Abstract
In the 21st century, thanks to advances in biotechnology and developing pharmaceutical technology, significant progress is being made in effective drug design. Drug targeting aims to ensure that the drug acts only in the pathological area; it is defined as the ability to accumulate selectively and quantitatively in the target tissue or organ, regardless of the chemical structure of the active drug substance and the method of administration. With drug targeting, conventional, biotechnological and gene-derived drugs target the body’s organs, tissues, and cells that can be selectively transported to specific regions. These systems serve as drug carriers and regulate the timing of release. Despite having many advantageous features, these systems have limitations in thoroughly treating complex diseases such as cancer. Therefore, combining these systems with nanoparticle technologies is imperative to treat cancer at both local and systemic levels effectively. The nanocarrier-based drug delivery method involves encapsulating target-specific drug molecules into polymeric or vesicular systems. Various drug delivery systems (DDS) were investigated and discussed in this review article. The first part discussed active and passive delivery systems, hydrogels, thermoplastics, microdevices and transdermal-based drug delivery systems. The second part discussed drug carrier systems in nanobiotechnology (carbon nanotubes, nanoparticles, coated, pegylated, solid lipid nanoparticles and smart polymeric nanogels). In the third part, drug targeting advantages were discussed, and finally, market research of commercial drugs used in cancer nanotechnological approaches was included.
1. Introduction
The majority of commonly used drugs do not exert their activity in the body by selective distribution in pathological organs, cells or tissues [1,2,3,4]. Generally, these drugs prefer to be distributed throughout the body. Moreover, drugs have to cross many biological barriers such as organs, cells and intracellular compartments to reach the site of action [2,5,6]. Meanwhile, drugs may accumulate in normal organs and tissues that are not involved in the pathological process. This results in the need for large amounts to be taken by the patient in order to reach therapeutic concentrations in the required body compartments, as well as many negative side effects. Drug targeting offers a solution to all these problems. Drug targeting is the ability of a drug to selectively and quantitatively accumulate in the target tissue or organ, independent of the chemical structure and mode of uptake of the active substance. In this way, the concentration of the drug will be high in diseased areas and will remain at a minimum level in other areas to prevent negative side effects [7,8]
Transport of the drug into the structure where it will act is one of the main problems in the pharmaceutical and biotechnological industries [9,10]. Therefore, drug delivery systems have always been an area of interest for researchers. New developments in biotechnology and research in other related sciences have helped in the discovery and rational design of many new drugs. However, most drugs are constrained by poor solubility, high toxicity, high dose, accumulation of drug due to poor solubility, nonspecific transport, in vivo degradation and short half-life [10,11,12,13,14]. Nowadays, many researchers from various disciplines are involved in the development of specific new drug delivery systems in order to minimize the increasing problems of drugs and to transform new developments into clinical efficacy. Targeted drug delivery is defined as the specific release of a bioactive agent into a certain structure at a certain rate. Targeted drug delivery systems deliver drugs more efficiently and practically than current drugs, improve patient compliance, prolong drug half-life and reduce healthcare costs. Therefore, the development of techniques that can selectively deliver drugs to pathologic cells, tissues or organs is one of the most important areas of drug research today.
Advances in nanotechnology have had a significant impact on the pharmaceutical industry, especially nanoparticles (NPs), which have many applications in the clinic. Nanotechnology focuses on the formulation of therapeutic agents in liposomes and nanocarriers (nanoparticles, nanocapsules, micelles and dendrimers, etc.). These formulations enable targeted drug delivery to the diseased structure. Since nanoparticles have the potential to be used in the diagnosis and treatment of many diseases, it is conceivable that they will play a greater role in drug delivery system technology in the near future. The process of nanotechnology applications in various branches of science, especially in the field of health, becoming widespread and new drugs replacing traditional drugs is accelerating. In this process, nanotechnology and biotechnology pave the way for the development of numerous drugs produced by pharmaceutical industries. Various active substance delivery systems and targeting systems have been developed to minimize active substance degradation and loss, prevent harmful side effects, and increase bioavailability and potency. Some of these systems include liposomes, nanoparticles, active substance-polymer conjugates and polymeric micelles [7,8,15,16,17,18,19].
The process of administering pharmaceuticals to humans or animals in order to achieve a therapeutic effect is known as drug delivery [20,21]. The pharmaceutical delivery system typically contains an appropriate dosage form that delivers the medication to the body; a mechanism for releasing the medication from the dosage form to the target cells following administration; and the device or technique used to create the dosage form [22,23,24]. Over the years, controlled drug delivery systems which dispense medications at predetermined rates for predetermined amounts of time have been developed. Unlike traditional dosage forms, controlled drug delivery systems prevent the drug from being released immediately and cause drug levels in the blood to fluctuate depending on the dosage form [25,26,27,28]. But it would be ideal if medications could be given in a way that perfectly meets the needs at the right periods and at the right location. It would have been very helpful if the active medicines could be administered through a system that detected the disease symptom, assessed its strength, and lastly acted to deliver the appropriate dosage of medication in response. The development of a smart drug delivery system like this would necessitate some kind of feedback mechanism to link the drug administration rate with physiological need [29,30,31,32,33]. Some medications that must enter the intracellular environment in order to have the desired effects are either incapable of being absorbed by cells or are wiped out by interaction with cytosol proteins. These issues have been resolved through advancements in nanotechnology and the development of new drug carriers.
The aim of drug targeting is to selectively transport, absorb and distribute the pharmacologic agent to the site of action. With this selective targeting, undesirable side effects are reduced, optimal therapeutic response is obtained, and substances with toxic effects at high doses can be used safely. With targeting, conventional, biotechnological and gene-derived drugs can be selectively delivered to specific parts of the body such as organs, tissues and cells. In the field of drug targeting [34], the liposomal formulation Doxil® (Doxurobicin, Baxter Healthcare Corporation, Deerfield, IL, USA) [35] and the nanoparticle formulation Abraxane® (Paclitaxel, Abraxis BioScience, Los Angeles, CA, USA) [36] have been approved by the US Food and Drug Administration (FDA) as new drug delivery systems. In addition, tumor targeting studies with specific monoclonal antibodies are currently being conducted. Monoclonal antibodies such as Erbitux® (Cetuximab, Eli Lilly and Company, Indianapolis, IN, USA) [37,38] used in colorectal cancer treatment, Vectibix® (Panitumumumab, Amgen Inc., Thousand Oaks, CA, USA) [39] and Trastuzumab (Herceptin®, Genentech, South San Francisco, CA, USA) [40] used in antiangiogenic therapy; small molecule tyrosine kinase inhibitors such as Imatinib (Gleevec®, Novartis Pharmaceuticals Corporation, Basel, Switzerland) [41], Erlotinib (Tarveca®, CHEPLAPHARM, Ziegelhof, Germany) [42], Sorafenib (Nexavar®, Bayer HealthCare Pharmaceuticals Inc., Whippany, Hanover, NJ, USA) [43,44], and Sunitinib (Sutent®, Pfizer, Hudson Boulevard East New York, NY, USA) [45] have received FDA approval and are used in the clinic.
