Green-Synthesized Nanomaterials from Edible and Medicinal Mushrooms: A Sustainable Strategy Against Antimicrobial Resistance
Abstract
1. Introduction
2. Methodology of the Review
3. Bioactive Compounds in Mushrooms for NP Synthesis
3.1. Polysaccharides: β-Glucans, Chitin, and Mannogalactans
3.2. Proteins and Peptides
3.3. Phenolics and Flavonoids
3.4. Terpenoids and Other Metabolites
3.5. Unique Bioactives: Homogentisic Acid
4. Antimicrobial Mechanisms of Myco-Nanoparticles
4.1. Direct Antimicrobial Effects
Differential Susceptibility of Gram-Positive and Gram-Negative Bacteria to Myco-NPs
4.2. Indirect Immunomodulatory Effects
4.3. Synergistic Enhancement with Conventional Antibiotics
4.4. Biofilm Disruption
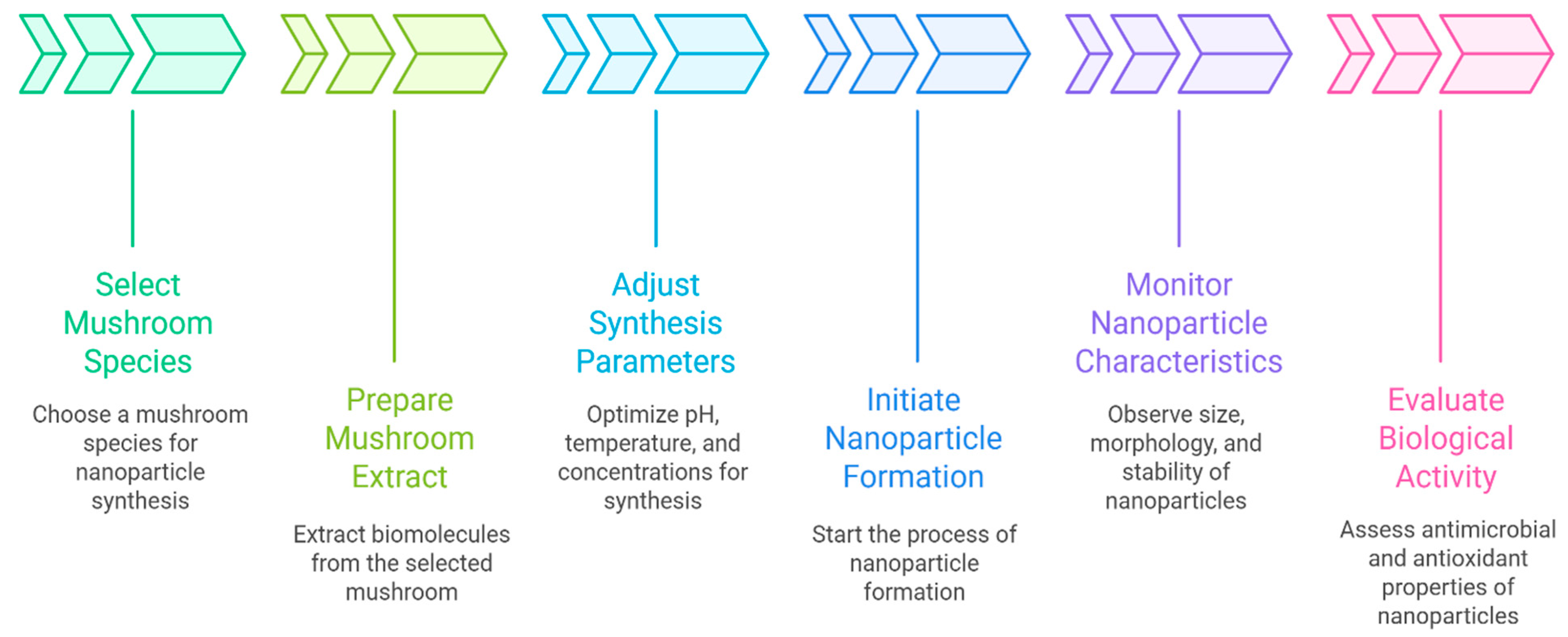
5. Green Synthesis of Myco-Nanomaterials
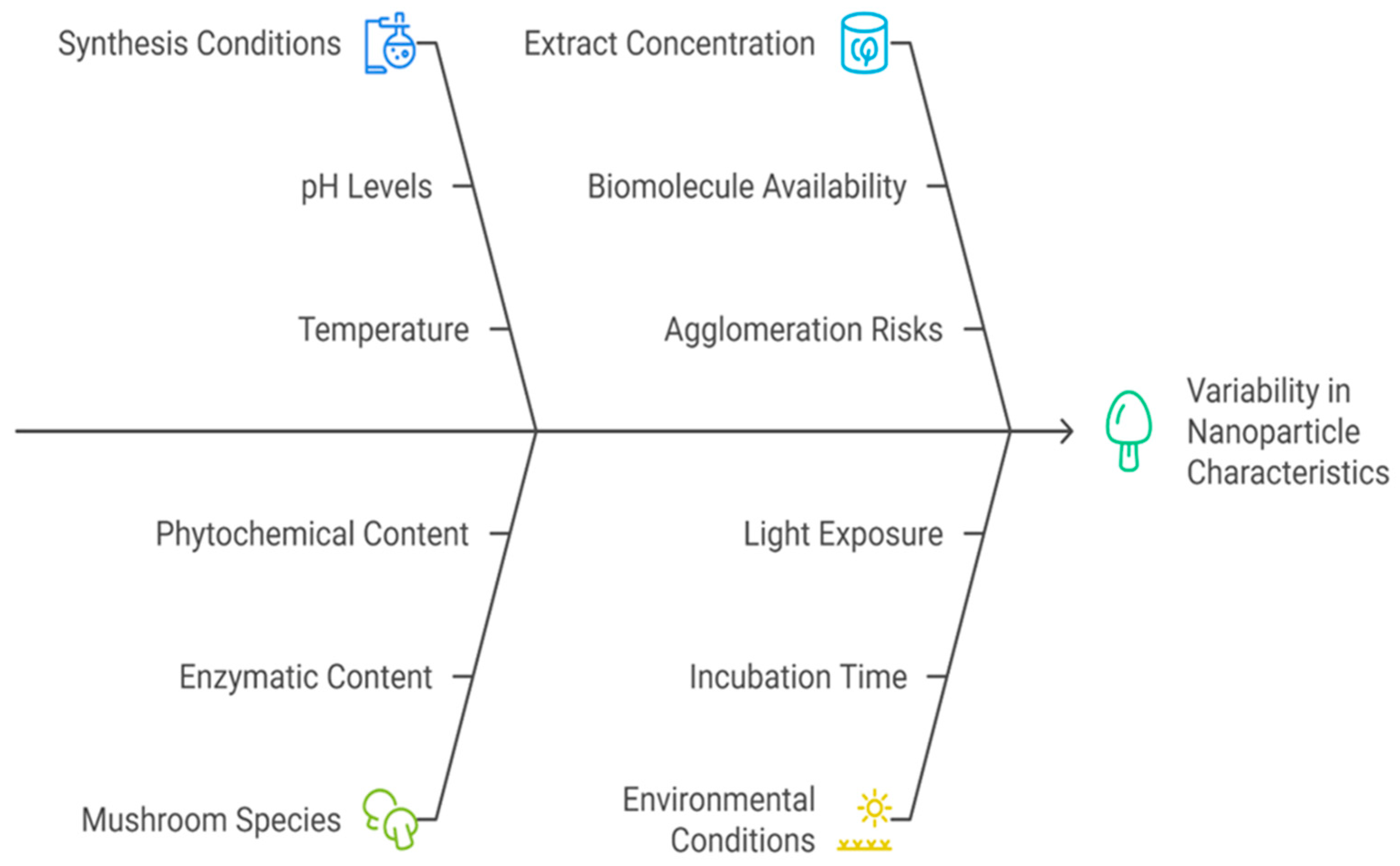
5.1. Optimization of Synthesis Parameters
5.2. Effect of Mushroom Species
- Pleurotus spp. (Oyster mushrooms) have been reported to produce silver nanoparticles (Ag-NPs) ranging from 2 to 100 nm, showing potent antimicrobial properties with applications in biomedicine and environmental remediation [73].
