Development and Characterization of Lyophilized Chondroitin Sulfate-Loaded Solid Lipid Nanoparticles: Encapsulation Efficiency and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanoparticles Manufacturing
2.2.2. Characterization of SLN Formulations
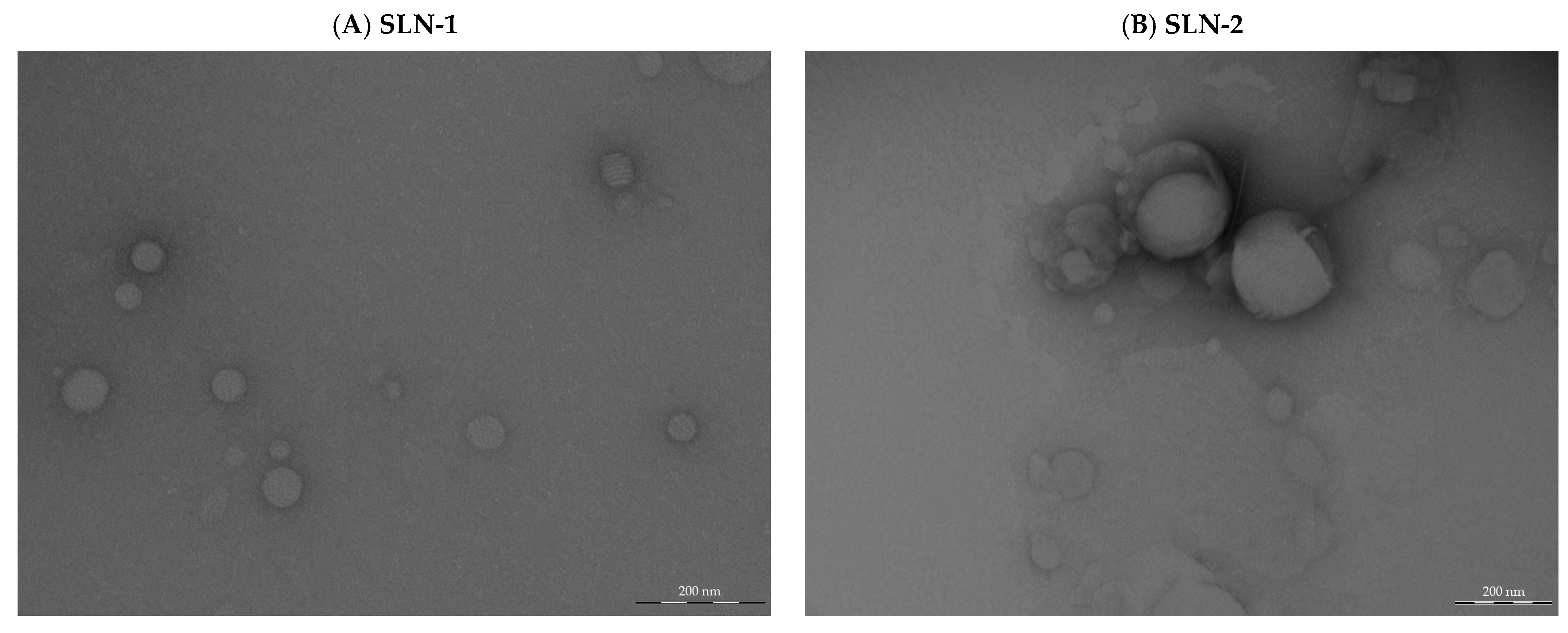
- Morphological analysis by transmission electron microscopy (TEM)
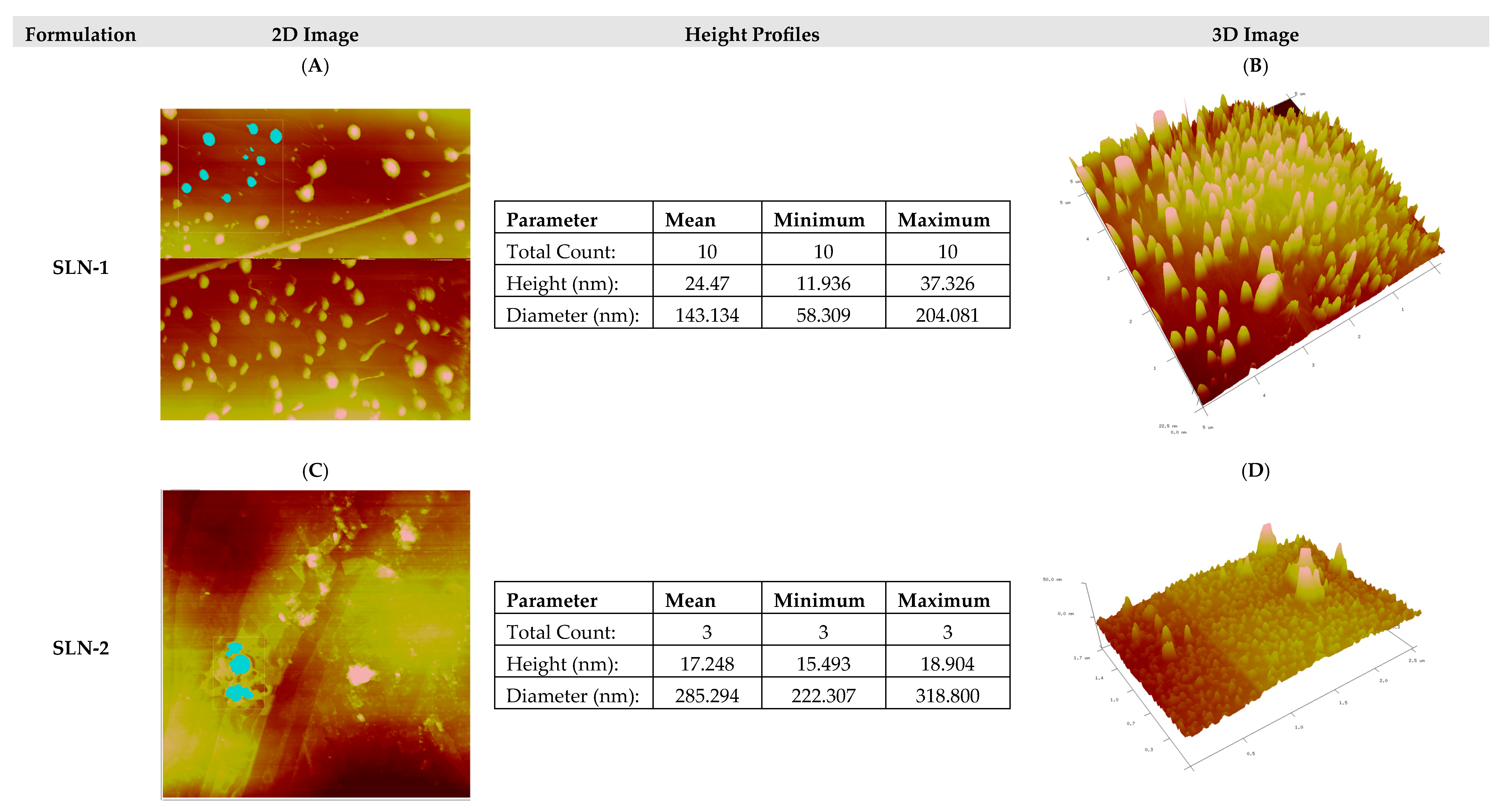
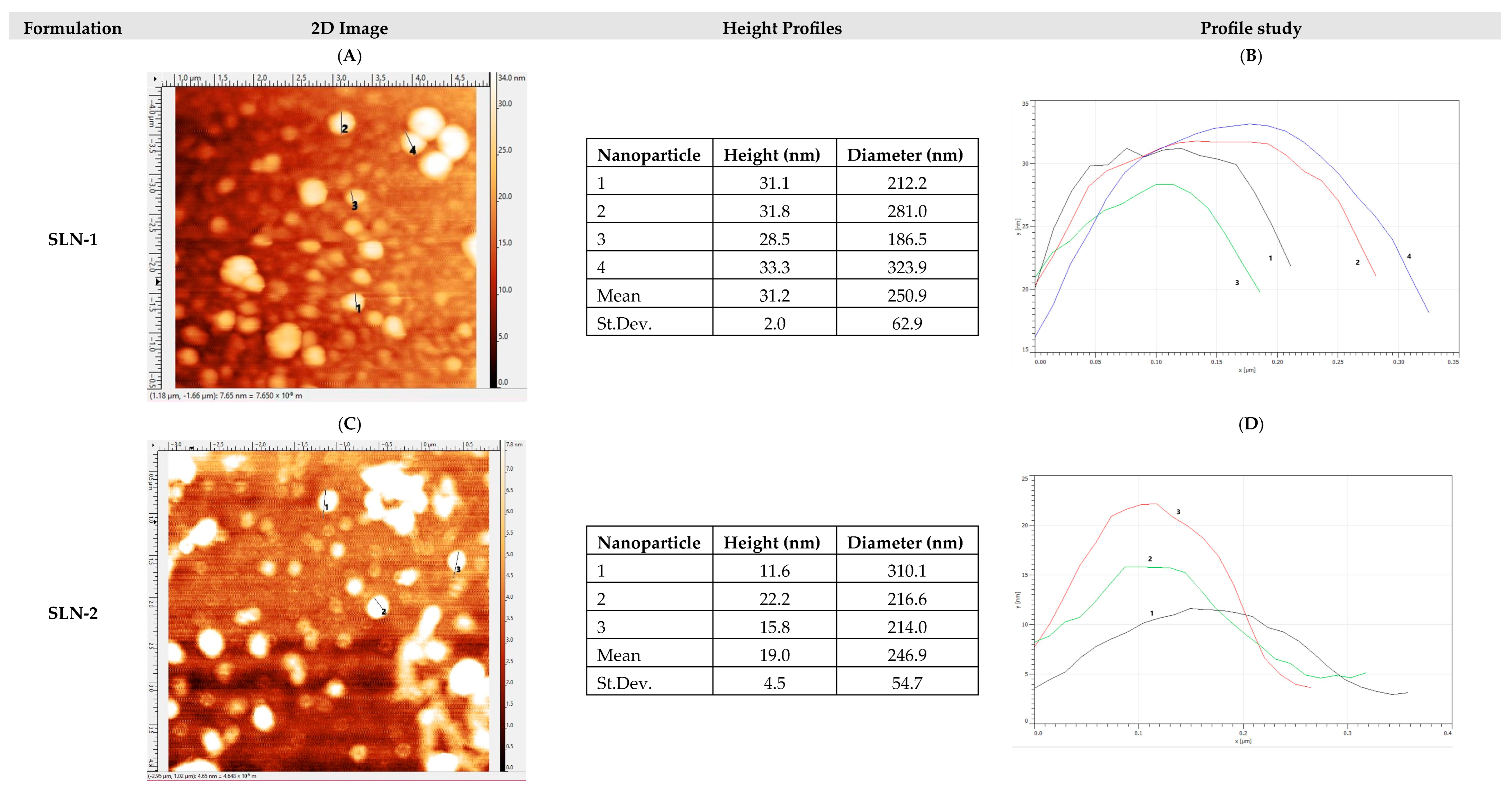
- Morphological analysis by atomic force microscopy (AFM)
- Determination of surface charge (zeta-potential) and particle size
- Differential scanning calorimetry (DSC)
