X-Ray Structures, Intermolecular Interactions, and Structural Transformations of Dihydroquercetin Solvates and Polymorphs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Screening
2.3. Preparation of Phases
2.4. SCXRD
2.5. Hirshfeld Surface Analysis and Packing Efficiency Analysis
2.6. Intermolecular Energy Calculation and Their Energy Frameworks
2.7. PXRD
2.8. DSC and TGA
2.9. FT-IR
2.10. Accelerated Stability Study
3. Results and Discussion
3.1. Crystal Structures
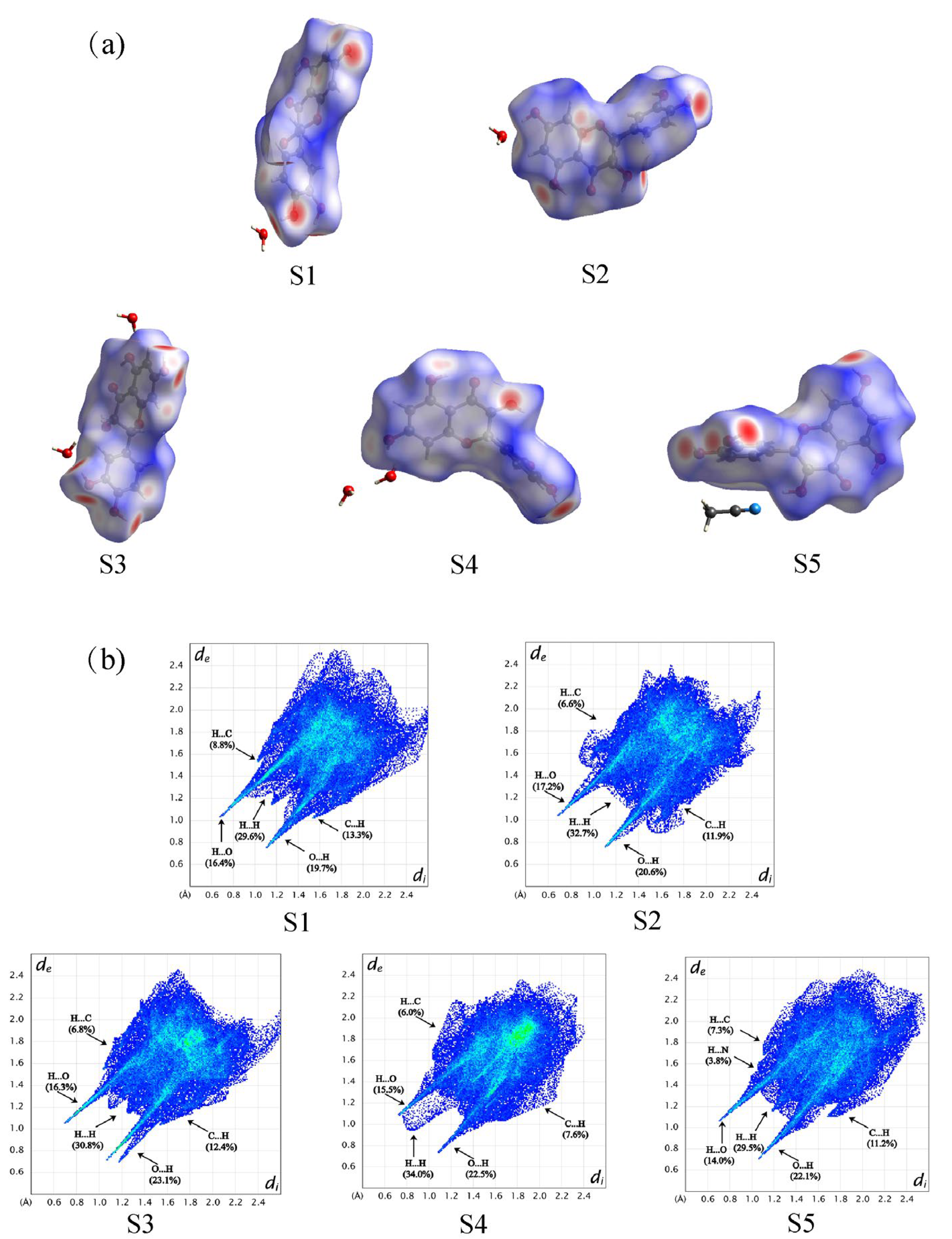
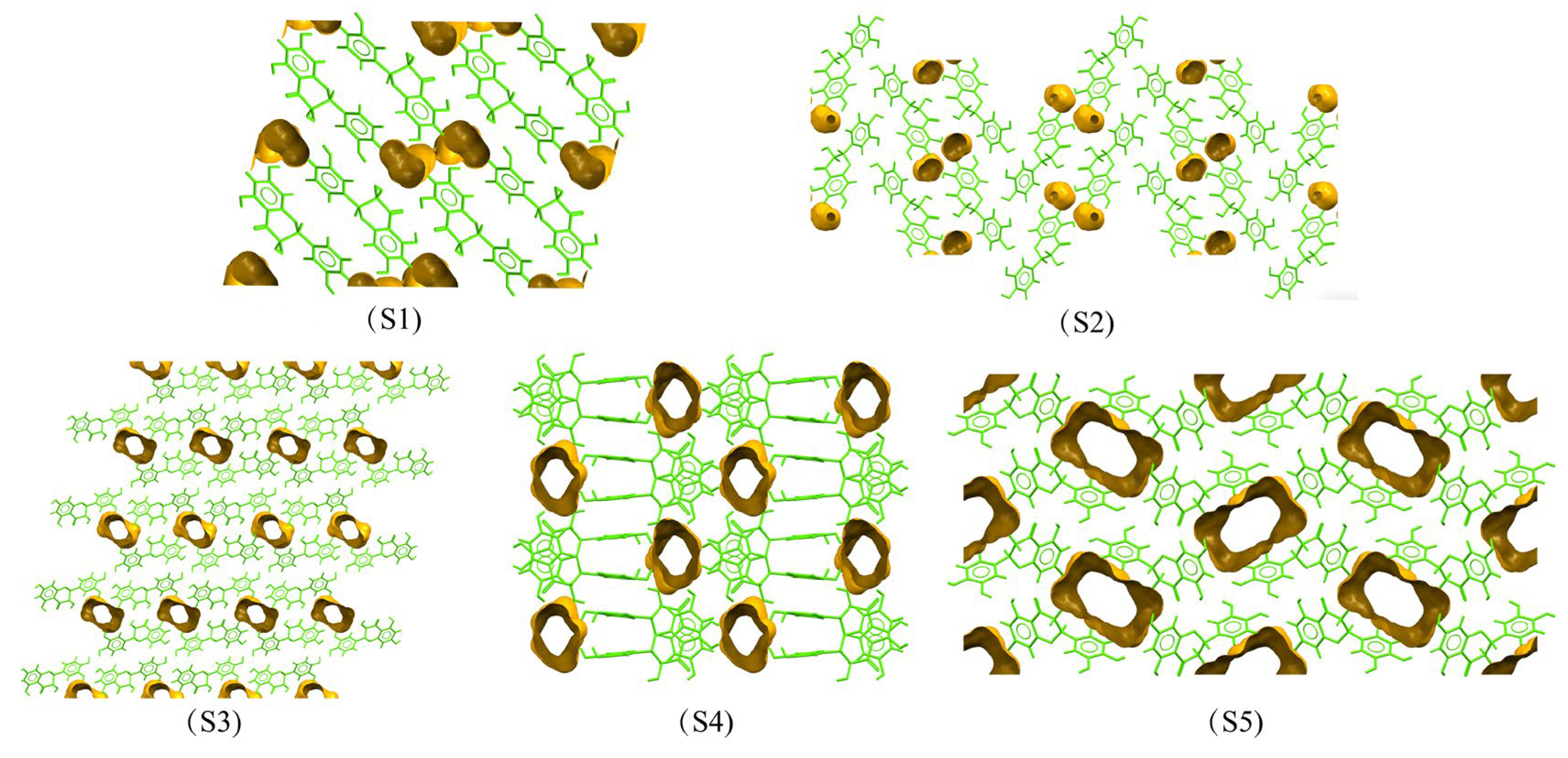
3.2. Hirshfeld Surface Investigation and Packing Efficiency Analysis
3.3. Intermolecular Energy Calculation and Energy Framework of DHQ Solvates
3.4. PXRD Analysis
3.5. DSC Analysis
3.6. TGA Analysis
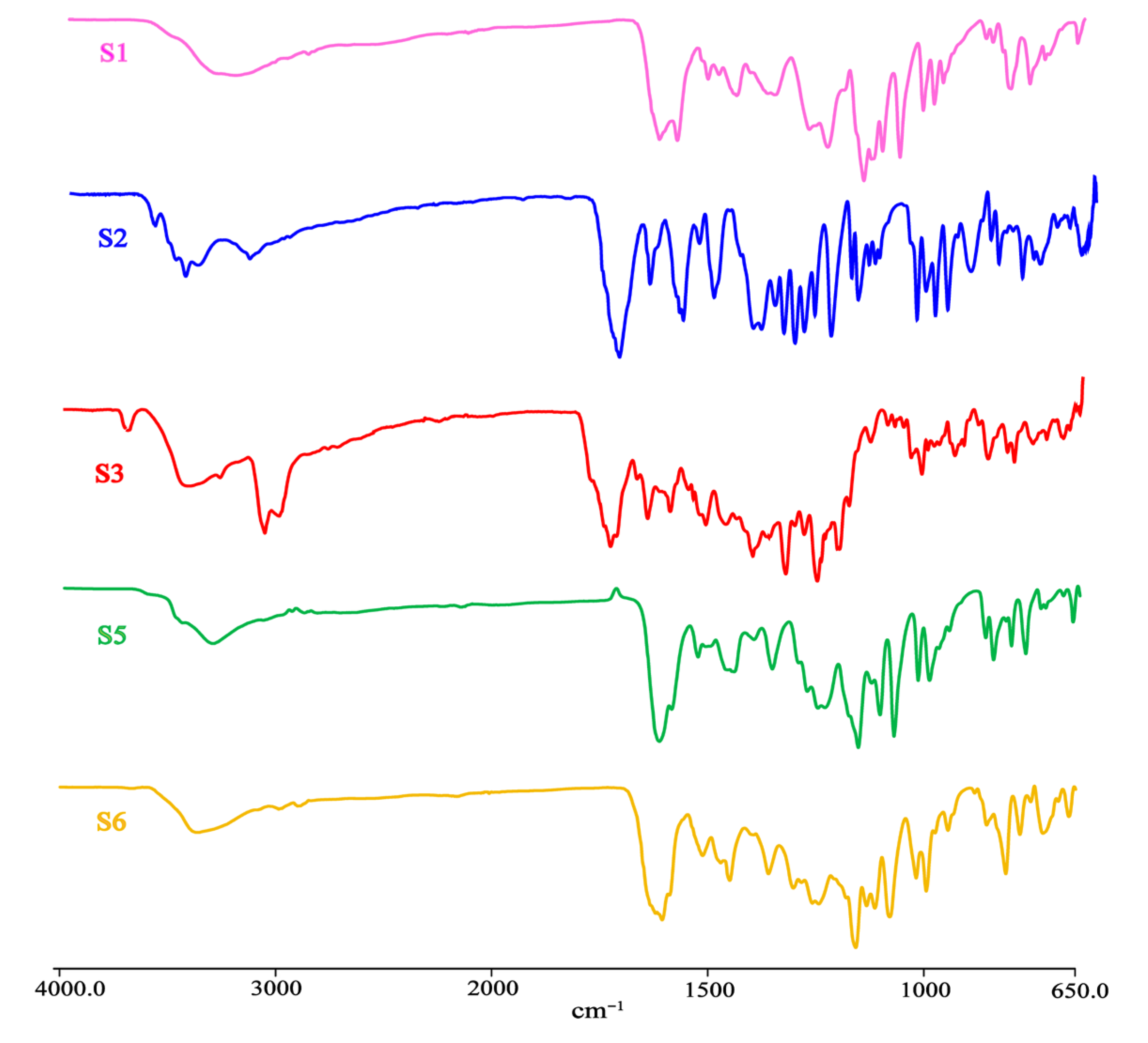
3.7. IR Analysis
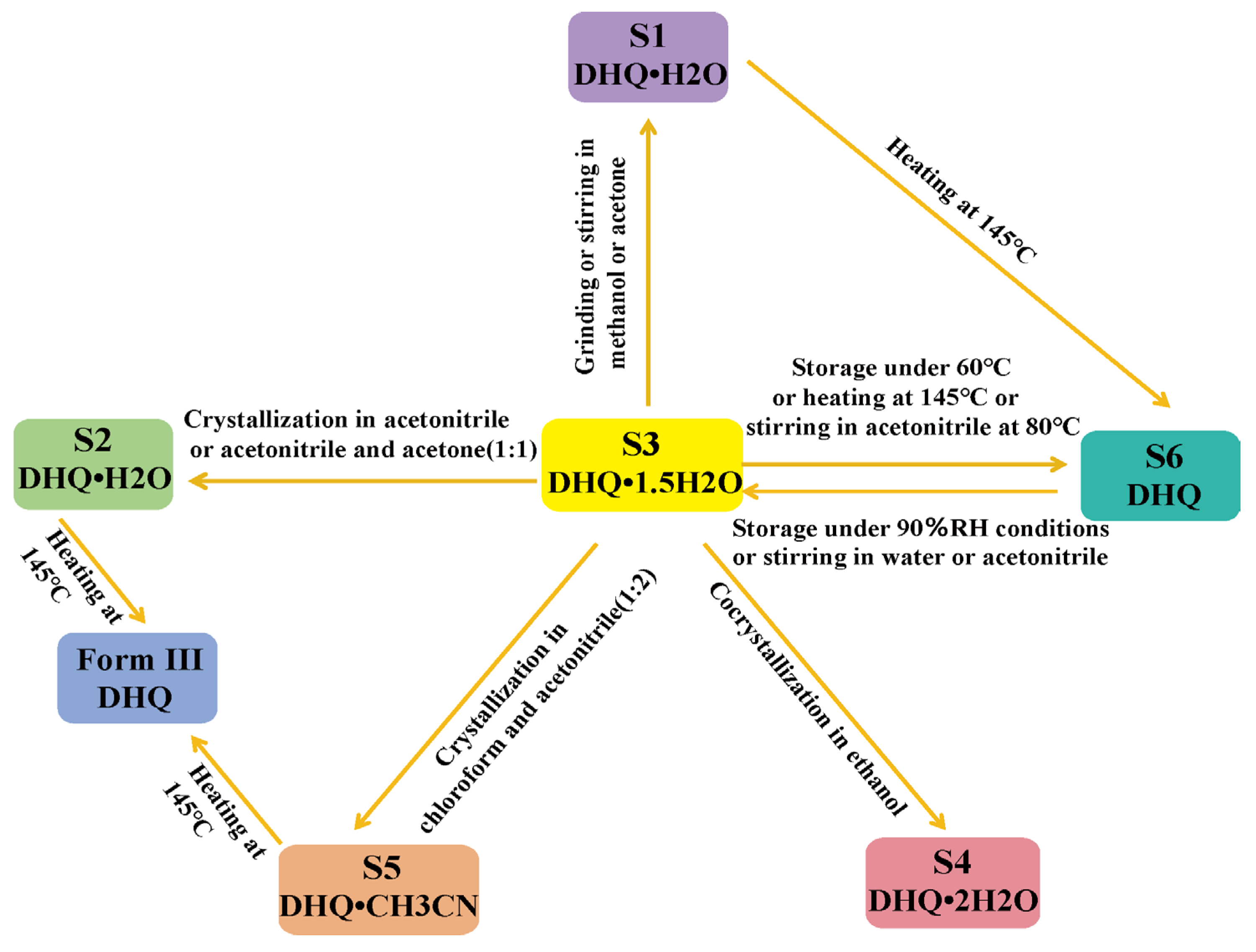
3.8. Phase Transformations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Yang, D.; Zhang, L.; Hu, K.; Yang, S.; Lu, Y.; Du, G. Experimental and Theoretical Investigation of Five Mosapride Forms. J. Mol. Struct. 2024, 1319, 139299. [Google Scholar] [CrossRef]
- Fael, H.; Barbas, R.; Prohens, R.; Ràfols, C.; Fuguet, E. Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile. Pharmaceutics 2021, 14, 49. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, I.S.; Rychkov, D.A.; Kil’MEt’EV, A.S.; Lomovskiy, I.O. Computational study of chemical phenol glycosylation mechanism in the gas phase for modeling direct glycoconjugate formation in raw plant material. Comput. Theor. Chem. 2023, 1225, 114182. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, X.; Lu, X. Crystallization of Asiaticoside from Total Triterpenoid Saponins of Centella Asiatica in a Methanol + Water System. Ind. Eng. Chem. Res. 2014, 53, 14022–14027. [Google Scholar] [CrossRef]
- Clements, M.; Blackie, M.; de Kock, C.; Lawrence, N.; Smith, P.; le Roex, T. Investigation into the Structures and Properties of Multicomponent Crystals Formed from a Series of 7-Chloroquinolines and Aromatic Acids. Cryst. Growth Des. 2019, 19, 1540–1549. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Liu, M.; Xing, C.; He, G.; Zhang, L.; Gong, N.; Lu, Y.; Du, G. Theoretical and experimental cocrystal screening of temozolomide with a series of phenolic acids, promising cocrystal coformers. Chin. Chem. Lett. 2023, 35, 109032. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sameena, J.; Saha, B.K. Solvates of Ajmaline and Two-Dimensional Isostructurality between Methanol and Ethanol Solvates. Cryst. Growth Des. 2011, 11, 905–909. [Google Scholar] [CrossRef]
