In Vivo Iterative Adjuvant Screening Identifies an Intranasal Vaccine Formulation for Elicitation of Protective Mucosal Immune Responses Against SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
3. Results
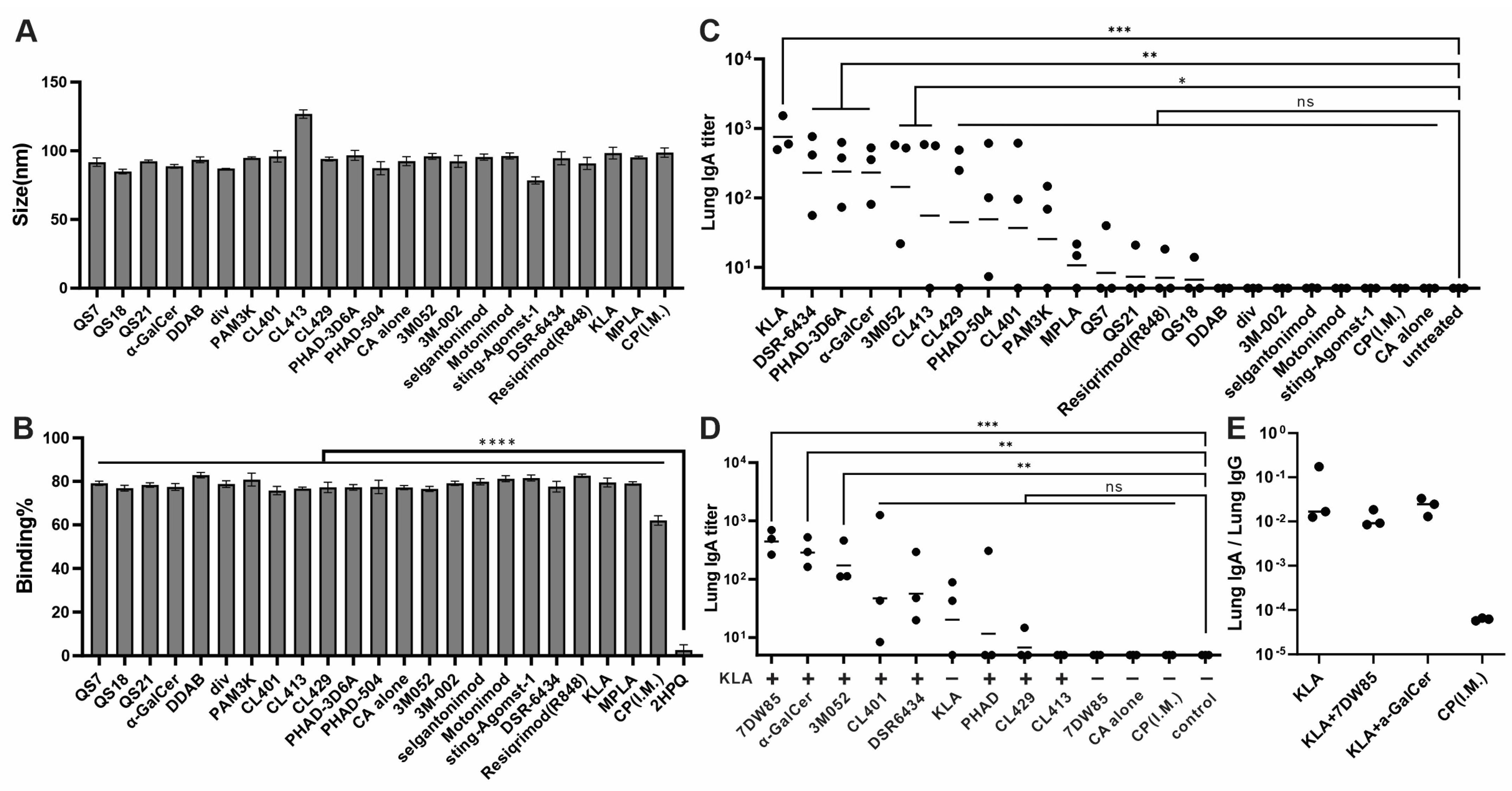
3.1. KLA/7DW8-5 and KLA/α-GalCer Formulation Vaccines Induced High Levels of Anti-RBD IgA Titer in Lung
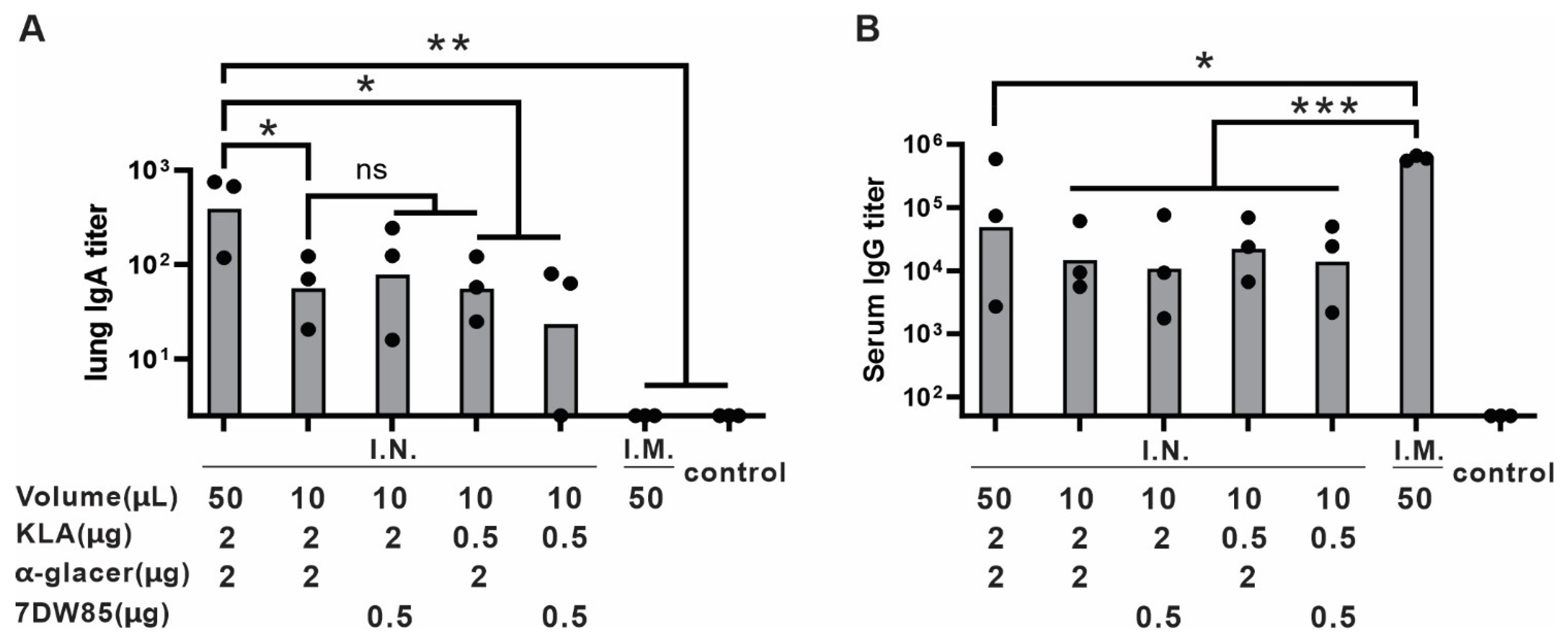
3.2. Volume Consideration for I.N. Vaccine Administration
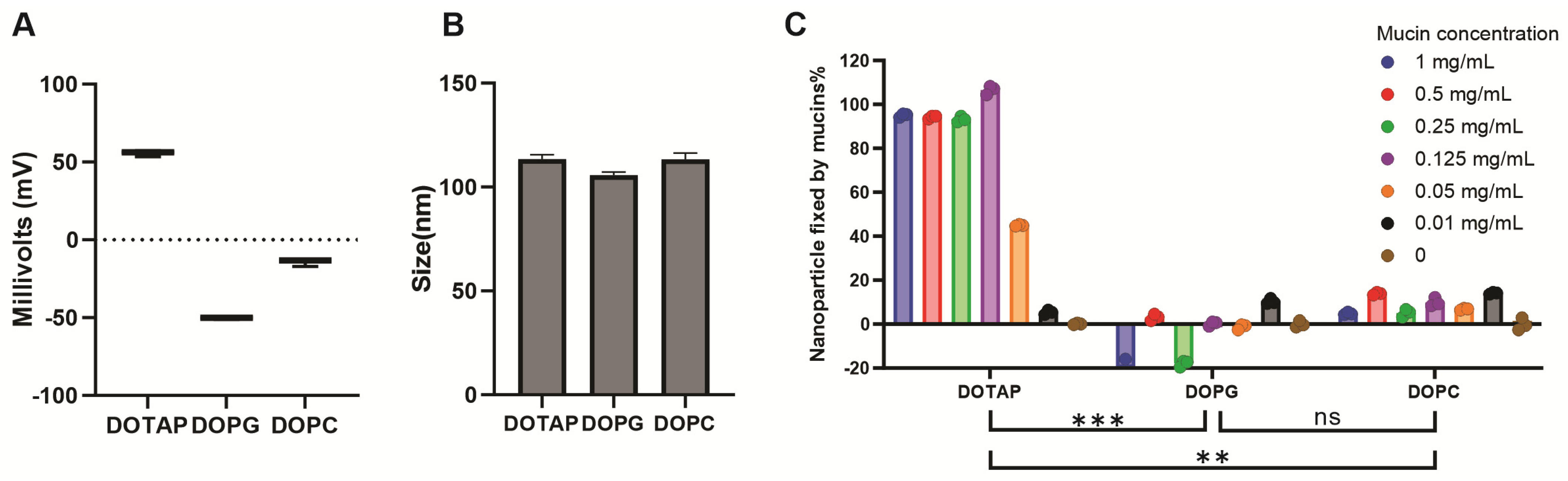
3.3. Cationic Lipid Formulations Provide Mucoadhesion and Maintain Immunogenicity
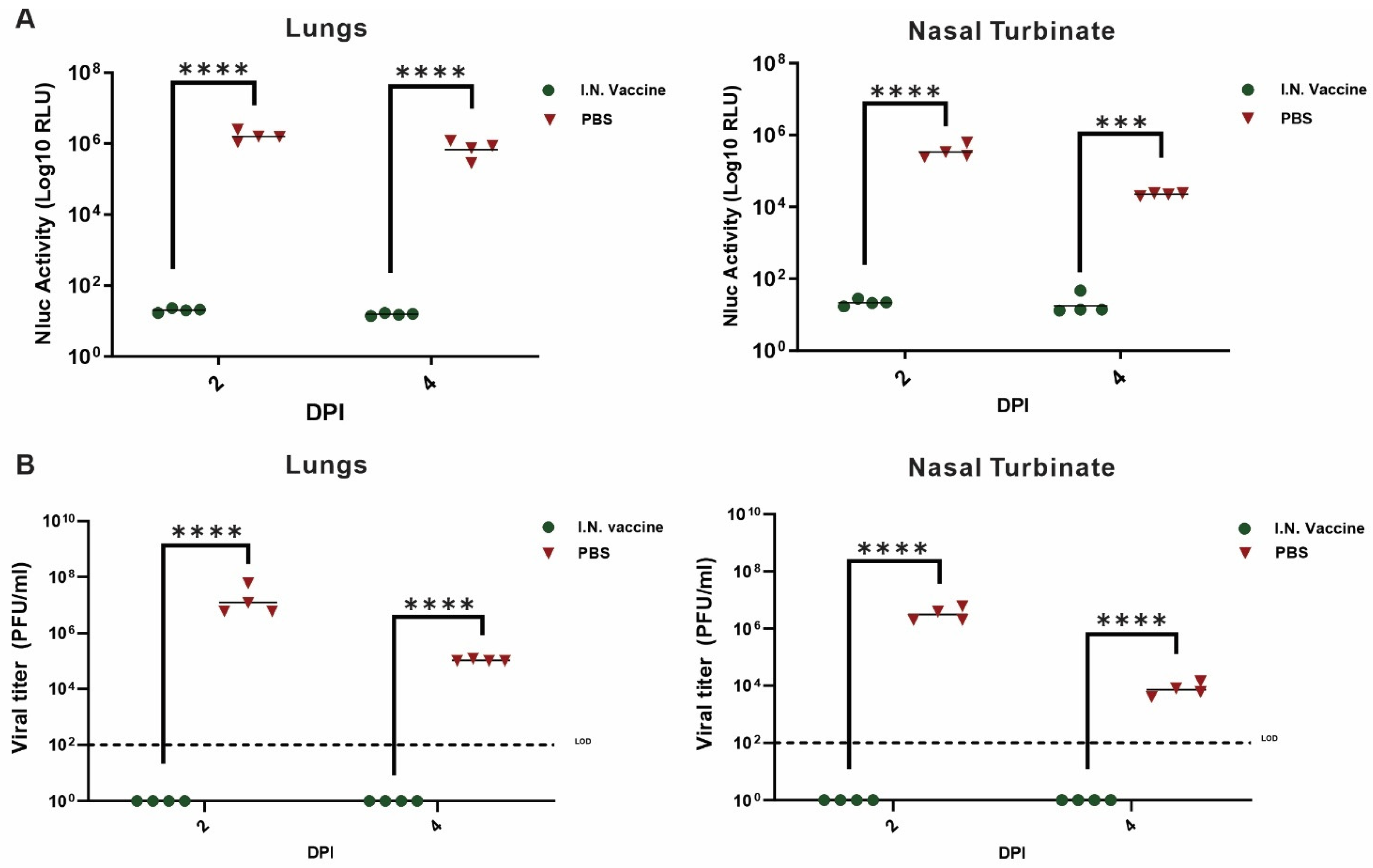
3.4. I.N. Vaccination Suppresses SARS-CoV-2 Replication and Prevents Disease Progression in K18-hACE2 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Tokunoh, N.; Tamiya, S.; Watanabe, M.; Okamoto, T.; Anindita, J.; Tanaka, H.; Ono, C.; Hirai, T.; Akita, H.; Matsuura, Y.; et al. A nasal vaccine with inactivated whole-virion elicits protective mucosal immunity against SARS-CoV-2 in mice. Front. Immunol. 2023, 14, 1224634. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv. Transl. Res. 2013, 3, 100–109. [Google Scholar] [CrossRef]
- Su, F.; Patel, G.B.; Hu, S.; Chen, W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccin. Immunother. 2016, 12, 1070–1079. [Google Scholar] [CrossRef]
- Baldeon Vaca, G.; Meyer, M.; Cadete, A.; Hsiao, C.J.; Golding, A.; Jeon, A.; Jacquinet, E.; Azcue, E.; Guan, C.M.; Sanchez-Felix, X.; et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci. Adv. 2023, 9, eadh1655. [Google Scholar] [CrossRef]
