In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Atorvastatin: A Focus on Cervical and Head and Neck Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Lines
2.2. Cell Antiproliferative MTT Assay
2.3. Cell Cycle Analysis Using a Flow Cytometer
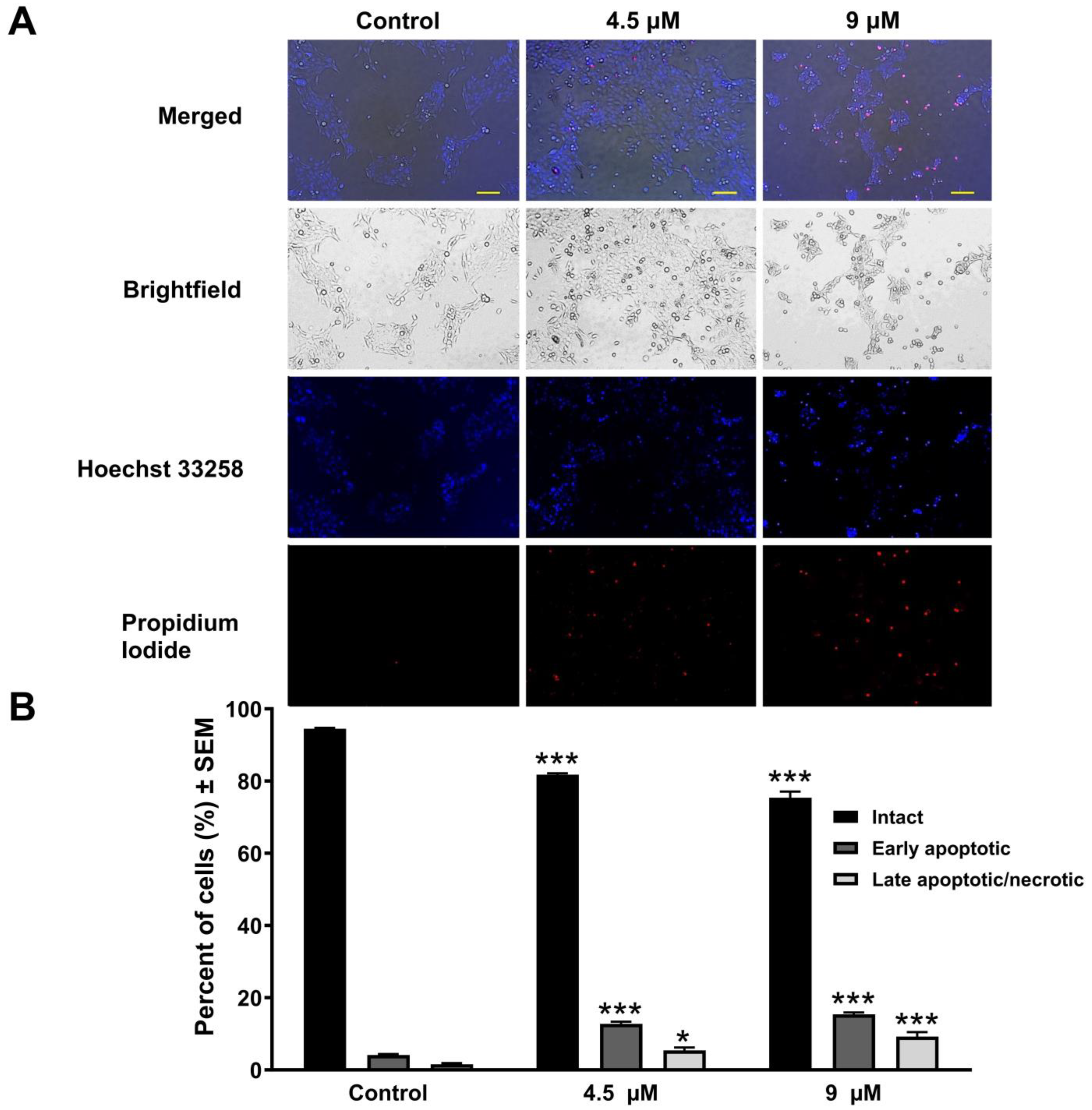
2.4. Hoechst 33258–Propidium Iodide Fluorescent Double Staining
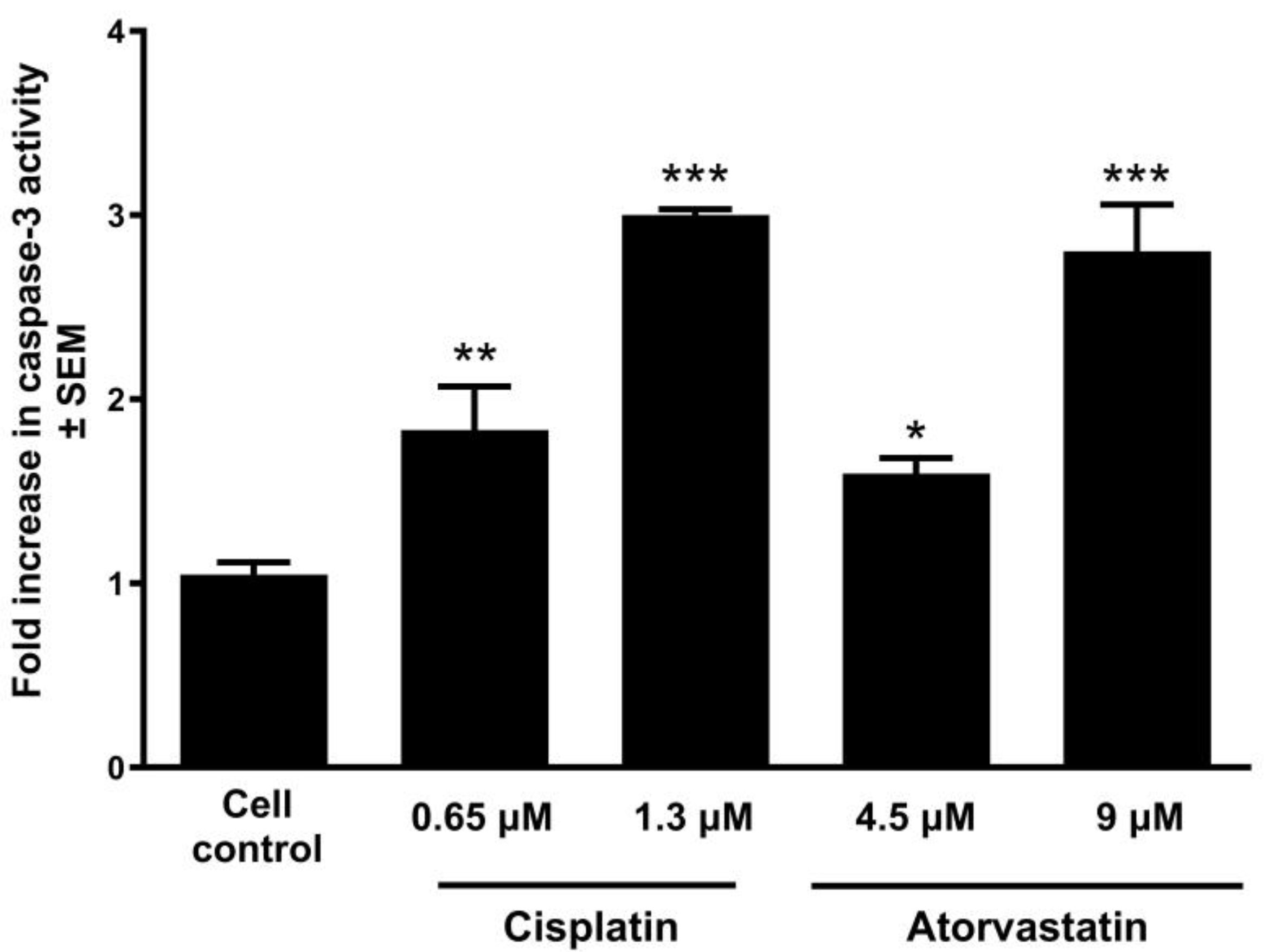
2.5. Determination of Caspase Activity
2.6. Measurement of Mitochondrial Membrane Potential
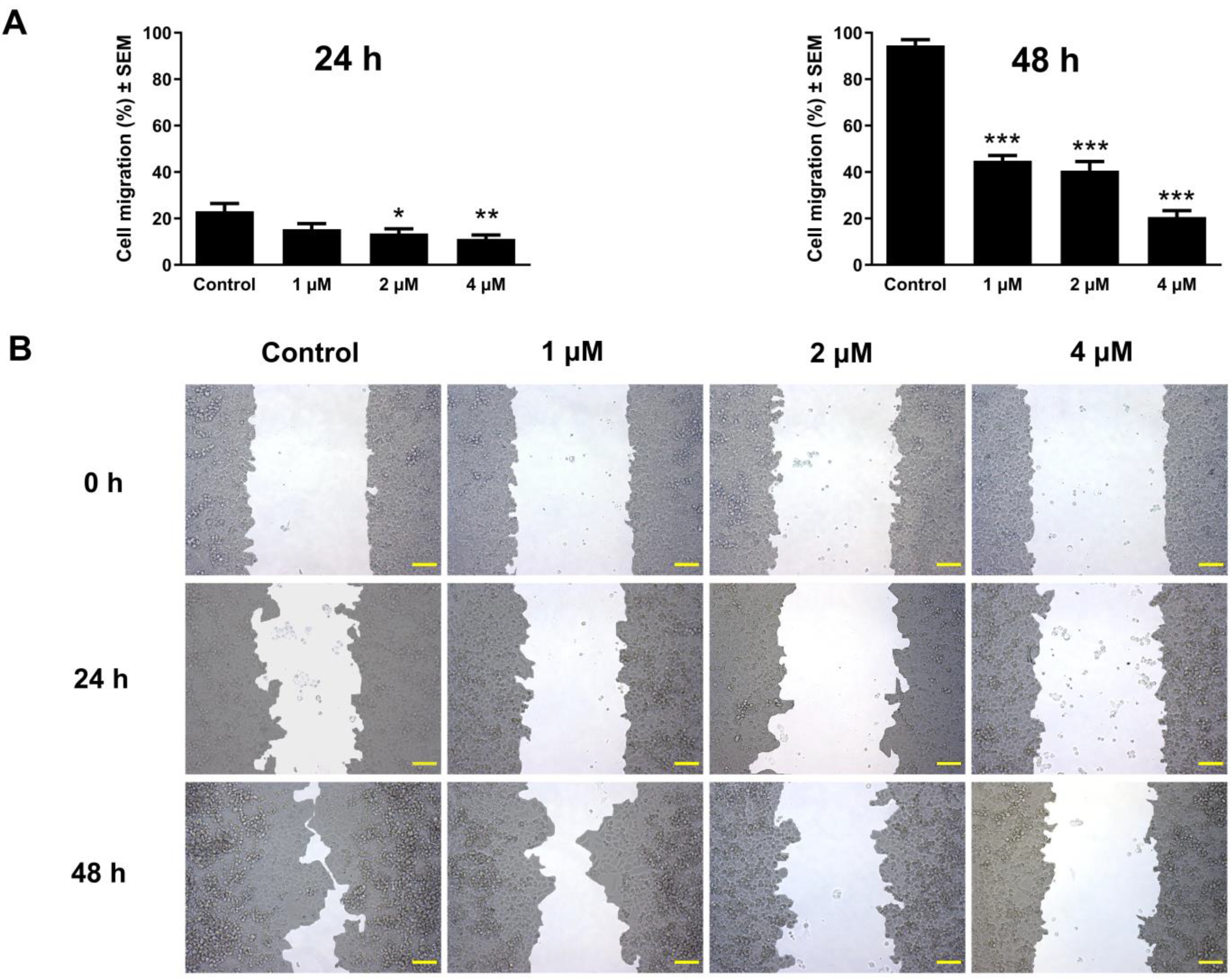
2.7. Antimigration Assay
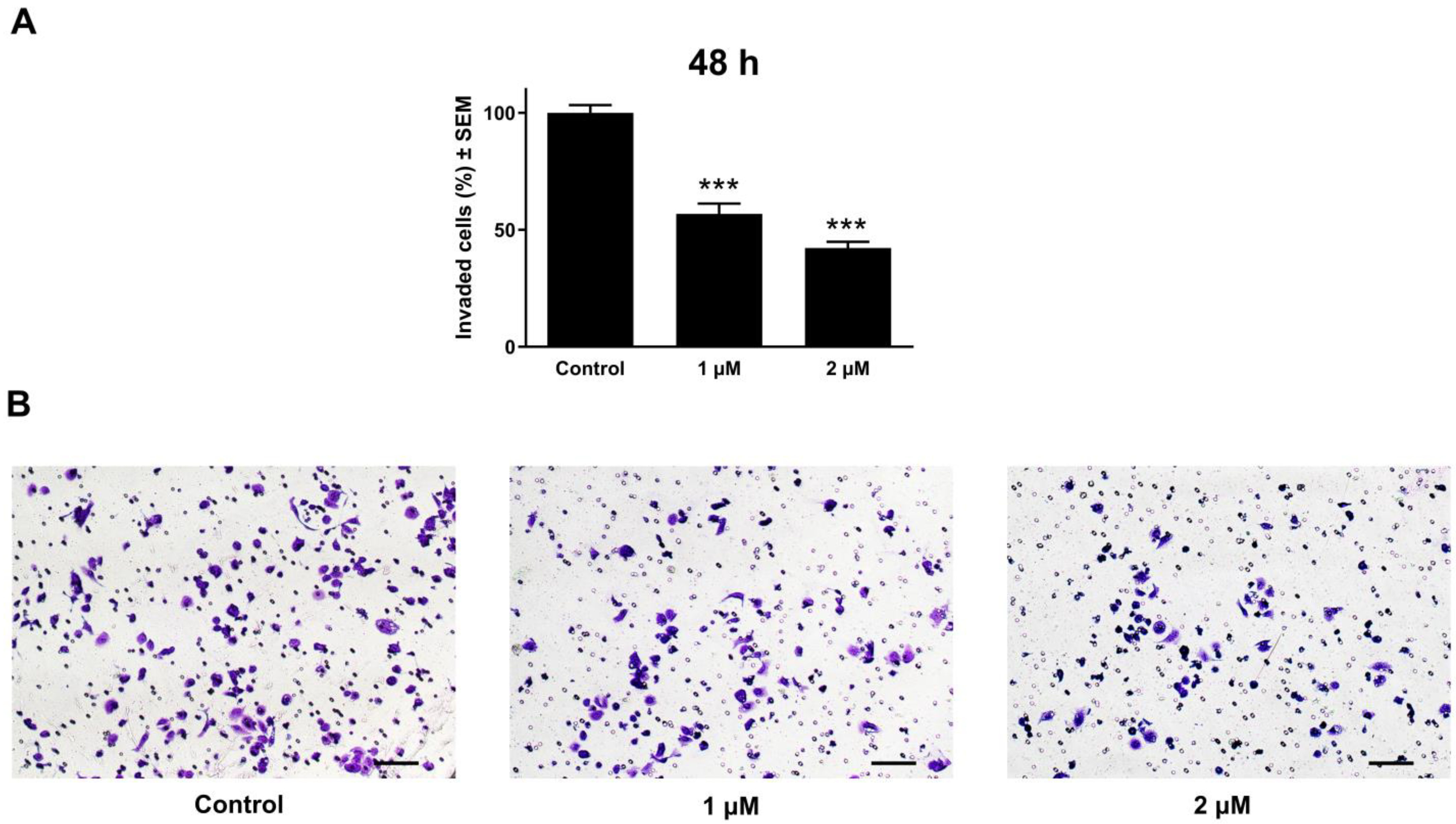
2.8. Anti-Invasion Assay
2.9. Statistical Analysis
3. Results
3.1. Atorvastatin Substantially Inhibited Cell Proliferation
3.2. Atorvastatin-Induced Cell Cycle Disturbances
3.3. Atorvastatin-Induced Cellular Apoptosis Visualized by Fluorescent Double Staining
3.4. Atorvastatin Promotes Apoptosis by Activating Caspase-3
3.5. Atorvastatin-Induced Mitochondrial Membrane Damage
3.6. Atorvastatin-Mediated Suppression of Cell Migration
3.7. Atorvastatin Demonstrated Substantial Anti-Invasive Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMG-CoA | 3-Hydroxy-3-methylglutaryl coenzyme A |
| EMT | Endothelial–mesenchymal transition |
| VEGF | Vascular endothelial growth factor |
| OSCC | Oral squamous cell carcinoma |
| DMSO | Dimethylsulfoxide |
| ECACC | European Collection of Authenticated Cell Cultures |
| ATCC | American Tissue Culture Collection |
| EMEM | Eagle’s Minimal Essential Medium |
| FBS | Fetal bovine serum |
| NEAA | Non-essential amino acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| ROS | Reactive oxygen species |
| MT1-MMP | Microglial membrane type 1 metalloproteinase |
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Kachalaki, S.; Ebrahimi, M.; Mohamed Khosroshahi, L.; Mohammadinejad, S.; Baradaran, B. Cancer Chemoresistance; Biochemical and Molecular Aspects: A Brief Overview. Eur. J. Pharm. Sci. 2016, 89, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The Cost of Drug Development: A Systematic Review. Health Policy 2011, 100, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H. Repurposing Approved Drugs for Cancer Therapy. Br. Med. Bull. 2021, 137, 13–27. [Google Scholar] [CrossRef]
