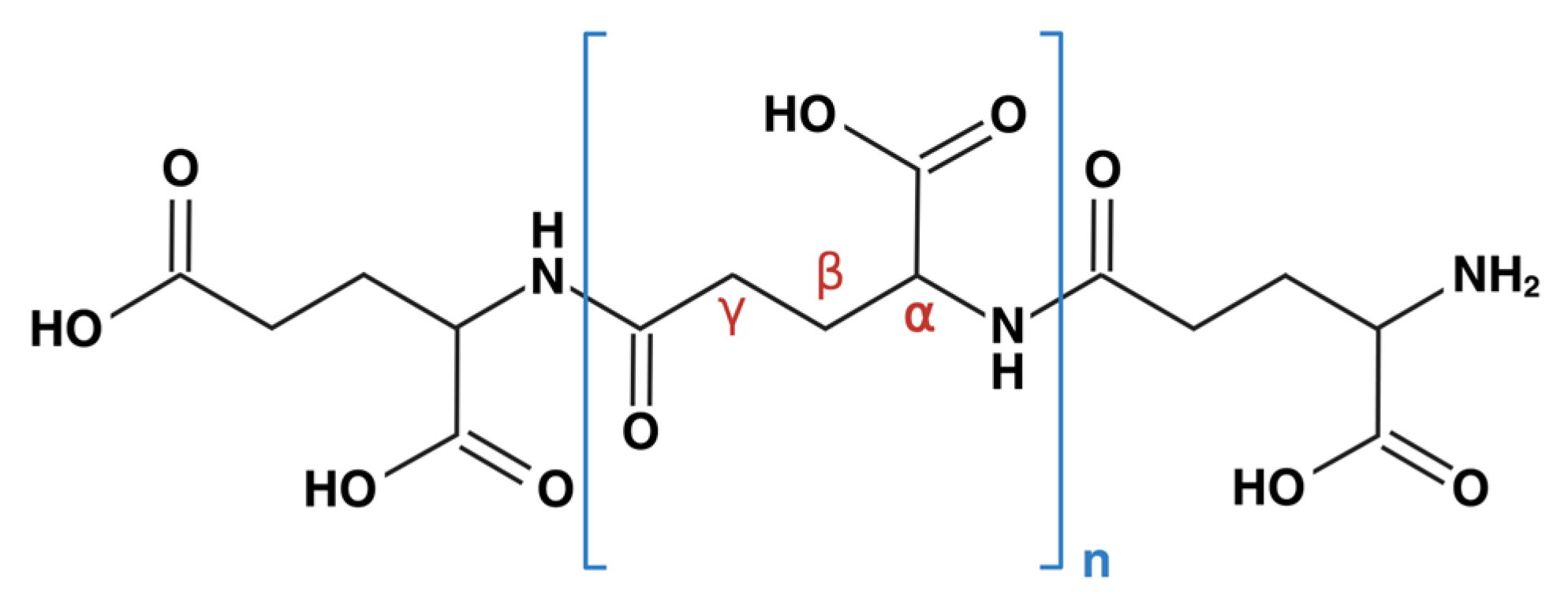
Polyglutamic Acid as an Antiviral Agent: Mechanistic and Structural Insights
Abstract
1. Introduction
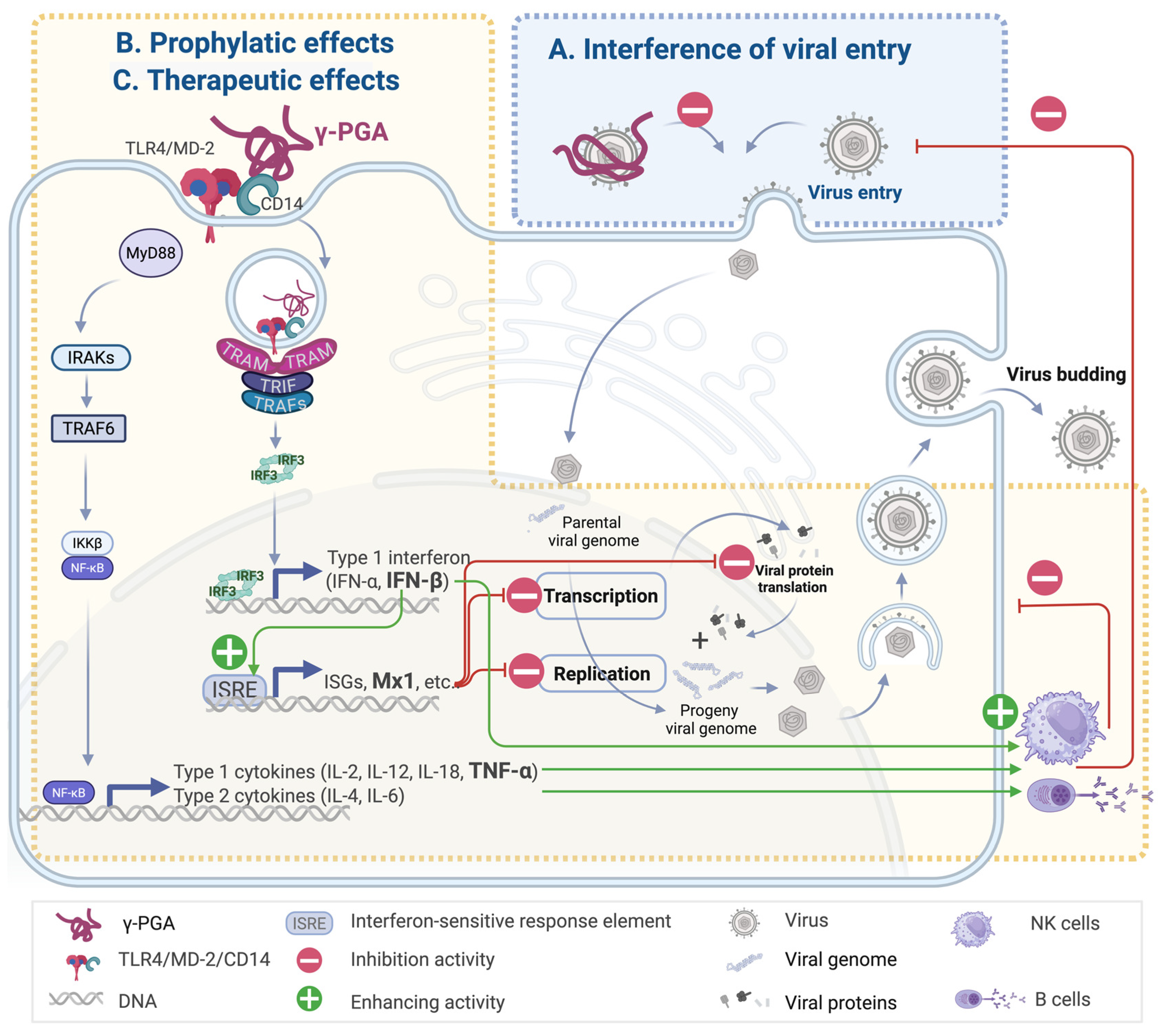
2. Antiviral Effects of γ-PGA
2.1. Interference in Viral Entry with γ-PGA Containing Exopolymers
2.2. Prophylactic Effect of γ-PGA
2.3. Therapeutic Potential of γ-PGA Following Viral Exposure
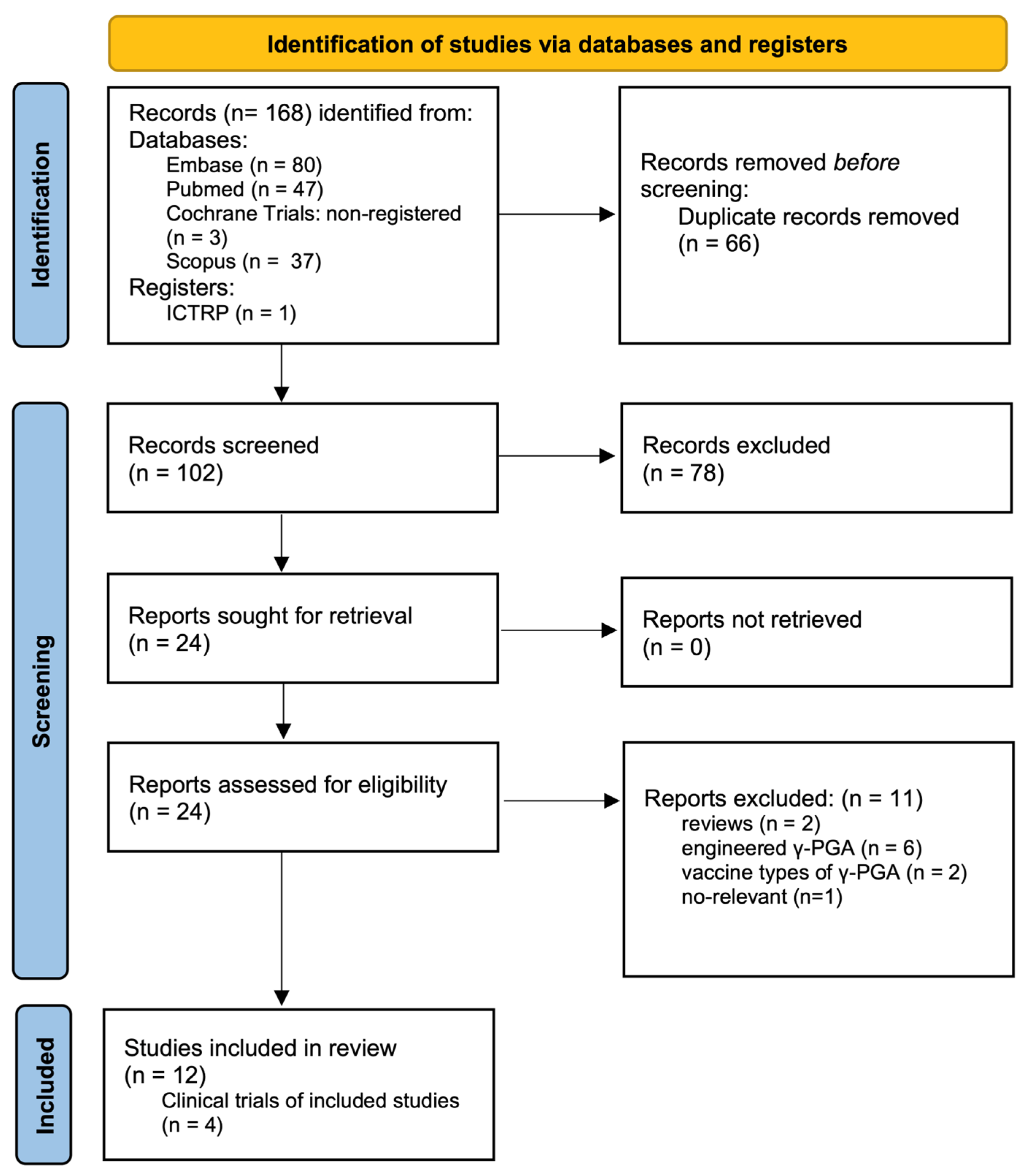
3. Clinical Trials
4. Pharmaceutical Considerations for γ-PGA
4.1. Stability and Bioavailability
4.2. Safety and Regulations
4.3. General Pharmackinetics
5. Downstream Molecular Signaling Pathways of γ-PGA
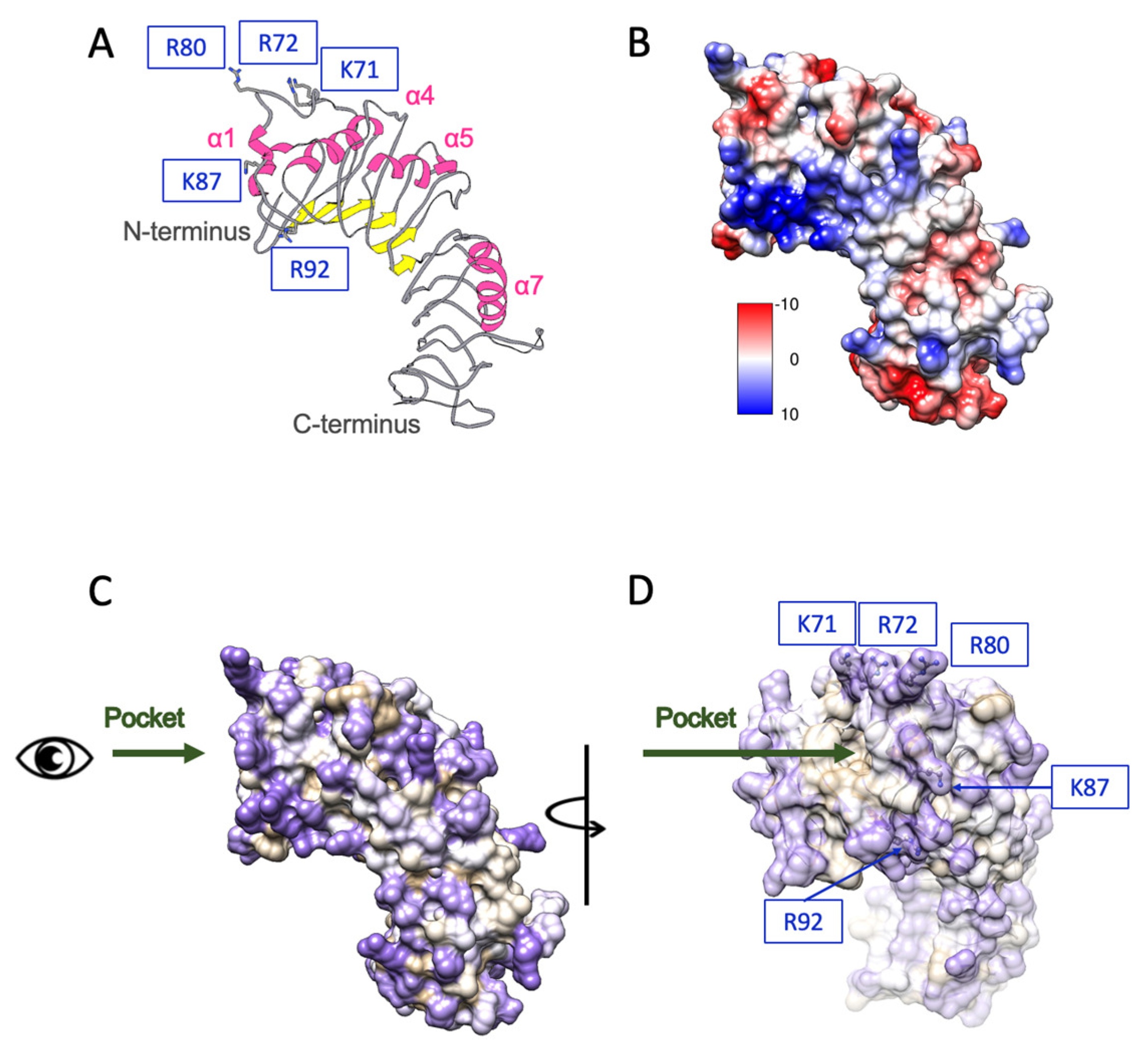
6. γ-PGA and TLR4-Mediated Immune Activation: Structural Biology Perspective
6.1. Structural Docking and Recognition of γ-PGA in TLR4 Activation
6.2. Structural Basis of Molecular Weight-Dependent γ-PGA Modulation of TLR4 Signaling
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| γ-PGA | γ-poly(glutamic acid) |
| CD14 | Cluster of differentiation 14 |
| H1N1 | Influenza A virus subtype H1N1 |
| H5N2 | Influenza A virus subtype H5N2 |
| HCV | Hepatitis C virus |
| Hek293 | Human embryonic kidney 293 cells |
| Hela | Human cervical cancer cells |
| Hep-2 | Human epithelial type 2 cells |
| HOG | Human oligodendroglioma cells |
| HPV | Human papillomavirus |
| HSV-1 | Herpes simplex virus type 1 |
| HSV-2 | Herpes simplex virus type 2 |
| Hu7 | Human hepatoma cells |
| IFN-α | Interferon-alpha |
| IFN-β | Interferon-beta |
| IFN-γ | Interferon-gamma |
| IKKβ | Iκb kinase β |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-12 | Interleukin-12 |
| IL-18 | Interleukin-18 |
| IRAKs | Interleukin-1 receptor–associated kinases |
| IRF3 | Interferon regulatory factor 3 |
| IRF7 | Interferon regulatory factor 7 |
| ISG(s) | Interferon-Stimulated Gene(s) |
| ISRE | Interferon-stimulated response element |
| Jurkat | Human T lymphocyte cells |
| kDa | Kilodalton |
| LPS | Lipopolysaccharide |
| MD-2 | Myeloid differentiation factor 2 |
| Mewo | Human melanoma cells |
| MNV | Murine norovirus |
| Mx1 | Myxovirus resistance protein 1 |
| MyD88 | Myeloid differentiation primary response 88 |
| NDV | Newcastle disease virus |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated b cells |
| NK cells | Natural killer cells |
| OAS | 2′-5′-oligoadenylate synthetase |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PRV | Pseudorabies virus |
