Correlations Between Rheology, In Situ Mucosal Retention and In Vivo Immunogenicity Reveal the Potential and Limitations of Mucoadhesive Excipients for Sublingual Vaccine Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Ethics
2.3. Preparation of Vaccine Formulations
2.4. In Vivo Immunization Study
2.4.1. Immunization Protocol
2.4.2. Sample Collection
2.4.3. Anti-OVA Antibody Quantification by ELISA
2.5. In Vivo Imaging of Mucosal In Situ Residence and Its Spatiotemporal Clearance Kinetics Using IVIS
2.6. Formulation Rheological Characterization
2.6.1. Viscosity Measurements
2.6.2. Mucoadhesion Measurements
2.7. Statistical Analysis
| ρ (Pearson’s r) | Effect Size | Interpretation |
| 0.10–0.29 | Small | Weak correlation |
| 0.30–0.49 | Medium | Moderate correlation |
| ≥0.50 | Large | Strong correlation |
| p-Value | Asterisks | Interpretation |
| p ≤ 0.05 | * | Significant |
| p ≤ 0.01 | ** | Very significant |
| p ≤ 0.001 | *** | Highly significant |
| p ≤ 0.0001 | **** | Extremely significant |
3. Results
3.1. Mucoadhesive Excipients as Key Modulators of Immunogenicity in Sublingual Vaccines

3.2. Impact of Mucoadhesive Excipients on In Situ Mucosal Retention of Sublingual Vaccines

3.3. Rheological Insights into Mucosal Retention of Sublingual Vaccine Formulations
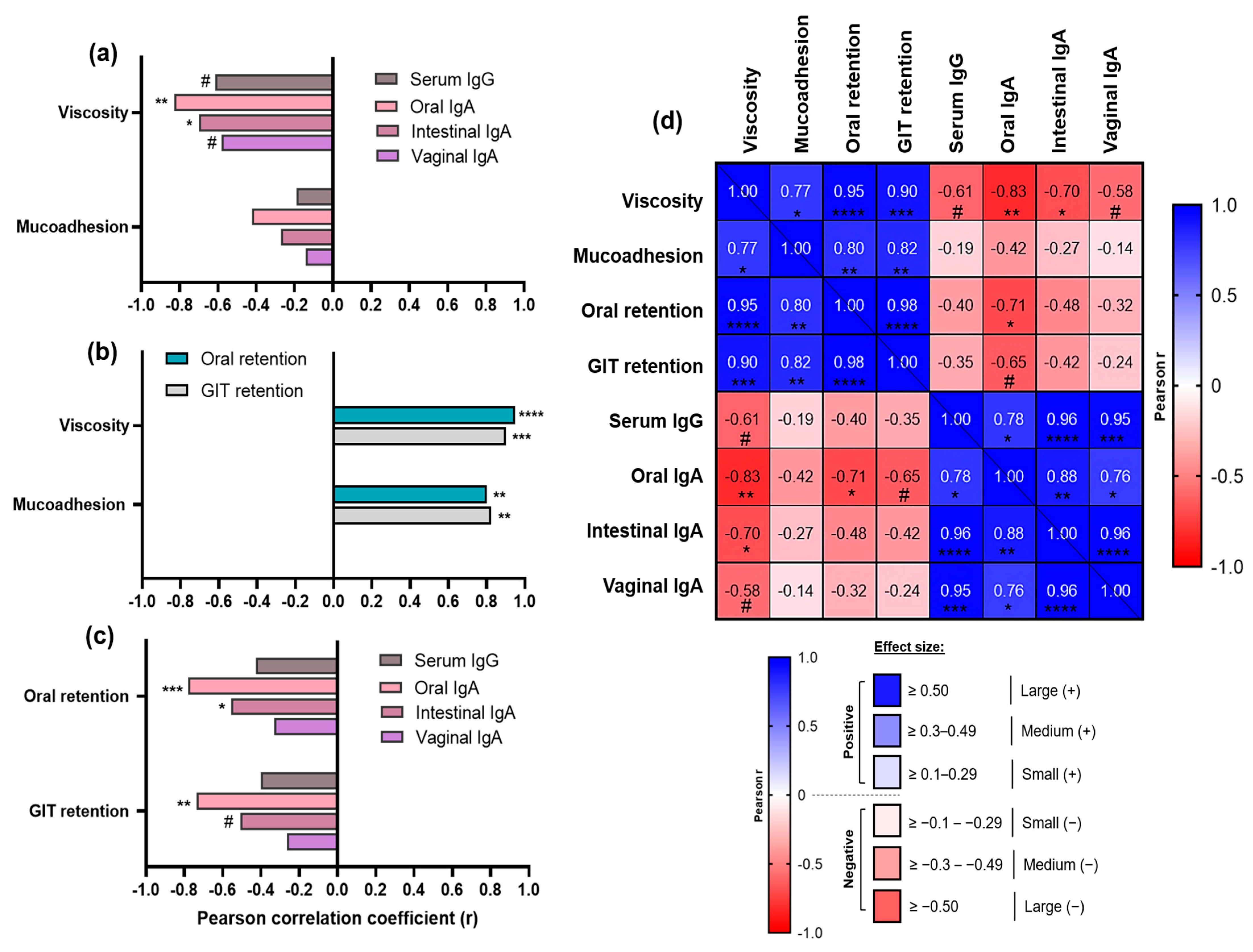
3.4. Correlation Analyses Uncover Key “Drivers” and “Brakes” of Sublingual Vaccine Efficacy
4. Discussion
4.1. Sublingual Immunization: Impact of Vaccine Formulation on Efficacy and Its Mechanistic Insights
4.2. HPMC: Immunological Trade-Offs of High Rheology and In Situ Retention
4.3. Chitosan: Balancing Permeation and Adjuvanticity for Optimal Sublingual Vaccine Performance
4.4. Strengths, Limitations, and Future Work
4.5. Implications for Sublingual Vaccine Formulation Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| AUC | Area under the curve |
| BSA | Bovine serum albumin |
| CTB | Cholera toxin B-subunit |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Foetal bovine serum |
| GALT | Gut-associated lymphoid tissue |
| GMT | Geometric mean titre |
| GIT | Gastrointestinal tract |
| HPMC | Hydroxypropyl methylcellulose |
| HRP | Horseradish peroxidase |
| Ig | Immunoglobulin |
| IVIS | In vivo imaging system |
| MALT | Mucosa-associated lymphoid tissue |
| MGC | Methyl glycol chitosan |
| OVA | Ovalbumin |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PBS-T | Phosphate-buffered saline with Tween 20 |
| PIB | Protease inhibitor buffer |
| PG | Propylene glycol |
| ROI | Region of interest |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| VALT | Vulvovaginal-associated lymphoid tissues |
References