- X-Ray Diffraction
- Entrapment efficiency of CHON (EE%)
- -
- Titration method:
- -
- UV-VIS spectroscopy method:
2.2.3. Stability Studies
3. Results and Discussion
3.1. Characterization of SLN Formulations
- Morphological analysis by transmission electron microscopy (TEM)
- Morphological analysis by atomic force microscopy (AFM)
- Determination of surface charge (zeta-potential) and particle size
- Differential scanning calorimetry (DSC)
- If the thermogram of the mixture is a superposition of the individual thermograms, then there is no interaction, and the species are considered compatible.
- If a new thermal phenomenon appears at a lower temperature than the first thermal phenomenon observed for any of the species separately, then interaction has taken place, and the species are considered incompatible.
- -
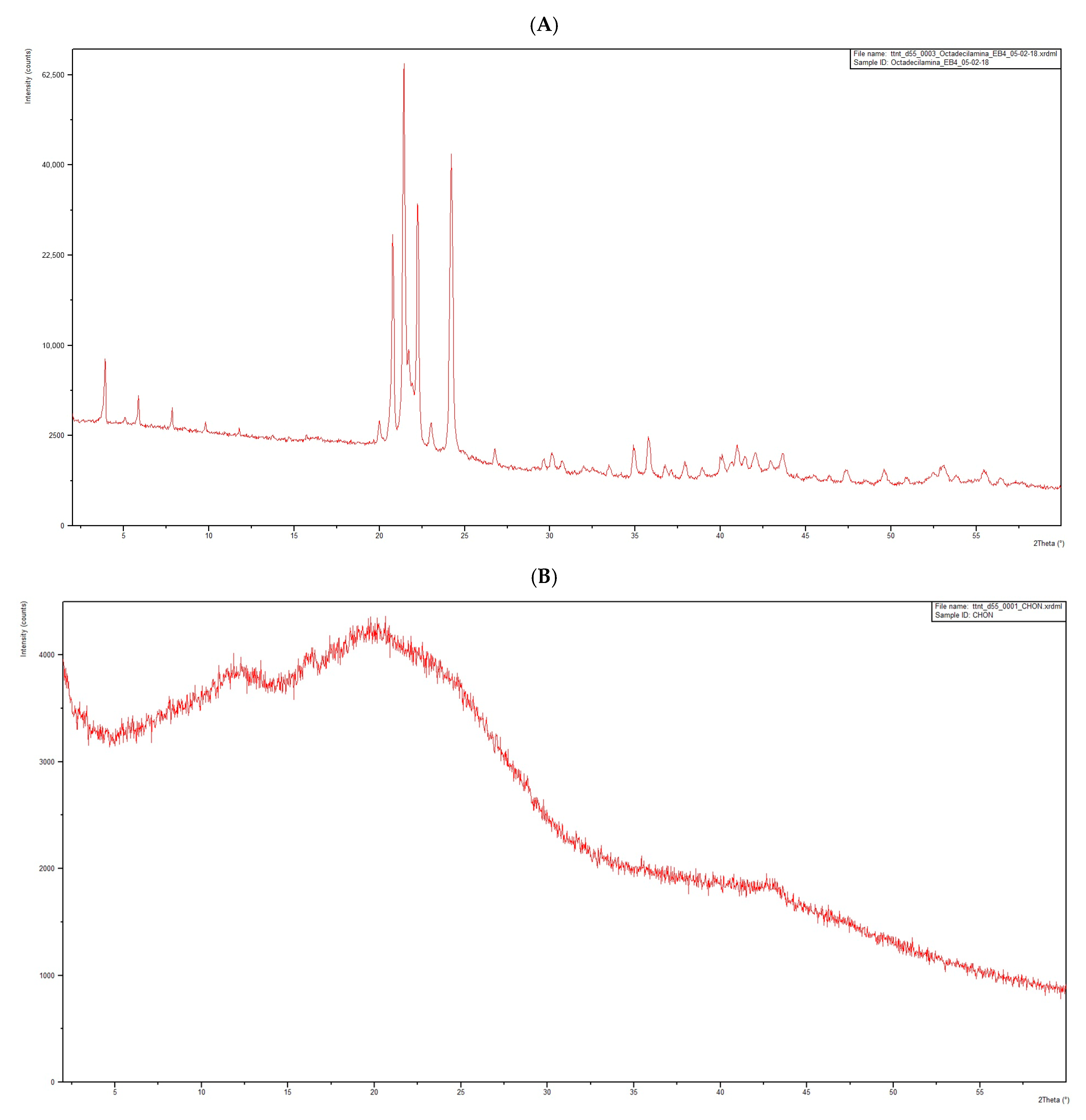
- The DSC thermogram of octadecylamine (Figure 4A) presents an initial endothermic phenomenon starting at 45 °C, with an associated heat of 261.69 J/g, and an exothermic phenomenon starting at 189 °C, with an associated heat of 45.76 J/g, corresponding to the degradation of octadecylamine when subjected to a high temperature.