| RAW 264.7 | Murine macrophage cells |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| TRAFs | Tumor necrosis factor receptor-associated factors |
| TRAM | TRIF-related adaptor molecule |
| TRIF | TIR-domain–containing adaptor inducing IFN-β |
| Vero | African green monkey kidney cells |
| VSV | Vesicular stomatitis virus |
| WISH | Human amnion cells |
References
- Connor, A.J.; Zha, R.H.; Koffas, M. Bioproduction of biomacromolecules for antiviral applications. Curr. Opin. Biotechnol. 2021, 69, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Parati, M.; Khalil, I.; Tchuenbou-Magaia, F.; Adamus, G.; Mendrek, B.; Hill, R.; Radecka, I. Building a circular economy around poly(D/L-γ-glutamic acid)—A smart microbial biopolymer. Biotechnol. Adv. 2022, 61, 108049. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Convers. Biorefin. 2023, 13, 4555–4573. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Kubler-Kielb, J.; Liu, T.Y.; Dai, Z.D.; Leppla, S.H.; Yergey, A.; Backlund, P.; Shiloach, J.; Majadly, F.; Robbins, J.B. Poly(gamma-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: A potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA 2003, 100, 8945–8950. [Google Scholar] [CrossRef]
- Buescher, J.M.; Margaritis, A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit. Rev. Biotechnol. 2007, 27, 1–19. [Google Scholar] [CrossRef]
- Halmschlag, B.; Steurer, X.; Putri, S.P.; Fukusaki, E.; Blank, L.M. Tailor-made poly-gamma-glutamic acid production. Metab. Eng. 2019, 55, 239–248. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Wang, Y.; Li, J.; Chen, Y.; Wen, Q.; Zheng, D.; Kang, W.; Quan, H. The Application and Functional Progress of gamma-Poly-Glutamic Acid in Food: A Mini-Review. Curr. Pharm. Des. 2020, 26, 5347–5352. [Google Scholar] [CrossRef]
- Liu, Z.; He, Y.; Ma, X. Preparation, Characterization and Drug Delivery Research of gamma-Polyglutamic Acid Nanoparticles: A Review. Curr. Drug Deliv. 2024, 21, 795–806. [Google Scholar] [CrossRef]
- Lee, T.Y.; Kim, Y.H.; Yoon, S.W.; Choi, J.C.; Yang, J.M.; Kim, C.J.; Schiller, J.T.; Sung, M.H.; Poo, H. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol. Immunother. 2009, 58, 1781–1794. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Okada, N.; Oda, A.; Matsuo, K.; Matsuo, K.; Kayamuro, H.; Ishii, Y.; Yoshinaga, T.; Akagi, T.; Akashi, M.; et al. Nanoparticles built by self-assembly of amphiphilic γ-PGA can deliver antigens to antigen-presenting cells with high efficiency:: A new tumor-vaccine carrier for eliciting effector T cells. Vaccine 2008, 26, 1303–1313. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, T.Y.; Bae, H.C.; Hahm, J.H.; Kim, Y.H.; Park, C.; Kang, T.H.; Kim, C.J.; Sung, M.H.; Poo, H. Oral administration of high molecular mass poly-gamma-glutamate induces NK cell-mediated antitumor immunity. J. Immunol. 2007, 179, 775–780. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) glutamic acid: A unique microbial biopolymer with diverse commercial applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Gao, Y.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Recent Advances in Microbial Synthesis of Poly-γ-Glutamic Acid: A Review. Foods 2022, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, P.; Cai, D.; Chen, S. Genetic and metabolic engineering for poly-γ-glutamic acid production: Current progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2022, 38, 208. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Han, Y.; Zheng, X.; Xue, B.; Zhang, X.; Mahmut, Z.; Wang, Y.; Dong, B.; Zhang, C.; Gao, D.; et al. Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials. Materials 2023, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Rahiman, N.; Cabral, H.; Quader, S.; Zirak, M.R.; Taghavizadeh Yazdi, M.E.; Jaafari, M.R.; Alavizadeh, S.H. Poly-γ-glutamic acid nanoparticles as adjuvant and antigen carrier system for cancer vaccination. J. Control. Release 2023, 362, 278–296. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and characterization of an exopolymer produced by Bacillus licheniformis: In vitro antiviral activity against enveloped viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Papaianni, E.; Maugeri, T.L.; Zammuto, V.; Spanò, A.; Nicolaus, B.; Poli, A.; Di Donato, P.; Mosca, C.; Mastino, A.; et al. Anti-herpes simplex virus 1 and immunomodulatory activities of a poly-γ- glutamic acid from Bacillus horneckiae strain APA of shallow vent origin. Appl. Microbiol. Biotechnol. 2017, 101, 7487–7496. [Google Scholar] [CrossRef]
- Poli, A.; Gugliandolo, C.; Spanò, A.; Taurisano, V.; Di Donato, P.; Maugeri, T.L.; Nicolaus, B.; Arena, A. Poly-γ-Glutamic Acid from Bacillus horneckiae Strain APA of Shallow Marine Vent Origin with Antiviral and Immunomodulatory Effects against Herpes Simplex Virus Type-2. J. Mar. Sci. Res. Dev. 2015, 5, 1000173. [Google Scholar] [CrossRef]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef]
