Application of Biomaterials in Diabetic Wound Healing: The Recent Advances and Pathological Aspects
Abstract
1. Introduction
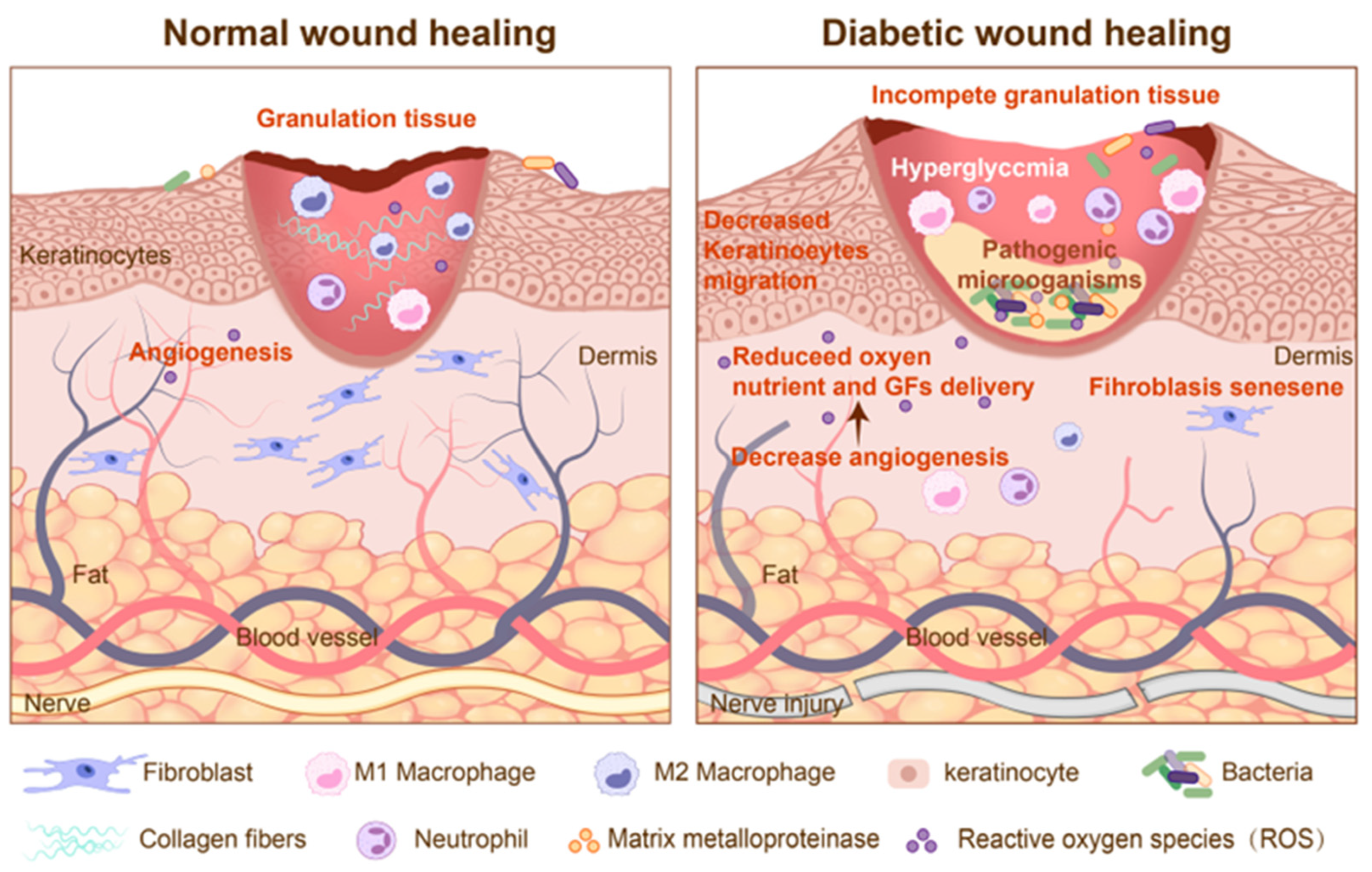
2. Pathological Mechanisms of Diabetic Wound Healing
2.1. Angiogenesis Disorders and Ischemic Microenvironment
2.2. Chronic Inflammation and Immune Dysfunction
2.3. Bacterial Infection and Biofilm Formation
2.4. Oxidative Stress and Cellular Damage
2.5. Neuropathy
3. Design and Application of Biomaterials in Diabetic Wounds
3.1. DNA Nanomaterials
3.1.1. Tetrahedral DNA Nanostructures
3.1.2. DNA Hydrogels
3.2. Peptide Hydrogels
3.3. Cells
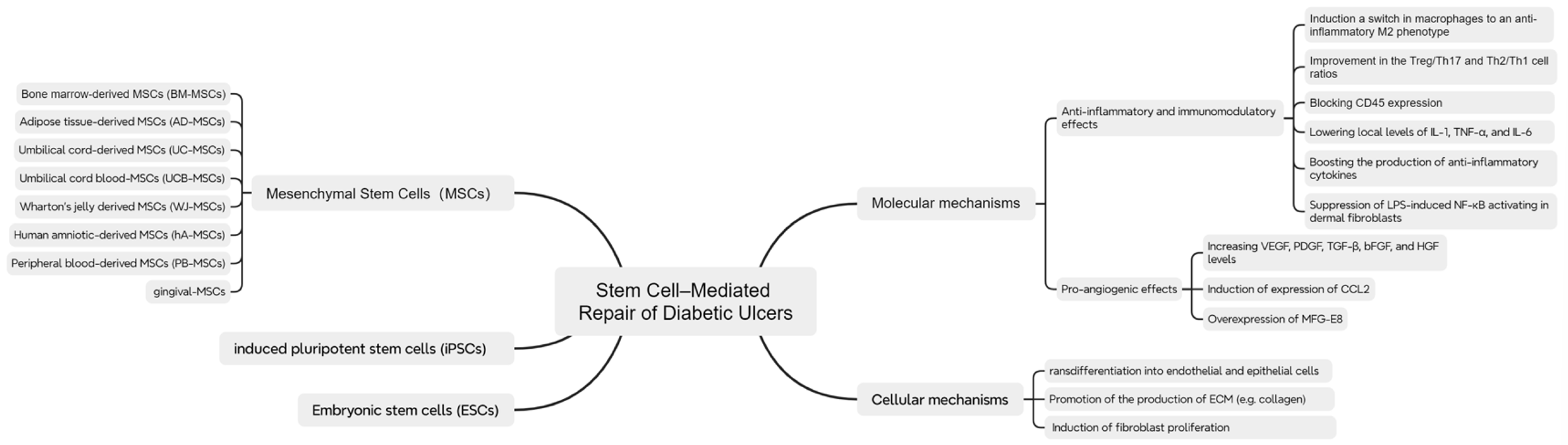
3.3.1. Mesenchymal Stem Cells (MSCs)
Bone Marrow Mesenchymal Stem Cells (BMSCs)
Adipose-Derived Mesenchymal Stem Cells (ADSCs)
Human Umbilical Cord-Derived Mesenchymal Stem Cells (hUCMSCs)
3.3.2. Induced Pluripotent Stem Cells (iPSCs)
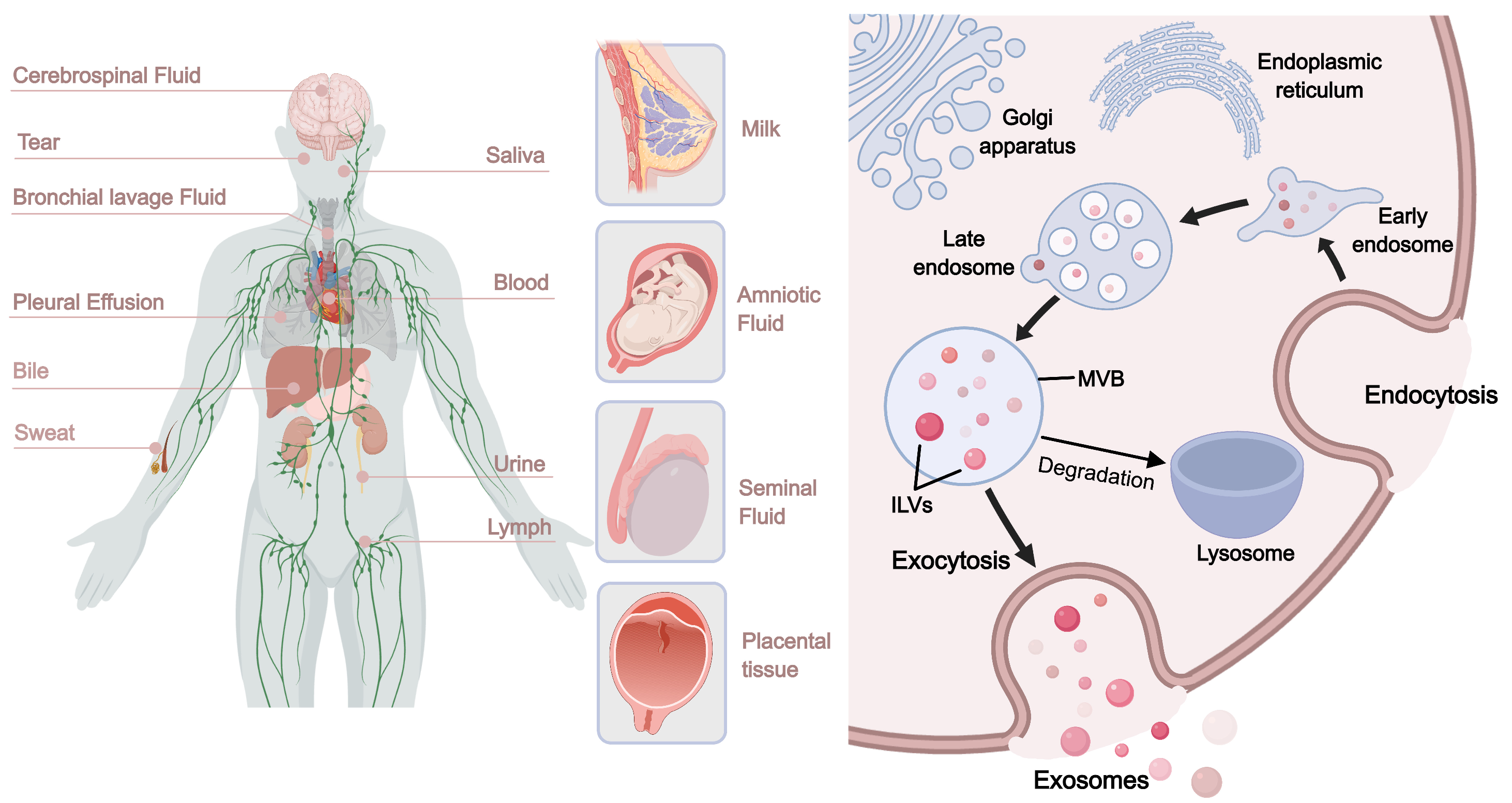
3.4. Extracellular Vesicles (EVs)
3.4.1. Cell-Derived Extracellular Vesicles
Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles (BMSC-EVs)
Adipose-Derived Stem Cell-Derived Extracellular Vesicles (ADSC-EVs)
Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles (hUCMSC-EVs)
Macrophage-Derived Extracellular Vesicles (M-EVs)
3.4.2. Tissue-Derived Extracellular Vesicles
Adipose Tissue-Derived Extracellular Vesicles
Plasma-Derived Extracellular Vesicles
Skin Tissue-Derived Extracellular Vesicles
3.4.3. Biomaterial-Assisted Extracellular Vesicle Delivery Systems
3.5. Cytokines
4. Challenges and Future Perspectives
4.1. Development Trends of Multifunctional Intelligent Biomaterials
4.2. Key Issues in Clinical Translation
4.3. Personalized Therapy and Multidisciplinary Integration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Foundation (IDF). Diabetes Atlas. 2025. Available online: http://www.diabetesatlas.org/ (accessed on 15 September 2025).
- Abate, M.C.M.O.; Aroucha, P.M.T.; Nóbrega, D.V.M.D.; Rocha, I.P.M.; Soares, S.D.; Reis, A.A.; Paliares, I.C.; Giuffrida, F.M.A.; Dib, S.A.; Reis, A.F.; et al. Cutaneous manifestations of diabetes mellitus: A narrative review. Einstein 2025, 23, eRW1193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Borah, P.; Dutta, P.P.; Sen, S. Evolving spectrum of diabetic wound: Mechanistic insights and therapeutic targets. World J. Diabetes 2022, 13, 696–716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 72–140. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desmet, C.M.; Préat, V.; Gallez, B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dörr, S.; Holland-Letz, A.K.; Weisser, G.; Chatzitomaris, A.; Lobmann, R. Bacterial Diversity, Antibiotic Resistance, and the Risk of Lower Limb Amputation in Younger and Older Individuals with Diabetic Foot Infection. Int. J. Low. Extrem. Wounds 2023, 22, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Lipsky, B.A.; Bakker, K.; International Working Group on the Diabetic Foot. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stitt, A.W.; Jenkins, A.J.; Cooper, M.E. Advanced glycation end products and diabetic complications. Expert. Opin. Investig. Drugs 2002, 11, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Kuroki, M.; Amano, S.; Tolentino, M.; Keough, K.; Kim, I.; Bucala, R.; Adamis, A.P. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J. Clin. Investig. 1998, 101, 1219–1224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamza, A.A.; Fikry, E.M.; Abdallah, W.; Amin, A. Mechanistic insights into the augmented effect of bone marrow mesenchymal stem cells and thiazolidinediones in streptozotocin-nicotinamide induced diabetic rats. Sci. Rep. 2018, 8, 9827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porel, P.; Kaur, M.; Sharma, V.; Aran, K.R. Understanding molecular mechanism of diabetic wound healing: Addressing recent advancements in therapeutic managements. J. Diabetes Metab. Disord. 2025, 24, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Li, Y.; Ke, C.; Jin, Y.; Lao, W.; Wu, Y.; Liu, Y.; Kong, X.; Qiao, J.; Zhai, A.; et al. HDAC4: An emerging target in diabetes mellitus and diabetic complications. Eur. J. Med. Res. 2025, 30, 429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of Endothelial Progenitor Cell: Past, Present, and Future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopes-Coelho, F.; Silva, F.; Gouveia-Fernandes, S.; Martins, C.; Lopes, N.; Domingues, G.; Brito, C.; Almeida, A.M.; Pereira, S.A.; Serpa, J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells 2020, 9, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiu, K.H.; Tse, H.F. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dong, L.; Wang, L.; Kang, L.; Xu, B. Advanced glycation end products impair function of late endothelial progenitor cells through effects on protein kinase Akt and cyclooxygenase-2. Biochem. Biophys. Res. Commun. 2009, 381, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zang, G.Y.; Huang, Y.; Sun, Z.; Zhang, L.L.; Qian, Y.J.; Yuan, W.; Wang, Z.Q. Advances in neovascularization after diabetic ischemia. World J. Diabetes 2022, 13, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.Q.; Cao, Q.; Zhang, J.J.; Huang, L.Y.; Sang, T.T.; Liu, F.; Chen, S.Y. Hydrogen peroxide induced impairment of endothelial progenitor cell viability is mediated through a FoxO3a dependant mechanism. Microvasc. Res. 2013, 90, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rosso, A.; Balsamo, A.; Gambino, R.; Dentelli, P.; Falcioni, R.; Cassader, M.; Pegoraro, L.; Pagano, G.; Brizzi, M.F. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J. Biol. Chem. 2006, 281, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.N.; Puchinyan, D.M.; Norkin, I.A. Vascular endothelial Barrier Function. Usp. Fiziol. Nauk. 2015, 46, 72–96. (In Russian) [Google Scholar] [PubMed]
- Gero, D. Hyperglycemia-induced endothelial dysfunction. In Endothelial Dysfunction—Old Concepts and New Challenges; Lenasi, H., Ed.; Intechopen: London, UK, 2018; pp. 179–210. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Venneri, M.A.; Fiore, D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: Therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J. Endocrinol. Investig. 2016, 39, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Shilo, S.; Roy, S.; Khanna, S.; Sen, C.K. MicroRNA in cutaneous wound healing: A new paradigm. DNA Cell Biol. 2007, 26, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Icli, B.; Feinberg, M.W. Emerging Roles for MicroRNAs in Diabetic Microvascular Disease: Novel Targets for Therapy. Endocr. Rev. 2017, 38, 145–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, S.; Cao, J.T.; Zhang, B.; Zhou, Q.; Shen, C.X.; Wang, C.Q. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J. Mol. Cell Cardiol. 2012, 53, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Caporali, A.; Emanueli, C. MicroRNA-503 and the extended microRNA-16 family in angiogenesis. Trends Cardiovasc. Med. 2011, 21, 162–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catrina, S.B.; Zheng, X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 179–185. [Google Scholar] [CrossRef] [PubMed]
