Improving In Vitro–In Vivo Correlation (IVIVC) for Lipid-Based Formulations: Overcoming Challenges and Exploring Opportunities
Abstract
1. Introduction
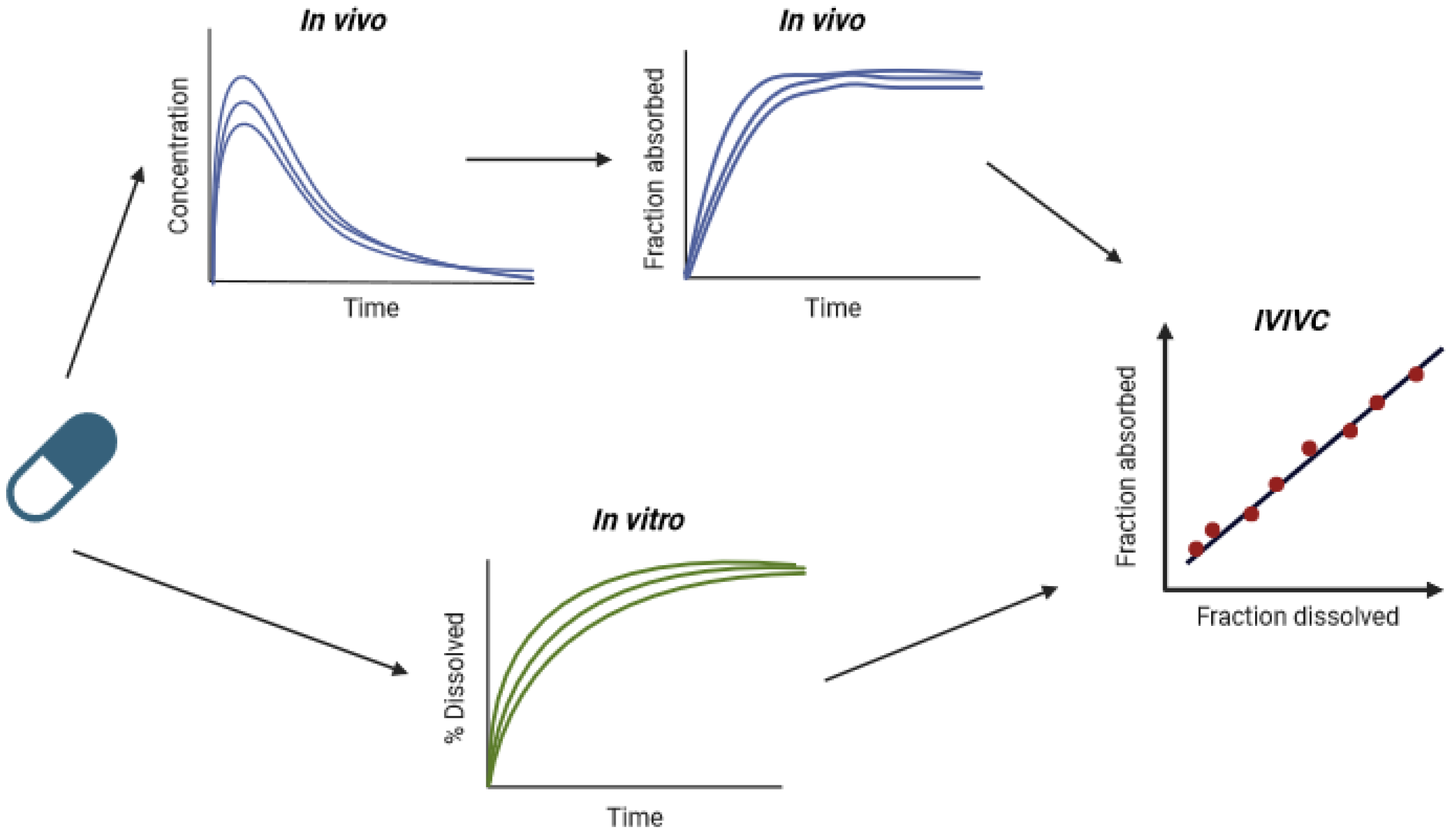
2. IVIVCs in the Context of Lipid-Based Formulations
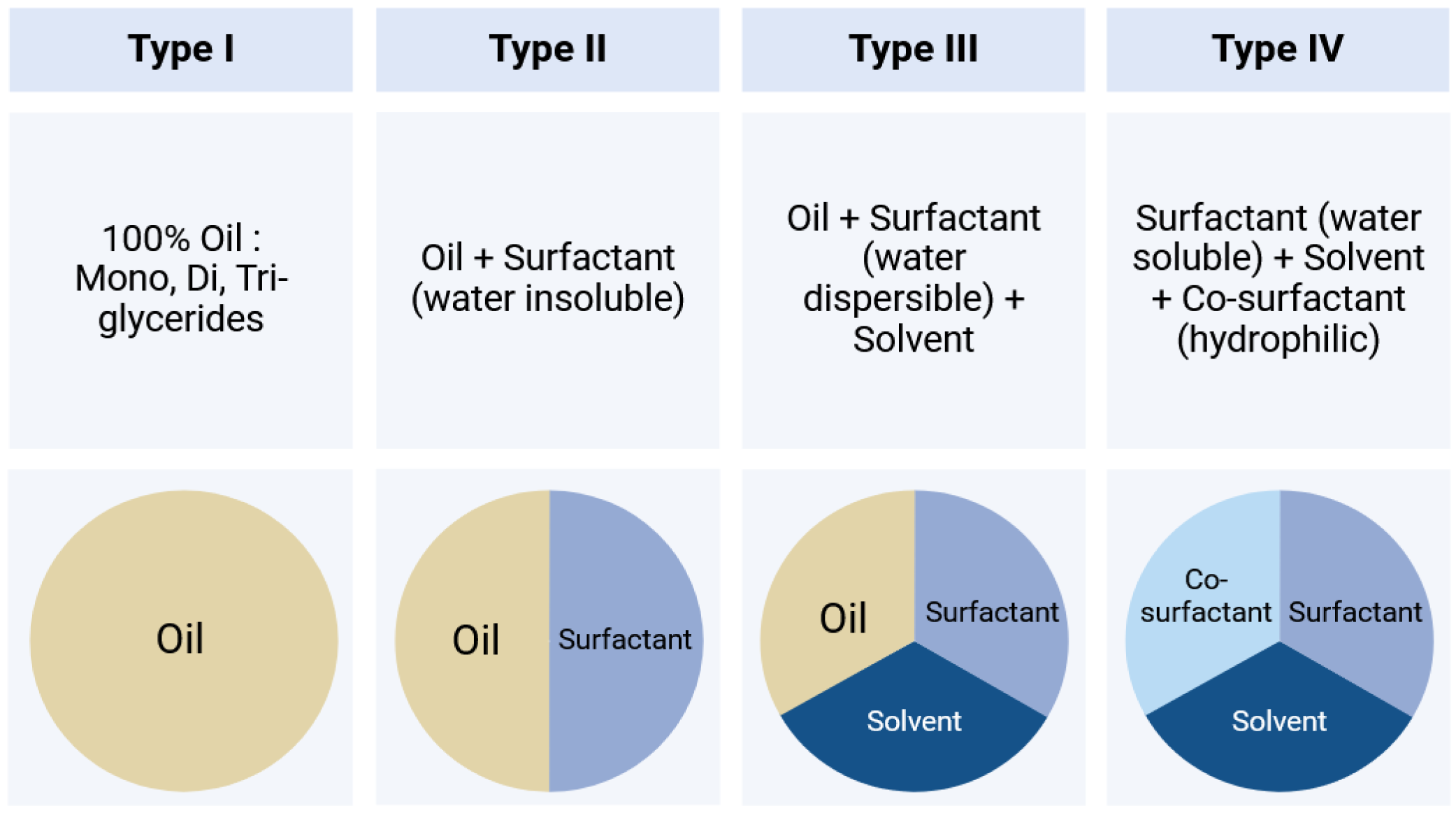
3. Specific Considerations for LBFs
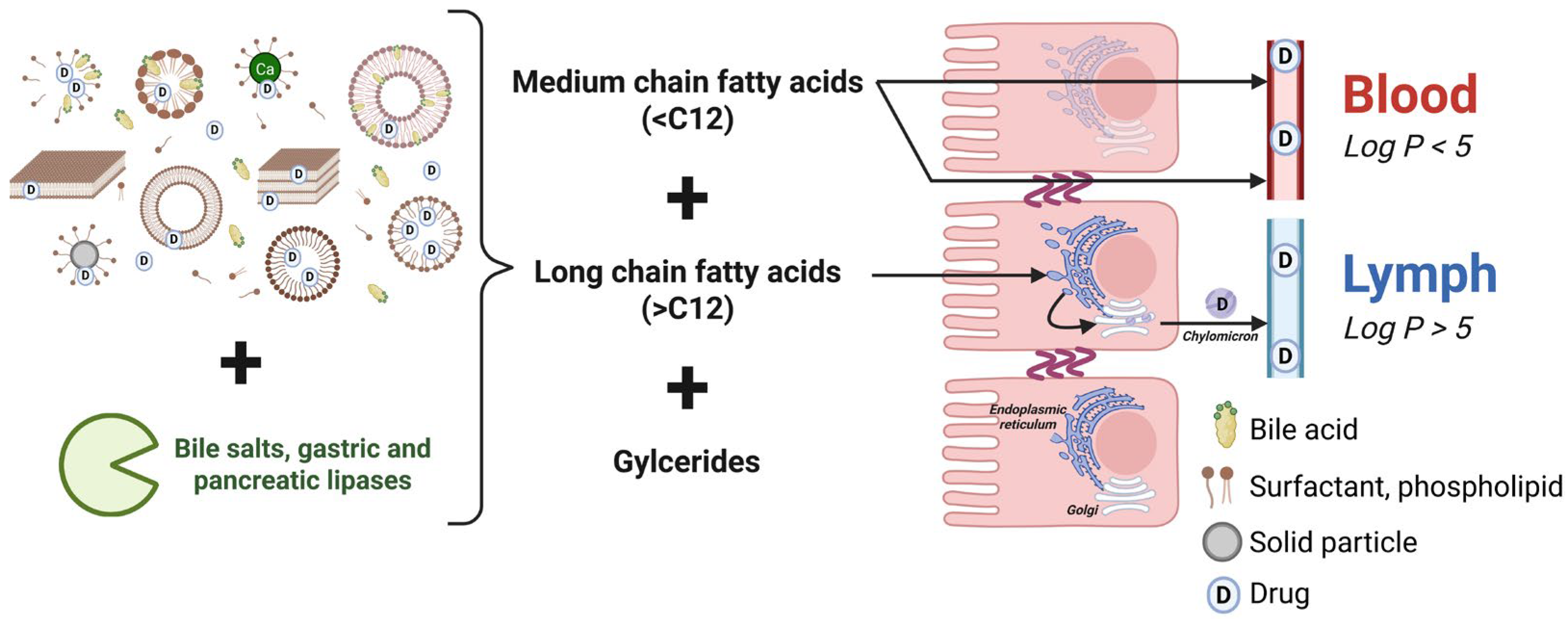
3.1. Dispersion/Digestion
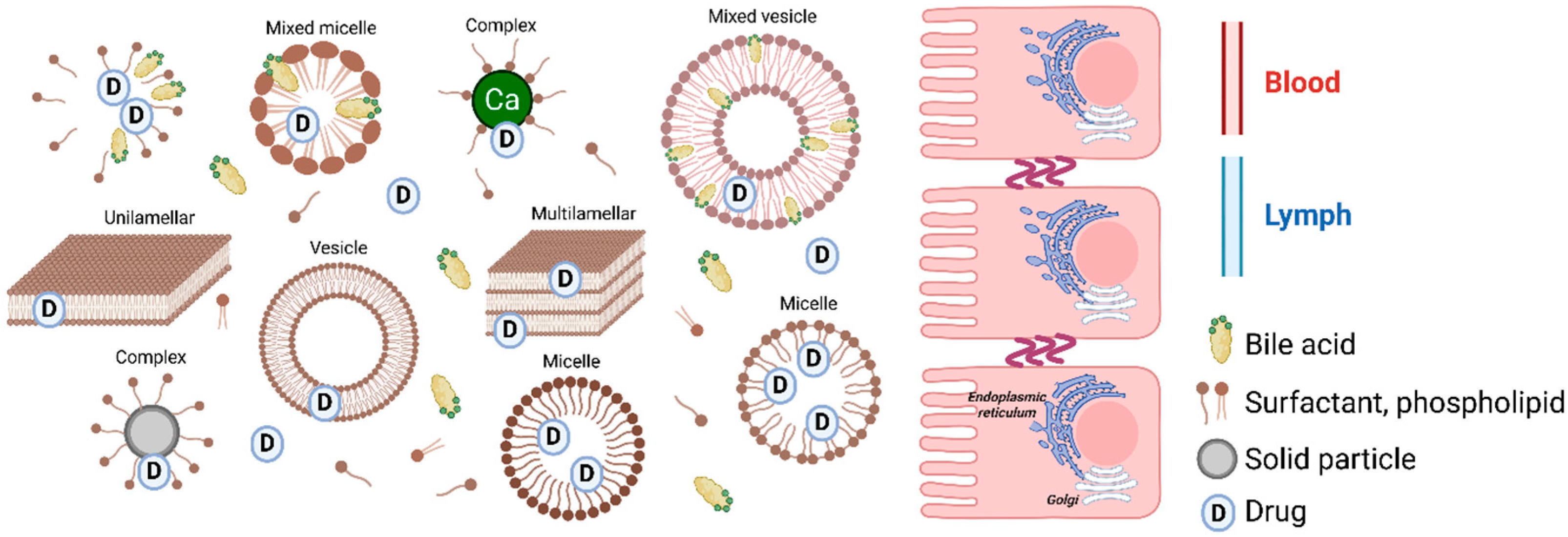
3.2. Absorption
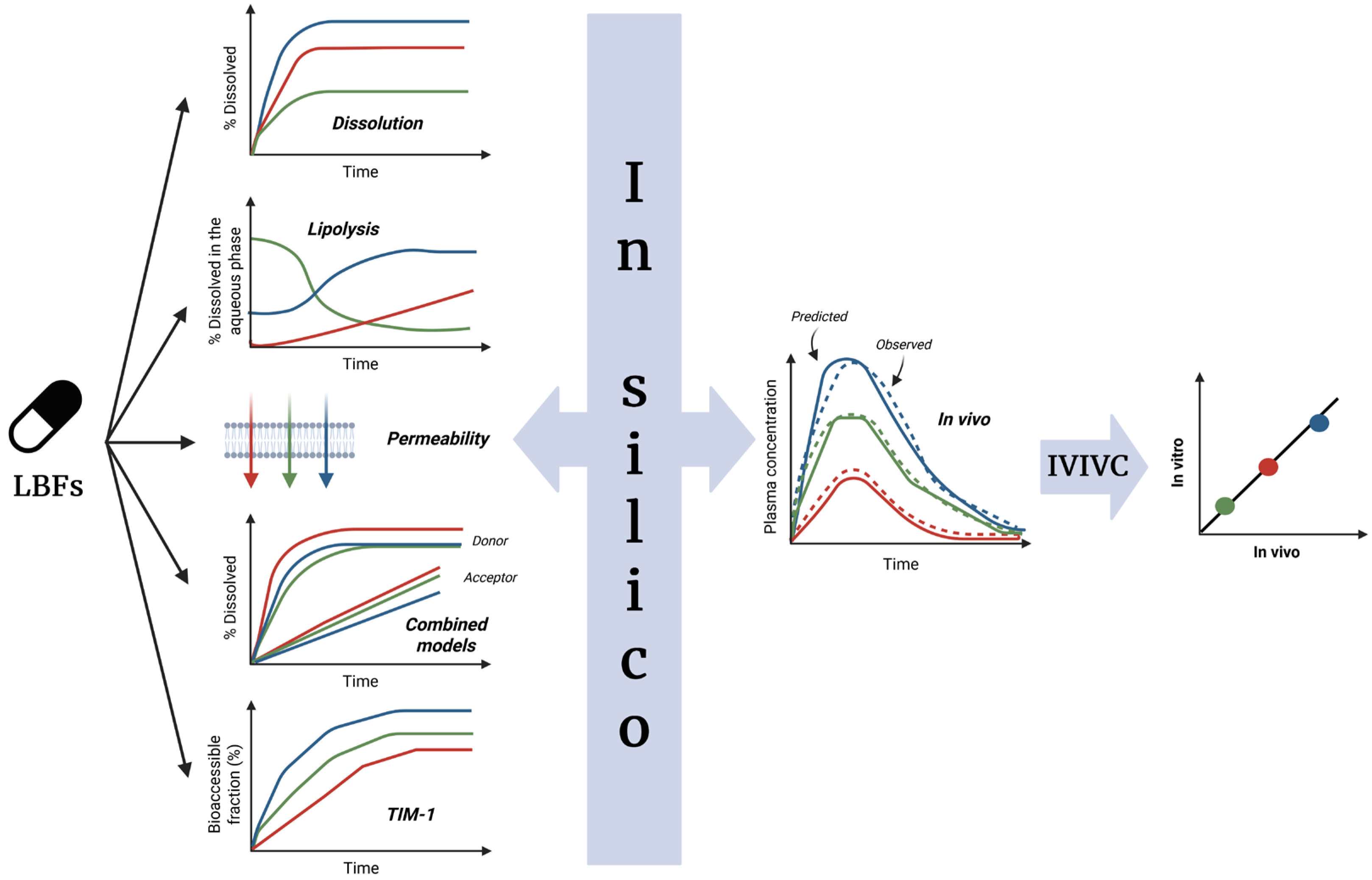
4. Available Data Sources for IVIVC Development
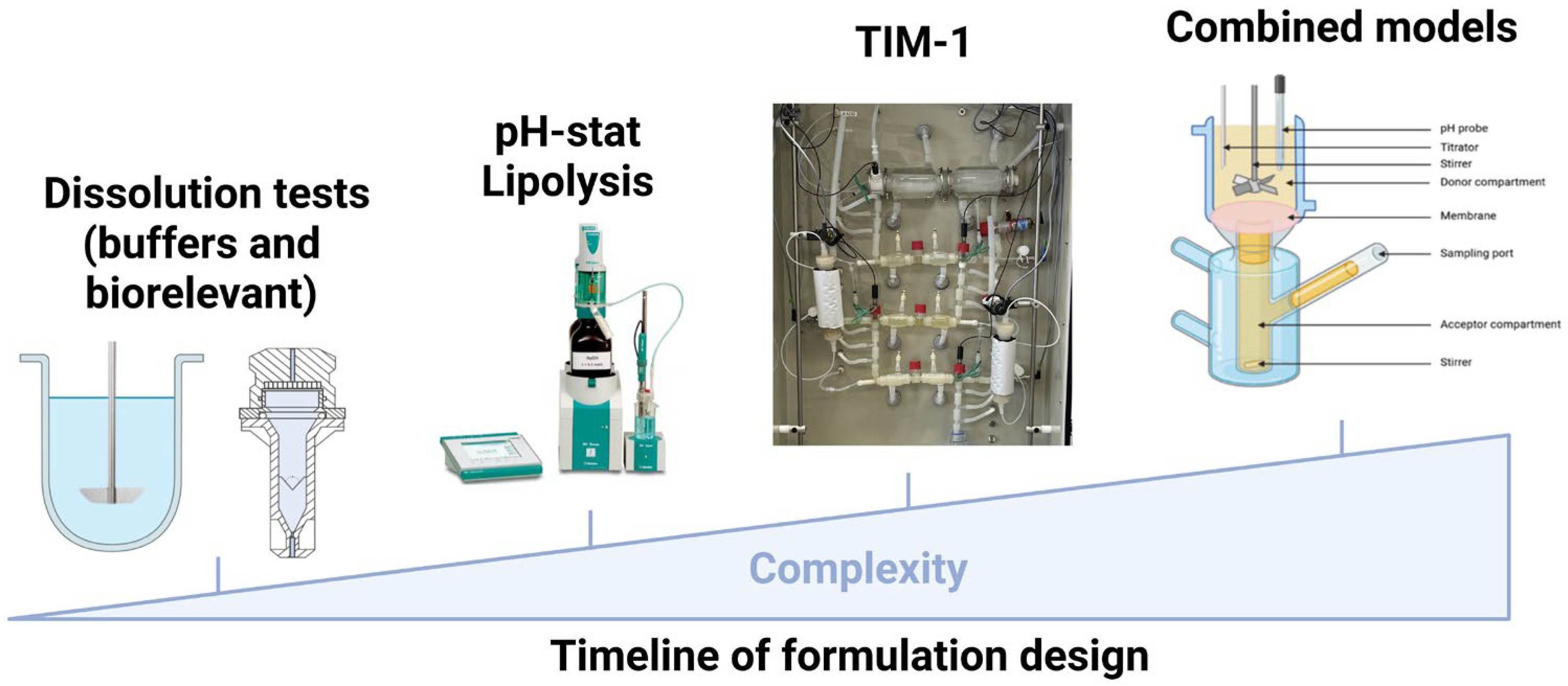
4.1. In Vitro Methods
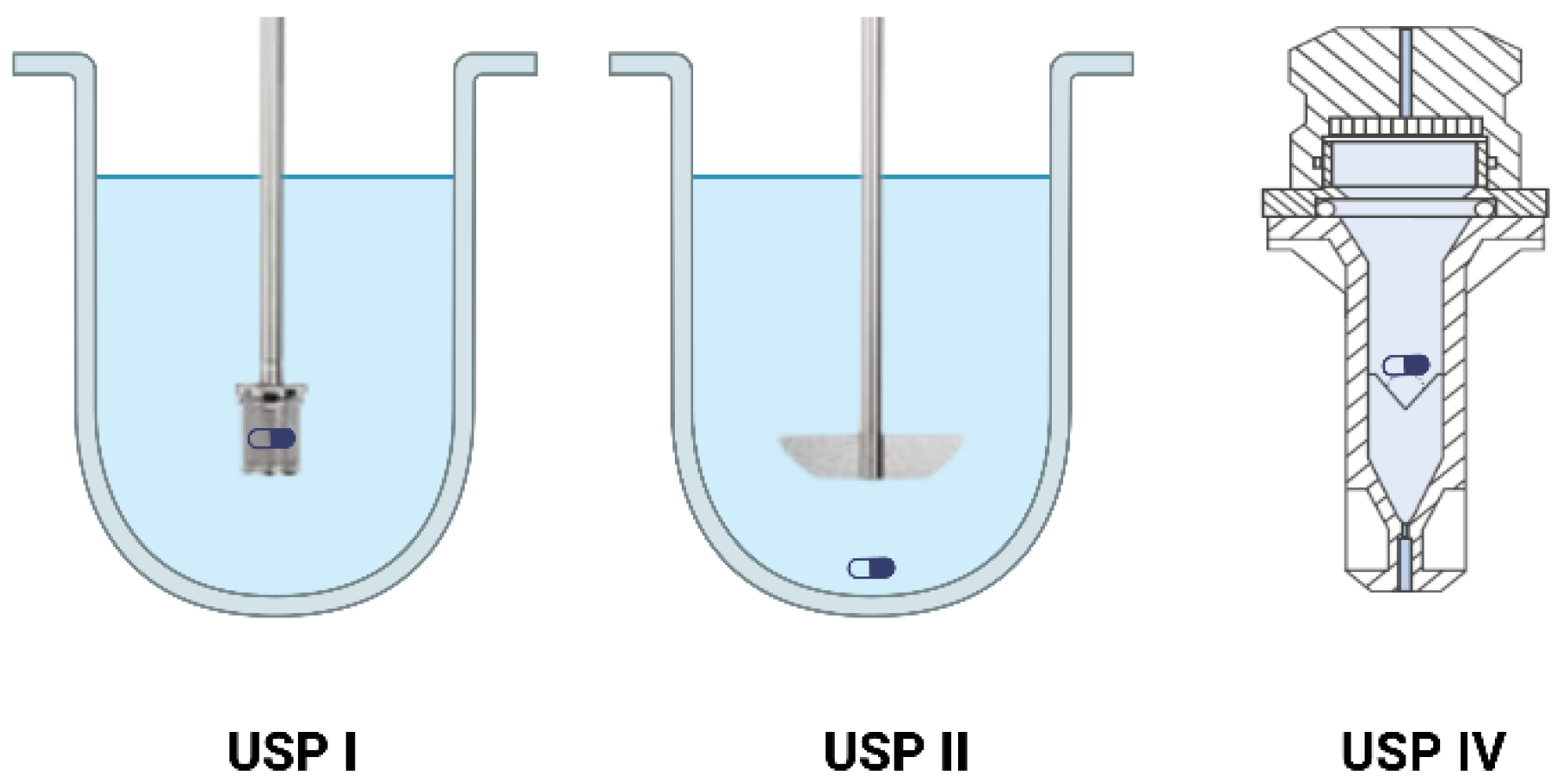
4.1.1. Dissolution
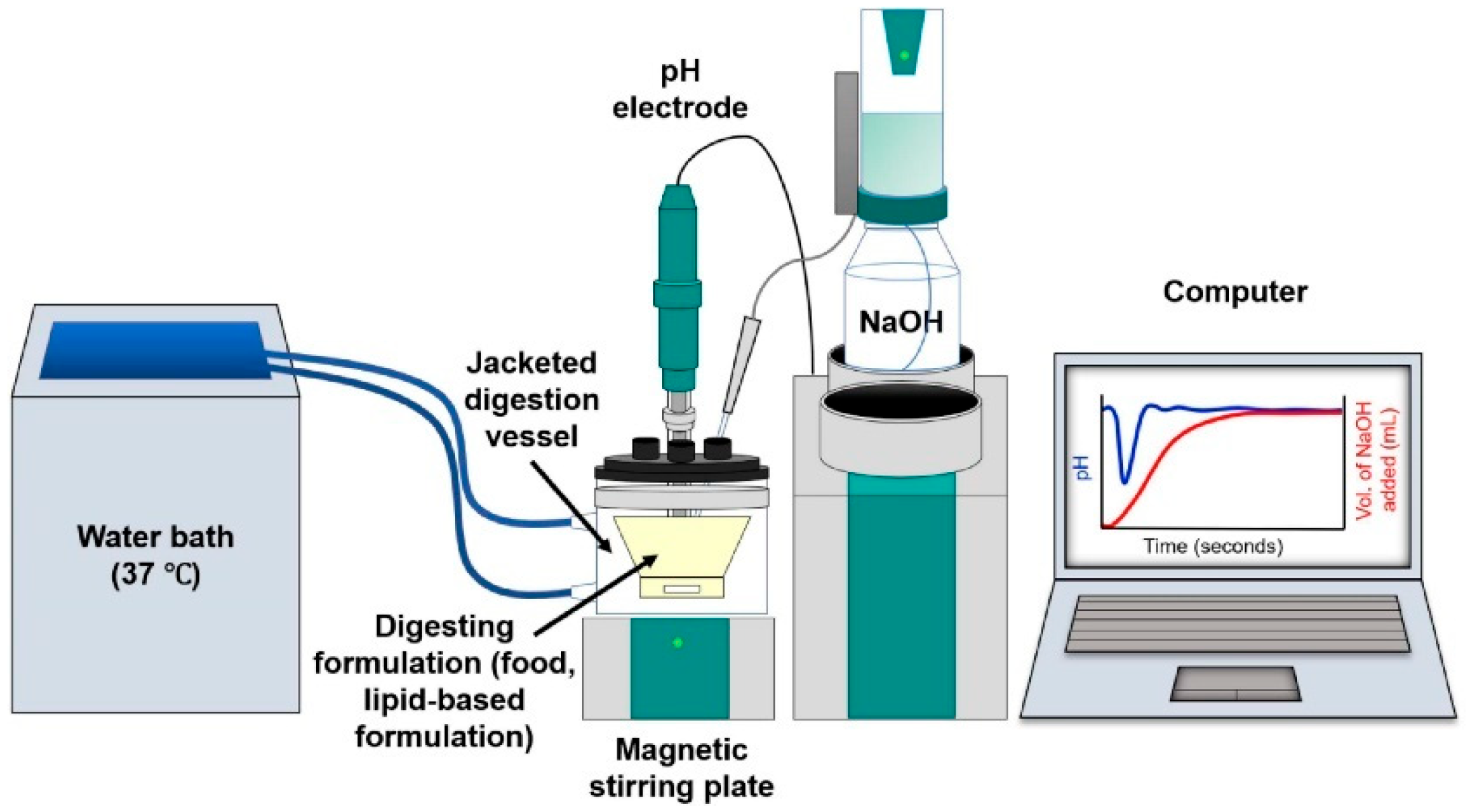
4.1.2. Lipolysis
4.1.3. Dynamic Digestion
4.1.4. Combined Models
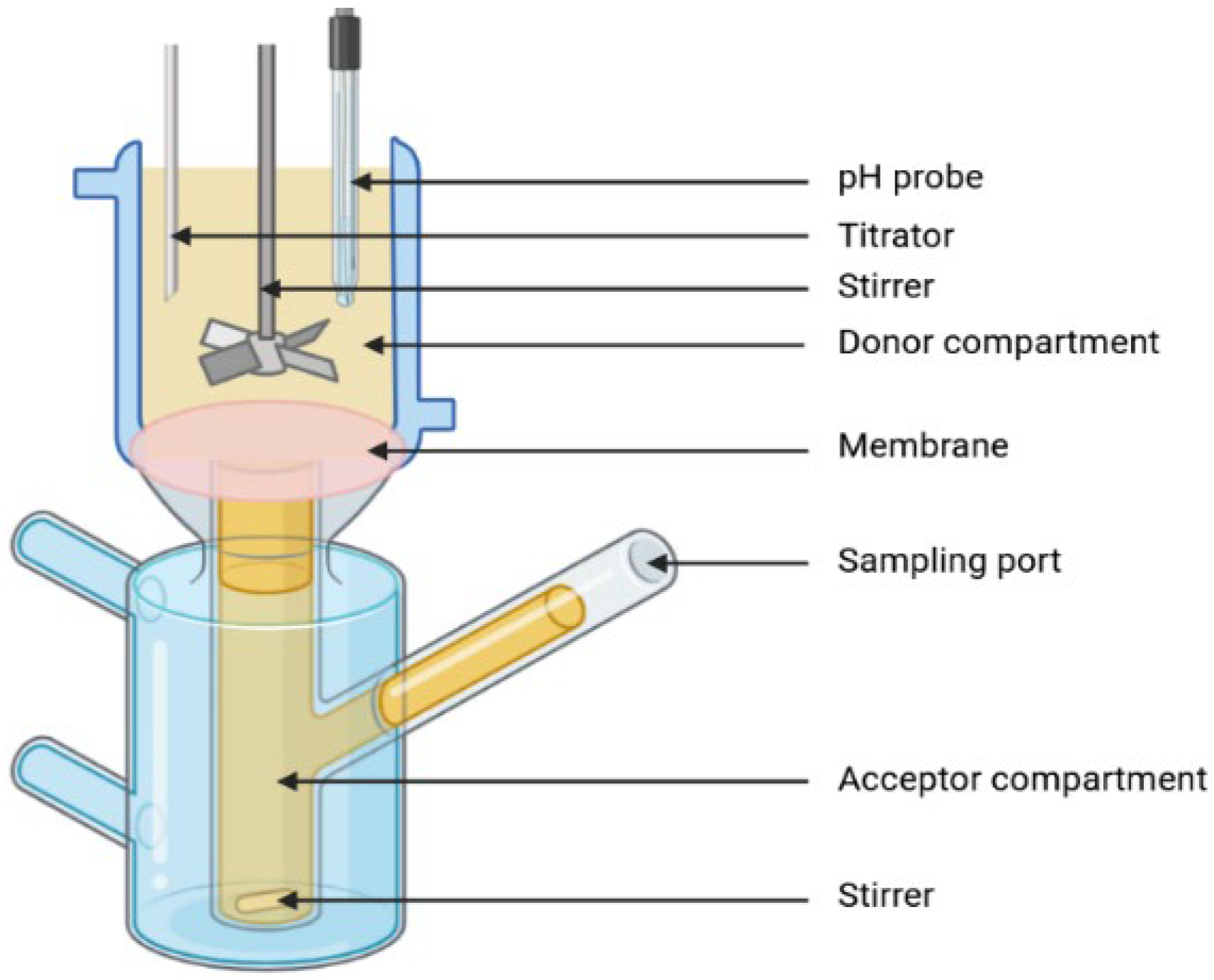
