Antidiabetic Potential of Mangiferin: An In Silico and In Vivo Approach
Abstract
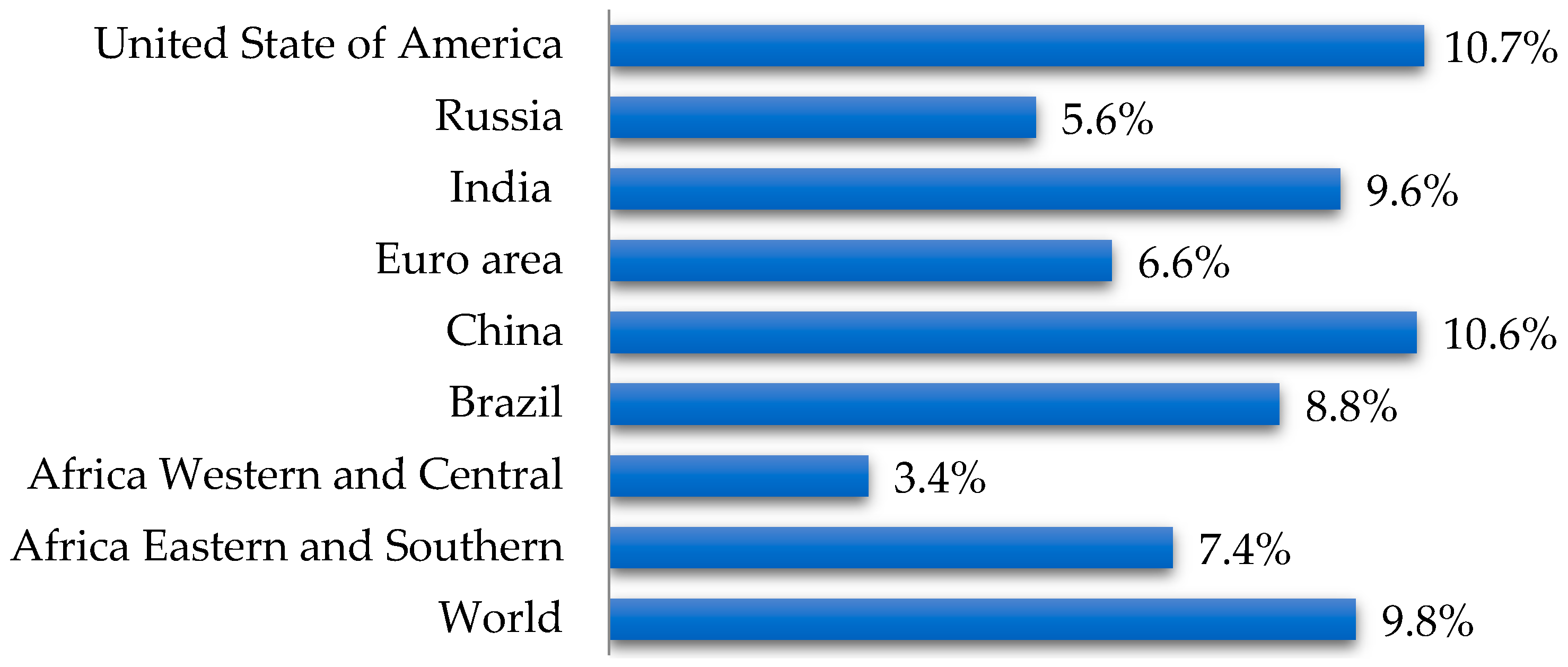
1. Introduction
2. Materials and Methods
2.1. In Silico Analysis
2.1.1. Prediction of Mangiferin’s Targeted Activity Spectrum in the Microcosm BioS System and Identification of Relevant Biotargets
2.1.2. Selection of 3D Models of Relevant Biotargets and Identification of Their Binding Sites
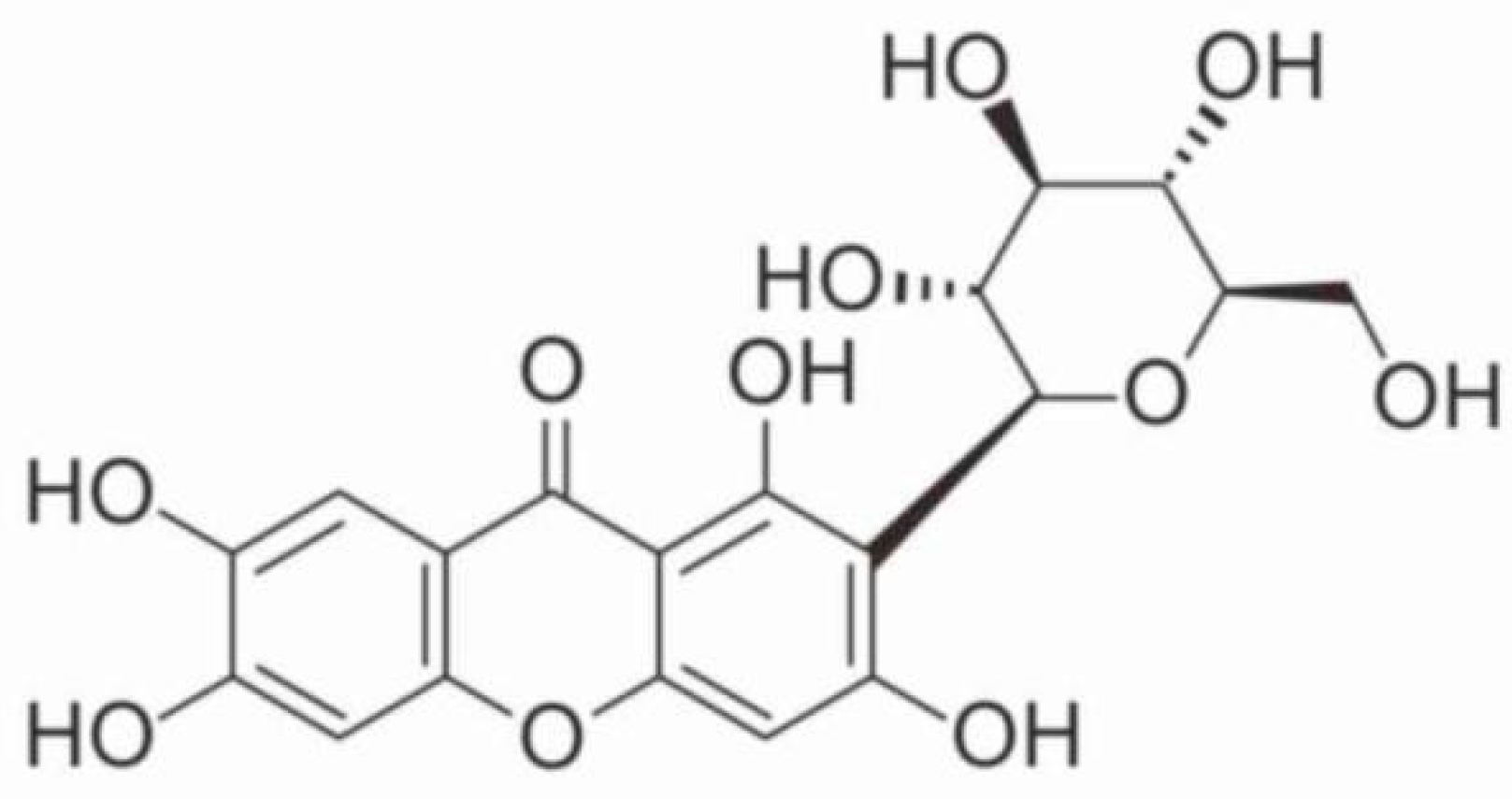
2.1.3. Construction of Optimized 3D Models of the Studied Compound
2.1.4. Ensemble Docking of the Studied Compound into the Binding Sites of Relevant Biotargets and Determination of the Most Affine to the Studied Compound
2.1.5. Analysis of the Molecular Mechanism of Binding of the Studied Compounds
2.1.6. Consensus Prediction of Antiglycation Activity in the IT Microcosm System
2.2. In Vivo Experiments
2.2.1. Design
2.2.2. Anti-Inflammatory Activity In Vivo
2.2.3. Antidiabetic Activity In Vivo
2.2.4. Hypocholesterolemic Activity In Vivo
2.3. Data Analysis
3. Results and Discussion
3.1. Results of In Silico Analysis
3.1.1. Prediction of the Spectrum of Targeted Mangiferin Activity in the Microcosm BioS System and Identification of Relevant Biotargets
3.1.2. Selection of 3D Models of Relevant Biotargets and Identification of Their Binding Sites
3.1.3. Construction of Optimized 3D Models of the Studied Compound
3.1.4. Ensemble Docking of the Studied Compound into the Binding Sites of Relevant Biotargets and Determination of the Most Affine to the Studied Compound