2. Drug Delivery Systems
Modern drug delivery systems have revolutionized therapeutic approaches by enabling precise, controlled, and efficient delivery of pharmaceutical compounds. These systems are designed to overcome traditional drug administration limitations, such as poor bioavailability, systemic toxicity, and lack of targeted delivery. Active and passive drug delivery mechanisms play pivotal roles in ensuring effective drug transport. While passive delivery relies on natural diffusion and concentration gradients, active delivery employs external stimuli or molecular recognition to achieve targeted release, enhancing therapeutic efficacy. Hydrogel-based drug delivery systems have gained attention due to their biocompatibility, high water content, and tunable properties, making them suitable for controlled and sustained drug release applications. Thermoplastic drug delivery systems leverage the versatility of thermoplastic polymers, offering robustness, flexibility, and adaptability for fabricating drug carriers that respond to physiological conditions. Microdevice-based delivery systems represent a breakthrough in precision medicine, utilizing miniaturized devices for localized drug administration, improving therapeutic outcomes while minimizing systemic effects. Transdermal patches provide a non-invasive alternative for drug administration, ensuring sustained release through the skin barrier, which improves patient compliance and maintains steady drug plasma levels. This section delves into these advanced drug delivery modalities, highlighting their principles, advantages, and potential for addressing current challenges in pharmaceutical science.
2.1. Active and Passive Drug Delivery
In some cases, drug targeting can be achieved in a simple way. The drug is administered directly to the pathologic site. Some of the successful examples of this approach are direct hormonal drug administration into the joints in the treatment of arthritis and direct administration of thrombolytic enzymes used in the treatment of myocardial infarction caused by thrombus directly into the coronary vessels [46,47]. Active targeting is defined as specific interactions between the drug delivery system and target cells, in short ligand-receptor interactions [48]. The essence of active targeting is based on the use of targeted ligands such as antibodies and peptides that can bind specifically to receptor structures directed to the target structure. Examples of targeted ligands from drug delivery systems used in active targeting to tumor cells include folate, transferrin, and galactosamine [49,50]. The success of active targeting is ensured by the correct selection of targeting vehicles that show high affinity for cell surface receptors and chemical modifications to form appropriate conjugation. Active targeting can be achieved by ligand-receptor, antigen-antibody interactions or by targeting aptamers to identify pathological cells with various molecules concentrated at the pathological site [51,52,53]. Aptamers are DNA or RNA oligonucleotide sequences that bind selectively with high affinity to the target utilized in the active targeting of therapeutics [54,55]. The targeted therapeutic agent prefers high drug accumulation in the pathological structure with the help of a carrier that can combine with a cell or tissue-specific ligand. Thus, besides having the ability to combine with different targeting ligands, nanosystems of varying sizes can offer excellent opportunities for overcoming physiological barriers and efficient cellular uptake of the drug. Various nanosystems can achieve higher concentrations in cellular uptake than normal drugs [56,57].
By integrating the drug into a macromolecule or nanoparticle that enters the target tissue passively, passive targeting is accomplished [16,58,59,60]. The length of circulation is determined, and the drug is ensured to reach the target organ with a coating around the nanoparticle. The surface of the nanoparticle can be made hydrophilic by the addition of a chemical such as as polyethylene glycol (PEG) [61,62,63,64,65], which allows the H2O molecules to interact with the O2 molecules through hydrogen bonding. After this interaction, the material becomes antiphagocytic. In a study for passive or active tumor targeting, poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) were produced in different compositions and combinations. Arg-Gly-Asp (RGD); chitosan; dopamine (DOPA); folic acid; hyaluronic acid; poly(ethylene glycol) (PEG); reticuloendothelial system (RES); transferrin (Tf); and vascular endothelial growth factor (VEGF) were loaded onto poly(lactic-co-glycolic acid) nanoparticles (NPs), demonstrating the passive and active tumor targeting capabilities of ligand-receptor interactions (Figure 1G) [66,67,68,69]. The enhanced permeability and retention (EPR) (Figure 1H) [61,70] effect is observed in cancerous tissues due to extensive vascular leakage and lack of lymphatic drainage. This enhanced vascular permeability effect in tumor sites compared to healthy tissues can be utilized for the delivery and accumulation of passive targeting nanocarriers in targeted tissues.
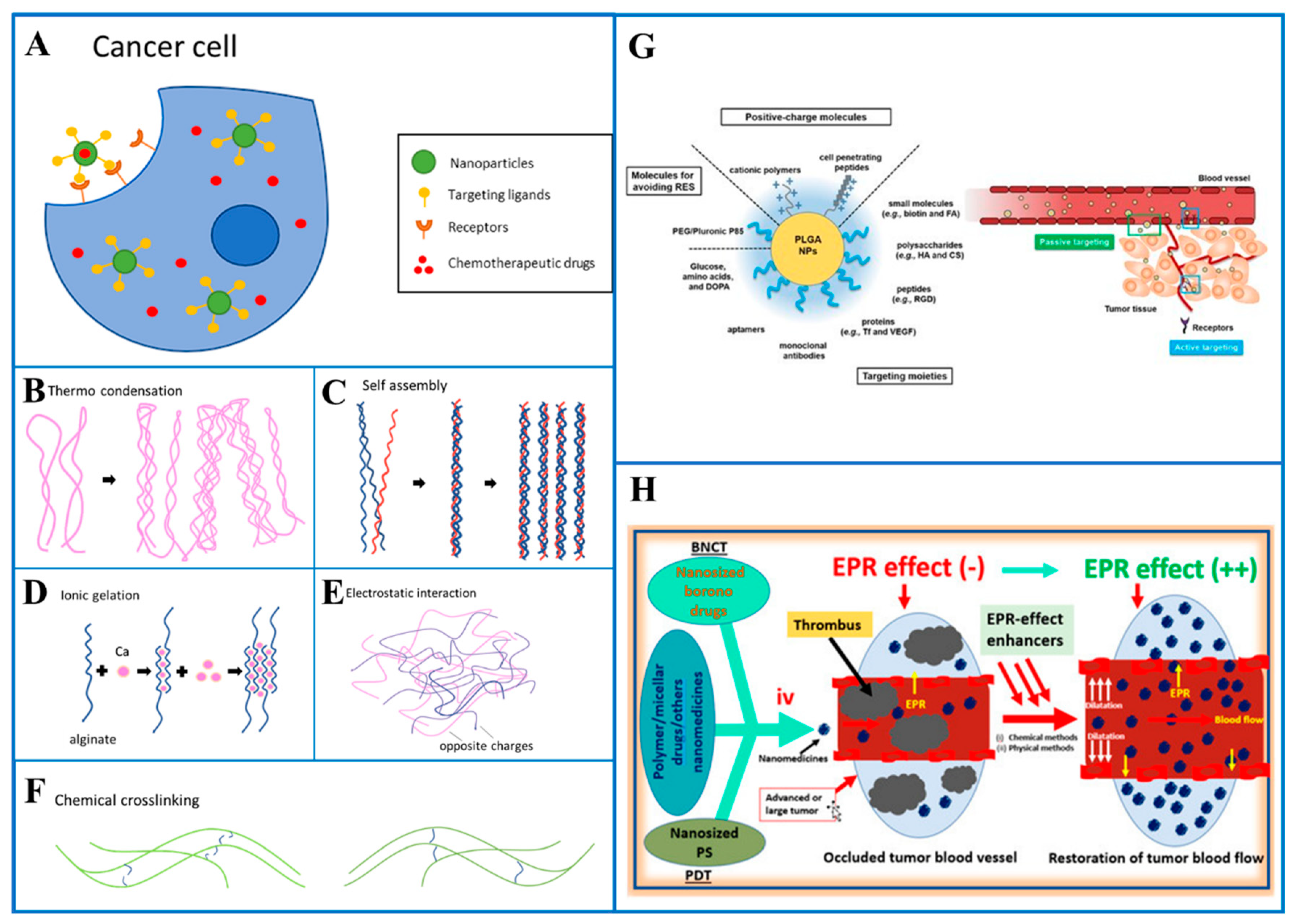
Figure 1.
