The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils
2.2. Determination of EO Components by Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. Cervicovaginal Sample Collection and Cytological Evaluation
2.4. Microbial Growth of Cervicovaginal Samples
2.5. Microorganisms
2.6. Microbial Identification of the Cervicovaginal Isolate and Evolutionary Analysis
2.7. Microbial Growth Conditions and Harvesting
2.8. Determination of Minimum Inhibitory Concentration (MIC), Minimum Fungicidal Concentration (MFC) and Zone of Inhibition (ZOI) of Tz and Ma EOs
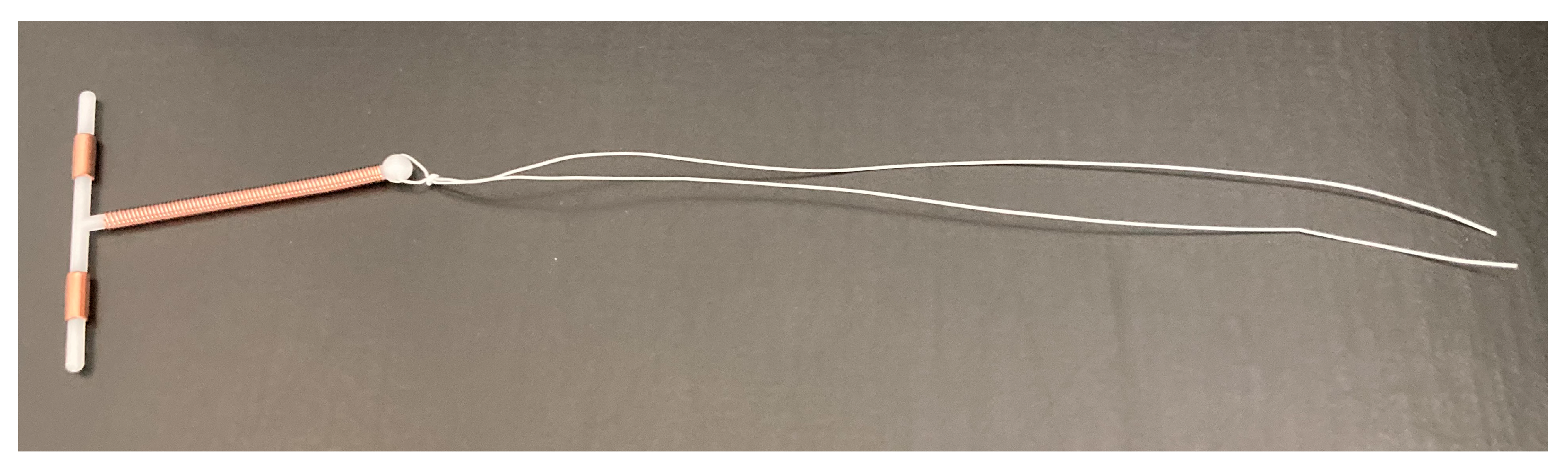
2.9. Preparation and Characterization of Tz EO-Coated IUD Strings
2.10. Antifungal Effect of Tz EO-Coated IUD Strings
2.11. Biofilm Inhibitory Effect of Tz EO-Coated IUD Strings
2.12. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Analysis
2.13. Statistical Analysis
2.14. Sequencing Data
3. Results
3.1. Components of Ma and Tz EOs Estimated by GC–MS Analysis
3.2. Cytological Evaluation of Cervicovaginal Sample and Tested Candida Strains
3.3. Antimicrobial Activity of Ma and Tz EOs
3.4. Antimicrobial and Biofilm Inhibitory Effect of Tz EO-Coated IUD Strings
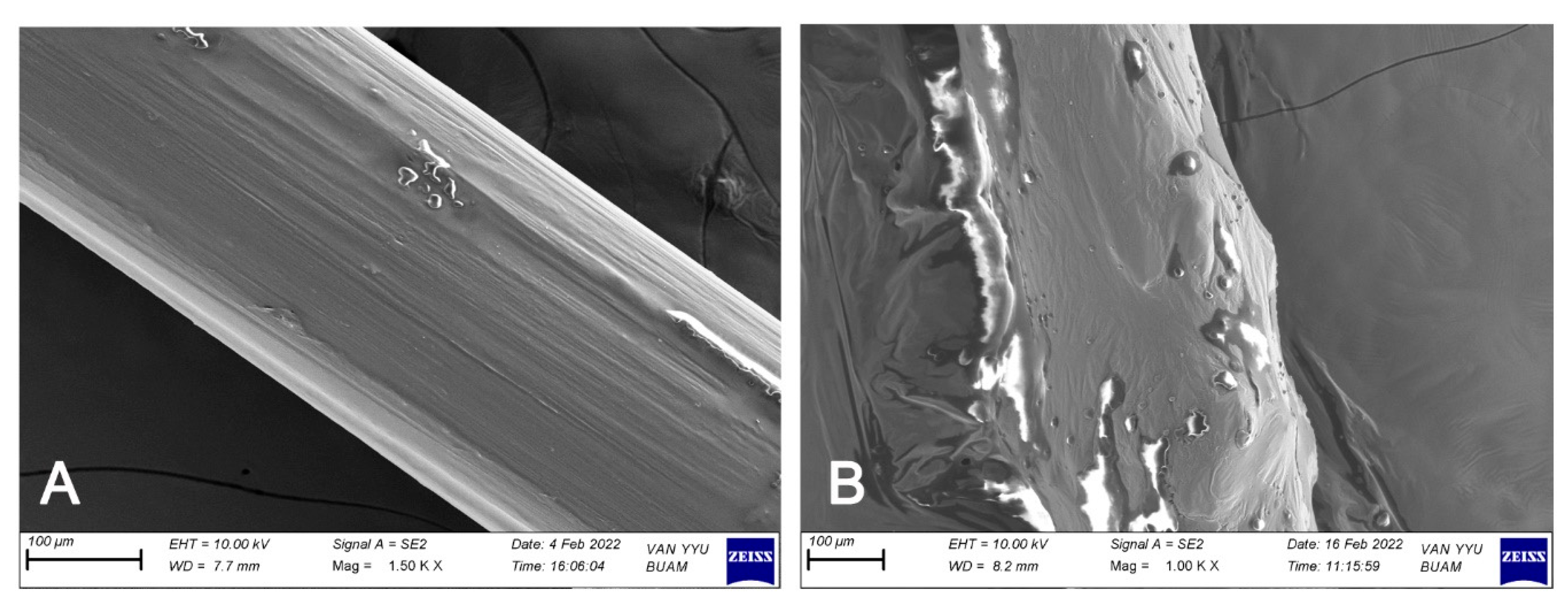