- Paris, A.; Colomb, E.; Verrier, B.; Anjuère, F.; Monge, C. Sublingual vaccination and delivery systems. J. Control. Release 2021, 332, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Verrier, B. Sublingual antigen delivery: A solution for needle-free HIV vaccination. Expert Rev. Vaccines 2021, 20, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J.-P. Buccal and sublingual vaccine delivery. J. Control. Release 2014, 190, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.-N. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine 2011, 54, 1–5. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, W.; Zhang, Y.; Li, X.; Que, H.; Wei, X. Mucosal immunity and vaccination strategies: Current insights and future perspectives. Mol. Biomed. 2025, 6, 57. [Google Scholar] [CrossRef]
- Wheeler, A.; Sharif, S. Sublingual delivery of vaccines: Can we enhance the immune response induced via this route? Eur. J. Pharm. Sci. 1996, 4, S39. [Google Scholar] [CrossRef]
- Shim, B.-S.; Choi, Y.K.; Yun, C.-H.; Lee, E.-G.; Jeon, Y.S.; Park, S.-M.; Cheon, I.S.; Joo, D.-H.; Cho, C.H.; Song, M.-S.; et al. Sublingual Immunization with M2-Based Vaccine Induces Broad Protective Immunity against Influenza. PLoS ONE 2011, 6, e27953. [Google Scholar] [CrossRef]
- Song, M.; Shim, B.-S. Sublingual immunization with M2-based vaccine induces broad protective immunity (P4301). J. Immunol. 2013, 190, 123.14. [Google Scholar] [CrossRef]
- Hervouet, C.; Luci, C.; Çuburu, N.; Cremel, M.; Bekri, S.; Vimeux, L.; Marañon, C.; Czerkinsky, C.; Hosmalin, A.; Anjuère, F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine 2010, 28, 5582–5590. [Google Scholar] [CrossRef]
- Song, J.-H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.-Y.; Park, S.-H.; Czerkinsky, C.; Kweon, M.-N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanji, M.; Mitsunaga, F.; Nakamura, S. SARS-CoV-2 sublingual vaccine with RBD antigen and poly(I:C) adjuvant: Preclinical study in cynomolgus macaques. Biol. Methods Protoc. 2023, 8, bpad017. [Google Scholar] [CrossRef]
- McMahan, K.; Wegmann, F.; Aid, M.; Sciacca, M.; Liu, J.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Powers, O.; Hope, D.; et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 2023, 626, 385–391. [Google Scholar] [CrossRef]
- Tobias, J.; Steinberger, P.; Wilkinson, J.; Klais, G.; Kundi, M.; Wiedermann, U. SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity. Vaccines 2024, 12, 795. [Google Scholar] [CrossRef]
- Sallard, E.; Aydin, M. Cutting-edge research frontiers in oral cavity vaccines for respiratory diseases: A roadmap for scientific advancement. Front. Cell. Infect. Microbiol. 2024, 14, 1388222. [Google Scholar] [CrossRef]
- Gulani, M.; Arte, T.; Ferguson, A.; Pasupuleti, D.; Adediran, E.; Harsoda, Y.; McCommon, A.N.; Gala, R.; D’souza, M.J. Recent Advancements in Non-Invasive Vaccination Strategies. Vaccines 2025, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Mascarell, L.; Lombardi, V.; Zimmer, A.; Louise, A.; Tourdot, S.; Van Overtvelt, L.; Moingeon, P. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+ T cells. Clin. Exp. Allergy 2009, 39, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Allam, J.; Duan, Y.; Winter, J.; Stojanovski, G.; Fronhoffs, F.; Wenghoefer, M.; Bieber, T.; Peng, W.; Novak, N. Tolerogenic T cells, Th1/Th17 cytokines and TLR2/TLR4 expressing dendritic cells predominate the microenvironment within distinct oral mucosal sites. Allergy 2010, 66, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, H.S.; Yorgensen, Y.M.; Morasse, A.; Evans, J.T.; Burkhart, D.J. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. J. Control. Release 2016, 223, 64–74. [Google Scholar] [CrossRef]
