Engineering Inorganic Nanoparticles to Induce Cuproptosis: A New Strategy for Cancer Therapy
Abstract
1. Introduction
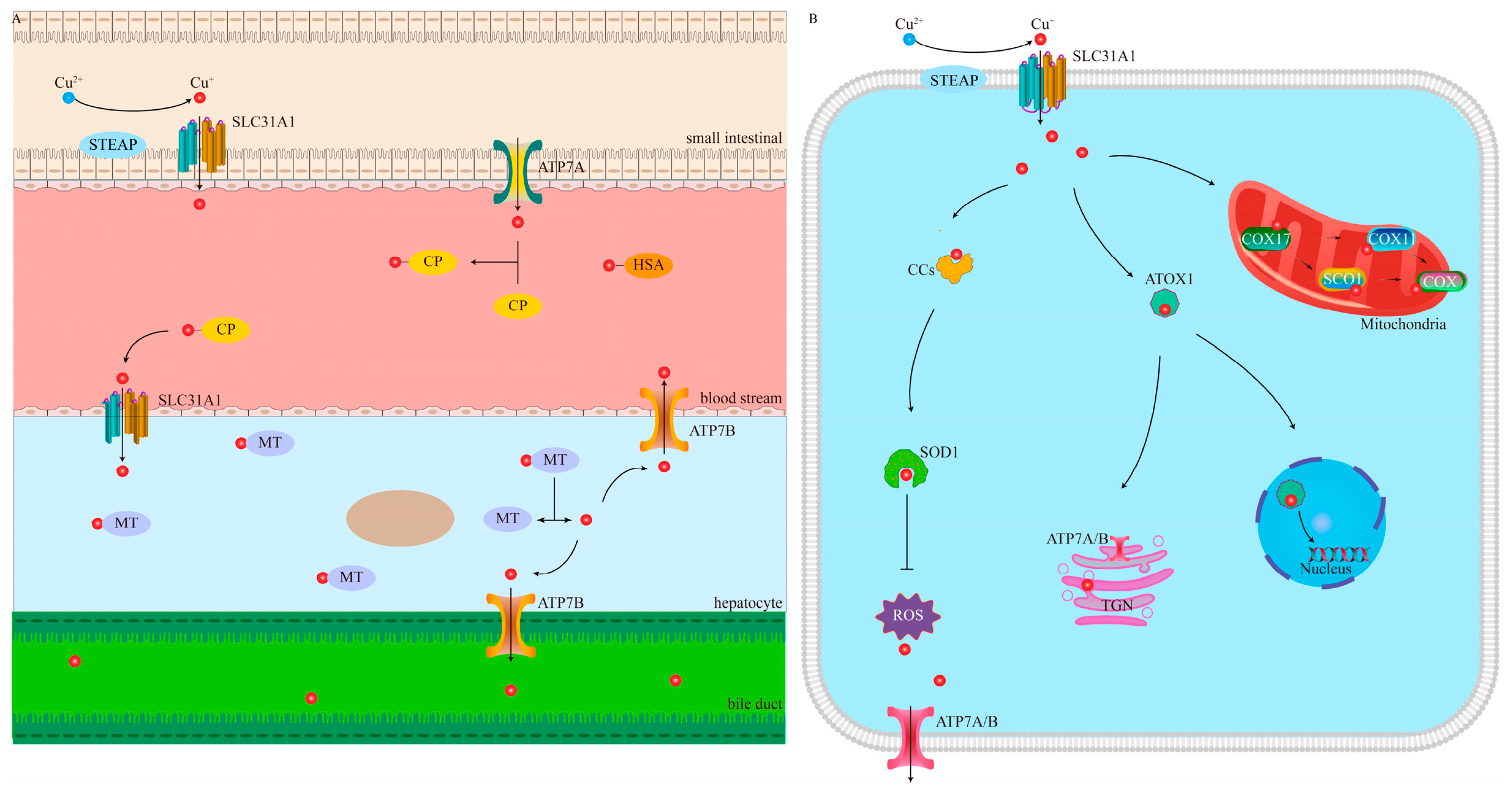
1.1. Cu Homeostasis
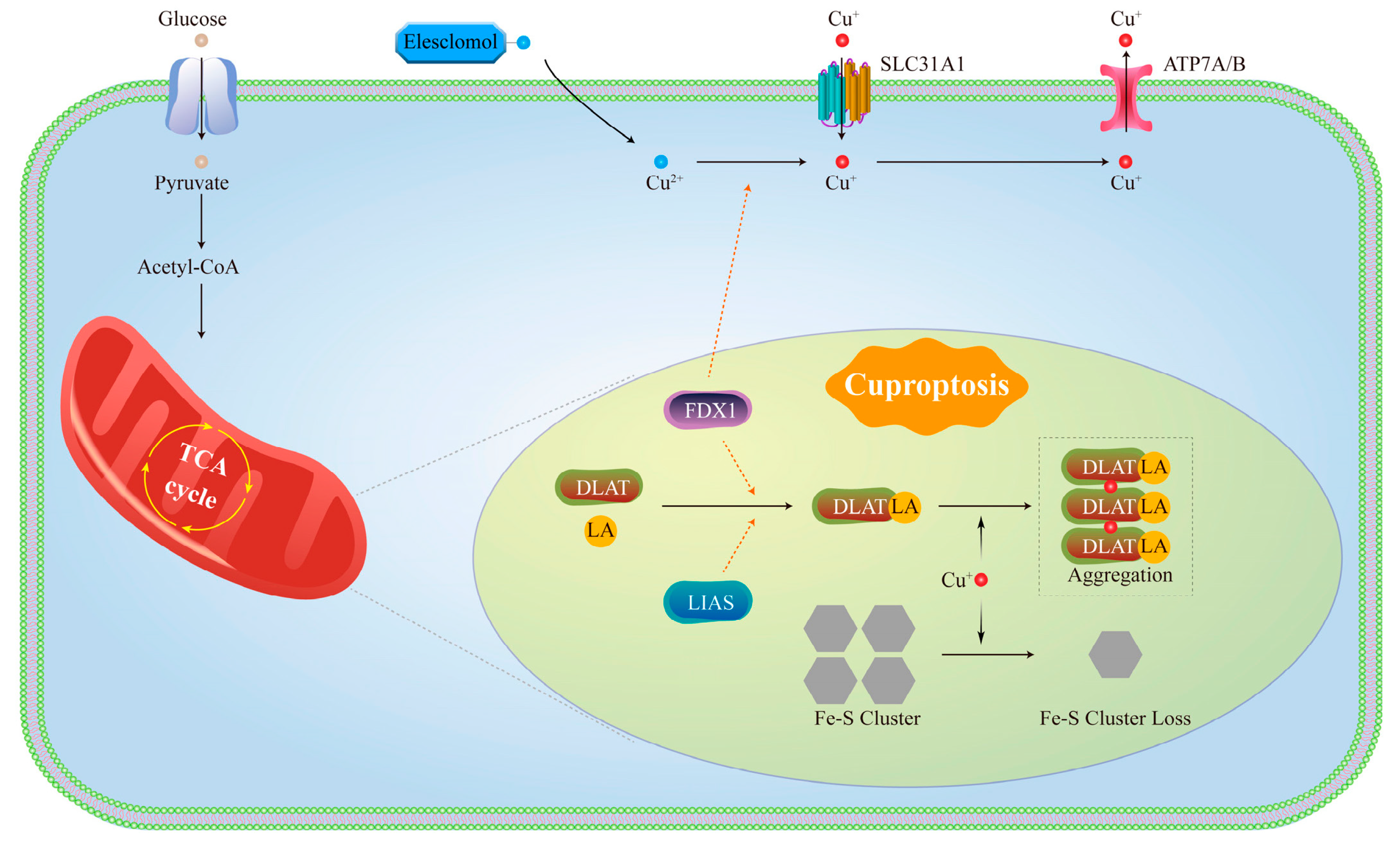
1.2. Molecular Mechanisms of Cuproptosis
2. Targeted Cuproptosis for Cancer Therapy
3. Strategies for Synergistic Induction of Cuproptosis Based on INPs
3.1. Enhancing Cu Delivery Efficiency
3.2. Increasing Cu+ Generation
3.3. Reducing Cu2+ Chelation
3.4. Metabolic Reprogramming
4. Synergistic Cuproptosis-Multimodal Antitumor Therapy Mediated by INPs
4.1. Cuproptosis Combined with IT
4.2. Cuproptosis Combined with PTT
4.3. Cuproptosis Combined with Sonodynamic Therapy
4.4. Cuproptosis Combined with RT
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 2021, 35, e21810. [Google Scholar] [CrossRef]
- Shan, D.; Song, J.; Ren, Y.; Zhang, Y.; Ba, Y.; Luo, P.; Cheng, Q.; Xu, H.; Weng, S.; Zuo, A.; et al. Copper in cancer: Friend or foe? Metabolism, dysregulation, and therapeutic opportunities. Cancer Commun. 2025, 45, 577–607. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Robinson, N.J.; Winge, D.R. Copper metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Keyes, W.R.; Kim, S.K.; Domek, J.M. Long-term high copper intake: Effects on copper absorption, retention, and homeostasis in men. Am. J. Clin. Nutr. 2005, 81, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.H.; Kama, J.A.; Lieberman, G.; Chopra, R.; Dorsey, K.; Chopra, V.; Volitakis, I.; Cherny, R.A.; Bush, A.I.; Hersch, S. Mechanisms of copper ion mediated Huntington’s disease progression. PLoS ONE 2007, 2, e334. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Simonelli, I.; Ventriglia, M.; Siotto, M.; Pasqualetti, P.; Rembach, A.; Doecke, J.; Bush, A.I. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 38, 809–822. [Google Scholar] [CrossRef]
- Ford, E.S. Serum copper concentration and coronary heart disease among US adults. Am. J. Epidemiol. 2000, 151, 1182–1188. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, T.; Li, L. Targeting cuproptosis for cancer therapy: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 68. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, C.; Ma, X.; Wang, Y.; Li, Z.; Xu, Y.; Chen, T.; Gao, C.; Ye, X.; Wu, A.; et al. Advances in cuproptosis harnessing copper-based nanomaterials for cancer therapy. J. Mater. Chem. B 2025, 13, 2978–2999. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.A.; Cherian, M.G. Differential regulation of signal transduction pathways in wild type and mutated p53 breast cancer epithelial cells by copper and zinc. Arch. Biochem. Biophys. 2004, 423, 351–361. [Google Scholar] [CrossRef]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [PubMed]
- Parr-Sturgess, C.A.; Tinker, C.L.; Hart, C.A.; Brown, M.D.; Clarke, N.W.; Parkin, E.T. Copper modulates zinc metalloproteinase-dependent ectodomain shedding of key signaling and adhesion proteins and promotes the invasion of prostate cancer epithelial cells. Mol. Cancer Res. 2012, 10, 1282–1293. [Google Scholar] [CrossRef]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef]
- Xie, H.; Kang, Y.J. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Lu, L.; Liu, B.; Ding, Y. Elesclomol: A copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res. 2022, 41, 271. [Google Scholar] [CrossRef]
- Gao, J.; Wu, X.; Huang, S.; Zhao, Z.; He, W.; Song, M. Novel insights into anticancer mechanisms of elesclomol: More than a prooxidant drug. Redox Biol. 2023, 67, 102891. [Google Scholar] [CrossRef]
- Wang, W.; Mo, W.; Hang, Z.; Huang, Y.; Yi, H.; Sun, Z.; Lei, A. Cuproptosis: Harnessing Transition Metal for Cancer Therapy. ACS Nano 2023, 17, 19581–19599. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G.; Kang, R. Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol. 2024, 21, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Huo, M.; Wang, L.; Chen, Y.; Chen, W.; Wang, B. Tumor-responsive copper-activated disulfiram for synergetic nanocatalytic tumor therapy. Nano Res. 2021, 14, 205–211. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.Y.; Zeng, L.; Ma, H.; Zhang, Y.; Yang, H.; Liu, Y.; Fang, S.; Zhao, J.; Xu, Y.; et al. An Enzyme-Engineered Nonporous Copper(I) Coordination Polymer Nanoplatform for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Mater. 2022, 34, e2204733. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in tumor immunotherapy: New strategies and challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Yang, J.L.; Koshy, P.; Unnikrishnan, A.; Sorrell, C.C. Inorganic nanoparticle-based advanced cancer therapies: Promising combination strategies. Drug Discov. Today 2022, 27, 103386. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, M.; Manifar, S.; Akkafan, R.; Mortazavi, H.; Sabour, S. Serum levels of ferritin, copper, and zinc in patients with oral cancer. Biomed. J. 2014, 37, 331–336. [Google Scholar] [CrossRef]
- Feng, J.F.; Lu, L.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W.; Wang, D. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int. J. Clin. Oncol. 2012, 17, 575–583. [Google Scholar] [CrossRef]
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, V.K.; Vaidya, M.P.; Roy, S.K.; Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 1993, 52, 172–175. [Google Scholar] [CrossRef]
- Mason, K.E. A conspectus of research on copper metabolism and requirements of man. J. Nutr. 1979, 109, 1979–2066. [Google Scholar] [CrossRef]
- Lönnerdal, B. Intestinal regulation of copper homeostasis: A developmental perspective. Am. J. Clin. Nutr. 2008, 88, 846s–850s. [Google Scholar] [CrossRef]
