Lysosomal Drug Sequestration Mediated by ABC Transporters and Drug Resistance
Abstract
1. Introduction
2. Material and Methods
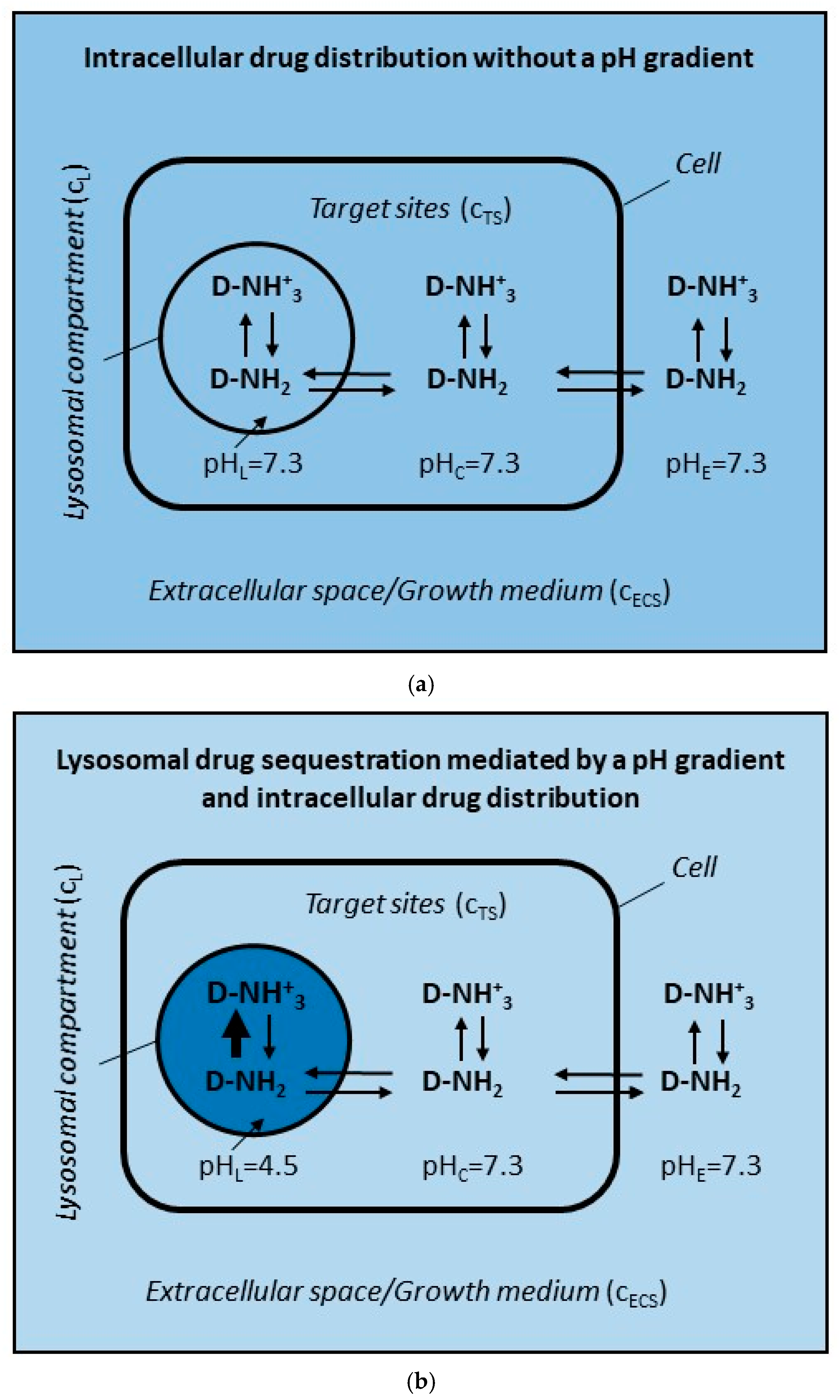
2.1. ABC Transporter in the Lysosomal Membrane Forms Drug Concentration Gradient Between Lysosomes and Target Sites
- (a)
- The volume of the drug is negligible and does not affect the total volume of the system (VT);
- (b)
- Drug is uncharged molecule that can pass freely through the cell membranes;
- (c)
- Only two interactions of the drug are considered: (i) the passive diffusion of uncharged molecules <=> and (ii) active transport mediated by ABC transporter (Figure 2a,b);
- (d)
- Unless otherwise stated, the number of cells is constant (total number of cells: 5 × 106; cell density: 0.5 × 106/mL);
- (e)
- Cells have an ideal spherical shape with an average diameter of 18 μm;
- (f)
- Cells contain one compartment—lysosomes, which volume represents 5% of the total cell volume (unless otherwise stated);
- (g)
- The volume of cells and lysosomes does not change with drug accumulation.