- Seth, A.; Kong, I.G.; Lee, S.-H.; Yang, J.-Y.; Lee, Y.-S.; Kim, Y.; Wibowo, N.; Middelberg, A.P.; Lua, L.H.; Kweon, M.-N. Modular virus-like particles for sublingual vaccination against group A streptococcus. Vaccine 2016, 34, 6472–6480. [Google Scholar] [CrossRef]
- Veazey, R.S.; Siddiqui, A.; Klein, K.; Buffa, V.; Fischetti, L.; Doyle-Meyers, L.; King, D.F.; Tregoning, J.S.; Shattock, R.J. Evaluation of mucosal adjuvants and immunization routes for the induction of systemic and mucosal humoral immune responses in macaques. Hum. Vaccines Immunother. 2015, 11, 2913–2922. [Google Scholar] [CrossRef]
- Spinner, J.L.; Oberoi, H.S.; Yorgensen, Y.M.; Poirier, D.S.; Burkhart, D.J.; Plante, M.; Evans, J.T. Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually. Vaccine 2015, 33, 5845–5853. [Google Scholar] [CrossRef]
- Buffa, V.; Klein, K.; Fischetti, L.; Shattock, R.J. Evaluation of TLR Agonists as Potential Mucosal Adjuvants for HIV gp140 and Tetanus Toxoid in Mice. PLoS ONE 2012, 7, e50529. [Google Scholar] [CrossRef] [PubMed]
- Appledorn, D.M.; Aldhamen, Y.A.; Godbehere, S.; Seregin, S.S.; Amalfitano, A. Sublingual Administration of an Adenovirus Serotype 5 (Ad5)-Based Vaccine Confirms Toll-Like Receptor Agonist Activity in the Oral Cavity and Elicits Improved Mucosal and Systemic Cell-Mediated Responses against HIV Antigens despite Preexisting Ad5 Immunity. Clin. Vaccine Immunol. 2011, 18, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, S.; Galvan, G.; Jagannath, C.; Sastry, K.J. Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice. Vaccines 2017, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, S.C.; Perduca, M.; Zipeto, D.; Bardi, G. Efficiency of Chitosan Nanocarriers in Vaccinology for Mucosal Immunization. Vaccines 2023, 11, 1333. [Google Scholar] [CrossRef]
- Vasquez-Martínez, N.; Guillen, D.; Moreno-Mendieta, S.A.; Sanchez, S.; Rodríguez-Sanoja, R. The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants. Polymers 2023, 15, 1615. [Google Scholar] [CrossRef]
- Jones, A.T.; Shen, X.; Walter, K.L.; LaBranche, C.C.; Wyatt, L.S.; Tomaras, G.D.; Montefiori, D.C.; Moss, B.; Barouch, D.H.; Clements, J.D.; et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 2019, 10, 798. [Google Scholar] [CrossRef]
- Desai, V.; Ashok, P.A.; Kulkarni, P.; Ahmed, K.A.; Raikar, P.K. Enhancing Drug Bioavailability Through Mucoadhesive Technologies: A Comprehensive Review of Delivery Systems. Biomed. Mater. Devices 2025, 1–19. [Google Scholar] [CrossRef]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles—Important step towards effective mucosal vaccines. J. Control. Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- White, J.A.; Blum, J.S.; Hosken, N.A.; Marshak, J.O.; Duncan, L.; Zhu, C.; Norton, E.B.; Clements, J.D.; Koelle, D.M.; Chen, D.; et al. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum. Vaccines Immunother. 2014, 10, 3611–3621. [Google Scholar] [CrossRef]