- Amare, G.G.; Meharie, B.G.; Belayneh, Y.M. A Drug Repositioning Success: The Repositioned Therapeutic Applications and Mechanisms of Action of Thalidomide. J. Oncol. Pharm. Pract. 2021, 27, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Harnden, K.; Blackwell, K. Routine Use of Zoledronic Acid in Early-Stage Breast Cancer. JNCCN J. Natl. Compr. Cancer Netw. 2015, 13, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A Repurposed Drug to Fight Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Liao, J.K. Isoprenoids as Mediators of the Biological Effects of Statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef][Green Version]
- Konstantinopoulos, P.A.; Karamouzis, M.V.; Papavassiliou, A.G. Post-Translational Modifications and Regulation of the RAS Superfamily of GTPases as Anticancer Targets. Nat. Rev. Drug Discov. 2007, 6, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Djulbegovic, B.; Hozo, I. Statins for Primary Prevention of Cardiovascular Disease. Ann. Intern. Med. 2019, 171, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Schelz, Z.; Muddather, H.F.; Zupkó, I. Repositioning of HMG-CoA Reductase Inhibitors as Adjuvants in the Modulation of Efflux Pump-Mediated Bacterial and Tumor Resistance. Antibiotics 2023, 12, 1468. [Google Scholar] [CrossRef]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer Efficacy of Simvastatin on Prostate Cancer Cells and Tumor Xenografts Is Associated with Inhibition of Akt and Reduced Prostate-Specific Antigen Expression. J. Pharmacol. Exp. Ther. 2011, 336, 496–505. [Google Scholar] [CrossRef]
- Hoque, A.; Chen, H.; Xu, X.C. Statin Induces Apoptosis and Cell Growth Arrest in Prostate Cancer Cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef]
- Toepfer, N.; Childress, C.; Parikh, A.; Rukstalis, D.; Yang, W. Atorvastatin Induces Autophagy in Prostate Cancer PC3 Cells through Activation of LC3 Transcription. Cancer Biol. Ther. 2011, 12, 691–699. [Google Scholar] [CrossRef]
- Wang, T.; Seah, S.; Loh, X.; Chan, C.W.; Hartman, M.; Goh, B.C.; Lee, S.C. Simvastatin-Induced Breast Cancer Cell Death and Deactivation of PI3K/Akt and MAPK/ERK Signalling Are Reversed by Metabolic Products of the Mevalonate Pathway. Oncotarget 2016, 7, 2532–2544. [Google Scholar] [CrossRef]
- Docrat, T.F.; Nagiah, S.; Krishnan, A.; Naidoo, D.B.; Chuturgoon, A.A. Atorvastatin Induces MicroRNA-145 Expression in HEPG2 Cells via Regulation of the PI3K/AKT Signalling Pathway. Chem. Biol. Interact. 2018, 287, 32–40. [Google Scholar] [CrossRef]
