Enhancing the Therapeutic Effect and Bioavailability of Irradiated Silver Nanoparticle-Capped Chitosan-Coated Rosuvastatin Calcium Nanovesicles for the Treatment of Liver Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of RC-Loaded Nanovesicles (RC. NVs)
2.4. Characterization of RC. NVs
2.4.1. EE%
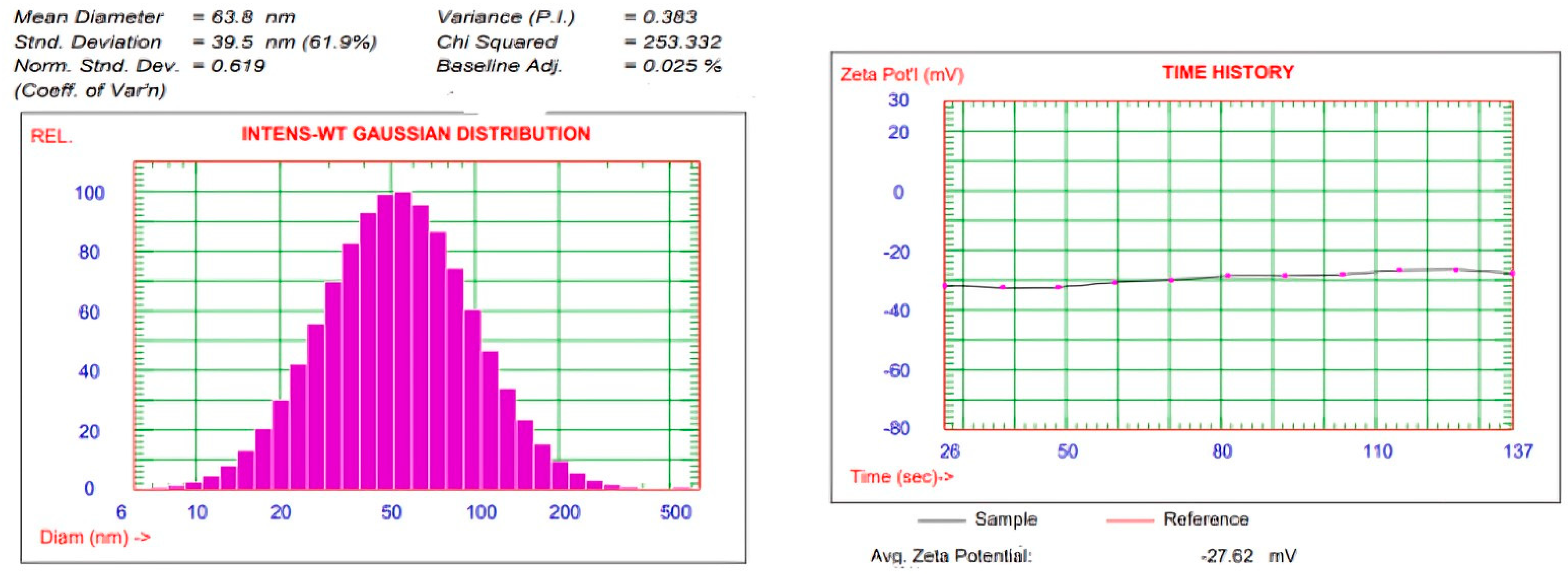
2.4.2. Evaluation of VS, PDI, and ZP
2.4.3. Release Study of RC from Designed Formulations (Q12)
2.4.4. Drug Release Kinetics
2.5. Selection of Nop
2.6. Preparation of CS.Nop
2.7. Characterization of CS.Nop
2.7.1. Transmission Electron Microscopy (TEM)
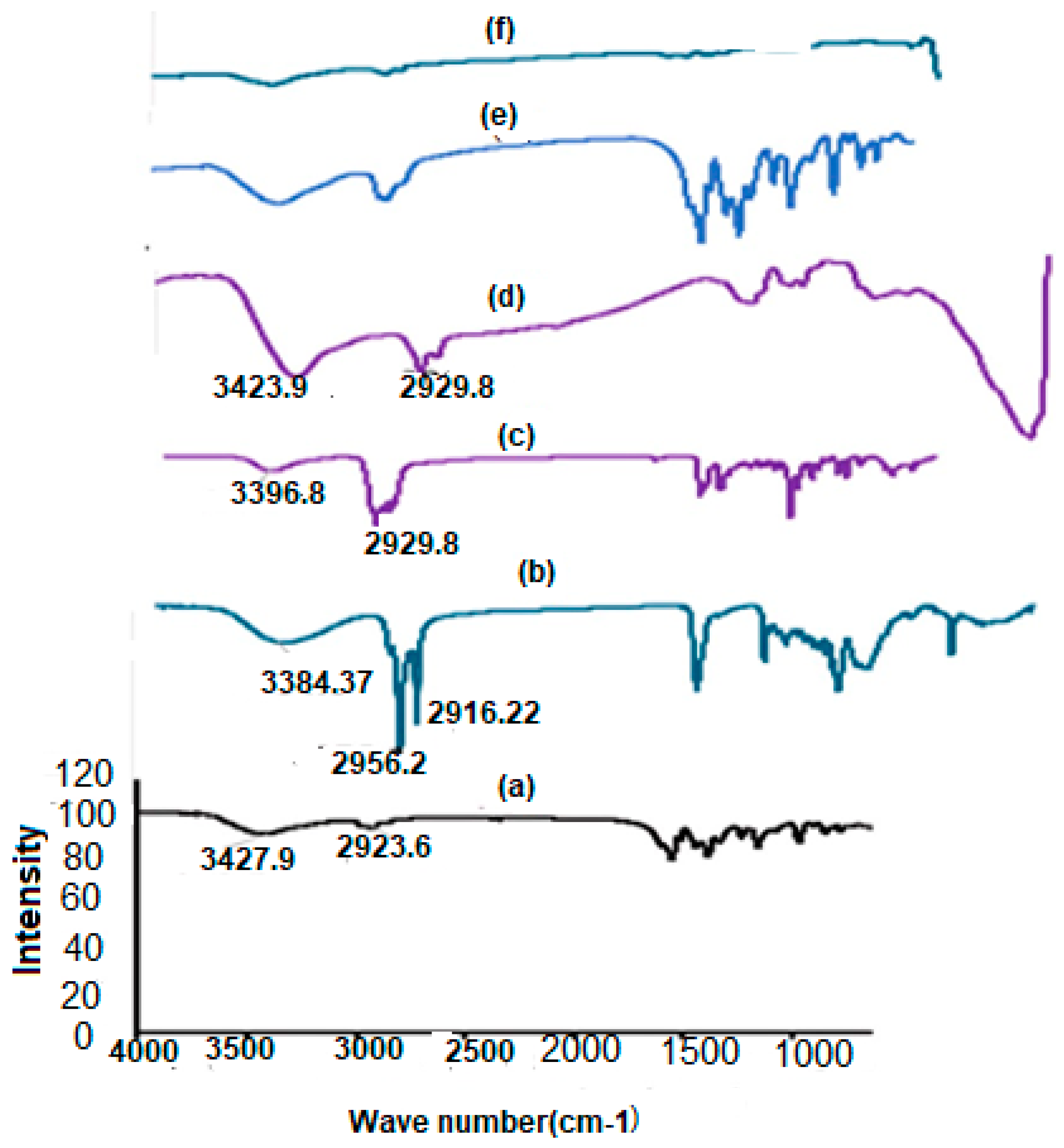
2.7.2. FTIR Analysis
2.7.3. DSC Analysis
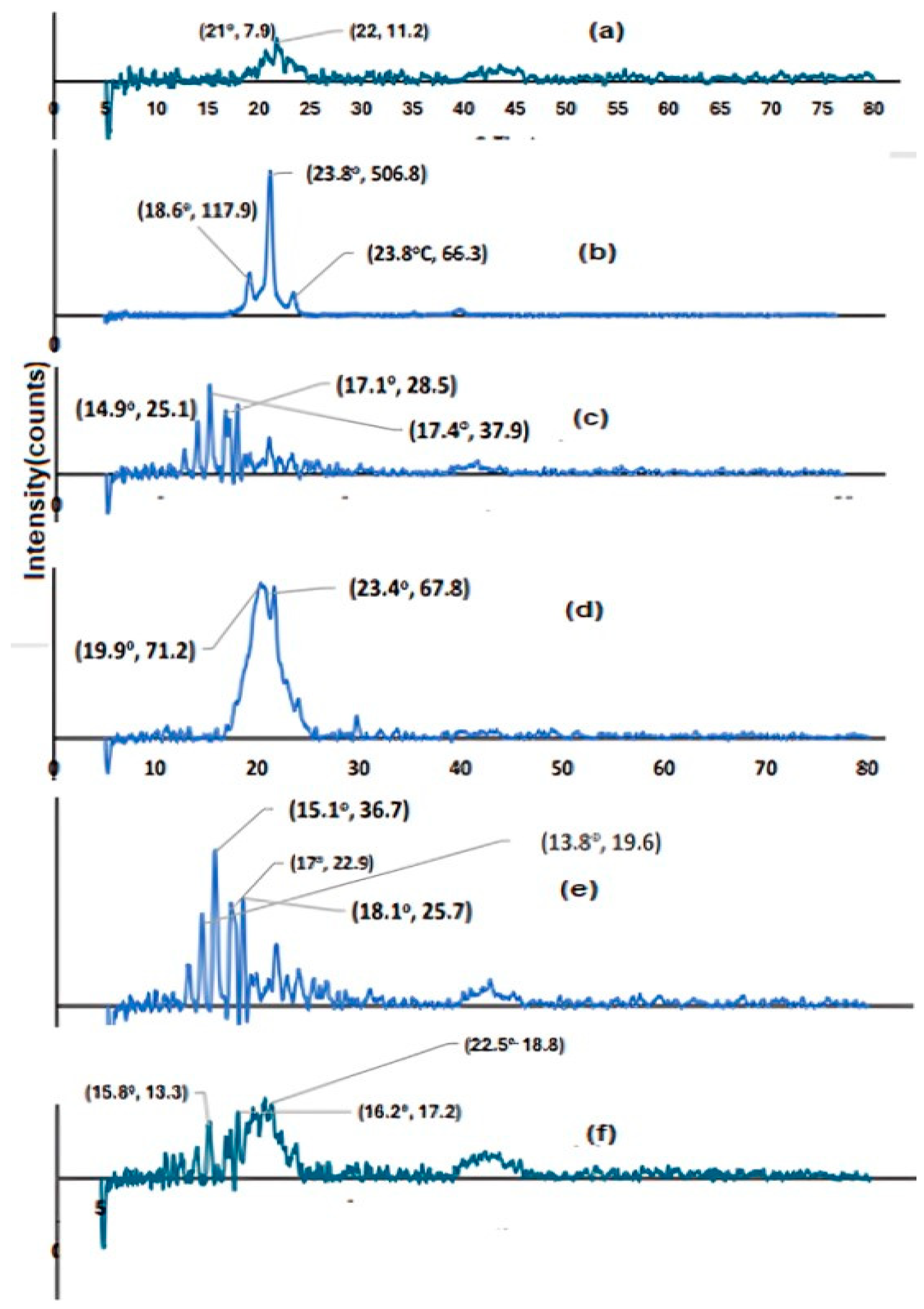
2.7.4. XRPD Analysis
2.7.5. Mucoadhesive Strength
2.7.6. In Vitro Drug Release
2.7.7. Drug Release Kinetics
2.7.8. Physical Stability Study
2.8. Synthesis of Ag NPs
Characterization of AgNPs
2.9. Preparation of Nop. AgNPs and CS.Nop. AgNPs
2.10. Preparation of Irradiated Formulations
2.11. Cytotoxicity Study
- Control 1 (Negative Control): This contained neither nanovesicles (NVs) nor silver nanoparticles (AgNPs), but rather the same solvent that was used to dissolve the active substances (such as ethanol, PBS, or water). This made sure that any biological effects that were seen were not caused by the solvent itself.
