CC-90009, a Cereblon E3 Ligase Modulator, Exhibits Antiviral Efficacy Against JEV In Vitro and In Vivo via Targeted Degradation of GSPT1 and Viral NS5 Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Chemicals and Antibodies
2.3. Small Interfering RNAs (siRNAs), Plasmids and Transfection
2.4. Immunofluorescence (IF) Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. Co-Immunoprecipitation (Co-IP)
2.8. Time-Window Compound Assay
2.9. Mouse Experiments
2.10. Statistical Analysis
3. Results
3.1. CC-90009 Potently Inhibits JEV Infection in SH-SY5Y Cells
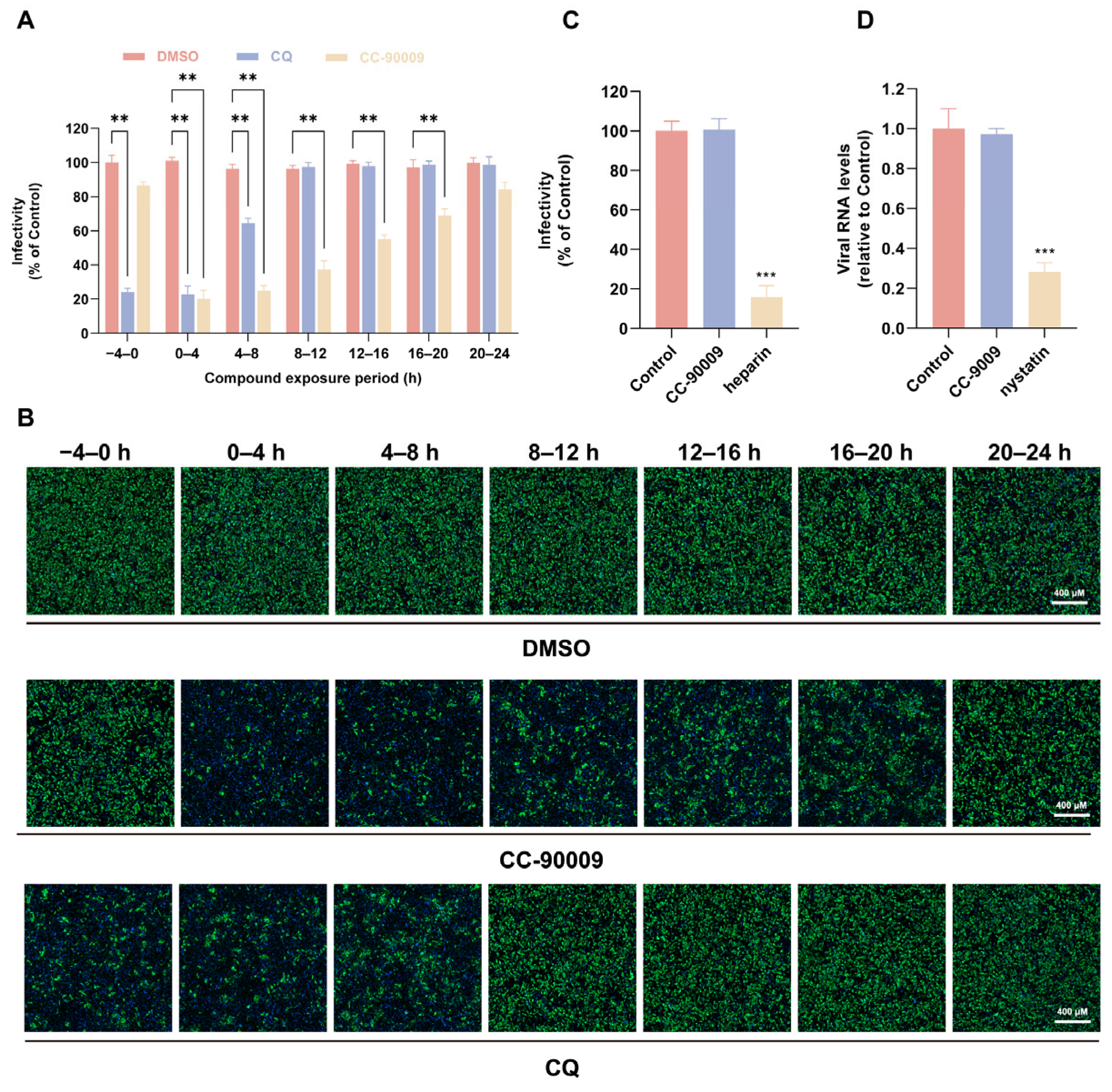
3.2. CC-90009 Does Not Affect JEV Binding or Endocytosis
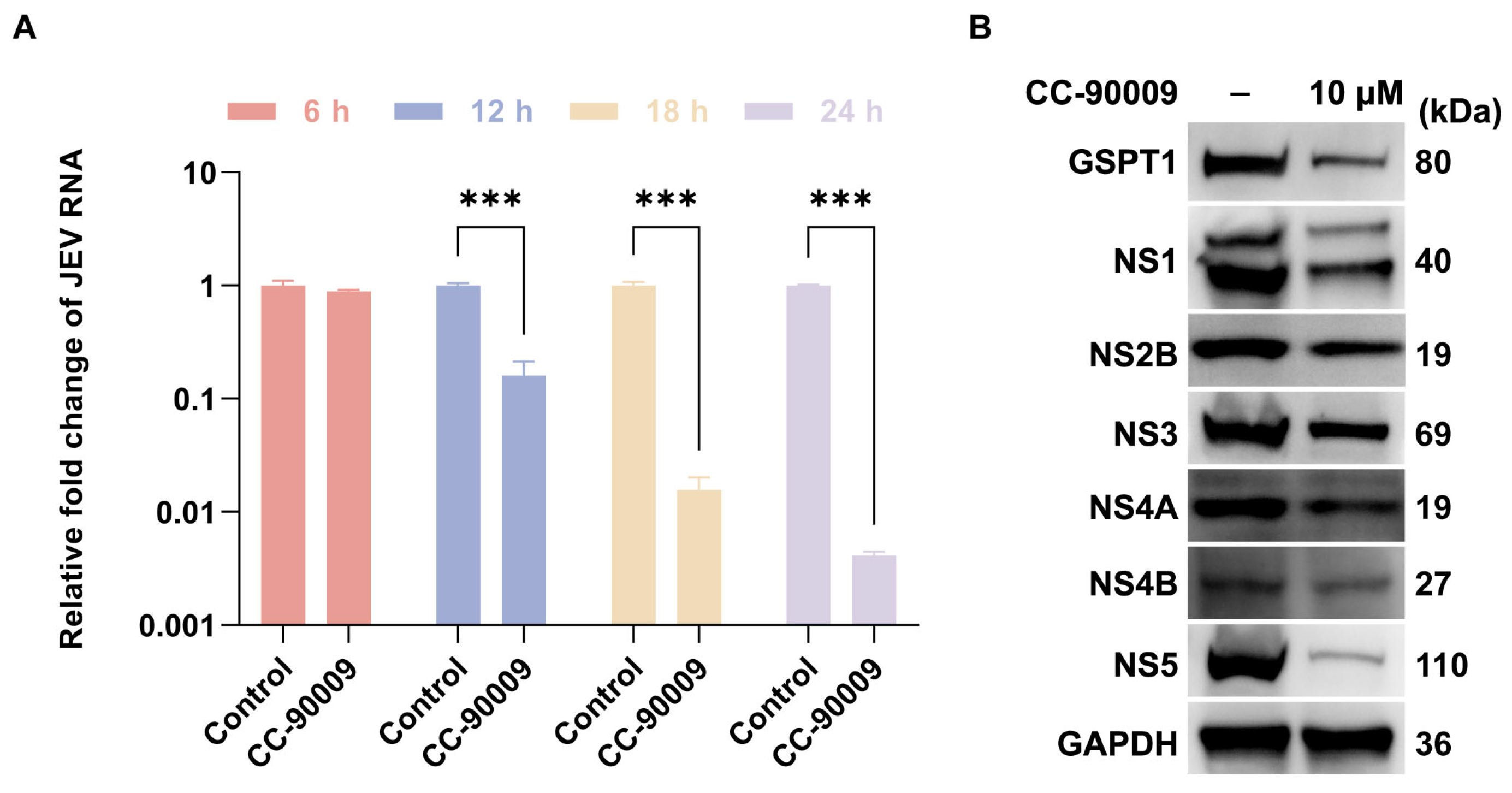
3.3. CC-90009 Impairs JEV Translation and Replication
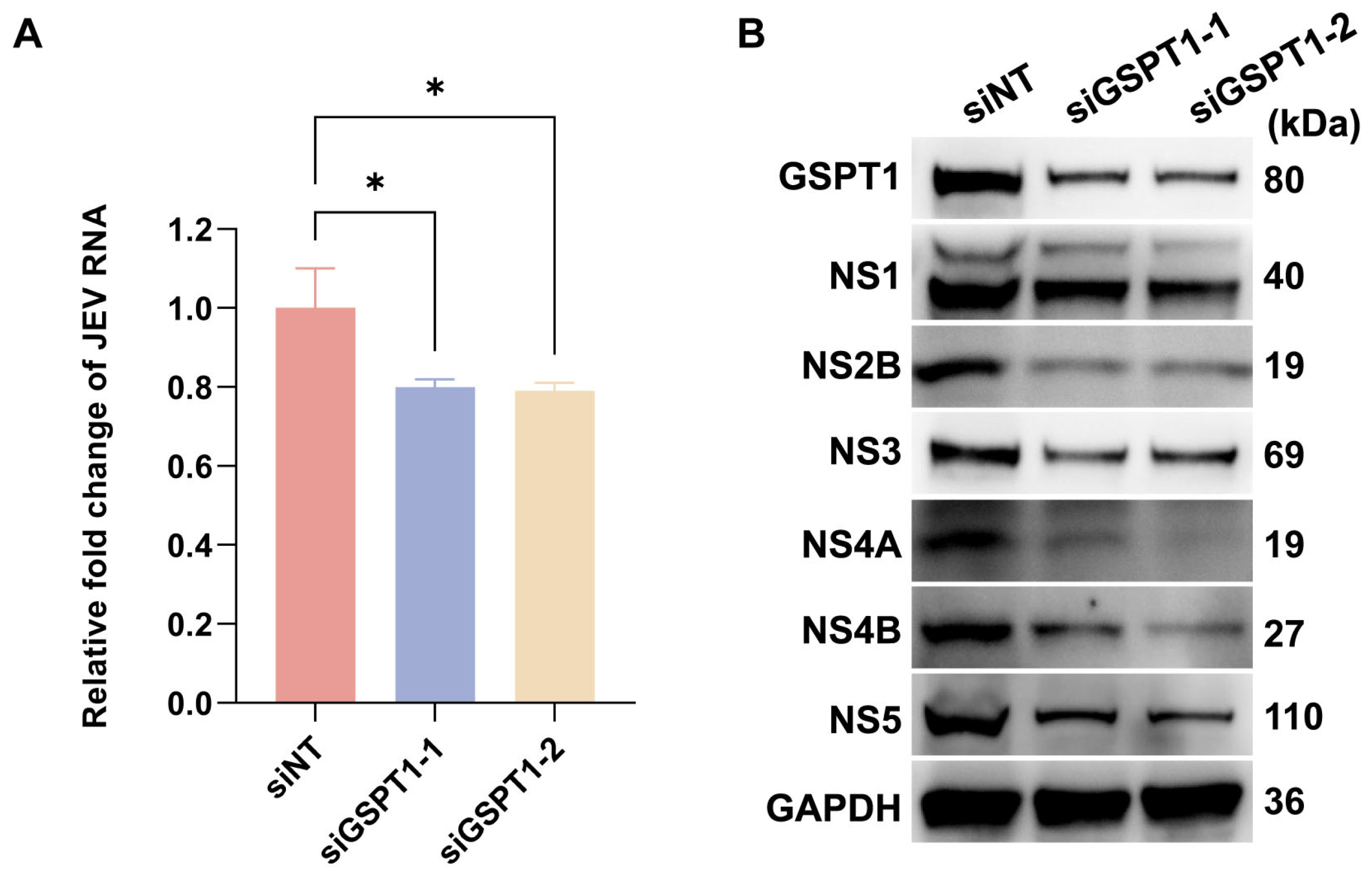
3.4. GSPT1 Facilitates JEV Non-Structural Protein Translation
3.5. CC-90009 Mediates the Degradation of GSPT1 and JEV NS5 via the Proteasome Pathway
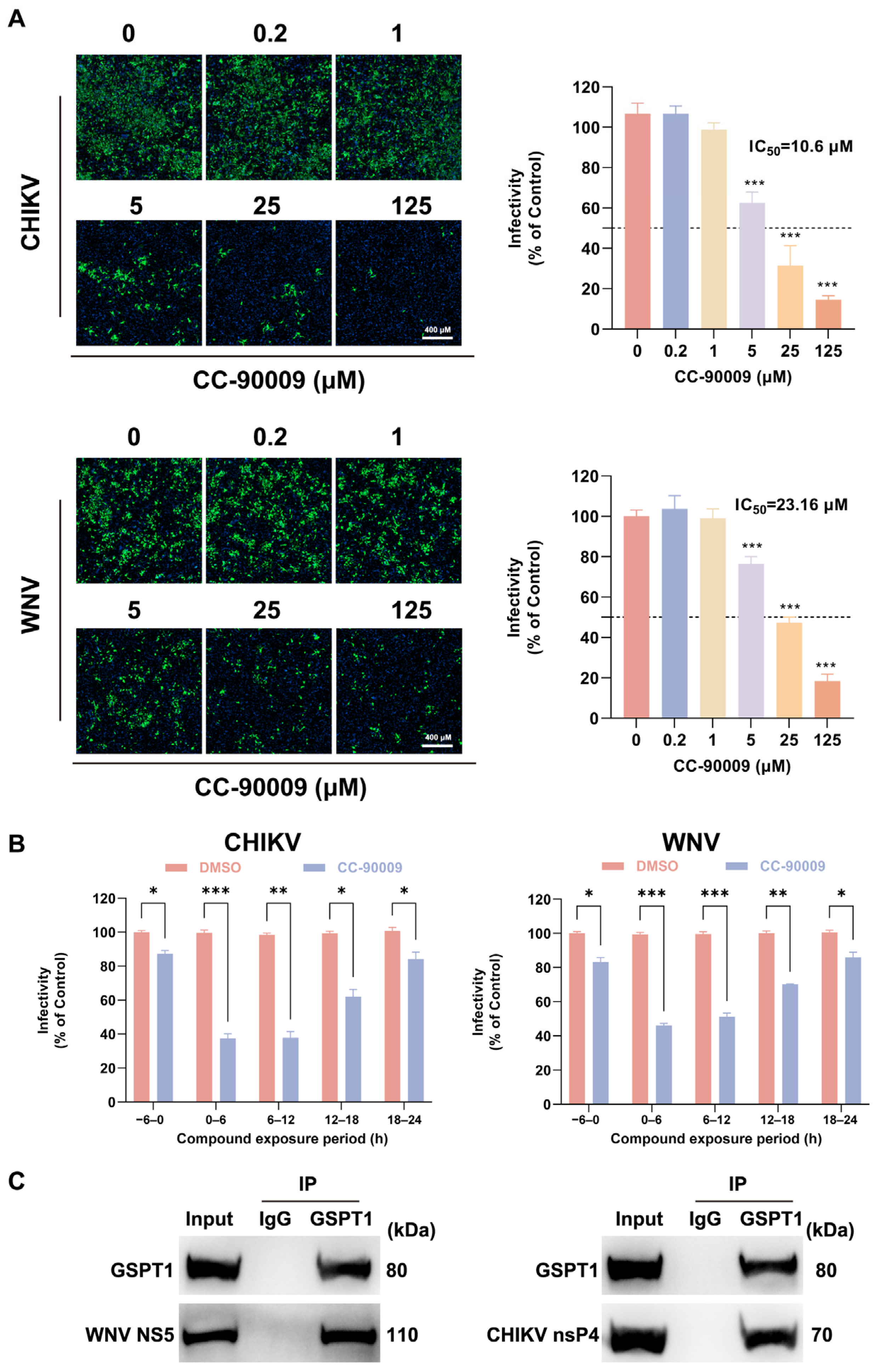
3.6. CC-90009 Exerts Broad-Spectrum Antiviral Activity Against Mosquito-Borne Viruses
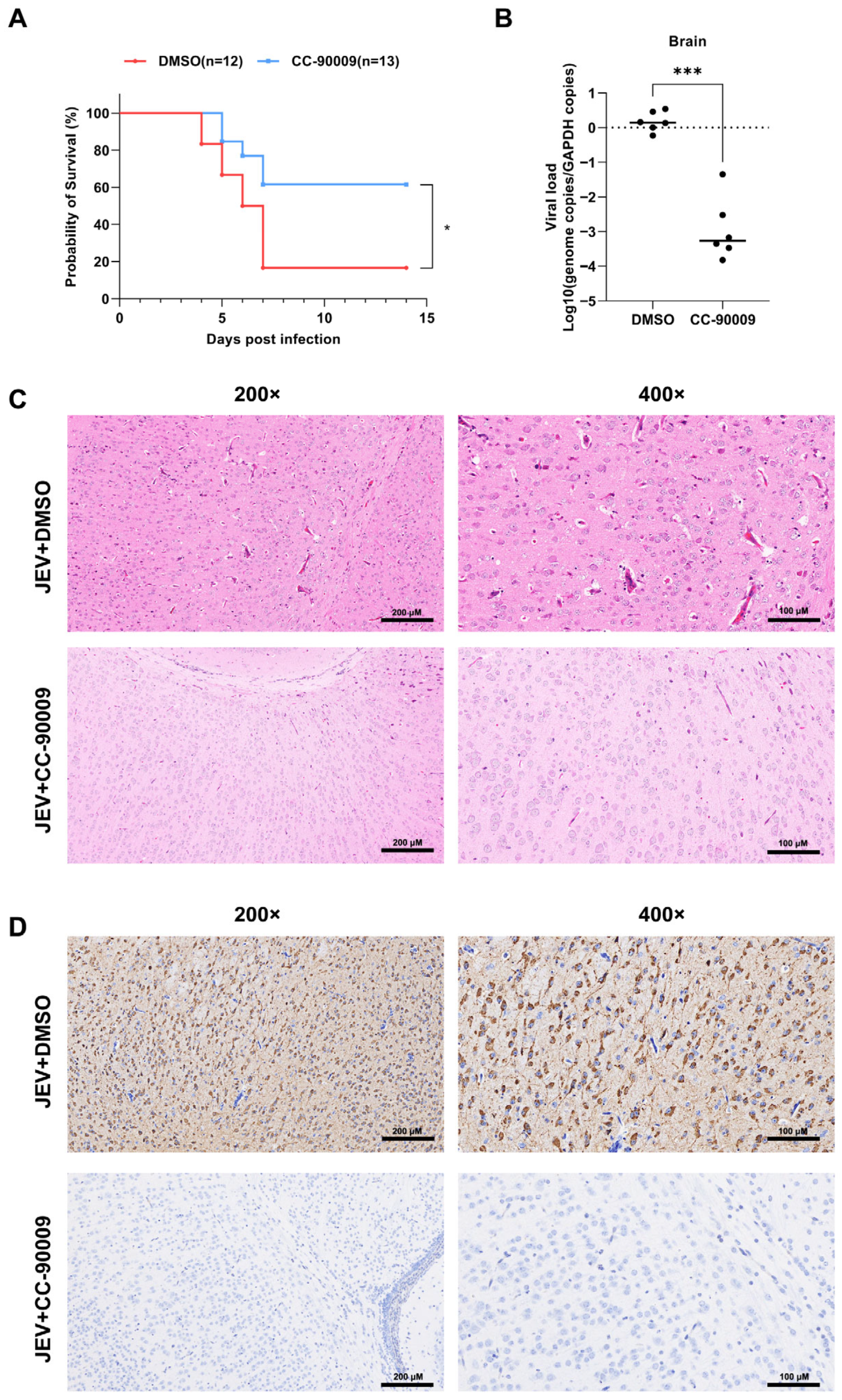
3.7. CC-90009 Confers Protection Against Lethal JEV Infection in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| JEV | Japanese encephalitis virus |
| NS5 | Non-structural proteins |
| RdRp | RNA-dependent RNA polymerase |
| TPD | Targeted protein degradation |