- You, H.Y.; Zhang, W.J.; Xie, X.M.; Zheng, Z.H.; Zhu, H.L.; Jiang, F.Z. Pitavastatin Suppressed Liver Cancer Cells in Vitro and in Vivo. OncoTargets Ther. 2016, 9, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Hong, E.M.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; Lee, J. Statin Induces Apoptosis of Human Colon Cancer Cells and Downregulation of Insulin-like Growth Factor 1 Receptor via Proapoptotic ERK Activation. Oncol. Lett. 2016, 12, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Qiu, P.; Rakariyatham, K.; Li, F.; Gao, Z.; Cai, X.; Wang, M.; Xu, F.; Zheng, J.; et al. Synergistic Chemopreventive Effects of Nobiletin and Atorvastatin on Colon Carcinogenesis. Carcinogenesis 2017, 38, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ahn, H.S.; Kim, H.J. Statin Use and Incidence and Mortality of Breast and Gynecology Cancer: A Cohort Study Using the National Health Insurance Claims Database. Int. J. Cancer 2022, 150, 1156–1165. [Google Scholar] [CrossRef]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef]
- Song, M.K.; Shin, B.S.; Ha, C.S.; Park, W.Y. Would Lipophilic Statin Therapy as a Prognostic Factor Improve Survival in Patients with Uterine Cervical Cancer? Int. J. Gynecol. Cancer 2017, 27, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Stokes, W.; Eguchi, M.; Hararah, M.; Amini, A.; Mueller, A.; Morgan, R.; Bradley, C.; Raben, D.; McDermott, J.; et al. Statin Use Associated with Improved Overall and Cancer Specific Survival in Patients with Head and Neck Cancer. Oral Oncol. 2019, 90, 54–66. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, P.; Wang, J.; Chen, P.; Fang, Z.; Luo, F. The Association of Statin Therapy and Cancer: A Meta-Analysis. Lipids Health Dis. 2023, 22, 192. [Google Scholar] [CrossRef]
- Jiao, X.F.; Li, H.; Zeng, L.; Yang, H.; Hu, Y.; Qu, Y.; Chen, W.; Sun, Y.; Zhang, W.; Zeng, X.; et al. Use of Statins and Risks of Ovarian, Uterine, and Cervical Diseases: A Cohort Study in the UK Biobank. Eur. J. Clin. Pharmacol. 2024, 80, 855–867. [Google Scholar] [CrossRef]
- Ravnskov, U.; Mccully, K.S.; Rosch, P.J. The Statin-Low Cholesterol-Cancer Conundrum. QJM Int. J. Med. 2012, 105, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic Statins Limit Cancer Cell Growth and Survival, via Involvement of Akt Signaling. PLoS ONE 2018, 13, e0197422. [Google Scholar] [CrossRef]
- Ricco, N.; Flor, A.; Wolfgeher, D.; Efimova, E.V.; Ramamurthy, A.; Appelbe, O.K.; Brinkman, J.; Truman, A.W.; Spiotto, M.T.; Kron, S.J. Mevalonate Pathway Activity as a Determinant of Radiation Sensitivity in Head and Neck Cancer. Mol. Oncol. 2019, 13, 1927–1943. [Google Scholar] [CrossRef]