- Control 2–6: These controls comprised 10% fetal calf serum in RPMI-1640 medium with the same amounts of vehicle solvents (such as ethanol and PBS) used to dissolve the test chemicals as in the experimental wells. These controls made sure that any cytotoxicity was attributable to the drug or formulation and helped assess any possible effects from the medium or vehicle alone.
2.12. In Vivo Study
2.13. In Vivo–In Vitro Correlation
3. Results and Discussion
3.1. Experimental Design
3.2. Characterization of RC.NVs
3.2.1. Drug Entrapment Efficiency % (EE%)
3.2.2. Measurements of VS, PDI, and ZP
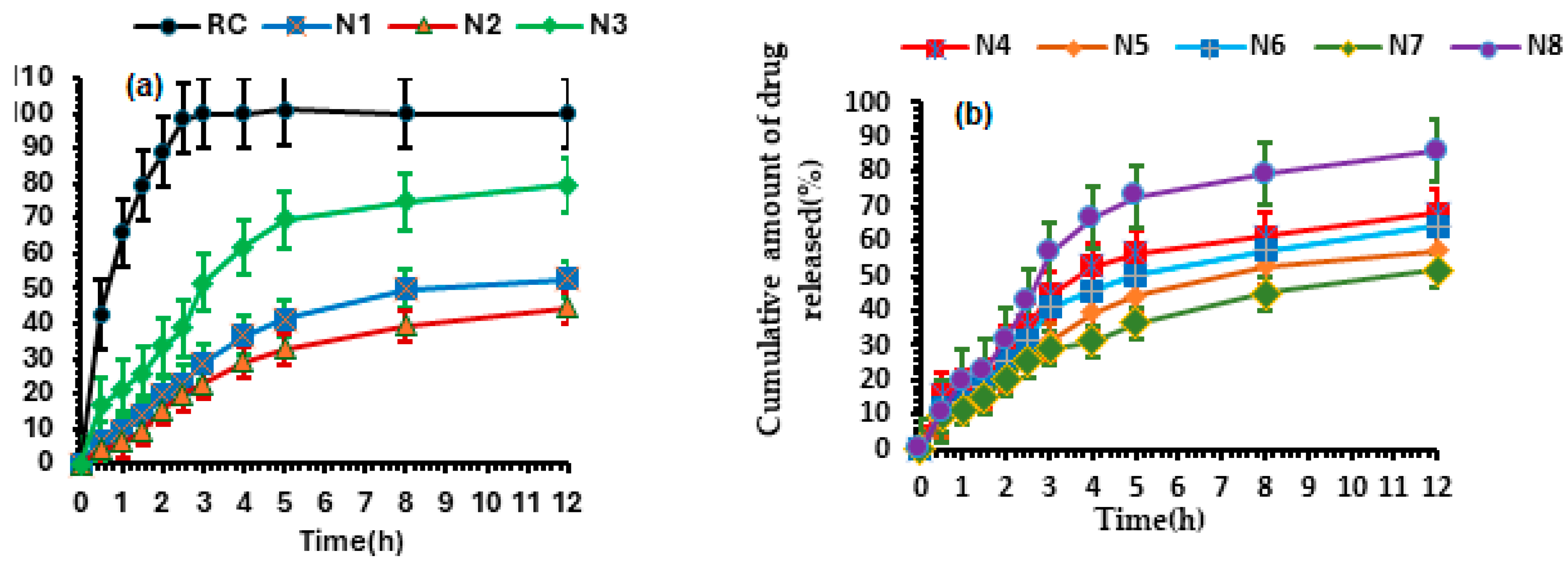
3.2.3. Release Study of Designed Formulations
3.2.4. Kinetics of Release Study
3.2.5. Determination of the Nop
3.3. Characterization of Nop and CS.Nop
3.3.1. Transmission Electron Microscopy (TEM)
3.3.2. FTIR Analysis
3.3.3. Differential Scanning Calorimetry (DSC)
3.3.4. XRPD
3.3.5. In Vitro Drug Release from CS.Nop at Different pH
3.3.6. Kinetics of Drug Release
- Mt is the amount of drug released at time t;
- M∞ is the total amount of drug to be release;
- k is the release rate constant;
- n is the release exponent, which helps determine the mechanism of drug release.