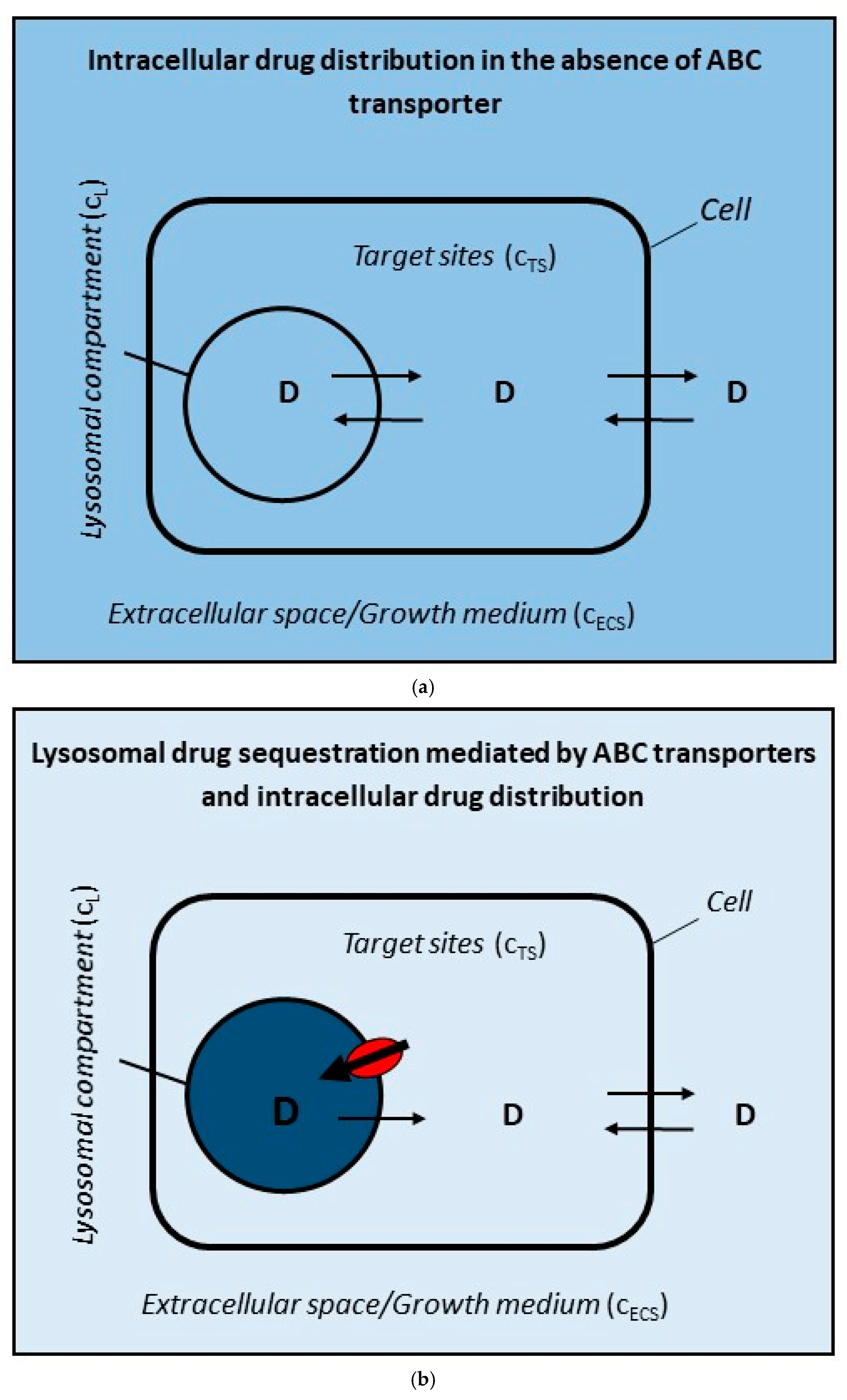
2.2. ABC Transporter in the Cell Membrane Forms Drug Concentration Gradient Between Extracellular Space and Target Sites
- (a)
- The volume of the drug is negligible and does not affect the total volume of the system (VT);
- (b)
- Drug is uncharged molecule that can pass freely through the cell membranes;
- (c)
- Only two interactions of the drug are considered: (i) the passive diffusion of uncharged molecules <=> and (ii) active transport mediated by ABC transporter (Figure 2a,c);
- (d)
- Unless otherwise stated, the number of cells is constant (total number of cells: 5 × 106; cell density: 0.5 × 106/mL);
- (e)
- Cells have an ideal spherical shape with an average diameter of 18 μm;
- (f)
- Cells contain no compartments (therefore, the total volume of cells = volume of the target sites).
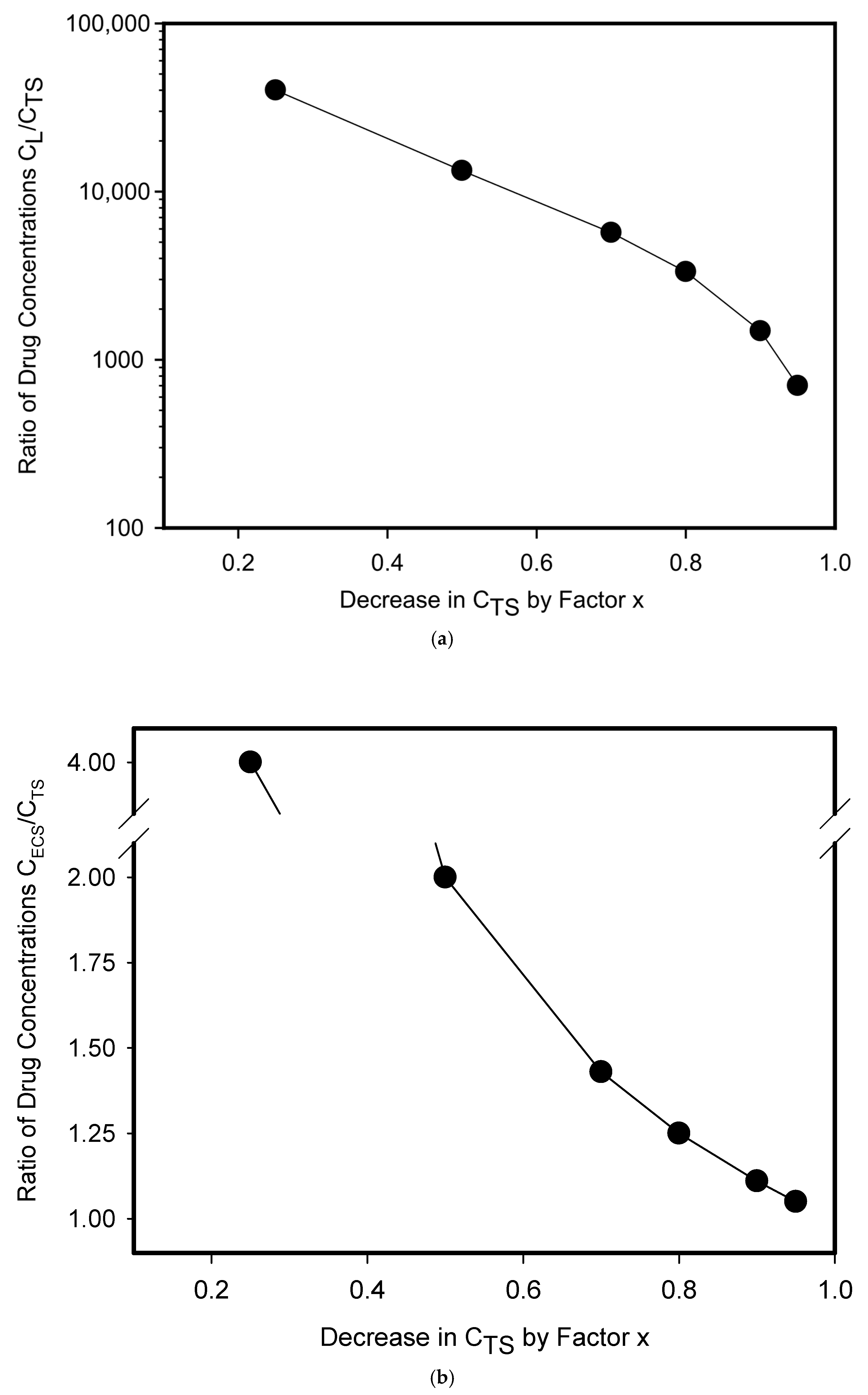
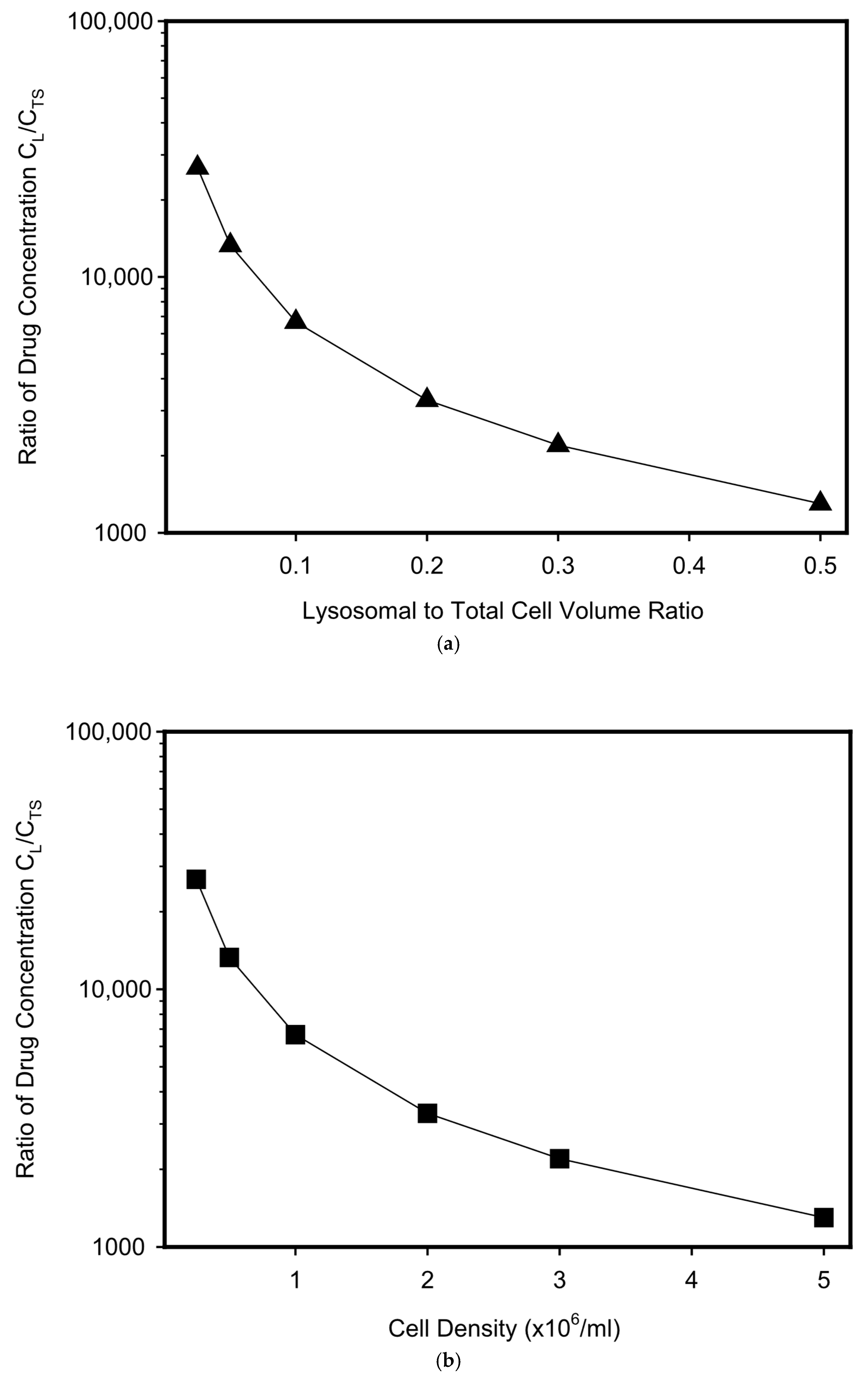
3. Results and Discussion

Funding
Data Availability Statement
Conflicts of Interest
References
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Fox, K.; Lee, P.; Yang, Y.D.; Ling, V. Functional intracellular P-glycoprotein. Int. J. Cancer 1998, 76, 857–864. [Google Scholar] [CrossRef]
- Ferrao, P.; Sincock, P.; Cole, S.; Ashman, L. Intracellular P-gp contributes to functional drug efflux and resistance in acute myeloid leukaemia. Leuk. Res. 2001, 25, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Sahni, S.; Sharp, D.M.; Arvind, A.; Jansson, P.J.; Richardson, D.R. P-glycoprotein mediates drug resistance via a novel mechanism involving lysosomal sequestration. J. Biol. Chem. 2013, 288, 31761–31771. [Google Scholar] [CrossRef]
