Liposomal Formulation of Hydroxychloroquine Can Inhibit Autophagy In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Liposome Preparation
2.3. Cu-Gluconate Liposomes and Sucrose–HEPES Liposomes
2.4. Drug Loading
2.5. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.6. In Vitro Liposomal Formulation Stability
2.7. High-Content Analysis of Viability and Autophagy
2.8. Western Blot Analysis
2.9. HCQ and Liposomal HCQ PK Study
2.10. Efficacy Study
2.11. Ethics Statement
2.12. Statistics
3. Results
3.1. HCQ Loading into Liposomes Depends on Transmembrane pH Gradient
3.2. Characterization of Liposomes with Encapsulated HCQ
3.3. In Vitro Inhibition of Autophagy as a Surrogate Measure of HCQ Release from Liposomes
3.4. PK of Liposomal HCQ
3.5. Efficacy of Liposome HCQ Formulations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breastcancer.org U.S. Breast Cancer Statistics. Available online: http://www.breastcancer.org/symptoms/understand_bc/statistics (accessed on 1 November 2024).
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M. Circumventing de Novo and Acquired Resistance to Trastuzumab: New Hope for the Care of ErbB2-Positive Breast Cancer. Clin. Breast Cancer 2008, 8 (Suppl. S3), S100–S113. [Google Scholar] [CrossRef] [PubMed]
- Tevaarwerk, A.J.; Gray, R.J.; Schneider, B.P.; Smith, M.L.; Wagner, L.I.; Fetting, J.H.; Davidson, N.; Goldstein, L.J.; Miller, K.D.; Sparano, J.A. Survival in Patients with Metastatic Recurrent Breast Cancer after Adjuvant Chemotherapy: Little Evidence of Improvement over the Past 30 Years. Cancer 2013, 119, 1140–1148. [Google Scholar] [CrossRef]
- Miglietta, F.; Bottosso, M.; Griguolo, G.; Dieci, M.V.; Guarneri, V. Major Advancements in Metastatic Breast Cancer Treatment: When Expanding Options Means Prolonging Survival. ESMO Open 2022, 7, 100409. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Feng, J.; Chang, Y.; Wang, Z.; Wen, A. Autophagy Facilitates the Lapatinib Resistance of HER2 Positive Breast Cancer Cells. Med. Hypotheses 2011, 77, 206–208. [Google Scholar] [CrossRef]
- Dragowska, W.H.; Weppler, S.A.; Wang, J.C.; Wong, L.Y.; Kapanen, A.I.; Rawji, J.S.; Warburton, C.; Qadir, M.A.; Donohue, E.; Roberge, M.; et al. Induction of Autophagy Is an Early Response to Gefitinib and a Potential Therapeutic Target in Breast Cancer. PLoS ONE 2013, 8, e76503. [Google Scholar] [CrossRef]
- Han, W.; Pan, H.; Chen, Y.; Sun, J.; Wang, Y.; Li, J.; Ge, W.; Feng, L.; Lin, X.; Wang, X.; et al. EGFR Tyrosine Kinase Inhibitors Activate Autophagy as a Cytoprotective Response in Human Lung Cancer Cells. PLoS ONE 2011, 6, e18691. [Google Scholar] [CrossRef]