3.3.7. Mucoadhesive Strength
3.3.8. Physical Stability Study
3.4. Characterization of AgNPs
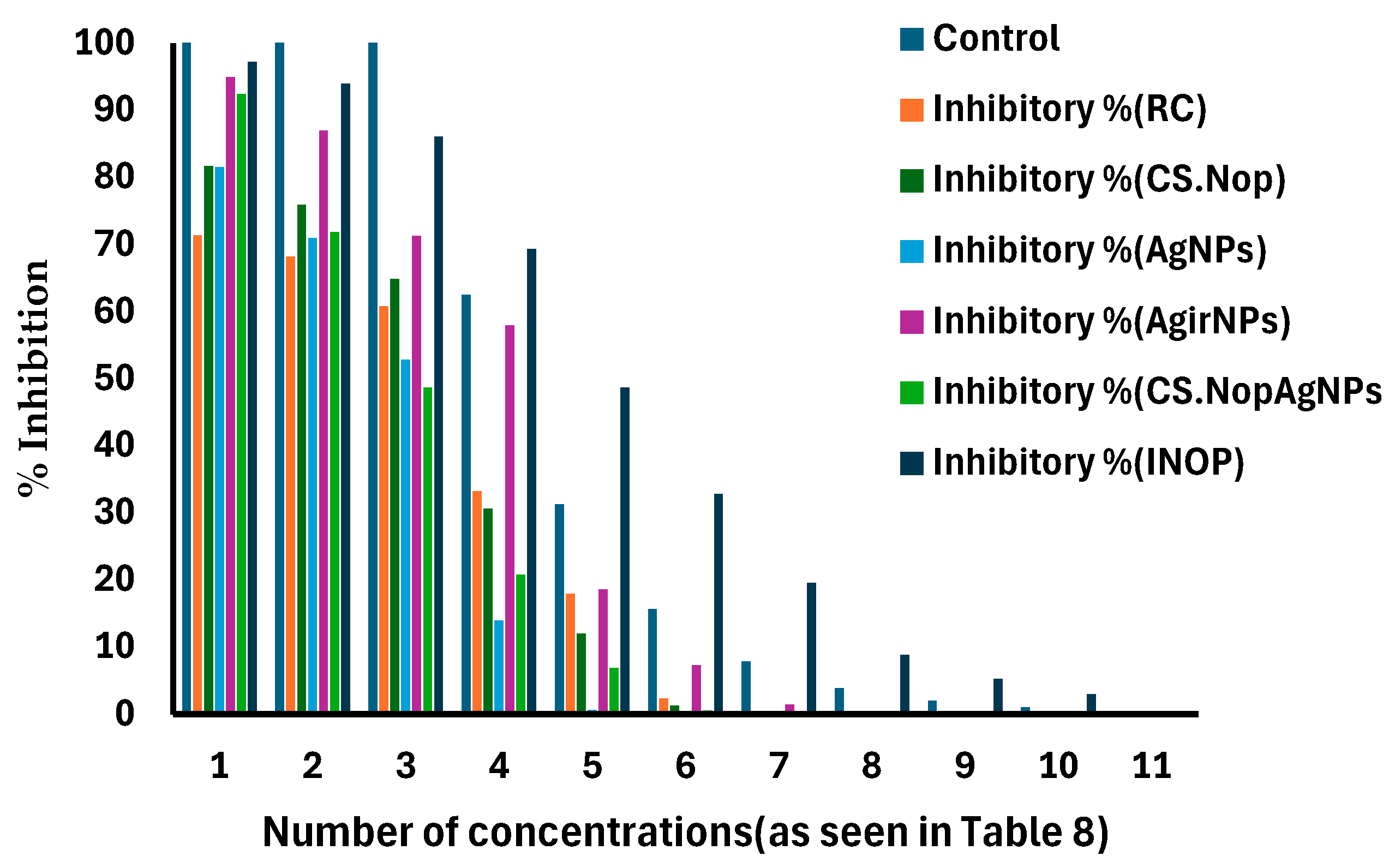
3.5. Cytotoxicity Study
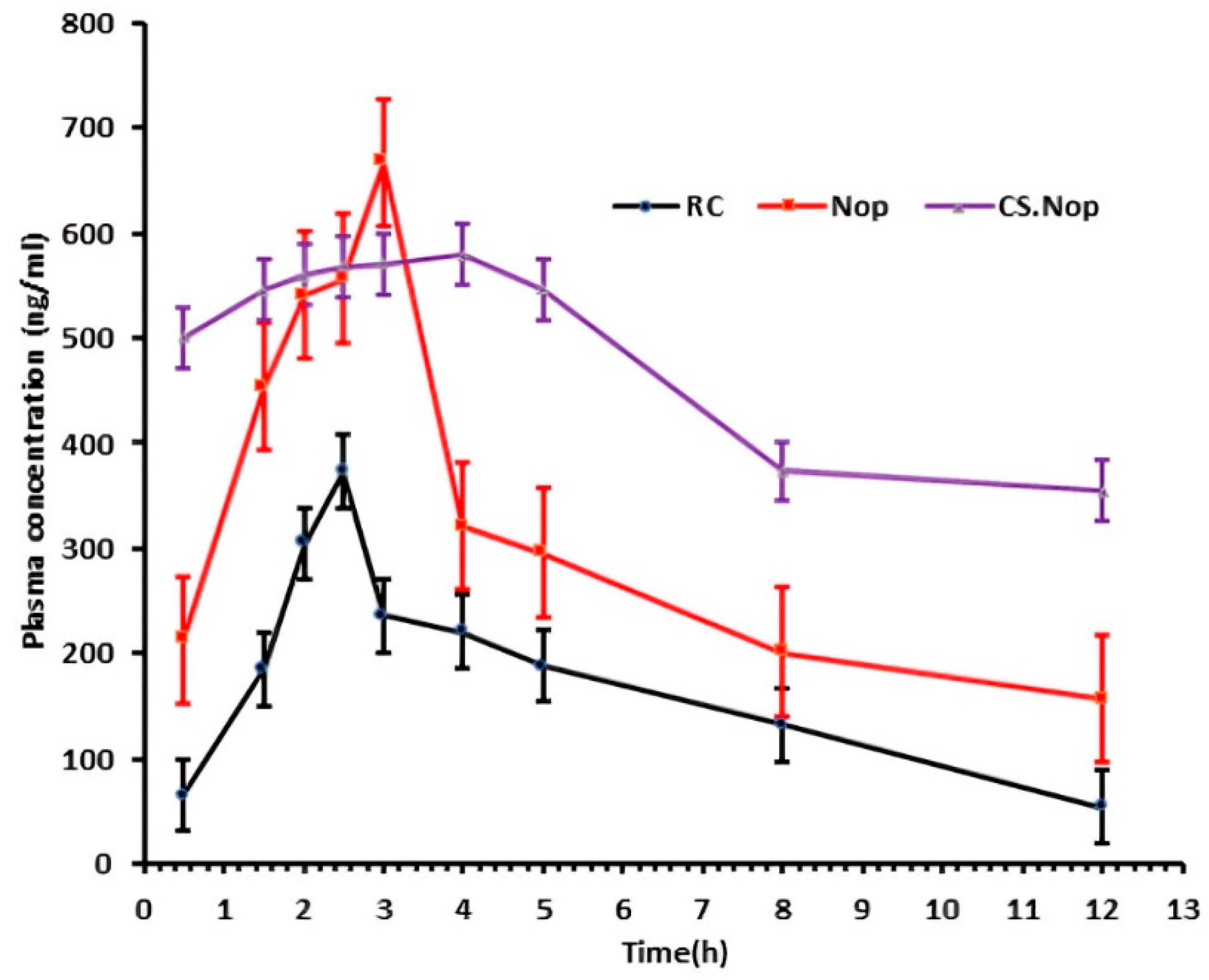
3.6. In Vivo Pharmacokinetic Study
3.7. In Vitro–In Vivo Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdoodt, F.; Hansen, M.K.; Kjaer, S.K.; Pottegård, A.; Friis, S.; Dehlendorff, C. Statin use and mortality among ovarian cancer patients: A population-based cohort study. Int. J. Cancer 2017, 141, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Doycheva, I.; Issa, D.; Watt, K.D.; Lopez, R.; Rifai, G.; Alkhouri, N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J. Clin. Gastroenterol. 2018, 52, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ren, X.; Zhang, B.; Lan, T.; Liu, B. A Systematic Review of Statins for the Treatment of Nonalcoholic Steatohepatitis: Safety, Efficacy, and Mechanism of Action. Molecules 2024, 29, 1859. [Google Scholar] [CrossRef]
- Ho, A.; Kiener, T.; Nguyen, Q.-N.; Le, Q.A. Effect of statin use on liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease (NAFLD). J. Clin. Lipidol. 2024, 18, 501. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Mortaja, M.; Son, H.G.; Zhao, X.; Sloat, L.M.; Azin, M.; Wang, J.; Collier, M.R.; Tummala, K.S.; Mandinova, A.; et al. Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression. Nat. Commun. 2024, 15, 4099. [Google Scholar] [CrossRef]
- Dispinzieri, G.; Becchetti, C.; Mazzarelli, C.; Airoldi, A.; Aprile, F.; Cesarini, L.; Cucco, M.; Perricone, G.; Stigliano, R.; Vangeli, M.; et al. Statin therapy: Improving survival in patients with hepatocellular carcinoma and portal hypertension is possible. Acta Gastro Enterol. Belg. 2024, 87, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Kim, J.; Cheong, J.Y.; Kim, S.S.; Lim, S.G.; Yang, M.J.; Noh, C.-K.; Lee, G.H.; Eun, J.W.; Park, B.; et al. The Impact of Statins on the Survival of Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib or Lenvatinib. Cancers 2024, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Nezasa, K.-I.; Higaki, K.; Matsumura, T.; Inazawa, K.; Hasegawa, H.; Nakano, M.; Koike, M. Liver-specific distribution of rosuvastatin in rats: Comparison with pravastatin and simvastatin. Drug Metab. Dispos. 2002, 30, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Bulhak, A.A.; Gourine, A.V.; Gonon, A.T.; Sjöquist, P.; Valen, G.; Pernow, J. Oral pre-treatment with rosuvastatin protects porcine myocardium from ischaemia/reperfusion injury via a mechanism related to nitric oxide but not to serum cholesterol level. Acta Physiol. Scand. 2005, 183, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Young, L.H.; Lefer, A.M. Rosuvastatin, a new HMG-CoA reductase inhibitor, protects ischemic reperfused myocardium in normocholesterolemic rats. J. Cardiovasc. Pharmacol. 2003, 41, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.O.; Scherrer-Crosbie, M.; Picard, M.H.; Nasseri, B.A.; MacGillivray, C.; Gannon, J.; Lian, Q.; Bloch, K.D.; Lee, R.T. Rosuvastatin reduces experimental left ventricular infarct size after ischemia-reperfusion injury but not total coronary occlusion. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1802–H1809. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Jain, R.; Jain, S.; Dhawan, A.; Khatri, S. Nanostructured Lipid Carriers (NLCs) for Lipophilic Drug Delivery: A Review. Int. J. Pharm. Investig. 2024, 14, 128. [Google Scholar] [CrossRef]
- Shah, A.; Kaur, M.; Tiwari, M.; Singh, R.; Roy, R.; Zong, C.; Ghosh, S. Emulsomes: New Lipidic Carriers for Drug Delivery with Special Mention to Brain Drug Transport. Future J. Pharm. Sci. 2024, 10, 33. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.; Lee, T. Microfluidic Synthesis of Lipid-Based Nanoparticles for Drug Delivery: Recent Advances and Opportunities. Lab Chip 2024, 24, 589. [Google Scholar] [CrossRef]
- Pereira, M.A.; Mosqueira, V.C.F.; Vilela, J.M.C.; Andrade, M.S.; Ramaldes, G.A.; Cardoso, V.N. PLA-PEG nanocapsules radiolabeled with 99mTechnetium-HMPAO: Release properties and physicochemical characterization by atomic force microscopy and photon correlation spectroscopy. Eur. J. Pharm. Sci. 2008, 33, 42–51. [Google Scholar] [CrossRef]
- Yurgel, V.; Collares, T.; Seixas, F. Developments in the use of nanocapsules in oncology. Braz. J. Med. Biol. Res. 2013, 46, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2024, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X.; Zhang, Z. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef]