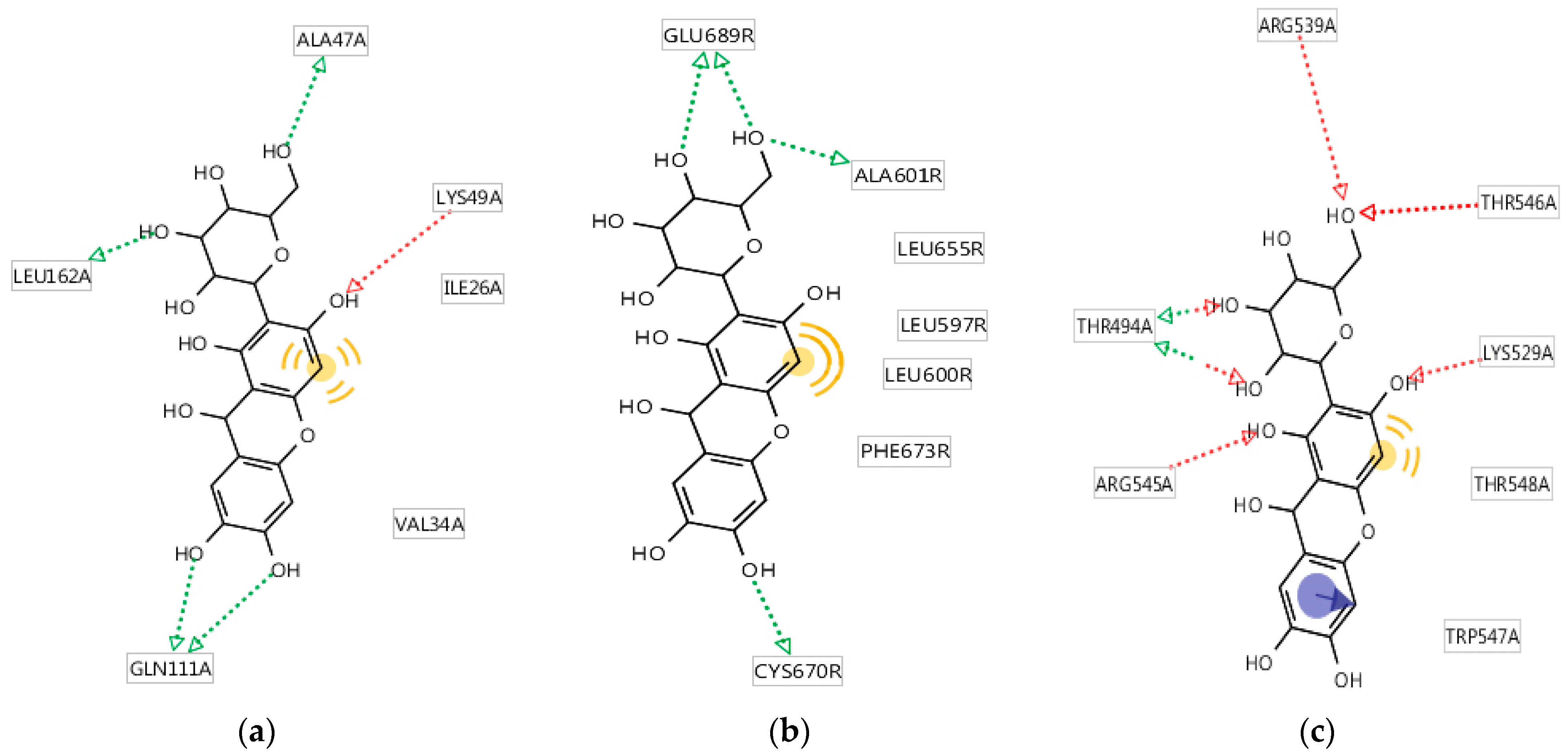
3.1.5. Analysis of the Molecular Mechanism of Binding of the Studied Compounds
3.1.6. Consensus Prediction of Antiglycation Activity Using the IT Microcosm System
3.2. Results of In Vivo Experiment
3.2.1. Anti-Inflammatory Activity In Vivo
3.2.2. Antidiabetic Activity In Vivo
3.2.3. Hypocholesterolemic Activity In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fokina, A.D.; Vesnina, A.D.; Frolova, A.S.; Chekushkina, D.Y.; Proskuryakova, L.A.; Aksenova, L.M. Bioactive Anti-Aging Substances: Geroprotectors. Food Process. Tech. Technol. 2024, 54, 423–435. [Google Scholar] [CrossRef]
- Vesnina, A.D.; Milentyeva, I.S.; Le, V.M.; Fedorova, A.M.; Altshuler, O.G.; Prosekov, A.Y. Quercetin isolated from Hedysarum neglectum Ledeb. as a preventer of metabolic diseases. Foods Raw Mater. 2025, 13, 192–201. [Google Scholar] [CrossRef]
- Vesnina, A.; Milentyeva, I.; Minina, V.; Kozlova, O.; Asyakina, L. Evaluation of the In Vivo Anti-Atherosclerotic Activity of Quercetin Isolated from the Hairy Roots of Hedysarum neglectum Ledeb. Life 2023, 13, 1706. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; El-Maraghy, N.; Reda, E.; Barakat, W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: Role of adiponectin and TNF-α. An. Da Acad. Bras. Cienc. 2014, 86, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Data Bank. Health Nutrition and Population. Available online: https://databank.worldbank.org/source/health-nutrition-and-population-statistics/Series/SH.STA.DIAB.ZS (accessed on 29 May 2025).