- Lee, W.; Kim, M.; Lee, S.H.; Jung, H.G.; Oh, J.W. Prophylactic efficacy of orally administered Bacillus poly-γ-glutamic acid, a non-LPS TLR4 ligand, against norovirus infection in mice. Sci. Rep. 2018, 8, 8667. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, J.S.; Choi, Y.K.; Park, J.Y.; Talactac, M.R.; Chowdhury, M.Y.; Poo, H.; Sung, M.H.; Lee, J.H.; Jung, J.U.; et al. Induction of type I interferon by high-molecular poly-γ-glutamate protects B6.A2G-Mx1 mice against influenza A virus. Antiviral Res. 2012, 94, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Lee, J.H.; Kang, I.J.; Shabir, N.; Khatun, A.; Yang, M.S.; Park, C.; Kim, B.; Kim, W.I. Effects of high molecular weight poly-γ-glutamic acid on pigs with porcine preproductive and respiratory syndrome virus (PRRSV) infection. Acta Vet. 2017, 67, 153–167. [Google Scholar] [CrossRef]
- Talactac, M.; Lee, J.S.; Moon, H.; Chowdhury, M.Y.; Kim, C.J. The Antiviral Effect of High-Molecular Weight Poly-Gamma-Glutamate against Newcastle Disease Virus on Murine Macrophage Cells. Adv. Virol. 2014, 2014, 301386. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.H.; Ahn, D.G.; Cho, H.; Sung, M.H.; Han, S.H.; Oh, J.W. The antiviral activity of poly-γ-glutamic acid, a polypeptide secreted by Bacillus sp., through induction of CD14-dependent type I interferon responses. Biomaterials 2013, 34, 9700–9708. [Google Scholar] [CrossRef]
- Kim, E.H.; Choi, Y.K.; Kim, C.J.; Sung, M.H.; Poo, H. Intranasal administration of poly-gamma glutamate induced antiviral activity and protective immune responses against H1N1 influenza A virus infection. Virol. J. 2015, 12, 160. [Google Scholar] [CrossRef]
- Araki, R.; Yamada, T.; Maruo, K.; Araki, A.; Miyakawa, R.; Suzuki, H.; Hashimoto, K. Gamma-Polyglutamic Acid-Rich Natto Suppresses Postprandial Blood Glucose Response in the Early Phase after Meals: A Randomized Crossover Study. Nutrients 2020, 12, 2374. [Google Scholar] [CrossRef]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Maruo, K.; Suzuki, H.; Hashimoto, K. The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study. Nutrients 2020, 12, 915. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.C.; Sung, M.H.; Kang, J.H.; Chang, M.J. High molecular weight poly-gamma-glutamic acid regulates lipid metabolism in rats fed a high-fat diet and humans. J. Microbiol. Biotechnol. 2011, 21, 766–775. [Google Scholar] [CrossRef]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Fairweather-Tait, S.J. Acute effect of poly-gamma-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef]