- Péter, F.; Wei, M.; He, S.; Yuan, W. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Liu, M.; Zhang, F. Nanocarriers Based on Niosomes for Improved Drug Delivery and Enhanced Stability. Nanoscale Adv. 2024, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.-F.; Zhou, X. Lipid-Based Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Yu, L.; Luo, Z.; Chen, T.; Ouyang, Y.; Xiao, L.; Liang, S.; Peng, Z.; Liu, Y.; Deng, Y. Bioadhesive Nanoparticles for Local Drug Delivery. Int. J. Mol. Sci. 2024, 23, 2370. [Google Scholar] [CrossRef]
- Castro, P.M.; Fonte, P.; Oliveira, A.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Mashoof, R.U.; Khanizadeh, S.; Ghaderi, E.; Farhadian, M.; Miresmaeili, A. Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs. those wearing conventional retainers: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 155–160. [Google Scholar] [CrossRef]
- Huq, M.A.; Akter, S. Bacterial Mediated Rapid and Facile Synthesis of Silver Nanoparticles and Their Antimicrobial Efficacy against Pathogenic Microorganisms. Materials 2021, 14, 2615. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Kanan, M.C.; El-Kadri, O.; Kanan, S.M. Recent advances in gold and silver nanoparticles: Synthesis and applications. J. Nanosci. Nanotechnol. 2014, 14, 4757–4780. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.A.; Yang, D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar]
- Singh, P.; Mijakovic, I. Green synthesis and antibacterial applications of gold and silver nanoparticles from Ligustrum vulgare berries. Sci. Rep. 2022, 12, 7902. [Google Scholar] [CrossRef] [PubMed]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Zharabad, S.H.; Mohammadian, M.; Aghdam, R.Z.; Dizaj, M.H.; Behrouzkia, Z. Increasing the radiation-induced cytotoxicity by silver nanoparticles and docetaxel in prostate cancer cells. Mol. Biol. Rep. 2024, 51, 633. [Google Scholar] [CrossRef]
- Fuster, M.G.; Montalbán, M.G.; Carissimi, G.; Lima, B.; Feresin, G.E.; Cano, M.; Giner-Casares, J.J.; López-Cascales, J.J.; Enriz, R.D.; Víllora, G. Antibacterial Effect of Chitosan-Gold Nanoparticles and Computational Modeling of the Interaction Between Chitosan and a Lipid Bilayer Model. Nanomaterials 2020, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Murugadoss, A.; Prasad, P.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Saber, W.I.A.; Zweil, A.M.; Bashir, S.I. An innovative green synthesis approach of chitosan nanoparticles and their inhibitory activity against phytopathogenic Botrytis cinerea on strawberry leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Shih, P.-Y.; Liao, Y.-T.; Tseng, Y.-K.; Deng, F.-S.; Lin, C.-H. A Potential Antifungal Effect of Chitosan Against Candida albicans Is Mediated via the Inhibition of SAGA Complex Component Expression and the Subsequent Alteration of Cell Surface Integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Chompoosor, A.; Han, G.; Rotello, V.M. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjug. Chem. 2008, 19, 1342–1345. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Lennox, R.B. Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir 2004, 20, 2867–2873. [Google Scholar] [CrossRef]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef]
- Hashem, A.H.; Shehabeldine, A.M.; Ali, O.M.; Salem, S.S. Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities. Polymers 2022, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Oransa, H.A.; Boughdady, M.F.; El-Sabbagh, H.M. Novel Mucoadhesive Chitosomes as a Platform for Enhanced Oral Bioavailability of Cinnarizine. Int. J. Nanomed. 2022, 17, 5641–5660. [Google Scholar] [CrossRef]
- Kamble, P.R.; Shaikh, K.S.; Chaudhari, P.D. Application of liquisolid technology for enhancing solubility and dissolution of rosuvastatin. Adv. Pharm. Bull. 2014, 4, 197–204. [Google Scholar]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.D.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box-Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2022, 32, 45–61. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- El-Menshawe, S.F.; Ali, A.; Rabeh, M.; Khalil, N.M. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: An approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018, 13, 307–318. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Kumari, S.; Nehra, A.; Gupta, K.; Puri, A.; Kumar, V.; Singh, K.P.; Kumar, M.; Sharma, A. Chlorambucil-Loaded Graphene-Oxide-Based Nano-Vesicles for Cancer Therapy. Pharmaceutics 2023, 15, 649. [Google Scholar] [CrossRef]