- Islam, S.; Dey, P.; Seth, S.K. Structural elucidation and various computational studies for quantitative investigation of intermolecular interactions in pyridine-2,6-dicarboxylic acid and its di-hydrate. J. Mol. Struct. 2024, 1311, 138433. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Products, N.A.A. (.E.P.O.D.; Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Statement on the safety of taxifolin-rich extract from Dahurian Larch (Larix gmelinii). EFSA J. 2017, 15, e05059. [Google Scholar] [CrossRef]
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant Activity of a Dihydroquercetin Isolated from Larix gmelinii (Rupr.) Rupr. Wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Z.; Cao, W.; Wang, Y.; Deng, X.; Zhou, Y. Identification of anti-nociceptive constituents from the pollen of Typha angustifolia L. using effect-directed fractionation. Nat. Prod. Res. 2018, 34, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Wang, W.-Y.; Chang, C.-C.; Liou, K.-T.; Sung, Y.-J.; Liao, J.-F.; Chen, C.-F.; Chang, S.; Hou, Y.-C.; Chou, Y.-C.; et al. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats throughits anti-oxidative effect and modulation of NF-kappa B activation. J. Biomed. Sci. 2006, 13, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Feng, X.; Liu, S.; Jiang, Y. Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Ruta graveolens L.: A Critical Review and Future Perspectives. Drug Des. Dev. Ther. 2024, 18, 6459–6485. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Oladejo, C.O.; Josiah, S.S.; Dele Famusiwa, C.; Ojo, O.B.; Olaleye, M.T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology 2018, 25, 365–371. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Gunesch, S.; Hoffmann, M.; Kiermeier, C.; Fischer, W.; Pinto, A.F.; Maurice, T.; Maher, P.; Decker, M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox Biol. 2020, 29, 101378. [Google Scholar] [CrossRef]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical Composition and Antibacterial Activity of Hydroalcoholic Extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus Isolates. BioMed Res. Int. 2016, 2016, 5201879. