Y.; Tian, Y.J.; Zhou, Z.X.; Tang, J.B.; Liu, X.R.; Jiang, H.P.; Shen, Y.Q. Enhanced water solubility, antioxidant activity, and oral absorption of hesperetin by D-α-tocopheryl polyethylene glycol 1000 succinate and phosphatidylcholine. J. Zhejiang Univ. Sci. B 2019, 20, 273–281. [Google Scholar] [CrossRef]
- Liu, L.G.; Chen, J. Solubility of hesperetin in various solvents from (288.2 to 323.2) K. J. Chem. Eng. Data 2008, 53, 1649–1650. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, W.; Zhang, R.; Wang, X.; Zhang, L. Improving curcumin bioavailability: Targeted delivery of curcumin and loading systems in intestinal inflammation. Food Res. Int. 2024, 96, 115079. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Y.; Cui, Z.; Hu, L. Nutraceutical delivery systems to improve the bioaccessibility and bioavailability of lycopene: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 6361–6379. [Google Scholar] [CrossRef]
- Lawson, M.K. Improvement of therapeutic value of quercetin with chitosan nanoparticle delivery systems and potential applications. Int. J. Mol. Sci. 2023, 24, 3293. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Arida, A.I.; Fahelelbom, K.M.; Sadek, B.; Saeed, D.A.; Abu Jarad, R.A.; Jawadi, J. Influence of capsule shell composition on the performance indicators of hypromellose capsule in comparison to hard gelatin capsules. Drug Dev. Ind. Pharm. 2015, 41, 1726–1737. [Google Scholar] [CrossRef]
- Ouellet, D.; Grossmann, K.F.; Limentani, G.; Nebot, N.; Lan, K.; Knowles, L.; Gordon, M.S.; Sharma, S.; Infante, J.R.; Lorusso, P.M.; et al. Effects of particle size, food, and capsule shell composition on the oral bioavailability of dabrafenib, a BRAF inhibitor, in patients with BRAF mutation-positive tumors. J. Pharm. Sci. 2013, 102, 3100–3109. [Google Scholar] [CrossRef]
- Liu, P.; Gan, N.; Li, Q.; Zeng, Z.; Zhong, J.; Wang, X.; Sun, Y.; Wu, D. Investigating the differences in β-Cyclodextrin derivatives/hyperoside inclusion complexes: Dissolution properties, thermal stability, and antioxidant activity. Food Chem. 2025, 481, 144044. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food Chem. 2013, 141, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Gnanaraj, C.; Traini, D.; Young, P.M.; Lee, W.H. Fabrication of polyphenol nanoparticles co-stabilized with different polyvinylpyrrolidone concentrations: Effects on particle stability drug release cellular uptake. J. Drug Deliv. Sci. Technol. 2023, 85, 104575. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Enhanced antioxidant and neuroprotective properties of pterostilbene (resveratrol derivative) in amorphous solid dispersions. Int. J. Mol. Sci. 2024, 25, 2774. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Myricetin amorphous solid dispersions-antineurodegenerative potential. Molecules 2024, 29, 1287. [Google Scholar] [CrossRef]
- Loftsson, T.; Masson, M. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 2004, 14, 35–43. [Google Scholar] [CrossRef]
- Ruan, L.P.; Yu, B.Y.; Fu, G.M.; Zhu, D.N. Improving the solubility of ampelopsin by solid dispersions and inclusion complexes. J. Pharm. Biomed. Anal. 2005, 38, 457–464. [Google Scholar] [CrossRef]
