Camptothecin in Cancer Therapy: Current Challenges and Emerging Strategies with Nanoemulsions
Abstract
1. Introduction
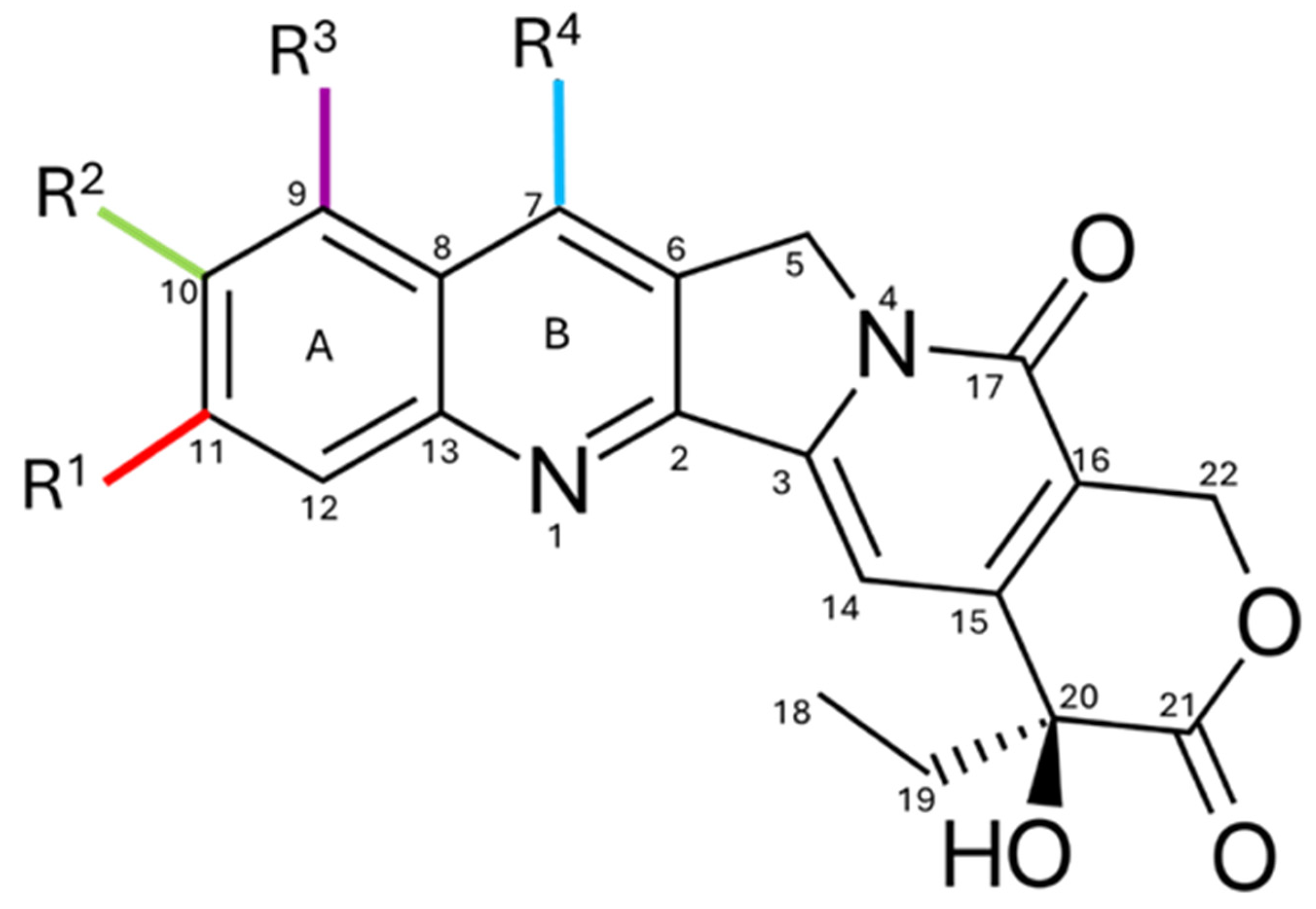
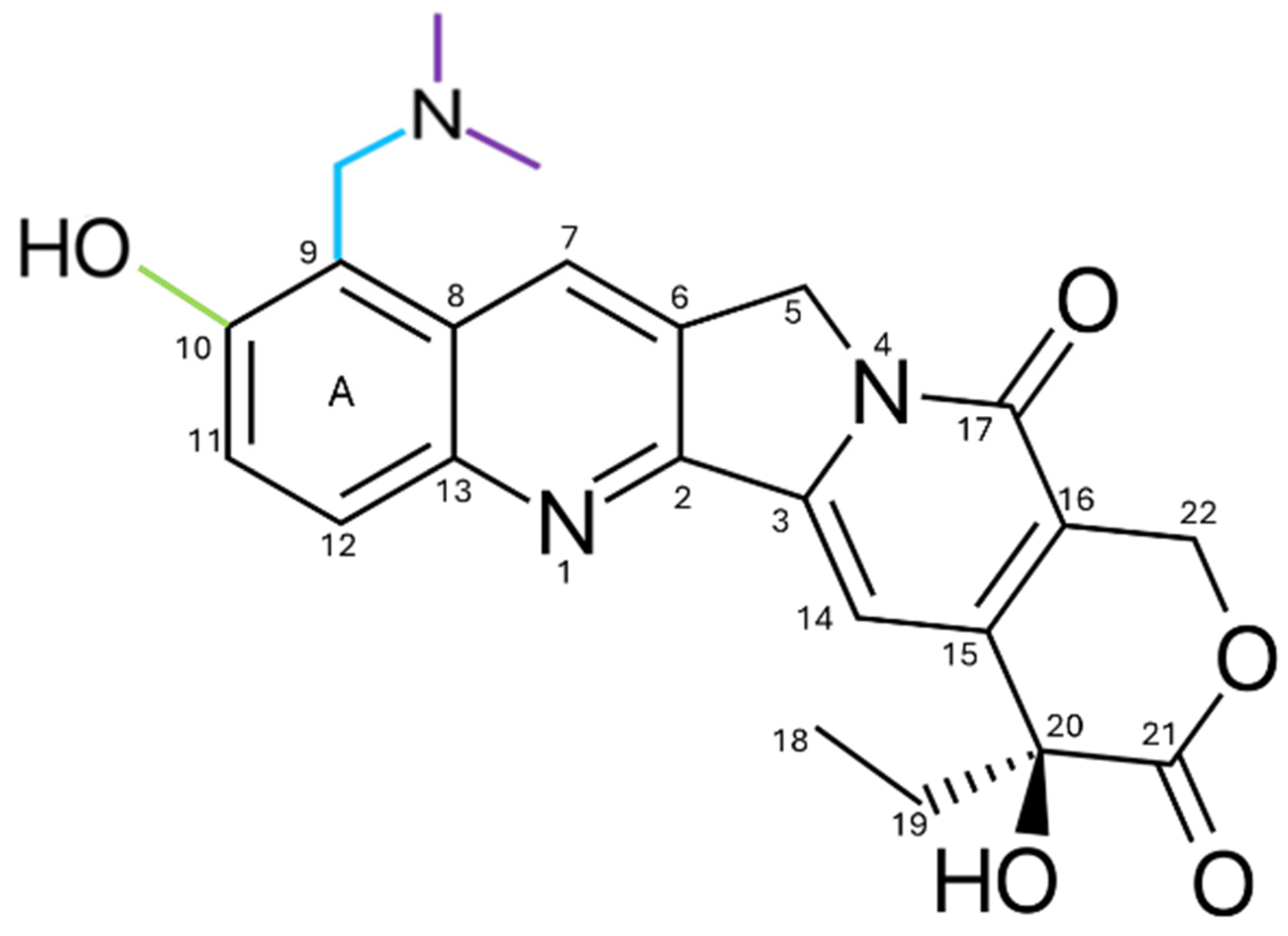
2. Chemical Structure and Physicochemical Properties
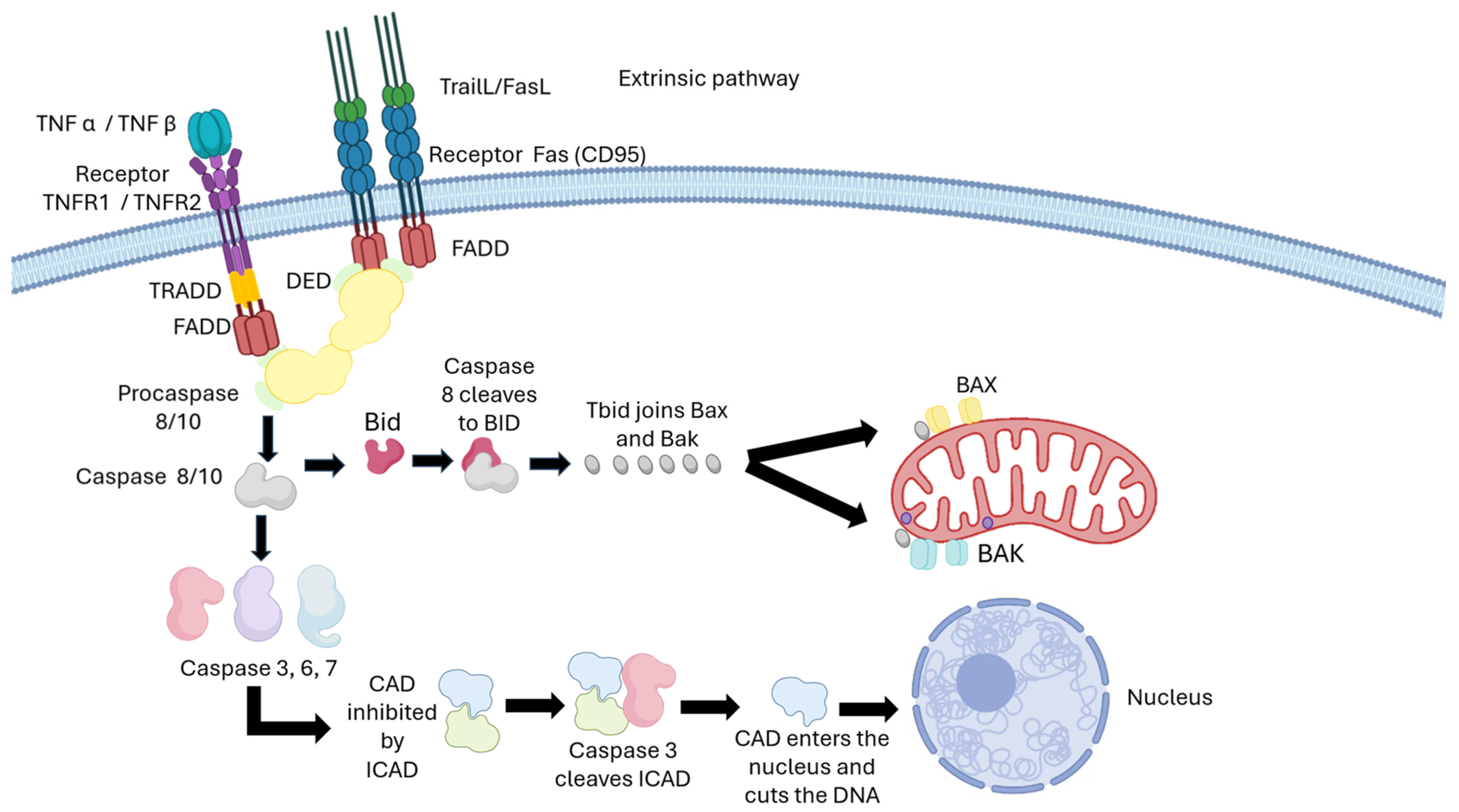
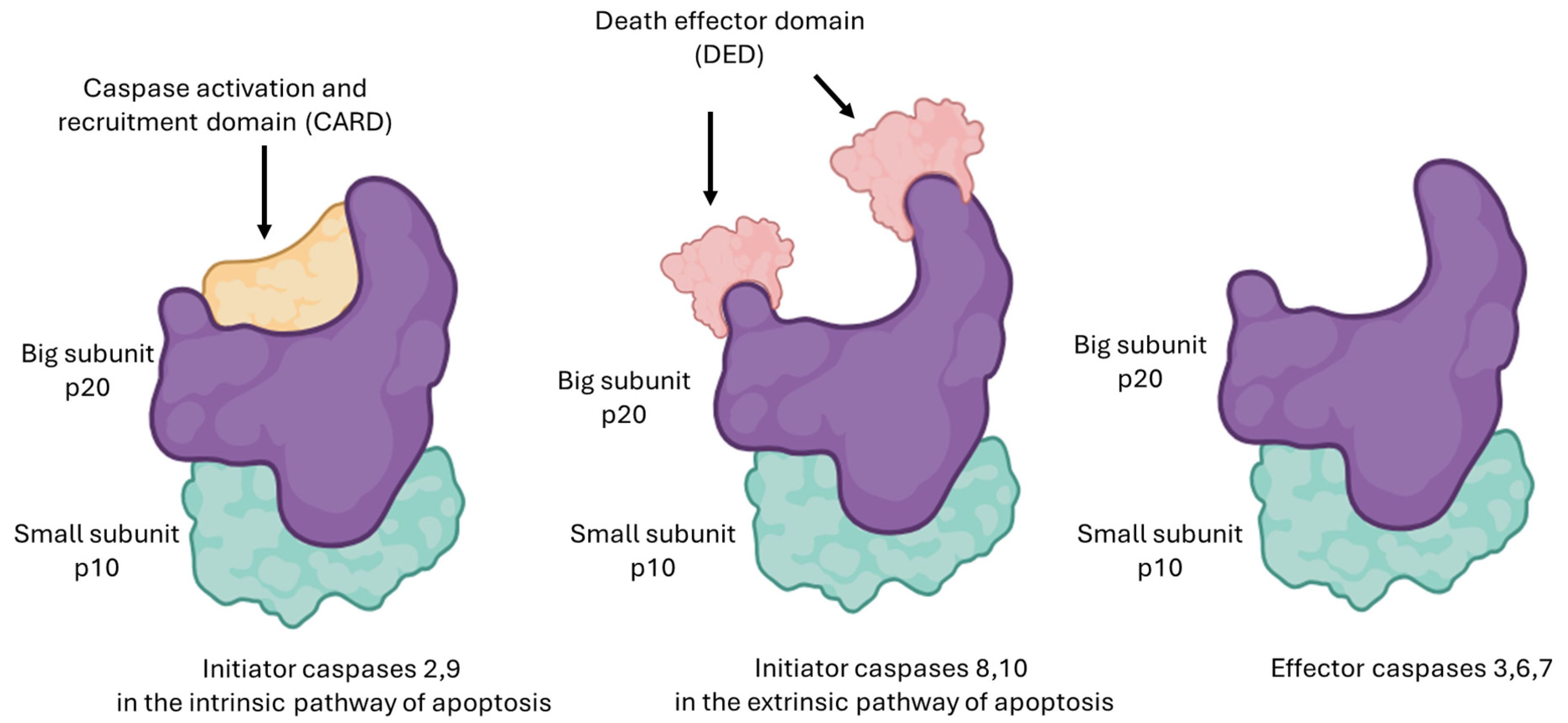
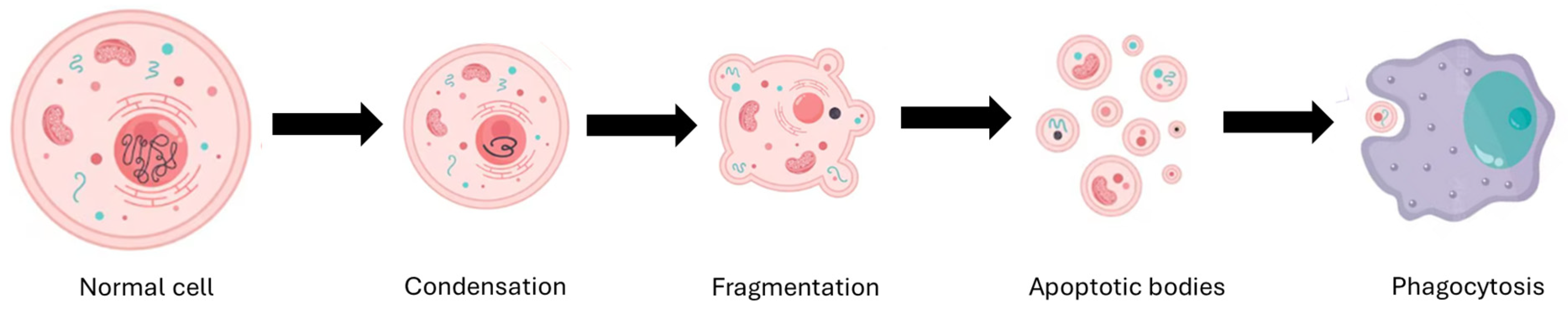
3. Mechanism of Action
4. Structural Changes in Camptothecin
4.1. Modifications on Rings A and B
4.2. Modifications on Rings C and D
4.3. Modifications on Ring E
5. FDA-Approved Camptothecin Analogs
5.1. Topotecan
5.2. Irinotecan
6. Unapproved Camptothecin Analogs
6.1. Belotecan
6.2. Exatecan
6.3. Lurtotecan
6.4. Rubitecan
7. Conjugates (ADC) Based on Camptothecin Derivatives
8. Resistance to Camptothecin
9. Management of Side Effects of Camptothecin
10. Research and Current Advances
10.1. Nanotechnology in Medicine
10.2. Types of Nanoparticles
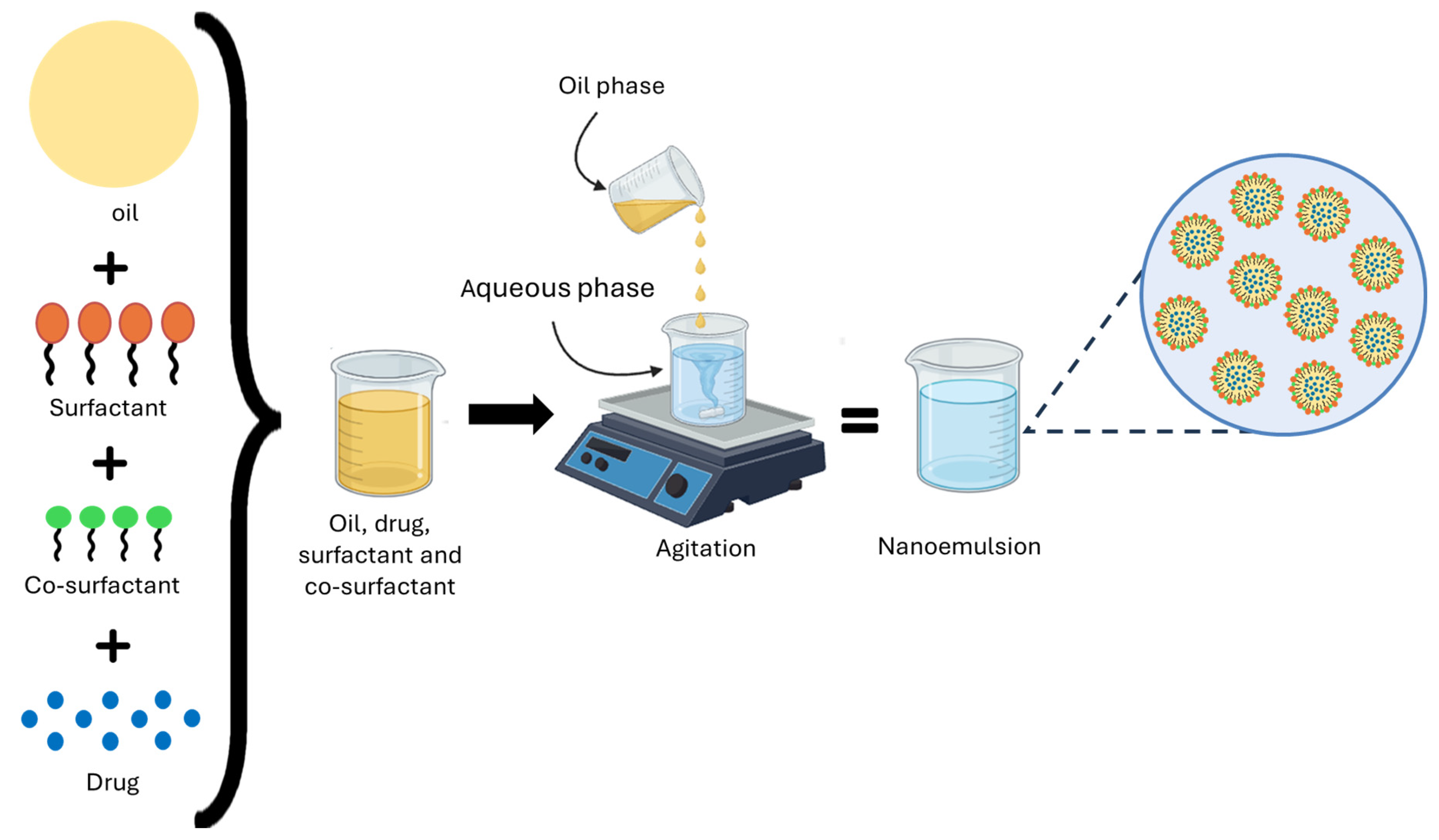
10.3. Formulation and Characterization of Nanoemulsions
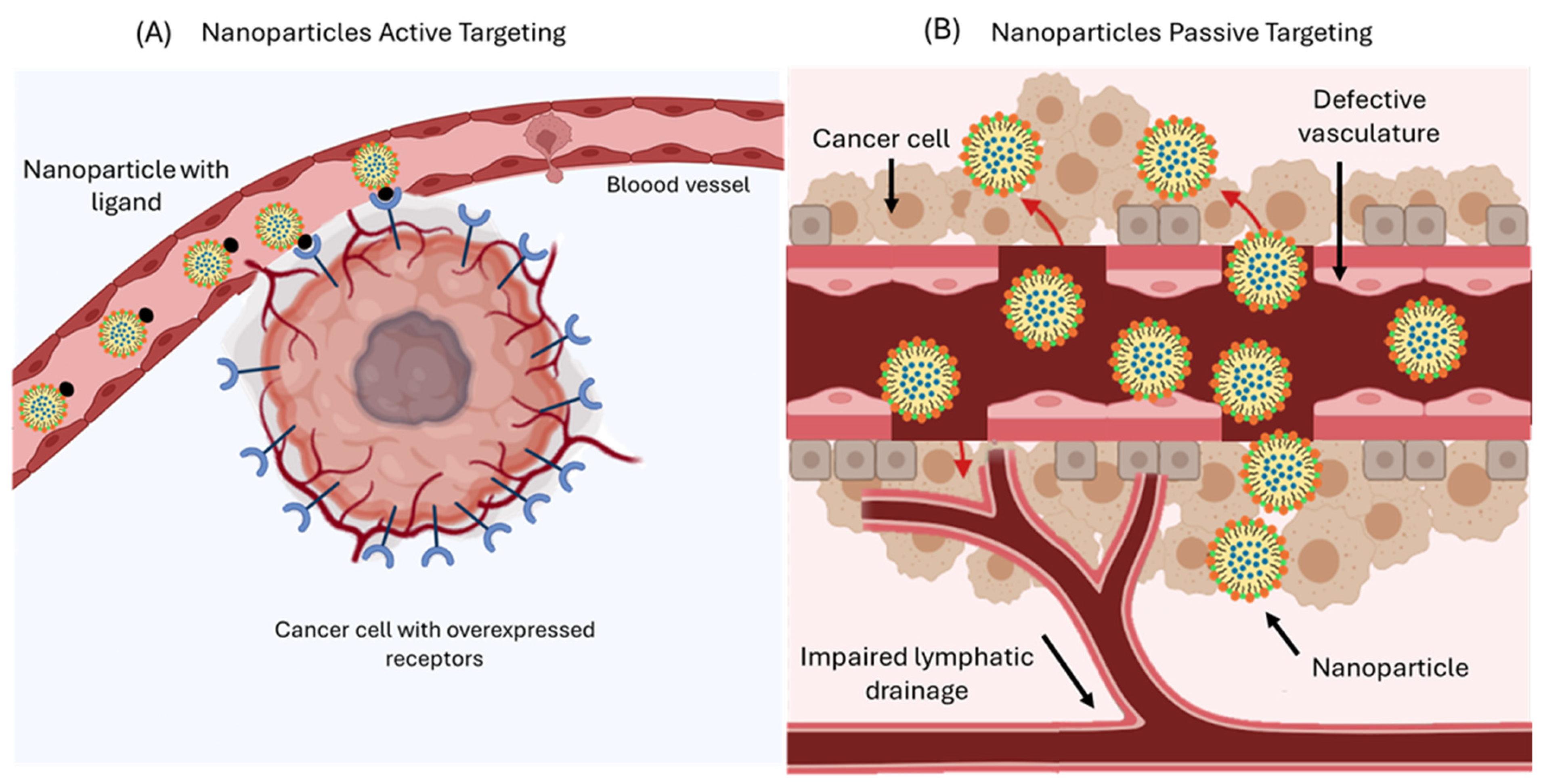
10.4. Targeted Delivery of Nanoemulsions
10.5. Nanotechnology in the Oncology Field
11. Prospects
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 10-HCPT | 10-hydroxycamptothecin |
| AAG | Alpha-1-acid glycoprotein |
| ABC | ATP-binding cassette |
| ADC | Antibody–drug conjugate |
| AIF | Apoptosis-inducing factor |
| Akt | AKT serine/threonine kinase |
| Apaf-1 | Apoptotic protease-activating factor 1 |
| AUC | Area under the curve |
| Bak | BCL-2 homologous antagonist/killer (pro-apoptotic effector) |
| Bax | BCL-2–associated X protein (pro-apoptotic effector) |
| BCL-2 | A proto-oncogene that promotes cell survival |
| BCRP | Breast cancer resistance protein |
| BI354 (IBI354) | Anti-HER2 antibody–drug conjugate (investigational code) |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| CAD | Caspase-activated DNase |
| CARD | Caspase recruitment domain |
| CES | Carboxylesterases |
| CI | Confidence interval |
| CKD-602 | Belotecan |
| cMOAT | Canalicular multispecific organic anion transporter |
| CPT | Camptothecin |
| CPT-11 | Irinotecan |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats–Cas9 |
| CYP3A4 | Cytochrome P450 3A4 |
| DCR | Disease control rate |
| DED | Death effector domain |
| DISC | Death-inducing signaling complex |
| DLS | Dynamic light scattering |
| DNA | Deoxyribonucleic acid |
| DoR | Duration of response |
| DS-8201 | Trastuzumab deruxtecan (development code) |
| DX-8951f | Exatecan |
| DXd | Deruxtecan payload |
| ECOG-PS | Eastern Cooperative Oncology Group Performance Status |
| Endo G | Endonuclease G |
| EPR | Enhanced permeation and retention |
| ERK | Extracellular signal-regulated kinase |
| FADD | Fas-associated death domain |
| FasL | Fas ligand |
| FDA | Food and Drug Administration |
| FL118 | Novel camptothecin derivative (research code) |
| FOLFIRI | Folinic acid, fluorouracil, and irinotecan |
| FOLFIRINOX | Fluorouracil, leucovorin, irinotecan, and oxaliplatin |
| FTIR | Fourier Transform Infrared Spectroscopy |
| G-CSF | Granulocyte colony-stimulating factor |
| GG211 | Lurtotecan |
| GI | Gastrointestinal tract |
| GLOBOCAN | International Agency for Research on Cancer (Global Cancer Observatory) |
| HAP1 | Near-haploid human cell line |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF | Hypoxia-inducible factor |
| HR | Hazard ratio |
| ICAD | Inhibitor of caspase-activated DNase |
| IMMU-132 (SG) | Sacituzumab govitecan |
| ISG15 | Interferon-stimulated gene 15 |
| Log P | Partition coefficient |
| MDR1 | Multidrug resistance protein 1 |
| MEK | Mitogen-activated extracellular signal-regulated kinase |
| mOS | Median overall survival |
| mPFS | Median progression-free survival |
| MC38 | Murine colon adenocarcinoma cell line |
| MRP1 | Multidrug resistance-associated protein 1 |
| MRP2 | Multidrug resistance-associated protein 2 |
| mTOR mTTP | Mechanistic Target of Rapamycin Median Time To Progression |
| Nal-IRI | Nanoliposomal irinotecan |
| NEs | Nanoemulsions |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Noxa | Phorbol-12-Myristate-13-Acetate-Induced Protein 1 (PMAIP1) |
| NPs | Nanoparticles |
| NSCLC | Non-small cell lung cancer |
| OH | Hydroxyl |
| OPN | Osteopontin |
| ORR | Overall/Objective response rate |
| O/W | Oil-in-water (emulsion) |
| p53 | Tumor suppressor protein |
| PFS | Progression-free survival |
| P-gp | P-glycoprotein |
| PK-tox | Pharmacokinetics–toxicity correlation |
| PNPs | Polymeric nanoparticles |
| PP | Progression parameters |
| RAD51 | RAD51 recombinase |
| RAIDD | Receptor-interacting protein with death domain |
| RFS 2000 | Rubitecan |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| SCLC | Small-cell lung cancer |
| SEM | Scanning electron microscopy |
| SG | Sacituzumab govitecan |
| SII | Systemic immune-inflammatory index |
| Smac | Second mitochondrial caspase activator |
| SN-38 | 7-ethyl-10-hydroxycamptothecin |
| SNEDDS | Self-nanoemulsifying drug delivery systems |
| ST1481 | Gimatecan |
| ST-1968 | Namitecan |
| tBid | Truncated Bid |
| T-DXd | Trastuzumab deruxtecan |
| TEM | Transmission electron microscopy |
| TKI | Tyrosine kinase inhibitors |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNFR2 | Tumor necrosis factor receptor 2 |
| Topo I | Topoisomerase I |
| TPT | Topotecan |
| TRADD | Tumor necrosis factor receptor type 1-associated death domain protein |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TROP-2 | Trophoblast Cell Surface Antigen 2 |
| UGT1A1 | UDP-glucuronosyltransferase 1A1 |
| VDAC | Voltage-dependent anion channel |
| WHO | World Health Organization |
| Wnt | Wingless-related integration site pathway |
References
- López-Plaza, B.; Loria-Kohen, V.; González-Rodríguez, L.G.; Fernández-Cruz, E. Alimentación y estilo de vida en la prevención del cáncer. Nutr. Hosp. 2022, 39, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Bishayee, A. Anticancer drug discovery based on natural products: From computational approaches to clinical studies. Cancers 2025, 17, 2507. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.; Sebastián, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When natural compounds meet nanotechnology: Nature-inspired nanomedicines for cancer immunotherapy. Pharmaceutics 2022, 14, 1589. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, Z.S.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural products/bioactive compounds as a source of anticancer drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Odeniran, P.O.; Madlala, P.; Mkhwanazi, N.P.; Soliman, M.E.S. Camptothecin and Its Derivatives from Traditional Chinese Medicine in Combination with Anticancer Therapy Regimens: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3802. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; Shi, Y.; Wang, G.; Wang, T. Predicting suitable habitats of Camptotheca acuminata considering both climatic and soil variables. Forests 2020, 11, 891. [Google Scholar] [CrossRef]