- Rajagopal, A.; Simon, S.M. Subcellular localization and activity of multidrug resistance proteins. Mol. Biol. Cell 2003, 14, 3389–3399. [Google Scholar] [CrossRef]
- Mlejnek, P.; Havlasek, J.; Pastvova, N.; Dolezel, P. Can image analysis provide evidence that lysosomal sequestration mediates daunorubicin resistance? Chem. Biol. Interact. 2020, 327, 109138. [Google Scholar] [CrossRef]
- Gigli, M.; Doglia, S.M.; Millot, J.M.; Valentini, L.; Manfait, M. Quantitative study of doxorubicin in living cell nuclei by microspectrofluorometry. Biochim. Biophys. Acta 1988, 950, 13–20. [Google Scholar] [CrossRef]
- Krumpochova, P.; Kocurova, A.; Dolezel, P.; Mlejnek, P. Assay for determination of daunorubicin in cancer cells with multidrug resistance phenotype. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1875–1880. [Google Scholar] [CrossRef]
- Katayama, K.; Kapoor, K.; Ohnuma, S.; Patel, A.; Swaim, W.; Ambudkar, I.S.; Ambudkar, S.V. Revealing the fate of cell surface human P-glycoprotein (ABCB1): The lysosomal degradation pathway. Biochim. Biophys. Acta 2015, 1853, 2361–2370. [Google Scholar] [CrossRef]
- Szakacs, G.; Abele, R. An inventory of lysosomal ABC transporters. FEBS Lett. 2020, 594, 3965–3985. [Google Scholar] [CrossRef]
- Laing, N.M.; Belinsky, M.G.; Kruh, G.D.; Bell, D.W.; Boyd, J.T.; Barone, L.; Testa, J.R.; Tew, K.D. Amplification of the ATP-binding cassette 2 transporter gene is functionally linked with enhanced efflux of estramustine in ovarian carcinoma cells. Cancer Res. 1998, 58, 1332–1337. [Google Scholar]