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Xia, T.; Mao, Q.; Zhong, L. Pretreatment with dihydroquercetin, a dietary flavonoid, protected against concanavalin A-induced immunological hepatic injury in mice and TNF-α/ActD-induced apoptosis in HepG2 cells. Food Funct. 2018, 9, 2341–2352. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Gu, Y.; Kapoor, A.; Peng, J.; Su, Y.; Wei, F.; Wang, Y.; Yang, C.; Gill, A.; et al. Taxifolin Inhibits Breast Cancer Growth by Facilitating CD8+ T Cell Infiltration and Inducing a Novel Set of Genes including Potential Tumor Suppressor Genes in 1q21.3. Cancers 2023, 15, 3203. [Google Scholar] [CrossRef]
- Unver, E.; Tosun, M.; Olmez, H.; Kuzucu, M.; Cimen, F.K.; Suleyman, Z. The Effect of Taxifolin on Cisplatin-Induced Pulmonary Damage in Rats: A Biochemical and Histopathological Evaluation. Mediat. Inflamm. 2019, 2019, 3740867. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sun, Y.; Zhou, N.; Wu, W.; Zheng, W.; Wang, Y. Dihydroquercetin Attenuates Silica-Induced Pulmonary Fibrosis by Inhibiting Ferroptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 845600. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Famusiwa, C.D.; Josiah, S.S.; Lawal, A.O.; Olaleye, M.T.; Akindahunsi, A.A. Dihydroquercetin improves rotenone-induced Parkinsonism by regulating NF-κB-mediated inflammation pathway in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23022. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, W.; Zheng, G.; Yin, Z.; Li, J.; Chen, L.; Zhang, Q. The anti-obesity and gut microbiota modulating effects of taxifolin in C57BL/6J mice fed with a high-fat diet. J. Sci. Food Agric. 2022, 102, 1598–1608. [Google Scholar] [CrossRef]
- Alpan, A.L.; Kızıldağ, A.; Özdede, M.; Karakan, N.C.; Özmen, Ö. The effects of taxifolin on alveolar bone in experimental periodontitis in rats. Arch. Oral Biol. 2020, 117, 104823. [Google Scholar] [CrossRef]