- Strzelecka, K.; Piotrowska, U.; Sobczak, M.; Oledzka, E. The advancement of biodegradable polyesters as delivery systems for camptothecin and its analogues—A status report. Int. J. Mol. Sci. 2023, 24, 1053. [Google Scholar] [CrossRef]
- Bacherikov, V.A. Total synthesis, mechanism of action, and antitumor efficacy of camptothecin and some of its analogues. Anticancer. Agents Med. Chem. 2022, 22, 3438–3465. [Google Scholar] [CrossRef]
- Peng, X.; Cai, X.; Tang, J.; Ge, J.; Chen, G. Rescuing and utilizing anticancer Nothapodytes species: Integrated studies from plant resources to natural medicines. Clin. Transl. Med. 2024, 14, e70110. [Google Scholar] [CrossRef]
- Fan, X.; Lin, X.; Ruan, Q.; Wang, J.; Yang, Y.; Sheng, M.; Zhou, W.; Kai, G.; Hao, X. Research progress on the biosynthesis and metabolic engineering of the anti-cancer drug camptothecin in Camptotheca acuminata. Ind. Crops Prod. 2022, 186, 115270. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Sharma, S.; Singh, S.; Saurabh Sarma, J.; Khare, D. Enhancing camptothecin yields: Innovative approaches for sustainable production of anticancer drugs. Biotechnol. Bioprocess Eng. 2025, 30, 423–445. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Nguyen, T.D.; Leung, Y.Y.; McConnachie, M.; Sannikov, O.; Xia, Z.; Dang, T.T. Discovering and harnessing oxidative enzymes for chemoenzymatic synthesis and diversification of anticancer camptothecin analogues. Commun. Chem. 2021, 4, 60. [Google Scholar] [CrossRef]
- Banadka, A.; Narasimha, S.W.; Dandin, V.S.; Naik, P.M.; Vennapusa, A.R.; Melmaiee, K.; Vemanna, R.S.; Al-Khayri, J.M.; Thiruvengadam, M.; Nagella, P. Biotechnological approaches for the production of camptothecin. Appl. Microbiol. Biotechnol. 2024, 108, 382. [Google Scholar] [CrossRef]
- Madkour, M.M.; Sebastian, A.; Ramadan, W.S.; Menon, V.; Lozon, L.; Srikanth, G.; Tarazi, H.; El-Gamal, M.I.; Al-Tel, T.H.; El-Awady, R. Synthesis and biological evaluation of new Camptothecin-like compounds as anticancer agents. J. Mol. Struct. 2025, 1328, 141271. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Cores, Á.; Martín-Cámara, O.; Orellana, K.; Cervera-Carrascón, V.; Michalska, P.; Olives, A.I.; León, R.; Martín, M.A.; Menéndez, J.C. Enhanced stability and bioactivity of natural anticancer topoisomerase I inhibitors through cyclodextrin complexation. Pharmaceutics 2021, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Purushotham, B.; Sinniah, U.R. Camptothecin: Occurrence, chemistry and mode of action. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 311–327. [Google Scholar] [CrossRef]
- Fan, S.; Cao, Y.-X.; Li, G.-Y.; Lei, H.; Attiogbe, M.K.I.; Yao, J.-C.; Yang, X.-Y.; Liu, Y.-J.; Hei, Y.-Y.; Zhang, H.; et al. F10, a new camptothecin derivative, was identified as a new orally–bioavailable, potent antitumor agent. Eur. J. Med. Chem. 2020, 202, 112528. [Google Scholar] [CrossRef]
- Nanavati, C.; Mager, D.E. Calculated Log D Is Inversely Correlated with Select Camptothecin Clearance and Efficacy in Colon Cancer Xenografts. J. Pharm. Sci. 2016, 105, 1561–1566. [Google Scholar] [CrossRef]
- Shaikh, R.; O’Brien, D.P.; Croker, D.M.; Walker, G.M. The development of pharmaceutical oral solid dosage forms. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 41, pp. 27–65. [Google Scholar] [CrossRef]
- Botella, P.; Rivero-Buceta, E. Safe approaches for camptothecin delivery: Structural analogues and nanomedicines. J. Control. Release 2017, 247, 28–54. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Attina, G.; Triarico, S.; Romano, A.; Maurizi, P.; Ruggiero, A. The DNA-topoisomerase inhibitors in cancer therapy. Biomed. Pharmacol. J. 2022, 15, 553–562. [Google Scholar] [CrossRef]
- Khuroo, T.; Khuroo, A.; Hussain, A.; Mirza, A.M.; Panda, A.K.; Wani, J. QbD-based and Box-Behnken design assisted oral delivery of stable lactone (active) form of topotecan as PLGA nanoformulation: Cytotoxicity, pharmacokinetic, in vitro, and ex vivo gut permeation studies. J. Drug Deliv. Sci. Technol. 2022, 77, 103850. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Dai, W.; Gemeinhart, R.A.; Zhang, Q.; Li, T. Pharmacokinetics and treatment efficacy of camptothecin nanocrystals on lung metastasis. Mol. Pharm. 2014, 11, 226–233. [Google Scholar] [CrossRef]
- Almeida, A.; Fernandes, E.; Sarmento, B.; Lúcio, M. A biophysical insight of camptothecin biodistribution: Towards a molecular understanding of its pharmacokinetic issues. Pharmaceutics 2021, 13, 869. [Google Scholar] [CrossRef]
- Yu, L.; Hua, Z.; Luo, X.; Zhao, T.; Liu, Y. Systematic interaction of plasma albumin with the efficacy of chemotherapeutic drugs. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188655. [Google Scholar] [CrossRef]
- Ahmed, H.; Bergmann, F.; Zeitlinger, M. Protein binding in translational antimicrobial development—Focus on interspecies differences. Antibiotics 2022, 11, 923. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Alshehri, M.M.; Alshehri, A.M.; Alshlali, O.M.; Mahzari, A.M.; Almalki, H.H.; Kulaybi, O.Y.; Alghazwni, M.K.; Kamal, M.; Imran, M. Camptothecin loaded nano-delivery systems in the cancer therapeutic domains: A critical examination of the literature. J. Drug Deliv. Sci. Technol. 2023, 79, 104034. [Google Scholar] [CrossRef]
- Adak, D.; Ray, P.; Setua, S. Unlocking therapeutic precision: Camptotheca acuminata, a traditional Chinese herb tailored for phytonano-cancer theranostics. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100447. [Google Scholar] [CrossRef]
- Behera, A.; Padhi, S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: A review. Environ. Chem. Lett. 2020, 18, 1557–1567. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Wang, N.; Xiao, W.; Shi, J. Unleashing the Potential of Camptothecin: Exploring Innovative Strategies for Structural Modification and Therapeutic Advancements. J. Med. Chem. 2024, 67, 3244–3273. [Google Scholar] [CrossRef]
- Fonseca, L.C.; De Sousa, M.; Maia, D.L.S.; Visani de Luna, L.; Alves, O.L. Understanding the driving forces of camptothecin interactions on the surface of nanocomposites based on graphene oxide decorated with silica nanoparticles. Nanoscale Adv. 2020, 2, 1290–3000. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Pandhi, S.; Mishra, S.; Barua, S.; Kurian, A.; Mahato, D.K.; Rasane, P.; Büsselberg, D.; Kumar, P.; Calina, D.; et al. Camptothecin and its derivatives: Advancements, mechanisms and clinical potential in cancer therapy. Med. Oncol. 2024, 41, 1–18. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Penumallu, N.R.; Shafi, S.; Kamal, A. Prospects of topoisomerase inhibitors as promising anti-cancer agents. Pharmaceuticals 2023, 16, 1456. [Google Scholar] [CrossRef] [PubMed]