- Stine, J.E.; Han, X.; Schointuch, M.; Zhou, C.; Gilliam, T.; Gehrig, P.A.; Bae-Jump, V.L. The HMG-CoA Reductase Inhibitor Simvastatin Exhibits Antitumorigenic and Antimetastatic Effects in Ovarian Cancer. Gynecol. Oncol. 2014, 133, 111–112. [Google Scholar] [CrossRef]
- Nübel, T.; Dippold, W.; Kleinert, H.; Kaina, B.; Fritz, G. Lovastatin Inhibits Rho-Regulated Expression of E-Selectin by TNFalpha and Attenuates Tumor Cell Adhesion. FASEB J. 2004, 18, 140–142. [Google Scholar] [CrossRef]
- Fan, Z.; Jiang, H.; Wang, Z.; Qu, J. Atorvastatin Partially Inhibits the Epithelial-Mesenchymal Transition in A549 Cells Induced by TGF-Β1 by Attenuating the Upregulation of SphK1. Oncol. Rep. 2016, 36, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Dworacka, M.; Iskakova, S.; Weso, A.; Zharmakhanova, G. Simvastatin Attenuates the Aberrant Expression of Angiogenic Factors Induced by Glucose Variability. Diabetes Res. Clin. Pract. 2018, 143, 245–253. [Google Scholar] [CrossRef]
- Asakage, M.; Tsuno, N.H.; Kitayama, J.; Kawai, K. 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitor (Pravastatin) Inhibits Endothelial Cell Proliferation Dependent on G1 Cell Cycle Arrest. Anticancer Drugs 2004, 15, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ghalali, A.; Wiklund, F.; Zheng, H.; Stenius, U.; Högberg, J. Atorvastatin Prevents ATP-Driven Invasiveness via P2X7 and EHBP1 Signaling in PTEN-Expressing Prostate Cancer Cells. Carcinogenesis 2014, 35, 1547–1555. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019, 30, 143–156.e5. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Gander, H.; Nussbaumer, O.; Nussbaumer, W.; Rahm, A.; Thurnher, M. IL-2 Costimulation Enables Statin-Mediated Activation of Human NK Cells, Preferentially through a Mechanism Involving CD56+ Dendritic Cells. Cancer Res. 2010, 70, 9611–9620. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar] [CrossRef]
- Kazanietz, M.G.; Caloca, M.J. The Rac GTPase in Cancer: From Old Concepts to New Paradigms. Cancer Res. 2017, 77, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.A.; Harvey, K.A.; Xu, Z.; Natarajan, S.K.; Davisson, V.J. Characterization of Lovastatin-Docosahexaenoate Anticancer Properties against Breast Cancer Cells. Bioorg. Med. Chem. 2014, 22, 1899–1908. [Google Scholar] [CrossRef]