- Saikumar, P.; Dong, Z.; Patel, Y.; Hall, K.; Hopfer, U.; Weinberg, J.M.; Venkatachalam, M.A. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 1998, 17, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Semenza, G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010, 86, 236–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bento, C.F.; Fernandes, R.; Ramalho, J.; Marques, C.; Shang, F.; Taylor, A.; Pereira, P. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS ONE 2010, 5, e15062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katavetin, P.; Miyata, T.; Inagi, R.; Tanaka, T.; Sassa, R.; Ingelfinger, J.R.; Fujita, T.; Nangaku, M. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J. Am. Soc. Nephrol. 2006, 17, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Trabold, O.; Wagner, S.; Wicke, C.; Scheuenstuhl, H.; Hussain, M.Z.; Rosen, N.; Seremetiev, A.; Becker, H.D.; Hunt, T.K. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair. Regen. 2003, 11, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Maan, Z.N.; Whittam, A.J.; Sorkin, M.; Hu, M.S.; Walmsley, G.G.; Baker, H.; Fischer, L.H.; Januszyk, M.; Wong, V.W.; et al. Fibroblast-Specific Deletion of Hypoxia Inducible Factor-1 Critically Impairs Murine Cutaneous Neovascularization and Wound Healing. Plast. Reconstr. Surg. 2015, 136, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, R.; Zayas, J.; Mohamed, M.F.; Aboonabi, A.; Delgado, K.; Wallace, J.; Bayat, M.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. IL-10 Dysregulation Underlies Chemokine Insufficiency, Delayed Macrophage Response, and Impaired Healing in Diabetic Wounds. J. Investig. Dermatol. 2022, 142 Pt A, 692–704.e14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meshkani, R.; Vakili, S. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clin. Chim. Acta 2016, 462, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Y.; Chen, Y.; Sun, X.; Zhang, Z. Epigenetic regulatory mechanism of macrophage polarization in diabetic wound healing (Review). Mol. Med. Rep. 2025, 31, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.; Zheng, C.; Ye, J.; Song, F.; Wang, X.; Liu, Y.; Tian, M.; Dong, J.; Lu, S. Effects of advanced glycation end products on neutrophil migration and aggregation in diabetic wounds. Aging 2021, 13, 12143–12159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Zheng, X.; Chen, Y.; Wu, L.; Yang, Z.; Chen, X.; Song, W. Role of matrix metalloproteinases in diabetic foot ulcers: Potential therapeutic targets. Front. Pharmacol. 2022, 13, 1050630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanna, S.; Biswas, S.; Shang, Y.; Collard, E.; Azad, A.; Kauh, C.; Bhasker, V.; Gordillo, G.M.; Sen, C.K.; Roy, S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 2010, 5, e9539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferracini, M.; Martins, J.O.; Campos, M.R.; Anger, D.B.; Jancar, S. Impaired phagocytosis by alveolar macrophages from diabetic rats is related to the deficient coupling of LTs to the Fc gamma R signaling cascade. Mol. Immunol. 2010, 47, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Y.; He, S.Y.; Hu, Q.Y.; Lu, Y.; Niu, Y.X.; Li, X.Y.; Zhang, H.M.; Qin, L.; Su, Q. Advanced Glycation End Products (AGEs) Inhibit Macrophage Efferocytosis of Apoptotic β Cells through Binding to the Receptor for AGEs. J. Immunol. 2022, 208, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Zayas, J.; Singh, S.K.; Delgado, K.; Wood, S.J.; Mohamed, M.F.; Frausto, D.M.; Albalawi, Y.A.; Price, T.P.; Estupinian, R.; et al. Overriding impaired FPR chemotaxis signaling in diabetic neutrophil stimulates infection control in murine diabetic wound. eLife 2022, 11, e72071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fadini, G.P.; Menegazzo, L.; Rigato, M.; Scattolini, V.; Poncina, N.; Bruttocao, A.; Ciciliot, S.; Mammano, F.; Ciubotaru, C.D.; Brocco, E.; et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes 2016, 65, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.S.; Sreejit, G.; Nagareddy, P.R.; Murphy, A.J. Attack of the NETs! NETosis primes IL-1β-mediated inflammation in diabetic foot ulcers. Clin. Sci. 2020, 134, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clayton, S.M.; Shafikhani, S.H.; Soulika, A.M. Macrophage and Neutrophil Dysfunction in Diabetic Wounds. Adv. Wound Care 2024, 13, 463–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thimmappa, P.Y.; Vasishta, S.; Ganesh, K.; Nair, A.S.; Joshi, M.B. Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: Implications in combating infections. Hum. Cell 2023, 36, 1265–1282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yano, H.; Kinoshita, M.; Fujino, K.; Nakashima, M.; Yamamoto, Y.; Miyazaki, H.; Hamada, K.; Ono, S.; Iwaya, K.; Saitoh, D.; et al. Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect. Immun. 2012, 80, 4409–4416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, M.; Qing, C.; Niu, Y.; Dong, J.; Cao, X.; Song, F.; Ji, X.; Lu, S. The Relationship Between Inflammation and Impaired Wound Healing in a Diabetic Rat Burn Model. J. Burn. Care Res. 2016, 37, e115–e124. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Rodrigues, J.; Gonçalves, M.; Amaral, C.; Lima, M.; Carvalho, E. Impaired T-cell differentiation in diabetic foot ulceration. Cell Mol. Immunol. 2017, 14, 758–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Liang, G.; Gui, L.; Li, Y.; Liu, M.; Bai, Y.; Zhang, X.; Hu, X.; Chen, J.; Huang, C.; et al. Weakened IL-15 Production and Impaired mTOR Activation Alter Dendritic Epidermal T Cell Homeostasis in Diabetic Mice. Sci. Rep. 2017, 7, 6028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Tan, Q.; Hou, Y.; Dou, H. Emerging Roles of Myeloid-Derived Suppressor Cells in Diabetes. Front. Pharmacol. 2021, 12, 798320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Feng, J.; Ni, Y.; Li, G.; Wang, Y.; Cao, Y.; Zhou, M.; Zhao, C. The role of SLC7A11 in diabetic wound healing: Novel insights and new therapeutic strategies. Front. Immunol. 2024, 15, 1467531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, K.I.; Syverson, A.L.; Kralik, R.M.; Choi, J.; DerGarabedian, B.P.; Chen, C.; Graves, D.T. Diabetes-Induced NF-κB Dysregulation in Skeletal Stem Cells Prevents Resolution of Inflammation. Diabetes 2019, 68, 2095–2106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, Y.; Huang, Y.; Zeng, G.; Hu, J.; Li, M.; Tian, M.; Lei, T.; Huang, R. Advanced glycation end products regulate macrophage apoptosis and influence the healing of diabetic foot wound through miR-361-3p/CSF1R and PI3K/AKT pathway. Heliyon 2024, 10, e24598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, F.M.; Tsoi, L.C.; Wasikowski, R.; denDekker, A.; Joshi, A.; Wilke, C.; Deng, H.; Wolf, S.; Obi, A.; Huang, S.; et al. Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight 2020, 5, e138443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karnam, K.; Sedmaki, K.; Sharma, P.; Routholla, G.; Goli, S.; Ghosh, B.; Venuganti, V.V.K.; Kulkarni, O.P. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1β secretion in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165903. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 2020, 9, 2228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gyurko, R.; Siqueira, C.C.; Caldon, N.; Gao, L.; Kantarci, A.; Van Dyke, T.E. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J. Immunol. 2006, 177, 7250–7256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivas-Santiago, B.; Trujillo, V.; Montoya, A.; Gonzalez-Curiel, I.; Castañeda-Delgado, J.; Cardenas, A.; Rincon, K.; Hernandez, M.L.; Hernández-Pando, R. Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 2012, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Zenilman, J.M.; Lazarus, G.S. Molecular microbiology: New dimensions for cutaneous biology and wound healing. J. Investig. Dermatol. 2010, 130, 38–48. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair. Regen. 2012, 20, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Oates, A.; Bowling, F.L.; Boulton, A.J.; McBain, A.J. Molecular and culture-based assessment of the microbial diversity of diabetic chronic foot wounds and contralateral skin sites. J. Clin. Microbiol. 2012, 50, 2263–2271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossana, C.; Meloni, M.; Giurato, L.; Lazaro-Martinez, J.L.; Aikaterini, A.; Valeria, R.; Bellia, A.; Lauro, D.; Uccioli, L. Microbiological and Clinical Characteristics of Infected Diabetic Foot Ulcers Managed in a Tertiary Level Diabetic Foot Service. Int. J. Low. Extrem. Wounds, 2023; 15347346231178642, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Durand, B.A.R.N.; Pouget, C.; Magnan, C.; Molle, V.; Lavigne, J.P.; Dunyach-Remy, C. Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review. Microorganisms 2022, 10, 1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J. Dynamic interactions of neutrophils and biofilms. J. Oral. Microbiol. 2014, 6, 26102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio 2016, 7, e01058-16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clinton, A.; Carter, T. Chronic Wound Biofilms: Pathogenesis and Potential Therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirumala, N. Bacteriological Profile of Diabetic Foot Ulcer with Special Reference to Biofilm Formation. Cureus 2025, 17, e80974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajab, A.A.H.; Hegazy, W.A.H. What’s old is new again: Insights into diabetic foot microbiome. World J. Diabetes 2023, 14, 680–704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stacey, H.J.; Clements, C.S.; Welburn, S.C.; Jones, J.D. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: A meta-analysis. Acta Diabetol. 2019, 56, 907–921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, Y.D.; Ou, S.J.; Zhang, W.; Li, J.X.; Xia, C.L.; Yang, Y.; Liu, J.B.; Ma, Y.F.; Jiang, N.; Wang, Y.Y.; et al. Microbiological profile of diabetic foot infections in China and worldwide: A 20-year systematic review. Front. Endocrinol. 2024, 15, 1368046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.S.; Kappler, F.; Brown, T.R. Identification of fructose 3-phosphate in the lens of diabetic rats. Science 1990, 247, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Kolm-Litty, V.; Sauer, U.; Nerlich, A.; Lehmann, R.; Schleicher, E.D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Investig. 1998, 101, 160–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helou, C.; Marier, D.; Jacolot, P.; Abdennebi-Najar, L.; Niquet-Léridon, C.; Tessier, F.J.; Gadonna-Widehem, P. Microorganisms and Maillard reaction products: A review of the literature and recent findings. Amino Acids 2014, 46, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-L.; Yang, M.H.; Chyau, C.C.; Chiu, C.H.; Wang, H.E.; Lin, Y.C.; Chiu, W.T.; Peng, R.Y. Kinetic analysis on the sensitivity of glucose- or glyoxal-induced LDL glycation to the inhibitory effect of Psidium guajava extract in a physiomimic system. Biosystems 2007, 88, 92–100. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J. Mol. Med. 2009, 87, 235–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, P.; Inoguchi, T.; Kern, T.S.; Engerman, R.L.; Oates, P.J.; King, G.L. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 1994, 43, 1122–1129. [Google Scholar] [CrossRef]
- Das Evcimen, N.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giardino, I.; Edelstein, D.; Brownlee, M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J. Clin. Investig. 1996, 97, 1422–1428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClain, D.A.; Crook, E.D. Hexosamines and insulin resistance. Diabetes 1996, 45, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model. Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengellér, Z.; Szabó, C.; Brownlee, M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003, 112, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, W.D.; Liu, G.L.; Wang, J.; Wang, H.; Zhang, J.N.; Zhang, F.; Ma, Y.; Ji, X.Y.; Li, C.; Zhang, M.X. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget 2016, 7, 35618–35631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassan, H.A.; Ahmed, H.S.; Hassan, D.F. Free radicals and oxidative stress: Mechanisms and therapeutic targets. Hum. Antibodies 2024, 32, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.E.; Dean, R.T.; Davies, M.J. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch. Biochem. Biophys. 2002, 403, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; Thomas, C.R.; Gopaul, N.; Dhir, S.; Anggård, E.E.; Poston, L.; Tribe, R.M. Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin-diabetic rat. Diabetologia 1998, 41, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, G.; Veves, A. Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides. Auton. Neurosci. 2020, 223, 102610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kansara, K.; Mansuri, A.; Kumar, A.; Bhatia, D. DNA Nano-Biomaterials Based Futuristic Technologies for Tissue Engineering and Regenerative Therapeutics. Small 2025, 21, e2504361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, N.; Zhou, M.; Li, S.; Cai, X. The Application of Tetrahedral Framework Nucleic Acids as a Drug Carrier in Biomedicine Fields. Curr. Stem Cell Res. Ther. 2021, 16, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, S.; Ravan, H.; Ebrahimi, A. DNA hydrogel-empowered biosensing. Adv. Colloid. Interface Sci. 2020, 275, 102060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, S.; Zhang, Q.; Li, S.; Zhang, T.; Wang, L.; Qin, X.; Zhang, M.; Shi, S.; Cai, X. Antioxidative and Angiogenesis-Promoting Effects of Tetrahedral Framework Nucleic Acids in Diabetic Wound Healing with Activation of the Akt/Nrf2/HO-1 Pathway. ACS Appl. Mater. Interfaces 2020, 12, 11397–11408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, H.; Tang, T.; Liu, L.; Pan, B.; Chen, J.; Cheng, D.; Cai, X.; Sun, Y.; Zhu, F.; et al. Tetrahedral framework nucleic acids promote diabetic wound healing via the Wnt signalling pathway. Cell Prolif. 