- Kostjukov, V.V. Theoretical analysis of lactone and carboxylate forms of camptothecin in aqueous solution: Electronic states, absorption spectra, and hydration. J. Mol. Liq. 2021, 344, 117804. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Hassan, W.H.B.; Sweilam, S.H.; Alqarni, M.H.S.; El Sayed, Z.I.; Abdel-Aal, M.M.; Abdelsalam, E.; Abdelaziz, S. Production, bioprocessing, and antiproliferative activity of camptothecin from Penicillium chrysogenum, a marine sponge endosymbiont, as a metabolically stable camptothecin producer. Molecules 2022, 27, 3033. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Kundu, B.; Sarkar, D.; Goon, S.; Mondal, M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022, 236, 114304. [Google Scholar] [CrossRef]
- Shen, G.; Li, S.; Zhu, Y.; Xu, Z.; Liu, X.; Lv, C.; Xing, Z.; Cui, L.; Li, W. Recent progress in topoisomerase inhibitors as anticancer agents: Research and design strategies for Topo I and II inhibitors via structural optimization. Bioorg. Chem. 2025, 165, 109040. [Google Scholar] [CrossRef]
- Tesauro, C.; Simonsen, A.K.; Bech Andersen, M.; Wandsoe Petersen, K.; Laust Kristoffersen, E.; Algreen, L. Topoisomerase I activity and sensitivity to camptothecin in breast cancer-derived cells: A comparative study. BMC Cancer 2019, 19, 1158. [Google Scholar] [CrossRef]
- Patil, M.A.; Sarkate, A.P.; Nirmal, N.P.; Sakhale, B.K. Alkaloids as potential anticancer agents. Recent Front. Phytochem. 2023, 203–224. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin Hassan, M.I.; Habib, S.; Islam, S. Apoptosis: Una descripción general completa de las vías de señalización, los cambios morfológicos y la importancia fisiológica y las implicaciones terapéuticas. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Dorstyn, L.; Kumar, S. The p53-caspase-2 axis in the cell cycle and DNA damage response. Exp. Mol. Med. 2021, 53, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Jentsch, M.; Snyder, P.; Sheng, C.; Cristiano, E.; Loewer, A. P53 dynamics in single cells are temperature-sensitive. Sci. Rep. 2020, 10, 1481. [Google Scholar] [CrossRef]
- Opferman, J.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qu, L.; Dai, S.; Li, Y.; Wang, H.; Feng, Y.; Chen, X.; Jiang, L.; Guo, M.; Li, J.; et al. Structural insight into the molecular mechanism of p53-mediated mitochondrial apoptosis. Nat. Commun. 2021, 12, 2280. [Google Scholar] [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-only proteins Noxa and Puma are key regulators of induced apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef]
- Hohorst, L.; Ros, U.; Garcia-Saez, A.J. Mitochondrial dynamics and pore formation in regulated cell death pathways. Trends Biochem. Sci. 2025, 1–14. [Google Scholar] [CrossRef]
- Park, H.H. Caspase recruitment domains for protein interactions in cellular signaling (Review). Int. J. Mol. Med. 2019, 43, 1119–1127. [Google Scholar] [CrossRef]
- Vigneswara, V.; Ahmed, Z. The role of caspase-2 in regulating cell fate. Cells 2020, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Dho, S.H.; Cho, M.; Woo, W.; Kim, D.; Park, S.; Lee, J.; Lee, H.; Choi, K.; Han, J. Caspases as master regulators of programmed cell death: Apoptosis, pyroptosis and beyond. Exp. Mol. Med. 2025, 57, 1121–1132. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E. Understanding apoptosis and apoptotic pathways: Targeted cancer therapies. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The nuclear envelope: Target and mediator of the apoptotic process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef]
- Gheyas, R.; Menko, A.S. The involvement of caspases in the process of nuclear removal during lens fiber cell differentiation. Cell Death Discov. 2023, 9, 386. [Google Scholar] [CrossRef]
- Choi, Y.N.; Seo, W.T.; Lee, T.Y.; Jeon, H.D.; Yoo, J.S. Nuclear endonuclease G regulates cell proliferation in ovarian cancer. FEBS Open Bio. 2023, 13, 655–669. [Google Scholar] [CrossRef]
- Schoeniger, A.; Wolf, P.; Edlich, F. How do hexokinases inhibit receptor-mediated apoptosis? Biology 2022, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Melloul, O.; Zabit, S.; Lichtenstein, M.; Duran, D.; Grunewald, M.; Lorberboum-Galski, H. Targeted and caspase-independent apoptosis induction with novel chimeric proteins for the treatment of solid cancers. Cancers 2025, 17, 1179. [Google Scholar] [CrossRef]
- Risso, V.; Lafont, E.; Le Gallo, M. Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases. Cell Death Dis. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for cancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Marikar, F.; Zi-Chun, H. Adapter protein—FADD bridges the apoptosis. Int. J. Med. Biochem. 2023, 6, 124–132. [Google Scholar] [CrossRef]
- Guerrache, A.; Micheau, O. TNF-related apoptosis-inducing ligand: Non-apoptotic signalling. Cells 2024, 13, 521. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Park, Y.H.; Han, C.W.; Jeong, M.S.; Jang, S.B. DED interaction of FADD and caspase-8 in the induction of apoptotic cell death. J. Microbiol. Biotechnol. 2022, 32, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Coria-Paredes, D.M.; Wilkins-Rodríguez, A.A.; Gutiérrez-Kobeh, L. Apoptosis: A key process that Trypanosoma cruzi modulates as a strategy to perpetuate infection. J. Parasitol. Res. 2025, 2025, 2093615. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.; Ansari, A.; Kumar, A.; Ranjan, K. Molecular perspective of proteases in modeling of cell death signaling. Preprints 2025, 2025010084. [Google Scholar] [CrossRef]
- Fu, Q.; Fu, T.-M.; Cruz, A.C.; Sengupta, P.; Thomas, S.K.; Wang, S.; Siegel, R.M.; Wu, H.; Chou, J.J. Structural basis and functional role of intramembrane trimerization of the Fas/CD95 death receptor. Mol. Cell 2016, 61, 602–613. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Liao, Y.; Zhou, M.; Xu, W.; Zou, Z. Caspase-8 in inflammatory diseases: A potential therapeutic target. Cell Mol. Biol. Lett. 2024, 29, 130. [Google Scholar] [CrossRef]
- Nadendla, E.K.; Tweedell, R.E.; Kasof, G.; Kanneganti, T.-D. Caspases: Structural and molecular mechanisms and functions in cell death, innate immunity, and disease. Cell Discov. 2025, 11, 42. [Google Scholar] [CrossRef]
- Tummers, B.; Mari, L.; Guy, C.S.; Heckmann, B.L.; Rodriguez, D.A.; Ruhl, S. Caspase-8-dependent inflammatory responses are controlled by its adaptor, FADD, and necroptosis. Immunity 2020, 52, 994–1006. [Google Scholar] [CrossRef]
- Han, Y.H.; Wang, Y.; Lee, S.J.; Jin, M.H.; Sun, H.N.; Kwon, T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal. 2023, 21, 227. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, W.; Lin, Z. Functional roles in cell signaling of adaptor protein TRADD from a structural perspective. Comput. Struct. Biotechnol. J. 2020, 18, 2867–2876. [Google Scholar] [CrossRef]