- Cabrera, A.; Alonzo, E.; Sauble, E.; Chu, Y.L.; Nguyen, D.; Linder, M.C.; Sato, D.S.; Mason, A.Z. Copper binding components of blood plasma and organs, and their responses to influx of large doses of 65Cu, in the mouse. Biometals 2008, 21, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S. Dynamic and cell-specific transport networks for intracellular copper ions. J. Cell Sci. 2021, 134, jcs240523. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Tsuchiya, Y.; García-Ruiz, J.P.; Lalioti, V.; Nielsen, S.; Cassio, D.; Sandoval, I.V. ATP7B copper-regulated traffic and association with the tight junctions: Copper excretion into the bile. Gastroenterology 2008, 134, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP proteins: From structure to applications in cancer therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef]
- Maia, C.J.; Socorro, S.; Schmitt, F.; Santos, C.R. STEAP1 is over-expressed in breast cancer and down-regulated by 17beta-estradiol in MCF-7 cells and in the rat mammary gland. Endocrine 2008, 34, 108–116. [Google Scholar] [CrossRef]
- Alves, P.M.S.; Faure, O.; Graff-Dubois, S.; Cornet, S.; Bolonakis, I.; Gross, D.-A.; Miconnet, I.; Chouaib, S.; Fizazi, K.; Soria, J.C.; et al. STEAP, a prostate tumor antigen, is a target of human CD8+ T cells. Cancer Immunol. Immunother. 2006, 55, 1515–1523. [Google Scholar] [CrossRef]
- Hubert, R.S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell, S.C.; Madraswala, R.; Zhou, Y.; Kuo, J.; Raitano, A.B.; et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 14523–14528. [Google Scholar] [CrossRef]
- Cai, Q.; Jing, C.; Wang, X.; Xing, X.; Liu, W. STEAP Proteins: Roles in disease biology and potential for therapeutic intervention. Int. J. Biol. Macromol. 2025, 309, 142797. [Google Scholar] [CrossRef]
- Casareno, R.L.; Waggoner, D.; Gitlin, J.D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Biol. Chem. 1998, 273, 23625–23628. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Z.; Zhao, J.; Lei, J.; Chen, Y.; Li, X.; Chen, H.; Tian, J.; Zhang, D.; Liu, C.; et al. The rational design of specific SOD1 inhibitors via copper coordination and their application in ROS signaling research. Chem. Sci. 2016, 7, 6251–6262. [Google Scholar] [CrossRef] [PubMed]
- Dodani, S.C.; Leary, S.C.; Cobine, P.A.; Winge, D.R.; Chang, C.J. A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J. Am. Chem. Soc. 2011, 133, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Horng, Y.C.; Cobine, P.A.; Maxfield, A.B.; Carr, H.S.; Winge, D.R. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem. 2004, 279, 35334–35340. [Google Scholar] [CrossRef]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef]
- La Fontaine, S.; Mercer, J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 149–167. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, M.J.; van Rooy, M.; Bester, M.J.; Serem, J.C.; Venter, C.; Oberholzer, H.M. Oxidative and haemostatic effects of copper, manganese and mercury, alone and in combination at physiologically relevant levels: An ex vivo study. Hum. Exp. Toxicol. 2019, 38, 419–433. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Sheu, J.Y.; Lin, T.H. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin. Biochem. 1999, 32, 131–136. [Google Scholar] [CrossRef]
- Díez, M.; Arroyo, M.; Cerdàn, F.J.; Muñoz, M.; Martin, M.A.; Balibrea, J.L. Serum and tissue trace metal levels in lung cancer. Oncology 1989, 46, 230–234. [Google Scholar] [CrossRef]
- Rizk, S.L.; Sky-Peck, H.H. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984, 44, 5390–5394. [Google Scholar] [PubMed]
- Carpentieri, U.; Myers, J.; Thorpe, L.; Daeschner, C.W., III; Haggard, M.E. Copper, zinc, and iron in normal and leukemic lymphocytes from children. Cancer Res. 1986, 46, 981–984. [Google Scholar] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Lin, W.R.; Yan, L.; Xu, W.H.; Yang, J. Copper homeostasis and cuproptosis in cancer immunity and therapy. Immunol. Rev. 2024, 321, 211–227. [Google Scholar] [CrossRef]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, e2004303. [Google Scholar] [CrossRef]
- Safaei, R.; Holzer, A.K.; Katano, K.; Samimi, G.; Howell, S.B. The role of copper transporters in the development of resistance to Pt drugs. J. Inorg. Biochem. 2004, 98, 1607–1613. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Yadav, A.A.; Patel, D.; Wu, X. The cytotoxicity of the anticancer drug elesclomol is due to oxidative stress indirectly mediated through its complex with Cu(II). J. Inorg. Biochem. 2014, 137, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, J.R.; He, S.; Balasubramanyam, V.; Kepros, J.; Yang, C.Y.; Zhang, M.; Du, Z.; Barsoum, J.; Bertin, J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 2008, 7, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.S.; Jung, K.H.; Park, J.W.; Lee, K.H. Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef]
- Morrison, B.W.; Doudican, N.A.; Patel, K.R.; Orlow, S.J. Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res. 2010, 20, 11–20. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, Y.; Chen, J.; Jin, Y. Disulfiram Chelated with Copper Promotes Apoptosis in Osteosarcoma via ROS/Mitochondria Pathway. Biol. Pharm. Bull. 2021, 44, 1557–1564. [Google Scholar] [CrossRef]
- Buccarelli, M.; D’Alessandris, Q.G.; Matarrese, P.; Mollinari, C.; Signore, M.; Cappannini, A.; Martini, M.; D’Aliberti, P.; De Luca, G.; Pedini, F.; et al. Elesclomol-induced increase of mitochondrial reactive oxygen species impairs glioblastoma stem-like cell survival and tumor growth. J. Exp. Clin. Cancer Res. 2021, 40, 228. [Google Scholar] [CrossRef]