- Esih, H.; Mezgec, K.; Billmeier, M.; Malenšek, Š.; Benčina, M.; Grilc, B.; Vidmar, S.; Gašperlin, M.; Bele, M.; Zidarn, M.; et al. Mucoadhesive film for oral delivery of vaccines for protection of the respiratory tract. J. Control. Release 2024, 371, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Borde, A.; Ekman, A.; Holmgren, J.; Larsson, A. Effect of protein release rates from tablet formulations on the immune response after sublingual immunization. Eur. J. Pharm. Sci. 2012, 47, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.H.; Opolot, E.E.; Wu, Y.; Cossette, B.; Varadhan, A.K.; Collier, J.H. Tabletized Supramolecular Assemblies for Sublingual Peptide Immunization. Adv. Healthc. Mater. 2021, 10, e2001614. [Google Scholar] [CrossRef] [PubMed]
- Kraan, H.; Soema, P.; Amorij, J.-P.; Kersten, G. Intranasal and sublingual delivery of inactivated polio vaccine. Vaccine 2017, 35, 2647–2653. [Google Scholar] [CrossRef]
- Yousif, M.D.; Felek, A.; Saydam, M.; Wilson, S.; Murdan, S.; Mawas, F. Sublingual immunisation with GBS serotype III capsular polysaccharide-tetanus toxoid conjugate vaccine induces systemic and mucosal antibody responses which are opsonophagocytic and inhibit GBS colonisation of vaginal epithelial cells. Vaccine 2022, 40, 6055–6063. [Google Scholar] [CrossRef]
- Iftimie, S.; Ladero-Palacio, P.; López-Azcona, A.F.; Pujol-Galarza, L.; Pont-Salvadó, A.; Gabaldó-Barrios, X.; Joven, J.; Camps, J.; Castro, A.; Pascual-Queralt, M. Evaluating the use of Uromune® autovaccine in recurrent urinary tract infections: A pilot unicenter retrospective study in Reus, Spain. BMC Infect. Dis. 2025, 25, 117. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Pintea, A.; Pintea, C.; Rédai, E.-M.; Antonoaea, P.; Bîrsan, M.; Ciurba, A. Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems. Pharmaceutics 2025, 17, 784. [Google Scholar] [CrossRef]
- Amanzholkyzy, A.; Zhumagaliyeva, S.; Sultanova, N.; Abilov, Z.; Ongalbek, D.; Donbayeva, E.; Niyazbekova, A.; Mukazhanova, Z. Hydrogel Delivery Systems for Biological Active Substances: Properties and the Role of HPMC as a Carrier. Molecules 2025, 30, 1354. [Google Scholar] [CrossRef]
- Tochikubo, K.; Isaka, M.; Yasuda, Y.; Kozuka, S.; Matano, K.; Miura, Y.; Taniguchi, T. Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine 1998, 16, 150–155. [Google Scholar] [CrossRef]
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera Toxin B: One Subunit with Many Pharmaceutical Applications. Toxins 2015, 7, 974–996. [Google Scholar] [CrossRef]