- Alarcon Martinez, T.; Zeybek, N.D.; Müftüoğlu, S. Evaluation of the Cytotoxic and Autophagic Effects of Atorvastatin on Mcf-7 Breast Cancer Cells. Balk. Med. J. 2018, 35, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.L.; Zhang, R.; Zeng, Y.; Zhu, Y.S.; Wang, G. Statins Induce Cell Apoptosis through a Modulation of AKT/FOXO1 Pathway in Prostate Cancer Cells. Cancer Manag. Res. 2019, 11, 7231–7242. [Google Scholar] [CrossRef]
- Ortiz, N.; Díaz, C. Mevalonate Pathway as a Novel Target for the Treatment of Metastatic Gastric Cancer. Oncol. Lett. 2020, 20, 320. [Google Scholar] [CrossRef]
- Göbel, A.; Zinna, V.M.; Dell’Endice, S.; Jaschke, N.; Kuhlmann, J.D.; Wimberger, P.; Rachner, T.D. Anti-Tumor Effects of Mevalonate Pathway Inhibition in Ovarian Cancer. BMC Cancer 2020, 20, 703. [Google Scholar] [CrossRef]
- Fang, Z.; Tang, Y.; Fang, J.; Zhou, Z.; Xing, Z.; Guo, Z.; Guo, X.; Wang, W.; Jiao, W.; Xu, Z.; et al. Simvastatin Inhibits Renal Cancer Cell Growth and Metastasis via AKT/MTOR, ERK and JAK2/STAT3 Pathway. PLoS ONE 2013, 8, e62823. [Google Scholar] [CrossRef]
- Zhou, Z.; Curtis, A.J.; Ernst, M.E.; Ryan, J.; Zoungas, S.; Wolfe, R.; McNeil, J.J.; Murray, A.M.; Reid, C.M.; Chowdhury, E.K.; et al. Comparison of Statins for Primary Prevention of Cardiovascular Disease and Persistent Physical Disability in Older Adults. Eur. J. Clin. Pharmacol. 2022, 78, 467–476. [Google Scholar] [CrossRef]
- Robinson, E.; Nandi, M.; Wilkinson, L.L.; Arrowsmith, D.M.; Curtis, A.D.M.; Richardson, A. Preclinical Evaluation of Statins as a Treatment for Ovarian Cancer. Gynecol. Oncol. 2013, 129, 417–424. [Google Scholar] [CrossRef]
- Kato, S.; Smalley, S.; Sadarangani, A.; Chen-Lin, K.; Oliva, B.; Brañes, J.; Carvajal, J.; Gejman, R.; Owen, G.I.; Cuello, M. Lipophilic but Not Hydrophilic Statins Selectively Induce Cell Death in Gynaecological Cancers Expressing High Levels of HMGCoA Reductase. J. Cell. Mol. Med. 2010, 14, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Rennert, G. Beyond Aspirin—Cancer Prevention with Statins, Metformin and Bisphosphonates. Nat. Rev. Clin. Oncol. 2013, 10, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Paoletti, R.; Corsini, A. Safety of Statins: Focus on Clinical Pharmacokinetics and Drug Interactions. Circulation 2004, 109, 50–57. [Google Scholar] [CrossRef]
- Thibault, A.; Samid, D.; Tompkins, A.C.; Figg, W.D.; Cooper, M.R.; Hohl, R.J.; Trepel, J.; Liang, B.; Patronas, N.; Venzon, D.J.; et al. Phase I Study of Lovastatin, an Inhibitor of the Mevalonate Pathway, in Patients with Cancer. Clin. Cancer Res. 1996, 2, 483–491. [Google Scholar]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; Simone, C.; Rosa, S.; et al. Pleiotropic Effects of Statins: A Focus on Cancer. BBA—Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef] [PubMed]