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934 (accessed on 3 May 2025).
- Monitoring, S.D. National Medical Research Center of Endocrinology of the Ministry of Health of the Russian Federation. Available online: https://sd.diaregistry.ru/#content. (accessed on 3 May 2025).
- Dedov, I.I.; Shestakova, M.V.; Vikulova, O.K.; Zheleznyakova, A.V.; Isakov, M.A.; Sazonova, D.V.; Mokrysheva, N.G. Diabetes mellitus in the Russian Federation: Dynamics of epidemiological indicators according to the Federal Register of Diabetes Mellitus for the period 2010–2022. Diabetes Mellit. 2023, 26, 104–123. (In Russian) [Google Scholar] [CrossRef]
- Sytaya, Y.S.; Mindlina, A.Y. Epidemiological Features of Obesity and Type 2 Diabetes Mellitus in the Russian Federation. Epidemiol. Vaccinal Prev. 2024, 23, 71–86. (In Russian) [Google Scholar] [CrossRef]
- Noh, J.W.; Lee, H.Y.; Lee, B.C. Mangiferin Ameliorates Obesity-Associated Inflammation and Autophagy in High-Fat-Diet-Fed Mice: In Silico and In Vivo Approaches. Int. J. Mol. Sci. 2022, 23, 15329. [Google Scholar] [CrossRef]
- Khunti, K.; Valabhji, J.; Misra, S. Diabetes and the COVID-19 pandemic. Diabetologia 2023, 66, 255–266. [Google Scholar] [CrossRef]
- Yin, S.; Niu, L.; Zhang, J.; Yang, W.; Liu, Y. Berry beverages: From bioactives to antidiabetes properties and beverage processing technology. Food Front. 2024, 5, 1445–1475. [Google Scholar] [CrossRef]
- Sukhikh, S.; Babich, O.; Prosekov, A.; Kalashnikova, O.; Noskova, S.; Bakhtiyarova, A.; Krol, O.; Tsvetkova, E.; Ivanova, S. Antidiabetic Properties of Plant Secondary Metabolites. Metabolites 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Movahednasab, M.; Dianat-Moghadam, H.; Khodadad, S.; Nedaeinia, R.; Safabakhsh, S.; Ferns, G.; Salehi, R. GLP-1-based therapies for type 2 diabetes: From single, dual and triple agonists to endogenous GLP-1 production and L-cell differentiation. Diabetol. Metab. Syndr. 2025, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, K.; Liu, S.; Yang, J.; Zhao, H.; Zheng, X.-H.; Tsai, M.-J.; Chang, C.-S.; Huang, L.; Weng, C.-F. Combination of plant metabolites hinders starch digestion and glucose absorption while facilitating insulin sensitivity to diabetes. Front. Pharmacol. 2024, 15, 1362150. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hao, J.; Tao, F.; Feng, Q.; Song, Y.; Zeng, B. Association of Metformin use with risk of dementia in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2025, 27, 1992–2001. [Google Scholar] [CrossRef]
- Imachueva, D.R.; Serebryanaya, F.K.; Zilfikarov, I.N. Quantitative determination of the amount of xanthones in terms of mangiferin in the aboveground organs of species of the genus kopechnik (Hedysarum L.) by UV spectrophotometry. Chem. Plant Raw Mater. 2020, 3, 179–186. (In Russian) [Google Scholar] [CrossRef]
- Yang, J.; Asyakina, L.K.; Babich, O.O.; Dyshlyuk, L.S.; Suhih, S.A.; Popov, A.D.; Kostyushnina, N.V. Physicochemical properties and biological activity of extracts of dried biomass of callus and suspension cells and in vitro root cultures. Food Process. Tech. Technol. 2020, 50, 480–492. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ramesh, K.; Kapusetti, G.; Ray, A.K.; Ray, B.; Misra, N. Mangiferin as chain transfer agent: Effect on the molecular weight of poly(methyl methacrylate) and polystyrene. Polym. Bull. 2015, 72, 1407–1416. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X. Preparative separation of mangiferin glycosides by high speed counter current chromatography and comparison of their antioxidant and antitumor activities. RSC Adv. 2020, 10, 25780–25785. [Google Scholar] [CrossRef]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef]
- Aswal, S.; Kumar, A.; Chauhan, A.; Semwal, R.B.; Kumar, A.; Semwal, D.K. A Molecular Approach on the Protective Effects of Mangiferin Against Diabetes and Diabetes-related Complications. Curr. Diabetes Rev. 2020, 16, 690–698. [Google Scholar] [CrossRef]
- Vo, T.H.T.; Nguen, C.Z.; Nguen, K.H.; Ushakova, N.A. Vydelenie iz list’ev mangovogo dereva Mangifera indica mangifirina i ocenka ego biologicheskoj aktivnosti po blokirovaniyu α-glyukozidazy. Him. Farm. Zhurnal 2017, 51, 44–48. [Google Scholar]
- Cheng, J.; Ren, C.; Cheng, R.; Li, Y.; Liu, P.; Wang, W.; Liu, L. Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-β/p38/MK2 signaling pathway. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2021, 25, 131–137. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Ganesan, K.; Ramkumar, K.M. Mangiferin Represses Inflammation in Macrophages Under a Hyperglycemic Environment Through Nrf2 Signaling. Int. J. Mol. Sci. 2024, 25, 11197. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yuan, H.; Guan, H.; Li, Y.; Niu, Y. Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPARα: Mangiferin Improved Insulin Resistance. J. Diabetes Res. 2019, 2019, 2052675. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, Y.S.; Denisova, S.V.; Beregovyh, G.V.; Halahin, V.V.; Suslov, N.I. Comparative study of different doses of Hedysarum alpinum L. extract Behavioral activity in the “Hanging by the tail test”. Natl. Health 2024, 2, 38–41. [Google Scholar]