- Boonstra, R.; Timmer-Bosscha, H.; van Echten-Arends, J.; van der Kolk, D.M.; van den Berg, A.; de Jong, B.; Tew, K.D.; Poppema, S.; de Vries, E.G. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 upregulation. Br. J. Cancer 2004, 90, 2411–2417. [Google Scholar] [CrossRef]
- Gotovdorj, T.; Lee, E.; Lim, Y.; Cha, E.J.; Kwon, D.; Hong, E.; Kim, Y.; Oh, M.Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cell-specific drug transporters with acquired cisplatin resistance in cisplatin sensitive cancer cells. J. Korean Med. Sci. 2014, 29, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Hose, C.D.; Monks, A.; Nagashima, K.; Han, B.; Newton, D.L.; Millione, A.; Shah, J.; Hollingshead, M.G.; Hite, K.M.; et al. Identification of CBX3 and ABCA5 as putative biomarkers for tumor stem cells in osteosarcoma. PLoS ONE 2012, 7, e41401. [Google Scholar] [CrossRef]
- Rakvács, Z.; Kucsma, N.; Gera, M.; Igriczi, B.; Kiss, K.; Barna, J.; Kovács, D.; Vellai, T.; Bencs, L.; Reisecker, J.M.; et al. The human ABCB6 protein is the functional homologue of HMT-1 proteins mediating cadmium detoxification. Cell. Mol. Life Sci. 2019, 76, 4131–4144. [Google Scholar] [CrossRef]
- Gong, J.P.; Yang, L.; Tang, J.W.; Sun, P.; Hu, Q.; Qin, J.W.; Xu, X.M.; Sun, B.C.; Tang, J.H. Overexpression of microRNA-24 increases the sensitivity to paclitaxel in drug-resistant breast carcinoma cell lines via targeting ABCB9. Oncol. Lett. 2016, 12, 3905–3911. [Google Scholar] [CrossRef]
- Moody, H.L.; Lind, M.J.; Maher, S.G. MicroRNA-31 Regulates Chemosensitivity in Malignant Pleural Mesothelioma. Mol. Ther. Nucleic Acids 2017, 8, 317–329. [Google Scholar] [CrossRef]