- Itaya, S.; Igarashi, K. Effects of Taxifolin on the Serum Cholesterol Level in Rats. Biosci. Biotechnol. Biochem. 1992, 56, 1492–1494. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Wei, Y.; Zheng, X.; Feng, W. Taxifolin improves disorders of glucose metabolism and water-salt metabolism in kidney via PI3K/AKT signaling pathway in metabolic syndrome rats. Life Sci. 2020, 263, 118713. [Google Scholar] [CrossRef]
- Alam, Q.; Krishnamurthy, S. Dihydroquercetin ameliorates LPS-induced neuroinflammation and memory deficit. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100091. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A Promising Therapy for Diabetic Encephalopathy through Inhibition of Hippocampal Ferroptosis. Phytomedicine 2023, 126, 154887. [Google Scholar] [CrossRef]
- Zeng, Y.-F.; Li, J.-Y.; Wei, X.-Y.; Ma, S.-Q.; Wang, Q.-G.; Qi, Z.; Duan, Z.-C.; Tan, L.; Tang, H. Preclinical evidence of reno-protective effect of quercetin on acute kidney injury: A meta-analysis of animal studies. Front. Pharmacol. 2023, 14, 1310023. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Xu, Q.; Yang, W.; Zhao, J.; Ren, Y.; Yu, Z.; Ma, L. Taxifolin Alleviates DSS-Induced Ulcerative Colitis by Acting on Gut Microbiome to Produce Butyric Acid. Nutrients 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, I.A.; Tyukavkina, N.A.; Kolesnik, Y.A.; Nesterov, V.N.; Kuleshova, L.N.; Khutoryanskii, V.A.; Bazhenov, B.N.; Saibotalov, M.Y. Study of the crystalline structure of dihydroquercetin. Pharm. Chem. J. 1999, 33, 222–224. [Google Scholar] [CrossRef]
- Nifant’eV, E.E.; Koroteev, M.P.; Kaziev, G.Z.; Uminskii, A.A.; Grachev, A.A.; Men’sHov, V.M.; Tsvetkov, Y.E.; Nifant’eV, N.E.; Bel’sKii, V.K.; Stash, A.I. On the problem of identification of the dihydroquercetin flavonoid. Russ. J. Gen. Chem. 2006, 76, 161–163. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Shylov, G.V.; Utenishev, A.N.; Porozov, Y.B. Taxifolin tubes: Crystal engineering and characteristics. Acta Crystallogr. Sect. B Struct. Sci. 2019, 75, 175–182. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, W.; Zu, Y.; Wang, L.; Zhang, Y.; Zhao, X. Preparation and Characterization of Taxifolin form II by Antisolvent Recrystallization. Chem. Eng. Technol. 2019, 42, 414–421. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Ilyasov, I.R.; Zhevlakova, A.K.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shylov, G.V.; Utenishev, A.N.; et al. Assembling the Puzzle of Taxifolin Polymorphism. Molecules 2020, 25, 5437. [Google Scholar] [CrossRef]