- Peppas, N.A. 1. Commentary on an exponential model for the analysis of drug delivery: Original research article: A simple equation for description of solute release: I II. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs, 1987. J. Control. Release 2014, 190, 31–32. [Google Scholar] [PubMed]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2016, 23, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; El Menshawe, S.F.; Abou-Taleb, H.A.; Elkarmalawy, M.H. Loratadine bioavailability via buccal transferosomal gel: Formulation, statistical optimization, in vitro/in vivo characterization, and pharmacokinetics in human volunteers. Drug Deliv. 2017, 24, 781–791. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Bawazir, A.O.; Alharbi, W.S.; Safo, M.K. Enhancement of Simvastatin ex vivo Permeation from Mucoadhesive Buccal Films Loaded with Dual Drug Release Carriers. Int. J. Nanomed. 2020, 15, 4001–4020. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbary, A.; Refai, H.; El-Nabarawi, M. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; El-Ghafar, O.A.A.; Farouk, H.O. Mitigation of Rheumatic Arthritis in a Rat Model via Transdermal Delivery of Dapoxetine HCl Amalgamated as a Nanoplatform: In vitro and in vivo Assessment. Int. J. Nanomed. 2020, 15, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Tekade, A.; Gattani, S.; Surana, S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech 2008, 9, 1262–1269. [Google Scholar] [CrossRef]
- Shalaby, T.I.; El-Refaie, W.M. Bioadhesive Chitosan-Coated Cationic Nanoliposomes with Improved Insulin Encapsulation and Prolonged Oral Hypoglycemic Effect in Diabetic Mice. J. Pharm. Sci. 2018, 107, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; El-Dahmy, R.M.; Elshafeey, A.H.; El Gawad, N.A.A.; El Gazayerly, O.N. Tripling the Bioavailability of Rosuvastatin Calcium Through Development and Optimization of an In-Situ Forming Nanovesicular System. Pharmaceutics 2019, 11, 275. [Google Scholar] [CrossRef]
- Kamuf, J.; Bardon, A.G.; Ziebart, A.; Ruemmler, R.; Schwab, J.; Dib, M.; Daiber, A.; Thal, S.C.; Hartmann, E.K. Influence of rosuvastatin treatment on cerebral inflammation and nitro-oxidative stress in experimental lung injury in pigs. BMC Anesthesiol. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.T.; Jiang, Y.; Debanne, S.M.; Labbato, D.; Juchnowski, S.; Ferrari, B.; Clagett, B.; Robinson, J.; Lederman, M.M.; McComsey, G.A. Rosuvastatin reduces vascular inflammation and T cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 68, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Veng-Pedersen, P. Goodness of fit evaluation of pharmacokinetic models. J. Pharmacokinet Biopharm. 1977, 5, 217–242. [Google Scholar]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Zhu, N.; Zhu, J.X.; Zhang, W.J.; Zhang, H.M.; Wang, Q.Q.; Wu, X.X.; Wang, X.; Zhang, J.; Hao, J.F. Self-Assembled Cubic Liquid Crystalline Nanoparticles for Transdermal Delivery of Paeonol. Med. Sci. Monit. 2015, 21, 3298–3310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Menshawe, S.F.; Hussein, A.K. Formulation and evaluation of meloxicam niosomes as vesicular carriers for enhanced skin delivery. Pharm. Dev. Technol. 2013, 18, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Titeva, S.; Rangelov, S.; Momekova, D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int. J. Pharm. 2019, 567, 118431. [Google Scholar] [CrossRef] [PubMed]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and characterization of niosomes containing ribavirin for liver targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobian, M.; Haeri, A.; Bolourchian, N.; Shahhosseni, S.; Dadashzadeh, S. The Impact of Surfactant Composition and Surface Charge of Niosomes on the Oral Absorption of Repaglinide as a BCS II Model Drug. Int. J. Nanomed. 2020, 15, 8767–8781. [Google Scholar] [CrossRef]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; Ramly, A.E. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: In-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv. 2015, 22, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Pochapski, D.J.; dos Santos, C.C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Naguib, I.A.; Alsantali, R.I.; Alsalahat, I.; Hegazy, A.M. Novel Chitosan-Coated Niosomal Formulation for Improved Management of Bacterial Conjunctivitis: A Highly Permeable and Efficient Ocular Nanocarrier for Azithromycin. J. Pharm. Sci. 2021, 110, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Chen, J.; Lai, J.; Sun, J.; Hu, F.; Wu, W. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int. J. Pharm. 