- Paranthaman, S.; Hani, U.; Osmani, R.A.M.; Bhosale, R.R.; Haider, N. Current Advances in Nanoparticle-Based Approaches for the Hepatocellular Carcinoma Treatment. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102508. [Google Scholar] [CrossRef]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, A.V. Effects of Simvastatin in Combination with Anticancer Drugs on Proliferation and Migration in Cholangiocarcinoma Cells. Indian J. Pharm. Sci. 2022, 84, 72–79. [Google Scholar] [CrossRef]
- Buranrat, B.; Suwannaloet, W.; Naowaboot, J. Simvastatin Potentiates Doxorubicin Activity against MCF-7 Breast Cancer Cells. Oncol. Lett. 2017, 14, 6243–6250. [Google Scholar] [CrossRef]
- Chen, M.-J.; Cheng, A.-C.; Lee, M.-F.; Hsu, Y.-C. Simvastatin Induces G1 Arrest by Up-Regulating GSK3β and down-Regulating CDK4/Cyclin D1 and CDK2/Cyclin E1 in Human Primary Colorectal Cancer Cells. J. Cell. Physiol. 2017, 233, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Lee, T.J.; Huang, C.C.; Chang, P.H.; Tsai, J.W.; Chuang, L.P.; Su Pang, J.H. Simvastatin Inhibits the Proliferation of HL-60 Clone 15- Derived Eosinophils by Inducing the Arrest of the Cell Cycle in the G1/S Phase. Eur. J. Pharmacol. 2019, 856, 172400. [Google Scholar] [CrossRef]
- Kobayashi, K.; Baba, K.; Kambayashi, S.; Okuda, M. Blockade of Isoprenoids Biosynthesis by Simvastatin Induces Autophagy-Mediated Cell Death via Downstream c-Jun N-Terminal Kinase Activation and Cell Cycle Dysregulation in Canine T-Cell Lymphoma Cells. Res. Vet. Sci. 2024, 169, 105174. [Google Scholar] [CrossRef]
- Denoyelle, C.; Vasse, M.; Ko, M.; Vannier, J.; Mishal, Z.; Ganne, F.; Soria, J.; Soria, C.; Difema, L.; Merci, G.D.R.; et al. Cerivastatin, an Inhibitor of HMG-CoA Reductase, Inhibits the Signaling Pathways Involved in the Invasiveness and Metastatic Properties of Highly Invasive Breast Cancer Cell Lines: An in Vitro Study. Carcinogenesis 2001, 22, 1139–1148. [Google Scholar] [CrossRef]