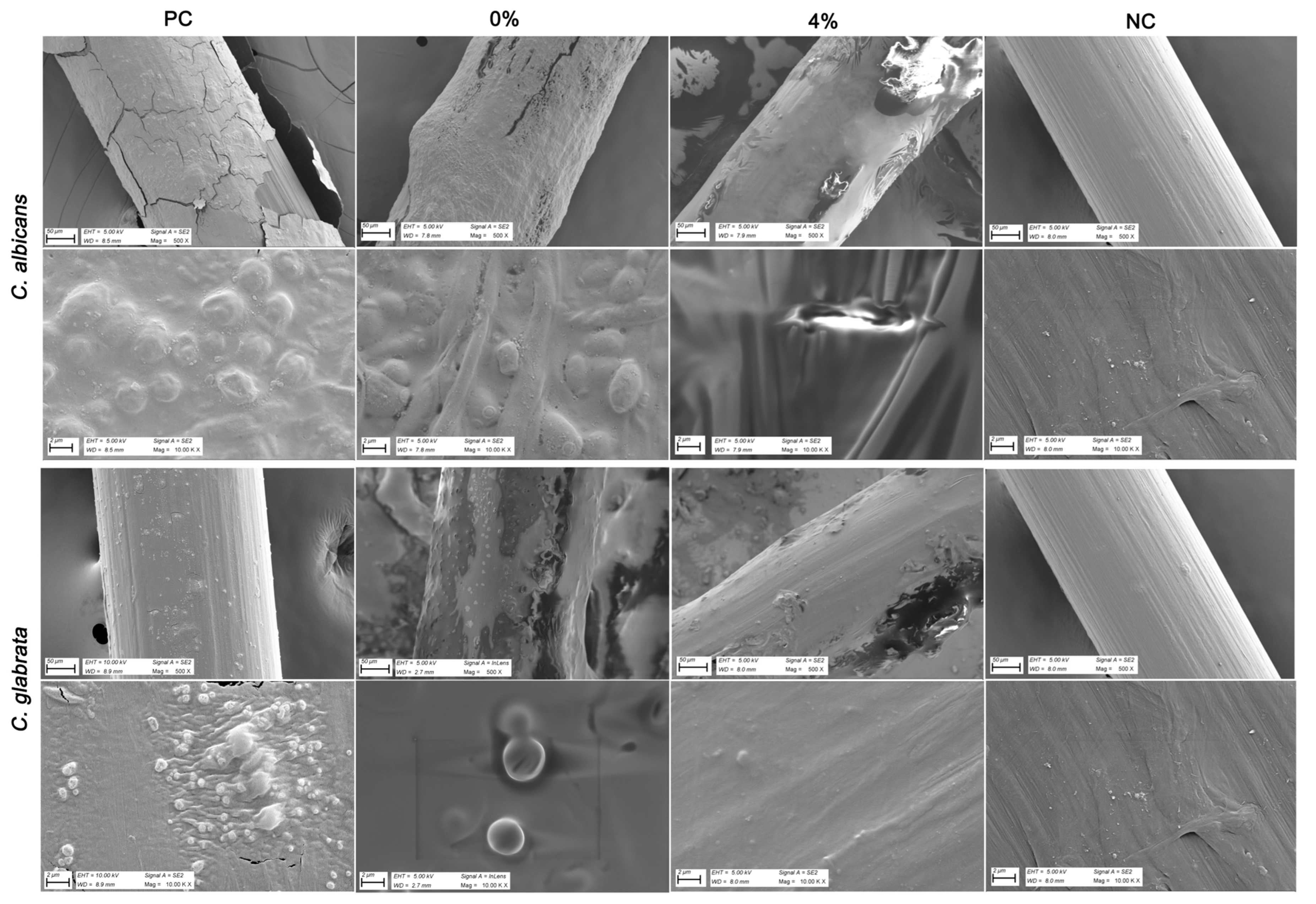
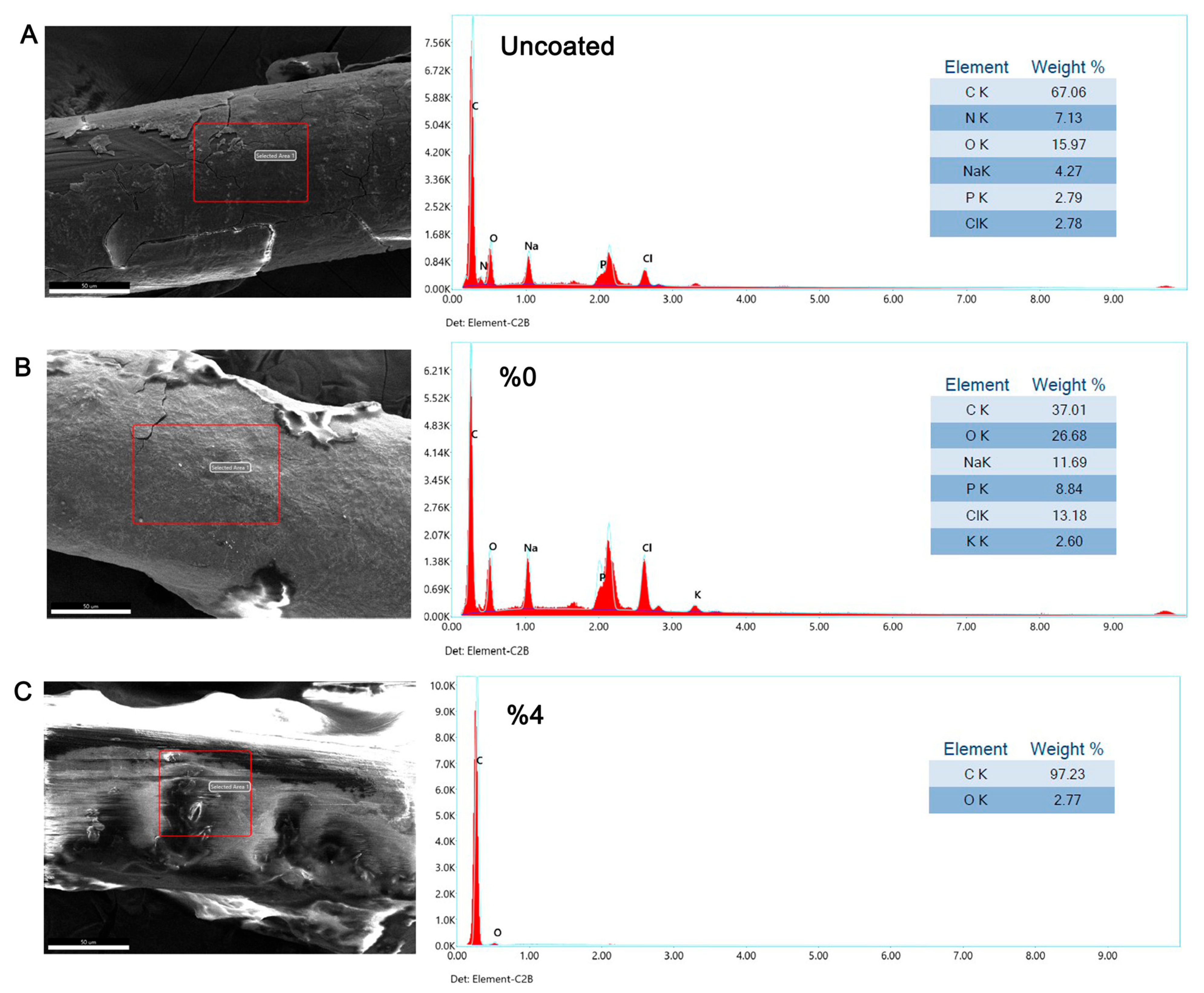
3.5. Inhibitory Effect of Tz EO-Coated IUD Strings by Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHI | Brain Heart Infusion |
| EDX | Energy Dispersive X-ray Spectroscopy |
| EO | Essential Oil |
| FNA | Formularium der Nederlandse Apothekers (Formulary of Dutch Pharmacists) |
| IUD | Intrauterine Device |
| Ma | Melaleuca alternifolia |
| MIC | Minimum Inhibitory Concentration |
| MFC | Minimum Fungicidal Concentration |
| SEM | Scanning Electron Microscopy |
| Tz | Thymus zygis |
| VVC | Vulvovaginal Candidiasis |
| ZOI | Zone of Inhibition |
References
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal Candidiasis: Epidemiology, Microbiology and Risk Factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Chassot, F.; Negri, M.F.N.; Svidzinski, A.E.; Donatti, L.; Peralta, R.M.; Svidzinski, T.I.E.; Consolaro, M.E.L. Can Intrauterine Contraceptive Devices Be a Candida albicans Reservoir? Contraception 2008, 77, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Fößleitner, P. Vulvovaginal candidosis. Die Gynäkologie 2025, 58, 93–100. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Livieratos, A.; Asimos, K.; Donders, F.; Donders, G.G.G. Fluconazole-Resistant Vulvovaginal Candidosis: An Update on Current Management. Pharmaceutics 2024, 16, 1555. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V.; Mitchell, A.P.; Andes, D.R. Fungal Biofilms, Drug Resistance, and Recurrent Infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Ceballos-Garzon, A.; Lebrat, J.; Holzapfel, M.; Josa, D.F.; Welsch, J.; Mercer, D. Antibiofilm activity of manogepix, ibrexafungerp, amphotericin B, rezafungin, and caspofungin against Candida spp. biofilms of reference and clinical strains. Antimicrob. Agents Chemother. 2025, 69, e0013725. [Google Scholar] [CrossRef] [PubMed]
- Fortney, J.A.; Feldblum, P.J.; Raymond, E.G. Intrauterine Devices. The Optimal Long-Term Contraceptive Method? J. Reprod. Med. 1999, 44, 269–274. [Google Scholar]
- Lanzola, E.L.; Ketvertis, K. Intrauterine Device. In StatPearls; 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557403/ (accessed on 28 July 2025).
- Drugs.com. Intrauterine Device. Available online: https://www.drugs.com/cg/intrauterine-device.html (accessed on 28 July 2025).