- Ganoderma spp. are noted for their high nanoparticle yield and safety profile, making them suitable for synthesizing various types of metal nanoparticles [71].
- Agaricus spp., widely appreciated for their nutritional and therapeutic properties, have shown potential in producing biologically active nanoparticles with enhanced antioxidant and antimicrobial activities [72].
- The biosynthesis mechanisms largely depend on the enzymes and phytochemicals secreted by mushrooms, which act as both reducing and stabilizing agents. Compounds such as flavones, phenolics, polysaccharides, and reductases contribute significantly to the reduction in metal ions and stabilization of the nanoparticles during formation [40,72].
- Further emphasizing the influence of species and cultivation is necessary, as both the mushroom strain and the method of cultivation play critical roles in determining nanoparticle characteristics. In their work, four species (Chlorophyllum agaricoides, Coriolopsis trogii, Ganoderma sp., and Lentinus tigrinus) were cultivated on potato dextrose agar (PDA) and used for AgNP synthesis. The resulting nanoparticles showed varying crystallite sizes (25.31 to 31.42 nm), reflecting the impact of species-specific biochemical profiles. The use of cultivated mushrooms under controlled conditions ensured reproducibility and consistency in nanoparticle production. Analytical characterization confirmed that bioactive molecules in the extracts were key to both the reduction and stabilization processes, and also contributed to the antimicrobial and antioxidant activities observed [74].
- Similarly, the use of A. bisporus as a biological reducing agent in the synthesis of silver nanoparticles. The study emphasized that the specific strain composition, rich in proteins, polysaccharides, and phenolics, played a vital role in both reducing Ag+ to Ag0 and stabilizing the nanoparticles. The authors highlighted that variations in the type and concentration of these biomolecules among mushroom strains directly affect the efficiency, size, and morphology of the resulting nanoparticles [18].
5.3. Effect of Mushroom Extract Concentration
5.4. Environmental Conditions
5.5. Advantages of Myco-Synthesis over Conventional Methods
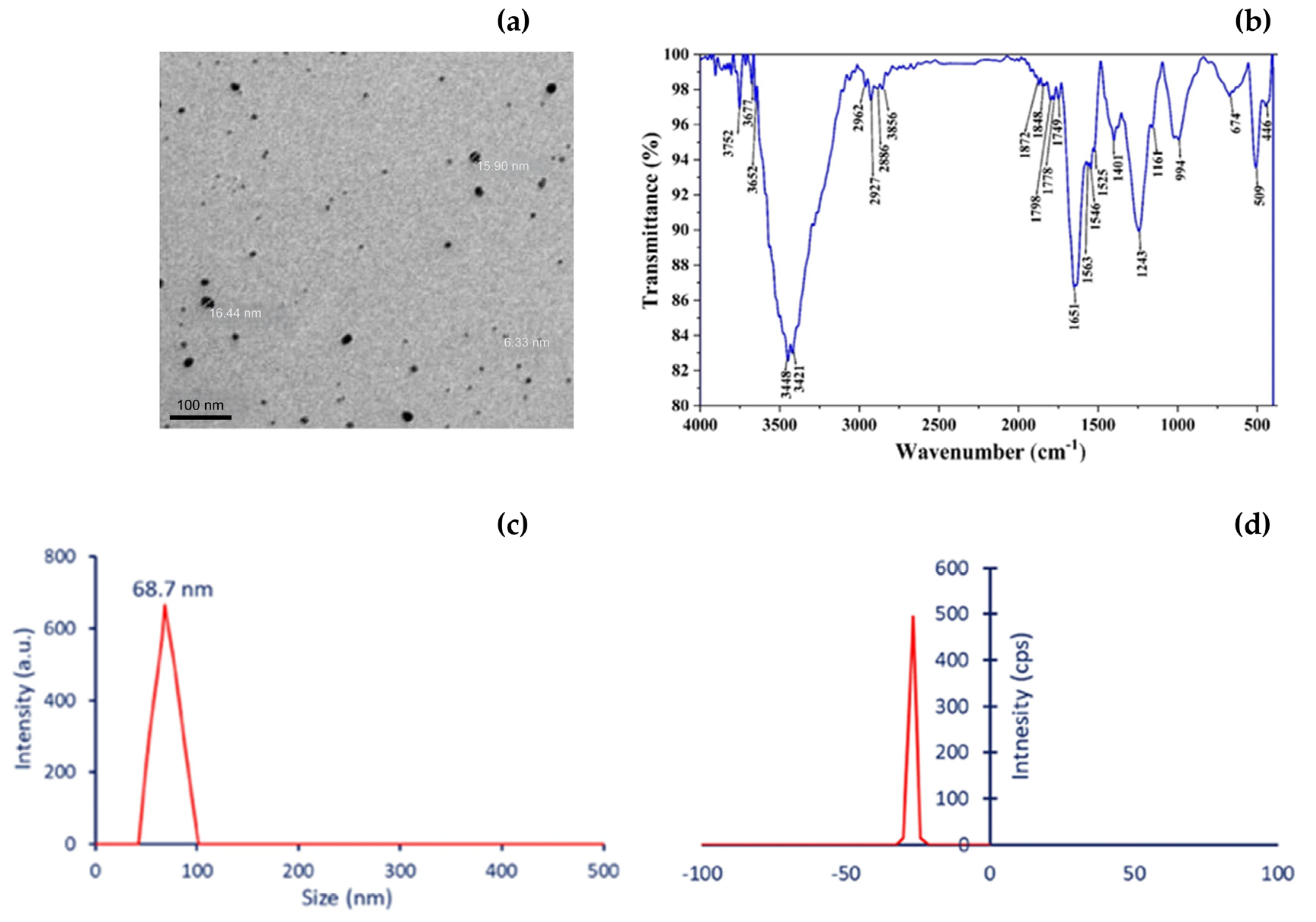
6. Characterization of Myco-Nanomaterials
6.1. Dynamic Light Scattering (DLS): Hydrodynamic Size and Surface Charge
6.2. Transmission and Scanning Electron Microscopy (TEM/SEM): Morphology and Core Size
6.3. X-Ray Diffraction (XRD): Crystallinity and Phase Identification
6.4. Fourier-Transform Infrared Spectroscopy (FTIR): Surface Functionalization and Capping Agents
6.5. UV-Visible Spectroscopy: Surface Plasmon Resonance (SPR) and Optical Monitoring
7. Safety and Regulatory Considerations
8. Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Barsola, B.; Saklani, S.; Pathania, D.; Kumari, P.; Sonu, S.; Rustagi, S.; Singh, P.; Raizada, P.; Moon, T.S.; Kaushik, A.; et al. Exploring Bio-Nanomaterials as Antibiotic Allies to Combat Antimicrobial Resistance. Biofabrication 2024, 16, 042007. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Al-Halawa, D.A.; Seir, R.A.; Qasrawi, R. Antibiotic Resistance Knowledge, Attitudes, and Practices among Pharmacists: A Cross-Sectional Study in West Bank, Palestine. J. Environ. Public Health 2023, 2023, 2294048. [Google Scholar] [CrossRef] [PubMed]
- Pilmis, B.; Le Monnier, A.; Zahar, J.-R. Gut Microbiota, Antibiotic Therapy and Antimicrobial Resistance: A Narrative Review. Microorganisms 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, T.; Wadhwa, R.; Thapliyal, N.; Gupta, R.; Hansbro, P.M.; Dua, K.; Maurya, P.K. Recent Trends of Nano-Material as Antimicrobial Agents. In Nanotechnology in Modern Animal Biotechnology; Springer: Singapore, 2019; pp. 173–193. ISBN 978-981-13-6003-9. [Google Scholar]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria-”A Battle of the Titans”. Front. Microbiol 2018, 9, 1441. [Google Scholar] [CrossRef]
- Giles, E.L.; Kuznesof, S.; Clark, B.; Hubbard, C.; Frewer, L.J. Consumer Acceptance of and Willingness to Pay for Food Nanotechnology: A Systematic Review. J. Nanopart. Res. 2015, 17, 467. [Google Scholar] [CrossRef]
- Ghebretatios, M.; Schaly, S.; Prakash, S. Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. Int. J. Mol. Sci. 2021, 22, 1942. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-Induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm (2020) 2023, 4, e327. [Google Scholar] [CrossRef]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Mahato, R.P.; Kumar, S. A Review on Green Approach toward Carbohydrate-Based Nanocomposite Synthesis from Agro-Food Waste to Zero Waste Environment. Nanotechnol. Environ. Eng. 2024, 9, 315–345. [Google Scholar] [CrossRef]
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable Production of Nanoparticles via Mycogenesis for Biotechnological Applications: A Critical Review. Environ. Res. 2022, 204, 111963. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.T.; Pandey, I.; Hachenberger, Y.; Krause, B.-C.; Haidar, R.; Laux, P.; Luch, A.; Singh, M.P.; Singh, A.V. Emerging Paradigm against Global Antimicrobial Resistance via Bioprospecting of Mushroom into Novel Nanotherapeutics Development. Trends Food Sci. Technol. 2020, 106, 333–344. [Google Scholar] [CrossRef]