2022, 55, e13316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Ma, W.; Zhu, Y.; Shi, S.; Li, Q.; Mao, C.; Zhao, D.; Zhan, Y.; Shi, J.; Li, W.; et al. Inhibiting Methicillin-Resistant Staphylococcus aureus by Tetrahedral DNA Nanostructure-Enabled Antisense Peptide Nucleic Acid Delivery. Nano Lett. 2018, 18, 5652–5659. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, S.; Zhang, Y.; Li, Q.; Xie, X.; Zhao, D.; Tian, T.; Shi, S.; Meng, L.; Lin, Y. Tetrahedral Framework Nucleic Acids Loading Ampicillin Improve the Drug Susceptibility against Methicillin-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 36957–36966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Li, S.; Liu, M.; Qin, X.; Chen, X.; Lin, Y. Tetrahedral Framework Nucleic Acids Deliver Antimicrobial Peptides with Improved Effects and Less Susceptibility to Bacterial Degradation. Nano Lett. 2020, 20, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Q.; Li, S.; Qin, X.; Cai, X.; Wang, H. Tetrahedral framework nucleic acids-based delivery promotes intracellular transfer of healing peptides and accelerates diabetic would healing. Cell Prolif. 2022, 55, e13279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
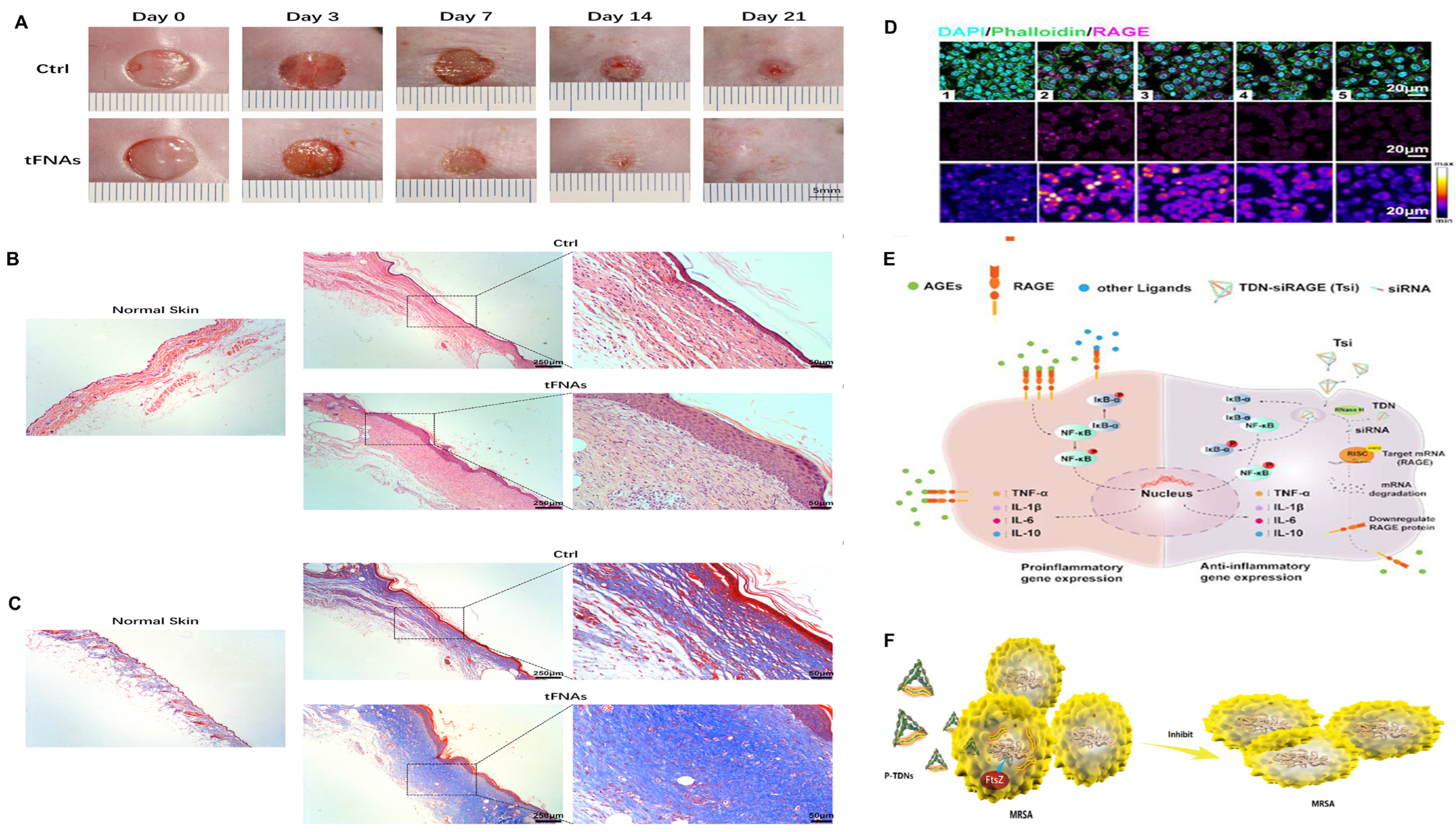
- Cai, Z.; Li, Y.; Bai, L.; Xu, J.; Liu, Z.; Zhang, T.; Gao, S.; Lin, Y. Tetrahedral Framework Nucleic Acids Based Small Interfering RNA Targeting Receptor for Advanced Glycation End Products for Diabetic Complications Treatment. ACS Nano 2023, 17, 22668–22683. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, Q.; Yao, Y.; Xin, Q.; Sun, J.; Chen, W.; Lin, Y.; Cai, X. Framework Nucleic Acids-Based VEGF Signaling Activating System for Angiogenesis: A Dual Stimulation Strategy. Adv. Sci. 2024, 11, e2308701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obuobi, S.; Tay, H.K.; Tram, N.D.T.; Selvarajan, V.; Khara, J.S.; Wang, Y.; Ee, P.L.R. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control. Release 2019, 313, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kiick, K.L.; Sullivan, M.O. VEGF-Encoding, Gene-Activated Collagen-Based Matrices Promote Blood Vessel Formation and Improved Wound Repair. ACS Appl. Mater. Interfaces 2023, 15, 16434–16447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Ma, X.; Gao, Q.; Zhang, M.; Hu, H. A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment. Molecules 2023, 28, 6788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, T.; Li, Y.; Lin, Y. Prospects and challenges of dynamic DNA nanostructures in biomedical applications. Bone Res. 2022, 10, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, J.W.; McLaughlin, C.K.; Castor, K.J.; Sleiman, H. DNA nanostructure serum stability: Greater than the sum of its parts. Chem. Commun. 2013, 49, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Cui, W.; Yang, X.; Lin, Y.; Ma, X.; Cai, X. Applications of tetrahedral DNA nanostructures in wound repair and tissue regeneration. Burns Trauma 2022, 10, tkac006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Liu, Y.; Zhang, T.; Lin, S.; Shi, S.; He, J.; Xie, Y.; Cai, X.; Tian, T.; Lin, Y. A Tetrahedral Framework DNA-Based Bioswitchable miRNA Inhibitor Delivery System: Application to Skin Anti-Aging. Adv. Mater. 2022, 34, e2204287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, X.; Ma, W.; Zhan, Y.; Mao, C.; Shao, X.; Lin, Y. Multi-targeted Antisense Oligonucleotide Delivery by a Framework Nucleic Acid for Inhibiting Biofilm Formation and Virulence. Nanomicro Lett. 2020, 12, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ge, Y.; Wang, Q.; Qin, X.; Li, S.; Liu, Z.; Lin, Y.; Li, X.; Cai, X. Tetrahedral Framework Nucleic Acids Connected with MicroRNA-126 Mimics for Applications in Vascular Inflammation, Remodeling, and Homeostasis. ACS Appl. Mater. Interfaces 2022, 14, 19091–19103. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA hydrogel-based gene editing and drug delivery systems. Adv. Drug Deliv. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, K.; Luo, S.; Li, F.; Zuo, X.; Fan, C.; Li, Q. Programmable DNA Hydrogels as Artificial Extracellular Matrix. Small 2022, 18, e2107640. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, Y.; Jiang, Y.; Gu, T.; Siow, L.; Gao, Y.; Zheng, Y.; Xing, K.; Zhou, S.; Zhang, C.; et al. DNA Hydrogels in Tissue Engineering: From Molecular Design to Next-Generation Biomedical Applications. Adv. Healthc. Mater. 2025, 14, e2500192. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, L.; Gu, Z.; Li, W.; Guo, L.; Ma, S.; Guo, L.; Zhang, W.; Han, B.; Chang, J. N-carboxymethyl chitosan/sodium alginate composite hydrogel loading plasmid DNA as a promising gene activated matrix for in-situ burn wound treatment. Bioact. Mater. 2021, 15, 330–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Xie, H.; Gou, L.; Zhou, Y.; Wang, H.; Li, R.; Zhang, Y.; Liu, S.; Liu, J.; Lu, Y.; et al. DNA-Based Hydrogels with Multidrug Sequential Release for Promoting Diabetic Wound Regeneration. JACS Au 2023, 3, 2597–2608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Li, W.; Gou, L.; Zhou, Y.; Peng, G.; Zhang, J.; Liu, J.; Li, R.; Ni, H.; Zhang, W.; et al. Biodegradable and Antioxidant DNA Hydrogel as a Cytokine Delivery System for Diabetic Wound Healing. Adv. Healthc. Mater. 2022, 11, e2200782. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Gou, L.; Zhou, Y.; Peng, L.; Liu, F.; Zhang, Y. Potential mechanism of the AgNCs-hydrogel in promoting the regeneration of diabetic infectious wounds. Analyst 2023, 148, 5873–5881. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, J.W.; Byun, J.H.; Lee, W.M.; Kim, M.H.; Wu, W.H. Comparison of wound healing effects between Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykiss-derived PDRN. Arch. Craniofacial Surg. 2018, 19, 20–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ansar, R.; Saqib, S.; Mukhtar, A.; Niazi, M.B.K.; Shahid, M.; Jahan, Z.; Kakar, S.J.; Uzair, B.; Mubashir, M.; Ullah, S.; et al. Challenges and recent trends with the development of hydrogel fiber for biomedical applications. Chemosphere 2022, 287 Pt 1, 131956. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lalhall, A.; Puri, S.; Wangoo, N. Design of Fmoc-Phenylalanine Nanofibrillar Hydrogel and Mechanistic Studies of Its Antimicrobial Action against Both Gram-Positive and Gram-Negative Bacteria. ACS Appl. Bio Mater. 2023, 6, 494–506. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Yoon, J.H.; Beaman, H.; Gosavi, P.; Lengyel-Zhand, Z.; Sternisha, A.; Centola, G.; Marshall, L.R.; Wehrman, M.D.; Schultz, K.M.; et al. Nine-Residue Peptide Self-Assembles in the Presence of Silver to Produce a Self-Healing, Cytocompatible, Antimicrobial Hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 17091–17099. [Google Scholar] [CrossRef] [PubMed]