| CRBN | Cereblon |
| CELMoD | Cereblon (CRBN) E3 ligase modulators |
| CRL4 | Cullin4-RING E3 ligase complex |
| PROTAC | Proteolysis-targeting chimera |
| GSPT1 | G1 to S phase transition 1 |
| SH-SY5Y | Human neuroblastoma cell line SH-SY5Y |
| BHK-21 | Baby hamster kidney cell line BHK-21 |
| HEK-293T | Human embryonic kidney cell line HEK-293T |
| CHIKV | Chikungunya virus |
| WNV | West Nile virus |
| ATCC | American Type Culture Collection |
| IgG | Immunoglobulin |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Co-IP | Co-immunoprecipitation |
| siRNA | Small interfering RNA |
| IF | Immunofluorescence |
| MOI | Multiplicity of infection |
| DMSO | Dimethyl sulfoxide |
References
- Zhu, Y.; Chen, S.; Lurong, Q.; Qi, Z. Recent Advances in Antivirals for Japanese Encephalitis Virus. Viruses 2023, 15, 33. [Google Scholar] [CrossRef]
- Guo, J.; Mi, Y.; Guo, Y.; Bai, Y.; Wang, M.; Wang, W.; Wang, Y. Current Advances in Japanese Encephalitis Virus Drug Development. Viruses 2024, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Edache, S.; Dixon, A.L.; Oliveira, A.R.S.; Cohnstaedt, L.W.; Mitzel, D.; Mire, C.E.; Cernicchiaro, N. Mosquito vector competence for Japanese encephalitis virus: A systematic review and meta-analysis update. Parasites Vectors 2025, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, Z.; Qi, Z. Virus-host Interactions in Early Japanese Encephalitis Virus Infection. Virus Res. 2023, 331, 199120. [Google Scholar] [CrossRef]