- Rowland, E.A.; Snowden, C.K.; Cristea, I.M. Protein lipoylation: An evolutionarily conserved metabolic regulator of health and disease. Curr. Opin. Chem. Biol. 2018, 42, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, J.; Kabin, E.; Järving, I.; Bragina, O.; Tõugu, V.; Plitz, T.; Palumaa, P. Copper(I)-binding properties of de-coppering drugs for the treatment of Wilson disease. α-Lipoic acid as a potential anti-copper agent. Sci. Rep. 2018, 8, 1463. [Google Scholar] [CrossRef]
- Brancaccio, D.; Gallo, A.; Piccioli, M.; Novellino, E.; Ciofi-Baffoni, S.; Banci, L. [4Fe-4S] Cluster Assembly in Mitochondria and Its Impairment by Copper. J. Am. Chem. Soc. 2017, 139, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Huang, C.; Radi, R.H.; Arbiser, J.L. Mitochondrial Metabolism in Melanoma. Cells 2021, 10, 3197. [Google Scholar] [CrossRef]
- Stubbins, R.J.; Maksakova, I.A.; Sanford, D.S.; Rouhi, A.; Kuchenbauer, F. Mitochondrial metabolism: Powering new directions in acute myeloid leukemia. Leuk. Lymphoma 2021, 62, 2331–2341. [Google Scholar] [CrossRef]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Palacios-Zambrano, S.; Moreno-Villa, M.R.; Ruiz-Valdepeñas, A.M.; Lendinez, C.; et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic. Biol. Med. 2019, 135, 167–181. [Google Scholar] [CrossRef]
- Denise, C.; Paoli, P.; Calvani, M.; Taddei, M.L.; Giannoni, E.; Kopetz, S.; Kazmi, S.M.; Pia, M.M.; Pettazzoni, P.; Sacco, E.; et al. 5-fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget 2015, 6, 41706–41721. [Google Scholar] [CrossRef] [PubMed]
- Gohil, V.M. Repurposing elesclomol, an investigational drug for the treatment of copper metabolism disorders. Expert Opin. Investig. Drugs 2021, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.M.; Soma, S.; Yuan, S.; Silva, A.; Zulkifli, M.; Snavely, T.C.; Greene, H.F.; Nunez, E.; Lynch, B.; De Ville, C.; et al. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science 2020, 368, 620–625. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Ren, Y.; Zhang, X. Disulfiram: A novel repurposed drug for cancer therapy. Cancer Chemother. Pharmacol. 2021, 87, 159–172. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef]
- Werlenius, K.; Kinhult, S.; Solheim, T.S.; Magelssen, H.; Löfgren, D.; Mudaisi, M.; Hylin, S.; Bartek, J., Jr.; Strandéus, M.; Lindskog, M.; et al. Effect of Disulfiram and Copper Plus Chemotherapy vs Chemotherapy Alone on Survival in Patients with Recurrent Glioblastoma: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e234149. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Zhang, M.; Lang, P.; Li, J.; Liu, Z.; Zhang, Z.; Li, L.; Zhang, L. Cuproptosis-immunotherapy using PD-1 overexpressing T cell membrane-coated nanosheets efficiently treats tumor. J. Control. Release 2023, 362, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- McGinnis, C.S.; Miao, Z.; Superville, D.; Yao, W.; Goga, A.; Reticker-Flynn, N.E.; Winkler, J.; Satpathy, A.T. The temporal progression of lung immune remodeling during breast cancer metastasis. Cancer Cell 2024, 42, 1018–1031.e1016. [Google Scholar] [CrossRef]
- Phuengkham, H.; Ren, L.; Shin, I.W.; Lim, Y.T. Nanoengineered Immune Niches for Reprogramming the Immunosuppressive Tumor Microenvironment and Enhancing Cancer Immunotherapy. Adv. Mater. 2019, 31, e1803322. [Google Scholar] [CrossRef]
- Yin, Y.; Feng, W.; Chen, J.; Chen, X.; Wang, G.; Wang, S.; Xu, X.; Nie, Y.; Fan, D.; Wu, K.; et al. Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: From bench to bedside. Exp. Hematol. Oncol. 2024, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Trivedi, R.; Lin, S.Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Jiang, A.; Luo, P.; Chen, M.; Fang, Y.; Liu, B.; Wu, Z.; Qu, L.; Wang, A.; Wang, L.; Cai, C. A new thinking: Deciphering the aberrance and clinical implication of copper-death signatures in clear cell renal cell carcinoma. Cell Biosci. 2022, 12, 209. [Google Scholar] [CrossRef]
- Yan, S.; Xue, P.; Sun, Y.; Bai, T.; Shao, S.; Zeng, X. Cupric Doping Hollow Prussian Blue Nanoplatform for Enhanced Cholesterol Depletion: A Promising Strategy for Breast Cancer Therapy and Metastasis Inhibition. Adv. Sci. 2025, 12, e2409967. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, e44. [Google Scholar] [CrossRef]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomedicine 2016, 12, 1663–1701. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Cheng, J.; Yuan, Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. Int. J. Environ. Res. Public Health 2016, 13, 1182. [Google Scholar] [CrossRef]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J. Hollow-structured mesoporous materials: Chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, S.; Li, C.; Yao, J.; Xie, Y.; Jiang, C.; Yuan, J.; Fei, K.; Zhang, P.; Wang, H.; et al. Cuproptosis and Serine Metabolism Blockade Triggered by Copper-Based Prussian Blue Nanomedicine for Enhanced Tumor Therapy. Small 2025, 21, e2406942. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Tong, B.; Zhang, W.; Wang, X.; Wang, Y.; Tian, G.; Xu, Z.; Zhang, G. Biomimetic Nano-Regulator that Induces Cuproptosis and Lactate-Depletion Mediated ROS Storm for Metalloimmunotherapy of Clear Cell Renal Cell Carcinoma. Adv. Healthc. Mater. 2024, 13, e2400204. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting. Sci. Transl. Med. 2011, 3, 64ra62. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, Q.; Song, J.; Li, S.; Li, X.L.; Kang, B.K.; Chen, H.Y.; Xu, J.J. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. Int. Ed. 2023, 62, e202213922. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, H.; Hong, R.; Gong, J.; Wan, Y.; Fu, Q.; Huang, K.; Li, Y.; Wang, N.; Zhao, P.; et al. A self-accelerating ‘copper bomb’ strategy activated innate and adaptive immune response against triple-negative breast cancer. Bioact. Mater. 2025, 49, 193–206. [Google Scholar] [CrossRef]