- Ali Syed, I.; Alvi, I.A.; Fiaz, M.; Ahmad, J.; Butt, S.; Ullah, A.; Ahmed, I.; Niaz, Z.; Khan, S.; Hayat, S.; et al. Synthesis of Silver Nanoparticles from Ganoderma Species and Their Activity against Multi Drug Resistant Pathogens. Chem. Biodivers. 2024, 21, e202301304. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps Sinensis: A Review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Mimi, X.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic Values and Nutraceutical Properties of Shiitake Mush-room (Lentinula edodes): A Review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Amr, M.; Abu-Hussien, S.H.; Ismail, R.; Aboubakr, A.; Wael, R.; Yasser, M.; Hemdan, B.; El-Sayed, S.M.; Bakry, A.; Ebeed, N.M.; et al. Utilization of Biosynthesized Silver Nanoparticles from Agaricus Bisporus Extract for Food Safety Application: Synthesis, Characterization, Antimicrobial Efficacy, and Toxicological Assessment. Sci. Rep. 2023, 13, 15048. [Google Scholar] [CrossRef]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and Characterization of Reishi Mushroom-Mediated Green Synthesis of Silver Nanoparticles for the Biochemical Applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef]
- Pothiraj, C.; Kumar, M.; Eyini, M.; Balaji, P. Mycosynthesis of Nanoparticles from Basidiomycetes Mushroom Fungi: Properties, Biological Activities, and Their Applications. In Materials Horizons: From Nature to Nanomaterials; Springer Nature: Singapore, 2022; pp. 315–337. ISBN 978-981-19-2638-9. [Google Scholar]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Ellah, N.H.A.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Eskandari-Nojedehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Hydrothermal Green Synthesis of Gold Nanoparticles Using Mushroom (Agaricus bisporus) Extract: Physico-Chemical Characteristics and Antifungal Activity Studies. Green Process. Synth. 2018, 7, 38–47. [Google Scholar] [CrossRef]
- Pandey, N.; Ahmad, F.; Singh, K.; Ahmad, S.; Sharma, R. Mushroom-Derived Nanoparticles in Drug Delivery Systems—Therapeutic Roles and Biological Functions. Precis. Nanomed. 2023, 6, 1157–1178. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag Nanoparticles Using Edible Mushroom Extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Karwa, A.S.; Gaikwad, S.; Rai, M.K. Mycosynthesis of Silver Nanoparticles Using Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (W. Curt.:Fr.) P. Karst. and Their Role as Antimicrobials and Antibiotic Activity Enhancers. Int. J. Med. Mushr. 2011, 13, 483–491. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green Synthesis of Nanoparticles with Extracellular and Intracellular Extracts of Basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Petrovic, P.; Vunduk, J.; Pavlovic, V.; Van Griensven, L.J.L.D. The Antimicrobial Activities of Silver Nanoparticles Synthesized from Medicinal Mushrooms. Int. J. Med. Mushrooms 2020, 22, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Sarwar, N.; Sadia, H.; Malik, K.; Javed, I.; Irshad, A.; Afzal, M.; Abbas, M.; Rizvi, H. Comprehensive Facts on Dynamic Antimicrobial Properties of Polysaccharides and Biomolecules-Silver Nanoparticle Conjugate. Int. J. Biol. Macromol. 2020, 145, 189–196. [Google Scholar] [CrossRef]
- Constantin, M.; Răut, I.; Suica-Bunghez, R.; Firinca, C.; Radu, N.; Gurban, A.-M.; Preda, S.; Alexandrescu, E.; Doni, M.; Jecu, L. Ganoderma lucidum-Mediated Green Synthesis of Silver Nanoparticles with Antimicrobial Activity. Materials 2023, 16, 4261. [Google Scholar] [CrossRef]
- Ekar, S.U.; Khollam, Y.B.; Koinkar, P.M.; Mirji, S.A.; Mane, R.S.; Naushad, M.; Jadhav, S.S. Biosynthesis of Silver Nanoparticles by Using Ganoderma-Mushroom Extract. Mod. Phys. Lett. B 2015, 29, 1540047. [Google Scholar] [CrossRef]
- Rehman, S.; Farooq, R.; Jermy, R.; Mousa Asiri, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.; Shah, M.A.; Reshi, Z.; Sabit, H.; et al. A Wild Fomes Fomentarius for Biomediation of One Pot Synthesis of Titanium Oxide and Silver Nanoparticles for Antibacterial and Anticancer Application. Biomolecules 2020, 10, 622. [Google Scholar] [CrossRef]
- Yuvarani, M.; Kiruthika, S.; Shiyamala, G.; Ashokkumar, L.; Prasannabalaji, N.; Matharasi, A.A.P. Mycosynthesis and Characterization of Zinc Oxide Nanoparticles from Shiitake Mushrooms (Lentinula edodes): Potential Antidiabetic, Antimicrobial and Antioxidant Efficacy. Asian J. Chem. 2024, 36, 2191–2196. [Google Scholar] [CrossRef]
- Madhanraj, R.; Eyini, M.; Balaji, P. Antioxidant Assay of Gold and Silver Nanoparticles from Edible Basidiomycetes Mushroom Fungi. Free. Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Smirnov, O.; Dzhagan, V.; Yeshchenko, O.; Kovalenko, M.; Kapush, O.; Vuichyk, M.; Dzhagan, V.; Mazur, N.; Kalynovskyi, V.; Skoryk, M.; et al. Effect of pH of Ganoderma Lucidum Aqueous Extract on Green Synthesis of Silver Nanoparticles. Adv. Nat. Sci: Nanosci. Nanotechnol. 2023, 14, 035009. [Google Scholar] [CrossRef]
- Vamanu, E.; Ene, M.; Biță, B.; Ionescu, C.; Crăciun, L.; Sârbu, I. In Vitro Human Microbiota Response to Exposure to Silver Nanoparticles Biosynthesized with Mushroom Extract. Nutrients 2018, 10, 607. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Mashwani, Z.-R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of Plant Terpenoids in the Synthesis of Colloidal Silver Nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, N.; Ahmed, S.; Habib, E.; Abdelhameed, R. B:Chemical Constituents and Biological Effects of Ganoderma Mushroom. Rec. Pharm. Biomed. Sci. 2020, 4, 25–35. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Singh, M.P.; Vamanu, E.; Petre, A. Biosynthesis and Bioapplications of Nanomaterials from Mushroom Products. Curr. Pharm. Des. 2023, 29, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Rauwel, P.; Rauwel, E. Antimicrobial Nanoparticles: Synthesis, Mechanism of Actions. In Antimicrobial Activity of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–202. ISBN 978-0-12-821637-8. [Google Scholar]