- Wyżewski, Z.; Gregorczyk-Zboroch, K.P.; Mielcarska, M.B.; Świtlik, W.; Niedzielska, A. Bid protein: A participant in the apoptotic network with roles in viral infections. Int. J. Mol. Sci. 2025, 26, 2385. [Google Scholar] [CrossRef]
- Makinwa, Y.; Luo, Y.; Musich, P.R.; Zou, Y. Canonical and non-canonical functions of BH3-only protein in apoptosis, oncogenesis, cancer therapeutics, and aging. Cancers 2024, 16, 2199. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Hohorst, L.; John, M.; Albert, M.; E King, L.; Beckmann, L.; Szabo, T.; Hertlein, V.; Luo, X.; Villunger, A.; et al. The BCL-2 family protein tBID acts as a BAX-like apoptosis effector. EMBO J. 2022, 41, e108690. [Google Scholar] [CrossRef]
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A long way to go: Caspase inhibitors in clinical use. Cell Death Dis. 2021, 12, 949. [Google Scholar] [CrossRef]
- Mandal, R.; Compte Barrón, J.; Kostova, I.; Becker, S.; Strebhardt, K. Caspase-8: The double-edged sword. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188357. [Google Scholar] [CrossRef]
- Hussar, P. Apoptosis regulators Bcl-2 and caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2021, 109, 121–141. [Google Scholar] [CrossRef]
- Moeed, A.; Thilmany, N.; Beck, F.; Puthussery, B.K.; Ortmann, N.; Haimovici, A.; Badr, M.T.; Haghighi, E.B.; Boerries, M.; Öllinger, R.; et al. The caspase-activated DNase drives inflammation and contributes to defense against viral infection. Cell Death Differ. 2024, 31, 924–937. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Cai, Z.; Cheng, Z.; Zhang, L.; Zhang, Y.; Shi, J.; Jin, J.; Mi, Z.; Yuan, Z.; Wu, Z. CAD hijacks STING to impair antitumor immunity and radiotherapy efficacy of colorectal cancer. Cell Death Dis. 2025, 16, 641. [Google Scholar] [CrossRef]
- Ahmad Bhat, I.; Maqsood Bhat, A.; Tasduq Abdullah, S. Apoptosis—Mechanisms, Regulation in Pathology, and Therapeutic potential. In Biochemistry; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Han, C.Z.; Juncadella, I.J.; Kinchen, J.M.; Buckley, M.W.; Klibanov, A.L.; Dryden, K.; Onengut-Gumuscu, S.; Erdbrügger, U.; Turner, S.D.; Shim, Y.M.; et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 2016, 539, 570–574. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Wang, Y.; Jiang, M.; Yao, L. The recent developments of camptothecin and its derivatives as potential anti-tumor agents. Eur. J. Med. Chem. 2023, 260, 115710. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Li, W.-Q.; Morris-Natschke, S.L.; Qian, K.; Yang, L.; Zhu, G.-X.; Wu, X.-B.; Chen, A.-L.; Zhang, S.-Y.; Nan, X.; et al. Perspectives on biologically active camptothecin derivatives. Med. Res. Rev. 2015, 35, 753–789. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, Y.; Wang, N.; Yu, P.; Teng, Y. Drug combinations of camptothecin derivatives promote the antitumor properties. Eur. J. Med. Chem. 2024, 279, 116872. [Google Scholar] [CrossRef]
- Dai, Y.; Qian, M.; Li, Y. Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions. Molecules 2023, 28, 4931. [Google Scholar] [CrossRef]
- Li, G.; Cai, C.; Qi, Y.; Tang, X. Hydroxyethyl starch–10-hydroxycamptothecin conjugate: Synthesis, pharmacokinetics, cytotoxicity and pharmacodynamic investigation. Drug Deliv. 2014, 23, 277–284. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. DNA intercalation and topoisomerase inhibition. In Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 325–387. [Google Scholar] [CrossRef]
- Pullaiah, T.; Raveendran, V. Camptothecin: Chemistry, biosynthesis, analogs, and chemical synthesis. In Camptothecin and Camptothecin Producing Plants: Botany, Chemistry, Anticancer Activity and Biotechnology; Academic Press: Cambridge, MA, USA, 2020; pp. 47–103. [Google Scholar] [CrossRef]
- Virupaksha, B.; Alpana, G. CoMFA QSAR models of camptothecin analogues based on the distinctive SAR features of combined ABC, CD and E ring substitutions. Comput. Biol. Med. 2012, 42, 890–897. [Google Scholar] [CrossRef]
- Liu, J.; Geng, G.; Liang, G.; Wang, L.; Luo, K.; Yuan, J.; Zhao, S. A novel topoisomerase I inhibitor DIA-001 induces DNA damage mediated cell cycle arrest and apoptosis in cancer cell. Ann. Transl. Med. 2020, 8, 89. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.-Z.; Yang, C.-J.; Yan, J.-X.; Wang, Z.-P.; Bai, Y.-P.; Peng, L.-Z.; Luo, H.-B.; Zhang, Z.-J.; Li, L.; et al. Improved anti-tumor activity of fluorinated camptothecin derivatives 9-fluorocamptothecin and 7-ethyl-9-fluorocamptothecin on hepatocellular carcinoma by targeting topoisomerase I. Bioorg. Chem. 2023, 139, 106652. [Google Scholar] [CrossRef]
- Zhu, L.; Zhuang, C.; Lei, N.; Guo, Z.; Sheng, C.; Dong, G.; Wang, S.; Zhang, Y.; Yao, J.; Miao, Z.; et al. Synthesis and preliminary bioevaluation of novel E-ring modified acetal analog of camptothecin as cytotoxic agents. Eur. J. Med. Chem. 2012, 56, 1–9. [Google Scholar] [CrossRef]
- Zi, C.-T.; Yang, L.; Xu, F.-Q.; Dong, F.-W.; Ma, R.-J.; Li, Y.; Zhou, J.; Ding, Z.-T.; Jiang, Z.-H.; Hu, J.-M. Synthesis and antitumor activity of biotinylated camptothecin derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2019, 29, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wu, G.; Zhang, Y.; Zhang, X.; Yin, R.; Qi, X.; Li, J.; Jiang, T. Design, synthesis, and in vitro/in vivo anti-cancer activities of novel (20S)-10,11-methylenedioxy-camptothecin heterocyclic derivatives. Int. J. Mol. Sci. 2020, 21, 8495. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Gerwick, W.H. Plant sources of drugs and chemicals. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 129–139. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Engineered liposomes bearing camptothecin analogue for tumour targeting: In vitro and ex-vivo studies. J. Liposome Res. 2020, 31, 326–341. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Morris-Natschke, S.L.; Liu, Y.; Guo, X.; Xu, X.; Goto, M.; Li, J.; Yang, G.; Lee, K. Biologically active quinoline and quinazoline alkaloids, part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.R.; Douhal, A. Robust inclusion complex of topotecan comprised within a rhodamine-labeled β-cyclodextrin: Competing proton and energy transfer processes. Pharmaceutics 2023, 15, 1620. [Google Scholar] [CrossRef]
- Kyle, A.H.; Baker, J.H.E.; Gandolfo, M.J.; Reinsberg, S.A.; Minchinton, A.I. Tissue penetration and activity of camptothecins in solid tumor xenografts. Mol. Cancer Ther. 2014, 13, 2727–2737. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Pharmacokinetic comparison of three different administration routes for topotecan hydrochloride in rats. Pharmaceuticals 2020, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.; Sharma, A.; Kaur, A.; Madan, J.; Pandey, R.S.; Jain, U.K.; Chandra, R. Natural plant-derived anticancer drugs nanotherapeutics: A review on preclinical to clinical success. In Nanostructures for Cancer Therapy; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 775–809. [Google Scholar] [CrossRef]