- Zhao, F.; Liang, L.; Wang, H.; Wang, C.; Su, D.; Ying, Y.; Li, W.; Li, J.; Zheng, J.; Qiao, L.; et al. H2S-Activated Ion-Interference Therapy: A Novel Tumor Targeted Therapy Based on Copper-Overload-Mediated Cuproptosis and Pyroptosis. Adv. Funct. Mater. 2023, 33, 2300941. [Google Scholar] [CrossRef]
- Cen, D.; Brayton, D.; Shahandeh, B.; Meyskens, F.L., Jr.; Farmer, P.J. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem. 2004, 47, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pan, Q.; Gao, W.; Pu, Y.; Luo, K.; He, B.; Gu, Z. Leveraging disulfiram to treat cancer: Mechanisms of action, delivery strategies, and treatment regimens. Biomaterials 2022, 281, 121335. [Google Scholar] [CrossRef]
- Zhao, W.N.; Li, H.; Sun, S.; Xu, Y. The construction of hierarchical assemblies with in situ generation of chemotherapy drugs to enhance the efficacy of chemodynamic therapy for multi-modal anti-tumor treatments. J. Mater. Chem. B 2023, 11, 11044–11051. [Google Scholar] [CrossRef]
- Xue, G.; Qin, B.; Ma, C.; Yin, P.; Liu, C.; Liu, K. Large-Area Epitaxial Growth of Transition Metal Dichalcogenides. Chem. Rev. 2024, 124, 9785–9865. [Google Scholar] [CrossRef]
- Sayed, M.; Qi, K.; Wu, X.; Zhang, L.; García, H.; Yu, J. Cu-based S-scheme photocatalysts. Chem. Soc. Rev. 2025, 54, 4874–4921. [Google Scholar] [CrossRef]
- Xia, J.; Hu, C.; Ji, Y.; Wang, M.; Jin, Y.; Ye, L.; Xie, D.; Jiang, S.; Li, R.; Hu, Z.; et al. Copper-Loaded Nanoheterojunction Enables Superb Orthotopic Osteosarcoma Therapy via Oxidative Stress and Cell Cuproptosis. ACS Nano 2023, 17, 21134–21152. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, C.; Tan, H.; Dong, S.; Ren, Y.; Chao, M.; Yan, H.; Yan, X.; Jiang, G.; Gao, F. A Stimulus-Responsive Ternary Heterojunction Boosting Oxidative Stress, Cuproptosis for Melanoma Therapy. Small 2024, 20, e2401147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, W.; Zhang, W.; Zhang, J.; Li, Z.; Guo, Y.; Chen, H.Y.; Xu, J.J. Nanoenhanced-Cuproptosis Results From the Synergy of Calcium Overload and GSH Depletion with the Increasing of Intracellular Ca/Mn/Cu Ions. Adv. Sci. 2025, 12, e2412067. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zu, H.; Wu, Y.; Meng, H.; Cheng, X.; Wang, Y.; Zhang, L.W.; Wang, Y. Tumor Metabolism Aiming Cu2−xS Nanoagents Mediate Photothermal-Derived Cuproptosis and Immune Activation. ACS Nano 2024, 18, 23941–23957. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, Y.; Weichselbaum, R.R.; Lin, W. Nanoparticles Synergize Ferroptosis and Cuproptosis to Potentiate Cancer Immunotherapy. Adv. Sci. 2024, 11, e2310309. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, J.; Wu, G.; Xiong, W.; Feng, J.; Yan, C.; Yang, J.; Li, Z.; Fan, Q.; Ren, B.; et al. A strategy of “adding fuel to the flames” enables a self-accelerating cycle of ferroptosis-cuproptosis for potent antitumor therapy. Biomaterials 2024, 311, 122701. [Google Scholar] [CrossRef]
- Deng, J.; Zhuang, H.; Shao, S.; Zeng, X.; Xue, P.; Bai, T.; Wang, X.; Shangguan, S.; Chen, Y.; Yan, S.; et al. Mitochondrial-Targeted Copper Delivery for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Healthc. Mater. 2024, 13, e2304522. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Zhu, Y.; Chen, H.; Tan, J.; Yang, Z.; Li, J.; Zheng, P.; Feng, L.; Wang, Q.; et al. One-Step Symbiosis of Bimetallic Peroxides Nanoparticles to Induce Ferroptosis/Cuproptosis and Activate cGAS-STING Pathway for Enhanced Tumor Immunotherapy. Adv. Mater. 2025, 37, e2500337. [Google Scholar] [CrossRef]
- Xie, L.; Gong, J.; He, Z.; Zhang, W.; Wang, H.; Wu, S.; Wang, X.; Sun, P.; Cai, L.; Wu, Z.; et al. A Copper-Manganese Based Nanocomposite Induces Cuproptosis and Potentiates Anti-Tumor Immune Responses. Small 2025, 21, e2412174. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Dong, Y.; Wang, B.; Wang, T.; Zhang, A.; Li, S.; Chen, R.; Su, Y.; Jiang, T.; Zhao, X. Dual Metal Nanoflower Oxygen Pump Microneedles Based on Cuproptosis and STING Pathway Activation for Cancer Immunotherapy. Small 2025, 21, e2409187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, H.; Zhao, H.; Yin, C.; Xing, Y.; Wang, J.; Chi, L.; Ye, L.; Gu, W. Tumor microenvironment-reprogrammable CpG-templated copper sulfide loaded with disulfiram for sensitized cuproptosis immunotherapy. Chem. Eng. J. 2024, 487, 150524. [Google Scholar] [CrossRef]
- Lu, X.; Chen, X.; Lin, C.; Yi, Y.; Zhao, S.; Zhu, B.; Deng, W.; Wang, X.; Xie, Z.; Rao, S.; et al. Elesclomol Loaded Copper Oxide Nanoplatform Triggers Cuproptosis to Enhance Antitumor Immunotherapy. Adv. Sci. 2024, 11, e2309984. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Zheng, X.; Wang, D.; Ni, H.; Chen, W.; Wang, K. A Smart Nanomedicine Unleashes a Dual Assault of Glucose Starvation and Cuproptosis to Supercharge αPD-L1 Therapy. Adv. Sci. 2025, 12, e2411378. [Google Scholar] [CrossRef]
- Qiao, L.; Ou, Y.; Li, L.; Wu, S.; Guo, Y.; Liu, M.; Yu, D.; Chen, Q.; Yuan, J.; Wei, C.; et al. H2S-driven chemotherapy and mild photothermal therapy induced mitochondrial reprogramming to promote cuproptosis. J. Nanobiotechnol. 2024, 22, 205. [Google Scholar] [CrossRef]