Lipolysis and Permeation
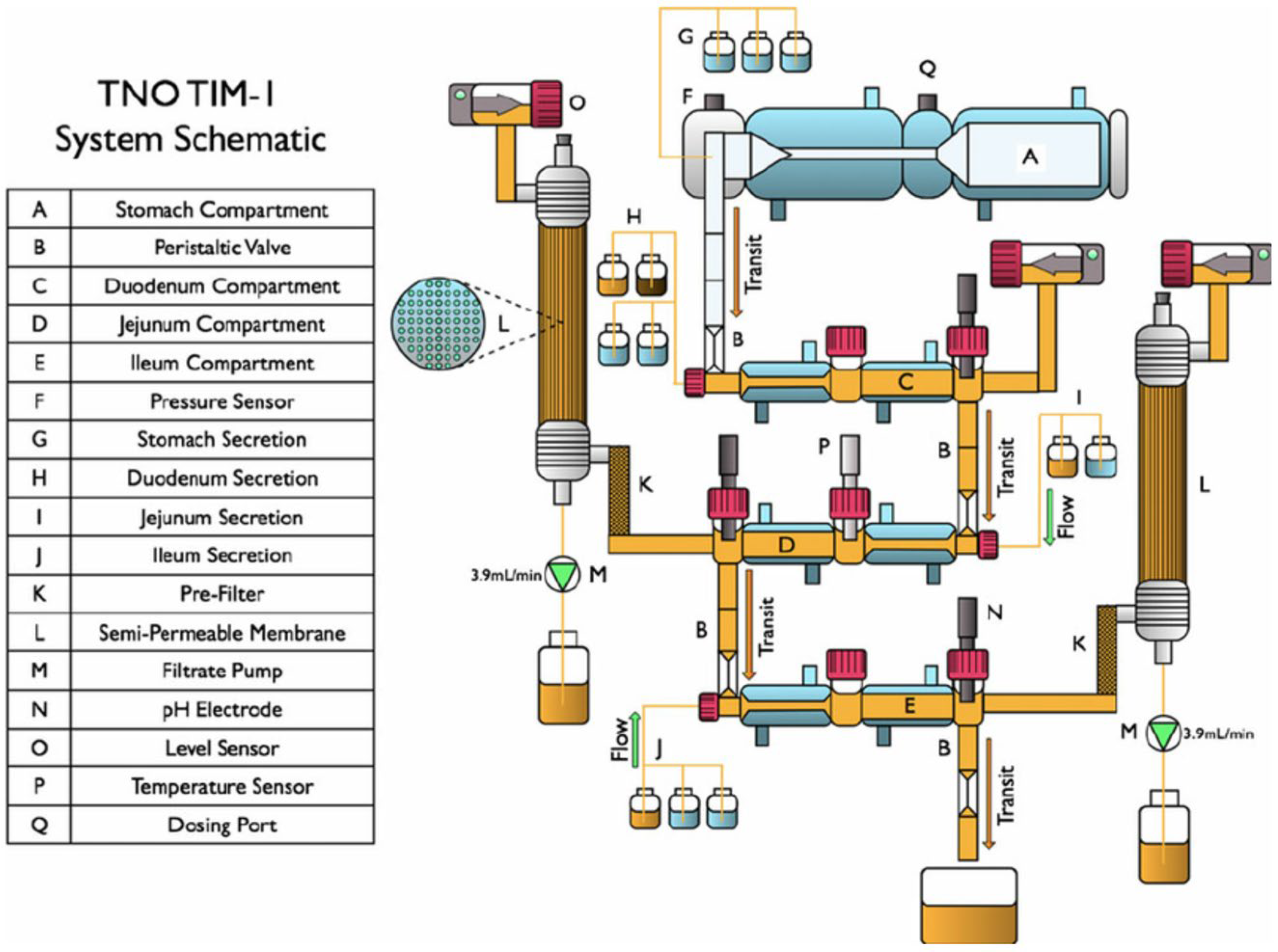
TNO TIM-1 and Permeation
4.2. In Vivo Data
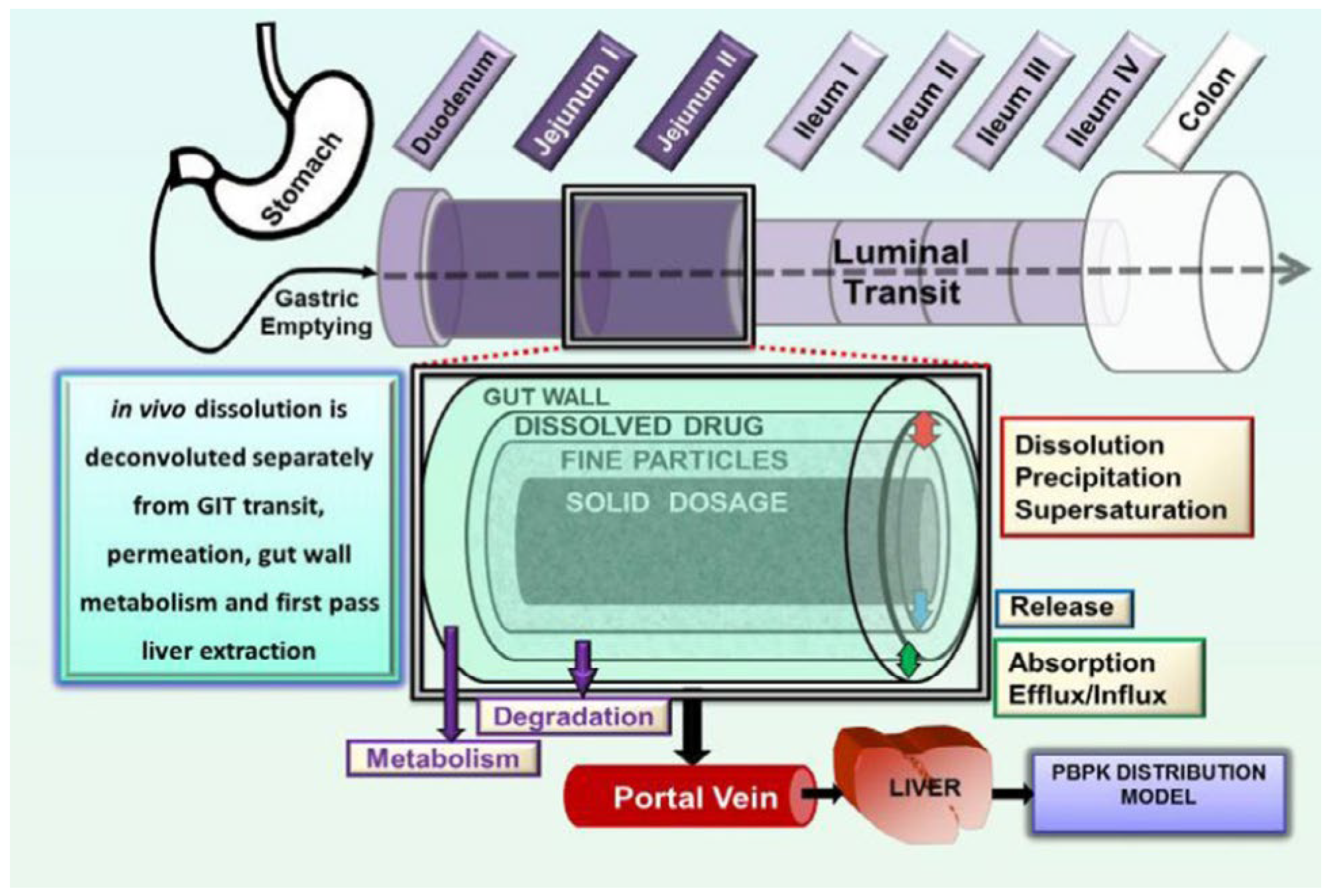
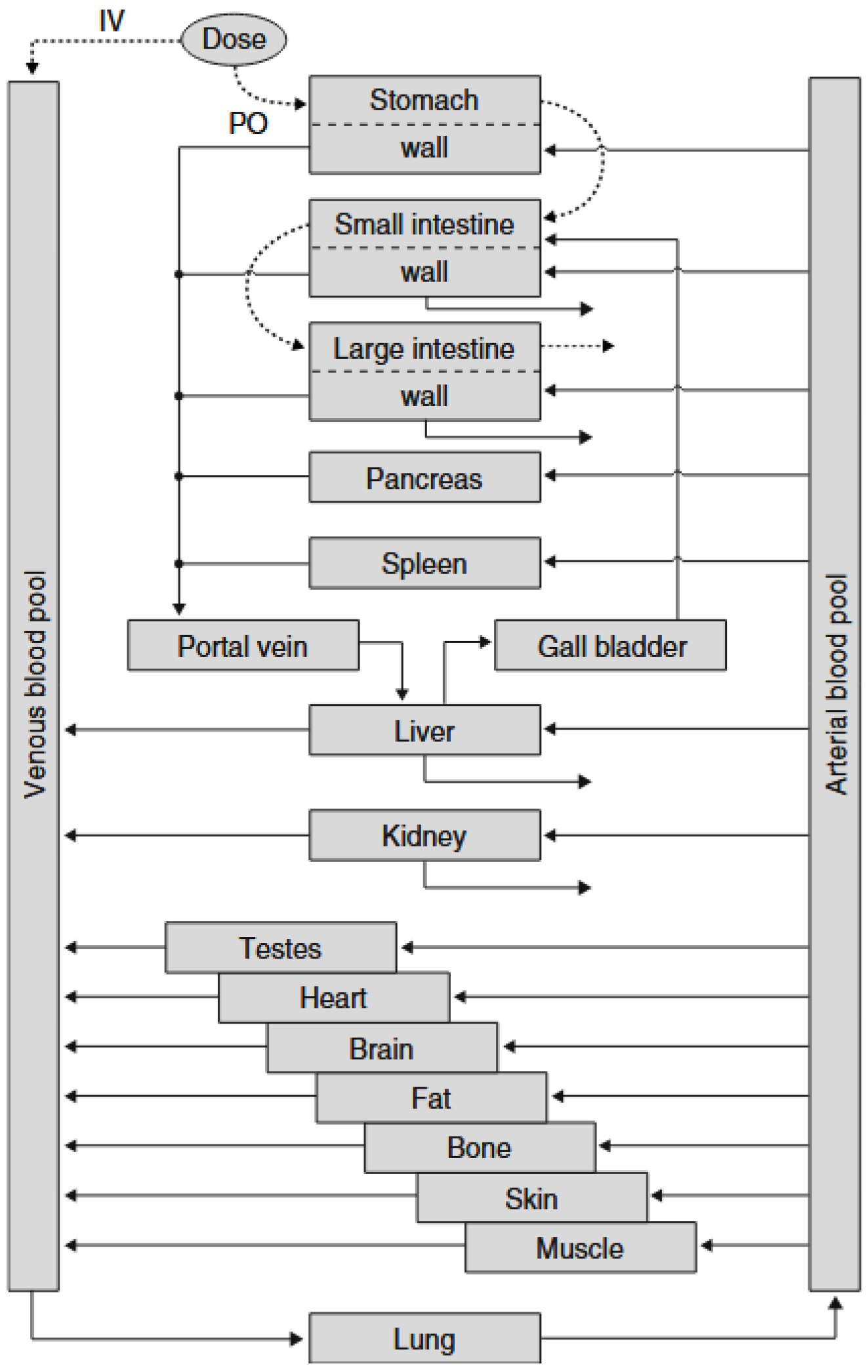
5. In Silico Models for Predicting In Vivo Performance
5.1. GastroPlus®
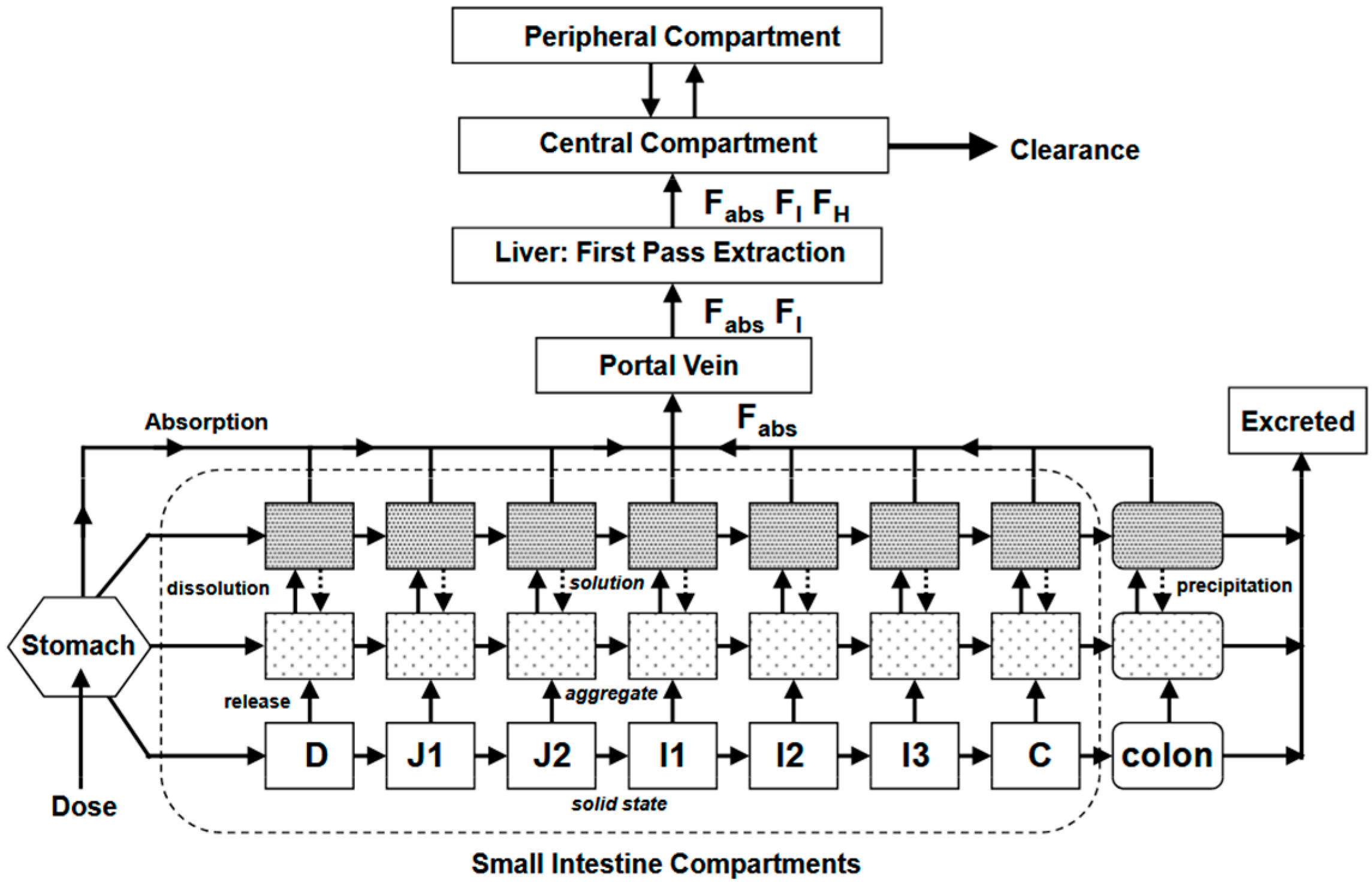
5.2. Simcyp®
5.3. gPROMS Formulated Products®
5.4. PK-Sim®
6. Proposed Strategy for IVIVC in LBFs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEDDS | Self-Emulsifying Drug Delivery System |
| SMEDDS | Self-Microemulsifying Drug Delivery System |
| SNEDDS | Self-Nanoemulsifying Drug Delivery System |
| LFCS | Lipid-Based Formulation Classification System |
| HLB | Hydrophilic–Lipophilic Balance |
| USP | United States Pharmacopeia |
| API | Active Pharmaceutical Ingredient |