- Atefyekta, S.; Blomstrand, E.; Rajasekharan, A.K.; Svensson, S.; Trobos, M.; Hong, J.; Webster, T.J.; Thomsen, P.; Andersson, M. Antimicrobial Peptide-Functionalized Mesoporous Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 1693–1702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Tan, K.; Xiang, S.; Zhang, Y.; Luo, F.; Liu, X.; Zhao, X.; Ouyang, L. Peptide loaded self-healing hydrogel promotes diabetic skin wound healing through macrophage orchestration and inflammation inhibition. Mater. Today Bio 2025, 32, 101690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanapalli, B.K.R.; Yele, V.; Singh, M.K.; Thumbooru, S.N.; Parvathaneni, M.; Karri, V.V.S.R. Human beta defensin-2 loaded PLGA nanoparticles impregnated in collagen-chitosan composite scaffold for the management of diabetic wounds. Biomed. Pharmacother. 2023, 161, 114540. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bao, X.; Wang, S.; Tang, C.; Wu, N.; Li, G.; Ren, K.; Yin, J.; Yan, S.; Xu, G. A biomimetic nanofiber composite hydrogel with tissue adhesion, self-healing and antibacterial ability for infected wound healing. Acta Biomater. 2025, 200, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Salick, D.A.; Kretsinger, J.K.; Pochan, D.J.; Schneider, J.P. Inherent antibacterial activity of a peptide-based beta-hairpin hydrogel. J. Am. Chem. Soc. 2007, 129, 14793–14799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Thompson, T.P.; Laverty, G. Self-assembling diphenylalanine peptide nanotubes selectively eradicate bacterial biofilm infection. Acta Biomater. 2018, 77, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, X.; Zhu, X.; Xu, M.; Liu, J. Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research. Biomaterials 2017, 141, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, R.T.C.; Riool, M.; Quarles van Ufford, H.L.C.; Zaat, S.A.J.; Kruijtzer, J.A.W.; Liskamp, R.M.J. Convenient Preparation of Bactericidal Hydrogels by Covalent Attachment of Stabilized Antimicrobial Peptides Using Thiol-ene Click Chemistry. ACS Macro Lett. 2014, 3, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Saklani, M.; Jha, C.B.; Baidya, A.T.K.; Singh, S.; Kumar, R.; Mathur, R.; Tiwari, A.K.; Varshney, R. Laminin mimetic angiogenic and collagen peptide hydrogel for enhance dermal wound healing. Biomater. Adv. 2024, 158, 213761. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Siddiqui, Z.; Acevedo-Jake, A.M.; Roy, A.; Choudhury, M.; Grasman, J.; Kumar, V. Angiogenic Hydrogels to Accelerate Early Wound Healing. Macromol. Biosci. 2022, 22, e2200067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Klingele, S.; Jünger, M.; Hasslacher, C.; Daeschlein, G.; Stemler, L.; Meyer-Pannwitt, U.; Schubert, K.; et al. Translational development of ABCB5+ dermal mesenchymal stem cells for therapeutic induction of angiogenesis in non-healing diabetic foot ulcers. Stem Cell Res. Ther. 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, S.; Cheng, Y.; Zhang, L.; Yin, Y.; Xue, J.; Li, B.; Gong, Z.; Gao, J.; Mu, Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, L.; Zeng, W.; Wu, Y.X.; Hou, C.L.; Chen, W.; Yang, M.C.; Li, L.; Zhang, Y.F.; Zhu, C.H. Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transplant. 2013, 22, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Santarella, F.; do Amaral, R.J.F.C.; Lemoine, M.; Kelly, D.; Cavanagh, B.; Marinkovic, M.; Smith, A.; Garlick, J.; O’Brien, F.J.; Kearney, C.J. Personalized Scaffolds for Diabetic Foot Ulcer Healing Using Extracellular Matrix from Induced Pluripotent Stem-Reprogrammed Patient Cells. Adv. Nanobiomed Res. 2022, 2, 2200052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorecka, J.; Gao, X.; Fereydooni, A.; Dash, B.C.; Luo, J.; Lee, S.R.; Taniguchi, R.; Hsia, H.C.; Qyang, Y.; Dardik, A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020, 15, 1277–1293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Q.; Chen, B.; Liang, Z. Mesenchymal Stem Cells as a Prospective Therapy for the Diabetic Foot. Stem Cells Int. 2016, 2016, 4612167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Gregorio, C.; Contador, D.; Díaz, D.; Cárcamo, C.; Santapau, D.; Lobos-Gonzalez, L.; Acosta, C.; Campero, M.; Carpio, D.; Gabriele, C.; et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlosser, S.; Dennler, C.; Schweizer, R.; Eberli, D.; Stein, J.V.; Enzmann, V.; Giovanoli, P.; Erni, D.; Plock, J.A. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc. Res. 2012, 83, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Brouki Milan, P.; Baino, F.; Kargozar, S. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair. Regen. 2022, 30, 421–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dos Santos, J.F.; Borçari, N.R.; da Silva Araújo, M.; Nunes, V.A. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: Towards an in vitro model of human epidermis. J. Cell Biochem. 2019, 120, 13141–13155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillén, M.I.; Platas, J.; Pérez Del Caz, M.D.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.Y.; Zheng, Z.H.; Li, X.Y.; Guo, J.; Zhang, Y.; Li, H.; Wang, Y.W.; Ren, J.; Wu, Z.B. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: Response and correction of immunological anomalies. Curr. Pharm. Des. 2013, 19, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, H.; Dong, X.; Wang, Z.; Yang, Z.; Shang, Q.; Wang, Q. Regulation of inflammation during wound healing: The function of mesenchymal stem cells and strategies for therapeutic enhancement. Front. Pharmacol. 2024, 15, 1345779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuang, S.; He, F.; Liu, G.; Sun, X.; Dai, J.; Chi, A.; Tang, Y.; Li, Z.; Gao, Y.; Deng, C.; et al. CCR2-engineered mesenchymal stromal cells accelerate diabetic wound healing by restoring immunological homeostasis. Biomaterials 2021, 275, 120963. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Herold, K.C. Immunotherapy: Building a bridge to a cure for type 1 diabetes. Science 2021, 373, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018, 2018, 6901983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Chen, C.; Wang, Y.; Yi, W.; Guo, P.; Yao, C.; Liu, J.; Wei, Y.; Hu, K.; Shang, X.; et al. A meta-analysis on application and prospect of cell therapy in the treatment of diabetes mellitus. Stem Cell Res. Ther. 2025, 16, 249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azizi, Z.; Abbaszadeh, R.; Sahebnasagh, R.; Norouzy, A.; Motevaseli, E.; Maedler, K. Bone marrow mesenchymal stromal cells for diabetes therapy: Touch, fuse, and fix? Stem Cell Res. Ther. 2022, 13, 348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, M.; Sun, L.; Huang, C.; Chen, B.C.; Zhou, Z. Induction of Macrophage M2b/c Polarization by Adipose Tissue-Derived Mesenchymal Stem Cells. J. Immunol. Res. 2019, 2019, 7059680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benabdellah, K.; Sánchez-Hernández, S.; Aguilar-González, A.; Maldonado-Pérez, N.; Gutierrez-Guerrero, A.; Cortijo-Gutierrez, M.; Ramos-Hernández, I.; Tristán-Manzano, M.; Galindo-Moreno, P.; Herrera, C.; et al. Genome-Edited Adult Stem Cells: The Next Generation of Advanced Therapeutic Medicinal Products. Stem Cells Transl. Med. 2020, 9, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Fu, R.H.; Shyu, W.C.; Liu, S.P.; Jong, G.P.; Chiu, Y.W.; Wu, H.S.; Tsou, Y.A.; Cheng, C.W.; Lin, S.Z. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transplant. 2013, 22, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Argentati, C.; Morena, F.; Bazzucchi, M.; Armentano, I.; Emiliani, C.; Martino, S. Adipose Stem Cell Translational Applications: From Bench-to-Bedside. Int. J. Mol. Sci. 2018, 19, 3475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, W.; Shi, J. Application of adipose-derived stem cells in ischemic heart disease: Theory, potency, and advantage. Front. Cardiovasc. Med. 2024, 11, 1324447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Li, J.; Guan, H.; Oishi, H.; Takahashi, S.; Zhang, C. Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: Applications and research advances. Int. J. Med. Sci. 2023, 20, 1492–1507. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Sundaravadivelu, P.K.; Raina, K.; Thool, M.; Ray, A.; Joshi, J.M.; Kaveeshwar, V.; Sudhagar, S.; Lenka, N.; Thummer, R.P. Tissue-Restricted Stem Cells as Starting Cell Source for Efficient Generation of Pluripotent Stem Cells: An Overview. Adv. Exp. Med. Biol. 2022, 1376, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Joshi, J.M.; Sundaravadivelu, P.K.; Raina, K.; Lenka, N.; Kaveeshwar, V.; Thummer, R.P. An Overview on Promising Somatic Cell Sources Utilized for the Efficient Generation of Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2021, 17, 1954–1974. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.; Klingenstein, M.; Liebau, S.; Linta, L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int. 2014, 2014, 768391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, W.; Li, Y.; Yu, M.; Ren, D.; Han, C.; Guo, S. Advances of exosomes in diabetic wound healing. Burns Trauma 2025, 13, tkae078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, T.; Wang, L.; Gao, C.; Jian, C.; Liu, Y.; Fu, Z.; Shi, C. Treg-Derived Extracellular Vesicles: Roles in Diseases and Theranostics. Mol. Pharm. 2024, 21, 2659–2672. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, M.; Jiang, X.; Li, H.; Zhang, C.; Zhang, Z.; Wu, C.; Zhang, J.; Hu, J.; Zhang, J. The role of mesenchymal stem cell-derived EVs in diabetic wound healing. Front. Immunol. 2023, 14, 1136098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguiar Koga, B.A.; Fernandes, L.A.; Fratini, P.; Sogayar, M.C.; Carreira, A.C.O. Role of MSC-derived small extracellular vesicles in tissue repair and regeneration. Front. Cell Dev. Biol. 2023, 10, 1047094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Bi, Y.; Wang, K.; Guo, X.; Liu, Z.; Li, J.; Wu, M. Stem Cell-Derived Extracellular Vesicles: Promising Therapeutic Opportunities for Diabetic Wound Healing. Int. J. Nanomed. 2024, 19, 4357–4375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Liu, D.; Wang, Z.; Zhu, Y.; Li, J.; Xu, K.; Li, F.; Wen, H.; Yang, R. Exosomal miR-4645-5p from hypoxic bone marrow mesenchymal stem cells facilitates diabetic wound healing by restoring keratinocyte autophagy. Burns Trauma 2024, 12, tkad058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, J.; Qian, S.; Zhao, Q.; Zhao, B.; Lu, B.; Zhang, L.; Mao, X.; Zhang, Y.; Cui, W.; Sun, X. Balancing macrophage polarization via stem cell-derived apoptotic bodies for diabetic wound healing. Med 2024, 5, 148–168.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Zhu, Y.Z.; Hu, X.; Nie, J.Y.; Wang, Z.H.; Wu, S.; Yi, Y.Y. Extracellular Vesicles Derived from Adipose-Derived Stem Cells Accelerate Diabetic Wound Healing by Suppressing the Expression of Matrix Metalloproteinase-9. Curr. Pharm. Biotechnol. 2022, 23, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Maqsood, M.; Zhu, M.; Zhou, Y.; Kang, M.; Zhou, J.; Chen, J. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. Int. J. Mol. Sci. 2022, 23, 10421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Q.; Wang, Y.; Ma, K.; Li, Q.; Li, B.; Hu, W.; Fu, X.; Zhang, C. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Diabetic Wound Healing Through MiR-17-5p-mediated Enhancement of Angiogenesis. Stem Cell Rev. Rep. 2022, 18, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, J.; Wu, B.; Tian, W. Human adipose tissue-derived small extracellular vesicles promote soft tissue repair through modulating M1-to-M2 polarization of macrophages. Stem Cell Res. Ther. 2023, 14, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, L.; Zhao, N.; Chen, X.; Zhang, W.; Lv, K.; Xu, Y. Platelet-rich plasma-derived exosomes accelerate the healing of diabetic foot ulcers by promoting macrophage polarization toward the M2 phenotype. Clin. Exp. Med. 2025, 25, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Wu, J.; Ren, K.; Zhang, Y.; Gao, F.; Chen, Y.; Chen, C.; Lu, J. Platelet-Rich Plasma-Derived Exosome-Encapsulated Hydrogels Accelerate Diabetic Wound Healing by Inhibiting Fibroblast Ferroptosis. ACS Appl. Mater. Interfaces 2025, 17, 27923–27936. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, L.; Chen, C.; Hu, Q.; Wang, J.; Shen, J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol. Int. 2021, 45, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Rasti, M.; Parniaei, A.H.; Dehghani, L.; Nasr Esfahani, S.; Mirhendi, H.; Yazdani, V.; Azimian Zavareh, V. Enhancing the wound healing process through local injection of exosomes derived from blood serum: An in vitro and in vivo assessment. Regen. Ther. 2024, 26, 281–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, Z.Y.; Xu, W.C.; Liang, Z.H. Bone marrow mesenchymal stem cell-derived exosomal miR-221-3p promotes angiogenesis and wound healing in diabetes via the downregulation of forkhead box P1. Diabet. Med. 2024, 41, e15386. [Google Scholar] [CrossRef] [PubMed]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen Md, G.; Wu Md, Y.; Zou Md, L.; Zeng Md, Y. Effect of MicroRNA-146a Modified Adipose-Derived Stem Cell Exosomes on Rat Back Wound Healing. Int. J. Low. Extrem. Wounds 2023, 22, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101 Pt B, 108282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, T.; Jiang, G.; Xu, X.; Yan, C.; Kang, Y.; Xiang, X.; Liu, S.; Nie, P.; Zhang, M.; et al. White adipose tissue-derived small extracellular vesicles: A new potential therapeutic reagent for accelerating diabetic wound healing. FASEB J. 2023, 37, e23314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wang, T.; Rapaport, J.A.; Talukder, M. Therapeutic Potential of Extracellular Vesicles (Exosomes) Derived from Platelet-Rich Plasma: A Literature Review. J. Cosmet. Dermatol. 