- Cheng, R.; Li, Z.; Luo, W.; Chen, H.; Deng, T.; Gong, Z.; Zheng, Q.; Li, B.; Zeng, Y.; Wang, H.; et al. A Copper-Based Photothermal-Responsive Nanoplatform Reprograms Tumor Immunogenicity via Self-Amplified Cuproptosis for Synergistic Cancer Therapy. Adv. Sci. 2025, 12, e2500652. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Tian, B.; Yang, M.; Dong, Y.; Zhou, B.; Gai, S.; Xie, Y.; Lin, J. A singular plasmonic-thermoelectric hollow nanostructure inducing apoptosis and cuproptosis for catalytic cancer therapy. Nat. Commun. 2024, 15, 7499. [Google Scholar] [CrossRef]
- Song, Y.; Zhan, G.; Zhou, S.-F. Design of Near Infrared Light-Powered Copper Phyllosilicate Nanomotors for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Funct. Mater. 2024, 34, 2314568. [Google Scholar] [CrossRef]
- Chen, W.; Xie, W.; Gao, Z.; Lin, C.; Tan, M.; Zhang, Y.; Hou, Z. Mild-Photothermal Effect Induced High Efficiency Ferroptosis-Boosted-Cuproptosis Based on Cu2O@Mn3Cu3O8 Nanozyme. Adv. Sci. 2023, 10, e2303694. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, L.; Hao, Y.; Yang, H.; Zhao, W.; Mao, C. Biodegradable CuMoO4 Nanodots with Multienzyme Activities for Multimodal Treatment of Tumor. Adv. Healthc. Mater. 2023, 12, e2300167. [Google Scholar] [CrossRef]
- Shang, W.; Xia, X.; Zhu, Y.; Chen, Q.; Rao, X.; Huang, L.; Tu, Y.; Chen, H.; Tian, H.; Lin, M.; et al. Three-Level Nanoparticle Rocket Strategy for Colorectal Cancer Therapeutics in Photothermal Therapy, Inflammation Modulation, and Cuproptosis Induction. Adv. Healthc. Mater. 2025, 14, e2403939. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, H.; Cao, X.; Tong, Q.; Yang, F.; Miao, Y.; Ye, D.; Fan, Q. Bioorthogonal Cu Single-Atom Nanozyme for Synergistic Nanocatalytic Therapy, Photothermal Therapy, Cuproptosis and Immunotherapy. Angew. Chem. Int. Ed. 2024, 63, e202405937. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xuan, W.; Ou, Y.; Li, L.; Wu, S.; Guo, Y.; Liu, M.; Yu, D.; Chen, Q.; Yuan, J.; et al. Tumor microenvironment activation amplify oxidative stress promoting tumor energy remodeling for mild photothermal therapy and cuproptosis. Redox Biol. 2024, 75, 103260. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Y.; Zhang, L.; Lan, X.; Ren, X.; Liang, W.; Wang, S.; Wang, Y.; Zhao, Y.; Zhang, Y.; et al. Defect-Engineered photothermal nanozyme with NIR-II absorption induces Cuproptosis-Apoptosis for synergized cancer immunotherapy and fast wound healing. Mater. Des. 2024, 237, 112568. [Google Scholar] [CrossRef]
- Ye, L.; Yu, C.; Xia, J.; Ni, K.; Zhang, Y.; Ying, X.; Xie, D.; Jin, Y.; Sun, R.; Tang, R.; et al. Multifunctional nanomaterials via cell cuproptosis and oxidative stress for treating osteosarcoma and OS-induced bone destruction. Mater. Today Bio 2024, 25, 100996. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Du, J.; Meng, Q.; Li, Y.; Miao, Y.; Miao, Q.; Wu, J. A p-n heterojunction sonosensitizer for improved sono-immunotherapy via induction of multimodal cell death mechanisms. Theranostics 2025, 15, 2737–2756. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Y.; You, Q.; Lei, Z.; Ji, J.; Zhang, F.; Dong, W.F.; Li, L. Metal-Doping Strategy for Carbon-Based Sonosensitizer in Sonodynamic Therapy of Glioblastoma. Adv. Sci. 2024, 11, e2404230. [Google Scholar] [CrossRef]
- Yan, L.; Chang, L.; Tian, Y.; Hu, J.; Cao, Z.; Guo, X.; Geng, B. Graphene Quantum Dot Sensitized Heterojunctions Induce Tumor-Specific Cuproptosis to Boost Sonodynamic and Chemodynamic Enhanced Cancer Immunotherapy. Adv. Sci. 2025, 12, e2410606. [Google Scholar] [CrossRef]
- Tang, W.; Wu, J.; Wang, L.; Wei, K.; Pei, Z.; Gong, F.; Chen, L.; Han, Z.; Yang, Y.; Dai, Y.; et al. Bioactive Layered Double Hydroxides for Synergistic Sonodynamic/Cuproptosis Anticancer Therapy with Elicitation of the Immune Response. ACS Nano 2024, 18, 10495–10508. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, D.; Gu, C.; Wang, X.; Zhu, S.; Zheng, Z.; Zhang, F.; Yan, J.; Gu, Z. A cuproptosis nanocapsule for cancer radiotherapy. Nat. Nanotechnol. 2024, 19, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Huang, W.; Ma, H.; Zhang, S.; Cai, Z.; Lin, W.; Zheng, J. Tumor Microenvironment Reprogrammed Bimetallic Hybrid Nanostimulator for Triggering Radio-Cuproptosis-Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2401902. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wen, W.; Morgans, A.K.; Pao, W.; Shu, X.O.; Zheng, W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015, 1, 88–96. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Zhu, J.; Chen, Z.; Yu, L.; Huang, X.; Dong, C.; Li, J.; Zhou, H.; Yang, Y.; et al. Enzyme Core Spherical Nucleic Acid That Enables Enhanced Cuproptosis and Antitumor Immune Response through Alleviating Tumor Hypoxia. J. Am. Chem. Soc. 2024, 146, 13805–13816. [Google Scholar] [CrossRef] [PubMed]
- Voli, F.; Valli, E.; Lerra, L.; Kimpton, K.; Saletta, F.; Giorgi, F.M.; Mercatelli, D.; Rouaen, J.R.C.; Shen, S.; Murray, J.E.; et al. Intratumoral Copper Modulates PD-L1 Expression and Influences Tumor Immune Evasion. Cancer Res. 2020, 80, 4129–4144. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, Y.; Li, D.; Wang, L.; Liang, Z.; Chen, Y.; Ma, G.; Wu, H.; Jiao, W.; Niu, H. Glucose metabolism involved in PD-L1-mediated immune escape in the malignant kidney tumour microenvironment. Cell Death Discov. 2021, 7, 15. [Google Scholar] [CrossRef]