- Yan, M.; Bo, X.; Zhang, X.; Zhang, J.; Liao, Y.; Zhang, H.; Cheng, Y.; Guo, J.; Cheng, J. Mangiferin Alleviates Postpartum Depression-Like Behaviors by Inhibiting MAPK Signaling in Microglia. Front. Pharmacol. 2022, 13, 840567. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.K.; Kisku, A.; Jangra, A. Mangiferin ameliorates intracerebroventricular-quinolinic acid-induced cognitive deficits, oxidative stress, and neuroinflammation in Wistar rats. Indian J. Pharmacol. 2020, 52, 296–305. [Google Scholar] [CrossRef]
- Frolova, A.S.; Fokina, A.D.; Vesnina, A.D.; Milent’eva, I.S.; Le, V.M.; Prosekov, A.Y.U.; Luzyanin, S.L. Study of the neuroprotective potential in vivo of the Hedysarum neglectum metabolite. Butlerite Messages 2024, 80, 59–67. Available online: https://butlerov.com/files/reports/2005/vol7/1/13_11_2024324-80-10-59--C24-9-4-2-.pdf (accessed on 25 September 2025). (In Russian).
- Frolova, A.; Milentyeva, I.; Fedorova, A.; Asyakina, L.; Prosekov, A. Assessment of the safety of a number of plant metabolites—Promising geroprotectors in an in vivo study. Sib. J. Life Sci. Agric. 2024, 16, 64–91. [Google Scholar] [CrossRef]
- Mihovich, Z.E.; Skrockaya, O.V.; Portnyagina, N.V. Morphogenesis features of the Alpine spearhead (Hedysarum alpinum L.) in in vitro culture. Samara Sci. Bull. 2023, 12, 87–92. (In Russian) [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Ganogpichayagrai, A.; Palanuvej, C.; Ruangrungsi, N. Antidiabetic and anticancer activities of Mangifera indica cv. Okrong leaves. J. Adv. Pharm. Technol. Res. 2017, 8, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, Y.S.; Suslov, N.I.; Kul’pin, P.V.; Melent’eva, Y.V.; Kosenko, K.K. Comparative analysis of biologically active substances Hedysarum alpinum L. and Hedysarum theinum Krasnob. by thin-layer chromatography. Bull. Sci. Educ. 2018, 16, 79–84. (In Russian) [Google Scholar]
- Shi, J.; Lv, H.; Tang, C.; Li, Y.; Huang, J.; Zhang, H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose- and hypoxia-induced RRCECs. Mol. Med. Rep. 2021, 23, 473. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wang, X.; Deng, L.; He, B.; Yi, X.; Li, J. Mangiferin alleviated poststroke cognitive impairment by modulating lipid metabolism in cerebral ischemia/reperfusion rats. Eur. J. Pharmacol. 2024, 977, 176724. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Zhang, H.; Fang, H.; Li, X.; Li, Z.; Huan, Z.; Zhang, Z.; Wang, Y.; Li, W.; et al. Application of interpretable machine learning algorithms to predict macroangiopathy risk in Chinese patients with type 2 diabetes mellitus. Sci. Rep. 2025, 15, 16393. [Google Scholar] [CrossRef]
- Imachueva, D.R.; Serebryanaya, F.K. The use of capillary electrophoresis in determining the quantitative content of mangiferin in the grass of species of the genus kopechnik (Hedysarum Caucasicum, M.Bieb., Hedysarum grandiflorum Pall., Hedysarum daghestanicum Rupr. ex Boiss.) flora of the North Caucasus. Dev. Regist. Med. 2021, 10, 90–96. [Google Scholar] [CrossRef]
- Kocupij, O.V.; Lobanova, I.E. Phenolic compounds in the leaves and inflorescences of Hedysarum alpinum L. and H. Flavescens Regel et Schmalh., introduced into the forest-steppe zone of Western Siberia. Chem. Plant Raw Mater. 2022, 1, 203–212. (In Russian) [Google Scholar] [CrossRef]
- Imachueva, D.R.; Serebryanaya, F.K.; Machs, E.M.; Koceruba, V.V. Using sequencing methods to identify species using the example of phylogenetic relationships within the genus Hedysarum L. Pharm. Pharmacol. 2021, 9, 506–518. (In Russian) [Google Scholar] [CrossRef]
- Huang, J.; She, Y.; Yue, J.; Chen, Y.; Li, Y.; Li, J.; Hu, Y.; Yang, D.; Chen, J.; Yang, L.; et al. Exploring the catalytic function and active sites of a novel C-glycosyltransferase from Anemarrhena asphodeloides. Synth. Syst. Biotechnol. 2022, 7, 621–630. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liu, Y.; Yan, Y.M.; Lu, X.F.; Cheng, Y.X. Phenolic Compounds from Belamcanda chinensis Seeds. Molecules 2018, 23, 580. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tang, D.; Zhang, N.; Li, Y.; Zhang, C.; Li, L.; Li, M. Phytochemicals and biological studies of plants in genus Hedysarum. Chem. Cent. J. 2013, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Matypov, B.D.; Sambueva, Z.G.; Nikolaeva, G.G. Choleretic activity of Hedysarum Alpinum L. extract in intact rats. Bull. Buryat State Univ. Med. Pharm. 2022, 2, 9–13. (In Russian) [Google Scholar] [CrossRef]