- Stacey, D.; Kazlauskas, A. Regulation of Ras Signaling by the Cell Cycle. Curr. Opin. Genet. Dev. 2002, 12, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Vosper, J.; Masuccio, A.; Kullmann, M.; Ploner, C.; Geley, S.; Hengst, L. Statin-Induced Depletion of Geranylgeranyl Pyrophosphate Inhibits Cell Proliferation by a Novel Pathway of Skp2 Degradation. Oncotarget 2015, 6, 2889–2902. [Google Scholar] [CrossRef][Green Version]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow Cytometry of Apoptotic Cell Death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Yu, X.; Pan, Y.; Ma, H.; Li, W. Simvastatin Inhibits Proliferation and Induces Apoptosis in Human Lung Cancer Cells. Oncol. Res. 2013, 20, 351–357. [Google Scholar] [CrossRef]
- Maksimova, E.; Yie, T.A.; Rom, W.N. In Vitro Mechanisms of Lovastatin on Lung Cancer Cell Lines as a Potential Chemopreventive Agent. Lung 2008, 186, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Shellman, Y.G.; Ribble, D.; Miller, L.; Gendall, J.; VanBuskirk, K.; Kelly, D.; Norris, D.A.; Dellavalle, R.P. Lovastatin-Induced Apoptosis in Human Melanoma Cell Lines. Melanoma Res. 2005, 15, 83–89. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.E.; Choi, Y.S. The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef]
- Infante, E.; Heasman, S.J.; Ridley, A.J. Statins Inhibit T-Acute Lymphoblastic Leukemia Cell Adhesion and Migration through Rap1b. J. Leukoc. Biol. 2011, 89, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Li, Y.; Li, W.; Chen, Y. Simvastatin Prevents Proliferation and Bone Metastases of Lung Adenocarcinoma in Vitro and in Vivo. Neoplasma 2013, 60, 240–246. [Google Scholar] [CrossRef]
- Collisson, E.A.; Kleer, C.; Wu, M.; De, A.; Gambhir, S.S.; Merajver, S.D.; Kolodne, M.S. Atorvastatin Prevents RhoC Isoprenylation, Invasion, and Metastasis in Human Melanoma Cells. Mol. Cancer Ther. 2003, 2, 941–948. [Google Scholar]
- Al-Haidari, A.A.; Syk, I.; Thorlacius, H. HMG-CoA Reductase Regulates CCL17-Induced Colon Cancer Cell Migration via Geranylgeranylation and RhoA Activation. Biochem. Biophys. Res. Commun. 2014, 446, 68–72. [Google Scholar] [CrossRef]
- Biselli-Chicote, P.M.; Lotierzo, A.T.; Biselli, J.M.; Paravino, É.C.; Goloni-Bertollo, E.M. Atorvastatin Increases Oxidative Stress and Inhibits Cell Migration of Oral Squamous Cell Carcinoma in Vitro. Oral Oncol. 2019, 90, 109–114. [Google Scholar] [CrossRef]
- Dorsch, M.; Kowalczyk, M.; Planque, M.; Heilmann, G.; Urban, S.; Dujardin, P.; Forster, J.; Ueffing, K.; Nothdurft, S.; Oeck, S.; et al. Statins Affect Cancer Cell Plasticity with Distinct Consequences for Tumor Progression and Metastasis. Cell Rep. 2021, 37, 110056. [Google Scholar] [CrossRef]
