Long-Term Therapeutic Effects of 225Ac-DOTA-E[c(RGDfK)]2 Induced by Radiosensitization via G2/M Arrest in Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Radiolabeled DOTA-E[c(RGDfK)]2
2.2. Cell Culture and Animal Model
2.3. Cell Viability Assay
2.4. FCM Analysis
2.5. Fluorescence Immunohistochemistry
2.6. In Vitro Combination Treatment
2.7. Biodistribution and SPECT/CT Imaging of 111In-DOTA-RGD2
2.8. Biodistribution and Alpha Camera Imaging of 225Ac-DOTA-RGD2
2.9. Therapy with 225Ac-DOTA-RGD2
2.10. Statistical Analyses
3. Results
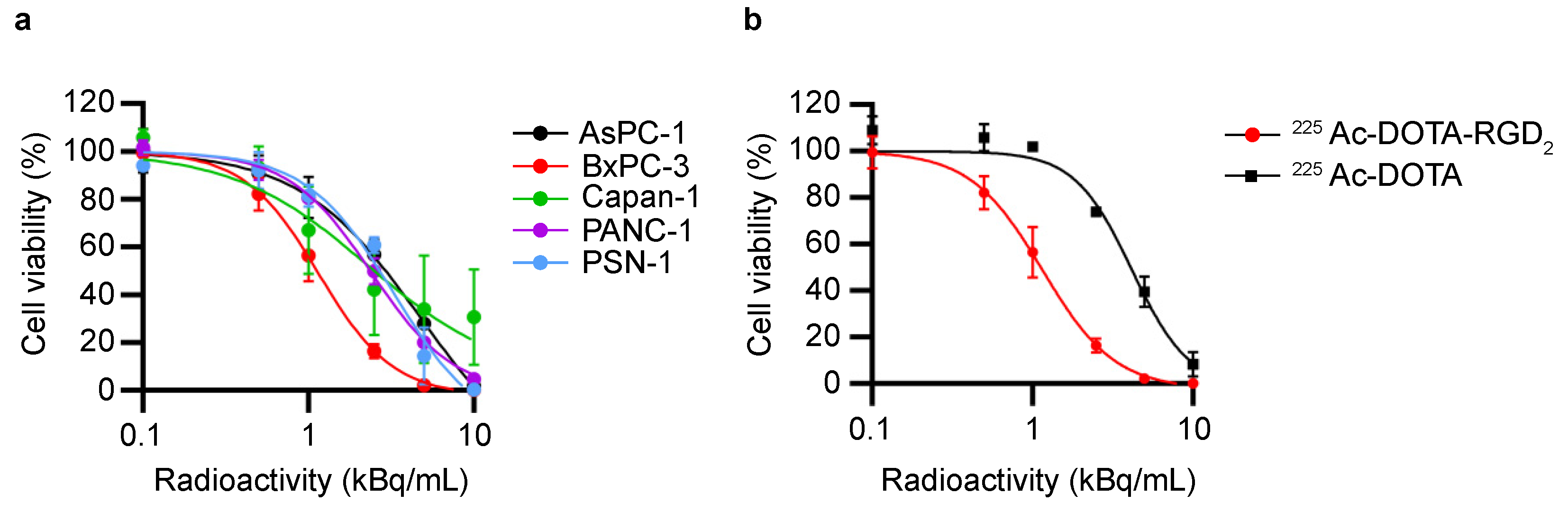
3.1. In Vitro Cytotoxicity Assessment by 225Ac-DOTA-RGD2 Binding
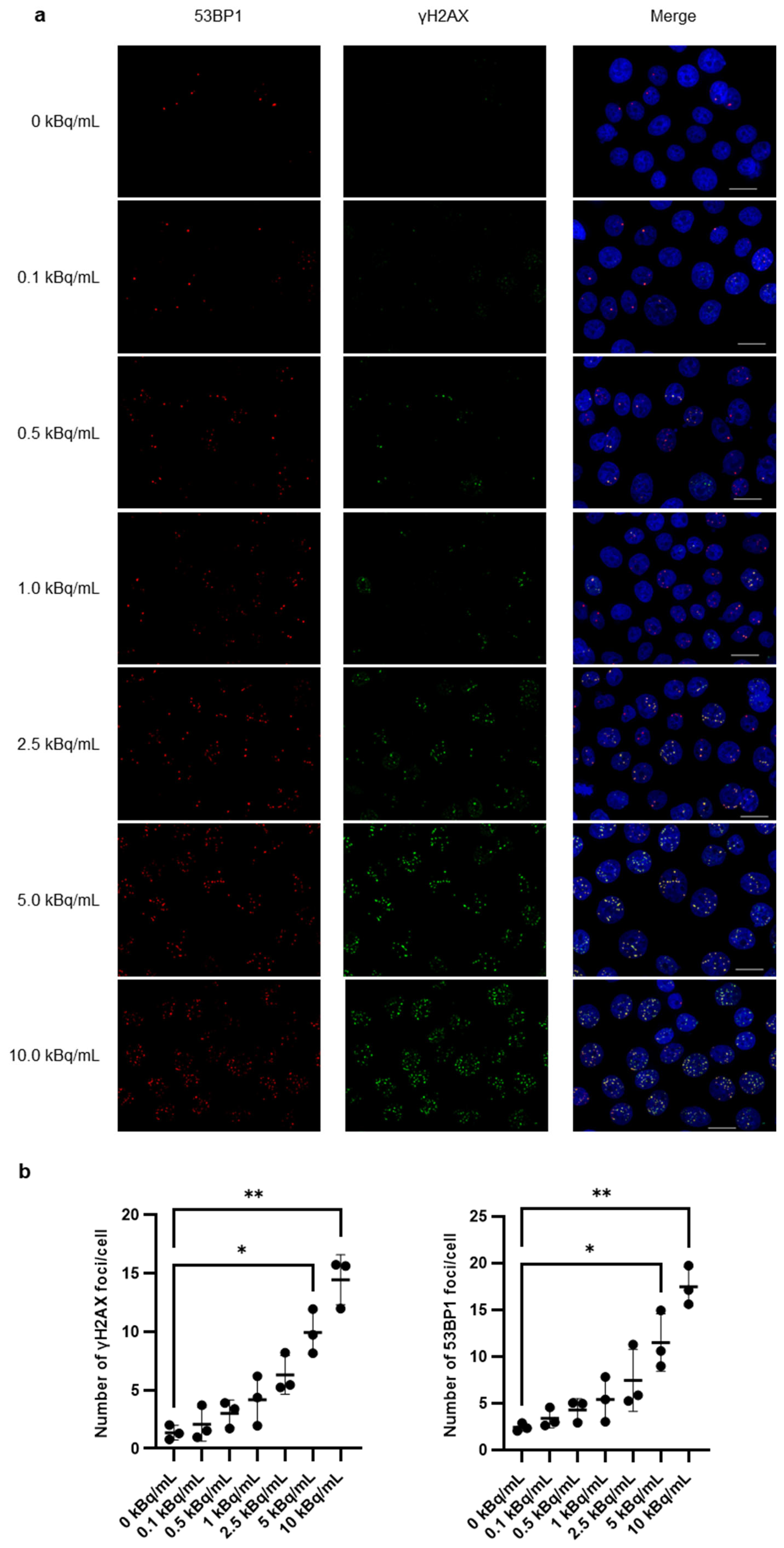
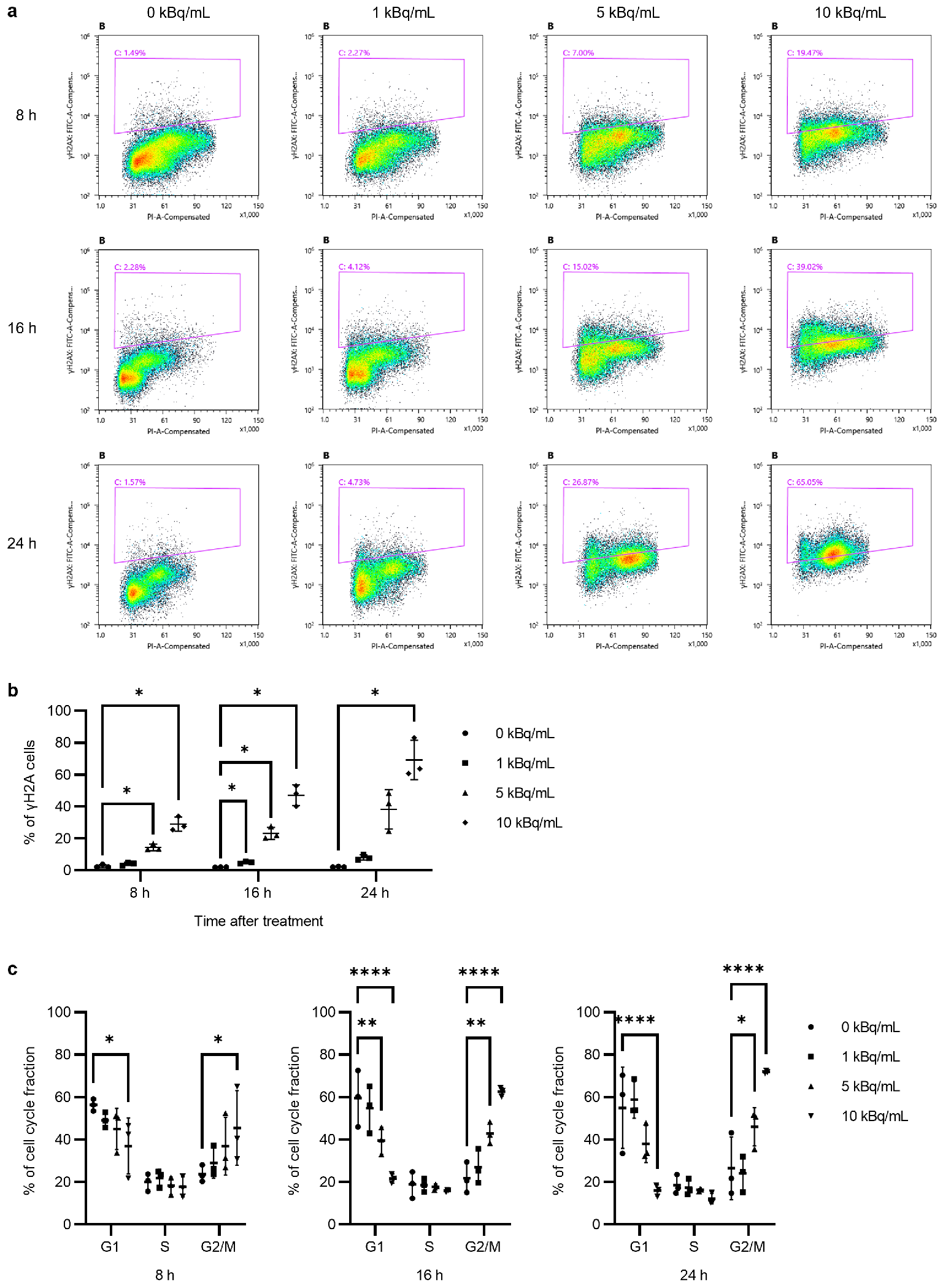
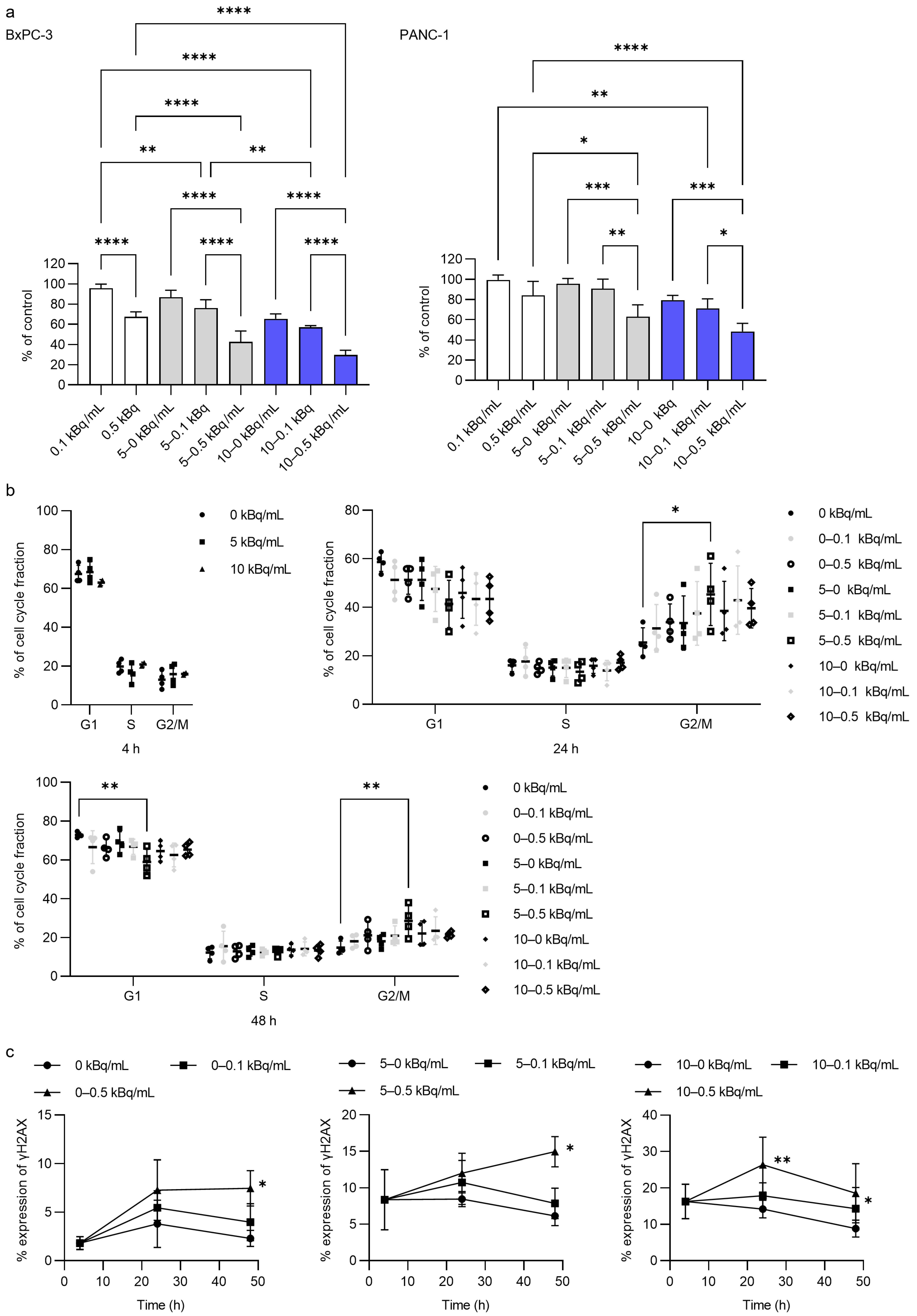
3.2. γH2AX/53BP1 Expression and G2/M Arrest After 225Ac-DOTA-RGD2
3.3. DOTA-RGD2 Pharmacokinetics and Intratumoral Distribution
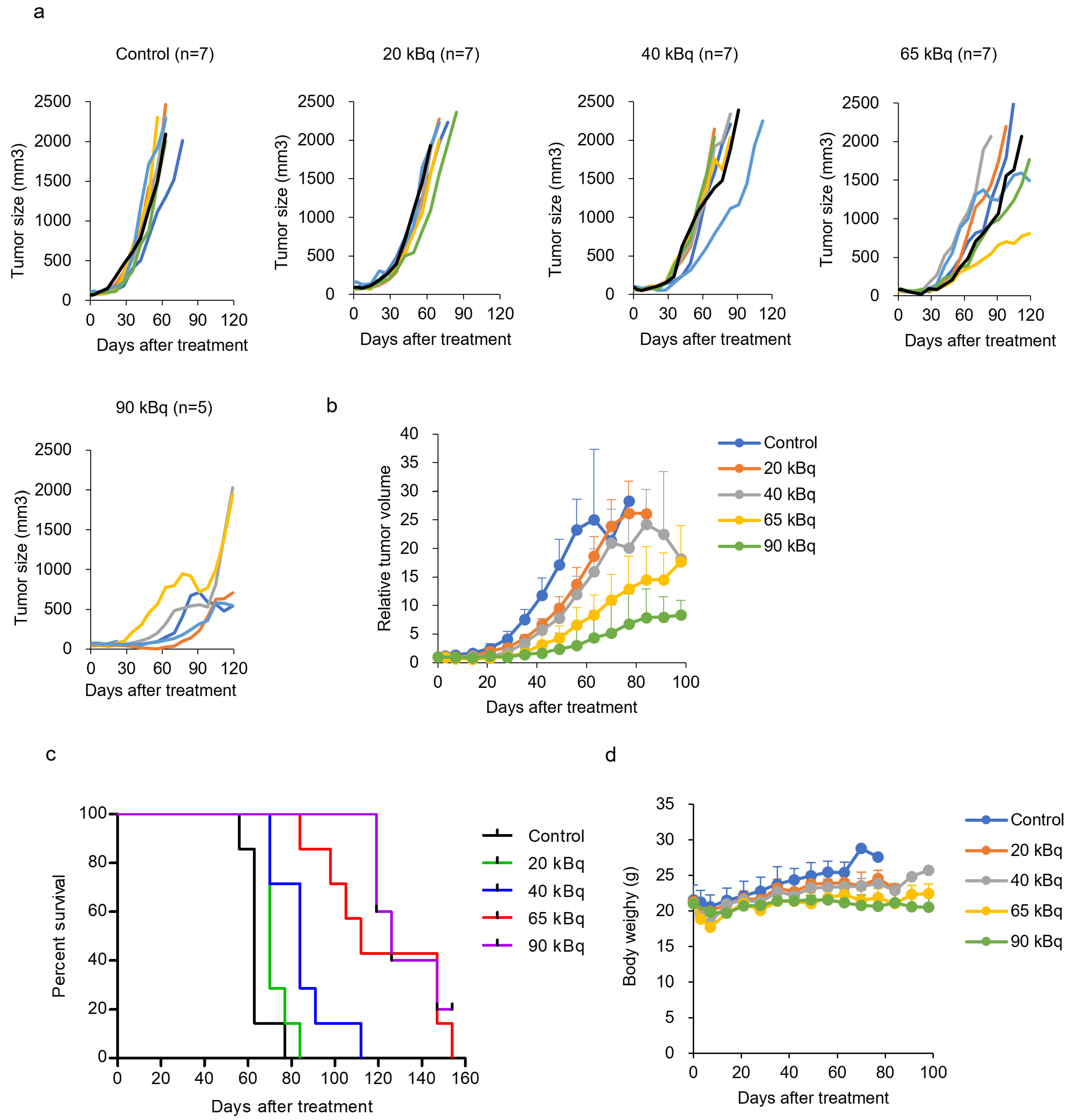
3.4. Treatment with 225Ac-DOTA-RGD2 in Mice Bearing BxPC-3
3.5. In Vitro Antitumor Activity of 225Ac-DOTA-RGD2 Combination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Xu, S.; Gao, Y.X.; Zhao, Z.M.; Zhao, G.D.; Hu, M.G.; Tan, X.L.; Lau, W.Y.; Liu, R. Early and late recurrence patterns of pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: A multicenter study. Int. J. Surg. 2023, 109, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, Y.; Lou, J.; Xu, L.; Shi, A.; Yang, L.; Fan, Y.; Yang, J.; Huang, J.; Wu, Y.; et al. Preoperative recurrence prediction in pancreatic ductal adenocarcinoma after radical resection using radiomics of diagnostic computed tomography. EClinicalMedicine 2022, 43, 101215. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer Zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Diep, C.H.; Jiang, P.; Watanabe, A.; LoBello, J.; Sima, C.; Hostetter, G.; Shepard, H.M.; Von Hoff, D.D.; Han, H. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 2015, 21, 3561–3568. [Google Scholar] [CrossRef]
- Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Linden, O.; Noordzij, W.; Park, J.; Saad, F. Targeted alpha therapy, an emerging class of cancer agents: A review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, R.; Kawaguchi, M.; Masui, T.; Koshiba, T.; Ida, J.; Fujimoto, K.; Wada, M.; Doi, R.; Imamura, M. Expression of integrin αVβ3 in pancreatic carcinoma: Relation to MMP-2 activation and lymph node metastasis. Pancreas 2002, 25, e30–e35. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.J.; Ho, J.C.; Moossa, A.R.; Bouvet, M. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas 2007, 35, 293–301. [Google Scholar] [CrossRef]
- Beer, A.J.; Haubner, R.; Goebel, M.; Luderschmidt, S.; Spilker, M.E.; Wester, H.J.; Weber, W.A.; Schwaiger, M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J. Nucl. Med. 2005, 46, 1333–1341. [Google Scholar]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar]
- Schnell, O.; Krebs, B.; Carlsen, J.; Miederer, I.; Goetz, C.; Goldbrunner, R.H.; Wester, H.J.; Haubner, R.; Popperl, G.; Holtmannspotter, M.; et al. Imaging of integrin αvβ3 expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009, 11, 861–870. [Google Scholar] [CrossRef]
- Hausner, S.H.; DiCara, D.; Marik, J.; Marshall, J.F.; Sutcliffe, J.L. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: Generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin αvβ6 expression with positron emission tomography. Cancer Res. 2007, 67, 7833–7840. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Hayakawa, T.; Mutoh, M.; Imai, T.; Tsuda, K.; Kimura, S.; Umeda, I.O.; Fujii, H.; Wakabayashi, K. In vivo SPECT imaging with 111In-DOTA-c(RGDfK) to detect early pancreatic cancer in a hamster pancreatic carcinogenesis model. J. Nucl. Med. 2012, 53, 765–771. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Ogawa, K.; Washiyama, K.; Shikano, N.; Mori, H.; Amano, R.; Kawai, K. αvβ3 integrin-targeting radionuclide therapy and imaging with monomeric RGD peptide. Int. J. Cancer 2008, 123, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Darzynkiewicz, Z. Cytometric assessment of histone H2AX phosphorylation: A reporter of DNA damage. Methods Mol. Biol. 2006, 314, 73–80. [Google Scholar] [CrossRef]
- Muslimovic, A.; Ismail, I.H.; Gao, Y.; Hammarsten, O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat. Protoc. 