- Chen, P.; Ma, Y.; Zheng, Z.; Wu, C.; Wang, Y.; Liang, G. Facile syntheses of conjugated polymers for photothermal tumour therapy. Nat. Commun. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, T.; Wang, Q.; Lv, X.; Song, X.; Ke, H.; Guo, Z.; Huang, X.; Hu, J.; Li, Z.; et al. Albumin-coordinated assembly of clearable platinum nanodots for photo-induced cancer theranostics. Biomaterials 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Li, X.; Shan, J.; Zhang, W.; Su, S.; Yuwen, L.; Wang, L. Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets. Small 2017, 13, 1602660. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Jacobson, O.; Wang, Z.; Liu, Y.; Lin, L.; Lin, J.; Lu, N.; Zhang, H.; Tian, R.; et al. Biomineralization-Inspired Synthesis of Copper Sulfide-Ferritin Nanocages as Cancer Theranostics. ACS Nano 2016, 10, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Le, W.; Lv, G.; Zhang, F.; Shi, L.; Wang, X.; Wang, J.; Wang, S.; Chang, J.; et al. Albumin-Bioinspired Gd:CuS Nanotheranostic Agent for In Vivo Photoacoustic/Magnetic Resonance Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Nano 2016, 10, 10245–10257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, C.; Zeng, J.; Sun, Q.; Wang, G.; Wang, Y.; Wu, Y.; Dou, S.; Gao, M.; Li, Z. Ambient Aqueous Synthesis of Ultrasmall PEGylated Cu2−xSe Nanoparticles as a Multifunctional Theranostic Agent for Multimodal Imaging Guided Photothermal Therapy of Cancer. Adv. Mater. 2016, 28, 8927–8936. [Google Scholar] [CrossRef]
- Chen, H.; Song, M.; Tang, J.; Hu, G.; Xu, S.; Guo, Z.; Li, N.; Cui, J.; Zhang, X.; Chen, X.; et al. Ultrahigh 19F Loaded Cu1.75S Nanoprobes for Simultaneous 19F Magnetic Resonance Imaging and Photothermal Therapy. ACS Nano 2016, 10, 1355–1362. [Google Scholar] [CrossRef]
- Li, M.; Lu, Z.; Zhang, J.; Chen, L.; Tang, X.; Jiang, Q.; Hu, Q.; Li, L.; Liu, J.; Huang, W. Near-Infrared-II Fluorophore with Inverted Dependence of Fluorescence Quantum Yield on Polarity as Potent Phototheranostics for Fluorescence-Image-Guided Phototherapy of Tumors. Adv. Mater. 2023, 35, e2209647. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Liu, X.; Wang, J.; Qiu, X.; Wei, W.; Zhao, J. Peptide-mediated Aqueous Synthesis of NIR-II Emitting Ag2S Quantum Dots for Rapid Photocatalytic Bacteria Disinfection. Angew. Chem. Int. Ed. 2023, 62, e202300085. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Chen, S.; Li, J.; Wu, T.; Ying, Y.; Zheng, J.; Zhang, Y.; Zhang, J.; Fan, X.; et al. Engineered NIR-II fluorophores with ultralong-distance molecular packing for high-contrast deep lesion identification. Nat. Commun. 2023, 14, 5017. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, J.; Guo, H.; Sun, A.; Chen, P.; Xi, L.; Huang, W.; Song, X.; Dong, X. Mo2C-Derived Polyoxometalate for NIR-II Photoacoustic Imaging-Guided Chemodynamic/Photothermal Synergistic Therapy. Angew. Chem. Int. Ed. 2019, 58, 18641–18646. [Google Scholar] [CrossRef]
- Lu, J.; Mao, Y.; Feng, S.; Li, X.; Gao, Y.; Zhao, Q.; Wang, S. Biomimetic smart mesoporous carbon nanozyme as a dual-GSH depletion agent and O2 generator for enhanced photodynamic therapy. Acta Biomater. 2022, 148, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gong, X.; Zhou, F.; Chen, B.; Tan, S.; Xu, H.; Pan, A.; Liang, S.; He, Y. Mesoporous peroxidase nanozyme for synergistic chemodynamic therapy and chemotherapy. Colloids Surf. B Biointerfaces 2022, 216, 112603. [Google Scholar] [CrossRef]
- Hu, T.; Xue, B.; Meng, F.; Ma, L.; Du, Y.; Yu, S.; Ye, R.; Li, H.; Zhang, Q.; Gu, L.; et al. Preparation of 2D Polyaniline/MoO3−x Superlattice Nanosheets via Intercalation-Induced Morphological Transformation for Efficient Chemodynamic Therapy. Adv. Healthc. Mater. 2023, 12, e2202911. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Tang, W.; Cui, X.; Wei, K.; Cheng, S.; Pei, Z.; Lei, H.; Liu, Z.; Cheng, L. Amplifying oxidation stress and T-cell activation by bioactive layered double hydroxide sonosensitizers for enhanced cancer immunotherapy. Mater. Today 2023, 68, 164–176. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, F.; Han, Z.; Lei, H.; Zhou, Y.; Cheng, S.; Yang, X.; Wang, T.; Wang, L.; Yang, N.; et al. Oxygen-Deficient Molybdenum Oxide Nanosensitizers for Ultrasound-Enhanced Cancer Metalloimmunotherapy. Angew. Chem. Int. Ed. 2023, 62, e202215467. [Google Scholar] [CrossRef]
- He, Z.; Du, J.; Miao, Y.; Li, Y. Recent Developments of Inorganic Nanosensitizers for Sonodynamic Therapy. Adv. Healthc. Mater. 2023, 12, e2300234. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, Y.; Liu, M.; Yu, W.; Yu, J.; Zheng, X.; Wang, L.; Wu, Y.; Wei, K.; Cheng, J.; et al. A Leaking-Proof Theranostic Nanoplatform for Tumor-Targeted and Dual-Modality Imaging-Guided Photodynamic Therapy. BME Front. 2023, 4, 0015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, D.; Dong, C.; Huang, H.; Feng, G.; Chen, Q.; Zheng, Y.; Tang, H.; Chen, Y.; Jing, X. Two-Dimensional MXene-Originated In Situ Nanosonosensitizer Generation for Augmented and Synergistic Sonodynamic Tumor Nanotherapy. ACS Nano 2022, 16, 9938–9952. [Google Scholar] [CrossRef]
- Gong, F.; Cheng, L.; Yang, N.; Betzer, O.; Feng, L.; Zhou, Q.; Li, Y.; Chen, R.; Popovtzer, R.; Liu, Z. Ultrasmall Oxygen-Deficient Bimetallic Oxide MnWO(X) Nanoparticles for Depletion of Endogenous GSH and Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2019, 31, e1900730. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Zhou, Z.; Wu, Z.; Li, H.; Peng, X.; Zhou, Y.; Tan, C.; Shen, J. Metallic phase enabling MoS2 nanosheets as an efficient sonosensitizer for photothermal-enhanced sonodynamic antibacterial therapy. J. Nanobiotechnol. 2022, 20, 136. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Bai, L.; Xu, J.; Gong, F.; Dong, Z.; Yang, Z.; Zeng, Z.; Liu, Z.; Cheng, L. Ultrafine Titanium Monoxide (TiO1+x) Nanorods for Enhanced Sonodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6527–6537. [Google Scholar] [CrossRef]