(A) Drug delivery with active targeting on a cancer cell, crosslinking mechanisms. (B) Thermo condensation, (C) Self-assembly, (D) Ionic gelation, (E) Electrostatic interaction, (F) Chemical crosslinking, (G) Various surface-engineered poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) for passive or active tumor targeting. Arg-Gly-Asp (RGD); reticuloendothelial system (RES); transferrin (Tf); vascular endothelial growth factor (VEGF) Reproduced from [71], MDPI, 2019. (H) Graphical abstract of enhanced permeability and retention (EPR) Reproduced from [72], MDPI, 2022.
Active targeting improves targeting such that a medicine becomes specialized to a target spot. The diseased tissue can be actively targeted by knowing the nature of the receptors on cells associated with it (Figure 1A). This makes the utilization of ligands, which allow the drug to specifically bind to the cells with the corresponding receptors, possible. Transferrin and RGD motif [73,74,75] are examples of cell-specific ligands which are utilized to target specific receptors on tumor cells. Magnetoliposomes, which are often used in Magnetic Resonance Imaging (MRI) as a contrast agent, can also be used in active targeting. Merging these liposomes with drugs allows magnetic placement to help the delivery of drugs to a specific area of the body. Redox potential is the basis for another triggering mechanism, hypoxia, which is one of the side effects of tumors and affects the redox potential in the tumor’s proximity. Different tumor types can be targeted specifically by particles via altering the redox potential that causes the release of the payload.
In general, passive targeting is the delivery of drugs to specific sites through natural physiological processes and factors. Passive targeting exploits anatomical differences between normal and pathological tissues to move drugs to the required site. Research has shown that in some cases (such as tumor cells) the permeability of blood vessel walls is increased. Due to the loose vascularity of the tumor, the drug delivery system spontaneously penetrates through the blood vessel walls into the interstitium. This is called the increased EPR effect (Figure 1H) [76,77,78]. The EPR effect has been observed not only in tumor cells but also in areas of inflammation. Maeda et al. demonstrated that excessive bradykinin release at sites of infection or inflammation elicits the EPR effect. The only difference between the EPR effect in an infection-based and tumor cell is the duration of drug retention time. When infection occurs in a normal cell, the retention time is shorter than in a cancer cell because the lymphatic drainage system is still functioning, so the infection can dissipate within a few days. In contrast, macromolecular or lipid drugs can take weeks to take hold in the cancer cell. The EPR effect has been utilized to transport various therapeutics to the site of action. Many studies have suggested findings supporting the mechanism of passive targeting. In the 1980s and 1990s, many nanocarriers based on passive targeting mechanisms were designed. For example, doxorubicin (DOXIL) designed in liposomal formulation was observed to be 6 times more effective compared to free doxorubicin [79,80,81].
2.2. Hydrogel Drug Delivery Systems
Selecting the ideal drug delivery material for the required clinical purpose might be challenging due to the diversity of the currently available polymers. Developments in polymer science resulted in the production of hydrogel systems which have been employed for different kinds of medication delivery applications over the years. Hydrogels are among the most promising biopolymers being used for drug delivery applications. They are made of tunable, injectable polymer-based networks which can absorb and hold large quantities of water. They can be produced from a variety of different polymers such as polyethylene glycol (PEG), poly(acrylic acid) (PAA), poly(N-isopropylacrylamide), amine terminated, and polypeptides as synthetic polymers and alginate, hyaluronic acid, collagen, chitosan, gelatin, dextran and silk as natural polymers (Table 1). Hydrogels can be created by chemical and physical crosslinking. One may readily produce hydrogels using physical mechanisms such as heat and ionic gelation, self-assembly, and electrostatic interactions. However, their qualities mostly rely on the inherent characteristics of the polymers and the hydrogels made using these procedures have a limited tolerance for fine-tuning. Contrarily, chemical crosslinking techniques enable more precise crosslinking but typically call for the alteration of the polymers, which may compromise their biofunctionality [82,83,84,85,86].
Physical crosslinking can be achieved by different methods. These methods include thermo condensation (Figure 1B), self-assembly (Figure 1C), ionic gelation (Figure 1D) and electrostatic interaction (Figure 1E). Thermo condensation happens when the polymer chains get physically entangled as a result of temperature fluctuations during the gelation process. Self-assembly depends on noncovalent bonds like hydrogen bonds, van der Waals forces, and hydrophobic interactions. Ionic and electrostatic interactions are caused by the interaction of the opposing charges. Chemical crosslinking (Figure 1F), on the other hand, is achieved by covalent bonding of chemically active polymers. Numerous techniques have been employed, including radical polymerization, aldehyde complementation, high-energy irradiation, click chemistry, carbodiimide chemistry, enzyme-enabled biochemistry and azide-alkyne reactions. When compared to physical processes, chemical crosslinking techniques result in greater stability in the hydrogel matrix. They also provide more control and flexibility of hydrogel production.
Collagen that has been partly hydrolyzed into gelatin may be treated and altered with ease utilizing various techniques and chemistries. Hydrogels are created by the heat condensation of gelatin taken from a variety of animal sources, a method which has been widely researched. Gelatin can also be modified with methacryloyl residues, which could be a good example of the chemical crosslinking of gelatin to produce materials [87,88,89,90]. The produced hydrogels were examined as prospective instruments for delivery of drugs and genes, as well as for the regeneration of various tissues. Through the use of enzymes, decellularized tissue extracellular matrix (dECM) may be converted into biomimetic hydrogels by being neutralized to temperature and pH. They may be extracted from any tissue, and depending on the source tissue and decellularization method, they produce hydrogels with distinct biochemical, architectural, and viscoelastic properties. This led to the creation of several dECM bioinks (Figure 2A) from a variety of organs and tissues. The use of dECM bioinks to control stem cell differentiation and produce biomimetic and functional tissues has enormous promise for use in disease modeling, drug monitoring and regenerative medicine [91,92,93].
The rate at which pharmaceuticals are released from DDSs can be regulated by factors such as the mesh size of the hydrogel network, degradation rate, and swelling ratio. The majority of these characteristics can be changed by varying concentration and crosslinking intensity. This allows them to be specifically engineered to deliver a variety of bioactive molecules [82,84]. Hydrogels can be made to respond to changes in their surroundings or other stimuli. However, cross-linked networks may hinder enzyme penetration, slowing down drug release and degradation. Developments in material science include hydrogels that are capable of repairing themselves after taking damage. The hydrogel’s structure and electrostatic forces stimulate the development of new bonds by non-covalent hydrogen bonding or reconstructive covalent dangling side chains. These characteristics, which resemble flesh, have encouraged research and development on self-healing hydrogels for use as scaffolds in passive and preventative applications as well as in reconstructive tissue engineering [94,95,96].

Table 1.
Components used in polymeric-based, drug-loaded hydrogel drug delivery systems, applications, methodology and advantages.
Table 1.