- Omran, E.A.; Youssef, N.E.S.; Abdelfattah, A.H.; Esmail, S.A.; Fouad, A.M. Copper IUD Increases Virulence of Non-albicans Candida Species Isolated from Women with Vulvovaginal Candidiasis. Eur. J. Contracept. Reprod. Health Care 2020, 25, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.C.F.; Donatti, L.; Patussi, E.V.; Svizdinski, T.I.E.; Lopes-Consolaro, M.E. Scanning Electron and Confocal Scanning Laser Microscopy Imaging of the Ultrastructure and Viability of Vaginal Candida albicans and Non-Albicans Species Adhered to an Intrauterine Contraceptive Device. Microsc. Microanal. 2010, 16, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Zahran, K.M.; Agban, M.N.; Ahmed, S.H.; Hassan, E.A.; Sabet, M.A. Patterns of Candida Biofilm on Intrauterine Devices. J. Med. Microbiol. 2015, 64, 375–381. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Prieto-Garcia, J.M.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy, 4th ed.; Elsevier Limited: Amsterdam, The Netherlands, 2024. [Google Scholar]
- European Directorate for the Quality of Medicines (EDQM). 5.30. Monographs of Essential Oils (Information Chapter). In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023; Available online: https://www.edqm.eu/en/-/essential-oils-revised-monograph-and-new-general-chapter-in-the-ph.-eur. (accessed on 28 July 2025).