- Chapuy, B.; Koch, R.; Radunski, U.; Corsham, S.; Cheong, N.; Inagaki, N.; Ban, N.; Wenzel, D.; Reinhardt, D.; Zapf, A.; et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia 2008, 22, 1576–1586. [Google Scholar] [CrossRef]
- Chapuy, B.; Panse, M.; Radunski, U.; Koch, R.; Wenzel, D.; Inagaki, N.; Haase, D.; Truemper, L.; Wulf, G.G. ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematologica 2009, 94, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, J.; Mohandoss, P.; Patel, R.; Gillan, H.; Li, M.; Kumar, K.; Nguyen, D.; Weygant, N.; Qu, D.; Pitts, K.; et al. DCLK1 Regulates Tumor Stemness and Cisplatin Resistance in Non-small Cell Lung Cancer via ABCD-Member-4. Mol. Ther. Oncolytics. 2020, 18, 24–36. [Google Scholar] [CrossRef]
- Eckford, P.D.; Sharom, F.J. ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 2009, 109, 2989–3011. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell. Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Duvvuri, M.; Krise, J.P. Intracellular drug sequestration events associated with the emergence of multidrug resistance: A mechanistic review. Front. Biosci. 2005, 10, 1499–1509. [Google Scholar] [CrossRef]
- Groth-Pedersen, L.; Jäättelä, M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 2013, 332, 265–274. [Google Scholar] [CrossRef]
- Noel, G.; Peterson, C.; Trouet, A.; Tulkens, P. Uptake and subcellular localization of daunorubicin and adriamycin in cultured fibroblasts. Eur. J. Cancer 1978, 14, 363–368. [Google Scholar] [CrossRef]