- Das, P.; Ghosh, S.; Nayak, B. Phyto-Fabricated Nanoparticles and Their Anti-Biofilm Activity: Progress and Current Status. Front. Nanotechnol. 2021, 3, 739286. [Google Scholar] [CrossRef]
- Kareem, A. Combination Effect of Edible Mushroom—Silver Nanoparticles and Antibiotics against Selected Multidrug Biofilm Pathogens. Int. J. Res. Ph. Sci. 2018, 9, 124–131. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, A.; Tejwan, N.; Bhardwaj, S.; Bhardwaj, P.; Nepovimova, E.; Shami, A.; Kalia, A.; Kumar, A.; Abd-Elsalam, K.A.; et al. Pleurotus Macrofungi-Assisted Nanoparticle Synthesis and Its Potential Applications: A Review. J. Fungi 2020, 6, 351. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Pooe, O.J.; Khoza, S.; Mongalo, I.N.; Khan, R.; Simelane, M.B.C. Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom. Appl. Sci. 2022, 12, 8563. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Kim, H.J.; Jo, J.W.; Rangarajulu, S.K. Macrofungal Mediated Biosynthesis of Silver Nanoparticles and Evaluation of Its Antibacterial and Wound-Healing Efficacy. Int. J. Mol. Sci. 2024, 25, 861. [Google Scholar] [CrossRef]
- Bin Ali, M.; Iftikhar, T.; Majeed, H. Green Synthesis of Zinc Oxide Nanoparticles for the Industrial Biofortification of (Pleurotus pulmonarius) Mushrooms. Heliyon 2024, 10, e37927. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohaimeed, A.M.; Al-Onazi, W.A.; El-Tohamy, M.F. Multifunctional Eco-Friendly Synthesis of ZnO Nanoparticles in Biomedical Applications. Molecules 2022, 27, 579. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Balasubramanian, S.; Perumal, E. Copper Oxide Nanoparticles Induced Reactive Oxygen Species Generation: A Systematic Review and Meta-Analysis. Chem.-Biol. Interact. 2025, 405, 111311. [Google Scholar] [CrossRef] [PubMed]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendhi, A. Green Chemistry Route of Biosynthesized Copper Oxide Nanoparticles Using Psidium Guajava Leaf Extract and Their Antibacterial Activity and Effective Removal of Industrial Dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Hayat, P.; Khan, I.; Rehman, A.; Jamil, T.; Hayat, A.; Rehman, M.U.; Ullah, N.; Sarwar, A.; Alharbi, A.A.; Dablool, A.S.; et al. Myogenesis and Analysis of Antimicrobial Potential of Silver Nanoparticles (AgNPs) against Pathogenic Bacteria. Molecules 2023, 28, 637. [Google Scholar] [CrossRef]
- Tufail, M.S.; Liaqat, I.; Andleeb, S.; Naseem, S.; Zafar, U.; Sadiqa, A.; Liaqat, I.; Ali, N.M.; Bibi, A.; Arshad, N.; et al. Biogenic Synthesis, Characterization and Antibacterial Properties of Silver Nanoparticles against Human Pathogens. J. Oleo Sci. 2022, 71, 257–265. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef]
- Krychowiak-Maśnicka, M.; Wojciechowska, W.; Bogaj, K.; Bielicka-Giełdoń, A.; Czechowska, E.; Ziąbka, M.; Narajczyk, M.; Kawiak, A.; Mazur, T.; Szafranek, B.; et al. The Substantial Role of Cell and Nanoparticle Surface Properties in the Antibacterial Potential of Spherical Silver Nanoparticles. Nanotechnol. Sci. Appl. 2024, 17, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, P.; Chen, H.; Qiao, M.; Yang, B.; Zhao, X. Impact of Reactive Oxygen Species on Cell Activity and Structural Integrity of Gram-Positive and Gram-Negative Bacteria in Electrochemical Disinfection System. Chem. Eng. J. 2023, 451, 138879. [Google Scholar] [CrossRef]
- Kumar, N.; Daniloski, D.; Pratibha; Neeraj; D’Cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate Peel Extract—A Natural Bioactive Addition to Novel Active Edible Packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef] [PubMed]
- Gimba, E.; Brum, M.; Nestal De Moraes, G. Full-Length Osteopontin and Its Splice Variants as Modulators of Chemoresistance and Radioresistance (Review). Int. J. Oncol. 2018, 54, 420–430. [Google Scholar] [CrossRef]
- Llauradó Maury, G.; Morris-Quevedo, H.J.; Heykers, A.; Lanckacker, E.; Cappoen, D.; Delputte, P.; Vanden Berghe, W.; Salgueiro, Z.; Cos, P. Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium. J. Fungi 2021, 7, 206. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, H.; Verma, A.; Gupta, P.; Chattopadhaya, A.; Singh, A.; Singh, S.; Kumar, B.; Mandal, A.; Kumar, R.; et al. Sustainable Synthesis of Novel Green-Based Nanoparticles for Therapeutic Interventions and Environmental Remediation. ACS Synth. Biol. 2024, 13, 1994–2007. [Google Scholar] [CrossRef]
- Sen, I.K.; Mandal, A.K.; Chakraborti, S.; Dey, B.; Chakraborty, R.; Islam, S.S. Green Synthesis of Silver Nanoparticles Using Glucan from Mushroom and Study of Antibacterial Activity. Int. J. Biol. Macromol. 2013, 62, 439–449. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12, 454. [Google Scholar] [CrossRef]
- Abdolhosseini, M.; Zamani, H.; Salehzadeh, A. Synergistic Antimicrobial Potential of Ciprofloxacin with Silver Nanoparticles Conjugated to Thiosemicarbazide against Ciprofloxacin Resistant Pseudomonas Aeruginosa by Attenuation of MexA-B Efflux Pump Genes. Biologia 2019, 74, 1191–1196. [Google Scholar] [CrossRef]