- Reddy, V.P.; Kandisa, R.V.; Varsha, P.V.; Satyam, S. Review on Thymus vulgaris Traditional Uses and Pharmacological Properties. Med. Aromat. Plants 2014, 3, 164. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules 2021, 26, 4937. [Google Scholar] [CrossRef]
- Tadtong, S.; Chantavacharakorn, R.; Khayankan, S.; Akachaipaibul, P.; Eiamart, W.; Samee, W. Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari against Malassezia furfur. Int. J. Mol. Sci. 2025, 26, 5601. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, D.; Xiang, Y.; Jiang, X.; Liu, J.; Bi, K.; Dong, X.; Wu, T.; Zhang, Y. Antifungal Activity of Essential Oils and Their Potential Synergistic Effect with Amphotericin B. Sci. Rep. 2024, 14, 31125. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef]
- Vukić, M.D.; Čmiková, N.; Hsouna, A.B.; Saad, R.B.; Garzoli, S.; Schwarzová, M.; Vuković, N.L.; Obradović, A.D.; Matić, M.M.; Waszkiewicz-Robak, B.; et al. Thymus zygis, valuable antimicrobial (in vitro and in situ) and antibiofilm agent with potential antiproliferative effects. Plants 2023, 12, 3920. [Google Scholar] [CrossRef]
- Radi, F.; Bouhrim, M.; Mechchate, H.; Al Zahrani, M. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Ind. Crops Prod. 2022, 177, 114369. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.G.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal Activity of Thymus Oils and Their Major Compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal Effects of Melaleuca alternifolia (Tea Tree) Oil and Its Components on Candida albicans, Candida glabrata and Saccharomyces Cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Salvatore, G.; Cassone, A. In Vitro and in Vivo Activity of Tea Tree Oil against Azole-Susceptible and Resistant Human Pathogenic Yeasts. J. Antimicrob. Chemother. 2003, 51, 1223–1229. [Google Scholar] [CrossRef]
- Beksac, K.; Sahal, G.; Donmez, H.G. Thyme Essential Oil as an Antimicrobial and Biofilm Inhibitory Agent against Abscesses with Proteus mirabilis Infections. J. Herb. Med. 2021, 28, 100446. [Google Scholar] [CrossRef]
- Donmez, H.G.; Sahal, G.; Akgör, U.; Cagan, M.; Ozgul, N.; Beksac, M.S. The Relationship between the Presence of HPV Infection and Biofilm Formation in Cervicovaginal Smears. Infection 2020, 48, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Wilbur, D.C. The Pap Test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 324–326. [Google Scholar] [CrossRef]
- Katz, D.S. The Streak Plate Protocol; American Society for Microbiology Microbe Library: Washington, DC, USA, 2008; pp. 1–10. [Google Scholar]
- Sahal, G.; Bilkay, I.S. Distribution of Clinical Isolates of Candida spp. and Antifungal Susceptibility of High Biofilm-Forming Candida Isolates. Rev. Inst. Med. Trop. São Paulo 2018, 60, 644–650. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and Biofilm Inhibitory Effect of Cymbopogon citratus (Lemongrass) Essential Oil on Biofilm Forming by Candida tropicalis Isolates: An in Vitro Study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef] [PubMed]
- Sahal, G.; Donmez, H.G.; Beksac, M.S. Chemical Characterization of Thymus zygis L. (Thyme) and Elettaria cardamomum L. (Cardamom) Essential Oils and Investigation of Their Inhibitory Potentials Against Cervicovaginal Escherichia coli Isolates. Eur. J. Integr. Med. 2024, 68, 102366. [Google Scholar] [CrossRef]
- Wijesinghe, G.; Dilhari, A.; Gayani, B.; Kottegoda, N.; Samaranayake, L.; Weerasekera, M. Influence of Laboratory Culture Media on In Vitro Growth, Adhesion, and Biofilm Formation of Pseudomonas aeruginosa and Staphylococcus aureus Mono- and Dual-Species Cultures. Med. Princ. Pract. 2019, 28, 28–35. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Mei, J.; Xie, J. Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure. Int. J. Mol. Sci. 2023, 24, 11066. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2012; pp. 1–13. [Google Scholar]