- Koohestanimobarhan, S.; Salami, S.; Imeni, V.; Mohammadi, Z.; Bayat, O. Lipophilic Statins Antagonistically Alter the Major Epithelial-to-Mesenchymal Transition Signaling Pathways in Breast Cancer Stem–like Cells via Inhibition of the Mevalonate Pathway. J. Cell. Biochem. 2019, 120, 2515–2531. [Google Scholar] [CrossRef] [PubMed]
- Yongjun, Y.; Shuyun, H.; Lei, C.; Xiangrong, C.; Zhilin, Y.; Yiquan, K. Atorvastatin Suppresses Glioma Invasion and Migration by Reducing Microglial MT1-MMP Expression. J. Neuroimmunol. 2013, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]






| Cell Lines | Atorvastatin | Rosuvastatin | Cisplatin | Atorvastatin Tumor Selectivity |
|---|---|---|---|---|
| IC50 (μM) ± SD | ||||
| MCF-7 | 61.01 ± 3.71 ** | 100< | 8.19 ± 0.20 | 0.80 |
| T47-D | 8.32 ± 1.52 * | 100< | 18.36 ± 1.24 | 5.85 |
| MDA-MB-231 | 2.57 ± 0.30 *** | 18.22 ± 1.12 | 19.12 ± 0.02 | 18.96 |
| HeLa | 20.27 ± 0.73 ** | 64.91 ± 1.80 | 12.43 ± 0.20 | 2.40 |
| SiHa | 12.02 ± 2.42 | 38.39 ± 0.30 | 4.80 ± 0.72 | 4.05 |
| C33A | 4.61 ± 0.15 | 31.63 ± 0.78 | 4.70 ± 1.64 | 10.54 |
| A2780 | 4.02 ± 0.78 * | 96.13 ± 0.72 | 1.34 ± 0.05 | 12.11 |
| UPCI-SCC-154 | 9.21 ± 1.68 * | 94.09 ± 0.86 | 1.29 ± 0.001 | 5.28 |
| UPCI-SCC-131 | 34.74 ± 1.63 ** | 94.16 ± 1.80 | 1.37 ± 0.21 | 1.40 |
| MRC-5 | 48.64 ± 3.24 ** | 100< | 4.74 ± 0.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muddather, H.F.; Bózsity, N.; Balogh, G.T.; Schelz, Z.; Zupkó, I. In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Atorvastatin: A Focus on Cervical and Head and Neck Cancers. Pharmaceutics 2025, 17, 1253. https://doi.org/10.3390/pharmaceutics17101253
Muddather HF, Bózsity N, Balogh GT, Schelz Z, Zupkó I. In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Atorvastatin: A Focus on Cervical and Head and Neck Cancers. Pharmaceutics. 2025; 17(10):1253. https://doi.org/10.3390/pharmaceutics17101253
Chicago/Turabian StyleMuddather, Hiba F., Noémi Bózsity, György T. Balogh, Zsuzsanna Schelz, and István Zupkó. 2025. "In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Atorvastatin: A Focus on Cervical and Head and Neck Cancers" Pharmaceutics 17, no. 10: 1253. https://doi.org/10.3390/pharmaceutics17101253
APA StyleMuddather, H. F., Bózsity, N., Balogh, G. T., Schelz, Z., & Zupkó, I. (2025). In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Atorvastatin: A Focus on Cervical and Head and Neck Cancers. Pharmaceutics, 17(10), 1253. https://doi.org/10.3390/pharmaceutics17101253