- Rodríguez-Suárez, J.M.; Gershenson, A.; Onuh, T.U.; Butler, C.S. The Heterogeneous Diffusion of Polystyrene Nanoparticles and the Effect on the Expression of Quorum-Sensing Genes and EPS Production as a Function of Particle Charge and Biofilm Age. Environ. Sci. Nano 2023, 10, 2551–2565. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive Polysaccharides from Medicinal Mushrooms: A Review on Their Isolation, Structural Characteristics and Antitumor Activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Gupta, N.; Abd-Elsalam, K.A. Green Synthesized Nanoparticles as a Promising Strategy for Controlling Microbial Biofilm. In Environmental and Microbial Biotechnology; Springer Nature: Singapore, 2021; pp. 1–28. ISBN 978-981-15-9915-6. [Google Scholar]
- Ozdal, M.; Gurkok, S. Recent Advances in Nanoparticles as Antibacterial Agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Guo, D.; Fernández-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Yang, H.; Liu, X.; Wang, Y.-C.; Zeng, Y.; et al. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharm. 2023, 20, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Sharma, S. Rhizobial-Metabolite Based Biocontrol of Fusarium Wilt in Pigeon Pea. Microb. Pathog. 2020, 147, 104278. [Google Scholar] [CrossRef]
- Prachumrak, N.; Prajudtasri, N.; Promarak, V.; Utara, S. Green Synthesized CaO Nanoparticles Using Various Mushroom Species Extracts: Structural Characterization, Antioxidant and Photodegradation Ability. Results Surf. Interfaces 2025, 19, 100503. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, X.; Yang, Y.; Zheng, L.; Xiao, D.; Ai, B.; Sheng, Z. Emerging Technologies in Reducing Dietary Advanced Glycation End Products in Ultra-processed Foods: Formation, Health Risks, and Innovative Mitigation Strategies. Comp. Rev. Food Sci. Food Safe 2025, 24, e70130. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Joel, D.; Singh, D.P.; Malek, R.A.; Elsayed, E.A.; Hanapi, S.Z.; Kumar, K. Mushrooms: New Biofactories for Nanomaterial Production of Different Industrial and Medical Applications. In Microbial Nanobionics; Prasad, R., Ed.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 87–126. ISBN 978-3-030-16382-2. [Google Scholar]
- Adebayo, E.A.; Azeez, M.A.; Alao, M.B.; Oke, M.A.; Aina, D.A. Mushroom Nanobiotechnology: Concepts, Developments and Potentials. In Microbial Nanobiotechnology; Lateef, A., Gueguim-Kana, E.B., Dasgupta, N., Ranjan, S., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 257–285. ISBN 978-981-334-776-2. [Google Scholar]
- Owaid, M.N. Green Synthesis of Silver Nanoparticles by Pleurotus (Oyster Mushroom) and Their Bioactivity. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100256. [Google Scholar] [CrossRef]
- Abdalrahman, A.S.; Suliaman, S.Q. Mushroom-Mediated Silver Nanoparticle Synthesis: Characterisation, Antimicrobial and Antioxidant Activities. Plant Sci. Today 2025, 12, 181–186. [Google Scholar] [CrossRef]
- Ali, D.; Arooj, N.; Muneer, I.; Bashir, F.; Hanif, M.; Ali, S. Sustainable Synthesis of ZnO Nanoparticles from Psathyrella Candolleana Mushroom Extract: Characterization, Antibacterial Activity, and Photocatalytic Potential. Inorg. Chem. Commun. 2023, 158, 111588. [Google Scholar] [CrossRef]
- Tudu, S.C.; Zubko, M.; Kusz, J.; Bhattacharjee, A. CdS Nanoparticles (<5 Nm): Green Synthesized Using Termitomyces Heimii Mushroom–Structural, Optical and Morphological Studies. Appl. Phys. A 2021, 127, 85. [Google Scholar] [CrossRef]
- Narasimha, G.; Praveen, B.; Mallikarjuna, K.; Deva, P.R.B. Mushrooms (Agaricus bisporus) Mediated Biosynthesis of Sliver Nanoparticles, Characterization and Their Antimicrobial Activity. Int. J. Nano Dimens. 2011, 2, 29–36. [Google Scholar]
- Kalia, A.; Kaur, G. Biosynthesis of Nanoparticles Using Mushrooms. In Biology of Macrofungi; Singh, B.P., Lallawmsanga Passari, A.K., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 351–360. ISBN 978-3-030-02621-9. [Google Scholar]
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Yadav, D.; Mohanta, T.K. Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Suliaman, S.Q.; AL-Abbasi, S.H.; Mahmood, Y.H.; AL-Azzawi, H.A. Antimicrobial Activity of Four Selected Wild Mushrooms in Iraq. Biochem. Cell. Arch 2021, 21, 4533–4537. [Google Scholar]
- Yang, B.; Chou, J.; Dong, X.; Qu, C.; Yu, Q.; Lee, K.J.; Harvey, N. Size-Controlled Green Synthesis of Highly Stable and Uniform Small to Ultrasmall Gold Nanoparticles by Controlling Reaction Steps and pH. J. Phys. Chem. C 2017, 121, 8961–8967. [Google Scholar] [CrossRef]
- Nurfadhilah, M.; Nolia, I.; Handayani, W.; Imawan, C. The Role of pH in Controlling Size and Distribution of Silver Nanoparticles Using Biosynthesis from Diospyros Discolor Willd. (Ebenaceae). IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012033. [Google Scholar] [CrossRef]
- Alves, M.F.; Murray, P.G. Biological Synthesis of Monodisperse Uniform-Size Silver Nanoparticles (AgNPs) by Fungal Cell-Free Extracts at Elevated Temperature and pH. J. Fungi 2022, 8, 439. [Google Scholar] [CrossRef]
- Søndergaard, M.; Bøjesen, E.D.; Christensen, M.; Iversen, B.B. Size and Morphology Dependence of ZnO Nanoparticles Synthesized by a Fast Continuous Flow Hydrothermal Method. Cryst. Growth Des. 2011, 11, 4027–4033. [Google Scholar] [CrossRef]
- Gurusamy, M.; Raju, R. Biosynthesis of Iron Nanoparticles from Pleurotus florida and Its Antimicrobial Activity against Selected Human Pathogens. Indian J. Pharm. Sci. 2021, 83, 45–51. [Google Scholar] [CrossRef]
- Padhi, A.; Magar, S.J.; Jena, M.K.; Kavale, A.N.; Sahu, S. Characterization of Zinc Nanoparticles Synthesized Using the Mushroom, Pleurotus sajor-caju. J. Adv. Biol. Biotechnol. 2024, 27, 484–492. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Ullah, K.; Almutairi, S.M.; AlMunqedhi, B.M.; Ragab AbdelGawwad, M. Mycosynthesis, Characterization of Zinc Oxide Nanoparticles, and Its Assessment in Various Biological Activities. Crystals 2023, 13, 171. [Google Scholar] [CrossRef]