- Samadarsi, R.; Augustin, L.; Kumar, C.; Dutta, D. In-silico and in-vitro studies on the efficacy of mangiferin against colorectal cancer. BMC Chem. 2022, 16, 42. [Google Scholar] [CrossRef]
- Olusola, A.J.; Famuyiwa, S.O.; Faloye, K.O.; Olatunji, O.E.; Olayemi, U.I.; Adeyemi, A.A.; Balogun, J.O.; Ogundele, S.B.; Babamuyiwa, B.O.; Patil, R.B. Neomangiferin, a Naturally Occurring Mangiferin Congener, Inhibits Sodium-Glucose Co-transporter-2: An In silico Approach. Bioinform. Biol. Insights 2024, 18, 11779322231223851. [Google Scholar] [CrossRef]
- Amraoui, A.; Djerrou, Z.; Ali Haimoud, S.; Zerouki, K.; Elmokli, S. Antihyperlipidemic and antioxidant potential of Olea europaea L. leaves: An experimental study in vivo, in vitro and in silico. Foods Raw Mater. 2025, 13, 35–45. [Google Scholar] [CrossRef]
- Faskhutdinova, E.R.; Sukhikh, A.S.; Le, V.M.; Minina, V.I.; Khelef, M.E.A.; Loseva, A.I. Effects of bioactive substances isolated from Siberian medicinal plants on the lifespan of Caenorhabditis elegans. Foods Raw Mater. 2022, 10, 340–352. [Google Scholar] [CrossRef]
- Vasilyev, P.M.; Luzina, O.A.; Babkov, D.A.; Appazova, D.T.; Salakhutdinov, N.F.; Spasov, A.A. Studying Dependences Between the Chemotype Structure of Some Natural Compounds and the Spectrum of Their Targeted Activities Correlated with the Hypoglycemic Effect. J. Struct. Chem. 2019, 60, 1827–1832. [Google Scholar] [CrossRef]
- Vasil’ev, P.M.; Kochetkov, A.N. Certificate of state registration of the computer program. IT “Microcosm” RU. No. 2011618547. 31 October 2011. [Google Scholar]
- Vassiliev, P.M.; Maltsev, D.V.; Spasov, A.A.; Perfilev, M.A.; Skripka, M.O.; Kochetkov, A.N. Consensus Ensemble Multitarget Neural Network Model of Anxiolytic Activity of Chemical Compounds and Its Use for Multitarget Pharmacophore Design. Pharmaceuticals 2023, 16, 731. [Google Scholar] [CrossRef]
- Open Targets. Available online: https://www.opentargets.org (accessed on 29 May 2025).
- Kubinyi, H. Similarity and Dissimilarity—A Medicinal Chemist’s View. Perspect. Drug Discov. Des 1998, 9, 225252. [Google Scholar] [CrossRef]
- UniProt (Switzerland, UK, USA). Available online: https://www.uniprot.org/uniprotkb/ (accessed on 29 May 2025).
- PDBe: Protein Data Bank in Europe. Available online: https://www.ebi.ac.uk/pdbe/ (accessed on 26 May 2025).
- Chem 3D Ultra. Available online: https://software.stanford.edu/software/chem3d-ultra (accessed on 25 September 2025).
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- MarvinSketch. Available online: https://chemaxon.com/marvin (accessed on 19 May 2025).
- MOPAC. Available online: http://openmopac.net (accessed on 20 May 2025).
- PyRx-Virtual Screening Tool. Available online: https://sourceforge.net/projects/pyrx/ (accessed on 20 May 2025).
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Inte:ligand. Available online: http://www.inteligand.com/ (accessed on 20 May 2025).
- Kainrad, T.; Hunold, S.; Seidel, T.; Langer, T. LigandScout Remote: A New User-Friendly Interface for HPC and Cloud Resources. J. Chem. Inf. Model. 2019, 59, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.N. (Ed.) Guidelines for Conducting Preclinical Studies of Medicines; Part 1; Grif and Ko: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medcina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef]
- Rabbani, S.I.; Devi, K.; Khanam, S. Protective role of glibenclamide against nicotinamide-streptozotocin induced nuclear damage in diabetic Wistar rats. J. Pharmacol. Pharmacother. 2010, 1, 18–23. [Google Scholar] [CrossRef]
- Nava-Molina, L.; Uchida-Fuentes, T.; Ramos-Tovar, H.; Fregoso-Padilla, M.; Rodríguez-Monroy, M.A.; Vega, A.V.; Navarrete-Vázquez, G.; Andrade-Jorge, E.; Villalobos-Molina, R.; Ortiz-Ortega, R.; et al. Novel CB1 receptor antagonist BAR-1 modifies pancreatic islet function and clinical parameters in prediabetic and diabetic mice. Nutr. Diabetes 2020, 10, 7. [Google Scholar] [CrossRef]
- Yoshimura, A.; Yamaguchi, T.; Kugita, M.; Kumamoto, K.; Shiogama, K.; Ogitsu, N.; Yoneda, M.; Miura, T.; Nagamura, Y.; Nagao, S. High Levels of Dietary Lard or Sucrose May Aggravate Lysosomal Renal Injury in Non-Obese, Streptozotocin-Injected CD-1 Mice Provided Isocaloric Diets. J. Nutr. Sci. Vitaminol. 2021, 67, 243–248. [Google Scholar] [CrossRef]
- Song, L.; Feng, S.; Yu, H.; Shi, S. Dexmedetomidine Protects Against Kidney Fibrosis in Diabetic Mice by Targeting miR-101-3p-Mediated EndMT. Dose-Response A Publ. Int. Hormesis Soc. 2022, 20, 15593258221083486. [Google Scholar] [CrossRef]
- Glushanko, V.S. Fundamentals of Medical Statistics; VGMU: Vitebsk, Belarus, 2012. (In Russian) [Google Scholar]
- GOST R ISO 16269-4-2017; Statistical Presentation of Data. Part 4: Identification and Treatment of Emissions. Standartinform: Moscow, Russia, 2017. Available online: https://docs.cntd.ru/document/1200146680 (accessed on 23 September 2025).