- Chugh, P.; Soni, S.; Ghanghas, N.; Kumar, S.; Mohan, H. Comprehensive insights into Japanese encephalitis virus: From molecular characterization to advanced detection and vaccine strategies. Antivir. Res. 2025, 243, 106268. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, M.; Song, H.; Chen, S.; Qian, X.; Zhao, P.; Ren, H.; Tang, H.; Wang, Y.; Wei, Y.; et al. Caveolin-1-mediated Japanese encephalitis virus entry requires a two-step regulation of actin reorganization. Future Microbiol. 2016, 11, 1227–1248. [Google Scholar] [CrossRef]
- Sarkar, R.; Chhabra, S.; Tanwar, M.; Agarwal, N.; Kalia, M. Japanese encephalitis virus hijacks ER-associated degradation regulators for its replication. J. Gen. Virol. 2024, 105, 1995. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.; Yadav, P.; Dubey, S.K.; Azhar, E.I.; Maitra, S.S.; Dwivedi, V.D. Molecular pathogenesis of Japanese encephalitis and possible therapeutic strategies. Arch. Virol. 2022, 167, 1739–1762. [Google Scholar] [CrossRef]
- Lu, G.; Gong, P. Crystal Structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PLoS Pathog. 2013, 9, e1003549. [Google Scholar] [CrossRef]
- Hinterndorfer, M.; Spiteri, V.A.; Ciulli, A.; Winter, G.E. Targeted protein degradation for cancer therapy. Nat. Rev. Cancer 2025, 25, 493–516. [Google Scholar] [CrossRef]