- Beltrán Pineda, M.E.; Lizarazo Forero, L.M.; Sierra, Y.C.A. Mycosynthesis of Silver Nanoparticles: A Review. Biometals 2023, 36, 745–776. [Google Scholar] [CrossRef]
- Mhammedsharif, R.M.; Jalil, P.J.; Piro, N.; Salih Mohammed, A.; Aspoukeh, P.K. Myco-Generated and Analysis of Magnetite (Fe3O4) Nanoparticles Using Aspergillus elegans Extract: A Comparative Evaluation with a Traditional Chemical Ap-proach. Heliyon 2024, 10, e31352. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.; Iqbal, D.; Alharbi, R.; Jahan, S.; Darwish, O.; Alshehri, B.; Banawas, S.; Palanisamy, M.; Ismail, A.; Aldosari, S.; et al. Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes. Biology 2023, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Sukumar, K.; Sabarimuthu, S.Q. Trends in Green Synthesis, Pharmaceutical and Medical Applications of Nano ZnO: A Review. Inorg. Chem. Commun. 2024, 169, 113002. [Google Scholar] [CrossRef]
- Szczepankowska, J.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M. Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology. Int. J. Mol. Sci. 2023, 24, 14984. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) Formation, Occurrence and Potential Health Concerns: Recent Developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- Hassan, S.; G K, K.; Singh, P.; Meenatchi, R.; Venkateswaran, A.S.; Ahmed, T.; Bansal, S.; Kamalraj, R.; Kiran, G.S.; Selvin, J. Implications of Myconanotechnology for Sustainable Agriculture-Applications and Future Perspectives. Biocatal. Agric. Biotechnol. 2024, 57, 103110. [Google Scholar] [CrossRef]
- Christos, R.E.; Anwar, H.; Lau, V.; Hadinata, E.; Syahputra, R.A.; Hardinsyah, H.; Taslim, N.A.; Tjandrawinata, R.R.; Kim, B.; Tsopmo, A.; et al. Harnessing Nanotechnology with Mushroom-Derived Bioactives: Targeting Inflammatory Pathways and miRNAs in Osteoarthritis. J. Agric. Food Res. 2025, 20, 101791. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Borkowski, M.; Barzowska, A.; Kobielusz, M.; Dąbrowski, J.M. Synthesis and Characterization of Size- and Charge-Tunable Silver Nanoparticles for Selective Anticancer and Antibacterial Treatment. ACS Appl. Mater. Interfaces 2022, 14, 14981–14996. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Häffner, S.; Malmsten, M. Membrane Interactions and Antimicrobial Effects of Inorganic Nanoparticles. Adv. Colloid Interface Sci. 2017, 248, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Studies on Interaction of Colloidal Silver Nanoparticles (SNPs) with Five Different Bacterial Species. Colloids Surf. B Biointerfaces 2011, 87, 129–138. [Google Scholar] [CrossRef]
- Carvalho Silva, R.; Alexandre Muehlmann, L.; Rodrigues Da Silva, J.; de Bentes Azevedo, R.; Madeira Lucci, C. Influence of Nanostructure Composition on Its Morphometric Characterization by Different Techniques. Microsc. Res. Tech. 2014, 77, 691–696. [Google Scholar] [CrossRef]
- Sayed, F.A.-Z.; Eissa, N.G.; Shen, Y.; Hunstad, D.A.; Wooley, K.L.; Elsabahy, M. Morphologic Design of Nanostructures for Enhanced Antimicrobial Activity. J. Nanobiotechnol. 2022, 20, 536. [Google Scholar] [CrossRef]
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A. Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review. Biomimetics 2022, 8, 1. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-Ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial Effects of Titanium Embedded with Silver Nanoparticles Based on Electron-Transfer-Induced Reactive Oxygen Species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tey, L.-H.; Razali, S.A.; Althubeiti, K.; Alkhammash, H.I.; Guha, S.K.; Ogawa, S.; Watanabe, A.; Shahiduzzaman, M.; et al. Muntingia calabura Leaves Mediated Green Synthesis of CuO Nanorods: Exploiting Phytochemicals for Unique Morphology. Materials 2021, 14, 6379. [Google Scholar] [CrossRef]
- Gade, A.; Gaikwad, S.; Duran, N.; Rai, M. Green Synthesis of Silver Nanoparticles by Phoma Glomerata. Micron 2014, 59, 52–59. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, K.G.; Cherkasov, V.R.; Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Surface Plasmon Resonance as a Tool for Investigation of Non-Covalent Nanoparticle Interactions in Heterogeneous Self-Assembly & Disassembly Systems. Biosens. Bioelectron. 2017, 88, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, X.-H.N. Synthesis and Characterization of Tunable Rainbow Colored Colloidal Silver Nanoparticles Using Single-Nanoparticle Plasmonic Microscopy and Spectroscopy. J. Mater. Chem. 2010, 20, 9867–9876. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, D.; Okram, G.S. Influence of Surfactant, Particle Size and Dispersion Medium on Surface Plasmon Resonance of Silver Nanoparticles. J. Phys. Condens. Matter 2020, 32, 145302. [Google Scholar] [CrossRef] [PubMed]
- Saroha, J.; Lalla, N.P.; Kumar, M.; Sharma, S.N. Ultrafast Transient Absorption Spectroscopic Studies on the Impact of Growth Time on Size, Stability, and Optical Characteristics of Colloidal Gold Nanoparticles. Optik 2022, 268, 169759. [Google Scholar] [CrossRef]
- Nassar, A.M.; Alanazi, A.H.; Alzaid, M.M.; Moustafa, S.M.N. Harnessing Wasted Mushroom Peel Aqueous Extract for Mycogenic Synthesis of Zinc Oxide Nanoparticles for Solar Photocatalysis and Antimicrobial Applications. Biomass Conv. Bioref. 2025, 15, 20991–21006. [Google Scholar] [CrossRef]