- Sun, W.L.; Chen, J.; Wang, Y.P.; Zheng, H. Autophagy Protects Breast Cancer Cells from Epirubicin-Induced Apoptosis and Facilitates Epirubicin-Resistance Development. Autophagy 2011, 7, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Chittaranjan, S.; Bortnik, S.; Dragowska, W.H.; Xu, J.; Abeysundara, N.; Leung, A.; Go, N.E.; DeVorkin, L.; Weppler, S.A.; Gelmon, K.; et al. Autophagy Inhibition Augments the Anticancer Effects of Epirubicin Treatment in Anthracycline-Sensitive and -Resistant Triple-Negative Breast Cancer. Clin. Cancer Res. 2014, 20, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy Modulation as a Potential Therapeutic Target for Diverse Diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and Chemotherapy Resistance: A Promising Therapeutic Target for Cancer Treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Piezzo, M.; Caputo, R.; Lauro, V.D.; Rella, F.D.; Fusco, G.; Capozzi, M.; Gioia, G.D.; Budillon, A.; et al. Targeting Autophagy in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 7836. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abedin, M.J.; Abe, A. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Yoshimori, T. The Origin of the Autophagosomal Membrane. Nat. Cell Biol. 2010, 12, 831–835. [Google Scholar] [CrossRef]
- Choi, K.S. Autophagy and Cancer. Exp. Mol. Med. 2012, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Masoud, V.; Pages, G. Targeted Therapies in Breast Cancer: New Challenges to Fight against Resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Aveic, S.; Tonini, G.P. Resistance to Receptor Tyrosine Kinase Inhibitors in Solid Tumors: Can We Improve the Cancer Fighting Strategy by Blocking Autophagy? Cancer Cell Int. 2016, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Oliveras-Ferraros, C.; Menendez, J.A. Autophagy Facilitates the Development of Breast Cancer Resistance to the Anti-HER2 Monoclonal Antibody Trastuzumab. PLoS ONE 2009, 4, e6251. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Anantha, M.; Backstrom, I.; Leung, A.; Chen, K.; Malhotra, A.; Edwards, K.; Bally, M.B. Nanoscale Reaction Vessels Designed for Synthesis of Copper-Drug Complexes Suitable for Preclinical Development. PLoS ONE 2016, 11, e0153416. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of Large Unilamellar Vesicles by a Rapid Extrusion Procedure: Characterization of Size Distribution, Trapped Volume and Ability to Maintain a Membrane Potential. Biochim. Biophys. Acta (BBA) Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)-Chloroquine and Hydroxychloroquine as Anti-Cancer Agents. ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Kwiatkowski, D.; Damodaran, S.; Kettner, N.M.; Ramirez, D.L.; Gombos, D.S.; Hunt, K.; Shen, Y.; Keyomarsi, K.; Tripathy, D. Phase I Safety and Efficacy Study of Autophagy Inhibition with Hydroxychloroquine to Augment the Antiproliferative and Biological Effects of Preoperative Palbociclib plus Letrozole for Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer (MBC). J. Clin. Oncol. 2021, 39, 1067. [Google Scholar] [CrossRef]
- Boya, P.; Gonzalez-Polo, R.A.; Poncet, D.; Andreau, K.; Vieira, H.L.; Roumier, T.; Perfettini, J.L.; Kroemer, G. Mitochondrial Membrane Permeabilization Is a Critical Step of Lysosome-Initiated Apoptosis Induced by Hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine Inhibits Autophagic Flux by Decreasing Autophagosome-Lysosome Fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Shou, J.; Deng, D.; Jiang, L.; Jing, Z.; Yao, J.; Li, H.; Xie, J.; Wang, Z.; Pan, Q.; et al. Crizotinib Induces Autophagy through Inhibition of the STAT3 Pathway in Multiple Lung Cancer Cell Lines. Oncotarget 2015, 6, 40268–40282. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting Autophagy in Cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Chude, C.I.; Amaravadi, R.K. Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors. Int. J. Mol. Sci. 2017, 18, 1279. [Google Scholar] [CrossRef] [PubMed]
- Bristol, M.L.; Emery, S.M.; Maycotte, P.; Thorburn, A.; Chakradeo, S.; Gewirtz, D.A. Autophagy Inhibition for Chemosensitization and Radiosensitization in Cancer: Do the Preclinical Data Support This Therapeutic Strategy? J. Pharmacol. Exp. Ther. 2013, 344, 544–552. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, K.; Zhang, L.; Hu, G.; Wan, J.; Tang, J.; Yin, S.; Duan, J.; Qin, M.; Wang, N.; et al. Significantly Enhanced Tumor Cellular and Lysosomal Hydroxychloroquine Delivery by Smart Liposomes for Optimal Autophagy Inhibition and Improved Antitumor Efficiency with Liposomal Doxorubicin. Autophagy 2016, 12, 949–962. [Google Scholar] [CrossRef]
- Yin, S.; Xia, C.; Wang, Y.; Wan, D.; Rao, J.; Tang, X.; Wei, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Dual Receptor Recognizing Liposomes Containing Paclitaxel and Hydroxychloroquine for Primary and Metastatic Melanoma Treatment via Autophagy-Dependent and Independent Pathways. J. Control. Release 2018, 288, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.M.; Crist, R.M.; Stern, S.T. Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine. Molecules 2020, 26, 175. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Hansen, C.B.; de Menezes, D.E.L. Pharmacokinetics of Long-Circulating Liposomes. Adv. Drug Deliv. Rev. 1995, 16, 267–284. [Google Scholar] [CrossRef]