2009, 376, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; El Mohamady, A.M.; El-Nabarawi, M.A.; Mohamed, A.I. Formulation and evaluation of niosomal vesicles containing ondansetron HCL for trans-mucosal nasal drug delivery. Drug Dev. Ind. Pharm. 2020, 46, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Ortiz, A.C.; Jerez, A.; Morales, J.; Arriagada, F. Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker. Pharmaceutics 2023, 15, 1590. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant-Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Alyahya, K.H.; Mahdi, W.A.; Qamar, W. Formulation and Evaluation of Luteolin-Loaded Nanovesicles: In Vitro Physicochemical Characterization and Viability Assessment. ACS Omega 2022, 7, 1048–1056. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Thomas, S.; Chong, Y.N.; Chaw, C.S. Preparation and characterization of enteric microparticles by coacervation. Drug Dev. Ind. Pharm. 2013, 39, 1142–1151. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.A.; El-Sabbagh, H.M. In vitro-in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef]
- Humphreys, C.J. The significance of Bragg’s law in electron diffraction and microscopy, and Bragg’s second law. Acta Crystallogr. A 2013, 69 Pt 1, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Gorain, B. Chitosan nanoparticles: A promising drug delivery system. Mar. Drugs 2019, 17, 466. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Equation for drug release from polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Vieira, A.C.; Chaves, L.L.; Pinheiro, S.; Pinto, S.; Pinheiro, M.; Lima, S.C.; Ferreira, D.; Sarmento, B.; Reis, S. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm. 2018, 536, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tila, D.; Yazdani-Arazi, S.N.; Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z. pH-sensitive, polymer modified, plasma stable niosomes: Promising carriers for anti-cancer drugs. EXCLI J. 2015, 14, 21–32. [Google Scholar]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009, 10, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, M.R.; Shoaib, M.; Imran, M.; Elhissi, A.M.A.; Ahmad, F.; Ali, I.; Shah, S.W.A. Development of a biocompatible creatinine-based niosomal delivery system for enhanced oral bioavailability of clarithromycin. Drug Deliv. 2016, 23, 3480–3491. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Schmeel, F.C.; Kim, Y.; Blaum-Feder, S.; Endo, T.; Schmidt-Wolf, I.G.H. In vitro efficacy of cinnarizine against lymphoma and multiple myeloma. Anticancer Res. 2015, 35, 835–841. [Google Scholar] [PubMed]
- Moazeni, E.; Gilani, K.; Sotoudegan, F.; Pardakhty, A.; Najafabadi, A.R.; Ghalandari, R.; Fazeli, M.R.; Jamalifar, H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J. Microencapsul. 2010, 27, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Safwat, S.; Ishak, R.A.H.; Hathout, R.M.; Mortada, N.D. Nanostructured lipid carriers loaded with simvastatin: Effect of PEG/glycerides on characterization, stability, cellular uptake efficiency and in vitro cytotoxicity. Drug Dev. Ind. Pharm. 2017, 43, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- AbuElfadl, A.; Boughdady, M.; Meshali, M. New Peceol™/Span™ 60 Niosomes Coated with Chitosan for Candesartan Cilexetil: Perspective Increase in Absolute Bioavailability in Rats. Int. J. Nanomed. 2021, 16, 5581–5601. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, F.; Li, Y.; Yang, X.; Huang, J.; Lv, T.; Zhang, Y.; Chen, J.; Chen, H.; Gao, Y.; et al. Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J. Nanotechnol. 2016, 7, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Lu, J.; Cao, Y.; Liu, J.Y.; Zhang, X.X.; Zhang, H.; Cui, J.H.; Cao, Q.R. Gastrointestinal stability, physicochemical characterization and oral bioavailability of chitosan or its derivative-modified solid lipid nanoparticles loading docetaxel. Drug Dev. Ind. Pharm. 2017, 43, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-P.; Song, R.-X.; Wang, T.; Sun, M.-J.; Liu, Y.; Chen, X.-G. Inducing sustained release and improving oral bioavailability of curcumin via chitosan derivatives-coated liposomes. Int. J. Biol. Macromol. 2018, 120 Pt A, 702–710. [Google Scholar] [CrossRef]
- Holm, R.; Olesen, N.E.; Hartvig, R.A.; Jørgensen, E.B.; Larsen, D.B.; Westh, P. Effect of cyclodextrin concentration on the oral bioavailability of danazol and cinnarizine in rats. Eur. J. Pharm. Biopharm. 2016, 101, 9–14. [Google Scholar] [CrossRef] [PubMed]






| Factors Levels | |||