- Stratmann, T. Cholera Toxin Subunit B as Adjuvant––An Accelerator in Protective Immunity and a Break in Autoimmunity. Vaccines 2015, 3, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 2022, 167, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Cole, H.; Bryan, D.; Lancaster, L.; Mawas, F.; Vllasaliu, D. Chitosan nanoparticle antigen uptake in epithelial monolayers can predict mucosal but not systemic in vivo immune response by oral delivery. Carbohydr. Polym. 2018, 190, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, J.; Weiner, D. Pharmacokinetic and Pharmacodynamic Data Analysis—Concepts and Applications, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Gloudemans, A.K.; Plantinga, M.; Guilliams, M.; Willart, M.A.; Ozir-Fazalalikhan, A.; van der Ham, A.; Boon, L.; Harris, N.L.; Hammad, H.; Hoogsteden, H.C.; et al. The Mucosal Adjuvant Cholera Toxin B Instructs Non-Mucosal Dendritic Cells to Promote IgA Production Via Retinoic Acid and TGF-β. PLoS ONE 2013, 8, e59822. [Google Scholar] [CrossRef]
- Haddadi, A.; Chaffey, A.; Ng, S.H.; Yalamati, D.; Wilson, H.L. Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice. Vaccines 2020, 8, 569. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Y.; Peng, L.; Dong, X.; Xu, Y.; Wang, Y.; Yang, Y. Effects of increasing sensitizing doses of ovalbumin on airway hyperresponsiveness in asthmatic mice. Immun. Inflamm. Dis. 2024, 12, e1225. [Google Scholar] [CrossRef]
- Rana, P.; Murthy, R.S.R. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef]
- McInnes, F.J.; O’Mahony, B.; Lindsay, B.; Band, J.; Wilson, C.G.; Hodges, L.A.; Stevens, H.N. Nasal residence of insulin containing lyophilised nasal insert formulations, using gamma scintigraphy. Eur. J. Pharm. Sci. 2007, 31, 25–31. [Google Scholar] [CrossRef]
- Kockisch, S.; Rees, G.D.; Young, S.A.; Tsibouklis, J.; Smart, J.D. A direct-staining method to evaluate the mucoadhesion of polymers from aqueous dispersion. J. Control. Release 2001, 77, 1–6. [Google Scholar] [CrossRef]
- Patel, D.; Smith, A.; Grist, N.; Barnett, P.; Smart, J. An in vitro mucosal model predictive of bioadhesive agents in the oral cavity. J. Control. Release 1999, 61, 175–183. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Çuburu, N.; Kweon, M.-N.; Anjuere, F.; Holmgren, J. Sublingual vaccination. Hum. Vaccines 2011, 7, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.Y.; Loh, J.M.S.; Fujihashi, K.; Kiyono, H. Mucosal vaccination: Onward and upward. Expert Rev. Vaccines 2023, 22, 885–899. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Pierce, N. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J. Exp. Med. 1978, 148, 195–206. [Google Scholar] [CrossRef]
- Lycke, N.; Lindholm, L.; Holmgren, J. IgA Isotype Restriction in the Mucosal but Not in the Extramucosal Immune Response after Oral Immunizations with Cholera Toxin or Cholera B Subunit. Int. Arch. Allergy Immunol. 1983, 72, 119–127. [Google Scholar] [CrossRef]
- Tamura, S.-I.; Samegai, Y.; Kurata, H.; Nagamine, T.; Aizawa, C.; Kurata, T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine 1988, 6, 409–413. [Google Scholar] [CrossRef]
- Smart, J. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Wheeler, W.; Elliott, G.; Cluff, C. Composition of Antigen and Glycolipid Adjuvant For Sublingual Administration. US8470331B2, 2013. Available online: https://patentimages.storage.googleapis.com/1b/d1/ab/60bb3716e38a0e/US8470331.pdf (accessed on 21 September 2025).