- Loetchutinat, C.; Priebe, W.; Garnier-Suillerot, A. Drug sequestration in cytoplasmic organelles does not contribute to the diminished sensitivity of anthracyclines in multidrug resistant K562 cells. Eur. J. Biochem. 2001, 268, 4459–4467. [Google Scholar] [CrossRef]
- Burger, H.; den Dekker, A.T.; Segeletz, S.; Boersma, A.W.; de Bruijn, P.; Debiec-Rychter, M.; Taguchi, T.; Sleijfer, S.; Sparreboom, A.; Mathijssen, R.H.; et al. Lysosomal Sequestration Determines Intracellular Imatinib Levels. Mol. Pharmacol. 2015, 88, 477–487. [Google Scholar] [CrossRef]
- Ruzickova, E.; Skoupa, N.; Dolezel, P.; Smith, D.A.; Mlejnek, P. The Lysosomal Sequestration of Tyrosine Kinase Inhibitors and Drug Resistance. Biomolecules 2019, 9, 675. [Google Scholar] [CrossRef]
- Mlejnek, P. Lysosomal-mediated drug resistance—Fact or illusion? Pharmacol. Res. 2024, 199, 107025. [Google Scholar] [CrossRef]
- Mlejnek, P. What Is the Significance of Lysosomal-Mediated Resistance to Imatinib? Cells 2023, 12, 709. [Google Scholar] [CrossRef]
- MacIntyre, A.C.; Cutler, D.J. The potential role of lysosomes in tissue distribution of weak bases. Biopharm. Drug Dispos. 1988, 9, 513–526. [Google Scholar] [CrossRef]
- Lieberman, A.P.; Puertollano, R.; Raben, N.; Slaugenhaupt, S.; Walkley, S.U.; Ballabio, A. Autophagy in lysosomal storage disorders. Autophagy 2012, 8, 719–730. [Google Scholar] [CrossRef]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Mlejnek, P.; Havlasek, J.; Pastvova, N.; Dolezel, P.; Dostalova, K. Lysosomal sequestration of weak base drugs, lysosomal biogenesis, and cell cycle alteration. Biomed. Pharmacother. 2022, 153, 113328. [Google Scholar] [CrossRef]