- Lam, J.H.; Shivhare, D.; Chia, T.W.; Chew, S.L.; Sinsinbar, G.; Aw, T.Y.; Wong, S.; Venkataraman, S.; Lim, F.W.I.; Vandepapeliere, P.; et al. Artificial Cell Membrane Polymersome-Based Intranasal Beta Spike Formulation as a Second Generation COVID-19 Vaccine. ACS Nano 2022, 16, 16757–16775. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 2022, 11, e12192. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID-19 vaccine (Covaxin®). npj Vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Chen, Q.; Lin, H.; He, T.; Zhang, R.; Ren, S.; Liu, L.; Wang, J.; Huang, H.; Wang, M.; et al. Short-term effectiveness of single-dose intranasal spray COVID-19 vaccine against symptomatic SARS-CoV-2 Omicron infection in healthcare workers: A prospective cohort study. eClinicalMedicine 2024, 67, 102374. [Google Scholar] [CrossRef]
- Chu, K.; Quan, J.; Liu, X.; Chen, Q.; Zang, X.; Jiang, H.; Liu, D.; Chu, X.; Zhuang, C.; Han, J.; et al. A randomized phase I trial of intranasal SARS-CoV-2 vaccine dNS1-RBD in children aged 3–17 years. npj Vaccines 2025, 10, 50. [Google Scholar] [CrossRef]
- Worzner, K.; Schmidt, S.T.; Zimmermann, J.; Tami, A.; Polacek, C.; Fernandez-Antunez, C.; Hartmann, K.T.; Jensen, R.F.; Hansen, J.S.; Illigen, K.; et al. Intranasal recombinant protein subunit vaccine targeting TLR3 induces respiratory tract IgA and CD8 T cell responses and protects against respiratory virus infection. eBioMedicine 2025, 113, 105615. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Lee, K.S.; Russ, B.P.; Horspool, A.M.; Kang, J.; Winters, M.T.; Allison Wolf, M.; Rader, N.A.; Miller, O.A.; Shiflett, M.; et al. Intranasal administration of BReC-CoV-2 COVID-19 vaccine protects K18-hACE2 mice against lethal SARS-CoV-2 challenge. NPJ Vaccines 2022, 7, 36. [Google Scholar] [CrossRef]