- Kuranov, S.; Luzina, O.; Khvostov, M.; Baev, D.; Kuznetsova, D.; Zhukova, N.; Vassiliev, P.; Kochetkov, A.; Tolstikova, T.; Salakhutdinov, N. Bornyl Derivatives of p-(Benzyloxy)Phenylpropionic Acid: In Vivo Evaluation of Antidiabetic Activity. Pharmaceuticals 2020, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An effective polyphenol in alleviating diabetes and diabetic complications. Crit. Rev. Food Sci. Nutr. 2022, 63, 9163–9186. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Hamid, H.K.; Obaid, M.A. Role of Quercetin Flavonoid as Antidiabetic: A Review. Int. J. Drug Deliv. Technol. 2021, 11, 1495–1500. Available online: https://impactfactor.org/PDF/IJDDT/11/IJDDT,Vol11,Issue4,Article65.pdf (accessed on 25 September 2025).
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Future J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef]
- Niture, N.T.; Ansari, A.A.; Naik, S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014, 52, 720–727. [Google Scholar]
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.X.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Zhou, L.; Zhong, Y.; Li, C.; Zhou, Y.; Liu, X.; Li, L.; Zou, Z.; Zhong, Z.; Ye, J. MAPK14 as a key gene for regulating inflammatory response and macrophage M1 polarization induced by ferroptotic keratinocyte in psoriasis. Inflammation 2024, 47, 1564–1584. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, H.; Liu, Y.; Cheng, L.; Wang, B.; Tian, X.; Fu, H.; Wu, C.; Li, Z.; Shen, C.; et al. Biased allosteric activation of ketone body receptor HCAR2 suppresses inflammation. Mol. Cell 2023, 83, 3171–3187. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, H.; Xing, C.; Zhang, L.; Zhu, H.; Deng, Z.; Yin, L.; Dong, E.; Wang, C.; Peng, H. Identification and validation of CALCRL-associated prognostic genes in acute myeloid leukemia. Gene 2022, 809, 146009. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, V.N.; Morozova, M.A.; Syroeshkin, A.V. Bioactive derivatives of xanthone C-glycosides—The QSAR approach. Dev. Regist. Med. 2023, 12, 21–33. (In Russian) [Google Scholar] [CrossRef]
- Chang, G.; Tian, S.; Luo, X.; Xiang, Y.; Cai, C.; Zhu, R.; Cai, H.; Yang, H.; Gao, H. Hypoglycemic Effects and Mechanisms of Polyphenols from Myrica rubra Pomace in Type 2 Diabetes (db/db) Mice. Mol. Nutr. Food Res. 2025, 69, e202400523. [Google Scholar] [CrossRef] [PubMed]
- Orlando, F.A.; Mainous, A.G. Editorial: Inflammation and chronic disease. Front. Med. 2024, 11, 1434533. [Google Scholar] [CrossRef]
- Khan, H.; Naseem, T.; Kaushik, P.; Narang, J.; Khan, R.; Panwar, S.; Parvez, S. Decoding paradoxical links of cytokine markers in cognition: Cross talk between physiology, inflammaging, and Alzheimer’s disease- related cognitive decline. Ageing Res. Rev. 2024, 101, 102535. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Ma, Y.; Jiang, N.; Wang, L.; Wang, B.; Niu, W.; Hu, Y.; Lin, Q.; Yu, B. Mangiferin Relieves Lipopolysaccharide-Induced Injury by Up-Regulating miR-181a via Targeting PTEN in ATDC5 Cells. Front. Pharmacol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Russell, W.M.N.; Bunch, R.L. The principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Bidzhieva, F.A. Biochemical features of alloxan-induced diabetes mellitus. Med. Alph. 2018, 2, 12–14. (In Russian) [Google Scholar]
- Cherkasova, O.P.; Kuznetsova, N.V.; Pal’chikova, N.A.; Selyatitskaya, V.G. Activity of the adrenocortical system in rats with experimental diabetes. Diabetes Mellit. 2011, 14, 37–40. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Volkhina, I.V.; Butolin, E.G. Oxidative stress and changes in liver sialoglycoconjugate metabolic parameters in rats with alloxanic diabetes mellitus. Diabetes Mellit. 2022, 25, 249–255. (In Russian) [Google Scholar] [CrossRef]
- Shaheen, A.; Akram, S.; Sharif, S.; Rashid, A.; Adnan, A.; Mushtaq, M. Fractionation of Xanthium strumarium L. foliage phenolics, in-vitro antioxidant activities, and in-vivo anti-diabetic potential. Front. Chem. 2023, 11, 1279729. [Google Scholar] [CrossRef] [PubMed]
- Amare, Y.E.; Dires, K.; Asfaw, T. Antidiabetic Activity of Mung Bean or Vigna radiata (L.) Wilczek Seeds in Alloxan-Induced Diabetic Mice. Evid. -Based Complement. Altern. Med. Ecam 2022, 2022, 6990263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, F.; Wang, L.; Du, J.; Li, Y. Plant polyphenols delay aging: A review of their anti-aging mechanisms and bioavailability. Food Res. Int. 2025, 218, 116900. [Google Scholar] [CrossRef] [PubMed]