- Cho, H.W.; Park, Y.C.; Sung, M.H.; Park, J.S.; Kim, T.J.; Seong, S.J.; Cho, C.H.; Lee, J.K. Short-term clinical and immunologic effects of poly-gamma-glutamic acid (γ-PGA) in women with cervical intraepithelial neoplasia 1 (CIN 1): A multicenter, randomized, double blind, phase II trial. PLoS ONE 2019, 14, e0217745. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, T.Y.; Hong, J.H.; Kim, A.; Kim, S.J.; Choi, J.C.; Sung, M.H.; Poo, H. A single-center, randomized double-blind placebo-controlled study evaluating the effects of poly-gamma-glutamate on human NK cell activity after an 8-week oral administration in healthy volunteers. Evid. Based Complement. Alternat. Med. 2013, 2013, 635960. [Google Scholar] [CrossRef]
- Gentilini, C.; Dong, Y.; May, J.R.; Goldoni, S.; Clarke, D.E.; Lee, B.H.; Pashuck, E.T.; Stevens, M.M. Functionalized poly(gamma-Glutamic Acid) fibrous scaffolds for tissue engineering. Adv. Healthc. Mater. 2012, 1, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chang, M.J.; Kim, S.H. Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats. Nutr. Res. Pract. 2010, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baik, I. Consumption of poly-gamma-glutamate-vitamin B6 supplement and urinary microbiota profiles in Korean healthy adults: A randomized, double-blinded, placebo-controlled intervention study. Nutr. Res. Pract. 2024, 18, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Haraguchi, T.; Ikegami, S.; Nishikawa, H.; Yoshida, M.; Ozeki, M.; Kawasaki, I.; Uchida, T. Preparation and Evaluation of Poly-gamma-glutamic Acid Hydrogel Mixtures with Amlodipine Besylate: Effect on Ease of Swallowing and Taste Masking. Chem. Pharm. Bull. 2019, 67, 1284–1292. [Google Scholar] [CrossRef]
- Sutherland, M.D.; Thorkildson, P.; Parks, S.D.; Kozel, T.R. In vivo fate and distribution of poly-gamma-D-glutamic acid, the capsular antigen from Bacillus anthracis. Infect. Immun. 2008, 76, 899–906. [Google Scholar] [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef]
- Poo, H.; Park, C.; Kwak, M.S.; Choi, D.Y.; Hong, S.P.; Lee, I.H.; Lim, Y.T.; Choi, Y.K.; Bae, S.R.; Uyama, H.; et al. New biological functions and applications of high-molecular-mass poly-gamma-glutamic acid. Chem. Biodivers. 2010, 7, 1555–1562. [Google Scholar] [CrossRef]