- Frank, N.; Dickinson, D.; Lovett, G.; Liu, Y.; Yu, H.; Cai, J.; Yao, B.; Jiang, X.; Hsu, S. Evaluation of Novel Nasal Mucoadhesive Nanoformulations Containing Lipid-Soluble EGCG for Long COVID Treatment. Pharmaceutics 2024, 16, 791. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, Y.; Lovell, J.F. Vaccine approaches for antigen capture by liposomes. Expert Rev. Vaccines 2023, 22, 1022–1040. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandao, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Mabrouk, M.T.; Zhou, L.; Baba, M.; Tachibana, M.; Torii, M.; Takashima, E.; Locke, E.; Plieskatt, J.; King, C.R.; et al. Vaccine co-display of CSP and Pfs230 on liposomes targeting two Plasmodium falciparum differentiation stages. Commun. Biol. 2022, 5, 773. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiem, K.; Martinez-Sobrido, L.; Lovell, J.F. Intranasal Immunization with Liposome-Displayed Receptor-Binding Domain Induces Mucosal Immunity and Protection against SARS-CoV-2. Pathogens 2022, 11, 1035. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhou, S.; He, X.; Chiem, K.; Mabrouk, M.T.; Nissly, R.H.; Bird, I.M.; Strauss, M.; Sambhara, S.; Ortega, J.; et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv. Mater. 2020, 32, 2005637. [Google Scholar] [CrossRef]
- Jiao, Y.; Huang, W.C.; Chiem, K.; Song, Y.; Sun, J.; Chothe, S.K.; Zhou, S.; Luo, Y.; Mabrouk, M.T.; Ortega, J.; et al. SARS-CoV-2 Protein Nanoparticle Vaccines Formed In Situ from Lyophilized Lipids. Small 2024, 20, e2304534. [Google Scholar] [CrossRef]
- Lovell, J.F.; Miura, K.; Baik, Y.O.; Lee, C.; Choi, Y.; Her, H.; Lee, J.Y.; Ylade, M.; Lee-Llacer, R.; De Asis, N.; et al. Interim safety and immunogenicity analysis of the EuCorVac-19 COVID-19 vaccine in a Phase 3 randomized, observer-blind, immunobridging trial in the Philippines. J. Med. Virol. 2024, 96, e29927. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Miura, K.; Baik, Y.O.; Lee, C.; Choi, Y.; Lee, J.Y.; Long, C.A.; Ylade, M.; Lee-Llacer, R.; De Asis, N.; et al. Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos. Vaccines 2025, 13, 776. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, S.; Song, Y.; Huang, W.C.; Wilding, G.E.; Jablonski, J.; Quinn, B.; Lovell, J.F. Iterative selection of lipid nanoparticle vaccine adjuvants for rapid elicitation of tumoricidal CD8+ T cells. Bioact. Mater. 2025, 48, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J.; Yan, A. Kdo2-lipid A: Structural diversity and impact on immunopharmacology. Biol. Rev. Camb. Philos. Soc. 2015, 90, 408–427. [Google Scholar] [CrossRef]
- Sims, K.; Haynes, C.A.; Kelly, S.; Allegood, J.C.; Wang, E.; Momin, A.; Leipelt, M.; Reichart, D.; Glass, C.K.; Sullards, M.C.; et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 2010, 285, 38568–38579. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.A.; Kronenberg, M. Activation or anergy: NKT cells are stunned by alpha-galactosylceramide. J. Clin. Investig. 2005, 115, 2328–2329. [Google Scholar] [CrossRef]
- Hosono, Y.; Tomiyasu, N.; Kasai, H.; Ishikawa, E.; Takahashi, M.; Imamura, A.; Ishida, H.; Compostella, F.; Kida, H.; Kumanogoh, A.; et al. Identification of α-galactosylceramide as an endogenous mammalian antigen for iNKT cells. J. Exp. Med. 2025, 222, e20240728. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Kronenberg, M.; Steinman, R.M. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat. Immunol. 2002, 3, 867–874. [Google Scholar] [CrossRef]
- Masimov, R.; Wasan, E.K. Chitosan non-particulate vaccine delivery systems. J. Pharm. Pharm. Sci. 2024, 27, 12921. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel-A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 2011, 3, 2307–2314. [Google Scholar] [CrossRef]