- Wenzhong, D.; Chengcheng, R.; Yumeng, Z.; Jiejie, W.; Jing, H.; Zihan, S.; Yue, S.; Jin, L. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Carlos Espín, J.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Suman, R.K.; Borde, M.K.; Mohanty, I.R.; Singh, H.K. Mechanism of Action of Natural Dipeptidyl Peptidase-IV Inhibitors (Berberine and Mangiferin) in Experimentally Induced Diabetes with Metabolic Syndrome. Int. J. Appl. Basic Med. Res. 2023, 13, 133–142. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, J.; Wang, Q.; Li, X.; Wei, H. Improvement in the solubility of mangiferin by HP-β-CD inclusion. Chin. Tradit. Pat. Med. 2008, 30, 1123–1126. [Google Scholar]
- Han, D.; Chen, C.; Zhang, C.; Zhang, Y.; Tang, X. Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. J. Pharm. Biomed. Anal. 2010, 51, 260–263. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Zhang, C.; Zhang, B. Gelucire44/14 as a novel absorption enhancer for drugs with different hydrophilicities: In vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 2011, 100, 3186–3196. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Sun, L.; Tong, L.; Zhang, T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014, 93, 54–61. [Google Scholar] [CrossRef]
- Leo, A.J.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Feng, M.; Wei, S.; Zhang, S.; Yang, Y. Anti-Inflammation and Anti-Pyroptosis Activities of Mangiferin via Suppressing NF-κB/NLRP3/GSDMD Signaling Cascades. Int. J. Mol. Sci. 2022, 23, 10124. [Google Scholar] [CrossRef]
- Melo-Betances, E.; Rodríguez-Bautista, C.C.; Núñez-Sellés, A.J. Synthesis of mangiferin derivatives, complexes, and carriers as potential therapeutic candidates for cancer treatment: An update. Front. Pharmacol. 2025, 16, 1598719. [Google Scholar] [CrossRef]
- Alharbi, H.M.; Alqahtani, T.; Alamri, A.H.; Kumarasamy, V.; Subramaniyan, V.; Babu, K.S. Nanotechnological synergy of mangiferin and curcumin in modulating PI3K/Akt/mTOR pathway: A novel front in ovarian cancer precision therapeutics. Front. Pharmacol. 2024, 14, 1276209. [Google Scholar] [CrossRef] [PubMed]



| Group Number | Number of Animals | Model | Injected Substance | |||
|---|---|---|---|---|---|---|
| Water, mL | Mangiferin, mg/kg | Diclofenac Sodium, mg/kg | GLB, mg/kg | |||
| 1 | 5 | A | 1.0 | - | - | - |
| 2 | 5 | A | - | 50.0 | - | - |
| 3 | 5 | A | - | 100.0 | - | - |
| 4 | 5 | A | - | - | 10.0 | - |
| 5 | 5 | B | 1.0 | - | - | - |
| 6 | 5 | B | - | 50.0 | - | - |
| 7 | 5 | B | - | 100.0 | - | - |
| 8 | 5 | B | - | - | 10.0 | - |
| 9 | 10 | - | - | - | - | - |
| 10 | 10 | C | 1.0 | - | - | - |
| 11 | 10 | C | - | 50.0 | - | - |
| 12 | 10 | C | - | 100.0 | - | - |
| 13 | 10 | C | - | - | - | 5.0 |
| 14 | 5 | - | - | - | - | - |
| 15 | 8 | D | 1.0 | - | - | - |
| 16 | 8 | D | - | 50.0 | - | - |
| 17 | 8 | D | - | 100.0 | - | - |
| Target | Gene 1 | Medium group | Mangiferin | Quercetin | Rutin | ||||
|---|---|---|---|---|---|---|---|---|---|
| T | Ind | T | Ind | T | Ind | T | Ind | ||
| Hypoxia-inducible factor prolyl hydroxylase 1 | EGLN2 | 0.26 | 3.80 | 0.24 | 4.8 ** | 0.27 | 3.1 | 0.27 | 3.5 |
| Mitogen-activated protein kinase 14 | MAPK14 | 0.55 | 3.57 | 0.39 | 2.3 ** | 0.68 | 3.7 | 0.59 | 4.7 |
| Basic fibroblast growth factor | FGF2 | 0.18 | 3.43 | 0.17 | 2.4 ** | 0.15 | 3.6 | 0.23 | 4.3 |
| Mitogen-activated protein kinase 10 | MAPK10 | 0.52 | 3.37 | 0.35 | 1.5 ** | 0.64 | 4.3 | 0.57 | 4.3 |
| Serine/threonine-protein kinase atr | ATR | 0.28 | 3.37 | 0.30 | 3.7 * | 0.29 | 2.8 | 0.26 | 3.6 |
| Hydroxycarboxylic acid receptor 2 | HCAR2 | 0.28 | 3.37 | 0.30 | 3.7 * | 0.29 | 2.8 | 0.26 | 3.6 |
| Fatty acid binding protein adipocyte | FABP4 | 0.24 | 3.30 | 0.26 | 3.6 | 0.24 | 3.0 | 0.21 | 3.3 |
| Calcitonin gene-related peptide type 1 receptor | CALCRL | 0.32 | 2.70 | 0.35 | −0.7 ** | 0.29 | 4.4 | 0.32 | 4.4 |
| Biotarget 1 | Name 2 | PDBe 3 | Code 4 | Key Amino Acids of the Site |
|---|---|---|---|---|
| EGLN2 | Prolyl hydroxylase EGLN2 | 1 | 5v1b | TYR287, TYR294, HIS297, ILE311, TYR313, ASN315, HIS358, VAL360, ARG367, ALA369 |
| MAPK14 | Mitogen-activated protein kinase 14 | 245 | 2bal | ALA51, LYS53, LEU104, THR106, HIS107, LEU108, MET109, GLY110, ALA111 |
| FGF2 | Fibroblast growth factor 2 | 22 | 2fgf | LYS26, ASN27, ASN101, LYS119, ARG120, LYS125, GLN134, LYS135, ALA136 |
| MAPK10 | Mitogen-activated protein kinase 10 | 60 | 2o0u | ILE70, VAL78, ALA91, LYS93, MET146, GLU147, LEU148, MET149, ASP150, ALA151, ASN152, GLN155, VAL196, LEU206 |
| ATR | Serine/threonine-protein kinase ATR | 1 | 5yz0 | LYS2308, ILE2377, TRP2379, VAL2380, ASN2381, THR2383, PRO2388, ASN2480, ILE2481, VAL2493, ASP2494 |
| HCAR2 | Hydroxycarboxylic acid receptor 2 | 13 | 7xk2 | LEU83, LEU104, LEU107, ALA108, ARG111, GLN112, LEU158, LEU162, SER179, PHE180, HIS189, MET192, PHE193, PHE277, LEU280, TYR284 |
| FABP4 | Fatty acid-binding protein, adipocyte | 240 | 2hnx | PHE16, TYR19, MET20, VAL25, ALA33, ALA36, PRO38, SER53, PHE57, ALA75, ILE104, VAL115 ARG108, TYR128, ARG130 |