- -
- The DSC thermogram of poloxamer (Figure 4B) shows an initial endothermic event starting at 45 °C, with an associated heat of 143.53 J/g, and an exothermic event starting at 152 °C, with an associated heat of 56.68 J/g, corresponding to the degradation of poloxamer when it is subjected to a high temperature.
- -
- The DSC thermogram of stearic acid (Figure 4C) shows an initial endothermic event starting at 54 °C, with an associated heat of 199.73 J/g, and an exothermic event starting at 165 °C, with an associated heat of 47.21 J/g, corresponding to the degradation of stearic acid when it is subjected to a high temperature.
- -
- The DSC thermogram of CHON (Figure 4D) shows an initial endothermic event starting at 191 °C, with an associated heat of 143.84 J/g, and an exothermic event starting at 217 °C, with an associated heat of 187.74 J/g, corresponding to the degradation of CHON when it is subjected to a high temperature.
- -
- The DSC thermogram of the mixture of the four substances in the same proportions as required in the manufacture of SLN (Figure 4E) shows an initial endothermic phenomenon starting at 47 °C, with an associated heat of 121.46 J/g, followed by an exothermic event (not evaluated since it is concomitant to the previous endothermic event) and an exothermic phenomenon starting at 191 °C, with an associated heat of 39.76 J/g. Since the percentage of CHON in the formulation mixture is only 5%, its melting phenomenon has not been observed, which, in addition, is also obscured by the superposition of the decomposition of the excipients in the heated mixture. Moreover, the exothermic event, which occurs concomitantly to the melting of the lowest melting excipient (the octadecylamine), can be attributed to a recrystallization event from the partially liquid mixture at around 50 °C. This phenomenon is followed by a broad endothermic event, probably as a consequence of the melting of the remaining solid excipients. Thus, according to the general rules previously described concerning the compatibility of excipients, we cannot discard a potential interaction between excipients upon heating. However, the low proportion of the active compound in the mixture precludes of a definitive conclusion.
- -
- For this reason, the possible interaction between CHON and the main excipient of the mixture, octadecylamine, was further studied (Figure 4F). The thermogram of the binary mixture, CHON–octadecylamine, shows an initial endothermic phenomenon from 44 °C, with an associated heat of 131.17 J/g; a second endothermic phenomenon from 71 °C, with an associated heat of 3.40 J/g; a third endothermic event starting at 148 °C, with an associated heat of 8.36 J/g; and a fourth endothermic event starting at 190 °C, with an associated heat of 94.92 J/g. Interestingly, the main melting events of both compounds appear essentially at the same temperature onsets. Moreover, secondary endothermic thermal events, which we have not studied in detail, can still be observed. Only the decomposition of the mixture is mostly altered. For all the foregoing, it is considered that the interaction is likely to be negligible.