- Moura, F.C.S.; Pinna, N.; Vivani, R.; Nunes, G.E.; Schoubben, A.; Bresolin, T.M.B.; Bechold, I.H.; Ricci, M. Exploring Taxifolin Polymorphs: Insights on Hydrate and Anhydrous Forms. Pharmaceutics 2021, 13, 1328. [Google Scholar] [CrossRef]
- Kumara, K.; Jyothi, M.; Kouser, S.; Kumar, A.U.; Warad, I.; Khanum, S.A.; Lokanath, N.K. Structural investigations and theoretical insights of a polymethoxy chalcone derivative: Synthesis, crystal structure, 3D energy frameworks and SARS CoV-2 docking studies. J. Mol. Struct. 2023, 1272, 134226. [Google Scholar] [CrossRef]
- Xing, W.; Yu, H.; Zhang, B.; Liu, M.; Zhang, L.; Wang, F.; Gong, N.; Lu, Y. Quantitative Analysis the Weak Non-Covalent Interactions of the Polymorphs of Donepezil. ACS Omega 2022, 7, 36434–36440. [Google Scholar] [CrossRef]
- Mukherjee, A. Building upon Supramolecular Synthons: Some Aspects of Crystal Engineering. Cryst. Growth Des. 2015, 15, 3076–3085. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 38. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Grabowsky, S.; Jayatilaka, D.; Spackman, M.A. Accurate and Efficient Model Energies for Exploring Intermolecular Interactions in Molecular Crystals. J. Phys. Chem. Lett. 2014, 5, 4249–4255. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, Y.; Wang, W.; An, Q.; Li, S.; Zhang, B.; Zhou, J.; Hu, K.; Zhang, L.; Yang, D.; et al. Characterization, Analysis, and Theoretical Calculation Studies of Solvates and Cocrystals of Betulin: An Exploration of the Boundary between Solvates and Cocrystals. Cryst. Growth Des. 2023, 23, 8694–8706. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, G.; Jin, G.; Du, G.; Lu, Y. Polymorphs and Versatile Solvates of 7-Hydroxyisoflavone. J. Pharm. Sci. 2016, 105, 1387–1397. [Google Scholar] [CrossRef]
- Jyothi, K.L.; Kumara, K.; Hema, M.K.; Gautam, R.; Row, T.G.; Lokanath, N.K. Structural elucidation, theoretical insights and thermal properties of three novel multicomponent molecular forms of gallic acid with hydroxypyridines. J. Mol. Struct. 2020, 1207, 127828. [Google Scholar] [CrossRef]