|---|---|---|---|
| X1 | Amounts of surfactant | 88 | 100 |
| X2 | Type of surfactant | S20 | S60 |
| X3 | Amount of cholesterol | 34 | 44 |
| Responses | Constraints | ||
| Y1: Vs (nm) | Minimize | ||
| Y2: PDI | Minimize | ||
| Y3: EE% | Maximize | ||
| Y4: ZP (mV) | Maximize | ||
| Y5: Q12 (%) | Maximize | ||
| Formulation | CH (mg) | Surfactant | Amount of Surfactant (mg) | VS (nm) | PDI | EE% | ZP (mV) (−) | Q12 |
|---|---|---|---|---|---|---|---|---|
| N1 | 44 | S60 | 88 | 374.4 ± 6.23 | 0.347 ± 0.02 | 92.4 ± 0.941 | −32.9 ± 2.13 | 52.7 ± 0.679 |
| N2 | 44 | S60 | 100 | 369.7 ± 7.25 | 0.284 ± 0.13 | 91.5 ± 1.641 | −31.5 ± 1.92 | 44.7 ± 1.14 |
| N3 | 34 | S20 | 100 | 356.1 ± 11.46 | 0.427 ± 0.08 | 73.3 ± 0.768 | −43.7 ± 3.65 | 79.6 ± 1.29 |
| N4 | 44 | S20 | 88 | 420.6 ± 8.35 | 0.505 ± 0.13 | 71.8 ± 0.210 | −40.5 ± 4.21 | 68.2 ± 1.03 |
| N5 | 34 | S60 | 100 | 221.5 ± 12.66 | 0.266 ± 0.07 | 85.6 ± 1.82 | −36.6 ± 2.91 | 57.2 ± 0.477 |
| N6 | 44 | S20 | 100 | 428.8 ± 18.31 | 0.510 ± 0.05 | 74.6 ± 0.463 | −42.8 ± 1.08 | 64.5 ± 0.794 |
| N7 | 34 | S60 | 88 | 231.7 ± 13.61 | 0.278 ± 0.32 | 80.5 ± 0.874 | −33.4 ± 2.15 | 51.8 ± 0.506 |
| N8 | 34 | S20 | 88 | 365.7 ± 15.71 | 0.439 ± 0.01 | 61.4 ± 0.734 | −41.6 ± 1.06 | 85.9 ± 0.104 |
| Responses | Model | R2 | Adjusted R2 | Predicted R2 | p-Value | F-Value | Adequate Precision |
|---|---|---|---|---|---|---|---|
| VS (nm) | Significant | 0.9764 | 0.9587 | 0.9055 | 0.001 | 55.11 | 17.7 |
| PDI | Significant | 0.9752 | 0.9566 | 0.9009 | 0.0011 | 52.49 | 17.5 |
| EE% | Significant | 0.9392 | 0.8936 | 0.7569 | 0.0068 | 20.60 | 11.9 |
| ZP (mV) | Significant | 0.9393 | 0.8973 | 0.7571 | 0.0068 | 20.63 | 11.0 |
| Q12 (%) | Significant | 0.9233 | 0.8658 | 0.6933 | 0.0107 | 16.05 | 10.1 |
| Formulation | Zero-Order (R2) | First-Order (R2) | Higuchi (R2) | Mechanism | R2 | n | Diffusion Type |
|---|---|---|---|---|---|---|---|
| N1 | 0.8427 | 0.8928 | 0.9477 | Higuchi | 0.9611 | 0.73 | Anomalous diffusion |
| N2 | 0.8635 | 9098 | 0.9602 | Higuchi | 0.8653 | 0.81 | Anomalous diffusion |
| N3 | 0.7866 | 0.8877 | 0.9053 | Higuchi | 0.9445 | 0.57 | Anomalous diffusion |
| N4 | 0.7877 | 0.8727 | 0.924 | Higuchi | 0.9102 | 0.56 | Anomalous diffusion |
| N5 | 0.8600 | 0.9156 | 0.9579 | Higuchi | 0.9720 | 0.64 | Anomalous diffusion |
| N6 | 0.8254 | 0.9058 | 0.9325 | Higuchi | 0.9362 | 0.62 | Anomalous diffusion |
| N7 | 0.9039 | 0.9505 | 0.9781 | Higuchi | 0.9698 | 0.57 | Anomalous diffusion |
| N8 | 0.7675 | 0.9138 | 0.8959 | First order | 0.9325 | 0.71 | First-order |
| Formulation | Composition | Responses | |||||
|---|---|---|---|---|---|---|---|
| CH (mg) | S 60 (mg) | VS (nm) | PDI | EE% | ZP (mV) | Q12 (%) | |
| Nop | 34 | 100 | 211.43 | 0.254 | 86.2 | −35.3 | 55.6 |
| Nexp | 34 | 100 | 223.2 | 1.000 | 84.8 | −51.3 | 57.2 |
| pH | Formulation | Zero-Order (R2) | First-Order (R2) | Higuchi Model (R2) | Mechanism | R2 | n | Diffusion Type |
|---|---|---|---|---|---|---|---|---|
| 1.2 | Nop | 0.9149 | 0.9268 | 0.9540 | Diffusion | 0.9732 | 0.72 | Anomalous diffusion |
| CS.Nop | 0.9132 | 0.9245 | 0.9524 | Diffusion | 0.9723 | 0.86 | Anomalous diffusion | |
| 5.4 | Nop | 0.9113 | 0.9243 | 0.9379 | Diffusion | 0.9559 | 0.64 | Anomalous diffusion |
| CS.Nop | 0.8992 | 0.9509 | 0.9648 | Diffusion | 0.9792 | 0.65 | Anomalous diffusion | |
| 7.4 | Nop | 0.8440 | 0.9001 | 0.9232 | Diffusion | 0.9547 | 0.59 | Anomalous diffusion |
| CS.Nop | 0.9666 | 0.9488 | 0.9666 | Diffusion | 0.9716 | 0.67 | Anomalous diffusion |
| Storage Temp (°C) | Parameters | Storage Period (Month) | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 4 ± 1 | VS | 529.3 ± 801.2 | 441.3 ± 92.4 | 433.0 ± 112.5 | 431.6 ± 112 |
| PDI | 1.000 | 0.528 | 0.664 | 0.891 | |
| ZP | +25.6 ± 1.10 | +24.11 ± 0.212 | +25.71 ± 0.614 | +24.23 ± 0.712 | |
| DR% | 99.91 ± 0.081 | 100.1 ± 0.00 | 99.9 ± 0.121 | 100 ± 0.00 | |
| 25 ± 2 | VS | 529.3 ± 801.2 | 560 ± 203.8 | 586.7 ± 312.9 | 598.8 ± 538.6 |
| PDI | 1.000 | 1.000 | 0.852 | 0.981 | |
| ZP | +25.26 ± 1.10 | +22.32 ± 0.961 | +23.61 ± 0.563 | +20.42 ± 0.851 | |
| DR% | 99.91 ± 0.081 | 99.64 ± 0.11 0 | 98.6 ± 1.02 | 97.9 ± 0.021 | |
| 37 ± 2 | VS | 529.3 ± 801.2 | 491.3 ± 71.4 | 486.8 ± 59.3 | 4851.8 ± 59.3 |
| PDI | 1.000 | 0.621 | 0.621 | 0.621 | |
| ZP | +25.6 ± 1.10 | +23.11 ± 0.212 | +26.14 ± 0.316 | +24.5 ± 0.612 | |
| DR% | 99.91 ± 0.081 | 99.6 ± 0.121 | 100.6 ± 0.043 | 99.4.6 ± 0.231 |
| A | ||||||
| Conc (µg/mL) | AgirNPs | CS.Nop.AgNPs | INop | |||
| Viability% | Inhibitory % | Viability% | Inhibitory % | Viability% | Inhibitory % | |