- Wang, Y.; Zeng, H.; Tian, J.; Zheng, Y.; Ban, X.; Zeng, J.; Mao, Y. In Vitro and In Vivo Activities of Essential Oil from the Seed of Anethum graveolens L. against Candida spp. Evid.-Based Complement. Altern. Med. 2011, 2011, 659704. [Google Scholar] [CrossRef]
- Sookto, T.; Srithavaj, T.; Thaweboon, S.; Thaweboon, B.; Shrestha, B. In vitro effects of Salvia officinalis L. essential oil on Candida albicans. Asian Pac. J. Trop. Biomed. 2013, 3, 376–380. [Google Scholar] [CrossRef]
- Russel, C.; Melhorn, S. Chapter 15. Oropharynx. In Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Le Brun, P., Crauste-Manciet, S., Krämer, I., Smith, J., Woerdenbag, H., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 341–342. [Google Scholar]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Farmacie (KNMP). (Royal Dutch Pharmacists Association), Hypromellosezalf 20% FNA (February 2016). In Formularium der Nederlandse Apothekers (FNA); KNMP: Den Haag, The Netherlands, 2016; Available online: https://kennisbank.knmp.nl (accessed on 28 July 2025).
- Hellier, S.D.; Wrynn, A.F. Beyond fluconazole: A review of vulvovaginal candidiasis diagnosis and treatment. Nurse Pract. 2023, 48, 33–39. [Google Scholar] [CrossRef]
- Özer, T.; Cengiz, T.; Yılmaz, H. Prevalence of Vulvovaginal Candidiasis and Treatment with Isoconazol Nitrate. J. Immunol. Clin. Microbiol. 2018, 3, 1. [Google Scholar] [CrossRef]
- de Cássia Orlandi Sardi, J.; Silva, D.R.; Anibal, P.C.; de Campos Baldin, J.J.C.M.; Ramalho, S.R.; Rosalen, P.L.; Macedo, M.L.R.; Hofling, J.F. Vulvovaginal Candidiasis: Epidemiology and Risk Factors, Pathogenesis, Resistance, and New Therapeutic Options. Curr. Fungal Infect. Rep. 2021, 15, 32–40. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Bekhof, A.M.W.; van de Koppel, S.; van Hunsel, F.P.A.M.; Woerdenbag, H.J. Safety Assessment and Adverse Drug Reaction Reporting of Tea Tree Oil (Melaleuca aetheroleum). Phytother. Res. 2023, 37, 1309–1318. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 40558. [Google Scholar] [CrossRef]
- Peixoto, P.M.C.; Júlio, A.A.; de Jesus, E.G.; Venancio, A.N.; Parreira, L.A.; Santos, M.F.C.; Menini, L. Fungicide Potential of Citronella and Tea Tree Essential Oils against Tomato-Cultivation Phytopathogen Fusarium oxysporum f. sp. lycopersici and Analysis of Their Chemical Composition by GC/MS. Nat. Prod. Res. 2024, 38, 667–672. [Google Scholar] [CrossRef]
- Rhind, J.P. Essential Oils: A Comprehensive Handbook for Aromatic Therapy, 3rd ed.; Singing Dragon: London, UK; Philadelphia, PA, USA, 2020; pp. 452–453 [Tz]; 488–493 [Ma]. [Google Scholar]
- Vasile, C.; Sivertsvik, M.; Miteluţ, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tănase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative analysis of the composition and active property evaluation of certain essential oils to assess their potential applications in active food packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Aslani, N.; Kokabi, R.; Moradi, F.; Abbasi, K.; Vaseghi, N.; Afsarian, M.H. Characterization of Candida Species Isolated from Vulvovaginal Candidiasis by MALDI-TOF with in vitro Antifungal Susceptibility Profiles. Curr. Med. Mycol. 2021, 7, 6–11. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y. Apoptotic Effects of Thymol, a Novel Monoterpene Phenol, on Different Types of Cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, N.L.; Ribbeck, K. Selected Antimicrobial Essential Oils Eradicate Pseudomonas spp. and Staphylococcus aureus Biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Šegvić Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal Activity of Thyme (Thymus vulgaris L.) Essential Oil and Thymol Against Moulds from Damp Dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents Against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Assessment Report on Thymus vulgaris L., Thymus zygis L., Aetheroleum. 8 July 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis-l-aetheroleum-revision-1_en.pdf (accessed on 28 July 2025).