2008, 3, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Mutoh, M.; Imai, T.; Tsuta, K.; Yanaka, A.; Fujii, H.; Yoshimoto, M. SPECT/CT of lung nodules using 111In-DOTA-c(RGDfK) in a mouse lung carcinogenesis model. Ann. Nucl. Med. 2013, 27, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.W.; Frost, S.H.; Frayo, S.L.; Kenoyer, A.L.; Santos, E.; Jones, J.C.; Green, D.J.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; et al. Quantitative single-particle digital autoradiography with α-particle emitters for targeted radionuclide therapy using the iQID camera. Med. Phys. 2015, 42, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Pandya, D.N.; Tichacek, C.J.; Budzevich, M.M.; Wang, Z.; Reff, J.N.; Engelman, R.W.; Boulware, D.C.; Chiappori, A.A.; Strosberg, J.R.; et al. Preclinical evaluation of [225Ac]Ac-DOTA-TATE for treatment of lung neuroendocrine neoplasms. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3408–3421. [Google Scholar] [CrossRef]
- Song, H.; Hobbs, R.F.; Vajravelu, R.; Huso, D.L.; Esaias, C.; Apostolidis, C.; Morgenstern, A.; Sgouros, G. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: Comparing efficacy with 213Bi and 90Y. Cancer Res. 2009, 69, 8941–8948. [Google Scholar] [CrossRef]
- Veach, D.R.; Storey, C.M.; Luckerath, K.; Braun, K.; von Bodman, C.; Lamminmaki, U.; Kalidindi, T.; Strand, S.E.; Strand, J.; Altai, M.; et al. PSA-targeted alpha-, beta-, and positron-emitting immunotheranostics in murine prostate cancer models and nonhuman primates. Clin. Cancer Res. 2021, 27, 2050–2060. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kong, Y.; Yang, Z.; Liu, Y.; Liu, R.; Geng, Y.; Luo, H.; Zhang, H.; Li, H.; Feng, S.; et al. Preliminary study on radiosensitivity to carbon ions in human breast cancer. J. Radiat. Res. 2020, 61, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Dukaew, N.; Konishi, T.; Chairatvit, K.; Autsavapromporn, N.; Soonthornchareonnon, N.; Wongnoppavich, A. Enhancement of radiosensitivity by eurycomalactone in human NSCLC cells through G2/M cell cycle arrest and delayed DNA double-strand break repair. Oncol. Res. 2020, 28, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein enhances the radiosensitivity of breast cancer cells via G2/M cell cycle arrest and apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef]
- Schwarz, K.; Dobiasch, S.; Nguyen, L.; Schilling, D.; Combs, S.E. Modification of radiosensitivity by curcumin in human pancreatic cancer cell lines. Sci. Rep. 2020, 10, 3815. [Google Scholar] [CrossRef]
- Buckley, A.M.; Lynam-Lennon, N.; O’Neill, H.; O’Sullivan, J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 298–313. [Google Scholar] [CrossRef]
- Shibata, A.; Conrad, S.; Birraux, J.; Geuting, V.; Barton, O.; Ismail, A.; Kakarougkas, A.; Meek, K.; Taucher-Scholz, G.; Lobrich, M.; et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011, 30, 1079–1092. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 196–207. [Google Scholar] [CrossRef]
- Bunting, S.F.; Callen, E.; Wong, N.; Chen, H.T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53BP1 inhibits homologous recombination in BRCA1-deficient cells by blocking resection of DNA breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef]