Components used in polymeric-based, drug-loaded hydrogel drug delivery systems, applications, methodology and advantages.
| Biocomposite | Applications | Methodology of Produced Hydrogel | Advantages | Ref. | ||
|---|---|---|---|---|---|---|
| Component 1 | Component 2 | Component 3 | ||||
| PVA(Polyvinyl Alcohol) | Alginate | Rosuvastatin (RSV) (Drug)-loaded Chitosan | Elimination of overdose, improves solubility of drugs | Solvent Casting Method | Elimination of overdose, improves solubility of drugs(PVA), Rate of drug release can be controlled by using different layers in hydrogels, nontoxicity, biodegradability, immunogenicity, biocompatilibilty. | [97] |
| PLA—Poly(Lactic) | PEG(Polyethylene Glycol) | Curcumin(Drug) | Wound dressing | Electrospinning | PLA increases the bioavailability and performance of curcumin in aqueous media, poly(ethylene glycol) (PEG) is widely used in pharmaceutical formulations due to its ability to increase the aqueous solubility of poorly soluble substances. This is owing to its specific properties, which include non-toxicity, great biocompatibility, and easy clearance from the human body. These qualities make PEG an important component in medicines, allowing the solubilization of otherwise weakly soluble chemicals, hence increasing their efficacy and bioavailability. | [98] |
| PNIPAAm | Curcumin(Drug) | - | Anti-cancer drug delivery | Free Radical Polymerization | PNIPAAM provides both pH- and temperature-sensitive drug release. It is biocompatible, and the loaded drug has the capacity to be released in response to the intracellular microenvironment. | [99] |
| Collagen | Recombinant Rat Nerve Growth Factor Beta (NGF-β) | - | Neuroregenerative Drug Delivery | Compression Molding | Favorable optical and mechanical properties to be applied in corneal stroma, collagen provided migration of host stromal cells | [100] |
| Chitosan Hydrogel | Graphene Oxide | Teriparatide | Regeneration of osteoporotic bone defects by utilizing photothermal responsive graphene oxide modified chitosan hydrogel | Electrodeposition | Reduced graphene oxide provides better photothermal conversion property. Also, this property enhanced the release of drug molecules | [101] |
2.3. Thermoplastic Drug Delivery Systems
When creating DDSs, thermoplastics provide a variety of benefits over other polymers [102,103,104]. They offer flexibility to modify mechanical characteristics and degradation to be used in different applications, and the ability to construct synthetic polymers into a variety of geometries using a variety of production techniques like casting, electrospinning, and 3D printing. Poly-(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) are some of the common thermoplastic polymers used in DDSs. These polymers are commonly hydrophobic and used for delivering hydrophobic drugs most of the time. One of their main benefits is maintaining stability while not requiring any crosslinking steps [105,106]. By changing PLA’s stereochemistry, one can change its thermal, mechanical and biological characteristics. Furthermore, by varying the proportion of PLA and PGA in the copolymer, further biodegradability in PLGA can be adjusted. Though local acidity increases resulting from decomposition may cause problems at the application site, PLGA is usually considered the “gold standard” among biodegradable polymers. According to in vitro release tests, the ratio of PLGA/PLA affected the rate at which DOX was released, with a higher PLA content. Additionally, it was shown that varying PEG concentrations can affect the release of DOX, since a rise in PEG corresponds to a marked rise in the rate of DOX release [107]. To create drug delivery systems, thermoplastic polymers can be used with a variety of fabrication processes. For instance, docetaxel was added to the poly(D,L-lactide) nanofibers by Ding et al. It was discovered that DTX may be released continuously from nanofibrous membranes in vitro for 24 days this way. In studies conducted on mice, it was observed that mice treated with drug-loaded membranes experienced a significantly lower rate of locoregional recurrence after the removal of the primary tumor compared to mice treated with systemically administered DTX or locally administered DTX. This suggests that drug-loaded membranes may be more effective in preventing local tumor recurrence in this experimental model. The absence of inflammation in the tissue surrounding these drug-loaded membranes underlined their high level of biocompatibility. In a distinct research study, nanofibrous membranes constructed from poly(L-lactic acid) and coated with 5-fluorouracil were designed to combat colorectal cancer in xenografted mice. The in vivo experiments revealed that these membranes were more effective at preventing tumor growth compared to intraperitoneal injection of 5-FU. This improved efficacy was attributed to the sustained and continuous release of 5-FU from the membranes, providing a more targeted and controlled approach to combating colorectal cancer [108]. In recent developments, 3D printing technology has been employed to fabricate implantable drug delivery systems using thermoplastic biopolymers. A recent innovative example in drug delivery involves the extrusion printing of a 3D patch made from a combination of PLGA and PCL, which is loaded with 5-fluorouracil. This 3D-printed patch is designed for the prevention of pancreatic cancer growth, showcasing the potential for tailored drug delivery systems specifically designed to address medical applications like cancer treatment. This approach combines advanced materials and 3D printing technology to develop more precise and effective drug delivery solutions [109,110]. In a different work, developed materials made of electrospun nanofibers that are loaded with drugs. Thermoplastic polyurethane (TPU) solutions at 8 and 10% (w/w) concentrations were electrospun to produce TPU ultrafine fiber mats that contain naproxen (NAP). The solutions comprised 10 and 20 percent NAP, respectively, based on the weight of TPU. The drug-loaded electrospun TPU fibers were collected at 5, 10, and 20 hours, and their properties were evaluated using Fourier transform infrared spectroscopy, differential scanning calorimetry, and thermogravimetric analysis. Average diameters of the NAP-loaded electrospun TPU fiber mats ranged from 523.66 to 723.50 nm, and they exhibited a smooth shape. To analyze the release characteristics of these fiber mats, the entire immersion method in a phosphate buffer solution at 37 °C was employed. It was observed that the duration of mat collection significantly influenced the release of NAP (Figure 2B) [102]. In a similar study, blend formulations for drug-eluting implants designed for prolonged loading have been developed. These formulations combine a high-modulus thermoplastic segmented polyurethane (TSPU) with poly(L-lactide) (PLLA). The production of various blend compositions was achieved by solution casting. This process is part of the development of drug-eluting implant materials for medical applications. Both strip- and tube-shaped samples underwent an elastic recoil test, and the tensile elastic recovery (TER) and relative elastic recovery (RER) were calculated. To assess the mechanical properties of various blend compositions and pure polymers, a uniaxial tensile test was performed [111]. In conclusion, by creating blends with various PLLA/TSPU ratios, a wide variety of DDSs with diverse extendibility, expandability, and elasticity can be created. An important benefit of using these polymer blends as implantable DDSs is the absence of the need for any plasticizer or compatibilizer.
Figure 2.
(A) 3D bioprinted breast tumor-stroma tumor models Reproduced with permission from [93], Elsevier, 2023. (B) naproxen-loaded thermoplastic polymer drug delivery system with electrospinning method Reproduced with permission from [102], Elsevier, 2016. (C) the antitumor effect of Dox combined with OPE and NR in vivo (xenograft tumor mouse model) Reproduced from [112], Elsevier, 2020. (D) zebrafish xenograft model Reproduced from [113], ACS publication, 2016 (E) schematic illustration of the 3D-printed microcontainers (Eudragit L100 and furosemide) that were appropriate as drug carriers Reproduced with permission from [114], Wiley Online library, 2023. (F) graphical abstract of transdermal patches embedded in nanostructured lipid carriers (NLCs) to improve transdermal delivery of capsaicin Reproduced with permission from [115], Elsevier, 2022. (G) in the in vivo wound model and in vivo persistence and histology images of resveratrol-loaded transdermal biomembrane Reproduced with permission from [116], Elsevier, 2023.