- X-Ray Diffraction
- Entrapment efficiency of CHON (EE%)
- -
- Titration method:
- -
- UV-VIS spectroscopy method:
3.2. Nanoparticles Manufacturing: Lyophilization Process
3.3. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals 2024, 17, 329. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Restaino, O.F.; Vassallo, V.; Cassese, E.; Finamore, R.; Ruosi, C.; Schiraldi, C. Chondroitin Sulfate in USA Dietary Supplements in Comparison to Pharma Grade Products: Analytical Fingerprint and Potential Anti-Inflammatory Effect on Human Osteoartritic Chondrocytes and Synoviocytes. Pharmaceutics 2021, 13, 737. [Google Scholar] [CrossRef]
- Shen, Q.; Guo, Y.; Wang, K.; Zhang, C.; Ma, Y. A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications. Molecules 2023, 28, 7093. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, H.; Dai, H.; Hua, S.; Long, X.; Li, H.; Ivanovski, S.; Xu, C. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio 2023, 19, 100597. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.E.B.; Nardi-Ricart, A.; Capmany, A.C.C.; Carmona, M.M. Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation. Int. J. Mol. Sci. 2024, 25, 10023. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. Saudi Pharm. J. 2017, 25, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Amis, T.M.; Renukuntla, J.; Bolla, P.K.; Clark, B.A. Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles. Pharmaceutics 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian. J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Regdon, G. Thermal Analysis in the Evaluation of Solid Lipid Microparticles in the Form of Aqueous Dispersion and Fine Powder. Appl. Sci. 2023, 13, 13282. [Google Scholar] [CrossRef]
- Gatto, M.S.; Najahi-Missaoui, W. Lyophilization of Nanoparticles, Does It Really Work? Overview of the Current Status and Challenges. Int. J. Mol. Sci. 2023, 24, 14041. [Google Scholar] [CrossRef] [PubMed]
- Pisano, R.; Fissore, D. New Trends in Freeze-Drying of Pharmaceutical Products. Pharmaceutics 2023, 15, 1975. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Deshmukh, N.S.; Telange, D.R.; Nangare, S.N.; Sonar, Y.Y.; Lakade, S.H.; Harde, M.T.; Pardeshi, C.V.; Gholap, A.; Deshmukh, P.K.; et al. Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: A state-of-the-art review. Futur. J. Pharm. Sci. 2023, 9, 99. [Google Scholar] [CrossRef]
- Bukke, S.P.N.; Venkatesh, C.; Rajanna, S.B.; Saraswathi, T.S.; Kusuma, P.K.; Goruntla, N.; Balasuramanyam, N.; Munishamireddy, S. Solid lipid nanocarriers for drug delivery: Design innovations and characterization strategies—A comprehensive review. Discov. Appl. Sci. 2024, 6, 279. [Google Scholar] [CrossRef]
- Makoni, P.A.; Kasongo, K.W.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef]
- Ball, R.; Bajaj, P.; Whitehead, K. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Guideline Q1A(R2): Stability Testing of New Drug Substances and Products–Scientific Guideline [Internet]. 2003. Available online: https://www.ich.org/page/quality-guidelines (accessed on 1 September 2022).
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Jain, K. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert. Rev. Mol. Diagn. 2003, 3, 153–161. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Targeted Drug Delivery in Cancer Therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef]
- Rehan, F.; Zhang, M.; Fang, J.; Greish, K. Therapeutic Applications of Nanomedicine: Recent Developments and Future Perspectives. Molecules 2024, 29, 2073. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.H.; van de Ven, A.L.; Godin, B.; Blanco, E.; Serda, R.E.; Grattoni, A.; Ziemys, A.; Bouamrani, A.; Hu, T.; Ranganathan, S.I.; et al. Enabling individualized therapy through nanotechnology. Pharmacol. Res. 2010, 62, 57–89. [Google Scholar] [CrossRef]
- Fàbregas, A.; Sánchez-Hernández, N.; Ticó, J.R.; García-Montoya, E.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Hernández-Munain, C.; Suñé, C.; Miñarro, M. A new optimized formulation of cationic solid lipid nanoparticles intended for gene delivery: Development, characterization and DNA binding efficiency of TCERG1 expression plasmid. Int. J. Pharm. 2014, 473, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia, E. European Directorate for the Quality of Medicines; EDQM Council of Europe: Strasbourg, France, 2017; Volume 01/2017. [Google Scholar]
- United States Pharmacopeia. Monograph for Chondroitin Sulfate Sodium. In USP 38–NF 33: The United States Pharmacopeia and National Formulary, 38th ed.; Pharmacopeial Convention: Rockville, MD, USA, 2015; p. 5970. [Google Scholar]
- Kosakai, M.; Yosizawa, Z. Study on the Factors Yielding High Color in the Carbazole Reaction with Hexuronic Acid-Containing Substances1. J. Biochem. 1978, 84, 779–785. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Somashekar, P.; Sathish, K.; Javali, C. Development and Validation of Spectrophotometric Method for the Estimation of Chondroitin Sulfate in Bulk Drug and Pharmaceutical Formulations. Chem. Sci. Trans. 2013, 2, 1427–1433. [Google Scholar] [CrossRef][Green Version]
- Myburgh, J.; Liebenberg, W.; Willers, C.; Dube, A.; Gerber, M. Investigation and Evaluation of the Transdermal Delivery of Ibuprofen in Various Characterized Nano-Drug Delivery Systems. Pharmaceutics 2023, 15, 2413. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]
- Sikora, A.; Bartczak, D.; Geißler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef]
- Craig, D.Q.M.; Reading, M. Thermal Analysis of Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar]
- Ford, J.L.; Timmins, P. Application of thermal analysis to compatibility studies for solid dosage forms. In Pharmaceutical Thermal Analysis; Rubinstein, M.H., Ed.; Ellis Horwood: Chichester, UK, 1989; pp. 238–247. [Google Scholar]
- Balestrieri, F.; Magri, A.D.; Magri, A.L.; Marini, D.; Sacchini, A. Application of differential scanning calorimetry to the study of drug-excipient compatibility. Termochimica Acta 1996, 285, 337–345. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Manderioli, A.; Furlanetto, S.; Pinzauti, S. Thermal Analysis as a Screening Technique in Preformulation Studies of Picotamide Solid Dosage Forms. Drug Dev. Ind. Pharm. 1998, 24, 747–756. [Google Scholar] [CrossRef]
- Feist, M. Thermal analysis: Basics, applications, and benefit. ChemTexts 2015, 1, 8. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1) [Internet]. 2005. Available online: https://somatek.com/wp-content/uploads/2014/06/sk140605h.pdf (accessed on 10 September 2022).