- Mayer, L.D.; Reamer, J.; Bally, M.B. Intravenous Pretreatment with Empty PH Gradient Liposomes Alters the Pharmacokinetics and Toxicity of Doxorubicin through In Vivo Active Drug Encapsulation. J. Pharm. Sci. 1999, 88, 96–102. [Google Scholar] [CrossRef]
- Embree, L.; Gelmon, K.; Tolcher, A.; Hudon, N.; Heggie, J.; Dedhar, C.; Logan, P.; Bally, M.B.; Mayer, L.D. Pharmacokinetic Behavior of Vincristine Sulfate Following Administration of Vincristine Sulfate Liposome Injection. Cancer Chemother. Pharmacol. 1998, 41, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.D.; Mayer, L.D.; Abraham, S.A.; Gallagher, R.C.; Cox, K.A.; Tardi, P.G.; Bally, M.B. Improved Retention of Idarubicin after Intravenous Injection Obtained for Cholesterol-Free Liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2002, 1561, 188–201. [Google Scholar] [CrossRef]
- Hare, J.I.; Neijzen, R.W.; Anantha, M.; Santos, N.D.; Harasym, N.; Webb, M.S.; Allen, T.M.; Bally, M.B.; Waterhouse, D.N. Treatment of Colorectal Cancer Using a Combination of Liposomal Irinotecan (Irinophore C™) and 5-Fluorouracil. PLoS ONE 2013, 8, e62349. [Google Scholar] [CrossRef] [PubMed]
- Balazsovits, J.A.; Mayer, L.D.; Bally, M.B.; Cullis, P.R.; McDonell, M.; Ginsberg, R.S.; Falk, R.E. Analysis of the Effect of Liposome Encapsulation on the Vesicant Properties, Acute and Cardiac Toxicities, and Antitumor Efficacy of Doxorubicin. Cancer Chemother. Pharmacol. 1989, 23, 81–86. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-Cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared with Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Li, C.; Cui, J.; Wang, C.; Li, Y.; Zhang, H.; Wang, J.; Li, Y.; Zhang, L.; Zhang, L.; Guo, W.; et al. Encapsulation of Mitoxantrone into Pegylated SUVs Enhances Its Antineoplastic Efficacy. Eur. J. Pharm. Biopharm. 2008, 70, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Nix, D.E.; Straubinger, R.M. Formulation and Efficacy of Liposome-Encapsulated Antibiotics for Therapy of Intracellular Mycobacterium Avium Infection. Antimicrob. Agents Chemother. 1995, 39, 2104–2111. [Google Scholar] [CrossRef]
- Rahman, Y.E.; Hanson, W.R.; Bharucha, J.; Ainsworth, E.J.; Jaroslow, B.N. Mechanisms of Reduction of Antitumor Drug Toxicity by Liposome Encapsulation. Ann. N. Y. Acad. Sci. 1978, 308, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Alnajim, J.; Anantha, M.; Zastre, J.; Yan, H.; Webb, M.; Waterhouse, D.; Bally, M. A Novel Liposomal Irinotecan Formulation with Significant Anti-Tumour Activity: Use of the Divalent Cation Ionophore A23187 and Copper-Containing Liposomes to Improve Drug Retention. Eur. J. Pharm. Biopharm. 