- Jin, S.; Haskins, M.M.; Deng, C.H.; Matos, C.R.M.O.; Zaworotko, M.J. Crystal engineering of ionic cocrystals comprising Na/K salts of hesperetin with hesperetin molecules and solubility modulation. IUCrJ 2023, 10, 329–340. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous inclusion complexes: Molecular interactions of hesperidin and hesperetin with H-B-CD and their biological effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Gosangari, S.; Dyakonov, T. Enhanced dissolution performance of curcumin with the use of supersaturatable formulations. Pharm. Dev. Technol. 2013, 18, 475–480. [Google Scholar] [CrossRef]
- Dong, Q.; Yuan, H.L.; Qian, J.J.; Zhang, C.Y.; Chen, W.D. Preparation and in vitro-in vivo characterization of trans-resveratrol nanosuspensions. Biomed. Mater. Eng. 2018, 29, 333–345. [Google Scholar] [CrossRef]
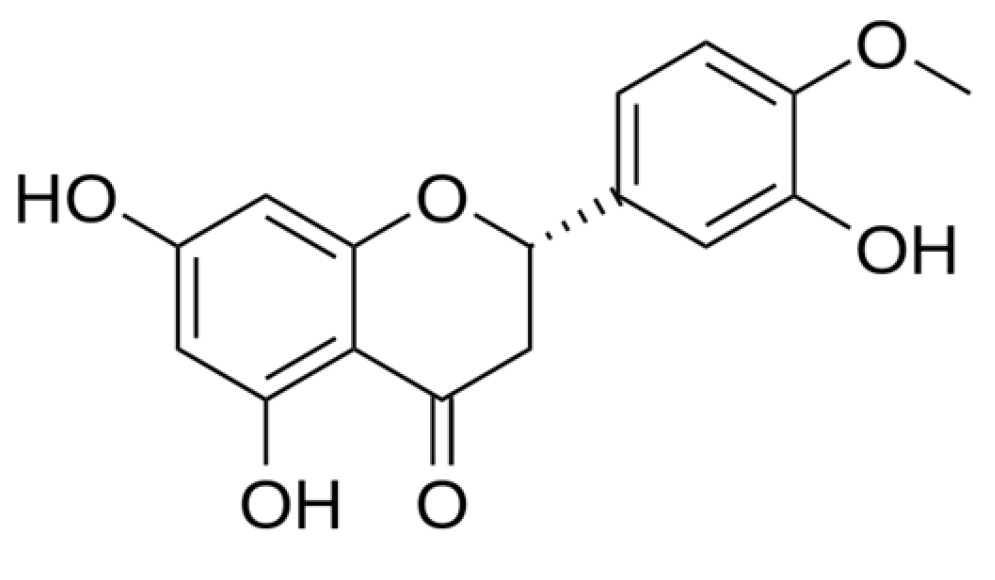
| Ratio | HSP (mg) | HPBCD (mg) | PVP-K30 (mg) |
|---|---|---|---|
| 1:15:5 | 20 | 300 | 100 |
| 1:15:12 | 20 | 300 | 240 |
| 1:15:15 | 20 | 300 | 300 |
| HSP: HPBCD:PVPK30 | Particle Size (nm) | Polydispersity Index |
|---|---|---|
| Hesperetin | >1000 | - |
| 1:15:5 | 13.51 ± 0.26 * | 0.255 ± 0.01 |
| 1:15:12 | 14.87 ± 0.49 * | 0.258 ± 0.01 |
| 1:15:15 | 14.03 ± 0.22 * | 0.226 ± 0.01 |
| HSP: HPBCD:PVPK30 | Water Solubility(μg/mL) | Encapsulation Efficiency (%) | Yield (%) |
|---|---|---|---|
| Hesperetin | 0.96 ± 0.12 * | - | - |
| 1:15:5 | 426.05 ± 19.70 * | 81.42 ± 1.41 | 85.23 ± 5.0 |
| 1:15:12 | 794.00 ± 25.73 * | 90.41 ± 1.32 | 88.55 ± 4.19 |
| 1:15:15 | 682.95 ± 8.379 * | 90.73 ± 1.32 | 83.78 ± 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-H.; Lan, Y.-Y.; Hsu, H.-E.; So, P.B.; Chen, Y.-Y.; Yen, F.-L. Hesperetin Nanoparticle Powder as a Potential Antioxidant Nutraceutical Ingredient: Fabrication, Characterization, and Comparative Dissolution in Vegetarian and Non-Vegetarian Capsules. Pharmaceutics 2025, 17, 1558. https://doi.org/10.3390/pharmaceutics17121558
Wu T-H, Lan Y-Y, Hsu H-E, So PB, Chen Y-Y, Yen F-L. Hesperetin Nanoparticle Powder as a Potential Antioxidant Nutraceutical Ingredient: Fabrication, Characterization, and Comparative Dissolution in Vegetarian and Non-Vegetarian Capsules. Pharmaceutics. 2025; 17(12):1558. https://doi.org/10.3390/pharmaceutics17121558
Chicago/Turabian StyleWu, Tzu-Hui, Yun-Yi Lan, Huai-En Hsu, Pamela Berilyn So, Yuan-Yu Chen, and Feng-Lin Yen. 2025. "Hesperetin Nanoparticle Powder as a Potential Antioxidant Nutraceutical Ingredient: Fabrication, Characterization, and Comparative Dissolution in Vegetarian and Non-Vegetarian Capsules" Pharmaceutics 17, no. 12: 1558. https://doi.org/10.3390/pharmaceutics17121558
APA StyleWu, T.-H., Lan, Y.-Y., Hsu, H.-E., So, P. B., Chen, Y.-Y., & Yen, F.-L. (2025). Hesperetin Nanoparticle Powder as a Potential Antioxidant Nutraceutical Ingredient: Fabrication, Characterization, and Comparative Dissolution in Vegetarian and Non-Vegetarian Capsules. Pharmaceutics, 17(12), 1558. https://doi.org/10.3390/pharmaceutics17121558