- Hansen, J.D.; Correa, M.; Nagy, M.A.; Alexander, M.; Plantevin, V.; Grant, V.; Whitefield, B.; Huang, D.; Kercher, T.; Harris, R.; et al. Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma. J. Med. Chem. 2020, 63, 6648–6676. [Google Scholar] [CrossRef]
- Bonazzi, S.; d’Hennezel, E.; Beckwith, R.E.J.; Xu, L.; Fazal, A.; Magracheva, A.; Ramesh, R.; Cernijenko, A.; Antonakos, B.; Bhang, H.C.; et al. Discovery and characterization of a selective IKZF2 glue degrader for cancer immunotherapy. Cell Chem. Biol. 2023, 30, 235–247.e12. [Google Scholar] [CrossRef] [PubMed]
- Surka, C.; Jin, L.; Mbong, N.; Lu, C.C.; Jang, I.S.; Rychak, E.; Mendy, D.; Clayton, T.; Tindall, E.; Hsu, C.; et al. CC-90009, a novel cereblon E3 ligase modulator, targets acute myeloid leukemia blasts and leukemia stem cells. Blood 2021, 137, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.B.; Neklesa, T.K.; Willard, R.R.; Gordon, D.A.; Pizzano, J.; Vitale, N.; Robling, K.; Dorso, M.A.; Moghrabi, W.; Landrette, S.; et al. Preclinical Evaluation of Bavdegalutamide (ARV-110), a Novel PROteolysis TArgeting Chimera Androgen Receptor Degrader. Mol. Cancer Ther. 2025, 24, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.M.; Flanagan, J.J.; Teh, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clin. Cancer Res. 2024, 30, 3549–3563. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Zhong, K.; Tong, A.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef]