2008, 68, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Madden, T.D.; Harrigan, P.R.; Tai, L.C.L.; Bally, M.B.; Mayer, L.D.; Redelmeier, T.E.; Loughrey, H.C.; Tilcock, C.P.S.; Reinish, L.W.; Cullis, P.R. The Accumulation of Drugs within Large Unilamellar Vesicles Exhibiting a Proton Gradient: A Survey. Chem. Phys. Lipids 1990, 53, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Chernov, L.; Chen, K.; Bally, M.B. PRCosomes: Pretty Reactive Complexes Formed in Liposomes. J. Drug Target. 2016, 24, 787–796. [Google Scholar] [CrossRef]
- Cobine, P.A.; Brady, D.C. Cuproptosis: Cellular and Molecular Mechanisms Underlying Copper-Induced Cell Death. Mol. Cell 2022, 82, 1786–1787. [Google Scholar] [CrossRef]
- Li, S.-R.; Bu, L.-L.; Cai, L. Cuproptosis: Lipoylated TCA Cycle Proteins-Mediated Novel Cell Death Pathway. Signal Transduct. Target. Ther. 2022, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; He, Y.; Fang, C.; Qiu, H.; Chen, Q.; Huang, F.; Wu, Z. Cuproptosis-Associated Genes and Immune Microenvironment Characterization in Breast Cancer. Medicine 2022, 101, e32301. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A Copper-Triggered Modality of Mitochondrial Cell Death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, F. Cuproptosis: A New Form of Programmed Cell Death. Cell. Mol. Immunol. 2022, 19, 867–868. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper Metabolism in Cell Death and Autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, Z.; Qi, X.; Zuo, T.; Wu, Z. Copper-Induced Tumor Cell Death Mechanisms and Antitumor Theragnostic Applications of Copper Complexes. Nanomedicine 2022, 17, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Balgi, A.D.; Fonseca, B.D.; Donohue, E.; Tsang, T.C.; Lajoie, P.; Proud, C.G.; Nabi, I.R.; Roberge, M. Screen for Chemical Modulators of Autophagy Reveals Novel Therapeutic Inhibitors of MTORC1 Signaling. PLoS ONE 2009, 4, e7124. [Google Scholar] [CrossRef]
- Fritze, A.; Hens, F.; Kimpfler, A.; Schubert, R.; Peschka-Süss, R. Remote Loading of Doxorubicin into Liposomes Driven by a Transmembrane Phosphate Gradient. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1633–1640. [Google Scholar] [CrossRef]