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Gustafsson, C.; Bonferoni, M.C.; Caramella, C.; Lennholm, H.; Nyström, C. Characterisation of particle properties and compaction behaviour of hydroxypropyl methylcellulose with different degrees of methoxy/hydroxypropyl substitution. Eur. J. Pharm. Sci. 1999, 9, 171–184. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Ju, R.T.C.; Nixon, P.R.; Patel, M.V. Diffusion Coefficients of Polymer Chains in the Diffusion Layer Adjacent to a Swollen Hydrophilic Matrix. J. Pharm. Sci. 1997, 86, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Pedacchia, A.; Adrover, A. Study of release kinetics and diffusion coefficients in swellable cellulosic thin films by means of a simple spectrophotometric technique. Chem. Eng. Res. Des. 2014, 92, 2550–2556. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef]
- Chaiya, P.; Rojviriya, C.; Pichayakorn, W.; Phaechamud, T. New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques. Pharmaceutics 2022, 14, 2299. [Google Scholar] [CrossRef]
- Cho, C.-S.; Hwang, S.-K.; Gu, M.-J.; Kim, C.-G.; Kim, S.-K.; Ju, D.-B.; Yun, C.-H.; Kim, H.-J. Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems. Tissue Eng. Regen. Med. 2021, 18, 693–712. [Google Scholar] [CrossRef]
- Diethart, B.; Emberlin, J.C.; Lewis, R.A. Hydroxypropylmethylcellulose gel application delays Der p 1 diffusion in vitro. Nat. Sci. 2010, 2, 79–84. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Commins, S.P. Mechanisms of Oral Tolerance. Pediatr. Clin. N. Am. 2015, 62, 1523–1529. [Google Scholar] [CrossRef]
- Faria, A.M.C.; Weiner, H.L. Oral tolerance. Immunol. Rev. 2005, 206, 232–259. [Google Scholar] [CrossRef]
- Gale, E.C.; Powell, A.E.; Roth, G.A.; Meany, E.L.; Yan, J.; Ou, B.S.; Grosskopf, A.K.; Adamska, J.; Picece, V.C.T.M.; d’Aquino, A.I.; et al. Hydrogel-Based Slow Release of a Receptor-Binding Domain Subunit Vaccine Elicits Neutralizing Antibody Responses Against SARS-CoV-2. Adv. Mater. 2021, 33, 2104362. [Google Scholar] [CrossRef]
- Ou, B.S.; Saouaf, O.M.; Yan, J.; Bruun, T.U.J.; Baillet, J.; Zhou, X.; King, N.P.; Appel, E.A. Broad and Durable Humoral Responses Following Single Hydrogel Immunization of SARS-CoV-2 Subunit Vaccine. Adv. Healthc. Mater. 2023, 12, e2301495. [Google Scholar] [CrossRef]
- Roth, G.A.; Gale, E.C.; Alcántara-Hernández, M.; Luo, W.; Axpe, E.; Verma, R.; Yin, Q.; Yu, A.C.; Hernandez, H.L.; Maikawa, C.L.; et al. Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Cent. Sci. 2020, 6, 1800–1812. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohashi-Doi, K.; Matsuhara, H.; Verhoog, L.; Lindholm, M.; Lawton, S.; Lund, K. Allergen Release Profiles of Fast-Dissolving Freeze-Dried Orodispersible Sublingual Allergy Immunotherapy Tablets. Curr. Ther. Res. 2022, 96, 100678. [Google Scholar] [CrossRef]
- Lund, K.; Kito, H.; Skydtsgaard, M.B.; Nakazawa, H.; Ohashi-Doi, K.; Lawton, S. The Importance of Tablet Formulation on Allergen Release Kinetics and Efficiency: Comparison of Freeze-dried and Compressed Grass Pollen Sublingual Allergy Immunotherapy Tablet Formulations. Clin. Ther. 2019, 41, 742–753. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs. 1: Influence of Molecular Weight and Degree of Acetylation on Drug Transport Across Human Intestinal Epithelial (Caco-2) Cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafieetehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef]
- Benediktsdóttir, B.E.; Baldursson, Ó.; Másson, M. Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application. J. Control. Release 2014, 173, 18–31. [Google Scholar] [CrossRef]