- Mahesha; Hema, M.K.; Karthik, C.S.; Pampa, K.J.; Mallu, P.; Lokanath, N.K. Solvent induced mononuclear and dinuclear mixed ligand Cu(II) complex: Structural diversity, supramolecular packing polymorphism and molecular docking studies†. New J. Chem. 2021, 44, 18048–18068. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.; Zhang, B.; Yang, D.; Lu, Y.; Du, G. Insights Into the Properties of Amygdalin Solvatomorphs: X-ray Structures, Intermolecular Interactions, and Transformations. ACS Omega 2022, 7, 8906–8918. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xing, W.; Zou, Y.; Hu, K.; Gong, N.; Lu, Y.; Du, G. Understanding solvent effects on solvatomorphisms of donepezil-maleic acid. J. Mol. Struct. 2024, 1296, 136898. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mondal, P.K.; Mirmehrabi, M.; Rohani, S. Desolvation of dasatinib methanolate: An improved anhydrous polymorph. CrystEngComm 2021, 23, 4272–4283. [Google Scholar] [CrossRef]
- Yuan, L.; Horosanskaia, E.; Engelhardt, F.; Edelmann, F.T.; Couvrat, N.; Sanselme, M.; Cartigny, Y.; Coquerel, G.; Seidel-Morgenstern, A.; Lorenz, H. Solvate Formation of Bis(demethoxy)curcumin: Crystal Structure Analyses and Stability Investigations. Cryst. Growth Des. 2018, 19, 854–867. [Google Scholar] [CrossRef]
- Liu, W.; Hou, B.; Huang, X.; Zong, S.; Zheng, Z.; Li, S.; Zhao, B.; Liu, S.; Zhou, L.; Hao, H. Influence of intermolecular interactions and crystal structure on desolvation mechanisms of solvates. CrystEngComm 2021, 23, 3557–3568. [Google Scholar] [CrossRef]
- Chęcińska, L.; Jóźwiak, A.; Ciechańska, M.; Paulmann, C.; Holstein, J.J.; Dittrich, B.; Małecka, M. Quantifying intermolecular interactions for isoindole derivatives: Substituent effect vs. crystal packing. Z. Krist. Mater. 2018, 233, 675–687. [Google Scholar] [CrossRef]
- Kaspiaruk, H.; Chęcińska, L. A comparison of three crystalline forms of miconazole: Solvent-free, ethanol monosolvate and hemihydrate. Acta Crystallogr. Sect. C Struct. Chem. 2022, 78 Pt 6, 343–350. [Google Scholar] [CrossRef]