- Morgan, M.A.; Parsels, L.A.; Zhao, L.; Parsels, J.D.; Davis, M.A.; Hassan, M.C.; Arumugarajah, S.; Hylander-Gans, L.; Morosini, D.; Simeone, D.M.; et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010, 70, 4972–4981. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Lee, S.T.; Gan, H.K.; Scott, A.M. Radiolabeled antibodies for cancer imaging and therapy. Cancers 2022, 14, 1545. [Google Scholar] [CrossRef] [PubMed]
- Merkx, R.I.J.; Lobeek, D.; Konijnenberg, M.; Jimenez-Franco, L.D.; Kluge, A.; Oosterwijk, E.; Mulders, P.F.A.; Rijpkema, M. Phase I study to assess safety, biodistribution and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3277–3285. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Boning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First clinical results for PSMA-targeted alpha-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Abbas, N.; Heyerdahl, H.; Bruland, O.S.; Borrebaek, J.; Nesland, J.; Dahle, J. Experimental α-particle radioimmunotherapy of breast cancer using 227Th-labeled p-benzyl-DOTA-trastuzumab. EJNMMI Res 2011, 1, 18. [Google Scholar] [CrossRef]
- Örbom, A.; Eriksson, S.E.; Elgstrom, E.; Ohlsson, T.; Nilsson, R.; Tennvall, J.; Strand, S.E. The intratumoral distribution of radiolabeled 177Lu-BR96 monoclonal antibodies changes in relation to tumor histology over time in a syngeneic rat colon carcinoma model. J. Nucl. Med. 