- Manning, T.J.; Leggett, T.; Jenkins, D.; Furtado, I.; Phillips, D.; Wylie, G.; Bythell, B.J.; Zhang, F.L. Structural and Some Medicinal Characteristics of the Copper(II)-Hydroxychloroquine Complex. Bioorg. Med. Chem. Lett. 2013, 23, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Yu, X.X.; Yan, L.J.; Xiao, H.T. Research Progress of Hydroxychloroquine and Autophagy Inhibitors on Cancer. Cancer Chemother. Pharmacol. 2017, 79, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bally, M.B.; Mayer, L.D.; Hope, M.J.; Nayar, R. Pharmacodynamics of Liposomal Drug Carriers: Methodological Considerations. In Liposome Technology, 2nd ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1993; Volume 3, pp. 27–42. [Google Scholar]
- Wabitsch, S.; McVey, J.C.; Ma, C.; Ruf, B.; Kamenyeva, O.; McCallen, J.D.; Diggs, L.P.; Heinrich, B.; Greten, T.F. Hydroxychloroquine Can Impair Tumor Response to Anti-PD1 in Subcutaneous Mouse Models. iScience 2021, 24, 101990. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.A.; Kwok, B.; Dragowska, W.H.; To, K.H.; Le, D.; Bally, M.B.; Gorski, S.M. Macroautophagy Inhibition Sensitizes Tamoxifen-Resistant Breast Cancer Cells and Enhances Mitochondrial Depolarization. Breast Cancer Res. Treat. 2008, 112, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, A.; Gitsov, I. Linear-Dendritic Supramolecular Complexes as Nanoscale Reaction Vessels for “Green” Chemistry. Diels-Alder Reactions between Fullerene C-60 and Polycyclic Aromatic Hydrocarbons in Aqueous Medium. Langmuir 2008, 24, 11431–11441. [Google Scholar] [CrossRef]
- Finbloom, D.S.; Silver, K.; Newsome, D.A.; Gunkel, R. Comparison of Hydroxychloroquine and Chloroquine Use and the Development of Retinal Toxicity. J. Rheumatol. 1985, 12, 692–694. [Google Scholar]
- Manic, G.; Obrist, F.; Kroemer, G.; Vitale, I.; Galluzzi, L. Chloroquine and Hydroxychloroquine for Cancer Therapy. Mol. Cell. Oncol. 2014, 1, e29911. [Google Scholar] [CrossRef]
- Gewirtz, D.A. The Challenge of Developing Autophagy Inhibition as a Therapeutic Strategy. Cancer Res. 2016, 76, 5610–5614. [Google Scholar] [CrossRef]
- Guo, S.; Pridham, K.J.; Virbasius, C.M.; He, B.; Zhang, L.; Varmark, H.; Green, M.R.; Sheng, Z. A Large-Scale RNA Interference Screen Identifies Genes That Regulate Autophagy at Different Stages. Sci. Rep. 2018, 8, 2822. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Im, J.S.; Cho, J.Y.; Bae, K.S.; Klein, T.A.; Yeom, J.S.; Kim, T.S.; Choi, J.S.; Jang, I.J.; Park, J.W. Pharmacokinetics of Hydroxychloroquine and Its Clinical Implications in Chemoprophylaxis against Malaria Caused by Plasmodium Vivax. Antimicrob. Agents Chemother. 