| CALCRL | Calcitonin gene-related peptide type 1 receptor | 23 | 8ax7 | ASP1071, TRP1074, TRP1084, ARG2038, ASP2070, GLY2071, TRP2072, TRP2121, THR2122, TYR2124 |
| Total | 605 |
| Compound | Docking Energy in Biotarget, kcal/mol | |||||||
|---|---|---|---|---|---|---|---|---|
| EGLN2 | MAPK14 | FGF2 | MAPK10 | ATR | HCAR2 | FABP4 | CALCRL | |
| Mangiferin | −8.8 | −8.9 | −5.6 | −10.0 | −7.3 | −10.1 | −7.4 | −10.1 |
| Quercetin | −9.2 | −8.8 | −5.5 | −9.3 | −7.0 | −9.1 | −8.7 | −8.4 |
| Rutin | −8.8 | −9.3 | −5.6 | −9.1 | −8.1 | −6.3 | −9.2 | −9.3 |
| Biotarget | Type of Binding | Number of Bonds | ||
|---|---|---|---|---|
| Mangiferin | Quercetin | Rutin | ||
| MAPK10 | HD | 4 | 0 | 7 |
| HA | 1 | 1 | 6 | |
| NS | 1 | 1 | 2 | |
| St | 0 | 0 | 0 | |
| HCAR2 | HD | 3 | 2 | — |
| HA | 0 | 3 | — | |
| NS | 1 | 1 | — | |
| St | 0 | 0 | — | |
| CALCRL | HD | 6 | — | 5 |
| HA | 2 | — | 3 | |
| NS | 1 | — | 2 | |
| St | 1 | — | 0 | |
| Compound | Assessment of the Activity Level by Strategy | |||
|---|---|---|---|---|
| Conservative | Normal | Risk | General | |
| Mangiferin | moderate | moderate | moderate | moderate |
| Quercetin | moderate | moderate | high | high? 1 |
| Rutin | moderate | moderate | low | moderate? |
| Group Number | Edema Size, μL | |
|---|---|---|
| After 3 h | After 4 h | |
| 1 | 480 ± 50 | 480 ± 20 |
| 2 | 600 ± 60 * | 600 ± 30 * |
| 3 | 610 ± 40 * | 740 ± 90 * |
| 4 | 340 ± 30 | 330 ± 40 |
| Group Number | Mass of Exudate, mg | Mass of Granulation Tissue, mg |
|---|---|---|
| 5 | 138.3 ± 10.9 | 40.6 ± 3.3 |
| 6 | 149.2 ± 13.8 * | 43.7 ± 3.4 * |
| 7 | 144.9 ± 9.7 * | 38.1 ± 2.7 * |
| 8 | 84.1 ± 5.3 | 22.9 ± 1.3 |
| Time Point | Animal Group Number | ||||
|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | |
| 0 | 242 ± 4 | 243 ± 1 | 243 ± 1 | 243 ± 1 | 243 ± 1 |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| 1 | 243 ± 4 | 246 ± 4 | 243 ± 4 | 243 ± 3 | 247 ± 3 |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| 2 | 247 ± 4 | 248 ± 2 | 246 ± 4 | 246 ± 4 | 248 ± 5 |
| (n = 10) | (n = 9) | (n = 9) | (n = 8) | (n = 9) | |
| 3 | 249 ± 3 | 251 ± 4 | 248 ± 4 | 249 ± 3 | 248 ± 4 |
| (n = 10) | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| 4 | 258 ± 3 | 260 ± 4 | 258 ± 5 | 257 ± 2 | 257 ± 4 |
| (n = 10) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Time Point | Animal Group Number | ||||
|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | |
| Glucose, mmol/L | |||||
| 0 | 5.26 ± 0.37 | 5.25 ± 0.16 | 5.25 ± 0.16 | 5.25 ± 0.16 | 5.25 ± 0.16 |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| 1 | 4.88 ± 0.30 | 22.62 ± 0.79 | 22.57 ± 0.52 | 21.67 ± 1.21 | 21.48 ± 0.62 |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| 2 | 4.34 ± 0.23 | 24.46 ± 0.70 | 25.36 ±0.79 | 25.91 ± 0.91 | 25.13 ± 0.58 |
| (n = 10) | (n = 9) | (n = 9) | (n = 8) | (n = 9) | |
| 3 | 4.53 ± 0.29 | 23.37 ± 0.72 | 23.70 ± 0.68 | 23.26 ± 0.57 | 22.86 ±0.32 |
| (n = 10) | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| 4 | 4.37 ± 0.38 | 20.09 ± 0.40 | 20.44 ± 0.28 * | 21.12 ± 0.43* | 13.02 ± 0.58 |
| (n = 10) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Cholesterol, mmol/L | |||||
| 0 | 1.63 ± 0.06 | 1.62 ± 0.06 | 1.63 ± 0.06 | 1.63 ± 0.06 | 1.62 ± 0.06 |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| 2 | 1.61 ±0.06 | 2.59 ± 0.09 | 2.62 ± 0.13 * | 2.61 ± 0.07 * | 2.58 ±0.04 |
| (n = 10) | (n = 9) | (n = 9) | (n = 8) | (n = 9) | |
| 3 | 1.54 ± 0.09 | 2.58 ± 0.07 | 2.46 ± 0.07 * | 2.53 ± 0.11 * | 2.55 ± 0.07 |
| (n = 10) | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| 4 | 1.58 ± 0.07 | 2.44 ±0.07 | 2.46 ± 0.10 * | 2.43 ± 0.14 * | 1.93 ± 0.04 |
| (n = 10) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Indicator, Units | Groups of Animals | |||
|---|---|---|---|---|
| 14 | 15 | 16 | 17 | |
| Cholesterol, mmol/l | 1.921 ± 0.199 | 9.923 ± 1.274 | 10.706 ± 1.023 * | 10.626 ± 1.489 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesnina, A.; Le, V.; Ivanova, S.; Prosekov, A. Antidiabetic Potential of Mangiferin: An In Silico and In Vivo Approach. Pharmaceutics 2025, 17, 1262. https://doi.org/10.3390/pharmaceutics17101262
Vesnina A, Le V, Ivanova S, Prosekov A. Antidiabetic Potential of Mangiferin: An In Silico and In Vivo Approach. Pharmaceutics. 2025; 17(10):1262. https://doi.org/10.3390/pharmaceutics17101262
Chicago/Turabian StyleVesnina, Anna, Violeta Le, Svetlana Ivanova, and Alexander Prosekov. 2025. "Antidiabetic Potential of Mangiferin: An In Silico and In Vivo Approach" Pharmaceutics 17, no. 10: 1262. https://doi.org/10.3390/pharmaceutics17101262
APA StyleVesnina, A., Le, V., Ivanova, S., & Prosekov, A. (2025). Antidiabetic Potential of Mangiferin: An In Silico and In Vivo Approach. Pharmaceutics, 17(10), 1262. https://doi.org/10.3390/pharmaceutics17101262