- USP. Chapter 1225: Validation of Compendial Procedures. In USP 40/NF 35; United States Pharmacopeia, 40th ed.; National Formulary, 35th ed.; The United States Pharmacopeia Convention; United Book Press, Inc.: Rockville, MD, USA, 2017. [Google Scholar]
- Jenkins, D.; Diallo, C.; Bethea, E.; Kaale, E.; Layloff, T. Method Validation Approaches for Pharmaceutical Assessments—Highlights with High Performance Thin Layer Chromatographic (HPTLC) Techniques. In Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen: London, UK, 2018. [Google Scholar]
- AOAC International Officers and Committees. J. AOAC Int. 1993, 76, 223–250. [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of Membranes in Anhydrobiotic Organisms: The Role of Trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, J.K.; Bhat, R. Why is Trehalose an Exceptional Protein Stabilizer? An Analysis of the Thermal Stability of Proteins in the Presence of the Compatible Osmolyte Trehalose. J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Hu, M.; Sun, Y.; Mao, B.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Effect of Trehalose and Lactose Treatments on the Freeze-Drying Resistance of Lactic Acid Bacteria in High-Density Culture. Microorganisms 2023, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, A.; Prieto-Sánchez, S.; Suñé-Pou, M.; Boyero-Corral, S.; Ticó, J.R.; García-Montoya, E.; Pérez-Lozano, P.; Miñarro, M.; Suñé-Negre, J.M.; Hernández-Munain, C.; et al. Improved formulation of cationic solid lipid nanoparticles displays cellular uptake and biological activity of nucleic acids. Int. J. Pharm. 2017, 516, 39–44. [Google Scholar] [CrossRef]
- Kamiya, M.; Matsumoto, M.; Yamashita, K.; Izumi, T.; Kawaguchi, M.; Mizukami, S.; Tsurumaru, M.; Mukai, H.; Kawakami, S. Stability Study of mRNA-Lipid Nanoparticles Exposed to Various Conditions Based on the Evaluation be-tween Physicochemical Properties and Their Relation with Protein Expression Ability. Pharmaceutics 2022, 14, 2357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehman, S.E.; Benkstein, K.D.; Cleveland, T.E.; Anderson, K.W.; Carrier, M.J.; Vreeland, W.N. Particle Me-trology Approach to Understanding How Storage Condition Affects Long-Term Liposome Stability. Langmuir 2023, 39, 12313–12323. [Google Scholar] [CrossRef]


| Composition (%) | Formulation | |
|---|---|---|
| SLN-1 | SLN-2 | |
| CHON | 5 | 5 |
| Stearic acid | 40 | 16 |
| Cholesteryl oleate | - | 24 |
| Octadecylamine | 48 | 48 |
| Poloxamer 188 | 8 | 8 |
| Total (%) | 100 | 100 |
| Formulation | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| SLN-1 | 121 ± 8 | −41.8 ± 4.3 |
| SLN-2 | 121 ± 6 | −41.9 ± 2.5 |
| Sample | SLN-CHON + NaOH (%) |
|---|---|
| 1 | 93.22 |
| 2 | 97.53 |
| 3 | 94.93 |
| 4 | 97.37 |
| 5 | 86.17 |
| Mean ± SEM | 93.84 ± 2.079 N = 5 |
| Sample | SLN-1 | SLN-2 |
|---|---|---|
| 1 | 77.6 | 88.1 |
| 2 | 76.4 | 86.2 |
| 3 | 78.8 | 81.4 |
| 4 | 83.4 | 82.3 |
| 5 | 80.2 | 87.0 |
| 6 | 82.5 | 87.2 |
| 7 | 74.9 | 84.8 |
| 8 | 83.7 | 82.1 |
| 9 | 83.7 | 98.3 |
| 10 | 83.1 | 81.6 |
| 11 | - | 91.5 |
| 12 | - | 93.6 |
| 13 | - | 89.1 |
| Mean (%) | 80.4 | 87.2 |
| Standard deviation | 3.3 | 5.1 |
| Standard error of the mean | 1.1 | 1.4 |