- European Medicines Agency (EMA). European Union Herbal Monograph on Thymus vulgaris L., Thymus zygis L., Aetheroleum. 8 July 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-thymus-vulgaris-l-thymus-zygis-l-aetheroleum-revision-1_en.pdf (accessed on 28 July 2025).
- European Medicines Agency (EMA). Assessment Report on Melaleuca alternifolia (Maiden & Betch) Cheel, M. linariifolia Smith, M. dissitiflora F. Mueller and/or Other Species of Melaleuca, Aetheroleum. 24 November 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-melaleuca-alternifolia-maiden-betch-cheel-m-linariifolia-smith-m/other-species-melaleuca-aetheroleum-first-version_en.pdf (accessed on 28 July 2025).
- European Medicines Agency (EMA). European Union Herbal Monograph on Melaleuca alternifolia (Maiden & Betch) Cheel, M. linariifolia Smith, M. dissitiflora F. Mueller and/or Other Species of Melaleuca, Aetheroleum. 24 November 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-melaleuca-alternifolia-maiden-betch-cheel-m-linariifolia-smith/other-species-melaleuca-aetheroleum-first-version_en.pdf (accessed on 28 July 2025).
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity Screening of Thymus vulgaris L. Essential Oil in Brine Shrimp Nauplii and Cancer Cell Lines. Sci. Rep. 2021, 11, 92679. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal Activity of Thymol and Carvacrol by Disrupting Ergosterol Biosynthesis and Membrane Integrity Against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S.F. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Safety Assessment of Hydroxypropyl Methylcellulose as a Food Ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Khosrowshahi Asl, A. Effect of Various Additives on the Properties of the Films and Coatings Derived from Hydroxypropyl Methylcellulose—A Review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef]
- Denstedt, J.D.; Wollin, T.A.; Reid, G. Biomaterials Used in Urology: Current Issues of Biocompatibility, Infection, and Encrustation. J. Endourol. 1998, 12, 493–500. [Google Scholar] [CrossRef]
- George, S.; Kishen, A.; Song, K.P. The Role of Environmental Changes on Monospecies Biofilm Formation on Root Canal Wall by Enterococcus faecalis. J. Endod. 2005, 31, 867–872. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.J.C.; Jamieson, H.E.; Cullimore, D.R. Formation of Nesquehonite and Other Minerals as a Consequence of Biofilm Dehydration. Geomicrobiol. J. 1997, 14, 25–27. [Google Scholar] [CrossRef]
- Casalini, S.; Montanari, F.; Baschetti, M.G. Diffusion of Thyme, Cinnamon and Oregano Essential Oils in Different Nanocellulose Matrices. Carbohydr. Polym. Technol. Appl. 2023, 5, 100271. [Google Scholar] [CrossRef]
- Sousa, I.; Parente, J.F.; Jos, C.; Marques, J.F.; Forte, M.A. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Croxatto, H.B.; Bardin, C.W. Mechanisms of Action of Intrauterine Devices. Obstet. Gynecol. Surv. 1996, 51, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Tekin, Y.B.; Guvendag Guven, E.S.; Acar Yazici, Z.; Kirbas, A.; Kir Sahin, F. Comparison of the Effects of Copper T and Levonorgestrel IUD on Proteoglycan Composition of Cervical Mucus. Gynecol. Obstet. Reprod. Med. 2014, 20, 159–162. [Google Scholar]