- Hansen, J.D.; Correa, M.; Alexander, M.; Nagy, M.; Huang, D.; Sapienza, J.; Lu, G.; LeBrun, L.A.; Cathers, B.E.; Zhang, W.; et al. CC-90009: A Cereblon E3 Ligase Modulating Drug That Promotes Selective Degradation of GSPT1 for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2021, 64, 1835–1843. [Google Scholar] [CrossRef]
- Fang, J.; Pietzsch, C.; Witwit, H.; Tsaprailis, G.; Crynen, G.; Cho, K.F.; Ting, A.Y.; Bukreyev, A.; Saphire, E.O.; de la Torre, J.C. Proximity interactome analysis of Lassa polymerase reveals eRF3a/GSPT1 as a druggable target for host-directed antivirals. Proc. Natl. Acad. Sci. USA 2022, 119, e2201208119. [Google Scholar] [CrossRef]
- Fang, J.; Pietzsch, C.; Tsaprailis, G.; Crynen, G.; Cho, K.F.; Ting, A.Y.; Bukreyev, A.; de la Torre, J.C.; Saphire, E.O. Functional interactomes of the Ebola virus polymerase identified by proximity proteomics in the context of viral replication. Cell Rep. 2022, 38, 110544. [Google Scholar] [CrossRef]
- He, Z.; Xia, B.; Zhao, T.; Zhao, P.; Ren, H.; Qi, Z.; Zhu, Y. Clathrin-Independent Carriers/Glycosylphosphatidylinositol-Anchored-Protein-Enriched Endosomal Compartment Endocytic Pathway Is Critical for Enterovirus A71 Entry Into Human Oral Epidermoid Carcinoma KB Cells. J. Med. Virol. 