| 500 | 5.12 ± 0.543 | 94.88 ± 0.456 | 7.59 ± 0.345 | 92.41 ± 0.675 | 2.81 ± 0.678 | 97.19 ± 0.567 |
| 250 | 13.06 ± 0.982 | 86.94 ± 0.895 | 28.14 ± 0.781 | 71.86 ± 0.567 | 6.04 ± 0.792 | 93.96 ± 0.345 |
| 125 | 28.74 ± 0.643 | 71.26 ± 0.346 | 51.36 ± 0.567 | 48.64 ± 0.452 | 13.96 ± 0.876 | 86.04 ± 0.378 |
| 62.5 | 42.05 ± 0.546 | 57.95 ± 0.671 | 79.28 ± 0.567 | 20.72 ± 0.668 | 30.65 ± 0.545 | 69.35 ± 0.872 |
| 31.25± | 81.43 ± 0.324 | 18.57 ± 0.876 | 93.17 ± 0.981 | 6.83 ± 0.678 | 51.38 ± 0.681 | 48.62 ± 0.456 |
| 15.6 | 92.73 ± 0.567 | 7.27 ± 0.678 | 99.51 ± 0.675 | 0.49 ± 0.345 | 67.19 ± 0.456 | 32.81 ± 0.632 |
| 7.8 | 98.60 ± 0.998 | 1.4 ± 0.654 | 100 ± 0.871 | 0 | 80.48 ± 0.341 | 19.52 ± 0.467 |
| 3.9 | 100 ± 0.341 | 0 | 100 ± 0.786 | 0 | 91.23 ± 0.467 | 8.77 ± 0.467 |
| 2 | 100 ± 0.234 | 0 | 100 ± 0.341 | 0 | 94.76 ± 0.567 | 5.24 ± 0.346 |
| 1 | 100± | 0 | 100 ± 0.678 | 0 | 97.02 ± 0.323 | 2.98 ± 0.567 |
| 0 | 100± | 0 | 100 ± 0.321 | 0 | 100 ± 0.678 | 0 |
| B | ||||||
| Conc (µg/mL) | RC | CS.Nop | Ag NPs | |||
| Viability% | Inhibitory % | Viability % | Inhibitory % | Viability % | Inhibitory % | |
| 500 | 7.34 ± 0.98 | 71.32 ± 0.419 | 6.73 ± 0.392 | 81.64 ± 0.818 | 7.84 ± 0.891 | 81.45 ± 0.671 |
| 250 | 20.63 ± 0.871 | 68.20 ± 0.532 | 14.05 ± 0.456 | 75.95 ± 0.865 | 29.03 ± 0.872 | 70.97 ± 0.881 |
| 125 | 39.24 ± 0.945 | 60.76 ± 0.498 | 35.19 ± 0.613 | 64.81 ± 0.873 | 47.21 ± 0.912 | 52.79 ± 0.756 |
| 62.5 | 66.83 ± 0.756 | 33.17 ± 0.653 | 69.42 ± 0.543 | 30.58 ± 0.765 | 86.09 ± 0.599 | 13.91 ± 0.786 |
| 31.25 | 82.14 ± 0.786 | 17.86 ± 0.498 | 88.06 ± 0.435 | 11.94 ± 0.791 | 99.42 ± 0.897 | 0.58 ± 0.121 |
| 15.6 | 97.69 ± 0.891 | 2.31 ± 0.521 | 98.75 ± 0.321 | 1.25 ± 0.668 | 100 ± 0.654 | 0 |
| 7.8 | 100 ± 0.651 | 0 | 100 ± 0.439 | 0 | 100 ± 0.664 | 0 |
| 3.9 | 100 ± 0.678 | 0 | 100 ± 0.348 | 0 | 100 ± 0.785 | 0 |
| 2 | 100 ± 0.976 | 0 | 100 ± 0.621 | 0 | 100 ± 0.899 | 0 |
| 1 | 100 ± 0.564 | 0 | 100 ± 0.342 | 0 | 100 ± 0.921 | 0 |
| 0 | 100 ± 0.432 | 0 | 100 ± 0.543 | 0 | 100 ± 0.782 | 0 |
| Pharmacokinetic Parameters | RC | Nop | CS.Nop |
|---|---|---|---|
| Kel (h−1) | 0.089 ± 0.621 | 0.093 ± 0.19 | 0.043 ± 0.039 |
| Cl (L/h) | 0.029 ± 0.091 | 0.015 ± 0.054 | 0.006 ± 0.061 |
| t1/2 (h) | 7.73 ± 0.731 | 8.47 ± 0.621 | 15.9 ± 0.921 |
| Cmax (ng/mL) | 372.61 ± 39.211 | 666.8 ± 31.362 | 579.6 ± 29.620 |
| AUC0–12 (ng·h/mL) | 1857.94 ± 211.367 | 3428.48 ± 68.113 | 5617.89 ± 301.043 |
| AUC0–∞ (ng·h/mL) | 2785.22 ± 299.625 | 5211.04 ± 411.482 | 13,787.47 ± 811.362 |
| AUMC0–12 (ng·h2/mL) | 9309.95 ± 945.402 | 16,957.69 ± 441.523 | 17,619.27 ± 568.428 |
| AUMC0–∞ (ng·h2/mL) | 30,748.83 ± 1621.628 | 18,7317.79 ± 1436.612 | 303,680.8 ± 1915.202 |
| MRT12 (h) | 5.01 ± 0.682 | 4.95 ± 0.521 | 3.14 ± 0.914 |
| MRT0–∞ | 11.04 ± 0.423 | 11.05 ± 0.325 | 22.03 ± 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, T.M.; Abdelfatah, M.M.; Omar, M.M.; Hasan, O.A.; Wali, S.M.; El-Mofty, M.S.; Ewees, M.G.; Salem, A.E.; Abd-El-Galil, T.I.; Mahmoud, D.M. Enhancing the Therapeutic Effect and Bioavailability of Irradiated Silver Nanoparticle-Capped Chitosan-Coated Rosuvastatin Calcium Nanovesicles for the Treatment of Liver Cancer. Pharmaceutics 2025, 17, 72. https://doi.org/10.3390/pharmaceutics17010072
Mahmoud TM, Abdelfatah MM, Omar MM, Hasan OA, Wali SM, El-Mofty MS, Ewees MG, Salem AE, Abd-El-Galil TI, Mahmoud DM. Enhancing the Therapeutic Effect and Bioavailability of Irradiated Silver Nanoparticle-Capped Chitosan-Coated Rosuvastatin Calcium Nanovesicles for the Treatment of Liver Cancer. Pharmaceutics. 2025; 17(1):72. https://doi.org/10.3390/pharmaceutics17010072
Chicago/Turabian StyleMahmoud, Tamer Mohamed, Mohamed Mahmoud Abdelfatah, Mahmoud Mohamed Omar, Omiya Ali Hasan, Saad M. Wali, Mohamed S. El-Mofty, Mohamed G. Ewees, Amel E. Salem, Tarek I. Abd-El-Galil, and Dina Mohamed Mahmoud. 2025. "Enhancing the Therapeutic Effect and Bioavailability of Irradiated Silver Nanoparticle-Capped Chitosan-Coated Rosuvastatin Calcium Nanovesicles for the Treatment of Liver Cancer" Pharmaceutics 17, no. 1: 72. https://doi.org/10.3390/pharmaceutics17010072
APA StyleMahmoud, T. M., Abdelfatah, M. M., Omar, M. M., Hasan, O. A., Wali, S. M., El-Mofty, M. S., Ewees, M. G., Salem, A. E., Abd-El-Galil, T. I., & Mahmoud, D. M. (2025). Enhancing the Therapeutic Effect and Bioavailability of Irradiated Silver Nanoparticle-Capped Chitosan-Coated Rosuvastatin Calcium Nanovesicles for the Treatment of Liver Cancer. Pharmaceutics, 17(1), 72. https://doi.org/10.3390/pharmaceutics17010072