- Ortiz, M.E.; Croxatto, H.B. Copper-T Intrauterine Device and Levonorgestrel Intrauterine System: Biological Bases of Their Mechanism of Action. Contraception 2007, 75, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.M.; Arisha, A.H.; Abo-Elmaaty, A.M.A.; Abdelgawad, F.E.; Metwally, M.M.M.; Saber, T.; Mansour, M.F. Thymol Alleviates Imidacloprid-Induced Testicular Toxicity by Modulating Oxidative Stress and Expression of Steroidogenesis and Apoptosis-Related Genes in Adult Male Rats. Ecotoxicol. Environ. Saf. 2021, 221, 112435. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]


| Components of Ma EO | Concentration |
|---|---|
| 1. (−)-alpha-Thujene | 1% |
| 2. (−)-alpha-Pinene | 2% |
| 3. (+)-Sabinene | 0.25% |
| 4. (−)-beta-Pinene | 0.63% |
| 5. beta-Myrcene | 0.67% |
| 6. (+)-alpha-Phellandrene | 0.32% |
| 7. 2-Carene | 7.38% |
| 8. p-Cymene | 6.54% |
| 9. Eucalyptol | 4.42% |
| 10. D-Limonene | 0.87% |
| 11. gamma-Terpinene | 16.72% |
| 12. Sabinene hydrate | 0.15% |
| 13. Cyclohexene, 1-methyl-4-(1-methyle thylidene)- | 3.29% |
| 14. 4-Thujanol | 0.41% |
| 15. 2-p-Menthen-1-ol | 0.46% |
| 16. 2-p-Menthen-1-ol | 0.33% |
| 17. Terpinen-4-ol | 39.99% |
| 18. Terpineol | 3.09% |
| 19. trans-Piperitol | 0.17% |
| 20. (3S,4S)-Hept-1-en-6-yne-3,4-diol | 0.17% |
| 21. Endo-2-bornyl carbanilate | 0.13% |
| 22. (−)-alpha-Gurjunene | 0.42% |
| 23. Caryophyllene | 0.45% |
| 24. 10s,11s-Himachala-3(12),4-diene | 1.27% |
| 25. (4-alpha,-5-beta,-6-alpha,-7-alpha,-10-alpha)-1-Aromadendrene | 0.35% |
| 26. (E)-2-epi-beta-Caryophyllene | 0.56% |
| 27. 1-Isopropyl-4,7-dimethyl-1,2,3,4,5,6-hexahydronaphthalene | 0.42% |
| 28. gamma-Muurolene | 0.28% |
| 29. (+)-Ledene | 1.54% |
| 30. alpha-Muurolene | 0.19% |
| 31. Calamenene | 0.25% |
| 32. Cadina-1(10),4-diene | 1.66% |
| 33. Cubenene | 0.20% |
| 34. (−)-Spathulenol | 0.24% |
| 35. (−)-Globulol | 0.32% |
| 36. 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.beta.,4a.beta.,7.alpha., 7a.beta.,7b.alpha.)]- | 0.12% |
| 37. Di-epi-1,10-cubenol | 0.21% |
| 38. Farnesol | 0.84% |
| 39. 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,11,15,19,23-heptamethyl-, (all-E)- | 1.69% |
| EOs | C. albicans V6 | C. glabrata V23 | |||||
|---|---|---|---|---|---|---|---|
| MIC (μL/mL) | MFC (μL/mL) | ZOI (mm) | MIC (μL/mL) | MFC (μL/mL) | ZOI (mm) | p+ (C. glabrata V23 vs. C. albicans V6) | |
| Ma | 0.49 | 1.95 | 91.3 ± 7.0 | 1.95 | 1.95 | 50.0 ± 9.2 | p < 0.001 a *** |
| Tz | ≤0.06 | 0.24 | 110 ± 6.0 | ≤0.06 | 0.24 | 84.0 ± 13.1 | p = 0.010 a * |
| p#(Ma vs. Tz) | 0.034 * b | 0.046 * b | p = 0.003 a ** | 0.034 * b | 0.037 * b | p = 0.003 a ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahal, G.; Donmez, H.G.; Woerdenbag, H.J.; Taner, A.; Beksac, M.S. The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings. Pharmaceutics 2025, 17, 1304. https://doi.org/10.3390/pharmaceutics17101304
Sahal G, Donmez HG, Woerdenbag HJ, Taner A, Beksac MS. The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings. Pharmaceutics. 2025; 17(10):1304. https://doi.org/10.3390/pharmaceutics17101304
Chicago/Turabian StyleSahal, Gulcan, Hanife Guler Donmez, Herman J. Woerdenbag, Abbas Taner, and Mehmet Sinan Beksac. 2025. "The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings" Pharmaceutics 17, no. 10: 1304. https://doi.org/10.3390/pharmaceutics17101304
APA StyleSahal, G., Donmez, H. G., Woerdenbag, H. J., Taner, A., & Beksac, M. S. (2025). The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings. Pharmaceutics, 17(10), 1304. https://doi.org/10.3390/pharmaceutics17101304