- Bai, S.; Yang, N.; Wang, X.; Gong, F.; Dong, Z.; Gong, Y.; Liu, Z.; Cheng, L. Ultrasmall Iron-Doped Titanium Oxide Nanodots for Enhanced Sonodynamic and Chemodynamic Cancer Therapy. ACS Nano 2020, 14, 15119–15130. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Bai, L.; Liu, B.; Yuan, M.; Ma, P.; Pang, M.; Cheng, Z.; Lin, J. Conferring Ti-Based MOFs with Defects for Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2021, 33, e2100333. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Xu, S.; Li, P.; Li, X.; Fang, F.; Pan, D.; Shen, L. Platinum Crosslinked Carbon Dot@TiO2−x p-n Junctions for Relapse-Free Sonodynamic Tumor Eradication via High-Yield ROS and GSH Depletion. Small 2022, 18, e2103528. [Google Scholar] [CrossRef]
- Geng, B.; Zhang, S.; Yang, X.; Shi, W.; Li, P.; Pan, D.; Shen, L. Cu2−xO@TiO2−y Z-scheme heterojunctions for sonodynamic-chemodynamic combined tumor eradication. Chem. Eng. J. 2022, 435, 134777. [Google Scholar] [CrossRef]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Pitroda, S.P.; Chmura, S.J.; Weichselbaum, R.R. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019, 20, e434–e442. [Google Scholar] [CrossRef]
- Nishiga, Y.; Drainas, A.P.; Baron, M.; Bhattacharya, D.; Barkal, A.A.; Ahrari, Y.; Mancusi, R.; Ross, J.B.; Takahashi, N.; Thomas, A.; et al. Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nat. Cancer 2022, 3, 1351–1366. [Google Scholar] [CrossRef]
- Petroni, G.; Cantley, L.C.; Santambrogio, L.; Formenti, S.C.; Galluzzi, L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 114–131. [Google Scholar] [CrossRef]
- Andratschke, N.; Willmann, J.; Appelt, A.L.; Alyamani, N.; Balermpas, P.; Baumert, B.G.; Hurkmans, C.; Høyer, M.; Langendijk, J.A.; Kaidar-Person, O.; et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus on re-irradiation: Definition, reporting, and clinical decision making. Lancet Oncol. 2022, 23, e469–e478. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhu, J.; Zhu, W.; Chen, L.; Li, M.; Shen, J.; Chen, M.; Wu, Y.; Pan, F.; Deng, Z.; et al. X-ray-Induced Release of Nitric Oxide from Hafnium-Based Nanoradiosensitizers for Enhanced Radio-Immunotherapy. Adv. Mater. 2023, 35, e2302220. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, J.; Xi, M.; Wang, C.; Fang, H.; Wu, X.; Zhang, C.; Sun, G.; Zhang, Y.; Shen, L.; et al. Biogenic platinum nanoparticles on bacterial fragments for enhanced radiotherapy to boost antitumor immunity. Nano Today 2022, 47, 101656. [Google Scholar] [CrossRef]
- Zhang, L.; Montesdeoca, N.; Karges, J.; Xiao, H. Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy. Angew. Chem. Int. Ed. 2023, 62, e202300662. [Google Scholar] [CrossRef]
- Lei, H.; Li, Q.; Pei, Z.; Liu, L.; Yang, N.; Cheng, L. Nonferrous Ferroptosis Inducer Manganese Molybdate Nanoparticles to Enhance Tumor Immunotherapy. Small 2023, 19, e2303438. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L.; Pan, H.; Cai, L. Biomimetic Active Materials Guided Immunogenic Cell Death for Enhanced Cancer Immunotherapy. Small Methods 2023, 7, e2201412. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Zhang, C.; An, J.-X.; Wang, Y.-Z.; Qiao, J.-Y.; Zeng, X.; Zhang, X.-Z. Metabolic Regulation of Tumor Microenvironment with Biohybrid Bacterial Bioreactor for Enhanced Cancer Chemo-Immunotherapy. Adv. Funct. Mater. 2023, 33, 2302728. [Google Scholar] [CrossRef]
- Wang, N.; Li, P.; Zhao, J.; Liu, Y.; Hu, X.; Ling, D.; Li, F. An anisotropic photocatalytic agent elicits robust photoimmunotherapy through plasmonic catalysis-mediated tumor microenvironment modulation. Nano Today 2023, 50, 101827. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, L.; Zhou, J.; Chen, J.; Ma, Y.; Han, Y. Low-Dose Cu Ions Assisted by Mild Thermal Stimulus Inducing Bacterial Cuproptosis-Like Death for Antibiosis and Biointegration. Adv. Funct. Mater. 2024, 34, 2308197. [Google Scholar] [CrossRef]
- Mei, J.; Xu, D.; Wang, L.; Kong, L.; Liu, Q.; Li, Q.; Zhang, X.; Su, Z.; Hu, X.; Zhu, W.; et al. Biofilm Microenvironment-Responsive Self-Assembly Nanoreactors for All-Stage Biofilm Associated Infection through Bacterial Cuproptosis-like Death and Macrophage Re-Rousing. Adv. Funct. Mater. 2023, 35, 2303432. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, T.; Song, S.; Hou, Y.; Feng, L.; Fan, C.; Li, M. Ferroptosis, Pyroptosis, and Cuproptosis in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3564–3587. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yao, C.; Tipper, J.; Yang, L.; Xu, X.; Chen, X.; Bao, G.; He, B.; Xu, X.; Zheng, Y. Nanostrategy of Targeting at Embryonic Trophoblast Cells Using CuO Nanoparticles for Female Contraception. ACS Nano 2023, 17, 25185–25204. [Google Scholar] [CrossRef]
- Yu, Y.; Ding, J.; Zhou, Y.; Xiao, H.; Wu, G. Biosafety chemistry and biosafety materials: A new perspective to solve biosafety problems. Biosaf. Health 2022, 4, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Tang, D.; Xu, L.; Zhang, M.; Dorfman, R.G.; Pan, Y.; Zhou, Q.; Zhou, L.; Wang, Y.; Li, Y.; Yin, Y.; et al. Metformin facilitates BG45-induced apoptosis via an anti-Warburg effect in cholangiocarcinoma cells. Oncol. Rep. 2018, 39, 1957–1965. [Google Scholar] [CrossRef]
- Yang, Z.; Su, W.; Wei, X.; Pan, Y.; Xing, M.; Niu, L.; Feng, B.; Kong, W.; Ren, X.; Huang, F.; et al. Hypoxia inducible factor-1α drives cancer resistance to cuproptosis. Cancer Cell 2025, 43, 937–954.e9. [Google Scholar] [CrossRef] [PubMed]
| Nanomaterials | Route of Administration | Chemical Modulators | Targeting Mechanism | Response | Enhancement Strategy | Disease | Reference |
|---|---|---|---|---|---|---|---|
| CuX-P | Iv a | Cu2+/Cu+/DSF | T cell membrane | - | Cu+ increase | Breast cancer | [84] |