- Ge, S.; Fu, M.; Cai, Z.; Gu, D.; Ma, Y.; Wang, H.; Ge, M.; Wang, Y. Study of the Polymorphic Transformation Mechanism and Crystal Habits Control of Peramivir from Dihydrate to Trihydrate. Cryst. Growth Des. 2024, 24, 7936–7947. [Google Scholar] [CrossRef]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Dong, X.-D.; Ji, H.-Y. Research on Characteristics, Antioxidant and Antitumor Activities of Dihydroquercetin and Its Complexes. Molecules 2017, 23, 20. [Google Scholar] [CrossRef]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuźniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. Drug Res. 2001, 58, 415–420. [Google Scholar]
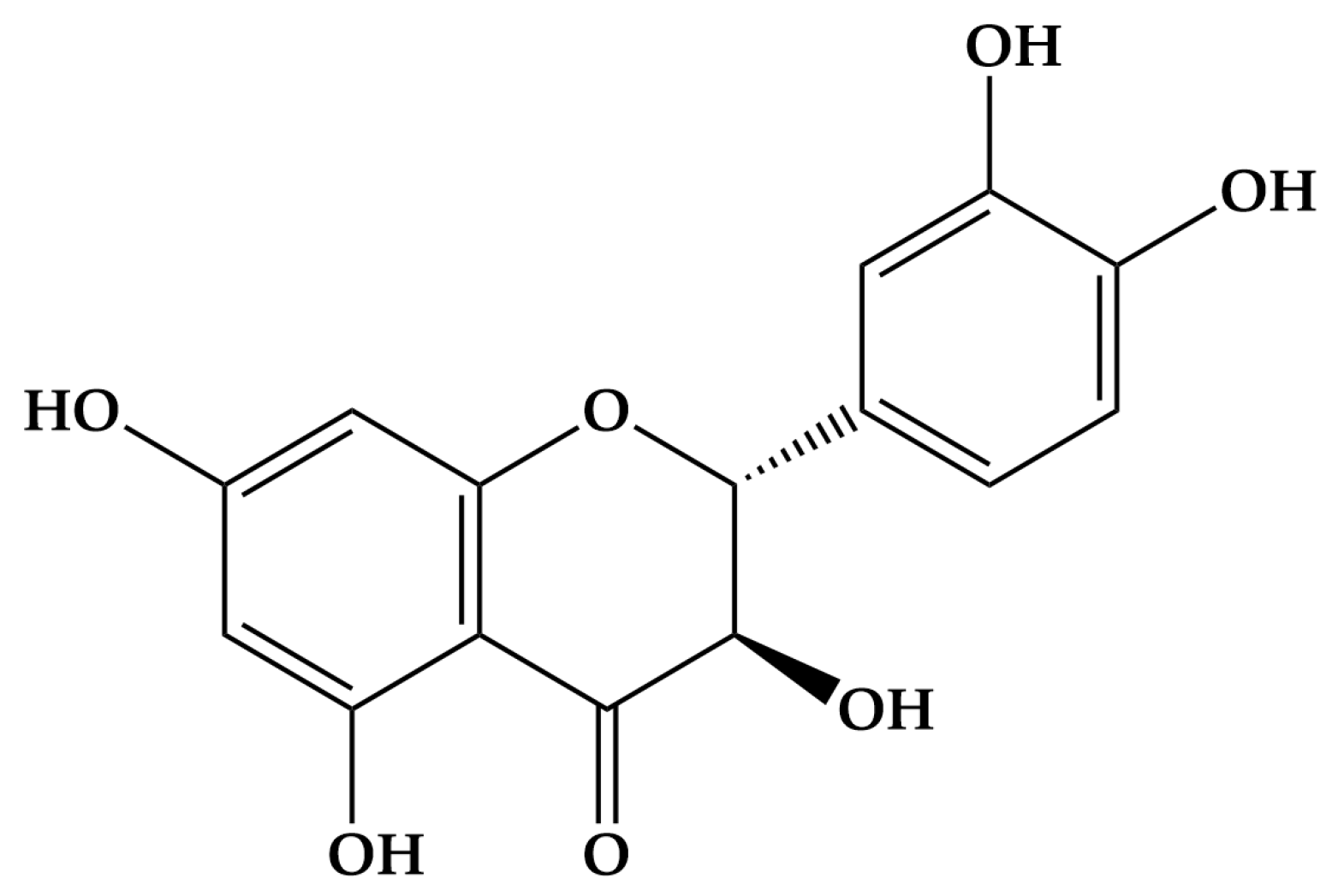





| Form Designation | Category | Chemical Composition | Crystal System/State |
|---|---|---|---|
| Form I | Reported | DHQ•2.5H2O | Monoclinic |
| Form Ia | Reported | DHQ•xH2O (pseudopolymorph, microtubes) | Monoclinic |
| Form II | Reported | DHQ | Crystalline |
| Form III | Reported | DHQ | Crystalline |
| Form IV | Reported | DHQ (microspheres) | Amorphous |
| Form V | Reported | DHQ (S6) | Monoclinic |
| Form VI | Reported | DHQ•1H2O | Crystalline |
| Form VII | Reported | DHQ | Triclinic |
| Form VIII | Newly Discovered | DHQ•1ACN (S5) | Monoclinic |
| Form IX | Newly Discovered | DHQ•1H2O (S1) | Triclinic |
| Form X | Newly Discovered | DHQ•1H2O (S2) | Monoclinic |
| Form XI | Newly Discovered | DHQ•1.5H2O (S3) | Monoclinic |
| Form XII | Newly Discovered | DHQ•2H2O (S4) | Monoclinic |
| Parameters | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| Formula | C15H12O7· H2O | C15H12O7· H2O | C15H12O7· 1.5(H2O) | C15H12O7· 2H2O | C15H12O7· C2H3N |
| Formula weight | 322.26 | 322.26 | 331.27 | 340.28 | 345.30 |
| Temperature/K | 293(2) | 293(2) | 293(2) | 293(2) | 293(2) |
| Crystal size (mm) | 0.05 × 0.12 × 0.22 | 0.11 × 0.14 × 0.27 | 0.04 × 0.09 × 0.41 | 0.21 × 0.23 × 0.25 | 0.05 × 0.12 × 0.22 |
| Description | plate | needle | plate | needle | plate |
| Crystal system | triclinic | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | P − 1 | P 21/n | C 2/c | P 21/c | P 21/c |
| a (Å) | 5.378(1) | 4.835(1) | 25.927(1) | 15.913(1) | 5.15(1) |
| b (Å) | 10.115(1) | 27.649(1) | 4.808(1) | 13.145(1) | 24.10 (1) |
| c (Å) | 13.260(1) | 10.293(1) | 23.251(1) | 7.119(1) | 12.63(1) |
| α (°) | 75.97(1) | 90 | 90 | 90 | 90 |
| β (°) | 82.90(1) | 95.51(1) | 100.89(1) | 93.71(1) | 90.90(1) |
| γ (°) | 79.06(1) | 90 | 90 | 90 | 90 |
| Volume (Å3) | 684.83(4) | 1369.62(6) | 2846.41(9) | 1486.01(5) | 1566.81(4) |