- Zhou, Q.; Lavorgna, A.; Bowman, M.; Hiscott, J.; Harhaj, E.W. Aryl Hydrocarbon Receptor Interacting Protein Targets IRF7 to Suppress Antiviral Signaling and the Induction of Type I Interferon. J. Biol. Chem. 2015, 290, 14729–14739. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, M.S.; Gad, H.H.; Thavachelvam, K.; Boesen, T.; Despres, P.; Hartmann, R. The 2′-5′-oligoadenylate synthetase 3 enzyme potently synthesizes the 2′-5′-oligoadenylates required for RNase L activation. J. Virol. 2014, 88, 14222–14231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, H.; Zhou, Q.; Li, Y.; Chen, H.; Ye, W.; Chen, D.; Fleming, J.; Shu, H.; Liu, Y. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell. Res. 2012, 22, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Akagi, T.; Yoshinaga, K.; Toyama, M.; Akashi, M.; Baba, M. The induction of innate and adaptive immunity by biodegradable poly(gamma-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 2011, 32, 5206–5212. [Google Scholar] [CrossRef]
- Isgor, C.; Aydin, C.; Oztan, O.; Libreros, S.; Iragavarapu-Charyulu, V. Inter-individual differences in immune profiles of outbred rats screened for an emotional reactivity phenotype. J. Neuroimmunol. 2020, 347, 577349. [Google Scholar] [CrossRef]
- Piasecka, B.; Duffy, D.; Urrutia, A.; Quach, H.; Patin, E.; Posseme, C.; Bergstedt, J.; Charbit, B.; Rouilly, V.; MacPherson, C.R.; et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc. Natl. Acad. Sci. USA 2018, 115, E488–E497. [Google Scholar] [CrossRef]
- Bell, J.K.; Mullen, G.E.; Leifer, C.A.; Mazzoni, A.; Davies, D.R.; Segal, D.M. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003, 24, 528–533. [Google Scholar] [CrossRef]
- Bella, J.; Hindle, K.L.; McEwan, P.A.; Lovell, S.C. The leucine-rich repeat structure. Cell. Mol. Life Sci. 2008, 65, 2307–2333. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef]
- Visintin, A.; Iliev, D.B.; Monks, B.G.; Halmen, K.A.; Golenbock, D.T. Md-2. Immunobiology 2006, 211, 437–447. [Google Scholar] [CrossRef]
- Mueller, M.; Lindner, B.; Kusumoto, S.; Fukase, K.; Schromm, A.B.; Seydel, U. Aggregates are the biologically active units of endotoxin. J. Biol. Chem. 2004, 279, 26307–26313. [Google Scholar] [CrossRef]
- Gabarin, R.S.; Li, M.; Zimmel, P.A.; Marshall, J.C.; Li, Y.; Zhang, H. Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: Avenues for Novel Therapeutic Strategies. J. Innate Immun. 2021, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, C.J.; Jin, M.S.; Lee, C.H.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J. Biol. Chem. 2005, 280, 11347–11351. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, R.L.; Munford, R.S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J. Biol. Chem. 1995, 270, 9904–9910. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Avalos, A.M.; Ploegh, H.L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 2012, 12, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.L.; Lukk, T.; Nair, S.K.; Tapping, R.I. The crystal structure of human soluble CD14 reveals a bent solenoid with a hydrophobic amino-terminal pocket. J. Immunol. 2013, 190, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Chen, J.T.; Wang, L.F.; Wu, S.; Zhang, G.Z.; Yu, H.Q.; Ye, X.D.; Shi, Q.S. Conformations and molecular interactions of poly-gamma-glutamic acid as a soluble microbial product in aqueous solutions. Sci. Rep. 2017, 7, 12787. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Pascual-Lucas, M.; Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J. Neurochem. 2014, 129, 448–462. [Google Scholar] [CrossRef]
- Rajaiah, R.; Perkins, D.J.; Ireland, D.D.; Vogel, S.N. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, 8391–8396. [Google Scholar] [CrossRef]
| Mechanisms | Authors | Target Virus | M.W., Dose of γ-PGA, and Formulations | Routes and Models | Cytokines and Signaling Pathways |
|---|---|---|---|---|---|
| Interference in viral entry | Sánchez-León et al. [17] | HSV-1, HSV-2, VSV, PRV | 5 kDa, 5 μg/mL Extracellular polymer comprising γ-PGA and teichoic acid | in vitro: γ-PGA was mixed with virus stock for 1 h and then added to cells for another hour. After two washes to remove unbound virus, viral titers were assessed 24 h post the viral inoculation (Vero, HOG, Mewo, Hela, Jurkat). | It exhibits a dose-dependent virucidal effect via blocking viral entry. |
| Interference in viral entry; therapeutic treatment | Poli et al. [19] | HSV-2 | 890–1000 kDa, 0.4 mg/mL Purified γ-PGA | in vitro: PBMCs were co-treated with γ-PGA and HSV-2 without washing. | It upregulates cytokines including IFN-γ, IFN-α, TNF-α, IL-12, and IL-18. |
| Prophylactic treatment | Moon et al. [22] | Influenza A (H1N1 and H5N2) | >3000 kDa, 1 mg/mL Commercially purified | in vitro: RAW 264.7 cells were pretreated with γ-PGA for 12 h prior to the infection. in vivo: Mice received intranasal γ-PGA 12 h prior to viral challenge. | It upregulates IFN-β, IFN-α, and TNF-α mRNA in lung tissue and increases ISG mRNA expression in macrophages. |
| Prophylactic treatment | Talactac et al. [24] | NDV | >3000 kDa, 1 mg/mL Commercially purified | in vitro: RAW 264.7 cells were pretreated with γ-PGA 12 h prior to viral challenge. | It upregulates IRF3, IFN-β, Mx1, and other ISGs and inhibits virus budding. Cytokine profile resembles LPS-treated cells. Inhibits virus budding. |