| AUC | Area Under Curve |
| CMC | Critical Micellar Concentration |
| FSM | Fed Stomach Model |
| DGM | Dynamic Gastric Model |
| PBPK | Physiologically Based PharmacoKinetic |
| MDCK | Madin–Darby Canine Kidney |
| PBBM | Physiologically Based Biopharmaceutics Modeling |
| NLCs | Nanostructured Lipid Carriers |
| ACAT | Advanced Compartmental and Transit |
| ADAM | Advanced Dissolution Absorption and Metabolism |
| COAS | Computational Oral Absorption Simulation |
| GIT | Gastrointestinal tract |
| GSA | Global System Analysis |
| CR | Controlled Release |
| IR | Immediate Release |
| Papp | Apparent Permeability |
| CSR | Corporate Social Responsibility |
| SLNs | Solid Lipid Nanoparticles |
References
- Ku, M.S. Use of the Biopharmaceutical Classification System in Early Drug Development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Mohsin, K.; Shahba, A.A.; Alanazi, F.K. Lipid based self emulsifying formulations for poorly water soluble drugs-an excellent opportunity. Indian J. Pharm. Educ. Res. 2012, 46, 88–96. [Google Scholar]
- Rajesh, B.V.; Reddy, T.K.; Srikanth, G.; Mallikarjun, V.; Nivethithai, P. Lipid based self-emulsifying drug delivery system (SEDDS) for poorly water-soluble drugs: A review. J. Glob. Pharma Technol. 2010, 2, 47–55. [Google Scholar]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S93–S98. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral lipid-based drug delivery systems—An overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef]
- Kollipara, S.; Gandhi, R.K. Pharmacokinetic aspects and in vitro–in vivo correlation potential for lipid-based formulations. Acta Pharm. Sin. B 2014, 4, 333–349. [Google Scholar] [CrossRef]
- Cardot, J.-M.; Beyssac, E.; Alric, M. In Vitro–In Vivo Correlation: Importance of Dissolution in IVIVC. Dissolution Technol. 2007, 14, 15–19. [Google Scholar] [CrossRef]
- U.S. Pharmacopoeia. In Vitro and In Vivo Evaluations of Dosage Forms; Mack Publishing Co.: Easton, PA, USA, 2004. [Google Scholar]
- FDA Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation and Application of In Vitro/In Vivo Correlation (Issued 9/1997, Posted 9/26/1997)—ECA Academy. Available online: https://www.gmp-compliance.org/guidelines/gmp-guideline/fda-guidance-for-industry-extended-release-oral-dosage-forms-development-evaluation-and-application-of-in-vitro-in-vivo-correlat (accessed on 16 October 2024).