| Z/Z′ | 2/1 | 4/1 | 8/1 | 4/1 | 4/1 |
| Density (g/cm3) | 1.563 | 1.563 | 1.546 | 1.521 | 1.464 |
| Independent reflections | 2601 | 2902 | 2779 | 2999 | 2989 |
| Reflections with I > 2σ(I) | 2512 | 2587 | 2301 | 2738 | 2506 |
| Final R, wR(F2) value | 0.050, 0.140 | 0.065, 0.177 | 0.083, 0.206 | 0.061, 0.148 | 0.082, 0.216 |
| GOOF | 1.070 | 1.059 | 1.042 | 1.039 | 1.091 |
| Rint | 0.0180 | 0.0907 | 0.0417 | 0.0203 | 0.0345 |
| CCDC number | 2,422,558 | 2,422,559 | 2,422,560 | 2,422,561 | 2,422,562 |
| Variable | Eele | Epol | Edis | Erep | Etot |
|---|---|---|---|---|---|
| S1 | −214.3 | −32.0 | −213.6 | 179.0 | −336.2 |
| S2 | −247.6 | −31.0 | −212.9 | 164.8 | −377.5 |
| S3 | −328.5 | −36.2 | −223.3 | 250.1 | −400.0 |
| S4 | −158.1 | −32.7 | −212.9 | 153.3 | −299.6 |
| S5 | −113.6 | −36.3 | −201.7 | 151.2 | −364.2 |
| Solvate | Desolvation Temperature (°C) | Theoretical Weight Loss (%) | Experimental Weight Loss (%) |
|---|---|---|---|
| S1 | 60–140 | 5.56 | 6.33 |
| S2 | 110–160 | 5.56 | 5.95 |
| S3 | 50–150 | 8.11 | 8.54 |
| S5 | 60–150 | 11.81 | 9.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zou, Y.; Yang, S.; Xing, C.; Gong, N.; Du, G.; Lu, Y. X-Ray Structures, Intermolecular Interactions, and Structural Transformations of Dihydroquercetin Solvates and Polymorphs. Pharmaceutics 2025, 17, 1512. https://doi.org/10.3390/pharmaceutics17121512
Meng X, Zou Y, Yang S, Xing C, Gong N, Du G, Lu Y. X-Ray Structures, Intermolecular Interactions, and Structural Transformations of Dihydroquercetin Solvates and Polymorphs. Pharmaceutics. 2025; 17(12):1512. https://doi.org/10.3390/pharmaceutics17121512
Chicago/Turabian StyleMeng, Xin, Yao Zou, Shiying Yang, Cheng Xing, Ningbo Gong, Guanhua Du, and Yang Lu. 2025. "X-Ray Structures, Intermolecular Interactions, and Structural Transformations of Dihydroquercetin Solvates and Polymorphs" Pharmaceutics 17, no. 12: 1512. https://doi.org/10.3390/pharmaceutics17121512
APA StyleMeng, X., Zou, Y., Yang, S., Xing, C., Gong, N., Du, G., & Lu, Y. (2025). X-Ray Structures, Intermolecular Interactions, and Structural Transformations of Dihydroquercetin Solvates and Polymorphs. Pharmaceutics, 17(12), 1512. https://doi.org/10.3390/pharmaceutics17121512