| Prophylactic treatment | Marino-Merlo et al. [18] | HSV-1 | 890–1000 kDa, 0.5 mg/mL Purified γ-PGA | in vitro: Hep-2 cells were treated with γ-PGA 2 h before viral challenge. | It upregulates cytokines including NF-κB, TNF-α, and IL-1β. |
| Prophylactic treatment | Lee et al. [21] | MNV | 2000 kDa, 100 nM, 50 mg/kg of body weight Commercially purified | in vitro: RAW 264.7 cells pretreated with γ-PGA 6 h before the infection. in vivo: Mice received daily oral γ-PGA for 5 days before viral challenge. | It blocks viral entry, induces IFN-γ, and elevates IFN-β in serum and ileum without triggering proinflammatory cytokines. |
| Prophylactic and therapeutic treatment | Lee et al. [25] | SARS-CoV, HCV | 2000 kDa, 100 μM Commercially purified | in vitro: Hek293-TLR4 cells were treated with γ-PGA 12 h after transfection with the SARS-CoV replicon. in vitro: Hu7 cells were transfected with the HCV genome for 24 h, followed by γ-PGA treatment for 18 h. | γ-PGA acts as a TLR4 ligand, requiring both CD14 and MD-2 to activate IFN-β mRNA expression. |
| Therapeutic treatment | Kim et al. [26] | Influenza A H1N1 | 2000 kDa, 100 μg in 20 μL PBS Commercially purified | in vivo: Mice received intranasal γ-PGA 24 h after viral inoculation. | It enhances antiviral cytokines (IFN-β, IL-12) and activates NK cells as well as influenza-specific cytotoxic T cells. |
| Therapeutic treatment | Seo et al. [23] | PRRSV | >2000 kDa, 20 mg/mL Commercially purified | in vivo: Pigs received intramuscular γ-PGA 7 days after viral challenge. | It upregulates cytokines including TNF-α, IFN-α, IFN-β, IFN-γ, IL-2, IL-4, and IL-6. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-N.; Wu, S.-R. Polyglutamic Acid as an Antiviral Agent: Mechanistic and Structural Insights. Pharmaceutics 2025, 17, 1296. https://doi.org/10.3390/pharmaceutics17101296
Wu Y-N, Wu S-R. Polyglutamic Acid as an Antiviral Agent: Mechanistic and Structural Insights. Pharmaceutics. 2025; 17(10):1296. https://doi.org/10.3390/pharmaceutics17101296
Chicago/Turabian StyleWu, Ya-Na, and Shang-Rung Wu. 2025. "Polyglutamic Acid as an Antiviral Agent: Mechanistic and Structural Insights" Pharmaceutics 17, no. 10: 1296. https://doi.org/10.3390/pharmaceutics17101296
APA StyleWu, Y.-N., & Wu, S.-R. (2025). Polyglutamic Acid as an Antiviral Agent: Mechanistic and Structural Insights. Pharmaceutics, 17(10), 1296. https://doi.org/10.3390/pharmaceutics17101296