- Fahmy, N.F.; Abdel-Kareem, M.M.; Ahmed, H.A.; Helmy, M.Z.; Mahmoud, E.A.-R. Evaluation of the Antibacterial and Antibiofilm Effect of Mycosynthesized Silver and Selenium Nanoparticles and Their Synergistic Effect with Antibiotics on Nosocomial Bacteria. Microb. Cell Fact. 2025, 24, 6. [Google Scholar] [CrossRef]
- Hassan, M.G.; Shahin, M.S.A.; Shafie, F.A.; Baraka, D.M.; Hamed, A.A. Myco-Fabricated CuONPs and Ch-CuONPs Conjugate Mediated by the Endophytic Fungus Aspergillus Fumigatus SM4 with in Vitro Antimicrobial, Antibiofilm, Antioxidant and Anticancer Activity. Inorg. Chem. Commun. 2025, 176, 114216. [Google Scholar] [CrossRef]
- Nassar, A.-R.A.; Atta, H.M.; Abdel-Rahman, M.A.; El Naghy, W.S.; Fouda, A. Myco-Synthesized Copper Oxide Nanoparticles Using Harnessing Metabolites of Endophytic Fungal Strain Aspergillus Terreus: An Insight into Antibacterial, Anti-Candida, Biocompatibility, Anticancer, and Antioxidant Activities. BMC Complement. Med. Ther. 2023, 23, 261, 20991–21006. [Google Scholar] [CrossRef]
- Selim, H.M.R.M.; Saleh, A.; Soliman, N.R.; Abdelkhalig, S.M.; Mattar, E.; Mahmoud, M.M.; Diab, N.S.; Hamed, A.A. Myco-Fabricated CuO NPs and Chitosan-Conjugated CuO Nanoparticles Using Endophytic Aspergillus Sp. JAWF3: Targeting E. Coli Outer Membrane for Enhanced Antibacterial and Biofilm Inhibition. Inorg. Chem. Commun. 2025, 178, 114361. [Google Scholar] [CrossRef]
- El-Fawal, M.F.; El-Fallal, A.A.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; El-Zahed, M.M. In Vitro Antibacterial Activity of Myco-Synthesized Selenium Nanoparticles against a Multidrug-Resistant E. Coli Isolated from Clinical Specimens. Discov. Mater. 2025, 5, 130. [Google Scholar] [CrossRef]
- EL-Zawawy, N.A.; Abou-Zeid, A.M.; Beltagy, D.M.; Hantera, N.H.; Nouh, H.S. Mycosynthesis of Silver Nanoparticles from Endophytic Aspergillus Flavipes AUMC 15772: Ovat-Statistical Optimiza-tion, Characterization and Biological Activities. Microb. Cell Factories 2023, 22, 228. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Shweqa, N.S.; Abdelmigid, H.M.; Alyamani, A.A.; Elshafey, N.; Soliman, H.M.; Heikal, Y.M. Myco-Biosynthesis of Silver Nanoparticles, Optimization, Characterization, and In Silico Anticancer Activities by Mo-lecular Docking Approach against Hepatic and Breast Cancer. Biomolecule 2024, 14, 1170. [Google Scholar] [CrossRef]
- Jain, A.; Bhise, K. Nano-Pharmacokinetics and Pharmacodynamics of Green-Synthesized ZnO Nanoparticles: A Pathway to Safer Therapeutic Applications. Xenobiotica 2025, 55, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.M.; Siccardi, M. Optimizing Nanomedicine Pharmacokinetics Using Physiologically Based Pharmacokinetics Modelling. Br. J. Pharmacol. 2014, 171, 3963–3979. [Google Scholar] [CrossRef]
- Al Qutaibi, M.; Kagne, S.R.; Bawazir, A.S.; Aateka Yahya, B. Mushroom-Mediated Biosynthesis of NPs: A Green Approach toward Antimicrobial Applications. CyTA-J. Food 2024, 22, 2413954. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B. Edible Mushroom Extract Engineered Ag NPs as Safe Antimicrobial and Antioxidant Agents with No Significant Cytotoxicity on Human Dermal Fibroblast Cells. Inorg. Chem. Commun. 2022, 139, 109362. [Google Scholar] [CrossRef]
- Sargin, I.; Karakurt, S.; Alkan, S.; Arslan, G. Live Cell Imaging With Biocompatible Fluorescent Carbon Quantum Dots Derived From Edible Mushrooms Agaricus bisporus, Pleurotus ostreatus, and Suillus luteus. J. Fluoresc. 2021, 31, 1461–1473. [Google Scholar] [CrossRef]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnology 2024, 22, 715. [Google Scholar] [CrossRef]
- Fröhlich, E. Role of Omics Techniques in the Toxicity Testing of Nanoparticles. J. Nanobiotechnol. 2017, 15, 84. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Pan, X.; Redding, J.E.; Wiley, P.A.; Wen, L.; McConnell, J.S.; Zhang, B. Mutagenicity Evaluation of Metal Oxide Nanoparticles by the Bacterial Reverse Mutation Assay. Chemosphere 2010, 79, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Mohd Yusoff, A.R. Flavonoids Mediated ‘Green’ Nanomaterials: A Novel Nanomedicine System to Treat Various Diseases—Current Trends and Future Perspective. Mater. Lett. 2018, 210, 26–30. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Arshadi, N.; Nouri, H.; Moghimi, H. Increasing the Production of the Bioactive Compounds in Medicinal Mushrooms: An Omics Perspective. Microb. Cell Fact. 2023, 22, 67. [Google Scholar] [CrossRef]



| Compound Class | Mushroom Source(s) | Key Molecules | Role in NP Synthesis | Nanoparticle Size (nm) | References |
|---|---|---|---|---|---|
| Polysaccharides | A. bisporus, Phellinus linteus, P. ostreatus, G. lucidum | β-glucans, chitin, mannogalactans | Reduction and capping agents; stabilization | 10–70 (AgNPs) | [27,28,29] |
| Proteins & Peptides | G. lucidum, Volvariella volvacea | Proteins (amide linkages, amino acids) | Reduction via amine and carboxylate groups; surface capping | 2–70 (AgNPs, AuNPs) | [24,25,30] |
| Phenolics & Flavonoids | L. edodes, Fomes fomentarius, Schizophyllum commune | Catechins, quercetin, phenolic acids | Antioxidant reduction in metal ions; surface stabilization | 10–20 (AgNPs), 35–200 (ZnO NPs) | [31,32,33] |
| Terpenoids | G. lucidum | Ganoderic acids, triterpenoids, steroids | Electron donation for reduction; surface functionalization | Not specified | [29,34] |
| Unique Phenolics | Lactarius piperatus | Homogentisic acid | Principal phenolic reducing agent; NP stabilization | 33–64 (AgNPs) | [35] |