- de Alvarenga, E.S. Characterization and Properties of Chitosan. In Biotechnology of Biopolymers; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2013, 10, 797–807. [Google Scholar] [CrossRef]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Fan, Q.; Hao, D.; Wu, J.; Ma, G.; Su, Z. Chitosan-based mucosal adjuvants: Sunrise on the ocean. Vaccine 2015, 33, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.; Davis, S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Riehl, M.; Harms, M.; Lucas, H.; Ebensen, T.; Guzmán, C.A.; Mäder, K. Dual dye in-vivo imaging of differentially charged PLGA carriers reveals antigen-depot effect, leading to improved immune responses in preclinical models. Eur. J. Pharm. Sci. 2018, 117, 88–97. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Verma, K.D.; Hu, Y.; Khera, E.; Priluck, A.; Smith, D.E.; Thurber, G.M. Oral Administration and Detection of a Near-Infrared Molecular Imaging Agent in an Orthotopic Mouse Model for Breast Cancer Screening. Mol. Pharm. 2018, 15, 1746–1754. [Google Scholar] [CrossRef]
- Usama, S.M.; Thapaliya, E.R.; Luciano, M.P.; Schnermann, M.J. Not so innocent: Impact of fluorophore chemistry on the in vivo properties of bioconjugates. Curr. Opin. Chem. Biol. 2021, 63, 38–45. [Google Scholar] [CrossRef]
- Martin, C.; Brachet, G.; Colas, C.; Allard-Vannier, E.; Kizlik-Masson, C.; Esnault, C.; Respaud, R.; Denevault-Sabourin, C.; Chourpa, I.; Gouilleux-Gruart, V.; et al. In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation. Pharmaceuticals 2019, 12, 176. [Google Scholar] [CrossRef]
- Hayashi-Takanaka, Y.; Stasevich, T.J.; Kurumizaka, H.; Nozaki, N.; Kimura, H. Evaluation of Chemical Fluorescent Dyes as a Protein Conjugation Partner for Live Cell Imaging. PLoS ONE 2014, 9, e106271. [Google Scholar] [CrossRef]
- Ksenofontova, K.V.; Kerner, A.A.; Ksenofontov, A.A.; Shagurin, A.Y.; Bocharov, P.S.; Lukanov, M.M.; Kayumov, A.R.; Zhuravleva, D.E.; Iskhakova, Z.I.; Molchanov, E.E.; et al. Amine-Reactive BODIPY Dye: Spectral Properties and Application for Protein Labeling. Molecules 2022, 27, 7911. [Google Scholar] [CrossRef]
- Takakura, H.; Sato, H.; Nakajima, K.; Suzuki, M.; Ogawa, M. In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers 2021, 13, 2245. [Google Scholar] [CrossRef]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef]
- Kulkarni, U.; Mahalingam, R.; Pather, I.; Li, X.; Jasti, B. Porcine buccal mucosa as in vitro model: Effect of biological and experimental variables. J. Pharm. Sci. 2010, 99, 1265–1277. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef]



| Formulation | OVA (µg) | CTB (µg) | MGC (µg) | HPMC (w/v %) | Immunogenicity | Sublingual Residence | Viscosity & Mucoadhesion |
|---|---|---|---|---|---|---|---|
| A | 50 | – | – | – | + | + | – |
| B | 50 | 10 | – | – | + | – | – |
| C | 50 | – | 25 | 3 | + | + | + |
| D | 10 | 2 | – | – | + | + | – |
| E | 10 | 2 | 25 | – | + | + | + |
| F | 10 | – | 25 | 3 | + | – | – |
| G | 10 | 2 | 25 | 3 | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousif, M.D.; Kubajewska, I.; Mawas, F.; Murdan, S. Correlations Between Rheology, In Situ Mucosal Retention and In Vivo Immunogenicity Reveal the Potential and Limitations of Mucoadhesive Excipients for Sublingual Vaccine Delivery. Pharmaceutics 2025, 17, 1456. https://doi.org/10.3390/pharmaceutics17111456
Yousif MD, Kubajewska I, Mawas F, Murdan S. Correlations Between Rheology, In Situ Mucosal Retention and In Vivo Immunogenicity Reveal the Potential and Limitations of Mucoadhesive Excipients for Sublingual Vaccine Delivery. Pharmaceutics. 2025; 17(11):1456. https://doi.org/10.3390/pharmaceutics17111456
Chicago/Turabian StyleYousif, Mohamed Deifallah, Ilona Kubajewska, Fatme Mawas, and Sudaxshina Murdan. 2025. "Correlations Between Rheology, In Situ Mucosal Retention and In Vivo Immunogenicity Reveal the Potential and Limitations of Mucoadhesive Excipients for Sublingual Vaccine Delivery" Pharmaceutics 17, no. 11: 1456. https://doi.org/10.3390/pharmaceutics17111456
APA StyleYousif, M. D., Kubajewska, I., Mawas, F., & Murdan, S. (2025). Correlations Between Rheology, In Situ Mucosal Retention and In Vivo Immunogenicity Reveal the Potential and Limitations of Mucoadhesive Excipients for Sublingual Vaccine Delivery. Pharmaceutics, 17(11), 1456. https://doi.org/10.3390/pharmaceutics17111456