- Patel, R.; Patel, A. In vivo–In Vitro correlation (IVIVC) in drug development: Bridging preclinical and clinical outcomes for regulatory approvals. World J. Adv. Res. Rev. 2024, 22, 2311–2328. [Google Scholar] [CrossRef]
- Emami, J. In vitro-in vivo correlation: From theory to applications. J. Pharm. Pharm. Sci. 2006, 9, 169–189. [Google Scholar]
- Cámara-Martinez, I.; Blechar, J.A.; Ruiz-Picazo, A.; Garcia-Arieta, A.; Calandria, C.; Merino-Sanjuan, V.; Langguth, P.; Gonzalez-Alvarez, M.; Bermejo, M.; Al-Gousous, J.; et al. Level A IVIVC for immediate release tablets confirms in vivo predictive dissolution testing for ibuprofen. Int. J. Pharm. 2022, 614, 121415. [Google Scholar] [CrossRef]
- Do, T.T.; Van Speybroeck, M.; Mols, R.; Annaert, P.; Martens, J.; Van Humbeeck, J.; Vermant, J.; Augustijns, P.; Van den Mooter, G. The conflict between in vitro release studies in human biorelevant media and the in vivo exposure in rats of the lipophilic compound fenofibrate. Int. J. Pharm. 2011, 414, 118–124. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.; Porter, C.J. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Richter, K.; Pedersen, T.B.; Holm, R.; Müllertz, A.; Rades, T. In vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014, 16, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, N.; Cheng, X.; Dapat, E.; Sassene, P.; Eisenbrand, G.; Fricker, G.; Müllertz, A. In vitro and in vivo evaluations of the performance of an indirubin derivative, formulated in four different self-emulsifying drug delivery systems. J. Pharm. Pharmacol. 2014, 66, 1567–1575. [Google Scholar] [CrossRef]
- Larsen, A.T.; Ohlsson, A.G.; Polentarutti, B.; Barker, R.A.; Phillips, A.R.; Abu-Rmaileh, R.; Dickinson, P.A.; Abrahamsson, B.; Østergaard, J.; Müllertz, A. Oral bioavailability of cinnarizine in dogs: Relation to SNEDDS droplet size, drug solubility and in vitro precipitation. Eur. J. Pharm. Sci. 2013, 48, 339–350. [Google Scholar] [CrossRef]
- Christophersen, P.C.; Christiansen, M.L.; Holm, R.; Kristensen, J.; Jacobsen, J.; Abrahamsson, B.; Müllertz, A. Fed and fasted state gastro-intestinal in vitro lipolysis: In vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur. J. Pharm. Sci. 2014, 57, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Holm, R.; Müllertz, A.; Rades, T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J. Control. Release 2012, 160, 25–32. [Google Scholar] [CrossRef]
- Klitgaard, M.; Beilles, S.; Sassene, P.J.; Berthelsen, R.; Müllertz, A. Adding a Gastric Step to the Intestinal In Vitro Digestion Model Improves the Prediction of Pharmacokinetic Data in Beagle Dogs of Two Lipid-Based Drug Delivery Systems. Mol. Pharm. 2020, 17, 3214–3222. [Google Scholar] [CrossRef]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Yeap, Y.Y.; Anby, M.U.; Pouton, C.W.; Porter, C.J.H. Lipid-based formulations and drug supersaturation: Harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm. Res. 2013, 30, 2976–2992. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Rege, B.D.; Amidon, G.L.; Polli, J.E. Surfactant-mediated dissolution: Contributions of solubility enhancement and relatively low micelle diffusivity. J. Pharm. Sci. 2004, 93, 2064–2075. [Google Scholar] [CrossRef]
- Williams, H.D.; Anby, M.U.; Sassene, P.; Kleberg, K.; Bakala-N’Goma, J.-C.; Calderone, M.; Jannin, V.; Igonin, A.; Partheil, A.; Marchaud, D.; et al. Toward the establishment of standardized in vitro tests for lipid-based formulations. 2. The effect of bile salt concentration and drug loading on the performance of type I, II, IIIA, IIIB, and IV formulations during in vitro digestion. Mol. Pharm. 2012, 9, 3286–3300. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Chapter 10—Hyperlipidemia in cardiovascular health and digestion. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Bagchi, D., Ohia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 141–150. ISBN 978-0-12-821232-5. [Google Scholar]
- Kesharwani, R.; Jaiswal, P.; Patel, D.K.; Yadav, P.K. Lipid-Based Drug Delivery System (LBDDS): An Emerging Paradigm to Enhance Oral Bioavailability of Poorly Soluble Drugs. Biomed. Mater. Devices 2023, 1, 648–663. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of surfactant excipients in oral drug formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef]
- Shiau, Y.F. Mechanism of intestinal fatty acid uptake in the rat: The role of an acidic microclimate. J. Physiol. 1990, 421, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hussain, M.M. Gut triglyceride production. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 727–735. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: Methods and studies. Fundam. Clin. Pharma 1999, 13, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Excipients for Solubility and Bioavailability Enhancement. Available online: https://www.gattefosse.com/fr/formulation-technologies/excipients-solubility-and-bioavailability-enhancement (accessed on 16 October 2024).
- McCartney, F.; Caisse, P.; Dumont, C.; Brayden, D.J. LabrafacTM MC60 is an efficacious intestinal permeation enhancer for macromolecules: Comparisons with Labrasol® ALF in ex vivo and in vivo rat studies. Int. J. Pharm. 2024, 661, 124353. [Google Scholar] [CrossRef]
- McCartney, F.; Jannin, V.; Chevrier, S.; Boulghobra, H.; Hristov, D.R.; Ritter, N.; Miolane, C.; Chavant, Y.; Demarne, F.; Brayden, D.J. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: Ex vivo and in vivo rat studies. J. Control Release 2019, 310, 115–126. [Google Scholar] [CrossRef]
- Garg, B.; Katare, O.P.; Beg, S.; Lohan, S.; Singh, B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf. B Biointerfaces 2016, 141, 611–622. [Google Scholar] [CrossRef]
- Yousef, M.; O’Croinin, C.; Le, T.S.; Park, C.; Zuo, J.; Bou Chacra, N.; Davies, N.M.; Löbenberg, R. In Vitro Predictive Model for Intestinal Lymphatic Uptake: Exploration of Additional Enhancers and Inhibitors. Pharmaceutics 2024, 16, 768. [Google Scholar] [CrossRef]
- Caliph, S.M.; Charman, W.N.; Porter, C.J. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J. Pharm. Sci. 2000, 89, 1073–1084. [Google Scholar] [CrossRef]
- Khoo, S.-M.; Shackleford, D.M.; Porter, C.J.H.; Edwards, G.A.; Charman, W.N. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm. Res. 2003, 20, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.A.; Wang, S.W.J.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Ryšánek, P.; Grus, T.; Šíma, M.; Slanar, O. Lymphatic Transport of Drugs after Intestinal Absorption: Impact of Drug Formulation and Physicochemical Properties. Pharm. Res. 2020, 37, 166. [Google Scholar] [CrossRef]
- Uddin, R.; Saffoon, N.; Sutradhar, K.B. Dissolution and dissolution apparatus: A review. Int. J. Curr. Biomed. Pharm. Res. 2011, 1, 201–207. [Google Scholar]
- Klein, S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010, 12, 397–406. [Google Scholar] [CrossRef]
- Fei, Y.; Kostewicz, E.S.; Sheu, M.-T.; Dressman, J.B. Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro-in silico-in vivo approach. Eur. J. Pharm. Biopharm. 2013, 85, 1274–1284. [Google Scholar] [CrossRef]
- Griffin, B.T.; Kuentz, M.; Vertzoni, M.; Kostewicz, E.S.; Fei, Y.; Faisal, W.; Stillhart, C.; O’Driscoll, C.M.; Reppas, C.; Dressman, J.B. Comparison of in vitro tests at various levels of complexity for the prediction of in vivo performance of lipid-based formulations: Case studies with fenofibrate. Eur. J. Pharm. Biopharm. 2014, 86, 427–437. [Google Scholar] [CrossRef]
- Nishimura, H.; Hayashi, C.; Aiba, T.; Okamoto, I.; Miyamoto, Y.; Nakade, S.; Takeda, K.; Kurosaki, Y. Application of the correlation of in vitro dissolution behavior and in vivo plasma concentration profile (IVIVC) for soft-gel capsules--a pointless pursuit? Biol. Pharm. Bull. 2007, 30, 2221–2225. [Google Scholar] [CrossRef]
- Singh, D.; Tiwary, A.K.; Bedi, N. Canagliflozin loaded SMEDDS: Formulation optimization for improved solubility, permeability and pharmacokinetic performance. J. Pharm. Investig. 2019, 49, 67–85. [Google Scholar] [CrossRef]
- Yang, S.-G. Biowaiver extension potential and IVIVC for BCS Class II drugs by formulation design: Case study for cyclosporine self-microemulsifying formulation. Arch. Pharm. Res. 2010, 33, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Ha, E.-S.; Choo, G.-H.; Baek, I.-H. Preparation and in vivo evaluation of a dutasteride-loaded solid-supersaturatable self-microemulsifying drug delivery system. Int. J. Mol. Sci. 2015, 16, 10821–10833. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Rajnikanth, P.S.; Kumar, M.; Chidambram, K. In-Vitro and In-Vivo Evaluation of Supersaturable Self-Nanoemulsifying Drug Delivery System (SNEDDS) of Dutasteride. Curr. Drug Deliv. 2020, 17, 74–86. [Google Scholar] [CrossRef]
- Donato, E.M.; Martins, L.A.; Fröehlich, P.E.; Bergold, A.M. Development and validation of dissolution test for lopinavir, a poorly water-soluble drug, in soft gel capsules, based on in vivo data. J. Pharm. Biomed. Anal. 2008, 47, 547–552. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.-B.; Choi, S.-H.; Nguyen, T.-T.-L.; Ahn, S.-H.; Moon, K.-S.; Cho, K.-H.; Sim, T.-Y.; Heo, E.-J.; Kim, S.T.; et al. Development and Evaluation of Self-Microemulsifying Drug Delivery System for Improving Oral Absorption of Poorly Water-Soluble Olaparib. Pharmaceutics 2023, 15, 1669. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Katare, O.P.; Saini, S.; Garg, B.; Khurana, R.K.; Singh, B. Solid self-nanoemulsifying systems of olmesartan medoxomil: Formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol. 2016, 294, 93–104. [Google Scholar] [CrossRef]
- Rossi, R.C.; Dias, C.L.; Donato, E.M.; Martins, L.A.; Bergold, A.M.; Fröehlich, P.E. Development and validation of dissolution test for ritonavir soft gelatin capsules based on in vivo data. Int. J. Pharm. 2007, 338, 119–124. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, V.H.; Pedersen, B.L.; Kristensen, H.G.; Müllertz, A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur. J. Pharm. Sci. 2005, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, J.; Li, J.; Cheng, G.; Zou, M.; Piao, H. In Vitro-In Vivo Correlation for Solid Dispersion of a Poorly Water-Soluble Drug Efonidipine Hydrochloride. AAPS PharmSciTech 2020, 21, 160. [Google Scholar] [CrossRef]
- Al Zahabi, K.H.; Ben Tkhayat, H.; Abu-Basha, E.; Sallam, A.S.; Younes, H.M. Formulation of Lipid-Based Tableted Spray-Congealed Microparticles for Sustained Release of Vildagliptin: In Vitro and In Vivo Studies. Pharmaceutics 2021, 13, 2158. [Google Scholar] [CrossRef]
- Markopoulos, C.; Andreas, C.J.; Vertzoni, M.; Dressman, J.; Reppas, C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: Choosing the appropriate test media. Eur. J. Pharm. Biopharm. 2015, 93, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.; Clulow, A.; Boyd, B. Formation of Self-Assembled Mesophases During Lipid Digestion. Front. Cell Dev. Biol. 2021, 9, 657886. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Chen, Z.; Wu, W.; Zhu, Q.; Lu, Y. In vitro and in vivo correlation for lipid-based formulations: Current status and future perspectives. Acta Pharm. Sin. B 2021, 11, 2469–2487. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Kaukonen, A.M.; Boyd, B.J.; Edwards, G.A.; Charman, W.N. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm. Res. 2004, 21, 1405–1412. [Google Scholar] [CrossRef]
- Larsen, A.; Holm, R.; Pedersen, M.L.; Müllertz, A. Lipid-based formulations for danazol containing a digestible surfactant, Labrafil M2125CS: In vivo bioavailability and dynamic in vitro lipolysis. Pharm. Res. 2008, 25, 2769–2777. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: The ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur. J. Pharm. Biopharm. 2007, 67, 96–105. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Kaukonen, A.M.; Taillardat-Bertschinger, A.; Boyd, B.J.; O’Connor, J.M.; Edwards, G.A.; Charman, W.N. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: Studies with halofantrine. J. Pharm. Sci. 2004, 93, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Hoffman, A. Use of a dynamic in vitro lipolysis model to rationalize oral formulation development for poor water soluble drugs: Correlation with in vivo data and the relationship to intra-enterocyte processes in rats. Pharm. Res. 2006, 23, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- De Prá, M.A.A.; Vardanega, R.; Loss, C.G. Lipid-based formulations to increase cannabidiol bioavailability: In vitro digestion tests, pre-clinical assessment and clinical trial. Int. J. Pharm. 2021, 609, 121159. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.L.; Trevaskis, N.L.; Edwards, G.A.; Perlman, M.E.; Ambler, C.M.; Mack, M.C.; Brockhurst, B.; Porter, C.J.H. In vitro-in vivo evaluation of lipid based formulations of the CETP inhibitors CP-529,414 (torcetrapib) and CP-532,623. Eur. J. Pharm. Biopharm. 2014, 88, 973–985. [Google Scholar] [CrossRef]
- Koziolek, M.; Görke, K.; Neumann, M.; Garbacz, G.; Weitschies, W. Development of a bio-relevant dissolution test device simulating mechanical aspects present in the fed stomach. Eur. J. Pharm. Sci. 2014, 57, 250–256. [Google Scholar] [CrossRef]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The Design, Operation, and Application of a Dynamic Gastric Model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Minekus, M. The TNO Gastro-Intestinal Model (TIM); Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Available online: https://library.oapen.org/bitstream/handle/20.500.12657/28028/1/1001968.pdf#page=54 (accessed on 16 October 2024).