| Cryoprotectants | Concentration | D (0.5) (nm) | Z-Potential (mV) | Macroscopic Aspect | Reconstitution |
|---|---|---|---|---|---|
| Povidone K30 | 5% | 668.474 | −5.17 | Collapsed yellowish | No aggregates; long reconstitution time |
| 251.000 | −4.63 | ||||
| 202.000 | −5.42 | ||||
| Povidone K30 | 10% | 1005.841 | −6.51 | Collapsed yellowish | No aggregates; long reconstitution time |
| 225.000 | −6.23 | ||||
| 175.000 | −6.85 | ||||
| Glycine | 5% | 97.694 | −45.0 | Correct and compact; the cake broke over time | Microscopic aggregates |
| 103.884 | −51.8 | ||||
| 116.957 | −46.3 | ||||
| Glycine | 10% | 104.384 | −56.4 | Correct and compact; the cake broke over time | Microscopic aggregates |
| 115.373 | −51.6 | ||||
| 142.348 | −57.8 | ||||
| Glycine | 5% | 72.297 | −2.85 | Correct and compact; the cake broke over time | Phase separation and aggregates |
| NaH2PO3 | 3.12% | 50.871 | −4.01 | ||
| Na2HPO3 | 3.56% | 49.355 | −4.16 | ||
| Glycine | 10% | 158.894 | −1.71 | Correct and compact; the cake broke over time. | Phase separation and aggregates |
| NaH2PO3 | 3.12% | 50.667 | −2.20 | ||
| Na2HPO3 | 3.56% | 50.587 | −3.06 | ||
| Mannitol | 5% | 123.497 | −38.1 | Unacceptable, brittle cake | Macroscopic aggregates |
| 115.142 | −37.0 | ||||
| 112.261 | −37.3 | ||||
| Mannitol | 10% | 127.939 | −43.6 | Unacceptable, brittle cake | Macroscopic aggregates |
| 107.501 | −41.8 | ||||
| 101.757 | −46.6 | ||||
| Trehalose Panreac® | 5% | 65.158 | −47.3 | Correct | No aggregates |
| 60.460 | −48.4 | ||||
| 58.672 | −49.1 | ||||
| Trehalose Panreac® | 10% | 68.672 | −30.5 | Correct | No aggregates |
| 61.978 | −28.6 | ||||
| 63.682 | −31.3 | ||||
| Trehalose Cerestar® | 5% | 59.561 | −38.4 | Correct | No aggregates |
| 63.408 | −39.6 | ||||
| 54.429 | −42.8 | ||||
| Trehalose Cerestar® | 10% | 84.113 | −38.9 | Correct | No aggregates |
| 69.195 | −44.7 | ||||
| 69.403 | −44.6 |
| SLN-1 | ||||||||||||||||||
| Day | Temperature 4 °C | 25 °C/60% RH | 40 °C/75% RH | |||||||||||||||
| Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | |
| 0 | 114 | 1.1 | −40.0 | 0.5 | 79.9 | 0.5 | 114 | 1.1 | −40.0 | 0.5 | 79.9 | 0.5 | 114 | 1.1 | −40.0 | 0.5 | 79.9 | 0.5 |
| 1 | 138 | 2.3 | −45.0 | 0.3 | 79.8 | 0.4 | 271 | 5.0 | −45.3 | 0.7 | 79.7 | 0.6 | 123 | 2.0 | −40.1 | 0.3 | 79.8 | 0.6 |
| 2 | 126 | 4.4 | −46.2 | 0.6 | 79.9 | 0.4 | 274 | 22.3 | −46.3 | 0.2 | 79.8 | 0.4 | 149 | 6.1 | −40.3 | 0.5 | 79.9 | 0.4 |
| 3 | 163 | 2.4 | −46.2 | 0.8 | 79.8 | 0.6 | 663 | 32.6 | −47.6 | 0.9 | ND | ND | 191 | 63.5 | −41.2 | 0.4 | 79.9 | 0.5 |
| 6 | 209 | 6.1 | −49.1 | 0.9 | 79.8 | 0.5 | 39,000 | 336.5 | −45.5 | 0.2 | ND | ND | 229 | 58.7 | −42.8 | 1.1 | 79.8 | 0.5 |
| 7 | 616 | 28.5 | −46.5 | 0.5 | ND | ND | 43,000 | 3265.4 | −44.4 | 0.3 | ND | ND | 278 | 31.1 | −44.8 | 0.9 | 79.8 | 0.4 |
| 9 * | 1335 | 216.6 | −45.9 | 1.0 | ND | ND | 47,000 | 3413.8 | −45.6 | 0.5 | ND | ND | 336 | 35.9 | −44.6 | 0.6 | 79.8 | 0.6 |
| SLN-2 | ||||||||||||||||||
| Day | Temperature 4 °C | 25 °C/60% RH | 40 °C/75% RH | |||||||||||||||
| Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | Size (nm) D [3.2] | SD | Pot Z | SD | % EE | SD | |
| 0 | 124 | 1.5 | −46.9 | 0.5 | 88.9 | 0.5 | 124 | 1.5 | −46.9 | 0.5 | 88.9 | 0.5 | 124 | 1.5 | −46.9 | 0.5 | 88.9 | 0.5 |