Figure 2.
(A) 3D bioprinted breast tumor-stroma tumor models Reproduced with permission from [93], Elsevier, 2023. (B) naproxen-loaded thermoplastic polymer drug delivery system with electrospinning method Reproduced with permission from [102], Elsevier, 2016. (C) the antitumor effect of Dox combined with OPE and NR in vivo (xenograft tumor mouse model) Reproduced from [112], Elsevier, 2020. (D) zebrafish xenograft model Reproduced from [113], ACS publication, 2016 (E) schematic illustration of the 3D-printed microcontainers (Eudragit L100 and furosemide) that were appropriate as drug carriers Reproduced with permission from [114], Wiley Online library, 2023. (F) graphical abstract of transdermal patches embedded in nanostructured lipid carriers (NLCs) to improve transdermal delivery of capsaicin Reproduced with permission from [115], Elsevier, 2022. (G) in the in vivo wound model and in vivo persistence and histology images of resveratrol-loaded transdermal biomembrane Reproduced with permission from [116], Elsevier, 2023.
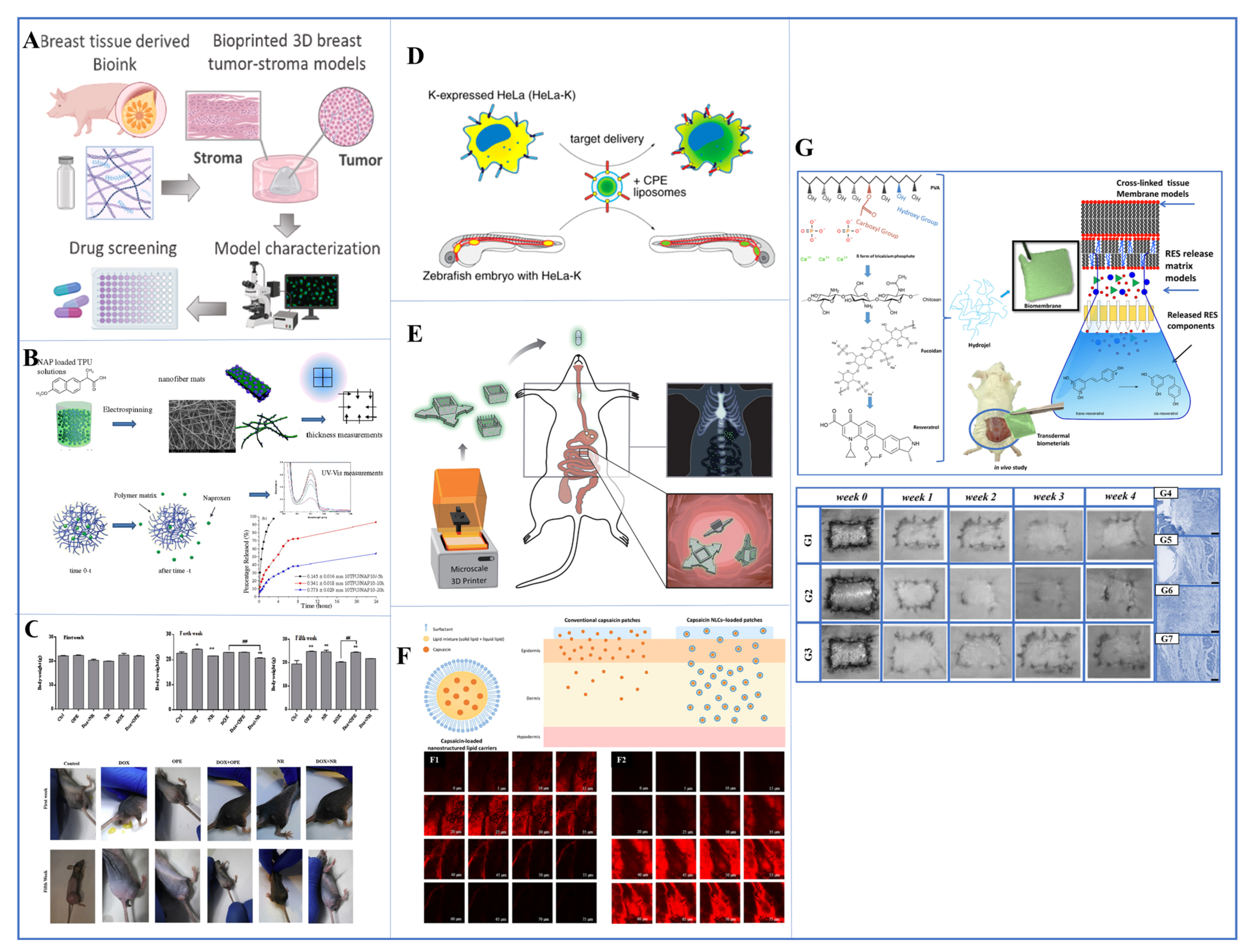
2.4. Microdevice Delivery Systems
Recent progress in the field of drug delivery has led to the development of integrated micro drug delivery devices [117,118,119,120,121]. These systems combine device technology with therapeutic molecules to create implanted devices that can provide disease therapy directly at the treatment site. Depending on their specific design, these microdevices offer various options, such as continuous or intermittent drug administration, and can function effectively for both short-term and long-term treatment regimens. This represents a significant step forward in the precision and effectiveness of drug delivery for various medical conditions. Similar to conventional drug delivery systems, these gadgets can either passively supply a medication or enable real-time drug dose changes through an outside stimulation. Jonas and his colleagues developed a tiny cylindrical device that uses micromachining to discharge a variety of medications using 16 distinct reservoirs around its periphery. The gadget was promptly put into tumors of various sorts utilizing a biopsy procedure, and it was left in place for 24 hours in order to quickly and simultaneously examine drug sensitivity in in vivo tumors. A coring biopsy needle was then used to remove a small portion of tissue from the device in order to assess each treatment’s potential anti-cancer effects. Three distinct methods were employed to alter the release rates of each medication in the reservoir. As a result, it was demonstrated that the device could administer a single drug or a combination of treatments into limited areas of cancer tissue. The researchers also assessed the pharmacokinetics of DOX release in breast cancer tumor xenografts (Figure 2C) and found that the medication delivered by the device can spread 200 to 300 nm into cancer tissue [112,113,122]. In another study, a significant effort was made by using photolithography to construct a temozolomide (TMZ)-based Microelectromechanical System Sensor (MEMS) microdevice that featured moving elements that could be controlled by an external magnet and was biocompatible and biodegradable. A mouse model was used to test in vivo the single-gear and Geneva drive variants of this device. A multireservoir gear was part of the single-gear system; one of the reservoirs contained iron oxide nanoparticles, while the others contained pharmaceuticals. Contrarily, the Geneva drive has two connected gears: a driven gear with six drug-filled chambers and a driving gear laced with iron oxide nanoparticles. The medicine was released from each reservoir when the top opening lined up with another opening on the uppermost part of the manufactured device, which was the same mechanism for both variants. An external magnet moved the gears, which turned them. Successful in vivo gear movement and controlled dye release were observed when the Geneva drive device was loaded with two different fluorescent dyes and subcutaneously implanted in a mouse model [123]. Another study involved a zebrafish xenograft (Figure 2D) that was injected next to E4-Lipo-TP3 or E4-Lipo-DOX into zebrafish xenografts of HeLa-K. As a result, E4-liposomes delivered TP3 to the implanted HeLa-K cells, and E4-Lipo-DOX could suppress cancer proliferation in the xenograft when compared to nontargeted conditions (i.e., zebrafish xenograft with free DOX injection). This resulted in enhanced cytotoxicity toward HeLa-K cells compared to free doxorubicin [113]. Another study described a technique for creating radiopaque microdevices with enhanced mucoadhesive geometries for testing and 3D printing. Three distinct microcontainer designs were developed and manufactured with the goal of extending gastrointestinal retention and managing the direction of one-way drug release. In order to determine if the 3D-printed microcontainers were appropriate as drug carriers for oral delivery, they were filled with the small molecule medication furosemide and coated with the pH-sensitive polymer Eudragit L100. The goal of the current work was to demonstrate that 3D-printed microcontainers can be used for oral medication delivery in a manner that is consistent with other types of microcontainers that have been previously reported. An Eudragit L100 coating was selected because of its suitable pH sensitivity and lack of enzymatic activity; it is commonly employed for oral medication administration to the small intestine. For an in vivo rat study, a comprehensive experimental design was developed, involving 24 rats. The study involved a 30-minute gastric phase, succeeded by an intestinal phase, lasting until complete furosemide [124] release was achieved. This release pattern resembled that of microcontainers produced using previous methods. Therefore, the 3D-printed microcontainers displayed promising results as an oral drug delivery system, particularly for targeted distribution to the small intestine when combined with a pH-sensitive polymeric coating (Figure 2E) [114].