2025, 97, e70369. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; He, Z.; Zhao, P.; Ren, H.; Qi, Z. Enterovirus 71 enters human brain microvascular endothelial cells through an ARF6-mediated endocytic pathway. J. Med. Virol. 2023, 95, e28915. [Google Scholar] [CrossRef]
- Liu, Y.G.; Chen, Y.; Wang, X.; Zhao, P.; Zhu, Y.; Qi, Z. Ezrin is essential for the entry of Japanese encephalitis virus into the human brain microvascular endothelial cells. Emerg. Microbes Infect. 2020, 9, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Hellen, C.U.T. Translation Termination and Ribosome Recycling in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2018, 10, a032656. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.Z.H.; De Hayr, L.; Khromykh, A.A.; Slonchak, A. The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines 2024, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Kumar, S.; Akshaya, S.; Singh, S.K. Drug repurposing toward the inhibition of RNA-dependent RNA polymerase of various flaviviruses through computational study. J. Cell. Biochem. 2023, 124, 127–145. [Google Scholar] [CrossRef]
- Malet, H.; Massé, N.; Selisko, B.; Romette, J.L.; Alvarez, K.; Guillemot, J.C.; Tolou, H.; Yap, T.L.; Vasudevan, S.; Lescar, J.; et al. The flavivirus polymerase as a target for drug discovery. Antivir. Res. 2008, 80, 23–35. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lello, L.S.; Liu, X.; Law, Y.S.; Kang, C.; Lescar, J.; Zheng, J.; Merits, A.; Luo, D. Crystal structures of alphavirus nonstructural protein 4 (nsP4) reveal an intrinsically dynamic RNA-dependent RNA polymerase fold. Nucleic Acids Res. 2022, 50, 1000–1016. [Google Scholar] [CrossRef]
- den Boon, J.A.; Nishikiori, M.; Zhan, H.; Ahlquist, P. Positive-strand RNA virus genome replication organelles: Structure, assembly, control. Trends Genet. 2024, 40, 681–693. [Google Scholar] [CrossRef]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef]
- Liu, C.; Peng, H.; Chen, P.; Li, Y.; Deng, Z.; Li, S.; Yang, T.; Liu, K.; Wang, Z.; Liu, L. GSPT1 degraders: Research progress, development strategies and challenges. Bioorg. Med. Chem. 2025, 131, 118390. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, Y.; Hong, Y.; Zhou, Y.; Chen, J.; Zeng, C. Research Progress in Targeting GSPT1: Molecular Glues, Bifunctional Degraders, and Antibody-Enabled Molecular Glues for Cancer Therapy. J. Med. Chem. 2025, 68, 13169–13185. [Google Scholar] [CrossRef]
- Zhao, N.; Ho, J.S.Y.; Meng, F.; Zheng, S.; Kurland, A.P.; Tian, L.; Rea-Moreno, M.; Song, X.; Seo, J.S.; Kaniskan, H.; et al. Generation of host-directed and virus-specific antivirals using targeted protein degradation promoted by small molecules and viral