2025, 24, e16709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Yang, H.; Wang, Z.; Tang, Q.; Cao, X.; Chen, C.; Dong, Y.; Xu, Z.; Lv, D.; Rong, Y.; et al. Epidermal Stem Cell Derived Exosomes Alleviate Excessive Autophagy Induced Endothelial Cell Apoptosis by Delivering miR200b-3p to Diabetic Wounds. J. Investig. Dermatol. 2024, 144, 1134–1147.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Theocharidis, G.; Vlachos, I.S.; Kounas, K.; Lobao, A.; Shu, B.; Wu, B.; Xie, J.; Hu, Z.; Qi, S.; et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J. Investig. Dermatol. 2022, 142, 2508–2517.e13. [Google Scholar] [CrossRef] [PubMed]
- Eerdekens, H.; Pirlet, E.; Willems, S.; Bronckaers, A.; Pincela Lins, P.M. Extracellular vesicles: Innovative cell-free solutions for wound repair. Front. Bioeng. Biotechnol. 2025, 13, 1571461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2022, 171, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, L.; Li, W.; Zhao, W.; Chen, W.; AlQranei, M.S.; Bi, J.; Huang, P. GelMA hydrogel-loaded extracellular vesicles derived from keratinocytes promote skin microvasculature regeneration and wound healing in diabetic mice through activation of the PDGF-induced PI3K/AKT pathway. Cell Biol. Toxicol. 2025, 41, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ju, Y.; Yang, P.; Liu, X.; Wu, R.; Shen, N.; Hsiung, N.; Yang, A.; Zhang, C.; Fang, B.; Liu, L. Antimicrobial dual-crosslinked hydrogel synergizes bioengineered extracellular vesicles for enhanced diabetic wound healing. Mater. Today Bio 2025, 32, 101870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, Y.; Wang, J.; Li, J.; Cheng, Y.; Zhou, S.; Zhang, Y.; Zhao, Y.; Zhou, M. Tβ4-Engineered ADSC Extracellular Vesicles Rescue Cell Senescence Through Separable Microneedle Patches for Diabetic Wound Healing. Adv. Sci. 2025, 12, e2505009. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ding, H.; Li, X.; Wu, Y.; Huang, X. Disulfiram-loaded nanovesicles hydrogel promotes healing of diabetic wound. J. Transl. Med. 2024, 22, 1066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenhalgh, D.G. The role of growth factors in wound healing. J. Trauma. 1996, 41, 159–167. [Google Scholar] [CrossRef] [PubMed]
- de Borst, M.H. Fibroblast growth factor 23 as a risk factor for incident diabetes. Curr. Opin. Nephrol. Hypertens. 2025, 34, 284–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omraninava, M.; Rezapour-Nasrabad, R.; Hosseini, M.; Kasiri, M.A.; Shahzamani, S.; Bahrami, M.; Sadrzadeh-Aghajani, Z.; Mirzaie, M.S. Promotion of skin regeneration in diabetic rats by collagen-based hydrogel incorporated with basic fibroblast growth factor: A histological, molecular, and tensiometrical study. Tissue Cell 2025, 96, 102983. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, L.; Atallah, P.; Werner, C.; Freudenberg, U. StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv. Healthc. Mater. 2016, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Emiroglu, D.B.; Singh, A.; Marco-Dufort, B.; Speck, N.; Rivano, P.G.; Oakey, J.S.; Nakatsuka, N.; deMello, A.J.; Labouesse, C.; Tibbitt, M.W. Granular Biomaterials as Bioactive Sponges for the Sequestration and Release of Signaling Molecules. Adv. Healthc. Mater. 2024, 13, e2400800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yang, J.; Zheng, W.; Chu, Z.; Wang, W.; Qian, H.; Xu, L. Comprehensive management of diabetic ulceration: Strategies and perspectives. J. Control. Release 2025, 385, 114058. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, C.; Liu, Y.; Gao, J.; Li, L.; Yin, C.; Yuan, X. Metal Nanoparticles: Advanced and Promising Technology in Diabetic Wound Therapy. Int. J. Nanomed. 2024, 19, 965–992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.; Yuan, W.; Chen, J.; Wu, L.P.; You, T. Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules 2023, 28, 1110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Liu, Y.; Wang, Z.; Zhao, H.; Zhan, L.; Gui, H.; Xu, X.; Ma, X.; Ma, B. pH-responsive and self-adaptive injectable sodium alginate/carboxymethyl chitosan hydrogel accelerates infected wound healing by bacteriostasis and immunomodulation. Carbohydr. Polym. 2025, 354, 123322. [Google Scholar] [CrossRef] [PubMed]
- Aldahish, A.; Shanmugasundaram, N.; Vasudevan, R.; Alqahtani, T.; Alqahtani, S.; Mohammad Asiri, A.; Devanandan, P.; Thamaraikani, T.; Vellapandian, C.; Jayasankar, N. Silk Fibroin Nanofibers: Advancements in Bioactive Dressings through Electrospinning Technology for Diabetic Wound Healing. Pharmaceuticals 2024, 17, 1305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Chen, L.; Yang, S.; Han, J.; Zheng, Y.; Chen, Z.; Shi, X.; Yang, J. Glucose and pH dual-responsive hydrogels with antibacterial, reactive oxygen species scavenging, and angiogenesis properties for promoting the healing of infected diabetic foot ulcers. Acta Biomater. 2024, 190, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, S.; Sun, M.; Guo, F.; Wang, P.; Jia, L.; Wang, D.; Bao, G.; Jiang, H.; Liu, X. Glycopeptide-based multifunctional nanofibrous hydrogel that facilitates the healing of diabetic wounds infected with methicillin-resistant Staphylococcus aureus. Acta Biomater. 2024, 181, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Wang, M.X.; Cai, C.; Cheng, W.W.; Cheng, Y.J.; Liu, W.L.; Huang, R.; Zhang, A.Q.; Qin, S.Y. Bacterial membrane-anchored lipopeptide/MXene nanoplatform for tri-modal therapy toward bacteria-infected diabetic wound. Biomater. Adv. 2025, 175, 214324. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zhang, L.; Wang, Z.; Dang, W.; Chen, H.; Li, T.; Liu, Y.; Tan, W. Molecular-Cellular Two-Pronged Reprogramming of Inflammatory Soft-Tissue Interface with an Immunosuppressive Pure DNA Hydrogel. Nano Lett. 2025, 25, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Xu, Z.; Li, L.; Guo, K.; Mi, J.; Wu, H.; Li, Y.; Xie, C.; Jin, J.; Xu, J.; et al. Hydrogels with programmed spatiotemporal mechanical cues for stem cell-assisted bone regeneration. Nat. Commun. 2025, 16, 3633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013. [Google Scholar] [CrossRef] [PubMed]