| Cu-HPB/C | Iv | Cu2+/ChOx | EPR | PH | ChOx, ROS increase, and Cu2+ chelation decrease | Breast cancer | [92] |
| NCT-503@Cu-HMPB | Iv | Cu2+/NCT-503 | EPR | PH/GSH | Disrupting serine metabolism, ROS increase, and Cu2+ chelation decrease | Breast cancer | [99] |
| mCGYL-LOx | Iv | Cu2+/LOx | Renca cell membrane | - | Lox, ROS increase, and Cu2+ chelation decrease | Kidney cancer | [100] |
| Au@MSN-Cu/PEG/DSF | Iv | Cu2+/Cu+/DSF | EPR | NIR-II | Cu+ increase | Breast cancer | [103] |
| CGNPs | Iv | Cu2+/GOx | tLyp-1 peptide and EPR | PH | GOx, metabolic reprogramming, ROS increase, and Cu2+ chelation decrease | Breast cancer | [105] |
| Cu2(PO4)(OH) | Iv | Cu2+/Cu+ | EPR | H2S | Cu+ increase | Colon cancer | [106] |
| GOx-CuCaP-DSF | Iv | Cu2+/Cu+/DSF/GOx | EPR | PH | GOx, metabolic reprogramming, ROS increase, Cu2+ chelation decrease, Cu+ increase, and calcium overload | Hepatocarcinoma | [109] |
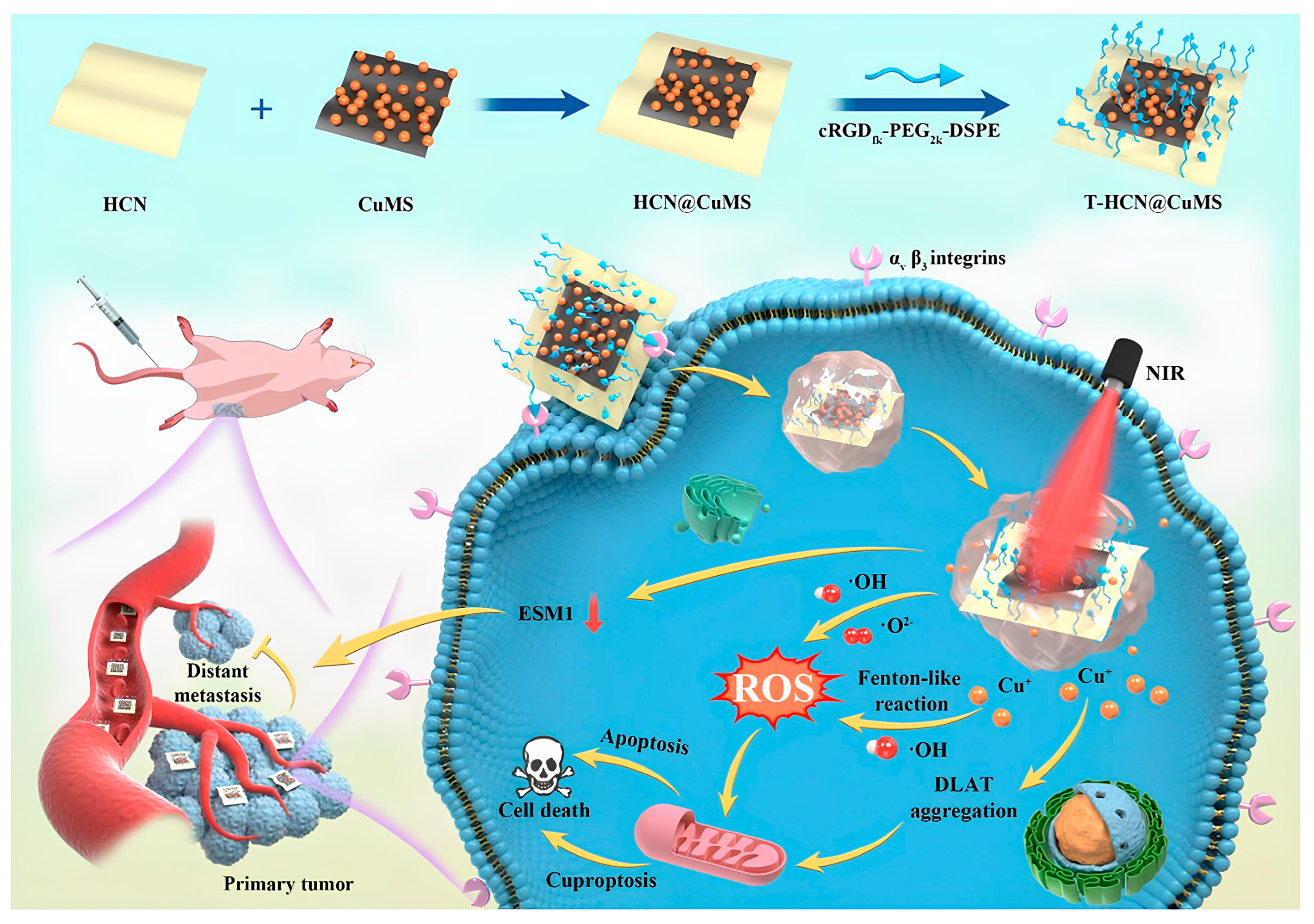
| T-HCN@CuMS | Iv | Cu2+ | cRGDfk and EPR | - | Heterojunction, ROS increase, and Cu2+ chelation decrease | Sarcoma of bone | [112] |
| HACT | Iv | Cu2+ | HA | PH/US | Heterojunction, ROS increase, and Cu2+ chelation decrease | Melanoma | [113] |
| CaCO3/Mn/Cu@lip-Apt | Iv | Cu2+/Mn*/Ca2+ | MCF-7-specific aptamer and EPR | PH | ROS increase, Cu2+ chelation decrease, and calcium overload | Breast cancer | [114] |
| MACuS | Iv | Cu2+ | GLUT-1 | - | Metabolic reprogramming | Breast cancer | [117] |
| CuP/Er | Iv | Cu2+/Er | EPR | PH | Er, metabolic reprogramming, ROS increase, and Cu2+ chelation decrease | Colon cancer/Breast cancer | [118] |
| DSF@HMCIS-PEG-FA | Iv | Cu2+/Cu+/DSF/Fe2+ | FA | PH | H2S, ROS increase, Cu2+ chelation decrease, and Cu+ increased | Breast cancer/Gastric cancer | [119] |
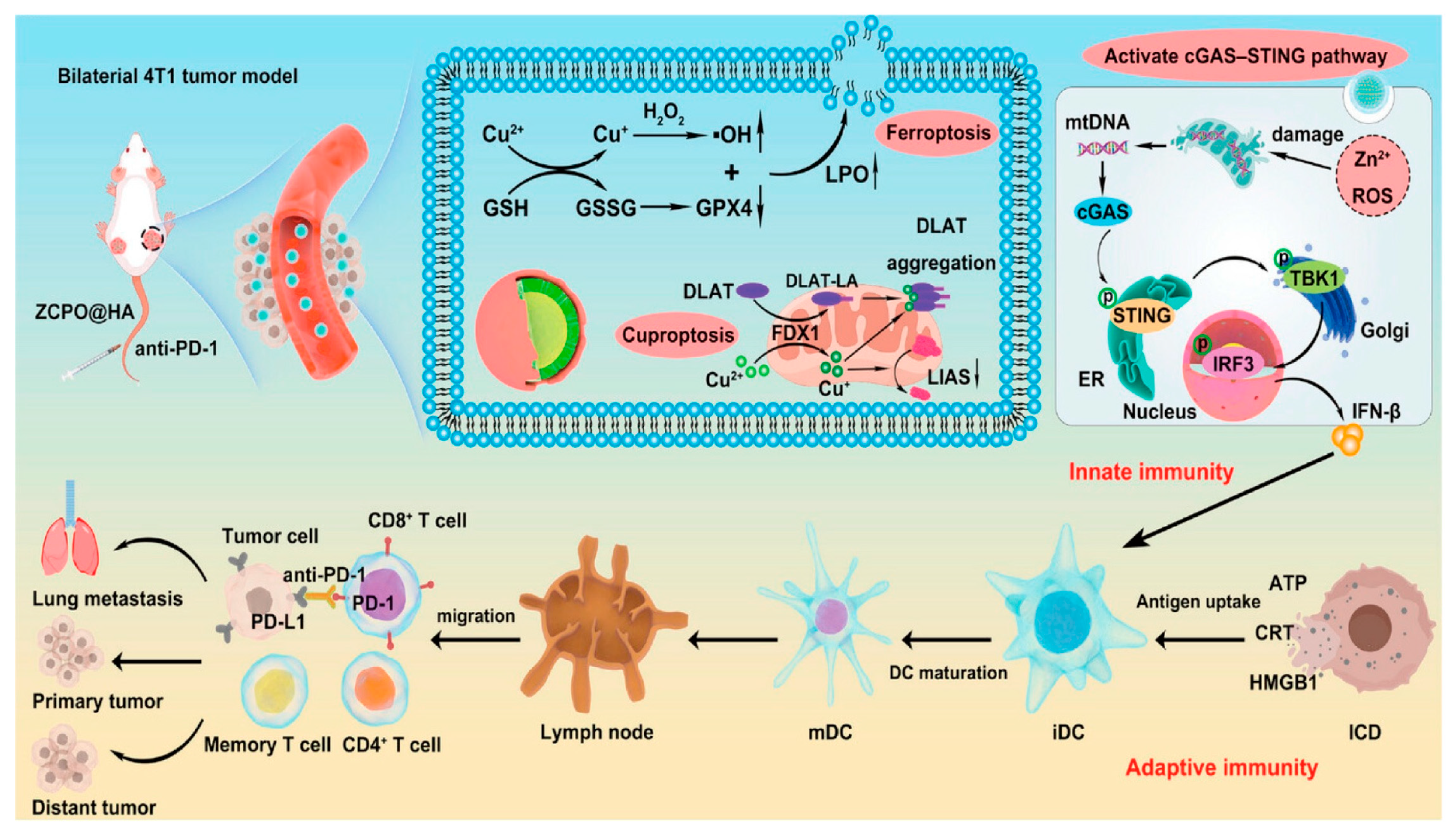
| ZCPO@HA | Iv | Cu2+/Zn2+ | HA | PH | ROS increase, and Cu2+ chelation decrease | Breast cancer | [121] |
| Cu2O-MnO@PEG | Iv | Cu2+/Cu+ | EPR | PH | Cu+ increase | Melanoma | [122] |
| OPMNs-ZCS@siPD-L1 | Microneedle patch | Cu2+ | Microneedle patch | PH | - | Melanoma | [123] |
| DSF/CuS-C | Iv | Cu2+/Cu+/DSF/CpG | EPR | TME | Cu+ increase | Breast cancer | [124] |
| ES@CuO | Iv | Cu2+/Cu+/Es | EPR | PH | Es | Melanoma | [125] |
| CMGCL | Iv | Cu2+/GOx | LLC membrane | PH | Gox, metabolic reprogramming, ROS increase, and Cu2+ chelation decrease | Lung cancer | [126] |
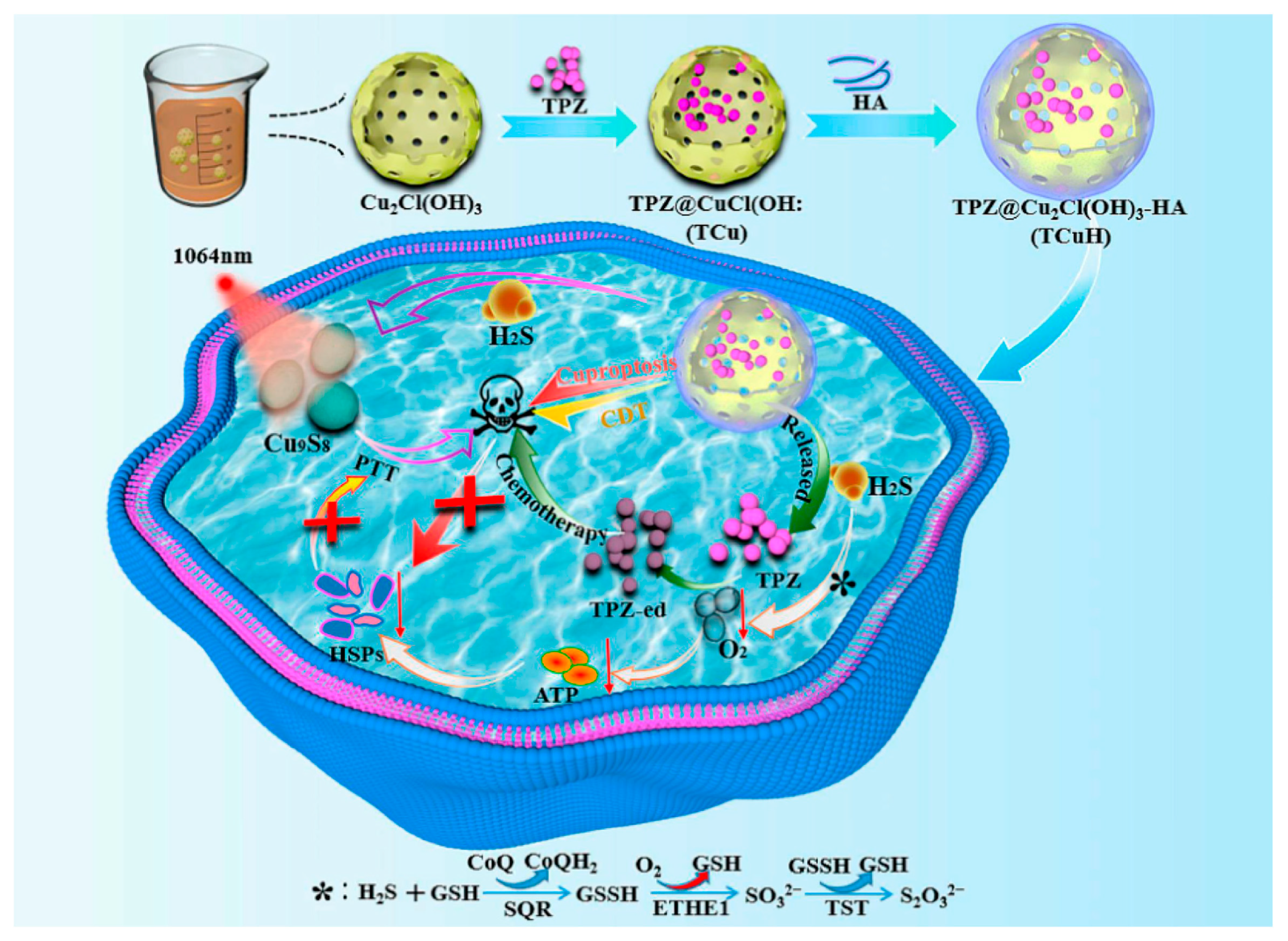
| TCuH | Iv | Cu2+/TPZ | HA and EPR | H2S | - | Colon cancer | [127] |
| CEL NP | Iv | Cu2+/Es | EPR | NIR-II/PH | Es | Colon cancer | [128] |
| Cu2−xSe HNSs | Iv | Cu2+ | EPR | NIR-II | Thermoelectrocatalysis, ROS increase, and Cu2+ chelation decrease | Colon cancer | [129] |
| CuSiO3@Au-Pd NMs | Iv | Cu2+ | EPR | PH | - | Breast cancer | [130] |
| CMCO | Iv | Cu2+/Cu+ | EPR | TME | Nanozyme, ROS increase, Cu2+ chelation decrease, and Cu+ increase | Colon cancer | [131] |
| CuMoO4 | Iv | Cu2+ | EPR | PH | Nanozyme, ROS increase, and Cu2+ chelation decrease | Breast cancer | [132] |
| Cu5.4O | Oral administration | Cu2+/Cu+ | EPR | - | Nanozyme, ROS increase, Cu2+ chelation decrease, and Cu+ increase | Colon cancer | [133] |
| CuSACO | Iv | Cu2+ | EPR | GSH | Nanozyme, ROS increase, and Cu2+ chelation decrease | Breast cancer | [134] |
| CDCuCDs | Iv | Cu2+/Cu+ | EPR | H2S | H2S, ROS increase, Cu2+ chelation decrease, and Cu+ increased | Colon cancer | [135] |
| Cu-BiSex | Iv | Cu2+ | EPR | - | Nanozyme, ROS increase, and Cu2+ chelation decrease | Prostate cancer | [136] |
| MCD | Iv | Cu2+/Cu+ | EPR | PH/GSH | ROS increase, Cu2+ chelation decrease, and Cu+ increase | Sarcoma of bone | [137] |
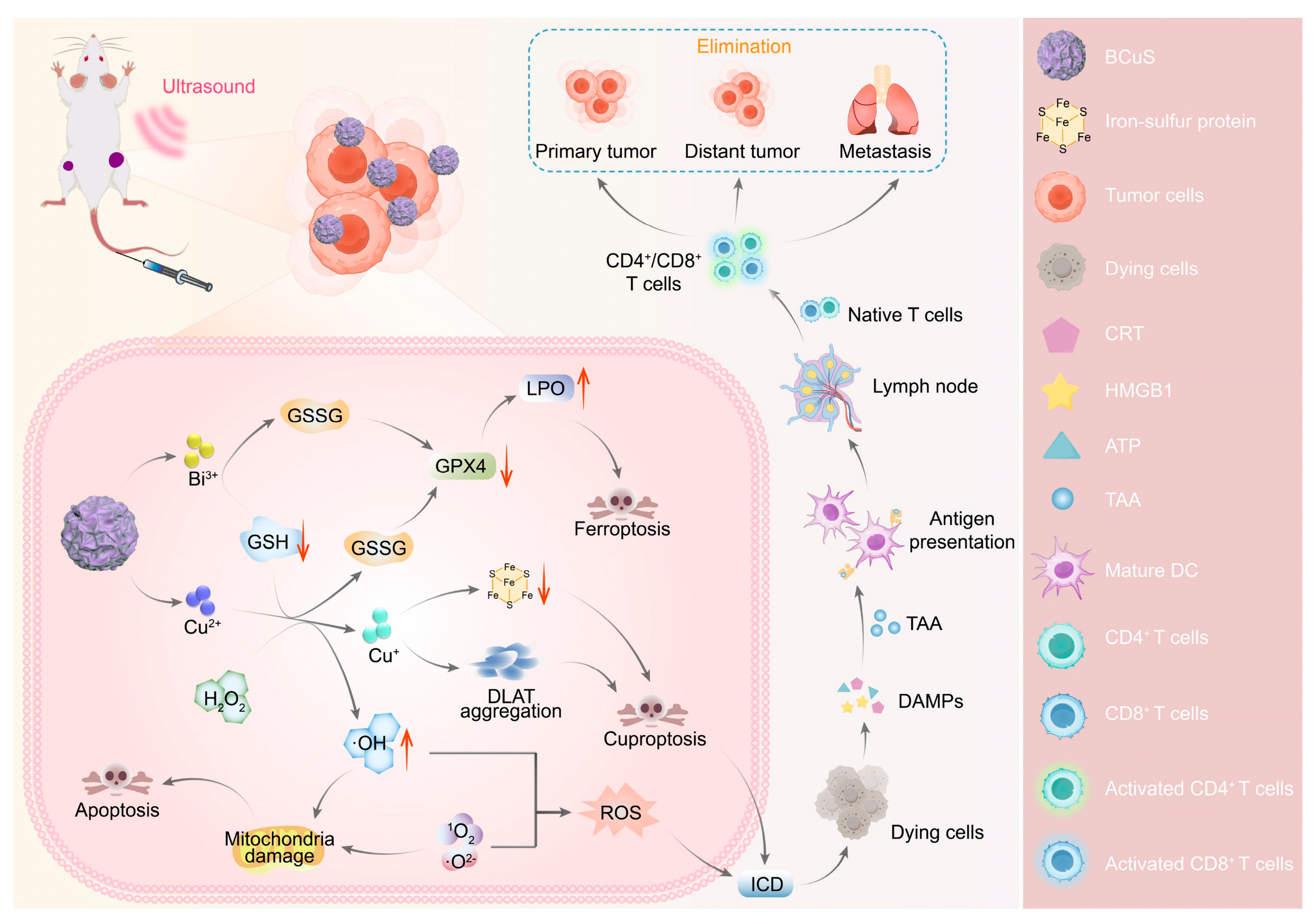
| BCuS | Iv | Cu2+ | EPR | TME/PH | Heterojunction, ROS increase, and Cu2+ chelation decrease | Breast cancer | [138] |
| Cu-CDs | Iv | Cu2+ | EPR | US | Heterojunction, ROS increase, and Cu2+ chelation decrease | Glioblastoma | [139] |
| GQD/Cu2O | Iv | Cu2+ | EPR | PH | Heterojunction, ROS increase, and Cu2+ chelation decrease | Breast cancer | [140] |
| ZCA NSs | intratumoral injection | Cu2+ | EPR | US | Jahn-Teller effect, ROS increase, and Cu2+ chelation decrease | Colon cancer/Breast cancer | [141] |
| PWCu | Iv | Cu2+/Cu+ | EPR | X-Ray | X-rays, FDX1 and LIAS expression upregulated, and Cu+ increased | Breast cancer | [142] |
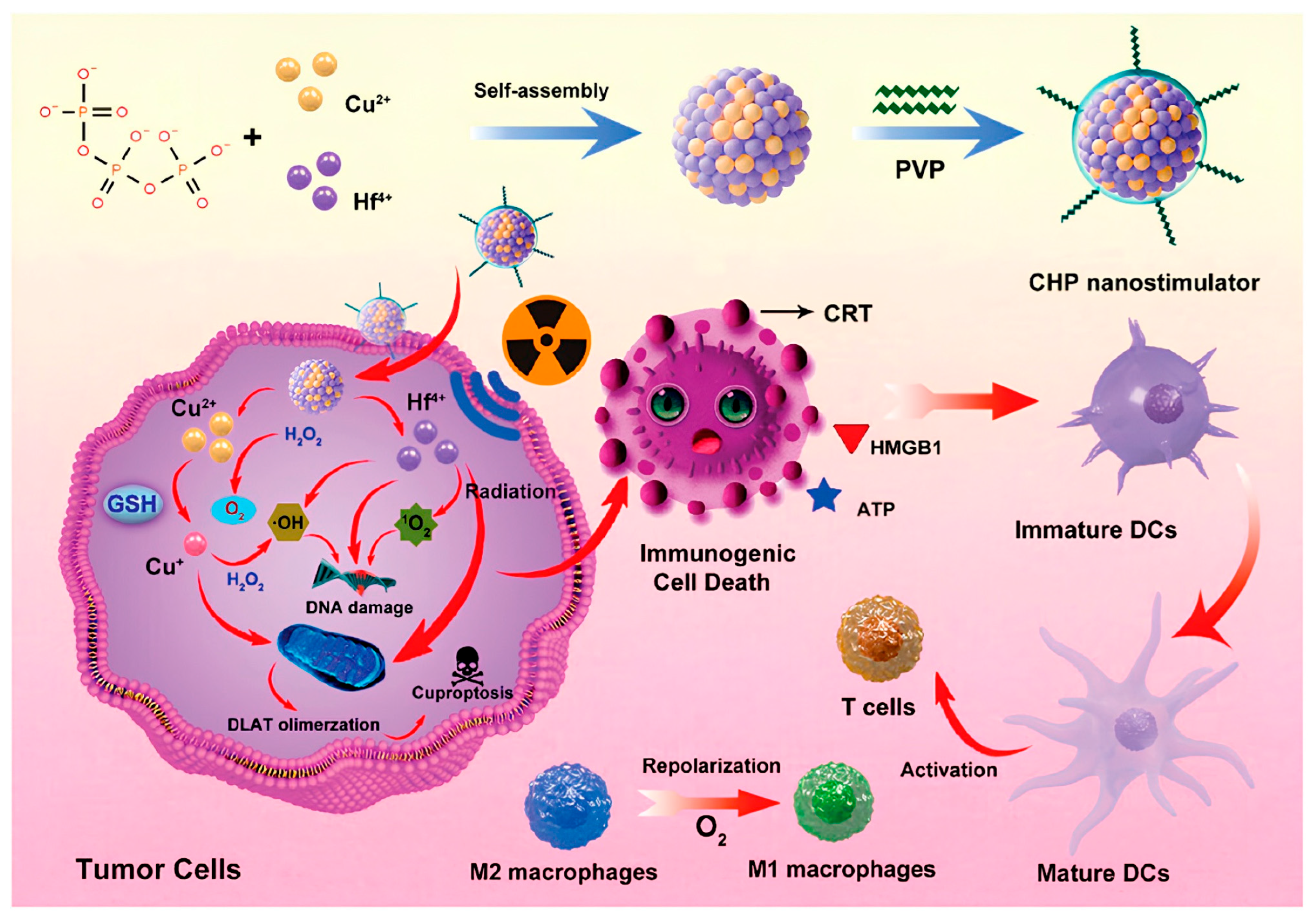
| CHP | Iv | Cu2+/Cu+/Hf4+ | EPR | TME/PH | X-rays, ROS increase, and Cu2+ chelation decrease | Breast cancer | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Dai, J.; Jiang, J.; Deng, S.; Gu, J.; Wang, J.; Chen, M.; Cai, W.; Wu, K.; Tao, K.; et al. Engineering Inorganic Nanoparticles to Induce Cuproptosis: A New Strategy for Cancer Therapy. Pharmaceutics 2025, 17, 1383. https://doi.org/10.3390/pharmaceutics17111383
Jiang Z, Dai J, Jiang J, Deng S, Gu J, Wang J, Chen M, Cai W, Wu K, Tao K, et al. Engineering Inorganic Nanoparticles to Induce Cuproptosis: A New Strategy for Cancer Therapy. Pharmaceutics. 2025; 17(11):1383. https://doi.org/10.3390/pharmaceutics17111383
Chicago/Turabian StyleJiang, Zhenxing, Jianwei Dai, Juanjuan Jiang, Shenghe Deng, Junnan Gu, Jun Wang, Mian Chen, Wentai Cai, Ke Wu, Kaixiong Tao, and et al. 2025. "Engineering Inorganic Nanoparticles to Induce Cuproptosis: A New Strategy for Cancer Therapy" Pharmaceutics 17, no. 11: 1383. https://doi.org/10.3390/pharmaceutics17111383
APA StyleJiang, Z., Dai, J., Jiang, J., Deng, S., Gu, J., Wang, J., Chen, M., Cai, W., Wu, K., Tao, K., Liu, K., & Cai, K. (2025). Engineering Inorganic Nanoparticles to Induce Cuproptosis: A New Strategy for Cancer Therapy. Pharmaceutics, 17(11), 1383. https://doi.org/10.3390/pharmaceutics17111383