RNA mimics. Cell Host Microbe 2023, 31, 1154–1169.e10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Chen, Y.; Xia, B.; Cheng, Z.; Zhao, P.; Qi, Z.; Zhu, Y. CC-90009, a Cereblon E3 Ligase Modulator, Exhibits Antiviral Efficacy Against JEV In Vitro and In Vivo via Targeted Degradation of GSPT1 and Viral NS5 Protein. Pharmaceutics 2025, 17, 1524. https://doi.org/10.3390/pharmaceutics17121524
He Z, Chen Y, Xia B, Cheng Z, Zhao P, Qi Z, Zhu Y. CC-90009, a Cereblon E3 Ligase Modulator, Exhibits Antiviral Efficacy Against JEV In Vitro and In Vivo via Targeted Degradation of GSPT1 and Viral NS5 Protein. Pharmaceutics. 2025; 17(12):1524. https://doi.org/10.3390/pharmaceutics17121524
Chicago/Turabian StyleHe, Zhiwei, Yibo Chen, Binghui Xia, Zimeng Cheng, Ping Zhao, Zhongtian Qi, and Yongzhe Zhu. 2025. "CC-90009, a Cereblon E3 Ligase Modulator, Exhibits Antiviral Efficacy Against JEV In Vitro and In Vivo via Targeted Degradation of GSPT1 and Viral NS5 Protein" Pharmaceutics 17, no. 12: 1524. https://doi.org/10.3390/pharmaceutics17121524
APA StyleHe, Z., Chen, Y., Xia, B., Cheng, Z., Zhao, P., Qi, Z., & Zhu, Y. (2025). CC-90009, a Cereblon E3 Ligase Modulator, Exhibits Antiviral Efficacy Against JEV In Vitro and In Vivo via Targeted Degradation of GSPT1 and Viral NS5 Protein. Pharmaceutics, 17(12), 1524. https://doi.org/10.3390/pharmaceutics17121524