2009, 53, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A. Chloroquine Analogues in Drug Discovery: New Directions of Uses, Mechanisms of Actions and Toxic Manifestations from Malaria to Multifarious Diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, D.C.; Steele, J.C.; Adagu, I.S.; Craig, J.C.; Cullander, C. Hydroxychloroquine Is Much Less Active than Chloroquine against Chloroquine-Resistant Plasmodium Falciparum, in Agreement with Its Physicochemical Properties. J. Antimicrob. Chemother. 2003, 52, 188–193. [Google Scholar] [CrossRef]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Ansell, S.; Maurer, N.; Cullis, P.R. Characterization of the Drug Retention and Pharmacokinetic Properties of Liposomal Nanoparticles Containing Dihydrosphingomyelin. Biochim. Biophys. Acta (BBA) Biomembr 2007, 1768, 1121–1127. [Google Scholar] [CrossRef]
- Abraham, S.A.; Edwards, K.; Karlsson, G.; Hudon, N.; Mayer, L.D.; Bally, M.B. An Evaluation of Transmembrane Ion Gradient-Mediated Encapsulation of Topotecan within Liposomes. J. Control. Release 2004, 96, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Leung, A.W.Y.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective—Can Copper Complexes Be Developed as a Novel Class of Therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Scherphof, G.L.; Kamps, J.A. The Role of Hepatocytes in the Clearance of Liposomes from the Blood Circulation. Prog. Lipid Res. 2001, 40, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, P.; Strambi, A.; Zipoli, C.; Hagg-Olofsson, M.; Buoncervello, M.; Linder, S.; Milito, A.D. Acidic Extracellular PH Neutralizes the Autophagy-Inhibiting Activity of Chloroquine: Implications for Cancer Therapies. Autophagy 2014, 10, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Gomes-de-Silva, L.C.; Dewitte, H.; Breckpot, K.; Fucikova, J.; Spisek, R.; Galluzzi, L.; Kepp, O.; Kroemer, G. Combinatorial Strategies for the Induction of Immunogenic Cell Death. Front. Immunol. 2015, 6, 187. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Rahim, R.; Strobl, J.S. Hydroxychloroquine, Chloroquine, and All-Trans Retinoic Acid Regulate Growth, Survival, and Histone Acetylation in Breast Cancer Cells. Anti-Cancer Drugs 2009, 20, 736–745. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Kivity, S.; Langevitz, P.; Shoenfeld, Y. Hydroxychloroquine: From Malaria to Autoimmunity. Clin. Rev. Allergy Immunol. 2012, 42, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective Autophagy Elicited by RAF→MEK→ERK Inhibition Suggests a Treatment Strategy for RAS-Driven Cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of ERK and Autophagy Inhibition as a Treatment Approach for Pancreatic Cancer. Nat. Med. 2019, 25, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Celano, S.L.; Solitro, A.R.; Gunaydin, H.; Scott, M.; O’Hagan, R.C.; Shumway, S.D.; Fuller, P.; MacKeigan, J.P. A Potent and Selective ULK1 Inhibitor Suppresses Autophagy and Sensitizes Cancer Cells to Nutrient Stress. iScience 2018, 8, 74–84. [Google Scholar] [CrossRef]