2013, 54, 1404–1410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimoto, M.; Washiyama, K.; Ohnuki, K.; Doi, A.; Inokuchi, M.; Kojima, M.; Miller, B.W.; Yoshii, Y.; Inaki, A.; Fujii, H. Long-Term Therapeutic Effects of 225Ac-DOTA-E[c(RGDfK)]2 Induced by Radiosensitization via G2/M Arrest in Pancreatic Ductal Adenocarcinoma. Pharmaceutics 2025, 17, 9. https://doi.org/10.3390/pharmaceutics17010009
Yoshimoto M, Washiyama K, Ohnuki K, Doi A, Inokuchi M, Kojima M, Miller BW, Yoshii Y, Inaki A, Fujii H. Long-Term Therapeutic Effects of 225Ac-DOTA-E[c(RGDfK)]2 Induced by Radiosensitization via G2/M Arrest in Pancreatic Ductal Adenocarcinoma. Pharmaceutics. 2025; 17(1):9. https://doi.org/10.3390/pharmaceutics17010009
Chicago/Turabian StyleYoshimoto, Mitsuyoshi, Kohshin Washiyama, Kazunobu Ohnuki, Ayano Doi, Miki Inokuchi, Motohiro Kojima, Brian W. Miller, Yukie Yoshii, Anri Inaki, and Hirofumi Fujii. 2025. "Long-Term Therapeutic Effects of 225Ac-DOTA-E[c(RGDfK)]2 Induced by Radiosensitization via G2/M Arrest in Pancreatic Ductal Adenocarcinoma" Pharmaceutics 17, no. 1: 9. https://doi.org/10.3390/pharmaceutics17010009
APA StyleYoshimoto, M., Washiyama, K., Ohnuki, K., Doi, A., Inokuchi, M., Kojima, M., Miller, B. W., Yoshii, Y., Inaki, A., & Fujii, H. (2025). Long-Term Therapeutic Effects of 225Ac-DOTA-E[c(RGDfK)]2 Induced by Radiosensitization via G2/M Arrest in Pancreatic Ductal Adenocarcinoma. Pharmaceutics, 17(1), 9. https://doi.org/10.3390/pharmaceutics17010009