- Dickinson, P.A.; Abu Rmaileh, R.; Ashworth, L.; Barker, R.A.; Burke, W.M.; Patterson, C.M.; Stainforth, N.; Yasin, M. An Investigation into the Utility of a Multi-compartmental, Dynamic, System of the Upper Gastrointestinal Tract to Support Formulation Development and Establish Bioequivalence of Poorly Soluble Drugs. AAPS J. 2012, 14, 196–205. [Google Scholar] [CrossRef]
- Verwei, M.; Minekus, M.; Zeijdner, E.; Schilderink, R.; Havenaar, R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016, 498, 178–186. [Google Scholar] [CrossRef]
- Havenaar, R.; Bellmann, S. Results from a Validated in vitro Gastrointestinal Model (TIM) used as input Data for in silico Modeling Give Highly Predictive Information for the Human Situation. Med. Res. Arch. 2022, 10, 9. [Google Scholar] [CrossRef]
- Naylor, T.; Connolly, P.C.; Martini, L.G.; Elder, D.; Minekus, M.; Havenaar, R.; Zeijdner, E.E. Use of a Gastro-Intestinal Model and GastroPLUSTM for the prediction of in vivo performance. Ind. Pharm. 2006, 12, 9–12. [Google Scholar]
- Ojala, K.; Schilderink, R.; Nykänen, P.; van Veen, B.; Malmström, C.; Juppo, A.; Korjamo, T. Predicting the effect of prandial stage and particle size on absorption of ODM-204. Eur. J. Pharm. Biopharm. 2020, 156, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Thakral, N.K.; Schwabe, R.; Li, L.; Chen, S. Using Tiny-TIM Dissolution and In Silico Simulation to Accelerate Oral Product Development of a BCS Class II Compound. AAPS PharmSciTech 2022, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-C.; Liu, J.; Nagapudi, K.; Wu, R.; Dolton, M.J.; Salehi, N.; Amidon, G. Evaluating the IVIVC by Combining Tiny-tim Outputs and Compartmental PK Model to Predict Oral Exposure for Different Formulations of Ibuprofen. J. Pharm. Sci. 2022, 111, 2018–2029. [Google Scholar] [CrossRef]
- Effinger, A.; McAllister, M.; Tomaszewska, I.; O’Driscoll, C.M.; Taylor, M.; Gomersall, S.; Heaton, J.; Smith, K.L.; Sarcevica, I.; Young, S.L.; et al. Investigating the Impact of Crohn’s Disease on the Bioaccessibility of a Lipid-Based Formulation with an In Vitro Dynamic Gastrointestinal Model. Mol. Pharm. 2021, 18, 1530–1543. [Google Scholar] [CrossRef]
- Schilderink, R.; Protopappa, M.; Fleth-James, J.; Vertzoni, M.; Schaefer, K.; Havenaar, R.; Kulla, I.; Metzger, M.; Reppas, C. On the usefulness of compendial setups and tiny-TIM system in evaluating the in vivo performance of oral drug products with various release profiles in the fasted state: Case example sodium salt of A6197. Eur. J. Pharm. Biopharm. 2020, 149, 154–162. [Google Scholar] [CrossRef]
- Keemink, J.; Mårtensson, E.; Bergström, C.A.S. Lipolysis-Permeation Setup for Simultaneous Study of Digestion and Absorption in Vitro. Mol. Pharm. 2019, 16, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Schulzen, A.; Andreadis, I.I.; Bergström, C.A.S.; Quodbach, J. Development and characterization of solid lipid-based formulations (sLBFs) of ritonavir utilizing a lipolysis and permeation assay. Eur. J. Pharm. Sci. 2024, 196, 106732. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, M.; Müllertz, A.; Berthelsen, R. Estimating the Oral Absorption from Self-Nanoemulsifying Drug Delivery Systems Using an In Vitro Lipolysis-Permeation Method. Pharmaceutics 2021, 13, 489. [Google Scholar] [CrossRef]
- Bibi, H.A.; Holm, R.; Bauer-Brandl, A. Simultaneous lipolysis/permeation in vitro model, for the estimation of bioavailability of lipid based drug delivery systems. Eur. J. Pharm. Biopharm. 2017, 117, 300–307. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Crum, M.F.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Porter, C.J.H. A new in vitro lipid digestion—In vivo absorption model to evaluate the mechanisms of drug absorption from lipid-based formulations. Pharm. Res. 2016, 33, 970–982. [Google Scholar] [CrossRef]
- Alskär, L.C.; Parrow, A.; Keemink, J.; Johansson, P.; Abrahamsson, B.; Bergström, C.A.S. Effect of lipids on absorption of carvedilol in dogs: Is coadministration of lipids as efficient as a lipid-based formulation? J. Control. Release 2019, 304, 90–100. [Google Scholar] [CrossRef]
- Sirvi, A.; Jadhav, K.; Sangamwar, A.T. Enabling superior drug loading in lipid-based formulations with lipophilic salts for a brick dust molecule: Exploration of lipophilic counterions and in vitro-in vivo evaluation. Int. J. Pharm. 2024, 656, 124108. [Google Scholar] [CrossRef] [PubMed]
- Mageshvaran, D.; Yadav, S.; Yadav, V.; Kuche, K.; Katari, O.; Jain, S. Enhancing oral bioavailability of dasatinib via supersaturable SNEDDS: Investigation of precipitation inhibition and IVIVC through in-vitro lipolysis-permeation model. Int. J. Pharm. 2025, 668, 125007. [Google Scholar] [CrossRef] [PubMed]
- Alvebratt, C.; Keemink, J.; Edueng, K.; Cheung, O.; Strømme, M.; Bergström, C.A.S. An in vitro dissolution–digestion–permeation assay for the study of advanced drug delivery systems. Eur. J. Pharm. Biopharm. 2020, 149, 21–29. [Google Scholar] [CrossRef]
- Higashino, H.; Develin, C.; Higashino, C.; Lim, T.; Martin, A.; Zhou, F.; Strab, R.; Patel, R.; Bhoopathy, S.; Hidalgo, I. In vitro digestion-diffusion model for predicting in vivo performance of lipid-based formulations. J. Drug Deliv. Sci. Technol. 2023, 83, 104439. [Google Scholar] [CrossRef]
- Déat, E.; Blanquet-Diot, S.; Jarrige, J.-F.; Denis, S.; Beyssac, E.; Alric, M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: Application to the assessment of lycopene and alpha-tocopherol bioavailability from a whole food. J. Agric. Food Chem. 2009, 57, 11314–11320. [Google Scholar] [CrossRef]
- Fadhlaoui, K.; Garrait, G.; Beyssac, E.; Laine, E.; Denis, S. Modèle Dynamique d’étude In Vitro de la Perméabilité Intestinale d’une Molécule d’intérêt Couplé en Continu à un Dispositif de Dissolution ou de Digestion 2023. WO2023242518A1, 21 December 2023. Available online: https://patents.google.com/patent/WO2023242518A1/en?q=(fadhlaoui)&inventor=khaled&oq=khaled+fadhlaoui+ (accessed on 2 September 2025).