- Samare-Najaf, M.; Samareh, A.; Jamali, N.; Abbasi, A.; Clark, C.C.T.; Khorchani, M.J.; Zal, F. Adverse effects and safety of etirinotecan pegol, a novel topoisomerase inhibitor, in cancer treatment: A systematic review. Curr. Cancer Ther. Rev. 2021, 17, 234–243. [Google Scholar] [CrossRef]
- Devriese, L.A.; Witteveen, P.E.; Mergui-Roelvink, M.; Smith, D.A.; Lewis, L.D.; Mendelson, D.S.; Bang, Y.; Chung, H.C.; Dar, M.M.; Huitema, A.D.R.; et al. Pharmacodynamics and pharmacokinetics of oral topotecan in patients with advanced solid tumors and impaired renal function. Br. J. Clin. Pharmacol. 2015, 80, 253–266. [Google Scholar] [CrossRef]
- Lambrecht, L.; Arnold, P.; Behr, J.; Mertsch, P.; Tufman, A.; Kauffmann-Guerrero, D. Topotecan in a real-world small-cell lung cancer cohort: Prognostic biomarkers improve selection of patients for second-line treatment. Diagnostics 2024, 14, 1572. [Google Scholar] [CrossRef]
- Tiseo, M.; Ardizzoni, A. Current status of second-line treatment and novel therapies for small cell lung cancer. J. Thorac. Oncol. 2007, 2, 764–772. [Google Scholar] [CrossRef]
- Tezuka, S.; Ueno, M.; Kobayashi, S.; Hamaguchi, T.; Yamachika, Y.; Oishi, R.; Nagashima, S.; Fukushima, T.; Morimoto, M.; Shin, M. Nal-IRI/5-FU/LV versus modified FOLFIRINOX and FOLFIRI as second-line chemotherapy for unresectable pancreatic cancer: A single center retrospective study. Pancreatology 2022, 22, 789–796. [Google Scholar] [CrossRef]
- Dai, W.; Habbous, S.; Saluja, R.; Beca, J.; Raphael, M.; Arias, J.; Gavura, S.; Earle, C.; Biagi, J.; Coburn, N.; et al. Comparative effectiveness of FOLFIRINOX versus gemcitabine and nab-paclitaxel in initially unresectable locally advanced pancreatic cancer: A population-based study to assess subsequent surgical resection and overall survival. Clin. Oncol. 2023, 35, 303–311. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.R.; Tian, H.; Yue, B.S.; Zhai, B.T.; Zhao, F. Research progress of SN38 drug delivery system in cancer treatment. Int. J. Nanomedicine 2024, 19, 945–964. [Google Scholar] [CrossRef]
- De Man, F.M.; Goey, A.K.; Van Schaik, R.H.; Mathijssen, R.H.; Bins, S. Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan: A key chemotherapeutic agent for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Mladenić, K.; Sedić, M. Chemoresistance mechanisms in colon cancer: Focus on conventional chemotherapy. Clin. Cancer Drugs. 2021, 8, 67–105. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Wang, Y.; Wu, Y.; Zhang, J. Managing irinotecan-induced diarrhea: A comprehensive review of therapeutic interventions in cancer treatment. Pharmaceuticals 2025, 18, 359. [Google Scholar] [CrossRef]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Dowlati, A.; Chen, Y.; Navarro, A.; Yang, J.C.-H.; Stojanovic, G.; Jove, M.; Rich, P.; Andric, Z.G.; Wu, Y.-L.; et al. RESILIENT Trial Investigators. RESILIENT Part 2: A randomized, open-label phase III study of liposomal irinotecan versus topotecan in adults with relapsed small cell lung cancer. J. Clin. Oncol. 2024, 42, 2317–2326. [Google Scholar] [CrossRef]
- Lemech, C.R.; Sun, Y.; Nagrial, A.; Wu, X.; Morris, M.F.; Ning, F.; Yang, J.; Pan, Y.; Cai, J.; Lu, P.; et al. IBI354 (anti-HER2 antibody-drug conjugate [ADC]) in patients with HER2-positive breast cancer and other solid tumors: Updates from a phase 1 study. J. Clin. Oncol. 2025, 43, 1029. [Google Scholar] [CrossRef]
- Chekerov, R.; Arndt, T.; Pietzner, K.; Canzler, U.; Wimberger, P.; Strauß, H.-G.; Mahner, S.; Woelber, L.; de Gregorio, N.; Stocker, G.; et al. Pazopanib with topotecan weekly for patients with platinum-resistant or intermediate-sensitive recurrent ovarian cancer: Results of a multicentre, open-label phase I/II study (TOPAZ). J. Cancer Res. Clin. Oncol. 2023, 149, 7637–7649. [Google Scholar] [CrossRef]
- Das, S.; Samaddar, S. Recent advances in the clinical translation of small-cell lung cancer therapeutics. Cancers 2025, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wen, J.; Ji, X.; Chen, J.; Yang, M.; Hong, M.; Deng, D. Preclinical development of a high affinity anti-exatecan monoclonal antibody and application in bioanalysis of antibody-exatecan conjugates. J. Pharm. Biomed. Anal. 2025, 262, 116843. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanotechnology toward treating cancer: A comprehensive review. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–256. [Google Scholar] [CrossRef]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional efficacy of biologically active nitro compounds included in medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Lee, K.-H.; Kim, D.-W.; Kim, S.-W.; Kim, H.R.; Kim, J.-H.; Choi, J.-H.; An, H.J.; Kim, J.-S.; Jang, J.-S.; et al. A randomized phase 2b study comparing the efficacy and safety of belotecan versus topotecan as monotherapy for sensitive-relapsed small-cell lung cancer. Br. J. Cancer. 2021, 124, 713–720. [Google Scholar] [CrossRef]
- Schöffski, P.; Wang, C.-C.; Schöffski, M.P.; Wozniak, A. Current role of topoisomerase I inhibitors for the treatment of mesenchymal malignancies and their potential future use as payload of sarcoma-specific antibody-drug conjugates. Oncol. Res. Treat. 2024, 47, 18–41. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, W.; Balthasar, J.P. Camptothecin-based anti-cancer therapies and strategies to improve their therapeutic index. Cancers 2025, 17, 1032. [Google Scholar] [CrossRef]
- Tonon, G.; Rizzolio, F.; Visentin, F.; Scattolin, T. Antibody drug conjugates for cancer therapy: From metallodrugs to nature-inspired payloads. Int. J. Mol. Sci. 2024, 25, 8651. [Google Scholar] [CrossRef]
- Han, S.; Lim, K.S.; Blackburn, B.J.; Yun, J.; Putnam, C.W.; Bull, D.A.; Won, Y.W. The Potential of Topoisomerase Inhibitor-Based Antibody-Drug Conjugates. Pharmaceutics 2022, 14, 1707. [Google Scholar] [CrossRef]
- Khan, S.; Jandrajupalli, S.B.; Bushara, N.Z.A.; Raja, R.D.P.; Mirza, S.; Sharma, K.; Verma, R.; Kumar, A.; Lohani, M. Targeting Refractory Triple-Negative Breast Cancer with Sacituzumab Govitecan: A New Era in Precision Medicine. Cells 2024, 13, 2126. [Google Scholar] [CrossRef]
- Indini, A.; Rijavec, E.; Grossi, F. Trastuzumab deruxtecan: Changing the destiny of HER2 expressing solid tumors. Int. J. Mol. Sci. 2021, 22, 4774. [Google Scholar] [CrossRef]
- Molina Trinidad, E.M.; Becerril Flores, M.A.; Imbert Palafox, J.L. Apoptosis y cáncer. Prensa. Med. Argent. 2020, 9, 124–130. [Google Scholar] [CrossRef]
- Beretta, L.; Gatti, G.; Perego, P.; Zaffaroni, N. Camptothecin resistance in cancer: Insights into the molecular mechanisms of a DNA-damaging drug. Curr. Med. Chem. 2013, 20, 1541–1556. [Google Scholar] [CrossRef]
- Stasiak, P.; Sopel, J.; Płóciennik, A.; Musielak, O.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Sterzyńska, K.; Korbecki, J.; Januchowski, R. Elacridar inhibits BCRP protein activity in 2D and 3D cell culture models of ovarian cancer and re-sensitizes cells to cytotoxic drugs. Int. J. Mol. Sci. 2025, 26, 5800. [Google Scholar] [CrossRef]