- Arabi, T.Z.; Almasry, Y.; Xue, A.; Eirin, A.; Lerman, A.; Zhu, X.Y.; Lerman, L.O. Immune rejection of human mesenchymal stem cells compared to extracellular vesicles in mice with renal artery stenosis. Stem Cells Transl. Med. 2025, 14, szaf015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Fang, Q.; Wang, J.; Li, M.; Li, Z.; Xu, H.; Huang, S.; Chen, J.; Guo, B. Glucose-Activated Programmed Hydrogel with Self-Switchable Enzyme-Like Activity for Infected Diabetic Wound Self-Adaptive Treatment. Adv. Mater. 2025, 37, e2419158. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakadia, B.M.; Qaed Ahmed, A.A.; Lamboni, L.; Shi, Z.; Mutu Mukole, B.; Zheng, R.; Pierre Mbang, M.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, R.; Zhu, Y.; Chen, Y.; Lin, Y.; Shi, S. Advances in DNA nanotechnology for chronic wound management: Innovative functional nucleic acid nanostructures for overcoming key challenges. J. Control. Release 2024, 375, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yao, J.; Zuo, H.; Jiao, Y.; Wu, J.; Meng, Z. Diverse-Origin Exosomes Therapeutic Strategies for Diabetic Wound Healing. Int. J. Nanomed. 2025, 20, 7375–7402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tabatabaei Hosseini, B.S.; Meadows, K.; Gabriel, V.; Hu, J.; Kim, K. Biofabrication of Cellulose-based Hydrogels for Advanced Wound Healing: A Special Emphasis on 3D Bioprinting. Macromol. Biosci. 2024, 24, e2300376. [Google Scholar] [CrossRef] [PubMed]
- Aminnezhad, S.; Hama, N.H.; Hasan, A.H.; Bagheri, F.; Alavi, M. Applications of biocompatible polymeric nanomaterials in three-dimensional (3D) scaffolds: Bacterial infections and diabetes. Int. J. Biol. Macromol. 2025, 301, 140331. [Google Scholar] [CrossRef] [PubMed]
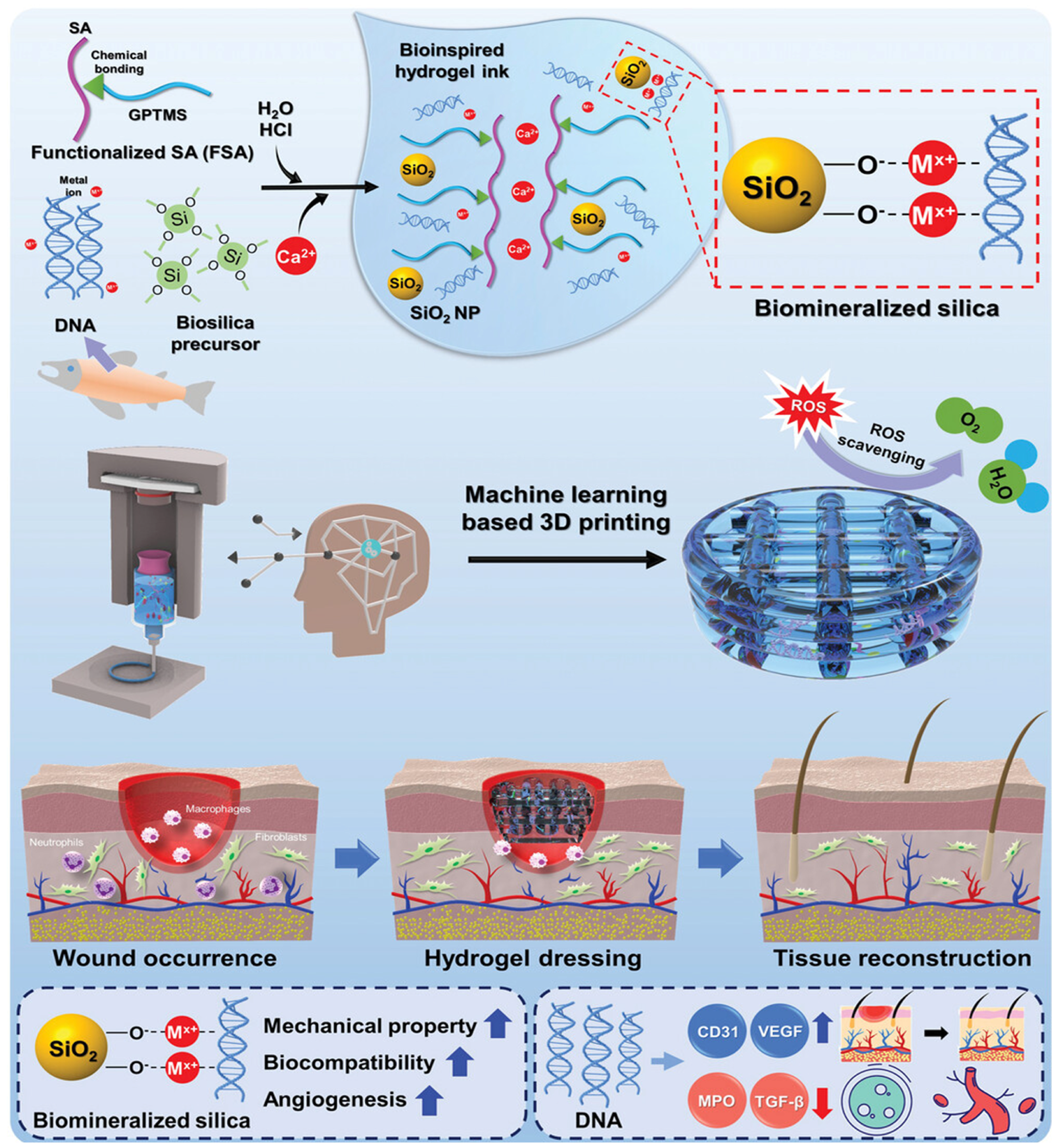
- Kim, N.; Lee, H.; Han, G.; Kang, M.; Park, S.; Kim, D.E.; Lee, M.; Kim, M.J.; Na, Y.; Oh, S.; et al. 3D-Printed Functional Hydrogel by DNA-Induced Biomineralization for Accelerated Diabetic Wound Healing. Adv. Sci. 2023, 10, e2300816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Biomaterials | Model | Results | Refs |
|---|---|---|---|
| TDNs Society | Diabetic Wistar rat full-thickness cutaneous wound | Accelerating vascularization, epithelialization, collagen deposition, and collagen alignment | [146] |
| TDNs | Diabetic mice (db/db) full-thickness cutaneous wound | Regeneration of the epidermis, capillaries, and collagen | [147] |
| TDN loaded with asPNAs | MRSA | Antibacterial activity | [148] |
| TDN loading Ampicillin | MRSA | Antibacterial activity | [149] |
| TDN loaded with GL13K | Pseudomonas gingivalis | Antibacterial activity | [150] |
| ASOs-tFNAs | S. mutans UA159 | Antibacterial activity | [151] |
| p@tFNA | Diabetic mice (db/db) full-thickness cutaneous wound | Promoting angiogenesis and antioxidant activity | [152] |
| tFNA-Apt02-DMOG | Diabetic mice (Balb/c) full-thickness cutaneous wound | Promoting angiogenesis | [153] |
| L12 loaded DNA hydrogels | Healthy mice (C57BL/6J) full-thickness cutaneous wound | Antibacterial activity | [154] |
| VEGF-GAHCM | Diabetes mice (C57BL/6J) full-thickness cutaneous wound | Promoting angiogenesis and collagen deposition | [155] |
| PEI/PDRN@SA/SCS hydrogels | Kunming mice full-thickness cutaneous wound | Promoting cell proliferation and Antibacterial activity | [156] |
| Biomaterials | Model | Results | Refs |
|---|---|---|---|
| Fmoc-Phenylalanine Nanofibrillar Hydrogel | S. aureus and E. coli | Antibacterial activity | [174] |
| L9-Ag Hydrogel | E. coli (ATCC 25922) | Antibacterial activity | [175] |
| Antimicrobial Peptide-Functionalized Mesoporous Hydrogels | Diabetic rat (SD) full-thickness cutaneous wound | Antibacterial activity | [176] |
| Peptide loaded self-healing hydrogel | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization, promoting asngiogenesis, and collagen deposition | [177] |
| HBD-2 COL-CS scaffold | Diabetic rat (Wistar) full-thickness cutaneous wound | Accelerating cell migration and angiogenesis; anti-inflammatory and antibacterial | [178] |
| PGF@ALG/PLGA hydrogel | Diabetic mice (db/db) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; Antibacterial activity | [179] |
| Cell Source | Model | Results | Refs |
|---|---|---|---|
| ABCB5 MSC | Diabetic foot patients | Promoting angiogenesis | [188] |
| ADSC | Diabetic rat (ZDF) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization | [189] |
| BMSC | Diabetes mice (C57BL/6J) full-thickness cutaneous wound | Promoting angiogenesis and cell migration | [190] |
| iPSC | DFU patient-matched fibroblasts | Promoting angiogenesis and ECM deposition | [191] |
| hiPSC-SMC | Diabetic mice full-thickness cutaneous wound | Promoting angiogenesis | [192] |
| EVs Source | Model | Results | Refs |
|---|---|---|---|
| BMSC-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting angiogenesis | [227] |
| BMSC-EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Induce keratinocyte autophagy; activate keratinocyte proliferation and migration | [228] |
| ADSC-ABs | Diabetic rat full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting angiogenesis | [229] |
| ADSC-EVs | Diabetic mice full-thickness cutaneous wound | Improve re-epithelialization and collagen; inhibit the expression of MMP-9 | [230] |
| hUCMSCs-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting angiogenesis; improve re-epithelialization and collagen | [231] |
| hUCMSCs-EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Promoting angiogenesis | [232] |
| M2-EVs | Diabetic mice (Balb/c) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting angiogenesis | [233] |
| AT-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting Adipose tissue regeneration | [234] |
| PRP-EVs | Diabetic mice (Balb/c) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; promoting angiogenesis | [235] |
| PRP-EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Inhibit fibroblast ferroptosis; enhancing M1-to-M2 macrophage polarization | [236] |
| Serum-EVs | Diabetic mice full-thickness cutaneous wound | Promote angiogenesis and ECM formation | [237] |
| EpiSC-EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Promote angiogenesis | [238] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Singla, R.K.; Wang, C. Application of Biomaterials in Diabetic Wound Healing: The Recent Advances and Pathological Aspects. Pharmaceutics 2025, 17, 1295. https://doi.org/10.3390/pharmaceutics17101295
Han C, Singla RK, Wang C. Application of Biomaterials in Diabetic Wound Healing: The Recent Advances and Pathological Aspects. Pharmaceutics. 2025; 17(10):1295. https://doi.org/10.3390/pharmaceutics17101295
Chicago/Turabian StyleHan, Chenglong, Rajeev K. Singla, and Chengshi Wang. 2025. "Application of Biomaterials in Diabetic Wound Healing: The Recent Advances and Pathological Aspects" Pharmaceutics 17, no. 10: 1295. https://doi.org/10.3390/pharmaceutics17101295
APA StyleHan, C., Singla, R. K., & Wang, C. (2025). Application of Biomaterials in Diabetic Wound Healing: The Recent Advances and Pathological Aspects. Pharmaceutics, 17(10), 1295. https://doi.org/10.3390/pharmaceutics17101295