2.5. Transdermal Patch Delivery Systems
Transdermal DDSs are designed to stick to the surface of the skin in order to permit the transfer of the active chemicals through the skin layers for either a local or systemic effect. Transdermal sprays, lotions, patches, and implantable devices can all be used to accomplish this. For transdermal patches, the medication is frequently housed in a reservoir with a porous membrane around it. Alternatively, it might be enclosed in an adhesive matrix that melts at body temperature to release the medication embedded therein [125,126,127,128,129]. In previous work, a transdermal DDS was developed for the first time to deliver prophylactic antibiotics. Solid lipid nanoparticles (SLNs) loaded with the vitamin E-based antibiotic cephalexin were created into a pressure-sensitive transdermal patch. After loading into TOS-based SLNs with different drug-to-lipid ratios, cephalexin was dispersed in PIB-based adhesive solutions using a weight percent ratio of 60:40 with PIB-B10 and PIB-B50. The incorporation of nanoparticles also resulted in an approximately twofold increase in drug loading. By using this optimal formulation, it is possible to reduce drug use by approximately 28% while maintaining antibacterial efficacy. In vitro drug release, antimicrobial activity and skin cell proliferation properties of transdermal patches were evaluated. It has been observed that the growth of human fibroblast skin cells in optimal patch-containing media occurs at approximately 25.5% proliferation [130]. In similar studies, they developed transdermal patches containing capsaicin nanostructured lipid carriers (NLCs), a drug used to treat skeletal muscle and neuropathic pain. These capsaicin-loaded NLCs were then incorporated into polyacrylic polymers to create transdermal patches with a consistent capsaicin concentration of 0.025 percent. In vivo studies showed that traditional capsaicin drug-loaded patches can cause skin irritation and redness, while capsaicin NLC-loaded patches exhibited lower skin side effects. Therefore, capsaicin NLC-loaded patches have been shown to be a potential transdermal delivery system for capsaicin, possibly reducing skin irritation (Figure 2F) [115]. In other similar studies, the design and production of transdermal membranes loaded with resveratrol and added with fucoidan and chitosan bioactive substances based on polyvinyl alcohol/β-tricalcium phosphate were carried out. In addition to in vitro biocompatibility (MTT test) and in vivo studies on resveratrol-loaded transdermal membranes, bioadhesion strength was also calculated. The shelf life of resveratrol-loaded transdermal membranes was also calculated using the Minitab program. In vivo wound healing in 4-week-old mice was estimated to be 98.75%, while the shelf life of resveratrol on transdermal membrane was estimated to be approximately 36 days. This study demonstrated that the innovative and novel transdermal biomaterial promoted tissue cell regeneration and cell proliferation in theranostic applications as wound dressings (Figure 2G) [116].
3. Nanobiotechnology Drug Delivery Systems
Materials at the nanoscale are employed to administer therapeutic drugs or as diagnostic instruments in nanomedicine, a field of study that is still relatively young but evolving quickly [131,132]. Nanomaterials can mimic the endocytosis mechanism used by viruses to get through a cell membrane [133]. To achieve their objectives, nanomaterials must possess specific characteristics. Each particle must circumvent two elimination systems that threaten its circulation time: renal clearance and phagocytosis. Therefore, it’s crucial to fine-tune the size and shape of these particles to optimize their half-life in circulation and their ability to enter cells. To prevent renal clearance, these particles should have a size greater than 10 nm, while to evade phagocytosis, their size should be smaller than 500 nm. This size range ensures they can effectively navigate both elimination systems [134].
3.1. Nanoparticle Internalization and Transport Mechanisms in Drug Delivery Systems
Before delving into the various nanomaterials utilized in nanobiotechnology-based drug delivery systems, it is essential to understand the mechanisms of nanoparticle internalization and transport. These processes, including endocytosis, clathrin-mediated endocytosis [135,136,137], caveolae-mediated endocytosis [138,139,140], and micropinocytosis [141,142,143], play a pivotal role in determining the efficiency and specificity of nanoparticle-mediated drug delivery. A thorough understanding of these mechanisms enables the rational design of nanocarriers capable of effectively targeting specific cells or organs while minimizing off-target effects.
Endocytosis represents the primary cellular mechanism for the internalization of nanoparticles and other extracellular substances [144,145]. This process involves the engulfment of particles through the formation of vesicles derived from the plasma membrane. Endocytosis is broadly categorized into phagocytosis and pinocytosis, with the latter being more relevant for nanoparticle uptake in non-phagocytic cells. Pinocytosis is further divided into clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis, each characterized by distinct cellular pathways and mechanisms.
3.1.1. Clathrin-Mediated Endocytosis
Clathrin-mediated endocytosis is one of the most extensively studied nanoparticle internalization mechanisms [135,137]. This process involves the formation of clathrin-coated pits on the plasma membrane, which invaginate to form vesicles. These vesicles transport nanoparticles to early endosomes and subsequently to lysosomes for further processing or degradation. The size, charge, and surface functionality of nanoparticles are critical factors influencing their uptake via this pathway. Nanoparticles functionalized with ligands targeting clathrin-associated receptors, such as transferrin receptors, exhibit significantly enhanced internalization efficiency [146,147,148].
3.1.2. Caveolae-Mediated Endocytosis
Caveolae-mediated endocytosis involves small, flask-shaped invaginations in the plasma membrane enriched with cholesterol, sphingolipids, and caveolin proteins [139,149]. Unlike clathrin-mediated pathways, caveolae-mediated endocytosis often bypasses lysosomal degradation, making it an advantageous route for the delivery of sensitive therapeutic agents such as proteins or nucleic acids [150,151,152]. Nanoparticles designed to exploit this pathway typically feature hydrophobic or amphiphilic surface modifications that mimic the lipid-rich environment of caveolae, enhancing their interaction and uptake.