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 238. [Google Scholar]
- Musther, H.; Olivares-Morales, A.; Hatley, O.J.D.; Liu, B.; Rostami Hodjegan, A. Animal versus human oral drug bioavailability: Do they correlate? Eur. J. Pharm. Sci. 2014, 57, 280–291. [Google Scholar] [CrossRef]
- Anand, O.; Pepin, X.J.H.; Kolhatkar, V.; Seo, P. The Use of Physiologically Based Pharmacokinetic Analyses—In Biopharmaceutics Applications -Regulatory and Industry Perspectives. Pharm. Res. 2022, 39, 1681–1700. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, N.; Zhao, X.; Wang, Z.; Liu, Z.; Cui, Y. Application of physiologically based pharmacokinetic modeling of novel drugs approved by the U.S. food and drug administration. Eur. J. Pharm. Sci. 2024, 200, 106838. [Google Scholar] [CrossRef] [PubMed]
- Reppas, C.; Kuentz, M.; Bauer-Brandl, A.; Carlert, S.; Dallmann, A.; Dietrich, S.; Dressman, J.; Ejskjaer, L.; Frechen, S.; Guidetti, M.; et al. Leveraging the use of in vitro and computational methods to support the development of enabling oral drug products: An InPharma commentary. Eur. J. Pharm. Sci. 2023, 188, 106505. [Google Scholar] [CrossRef] [PubMed]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50 (Suppl. 1), S41–S67. [Google Scholar] [CrossRef]
- Bolger, M.B. GastroPlus: Mechanistic Deconvolution and the Future Role of Physiological Modeling in IVIVC; Simulations Plus, Inc.: Lancaster CA, USA, 2012. [Google Scholar]
- Chow, E.C.Y.; Pang, K.S. Why we need proper PBPK models to examine intestine and liver oral drug absorption. Curr. Drug Metab. 2013, 14, 57–79. [Google Scholar] [CrossRef]
- Ali, H.; Verma, P.R.P.; Dubey, S.K.; Venkatesan, J.; Seo, Y.; Kim, S.-K.; Singh, S.K. In vitro–in vivo and pharmacokinetic evaluation of solid lipid nanoparticles of furosemide using GastroplusTM. RSC Adv. 2017, 7, 33314–33326. [Google Scholar] [CrossRef]
- Aljurbui, S.J.; Hussain, A.; Yusuf, M.; Ramzan, M.; Afzal, O.; Almohaywi, B.; Yasmin, S.; Altamimi, A.S.A. Impact of Composition and Morphology of Ketoconazole-Loaded Solid Lipid Nanoparticles on Intestinal Permeation and Gastroplus-Based Prediction Studies. ACS Omega 2022, 7, 22406–22420. [Google Scholar] [CrossRef]
- O’Shea, J.P.; Faisal, W.; Ruane-O’Hora, T.; Devine, K.J.; Kostewicz, E.S.; O’Driscoll, C.M.; Griffin, B.T. Lipidic dispersion to reduce food dependent oral bioavailability of fenofibrate: In vitro, in vivo and in silico assessments. Eur. J. Pharm. Biopharm. 2015, 96, 207–216. [Google Scholar] [CrossRef]
- Hussain, A.; Shakeel, F.; Singh, S.K.; Alsarra, I.A.; Faruk, A.; Alanazi, F.K.; Peter Christoper, G.V. Solidified SNEDDS for the oral delivery of rifampicin: Evaluation, proof of concept, in vivo kinetics, and in silico GastroPlusTM simulation. Int. J. Pharm. 2019, 566, 203–217. [Google Scholar] [CrossRef]
- Ćetković, Z.; Cvijić, S.; Vasiljević, D. Formulation and characterization of novel lipid-based drug delivery systems containing polymethacrylate polymers as solid carriers for sustained release of simvastatin. J. Drug Deliv. Sci. Technol. 2019, 53, 101222. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; AbuRuz, S. Development and In Vitro Evaluation of Controlled Release Viagra® Containing Poloxamer-188 Using GastroplusTM PBPK Modeling Software for In Vivo Predictions and Pharmacokinetic Assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Pettarin, M.; Bolger, M.B.; Chronowska, M.; Kostewicz, E.S. A combined in vitro in-silico approach to predict the oral bioavailability of borderline BCS Class II/IV weak base albendazole and its main metabolite albendazole sulfoxide. Eur. J. Pharm. Sci. 2020, 155, 105552. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Aarons, L.; Bergstrand, M.; Bolger, M.B.; Galetin, A.; Hatley, O.; Jamei, M.; Lloyd, R.; Pepin, X.; Rostami-Hodjegan, A.; et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Simcyp PBPK. Available online: https://www.certara.com/software/simcyp-pbpk/ (accessed on 16 October 2024).
- Hens, B.; Pathak, S.M.; Mitra, A.; Patel, N.; Liu, B.; Patel, S.; Jamei, M.; Brouwers, J.; Augustijns, P.; Turner, D.B. In Silico Modeling Approach for the Evaluation of Gastrointestinal Dissolution, Supersaturation, and Precipitation of Posaconazole. Mol. Pharm. 2017, 14, 4321–4333. [Google Scholar] [CrossRef]
- Howgate, E.M.; Rowland Yeo, K.; Proctor, N.J.; Tucker, G.T.; Rostami-Hodjegan, A. Prediction of in vivo drug clearance from in vitro data. I: Impact of inter-individual variability. Xenobiotica 2006, 36, 473–497. [Google Scholar] [CrossRef]
- Mechanistic IVIVC Using the Simcyp ADAM Model. Available online: https://pqri.org/wp-content/uploads/2015/08/pdf/Turner.pdf (accessed on 23 October 2024).
- Rakhit, A.; Pantze, M.P.; Fettner, S.; Jones, H.M.; Charoin, J.-E.; Riek, M.; Lum, B.L.; Hamilton, M. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: Computer-based simulation (SimCYPTM) predicts in vivo metabolic inhibition. Eur. J. Clin. Pharmacol. 2008, 64, 31–41. [Google Scholar] [CrossRef]
- Youdim, K.A.; Zayed, A.; Dickins, M.; Phipps, A.; Griffiths, M.; Darekar, A.; Hyland, R.; Fahmi, O.; Hurst, S.; Plowchalk, D.R.; et al. Application of CYP3A4 in vitro data to predict clinical drug–drug interactions; predictions of compounds as objects of interaction. Br. J. Clin. Pharma 2008, 65, 680–692. [Google Scholar] [CrossRef]
- Hyland, R.; Dickins, M.; Collins, C.; Jones, H.; Jones, B. Maraviroc: In vitro assessment of drug–drug interaction potential. Br. J. Clin. Pharma 2008, 66, 498–507. [Google Scholar] [CrossRef]
- Arora, S.; Pansari, A.; Kilford, P.; Jamei, M.; Gardner, I.; Turner, D.B. Biopharmaceutic In Vitro In Vivo Extrapolation (IVIV_E) Informed Physiologically-Based Pharmacokinetic Model of Ritonavir Norvir Tablet Absorption in Humans Under Fasted and Fed State Conditions. Mol. Pharm. 2020, 17, 2329–2344. [Google Scholar] [CrossRef]
- gPROMS FormulatedProducts. Available online: https://www.siemens.com/global/en/products/automation/industry-software/gproms-digital-process-design-and-operations/gproms-modelling-environments/gproms-formulatedproducts.html (accessed on 16 October 2024).
- Bahr, M.N.; Modi, D.; Patel, S.; Campbell, G.; Stockdale, G. Understanding the Role of Sodium Lauryl Sulfate on the Biorelevant Solubility of a Combination of Poorly Water-Soluble Drugs Using High Throughput Experimentation and Mechanistic Absorption Modeling. J. Pharm. Pharm. Sci. 2019, 22, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Louit, G.; Ibrahim, F. The Use of Global Sensitivity Analysis to Assess the Oral Absorption of Weakly Basic Compounds: A Case Example of Dipyridamole. Pharm. Res. 2024, 41, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Thelen, K.; Jantratid, E. Towards quantitative prediction of oral drug absorption. Clin. Pharmacokinet. 2008, 47, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Willmann, S.; Thelen, K.; Lippert, J. Integration of dissolution into physiologically-based pharmacokinetic models III: PK-Sim®. J. Pharm. Pharmacol. 2012, 64, 997–1007. [Google Scholar] [CrossRef]
- Willmann, S.; Thelen, K.; Becker, C.; Dressman, J.B.; Lippert, J. Mechanism-based prediction of particle size-dependent dissolution and absorption: Cilostazol pharmacokinetics in dogs. Eur. J. Pharm. Biopharm. 2010, 76, 83–94. [Google Scholar] [CrossRef]
- Willmann, S.; Schmitt, W.; Keldenich, J.; Dressman, J.B. A physiologic model for simulating gastrointestinal flow and drug absorption in rats. Pharm. Res. 2003, 20, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Willmann, S.; Edginton, A.N.; Dressman, J.B. Development and validation of a physiology-based model for the prediction of oral absorption in monkeys. Pharm. Res. 2007, 24, 1275–1282. [Google Scholar] [CrossRef]
- Thelen, K.; Jantratid, E.; Dressman, J.B.; Lippert, J.; Willmann, S. Analysis of nifedipine absorption from soft gelatin capsules using PBPK modeling and biorelevant dissolution testing. J. Pharm. Sci. 2010, 99, 2899–2904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourderi-Cambon, A.; Fadhlaoui, K.; Garrait, G.; Lainé, E.; Dhifallah, I.; Rossano, M.; Caisse, P.; Beyssac, E. Improving In Vitro–In Vivo Correlation (IVIVC) for Lipid-Based Formulations: Overcoming Challenges and Exploring Opportunities. Pharmaceutics 2025, 17, 1310. https://doi.org/10.3390/pharmaceutics17101310
Bourderi-Cambon A, Fadhlaoui K, Garrait G, Lainé E, Dhifallah I, Rossano M, Caisse P, Beyssac E. Improving In Vitro–In Vivo Correlation (IVIVC) for Lipid-Based Formulations: Overcoming Challenges and Exploring Opportunities. Pharmaceutics. 2025; 17(10):1310. https://doi.org/10.3390/pharmaceutics17101310
Chicago/Turabian StyleBourderi-Cambon, Arnaud, Khaled Fadhlaoui, Ghislain Garrait, Emmanuelle Lainé, Imen Dhifallah, Manon Rossano, Philippe Caisse, and Eric Beyssac. 2025. "Improving In Vitro–In Vivo Correlation (IVIVC) for Lipid-Based Formulations: Overcoming Challenges and Exploring Opportunities" Pharmaceutics 17, no. 10: 1310. https://doi.org/10.3390/pharmaceutics17101310
APA StyleBourderi-Cambon, A., Fadhlaoui, K., Garrait, G., Lainé, E., Dhifallah, I., Rossano, M., Caisse, P., & Beyssac, E. (2025). Improving In Vitro–In Vivo Correlation (IVIVC) for Lipid-Based Formulations: Overcoming Challenges and Exploring Opportunities. Pharmaceutics, 17(10), 1310. https://doi.org/10.3390/pharmaceutics17101310