- Dower, C.M.; Bhat, N.; Gebru, M.T.; Chen, L.; Wills, C.A.; Miller, B.A.; Wang, H.G. Targeted Inhibition of ULK1 Promotes Apoptosis and Suppresses Tumor Growth and Metastasis in Neuroblastoma. Mol. Cancer Ther. 2018, 17, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Bosc, D.; Vezenkov, L.; Bortnik, S.; An, J.; Xu, J.; Choutka, C.; Hannigan, A.M.; Kovacic, S.; Loo, S.; Clark, P.G.K.; et al. A New Quinoline-Based Chemical Probe Inhibits the Autophagy-Related Cysteine Protease ATG4B. Sci. Rep. 2018, 8, 11653. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Pedro, J.M.B.-S.; Galluzzi, L.; Kroemer, G. Autophagy in Natural and Therapy-Driven Anticancer Immunosurveillance. Autophagy 2017, 13, 2163–2170. [Google Scholar] [CrossRef]
- Ma, Y.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Autophagy and Cellular Immune Responses. Immunity 2013, 39, 211–227. [Google Scholar] [CrossRef] [PubMed]
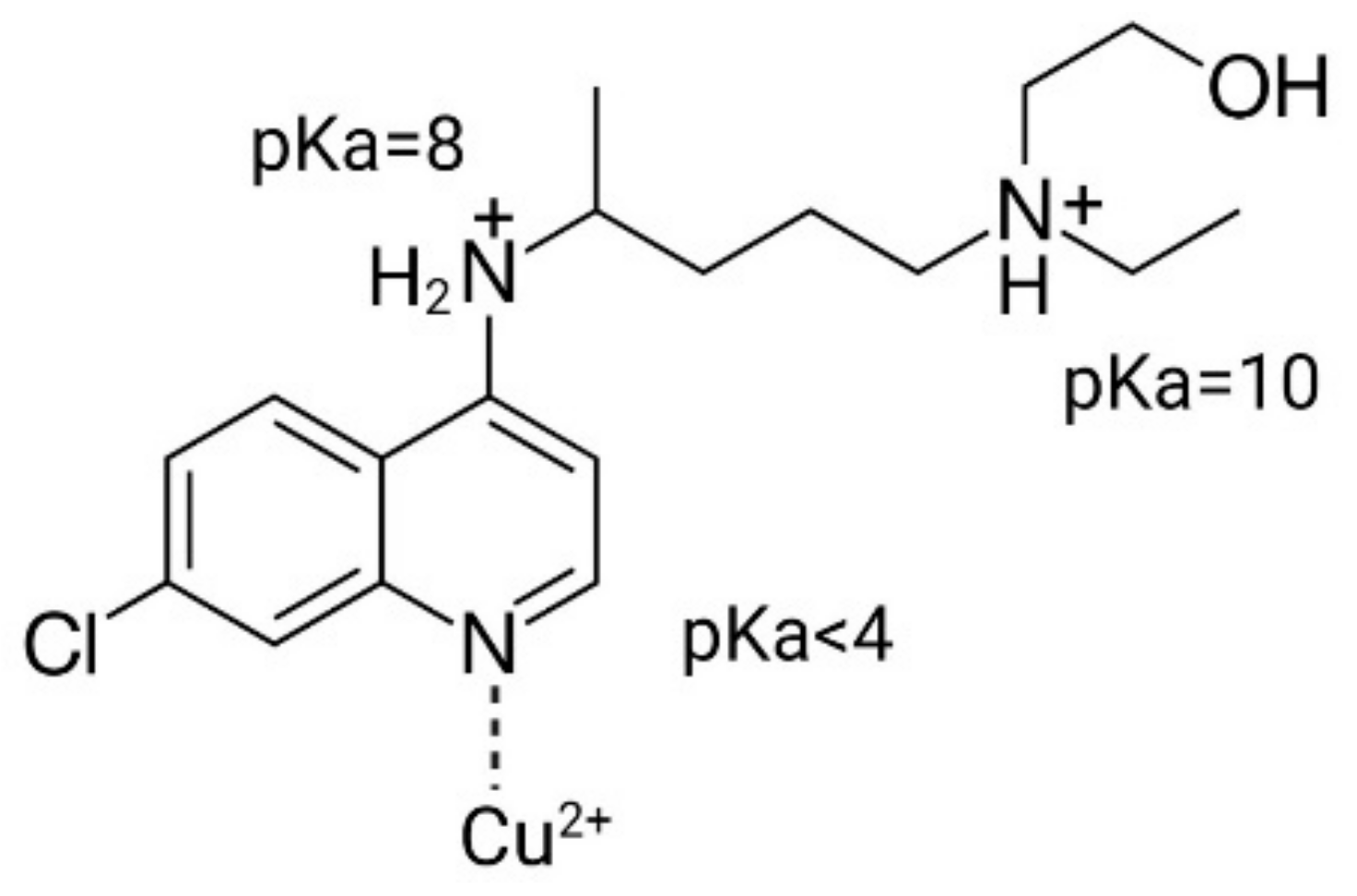
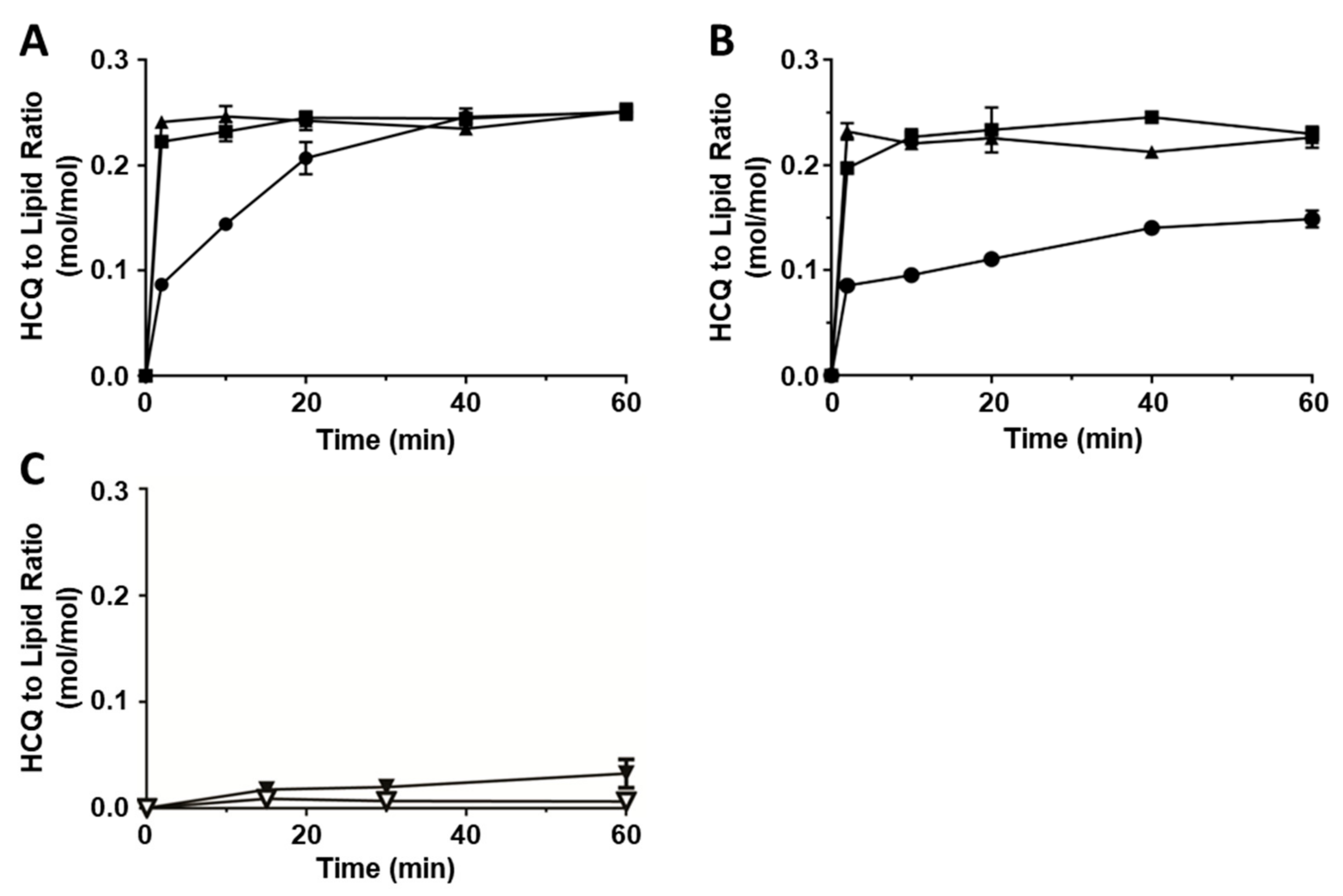

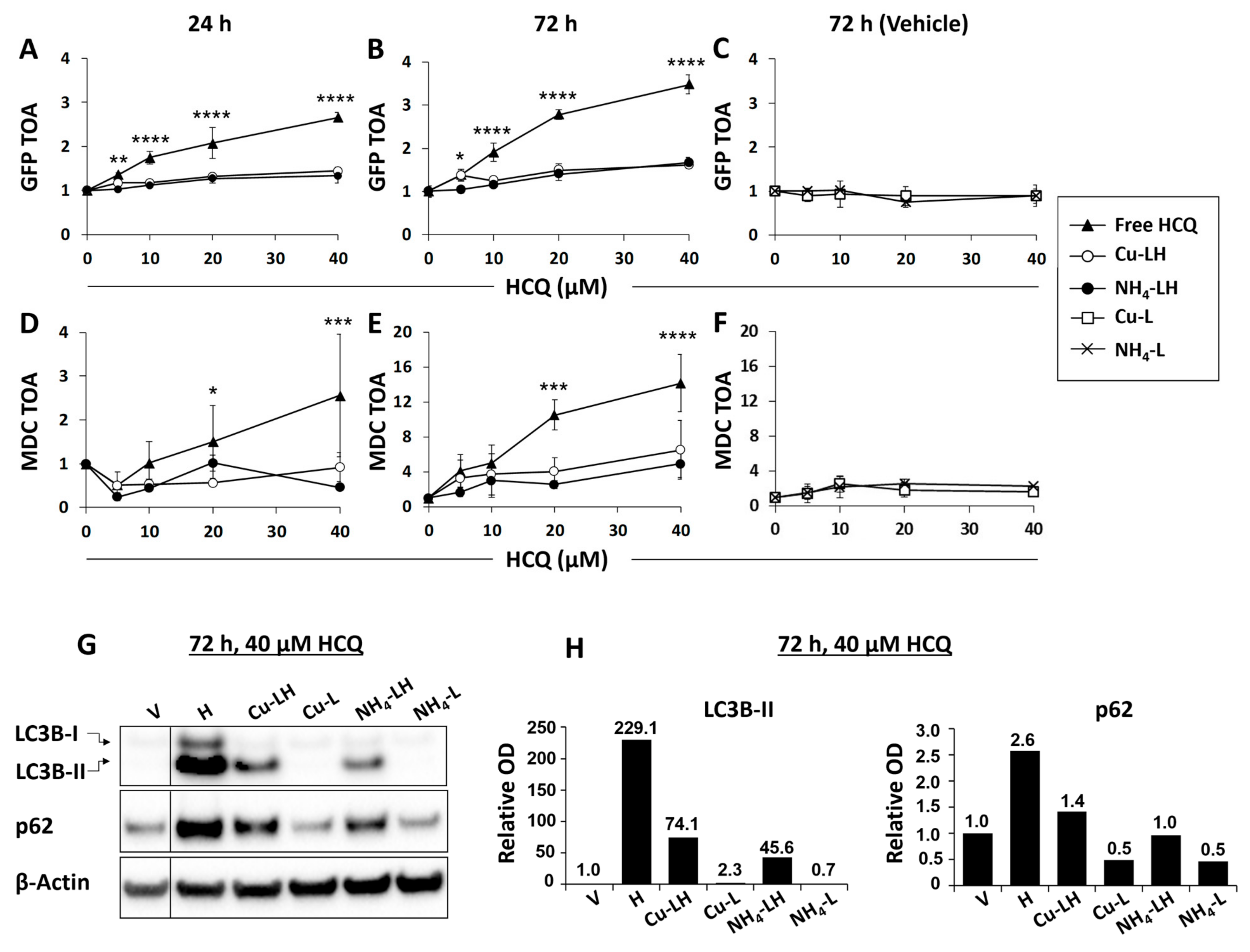


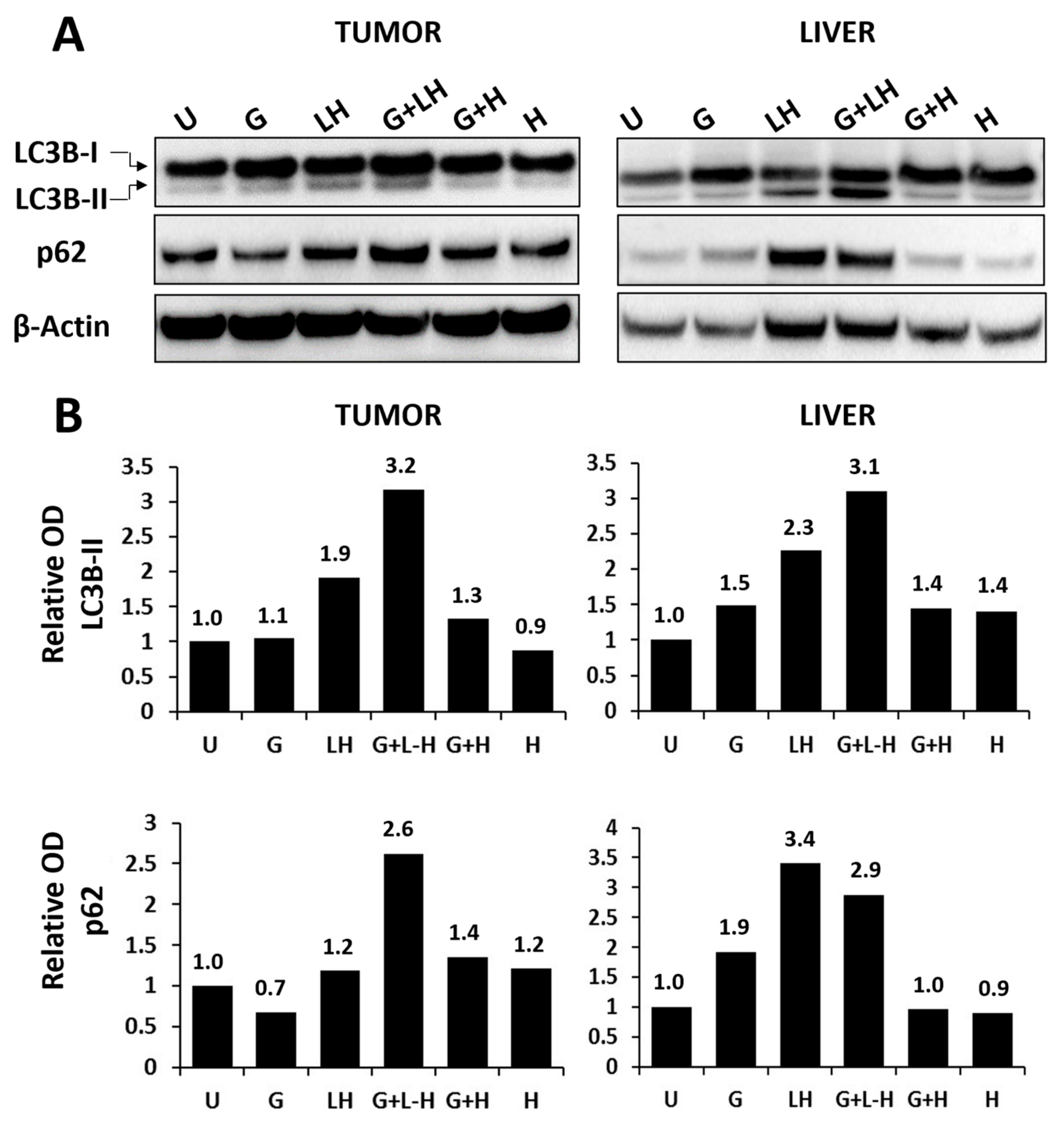
| Formulation | Particle Size (nm) | Polydispersity |
|---|---|---|
| (NH4)2SO4 Liposomes | 124 ± 0.8 | 0.122 |
| HCQ-(NH4)2SO4 | 114 ± 1.5 | 0.09 |
| CuSO4 Liposomes | 101 ± 1.5 | 0.038 |
| HCQ-CuSO4 | 106 ± 0.7 | 0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragowska, W.H.; Singh, J.; Wehbe, M.; Anantha, M.; Edwards, K.; Gorski, S.M.; Bally, M.B.; Leung, A.W.Y. Liposomal Formulation of Hydroxychloroquine Can Inhibit Autophagy In Vivo. Pharmaceutics 2025, 17, 42. https://doi.org/10.3390/pharmaceutics17010042
Dragowska WH, Singh J, Wehbe M, Anantha M, Edwards K, Gorski SM, Bally MB, Leung AWY. Liposomal Formulation of Hydroxychloroquine Can Inhibit Autophagy In Vivo. Pharmaceutics. 2025; 17(1):42. https://doi.org/10.3390/pharmaceutics17010042
Chicago/Turabian StyleDragowska, Wieslawa H., Jagbir Singh, Mohamed Wehbe, Malathi Anantha, Katarina Edwards, Sharon M. Gorski, Marcel B. Bally, and Ada W. Y. Leung. 2025. "Liposomal Formulation of Hydroxychloroquine Can Inhibit Autophagy In Vivo" Pharmaceutics 17, no. 1: 42. https://doi.org/10.3390/pharmaceutics17010042
APA StyleDragowska, W. H., Singh, J., Wehbe, M., Anantha, M., Edwards, K., Gorski, S. M., Bally, M. B., & Leung, A. W. Y. (2025). Liposomal Formulation of Hydroxychloroquine Can Inhibit Autophagy In Vivo. Pharmaceutics, 17(1), 42. https://doi.org/10.3390/pharmaceutics17010042