- Park, J.-G.; Oladunni, F.S.; Chiem, K.; Ye, C.; Pipenbrink, M.; Moran, T.; Walter, M.R.; Kobie, J.; Martinez-Sobrido, L. Rapid in vitro assays for screening neutralizing antibodies and antivirals against SARS-CoV-2. J. Virol. Methods 2021, 287, 113995. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Sun, R.; Song, G.; Zhu, S.; Nie, Z.; Lin, L.; Yi, R.; Wu, S.; Wang, G.; He, Y.; et al. A Glycolipid α-GalCer Derivative, 7DW8-5 as a Novel Mucosal Adjuvant for the Split Inactivated Influenza Vaccine. Viruses 2022, 14, 1174. [Google Scholar] [CrossRef] [PubMed]
- Padte, N.N.; Boente-Carrera, M.; Andrews, C.D.; McManus, J.; Grasperge, B.F.; Gettie, A.; Coelho-dos-Reis, J.G.; Li, X.; Wu, D.; Bruder, J.T.; et al. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS ONE 2013, 8, e78407. [Google Scholar] [CrossRef]
- Jangra, S.; Landers, J.J.; Laghlali, G.; Rathnasinghe, R.; Warang, P.; Park, S.C.; O’Konek, J.J.; Singh, G.; Janczak, K.W.; Garcia-Sastre, A.; et al. Multicomponent intranasal adjuvant for mucosal and durable systemic SARS-CoV-2 immunity in young and aged mice. NPJ Vaccines 2023, 8, 96. [Google Scholar] [CrossRef]
- Kawai, A.; Tokunoh, N.; Kawahara, E.; Tamiya, S.; Okamura, S.; Ono, C.; Anindita, J.; Tanaka, H.; Akita, H.; Yamasaki, S.; et al. Intranasal immunization with an RBD-hemagglutinin fusion protein harnesses preexisting immunity to enhance antigen-specific responses. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef]