- Godbey, W.T. Cellular transport. In An Introduction to Biotechnology, 1st ed.; Academic Press: London, UK, 2014; pp. 35–64. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Miura, T.; Terashima, J.; Habano, W. Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: A review. Cancer Drug Resist. 2021, 4, 946–964. [Google Scholar] [CrossRef] [PubMed]
- Bircsak, K.M.; Aleksunes, L.M. Interaction of isoflavones with the drug transporter BCRP/ABCG2. Curr. Drug Metab. 2015, 16, 124–140. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Kundu, M.K.; Dey, A.; Rahman, M.H.; et al. Multidrug resistance in cancer cells: Focus on a possible strategy plan to address colon carcinoma cells. Life 2022, 12, 811. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.Y.; Kim, H.S.; Yoon, H. Establishment of acquired cisplatin resistance in ovarian cancer cell lines characterized by enriched metastatic properties with increased Twist expression. Int. J. Mol. Sci. 2020, 21, 7613. [Google Scholar] [CrossRef]
- Ricci, J.W.; Lovato, D.M.; Severns, V.; Sklar, L.A.; Larson, R.S. Novel ABCG2 antagonists reverse topotecan-mediated chemotherapeutic resistance in ovarian carcinoma xenografts. Mol. Cancer Ther. 2016, 15, 2853–2862. [Google Scholar] [CrossRef]
- Ando, K.; Shah, A.K.; Sachdev, V.; Kleinstiver, B.P.; Taylor-Parker, J.; Welch, M.M.; Hu, Y.; Salgia, R.; White, F.M.; Parvin, J.D.; et al. Camptothecin resistance is determined by topoisomerase I degradation regulation through the ubiquitin proteasome pathway. Oncotarget 2017, 8, 43733–43751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sherman, M.Y. Resistance to TOP-1 inhibitors: Good old drugs still can surprise us. Int. J. Mol. Sci. 2023, 24, 7233. [Google Scholar] [CrossRef] [PubMed]
- Saurav, S.; Karfa, S.; Vu, T.; Liu, Z.; Datta, A.; Manne, U.; Samuel, T.; Datta, P.K. Overcoming irinotecan resistance by targeting its downstream signaling pathways in colon cancer. Cancers 2024, 16, 3491. [Google Scholar] [CrossRef]
- Sharma, N.K.; Bahot, A.; Sekar, G.; Bansode, M.; Khunteta, K.; Sonar, P.V.; Hebale, A.; Salokhe, V.; Sinha, B.K. Understanding Cancer’s Defense against Topoisomerase-Active Drugs: A Comprehensive Review. Cancers 2024, 16, 680. [Google Scholar] [CrossRef]
- Jado, J.C.; Dow, M.; Carolino, K.; Klie, A.; Fonseca, G.J.; Ideker, T.; Carter, H.; Winzeler, E.A. In vitro evolution and whole genome analysis to study chemotherapy drug resistance in haploid human cells. Sci. Rep. 2024, 14, 13989. [Google Scholar] [CrossRef]
- Tomicic, M.T.; Kaina, B. Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors. Biochim. Biophys. Acta Rev. Cancer 2013, 1835, 11–27. [Google Scholar] [CrossRef]
- Álvarez-Fernández, L.; Millán-García, A.; Merino, G.; Blanco-Paniagua, E. ABCG2 transporter: From structure to function—Current insights and open questions. Int. J. Mol. Sci. 2025, 26, 6119. [Google Scholar] [CrossRef]
- Gavande, N.S.; VanderVere-Carozza, P.S.; Hinshaw, H.D.; Jalal, S.I.; Sears, C.R.; Pawelczak, K.S.; Turchi, J.J. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol. Ther. 2016, 160, 65–83. [Google Scholar] [CrossRef]
- Steurer, B.; Marteijn, J.A. Traveling rocky roads: The consequences of transcription-blocking DNA lesions on RNA polymerase II. J. Mol. Biol. 2017, 429, 3146–3155. [Google Scholar] [CrossRef]
- Chen, Y.F.; Xu, Y.Y.; Shao, Z.M.; Yu, K.D. Resistance to antibody-drug conjugates in breast cancer: Mechanisms and solutions. Cancer Commun. 2023, 43, 295–412. [Google Scholar] [CrossRef]
- Skok, Ž.; Zidar, N.; Kikelj, D.; Ilaš, J. Dual inhibitors of human DNA topoisomerase II and other cancer-related targets. J. Med. Chem. 2020, 63, 884–904. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Tsukamoto, M.; Imai, R.; Muramatsu, H.; Nakagawa, H. rs2231142 (421 C>A, Q141K) Is More Functionally Influential than rs2231137 (34 G>A, V12M) on Anticancer Drug Resistance Mediated by the ABCG2 Haplotype In Vitro. Int. J. Mol. Sci. 2025, 26, 7428. [Google Scholar] [CrossRef]
- Cravo, D.L.d.M.; Pimentel, P.A.B.; Garcia, A.P.V.; Junqueira, A.L.d.M.; Soares, F.S.; Giuliano, A.; Almendros, A.; Horta, R.d.S. Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals. Vet. Sci. 2025, 12, 747. [Google Scholar] [CrossRef]
- Bianchi, N.; Ancona, P.; Aguiari, G. Molecular Mechanisms of Drug Resistance in Clear Cell Renal Cell Carcinoma. Cancers 2025, 17, 1613. [Google Scholar] [CrossRef]
- Gongora, C.; Vezzio-Vie, N.; Tuduri, S.; Denis, V.; Causse, A.; Auzanneau, C.; Collod-Beroud, G.; Coquelle, A.; Pasero, P.; Pourquier, P.; et al. New topoisomerase I mutations are associated with resistance to camptothecin. Mol. Cancer 2011, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Arakawa, Y.; Ozaki, K.; Okawa, Y.; Yamada, H. Three missense mutations of DNA topoisomerase I in highly camptothecin-resistant colon cancer cell sublines. Oncol. Rep. 2013, 30, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Al Qadire, M.; Ballad, C.A.C.; Aljezawi, M.; Al Omari, O.; Alaloul, F.; Musa, A.; Al Sabei, S.; Khalaf, A. Nurses’ knowledge of chemotherapy-induced neutropenia and its management: A cross-sectional survey. J. Cancer Res. Clin. Oncol. 2023, 149, 2893–2901. [Google Scholar] [CrossRef]
- Pisani, R.P.d.L.; Altieri, G.; Stasio, R.C.; Lazzano, P.; Reni, M.; Falconi, M.; Vanella, G.; Arcidiacono, P.G.; Capurso, G. Gastrointestinal symptoms in the journey of pancreatic cancer patients. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 883–900. [Google Scholar] [CrossRef]
- Karlović, D.; Klarica, L.; Kršul, D.; Simičić, N.; Jerković, A.; Milotić, M.; Zelić, M. Fecal Incontinence: How to Approach and Treat? IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Y.; He, X.; Jiang, L.; Zhang, W.; Zheng, W.; Hu, M.; Zhu, C. Important role of gut microbiota in chemotherapy-induced diarrhea and therapeutics. J. Cancer 2025, 16, 648–659. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Chen, P. Restore intestinal steady-state: New advances in the clinical management of chemotherapy-associated diarrhea and constipation. J. Mol. Histol. 2025, 56, 101. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.; Zhong, W.; Chan, V.; Leslie, S. Successful management of severe refractory haemorrhagic cystitis secondary to cyclophosphamide and BK virus with cystotomy and alum infusion. Urol. Case Rep. 2021, 39, 101781. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Koyfman, A. Red blood cell transfusion in the emergency department. J. Emerg. Med. 2016, 51, 120–130. [Google Scholar] [CrossRef]
- American Cancer Society. Náuseas y Vómitos por Quimioterapia. Atlanta: American Cancer Society. Available online: https://www.cancer.org/es/cancer/como-sobrellevar-el-cancer/efectos-secundarios/problemas-alimentarios/nauseas-y-vomito/quimio-nauseas-vomitos.html (accessed on 28 August 2024).