| Transporter | Weak-Base Drugs | Hydrophobic Drugs | Other Drugs/Heavy Metals |
|---|---|---|---|
| ABCA2 | mitoxantrone * [16] | Estradiol *, estramustine [15] | Cisplatin * [17] |
| ABCA3 | daunorubicin *, mitoxantrone *, vincristine *, imatinib [22] | etoposide * [22] | - |
| ABCA5 | dasatinib * [18] | - | thiosemicarbazone derivative * [18] |
| ABCB1 † | daunorubicin, doxorubicin, vincristine, vinblastine, imatinib, gefitinib [25] | tacrolimus, colchicine, teniposide, podophyllotoxin, paclitaxel [25] | - |
| ABCB6 | - | - | cadmium [19] |
| ABCB9 | - | paclitaxel * [20] | - |
| ABCC1 † | anthracyclines, vincristine, vinblastine, imatinib, gefitinib [25] | flutamide, cochicine, teniposide, paclitaxel [25] | arsenate, arsenite [25] |
| ABCD4 | - | - | cisplatin * [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlejnek, P. Lysosomal Drug Sequestration Mediated by ABC Transporters and Drug Resistance. Pharmaceutics 2025, 17, 1255. https://doi.org/10.3390/pharmaceutics17101255
Mlejnek P. Lysosomal Drug Sequestration Mediated by ABC Transporters and Drug Resistance. Pharmaceutics. 2025; 17(10):1255. https://doi.org/10.3390/pharmaceutics17101255
Chicago/Turabian StyleMlejnek, Petr. 2025. "Lysosomal Drug Sequestration Mediated by ABC Transporters and Drug Resistance" Pharmaceutics 17, no. 10: 1255. https://doi.org/10.3390/pharmaceutics17101255
APA StyleMlejnek, P. (2025). Lysosomal Drug Sequestration Mediated by ABC Transporters and Drug Resistance. Pharmaceutics, 17(10), 1255. https://doi.org/10.3390/pharmaceutics17101255