3.1.3. Macropinocytosis
Macropinocytosis is a non-specific internalization mechanism characterized by the formation of large vesicles known as macropinosomes [141,153,154,155]. This process is often induced by cellular signaling events that trigger membrane ruffling, leading to the engulfment of extracellular fluid and particles. While macropinocytosis lacks the specificity of receptor-mediated pathways, it provides a high-capacity route for the uptake of larger nanoparticles or aggregates. Surface properties, such as hydrophilicity and charge, can significantly influence the efficiency of macropinocytic uptake [156,157,158].
3.1.4. Implications for Nanocarrier Design
The choice of internalization pathway has profound implications for nanocarrier design. For instance, nanoparticles targeting clathrin-mediated endocytosis necessitate precise ligand-receptor interactions, while those leveraging caveolae-mediated pathways benefit from structural mimicry of lipid rafts [159,160,161]. Furthermore, the size and morphology of nanoparticles must be optimized to facilitate interactions with specific endocytic pathways. Particles in the 10–100 nm range are generally ideal for clathrin-mediated endocytosis, whereas larger particles are more likely to engage macropinocytosis. Morphological features, such as spherical, rod-like, or platelet shapes, further influence the efficiency of cellular uptake and the selection of internalization pathways.
In conclusion, a comprehensive understanding of nanoparticle internalization and transport mechanisms is fundamental to advancing nanobiotechnology-based drug delivery systems. By leveraging these pathways, researchers can design nanocarriers with enhanced specificity, efficiency, and therapeutic potential. Such knowledge allows for the rational development of tailored nanomaterials for specific biological applications, ultimately improving the efficacy and precision of modern therapeutics.
3.2. Carbon Nanotubes for Targeted Drug Delivery
A carbon nanotube (CNT) is a structure that looks like a tube formed by rolling a graphene sheet. Its length and diameter are roughly a few hundred micrometers and a few nanometers, respectively. The primary advantages of CNTs are their high surface area for transporting several medications, sp2-hybridized structure, which may draw drug molecules and targeting agents, and ability to penetrate cells [162,163,164,165].
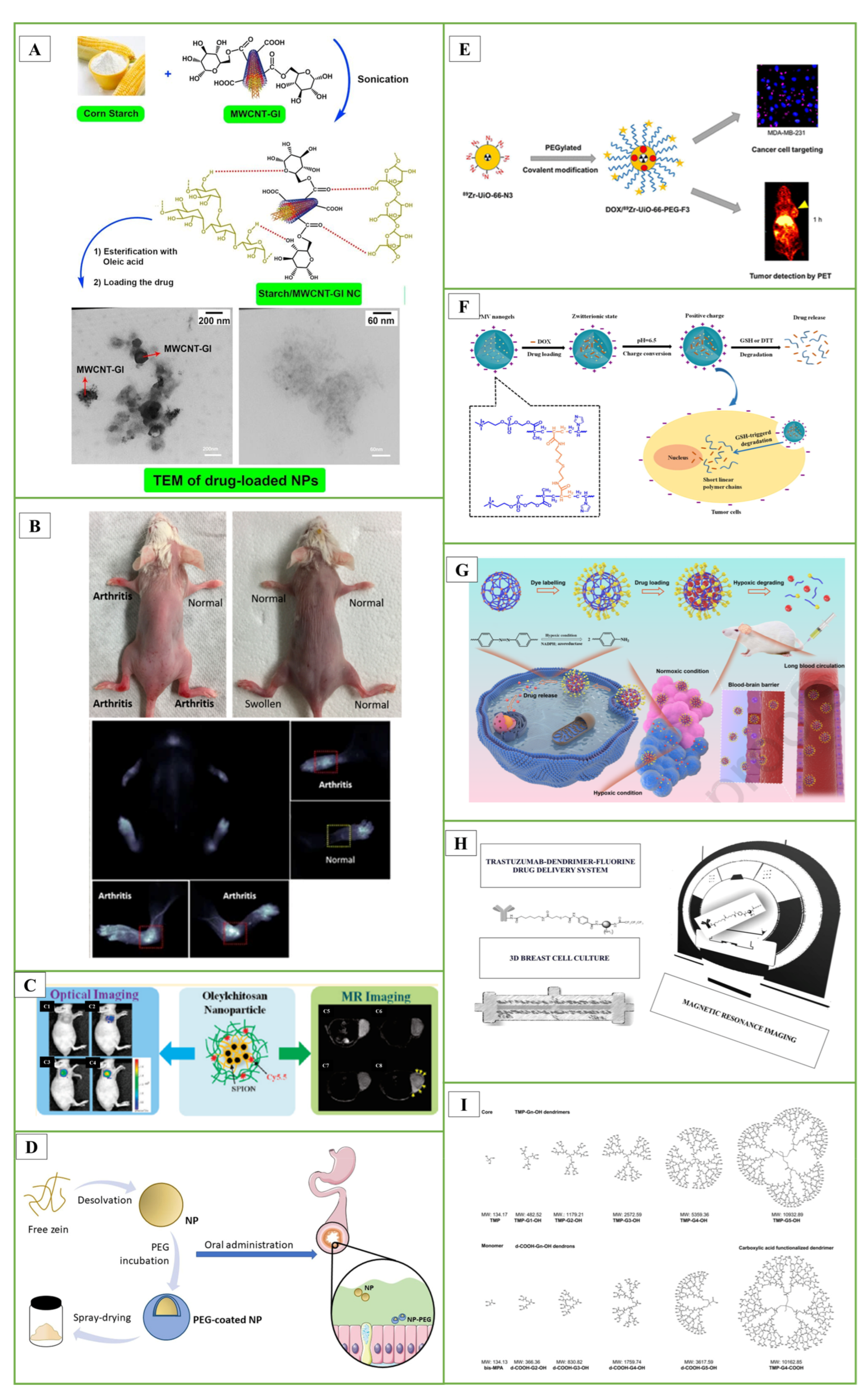
In the studies, starch nanocomposite (NC) films containing carbon nanotubes (MWCNTs) were designed. To increase the hydrophilicity of MWCNTs, its surface was modified with the D-glucose (Gl) biomolecule. Starch was loaded onto MWCNT-G1 NC by sonochemical method. Then, pure starch and starch/MWCNT-Gl NCs were reacted with oleic acid to obtain amphiphilic (Amph) esters. The hydrophobic anti-insomnia drug zolpidem was loaded onto NCs for drug delivery. The entrapment efficiency, loading capacity and in vitro release study of zolpidem as a hydrophobic drug model were performed (Figure 3A) [166].