| Mixed Compounds | G. lucidum | Steroids, alkaloids, sesquiterpenoids, vitamins | Synergistic contribution to reduction and capping | Not specified | [29] |
| Nanoparticle | Mushroom Species | Extract Concentration | Key NP Properties | Ref. |
|---|---|---|---|---|
| AgNPs | Pleurotus spp. | Not specified | 2–100 nm; spherical; antimicrobial, anticancer, dye degradation | [73] |
| FeNPs | Pleurotus florida | Not specified | Color change; antimicrobial vs. bacteria & fungi; comparable to antibiotics | [85] |
| Metal NPs | Pleurotus spp. | Not specified | Intra-/extracellular routes; eco-friendly; antimicrobial, anticancer, antioxidant | [44] |
| ZnNPs | Pleurotus sajor caju | 1 mM ZnSO4 | SPR: 300 nm; spherical; 7–13 nm; FTIR: 675–3675 cm−1 | [86] |
| ZnO NPs | Daedalea sp. | 10 g extract + Zn acetate (1.834 g/100 mL) | 14.6 nm; irregular, agglomerated; hexagonal (XRD); FTIR phenolics; biocompatible | [87] |
| ZnO NPs | Psathyrella candolleana | Varying concentrations | 19–51 nm; sword-like (TEM); antibacterial; photocatalytic MB degradation (80%/60 min) | [75] |
| Criteria | Myco-Synthesis of NPs | Conventional Methods of NPs | Refs. |
|---|---|---|---|
| Methodology | Uses fungi in the live cells or extracts as reducing or stabilizing agents | In chemical methods, it relies on reducing agents (e.g., NaBH4, citrate, vitamin C). In physical methods by laser ablation, ball milling, grinding, or evaporation–condensation | [89,90] |
| Reducing agents | By fungal exudated biomolecules such as amino acids, proteins, or enzymes (mainly reductases), metabolites, and polyphenolic as the reducing agents during the formation of NPs | Mainly in the chemical methods: harsh chemicals (e.g., hydrazine, sodium citrate, ascorbic acid) | [91,92] |
| Yield, stability & NPs size control | Moderate (depends on fungal strain, culture conditions), free from impurities with higher yields, eco-friendly, simple, and cost-effective | High (precise control via concentration/pH/temperature), contamination of the final NPs with chemicals could be observed, along with the production of hazardous by-products. | [92,93] |
| Solvents and stabilizing agents | Aqueous (water-based), natural fungal biomolecules (proteins, polysaccharides) | Often, organic solvents (e.g., toluene, hexane) are used. Synthetic surfactants/polymers (e.g., PVP, PEG) | [94] |
| Costs+ energy requirements | Low costs as they use biomass or waste substrates Low energy requirement as it uses the ambient temperature and pressure | High costs due to the expensive chemicals, energy-intensive, and others High energy requirements, such as high temperature, pressure, and vacuum, for physical methods | [70] |
| Environmental impacts | Safe product, competitive advantages, biodegradable byproducts, economical, eco-friendly as lesser waste | Toxic waste may contain heavy metals, solvents, and non-degradable materials, posing environmental pollution and adverse effects on ecosystems. | [70,91] |
| Scalability | Challenging for large-scale production | Well-established for industrial scale. | [70] |
| Applications | Biochemical, biomedicine, drug delivery, antimicrobials, eco-remediation, photodegradation ability, industrial biofortification, | Electronics, catalysis, optics, biomedical imaging and healthcare applications, energy storage applications, | [70,95,96,97] |
| Mushroom Species | NPs | Size (nm) | Shape | Effective Against | Ref. |
|---|---|---|---|---|---|
| A. bisporus | ZnO-NPs | 15–25 | Spherical/hexagonal | B. cereus, E. coli, E. faecium, P. aeruginosa, A. niger, P. polonicum, P. ultimum, V. dahliae | [115] |
| A. carneus MAK259 | Ag-NPs | 5–26 | Spherical | P. aeruginosa | [116] |
| A. fumigatus SM4 | CuO-NPs | 20–150 | Spherical | S. aureus, B. subtilis, P. aeruginosa, E. coli, C. albicans | [117] |
| A. terreus | Cu-NPs | 4–45 | Spherical | Klebsiella, E. coli | [118] |
| Aspergillus sp. JAWF3 | CuO-NPs | 35–130 | Cuboid | E. coli, P. aeruginosa, S. aureus, B. subtilis | [119] |
| F. fujikuroi MED14 | Se-NPs | 10–19 | Spherical | E. coli, B. cereus | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; El-Ramady, H.; Nguyen, D.H.H.; Alibrahem, W.; Kharrat Helu, N.; Atieh, R.; Muthu, A.; Jevcsák, S.; Semsey, D.; Abdalla, N.; et al. Green-Synthesized Nanomaterials from Edible and Medicinal Mushrooms: A Sustainable Strategy Against Antimicrobial Resistance. Pharmaceutics 2025, 17, 1388. https://doi.org/10.3390/pharmaceutics17111388
Törős G, El-Ramady H, Nguyen DHH, Alibrahem W, Kharrat Helu N, Atieh R, Muthu A, Jevcsák S, Semsey D, Abdalla N, et al. Green-Synthesized Nanomaterials from Edible and Medicinal Mushrooms: A Sustainable Strategy Against Antimicrobial Resistance. Pharmaceutics. 2025; 17(11):1388. https://doi.org/10.3390/pharmaceutics17111388
Chicago/Turabian StyleTörős, Gréta, Hassan El-Ramady, Duyen H. H. Nguyen, Walaa Alibrahem, Nihad Kharrat Helu, Reina Atieh, Arjun Muthu, Szintia Jevcsák, Dávid Semsey, Neama Abdalla, and et al. 2025. "Green-Synthesized Nanomaterials from Edible and Medicinal Mushrooms: A Sustainable Strategy Against Antimicrobial Resistance" Pharmaceutics 17, no. 11: 1388. https://doi.org/10.3390/pharmaceutics17111388
APA StyleTörős, G., El-Ramady, H., Nguyen, D. H. H., Alibrahem, W., Kharrat Helu, N., Atieh, R., Muthu, A., Jevcsák, S., Semsey, D., Abdalla, N., Elsakhawy, T., Tóth, A. F., Nagy, P. T., & Prokisch, J. (2025). Green-Synthesized Nanomaterials from Edible and Medicinal Mushrooms: A Sustainable Strategy Against Antimicrobial Resistance. Pharmaceutics, 17(11), 1388. https://doi.org/10.3390/pharmaceutics17111388