- Fischer, O.W.; Justesen, T.F.; Gögenur, D.S.; Madsen, M.T.; Mortensen, M.B.; Gögenur, I.; Orhan, A. Long-term oncological outcomes of granulocyte colony-stimulating factor (G-CSF) treatment in gastrointestinal cancers: A systematic review and meta-analysis. Cancers 2025, 17, 1313. [Google Scholar] [CrossRef]
- Aslam, S.; Li, E.; Bell, E.; Lal, L.; Anderson, A.J.; Peterson-Brandt, J.; Lyman, G. Risk of chemotherapy-induced febrile neutropenia in intermediate-risk regimens: Clinical and economic outcomes of granulocyte colony-stimulating factor prophylaxis. J. Manag. Care Spec. Pharm. 2023, 29, 128–138. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Kaceli, T.; Mondal, A.; Hosein Farzaei, M.; Bishayee, A. Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef]
- Fortune, A.; Aime, A.; Raymond, D.; Kumar, S. Nanotechnology in medicine: A double-edged sword for health outcomes. Health Nanotechnol. 2025, 1, 9. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Soto Vazquez, R.; Záyago Lau, E.; López Maldonado, L.A. Gobernanza de la nanomedicina: Una revisión sistemática. Mundo Nano. 2021, 15, 1–25. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; El Andaloussi, S.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and nanogels: Pioneering the future of advanced drug delivery systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Kazi, R.N.A.; Hasani, I.W.; Khafaga, D.S.R.; Kabba, S.; Farhan, M.; Aatif, M.; Muteeb, G.; Fahim, Y.A. Nanomedicine: The effective role of nanomaterials in healthcare from diagnosis to therapy. Pharmaceutics 2025, 17, 987. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Al Siraj, A.; Lowry, I.; Ruan, E.; Patel, R.; Gao, W.; Kalin, T.V.; Kalinichenko, V.V. Nanoparticle-based delivery systems for synergistic therapy in lung cancers. Bioengineering 2025, 12, 968. [Google Scholar] [CrossRef]
- Russell, J.W.; Li, Y.; Yang, G.; Chun-Xia, Z. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Holm, R.; Kuentz, M.; Ilie-Spiridon, A.R.; Griffin, B.T. Lipid based formulations as supersaturating oral delivery systems: From current to future industrial applications. Eur. J. Pharm. Sci. 2023, 189, 106556. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Saharan, R.; Narwal, S.; Malik, R.; Gahlot, V.; Khalid, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; et al. Exploring lipids for their potential to improve bioavailability of lipophilic drug candidates: A review. Saudi Pharm. J. 2023, 31, 101870. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, B.; Armanetti, P.; Sanità, G.; Vitiello, G.; Lamberti, A.; Calì, G.; Pezzella, A.; Luciani, G.; Menichetti, L.; Luin, S.; et al. Silver nanoparticles as plasmon-resonant enhancers for eumelanin’s photoacoustic signal in a self-structured hybrid nanoprobe. Mater. Sci. Eng. C 2019, 102, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Cueto, Y.L.; Ortega, W.L.; Sotomayor, R.G. Sistemas de entrega de fármacos autoemulsificables: Una plataforma de desarrollo alternativa para la industria farmacéutica colombiana. Rev. Colomb. Cienc. Quím. Farm. 2019, 48, 260–313. [Google Scholar] [CrossRef]
- Mohite, P.; Singh, S.; Pawar, A.; Sangale, A.; Prajapati, B.G. Lipid-based oral formulation in capsules to improve the delivery of poorly water-soluble drugs. Front. Drug Deliv. 2023, 3, 1232012. [Google Scholar] [CrossRef]
- Silva, A.C.; Moreira, J.N.; Sousa Lobo, J.M. Current insights on lipid-based nanosystems 2023. Pharmaceuticals 2023, 16, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019, 69, 5–23. [Google Scholar] [CrossRef]
- Almeida, A.; Castro, F.; Resende, C.; Lucio, M.; Schwartz, S., Jr.; Sarmento, B. Oral delivery of camptothecin-loaded multifunctional chitosan-based micelles is effective in reduce colorectal cancer. J. Control Release. 2022, 349, 731–743. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomedicine 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Czerniel, J.; Gostyńska, A.; Jańczak, J.; Stawny, M. A critical review of the novelties in the development of intravenous nanoemulsions. Eur. J. Pharm. Biopharm. 2023, 191, 36–56. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Nanopartículas basadas en lípidos: Aplicación y avances recientes en el tratamiento del cáncer. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.M.; Kim, D.H. Functional ligands for improving anticancer drug therapy: Current status and applications to drug delivery systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Vagena, I.A.; Malapani, C.; Gatou, M.A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR Effect for Passive Tumor Targeting: Current Status and Future Perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Advanced drug delivery systems in the treatment of ovarian cancer. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 127–139. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Li, Y.; Rui, Y.; Zhang, P. Enhancing the efficacy of active pharmaceutical ingredients in medicinal plants through nanoformulations: A promising field. Nanomaterials 2024, 14, 1598, Erratum in Nanomaterials 2025, 15, 1442. [Google Scholar] [CrossRef]
- Alrushaid, N.; Khan, F.A.; Al-Suhaimi, E.A.; Elaissari, A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics 2023, 15, 1025. [Google Scholar] [CrossRef]
- Farhoudi, L.; Hosseinikhah, S.M.; Vahdat-Lasemi, F.; Sukhorukov, V.N.; Kesharwani, P.; Sahebkar, A. Polymeric micelles paving the way: Recent breakthroughs in camptothecin delivery for enhanced chemotherapy. Int. J. Pharm. 2024, 659, 124292. [Google Scholar] [CrossRef] [PubMed]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A promising approach for cancer diagnosis, therapeutics and theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Amiji, M.M. Application of nanotechnology in medical diagnosis and imaging. Curr. Opin. Biotechnol. 2022, 74, 241–246. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent progress in nanostructured smart drug delivery systems for cancer therapy: A review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Kher, C.; Kumar, S. The application of nanotechnology and nanomaterials in cancer diagnosis and treatment: A review. Cureus 2022, 14, e29059. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibáñez, E.; Cifuentes, A.; Lu, W. Microfluidic Biosensors for Biomarker Detection in Body Fluids: A Key Approach for Early Cancer Diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Karzai, F.; Bilusic, M.; Cordes, L.M.; Chau, C.H.; Peer, C.J.; Wroblewski, S.; Huitema, A.D.R.; Schellens, J.H.M.; Gulley, J.L.; et al. A single-arm phase II study combining NLG207, a nanoparticle camptothecin, with enzalutamide in advanced metastatic castration-resistant prostate cancer post-enzalutamide. Oncol. 2022, 27, 718-e694. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, J.C.; Munster, P.; Northfelt, D.W.; Han, H.S.; Ma, C.; Maxwell, F.; Wang, T.; Belanger, B.; Zhang, B.; Moore, Y.; et al. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: Findings from the expansion phase. Breast Cancer Res. Treat. 2021, 185, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, J.; Gan, Y.; Liang, X.J. Innovative irinotecan-loaded nanomicelles will enter Phase I clinical trial in 2021. Transl. Patent 2020, 1, 100057. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Moon, D.H.; Moore, D.T.; Boles, J.; Bui, C.; Blackstock, W.; O’Neil, B.H.; Subramaniam, S.; McRee, A.J.; Carlson, C.; et al. Phase I/II trial of CRLX101 nanocamptothecin with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomedicine 2019, 18, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Webster, T.J.; Kia, P.; Kalantari, K.; Misran, M.; Rasouli, E. Nanoemulsions based therapeutic strategies: Enhancing targeted drug delivery against breast cancer cells. Int. J. Nanomed. 2025, 20, 6133–6162. [Google Scholar] [CrossRef]
- Amparo, T.R.; Almeida, T.C.; Sousa, L.R.D.; Xavier, V.F.; da Silva, G.N.; Brandão, G.C.; dos Santos, O.D.H. Nanostructured formulations for a local treatment of cancer: A mini review about challenges and possibilities. Pharmaceutics 2025, 17, 205. [Google Scholar] [CrossRef]
- Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules 2025, 30, 3177. [Google Scholar] [CrossRef]
- Galatage Sunil, T.; Trivedi, R.; Durgacharan, A.B. Oral self-emulsifying nanoemulsion systems for enhancing dissolution, bioavailability and anticancer effects of camptothecin. J. Drug Deliv. Sci. Technol. 2022, 78, 103929. [Google Scholar] [CrossRef]
- Martínez Torreblanca, A.; Tirado Hernández, J.; Villalpando Castro, D.; Villapudua Rodríguez, G. Nanomedicina desde una perspectiva tecnológica: Revisión de literatura. Rev. Investig. Tecnol. Inf. 2020, 8, 56–65. [Google Scholar] [CrossRef]








| Drug | Cancer Type | Clinical Phase | Result | Toxicity Profile | Ref. |
|---|---|---|---|---|---|
| Nal-IRI (nanoliposomal Irinotecan) vs. Topotecan | Small Cell Lung Cancer (SCLC) relapsed | Phase III, randomized | Similar OS and PFS; Nal-IRI showed higher ORR in some analyses; did not consistently achieve superiority | Nal-IRI: neutropenia, diarrhea (like irinotecan); topotecan: intense myelosuppression | [126] |
| BI354 (ADC: trastuzumab- CPT derivative) | HER2+ metastatic breast cancer and solid tumors | Phase I, global | ORR 58%; DCR 90.9%; DoR 12 m: 71.8%; PFS/OS not achieved; OS 9 m: 96.2% | Myelosuppression, anemia, nausea | [127] |
| Pazopanib + Topotecan | Platinum-resistant ovarian cancer | Phase II, randomized | Improved ORR in PP but with greater toxicity; pazopanib reduced bioavailability (−18.8%) but without impact on AUC; no PK-tox correlation | Dose-limiting toxicity in 23% (hematological and hepatic) in combination | [128] |
| Drug | Cancer Type | Clinical Phase | Result | Toxicity Profile | Ref. |
|---|---|---|---|---|---|
| Belotecan | Sensitive-relapsed small-cell lung cancer (SCLC) | Phase II | ORR 33%; DCR 85%; median OS 13.2 months; PFS not significantly different | Acceptable safety profile; higher treatment completion rate (53% vs. 35%); well tolerated in patients < 65 years | [133] |
| Exatecan | Solid tumors, including sarcomas | Phase I | Some antitumor activity: partial responses reported in solid tumors, including a patient with sarcoma | Neutropenia, Thrombocytopenia hematological, nausea, vomiting, diarrhea, elevated hepatic transaminases, asthenia, alopecia | [134] |
| Exatecan mesylate | Advanced soft tissue sarcomas (STS) | Phase II | CBR: 60% leiomyosarcoma; 53% non-leiomyosarcoma; 3-month PFS: 56% leiomyosarcoma; 26% non leiomyosarcoma; ORR 0% | Neutropenia, thrombocytopenia, anemia, dyspnea, fatigue | [134] |
| Rubitecan | Advanced soft tissue sarcomas (STS) | Phase I/II | Chordoma ORR 7%, mTTP 9.9 weeks. soft tissue sarcomas ORR 4%, mTTP 8.0 weeks; gastrointestinal stromal tumors mTTP 8.3 weeks | Anemia, hyperglycemia, nausea, leukopenia | [134] |
| Drug & Sponsor | Target | Clinical Trial | Cancer Type | Toxicity Profile | Ref. |
|---|---|---|---|---|---|
| NLG207 (Camptothecin nanoparticle–drug conjugate) + Enzalutamide NewLink Genetics Corporation | Topoisomerase I Androgen receptor inhibitor | Phase II | Metastatic castration-resistant prostate cancer (mCRPC) post-enzalutamide | Noninfectious cystitis (Grade 3) and myelosuppression; poor tolerability at 12 mg/m2 | [216] |
| Liposomal Irinotecan Ipsen Biopharmaceuticals Servier | Topoisomerase I | Phase I | Metastatic breast cancer, including active brain metastases | Diarrhea (27.6%), nausea (17.2%), fatigue (13.8%), asthenia (10.3%), hypokalemia (10.3%); no treatment-related deaths | [217] |
| IH-NM (Irinotecan-loaded nanomicelles) National Center for Nanoscience and Technology of China | Topoisomerase I | Phase I | Colorectal cancer (CRC) | Improved tolerability (1.56× higher MTD vs. free CPT-11), reduced systemic toxicity, and no hematologic toxicity observed | [218] |
| CRLX101 (Camptothecin nanoparticle–drug conjugate) Cerulean Pharma Inc. | Topoisomerase I | Phase I/II | Locally advanced rectal cancer (with chemoradiotherapy) | Lymphopenia (1 Grade 4 case); overall excellent safety and feasibility | [219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ortega, H.U.; Córdova-Espíritu, R.R.; Cano-Serrano, S.; García-González, E.; Bravo-Sánchez, M.G.; Orozco-Mosqueda, M.d.C.; Jiménez-Islas, H.; Luna-Bárcenas, G.; Villaseñor-Ortega, F. Camptothecin in Cancer Therapy: Current Challenges and Emerging Strategies with Nanoemulsions. Pharmaceutics 2025, 17, 1414. https://doi.org/10.3390/pharmaceutics17111414
Pérez-Ortega HU, Córdova-Espíritu RR, Cano-Serrano S, García-González E, Bravo-Sánchez MG, Orozco-Mosqueda MdC, Jiménez-Islas H, Luna-Bárcenas G, Villaseñor-Ortega F. Camptothecin in Cancer Therapy: Current Challenges and Emerging Strategies with Nanoemulsions. Pharmaceutics. 2025; 17(11):1414. https://doi.org/10.3390/pharmaceutics17111414
Chicago/Turabian StylePérez-Ortega, Heber Uriel, Rubén Ricardo Córdova-Espíritu, Sebastian Cano-Serrano, Eduardo García-González, Micael Gerardo Bravo-Sánchez, Ma. del Carmen Orozco-Mosqueda, Hugo Jiménez-Islas, Gabriel Luna-Bárcenas, and Francisco Villaseñor-Ortega. 2025. "Camptothecin in Cancer Therapy: Current Challenges and Emerging Strategies with Nanoemulsions" Pharmaceutics 17, no. 11: 1414. https://doi.org/10.3390/pharmaceutics17111414
APA StylePérez-Ortega, H. U., Córdova-Espíritu, R. R., Cano-Serrano, S., García-González, E., Bravo-Sánchez, M. G., Orozco-Mosqueda, M. d. C., Jiménez-Islas, H., Luna-Bárcenas, G., & Villaseñor-Ortega, F. (2025). Camptothecin in Cancer Therapy: Current Challenges and Emerging Strategies with Nanoemulsions. Pharmaceutics, 17(11), 1414. https://doi.org/10.3390/pharmaceutics17111414