- Biburger, M.; Tiegs, G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J. Immunol. 2005, 175, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Artiaga, B.L.; Madden, D.; Kwon, T.; McDowell, C.; Keating, C.; Balaraman, V.; de Carvahlo Madrid, D.M.; Touchard, L.; Henningson, J.; Meade, P.; et al. Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist alpha-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs. Vaccines 2024, 12, 1068. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Mahmoud, S.H.; Ye, C.; Luo, Y.; Huang, W.-C.; Li, Q.; Zhou, S.; Song, Y.; Tsuji, M.; Martinez-Sobrido, L.; et al. In Vivo Iterative Adjuvant Screening Identifies an Intranasal Vaccine Formulation for Elicitation of Protective Mucosal Immune Responses Against SARS-CoV-2. Pharmaceutics 2025, 17, 1422. https://doi.org/10.3390/pharmaceutics17111422
Jiao Y, Mahmoud SH, Ye C, Luo Y, Huang W-C, Li Q, Zhou S, Song Y, Tsuji M, Martinez-Sobrido L, et al. In Vivo Iterative Adjuvant Screening Identifies an Intranasal Vaccine Formulation for Elicitation of Protective Mucosal Immune Responses Against SARS-CoV-2. Pharmaceutics. 2025; 17(11):1422. https://doi.org/10.3390/pharmaceutics17111422
Chicago/Turabian StyleJiao, Yang, Sara H. Mahmoud, Chengjin Ye, Yuan Luo, Wei-Chiao Huang, Qinzhe Li, Shiqi Zhou, Yiting Song, Moriya Tsuji, Luis Martinez-Sobrido, and et al. 2025. "In Vivo Iterative Adjuvant Screening Identifies an Intranasal Vaccine Formulation for Elicitation of Protective Mucosal Immune Responses Against SARS-CoV-2" Pharmaceutics 17, no. 11: 1422. https://doi.org/10.3390/pharmaceutics17111422
APA StyleJiao, Y., Mahmoud, S. H., Ye, C., Luo, Y., Huang, W.-C., Li, Q., Zhou, S., Song, Y., Tsuji, M., Martinez-Sobrido, L., & Lovell, J. F. (2025). In Vivo Iterative Adjuvant Screening Identifies an Intranasal Vaccine Formulation for Elicitation of Protective Mucosal Immune Responses Against SARS-CoV-2. Pharmaceutics, 17(11), 1422. https://doi.org/10.3390/pharmaceutics17111422