| 3 | 129 | 2.9 | −50.0 | 0.2 | 88.7 | 0.4 | 118 | 0.6 | −49.8 | 0.5 | 89.0 | 0.5 | 128 | 12.7 | −51.3 | 0.3 | 88.7 | 0.6 |
| 7 | 129 | 2.8 | −48.1 | 1.4 | 88.8 | 0.5 | 125 | 3.2 | −51.6 | 1.0 | 88.9 | 0.5 | 125 | 3.2 | −48.4 | 0.2 | 88.8 | 0.6 |
| 14 | 124 | 2.6 | −50.8 | 0.2 | 88.7 | 0.6 | 127 | 2.3 | −47.6 | 1.7 | 88.7 | 0.6 | 131 | 10.4 | −40.4 | 1.0 | 88.6 | 0.5 |
| 21 | 143 | 1.7 | −26.9 | 0.4 | 88.6 | 0.6 | 130 | 3.1 | −47.6 | 0.7 | 88.8 | 0.4 | 123 | 2.9 | −45.4 | 1.0 | 88.7 | 0.5 |
| 23 | 128 | 2.0 | −31.4 | 0.7 | 88.7 | 0.5 | 130 | 3.5 | −25.8 | 0.9 | 88.9 | 0.4 | 162 | 21.2 | −48.5 | 1.2 | 88.7 | 0.5 |
| 28 | 213 | 4.5 | −48.1 | 0.8 | 88.9 | 0.5 | 128 | 1.7 | −42.4 | 0.7 | 88.8 | 0.5 | 139 | 1.0 | −47.1 | 1.2 | 88.8 | 0.4 |
| SLN-1 | ||||||
|---|---|---|---|---|---|---|
| Date/Condition | Size (nm) | SD | Pot Z | SD | EE% | SD |
| Day 1_aquous medium | 114 | 1.1 | −40 | 0.5 | 79.9 | 0.5 |
| Day 1_Freeze-dried | 183 | 43.3 | −39.3 | 1.2 | 79.8 | 0.6 |
| Month 6_Freeze-dried | 164 | 24.7 | −43.8 | 1.7 | 79.7 | 0.4 |
| Month 8_Freeze-dried | 391.2 | 4.9 | −32.55 | 1.08 | 79.6 | 0.4 |
| SLN-2 | ||||||
| Date/Condition | Size (nm) | SD | Pot Z | SD | EE% | SD |
| Day 1_aquous medium | 124 | 1.5 | −46.9 | 0.5 | 88.9 | 0.5 |
| Day 1_Freeze-dried | 137 | 4.2 | −33.2 | 0.6 | 88.7 | 0.5 |
| Month 6_Freeze-dried | 179 | 9.3 | −25.8 | 0.7 | 88.7 | 0.5 |
| Month 8_Freeze-dried | 400.7 | 4.9 | −22.76 | 1.29 | 88.6 | 0.4 |
| Sample | Time | Mean (nm) | D90 (nm) | Concentration (Particle/mL) |
|---|---|---|---|---|
| SLN-1 | Month 4 | 149.2 ± 4.5 | 215.3 ± 12.8 | 1.55 × 1011 ± 1.40 × 1010 |
| Month 11 | 152.7 ± 2.0 | 267.1 ± 9.0 | 8.87 × 109 ± 1.41 × 108 | |
| SLN-2 | Month 4 | 235.0 ± 3.4 | 319.9 ± 12.1 | 4.30 × 1011 ± 1.06 × 1010 |
| Month 11 | 276.4 ± 5.9 | 377.6 ± 18.2 | 1.59 × 1011 ± 7.18 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos Araya, M.E.; Ricart, A.N.; Calpena Campmany, A.C.; Prohens, R.; Miñarro Carmona, M. Development and Characterization of Lyophilized Chondroitin Sulfate-Loaded Solid Lipid Nanoparticles: Encapsulation Efficiency and Stability. Pharmaceutics 2025, 17, 86. https://doi.org/10.3390/pharmaceutics17010086
Bustos Araya ME, Ricart AN, Calpena Campmany AC, Prohens R, Miñarro Carmona M. Development and Characterization of Lyophilized Chondroitin Sulfate-Loaded Solid Lipid Nanoparticles: Encapsulation Efficiency and Stability. Pharmaceutics. 2025; 17(1):86. https://doi.org/10.3390/pharmaceutics17010086
Chicago/Turabian StyleBustos Araya, Marta E., Anna Nardi Ricart, Ana C. Calpena Campmany, Rafel Prohens, and Montserrat Miñarro Carmona. 2025. "Development and Characterization of Lyophilized Chondroitin Sulfate-Loaded Solid Lipid Nanoparticles: Encapsulation Efficiency and Stability" Pharmaceutics 17, no. 1: 86. https://doi.org/10.3390/pharmaceutics17010086
APA StyleBustos Araya, M. E., Ricart, A. N., Calpena Campmany, A. C., Prohens, R., & Miñarro Carmona, M. (2025). Development and Characterization of Lyophilized Chondroitin Sulfate-Loaded Solid Lipid Nanoparticles: Encapsulation Efficiency and Stability. Pharmaceutics, 17(1), 86. https://doi.org/10.3390/pharmaceutics17010086