In another study, they evaluated single-walled CNTs, specifically HiPco-SWCNT and carboxyl-SWCNT, as carriers for delivery of an anti-inflammatory drug, methotrexate, and siRNA targeting the NOTCH1 gene. Methotrexate (MTX) is frequently used to treat rheumatoid arthritis (RA). However, MTX is toxic, and a large proportion of patients receiving MTX alone or in combination with other medications experienced gastrointestinal side effects and hepatotoxicity. HiPco-SWCNTs or carboxyl-SWCNTs were combined with a NOTCH1 siRNA and/or MTX to create 12 different nanotube products. The products were then subjected to many tests to see if they could be used as a drug delivery mechanism to reduce the side effects of MTX. According to in vivo studies, HiPco-SWCNTs assemble in arthritic joints. The %CE of MTX for HiPco-SWCNTs was between 77% and 79%; for siRNA binding efficiency, this rate was up to 97%. SWCNTs showed dose-dependent interactions with monocytes, neutrophils, and to a lesser extent B cells when cultured with human blood. Notably, the incorporation of siRNA increased the adsorption efficiency of HiPco-SWCNTs. These findings indicate that HiPco-SWCNTs are effective drug delivery systems that can be used in the treatment of RA (Figure 3B) [167].
3.3. Nanoparticle Drug Delivery
Nanoparticles are matrix systems prepared with natural or synthetic polymers, ranging in size from 10–1000 nm, called nanospheres or nanocapsules depending on the preparation method, in which the active substance is dissolved in the particle, entrapped and/or adsorbed or bound to the surface. Nanocapsules are vesicular systems, in which the drug is entrapped in a cavity and surrounded by a polymer membrane. Nanospheres are matrix systems where the drug is physically and uniformly dispersed [8,131]. The advantages of nanoparticles, which are obtained by using natural or synthetic polymers and used for targeting drugs as well as proteins, peptides and genes to the relevant tissue, stem from two main features. The first is that nanoparticles have small particle sizes. Thus, they are taken into the cells by passing through small capillaries and provide accumulation of active substance in the target area. The second is the use of biodegradable materials in the preparation of nanoparticles. Biodegradable materials provide controlled release of active substances in the target tissue over periods of days or even weeks. In addition to all these, nanoparticles increase the stability of drugs, proteins or peptides; can be easily sterilized; have a high active substance loading capacity; and thus increase the intracellular distribution of the active substance. In this way, the release and bioavailability of the drug given as nanoparticles in oral drug administration increases [131].
Advanced synthesis techniques have made it possible to use a variety of materials and produce a large range of nanoparticles in different sizes and shapes. Nanoparticles can be categorized based on various physical and chemical attributes. Different types of nanostructures, including metalloid, organic, inorganic, and polymeric ones like dendrimers, micelles, and liposomes, are commonly considered when designing drug delivery systems for specific targets. These nanoparticles are particularly useful for enhancing the delivery of drugs with poor absorption and limited solubility. However, the effectiveness of these nanostructures as drug carriers can vary significantly based on factors such as their size, shape, and other inherent biophysical and chemical properties. Polymeric nanoparticles with a diameter of 10 to 1000 nm have characteristics that make them superior delivery devices.
A wide array of synthetic polymers, such as polyvinyl alcohol, poly-L-lactic acid, polyethylene glycol, and poly(lactic-co-glycolic acid), as well as natural polymers like alginate and chitosan, are extensively employed in the fabrication of nanoparticles [168,169,170,171]. Their use is driven by their excellent biocompatibility and biodegradability. These polymeric nanoparticles, both nanospheres and nanocapsules, offer effective drug delivery methods. Additionally, lipid-based nanostructures, including compact lipid nanoparticles and phospholipids, are highly valuable for targeted drug delivery, much like liposomes and micelles [172,173,174].
3.3.1. Coated Nanoparticles
By coating nanoparticles with surfactants or physiological elements like serum complement factors, one can change the dispersion of the particles within the body. To target binding drugs to tumors, the brain, and inflammatory bodily regions, different coated nanoparticles can be employed [175,176,177].
In previous studies, they encapsulated oleic acid-coated FeO nanoparticles in oleic acid-conjugated chitosan (oleyl-chitosan) to investigate the uptake of nanoparticles by tumor cells. They aimed to study the in vivo penetration and retention of these nanoparticles for analytical applications using near-infrared and magnetic resonance imaging techniques. After intravenous administration of cyanine-5-linked oleyl-chitosan nanoparticles, both imaging modalities exhibited a noticeable increase and improvement in signal intensity in tumor tissues, primarily due to the increased EPR effect. This research demonstrated the potential utility of these nanoparticles for imaging and analytical purposes (Figure 3C) [178].
In another study, a biomimetic nanoparticle formulation designed to target inflammation by exploiting the specific interaction between very late antigen-4 (VLA-4) and VCAM-1 was developed. In this approach, polymeric nanoparticle cores are surrounded by plasma membrane from cells genetically modified to consistently express VLA-4. Consequently, these cell membrane-coated nanoparticles exhibit enhanced affinity towards target cells overexpressing VCAM-1, as demonstrated in vitro. Additionally, this formulation involves the encapsulation of dexamethasone, a well-known anti-inflammatory drug. This improves drug delivery to inflamed lung tissues and greatly increases its therapeutic efficacy in vivo. The overall goal of this research is to exploit naturally occurring target-ligand interactions and take advantage of the different properties of biological membrane coatings to develop nanoparticles with greater targeting specificity [179].
3.3.2. Pegylated Nanoparticles
The target molecule is commonly PEGylated by being incubated with a reactive PEG derivative [180,181]. By increasing the agent’s size in solution and lengthening its circulation duration by reducing renal clearance, covalent attachment of PEG to a medication or therapeutic protein may “hide” the agent from the host’s immune system, reducing immunogenicity and antigenicity while increasing its size in solution. PEGylation is able to make hydrophobic proteins and drugs soluble in water. PEGylation technology is expanding quickly and has proven to have therapeutic effects [182].
In a study, PEG-coated nanoparticles (NP-PEG) with mucus-permeable properties were produced for oral drug delivery. For this purpose, zein nanoparticles were prepared by desolvation and then coated by incubation with PEG 35,000. The hydrophobic surface of zein nanoparticles (NP) was significantly reduced due to their coating with PEG. This increase in the hydrophilicity of PEG-coated nanoparticles was associated with a significant increase in their mobility in porcine intestinal mucus. After oral administration, NP appeared to remain trapped in the mucus network, whereas NP-PEG was able to penetrate the protective mucus layer and reach the epithelium. It was concluded that PEG-coated zein nanoparticles could be adequate carriers to promote the oral bioavailability of biomacromolecules and other biologically active compounds with low permeability properties (Figure 3D) [183].
In another important study, a zirconium-based DOX UiO-66-N3 was developed, and its surface was covalently functionalized with PEG containing alkynes using azide-alkyne click chemistry. The F3 peptide was then incorporated to specifically target cancer cells. Positron-emitting zirconium-89 was added to UiO-66-N3 to monitor pharmacokinetic behavior in vivo. A corresponding PEGylated F3 peptide was conjugated to provide 89Zr-UiO-66-PEG-F3. Serial PET imaging demonstrated that 89Zr-UiO-66-PEG-F3 accumulated preferentially in MDA-MB-231 tumors and was cleared from the liver more quickly than PEGylated UiO-66 using non-covalent techniques (Figure 3E) [184].
Advantages of PEG-Activity Conjugates
- PEG masks the protein surface by steric hindrance and can be used to protect against renal damage;
- It increases the molecular size of the polypeptide, and as a result, renal ultrafiltration is reduced;
- The contact of the antibody or antigen processing cells with PEG chains is also inhibited;
- Protein immunogenicity is reduced or eliminated;
- PEG carries its physicochemical properties to the peptide or nonpeptide molecule to which it binds, and thus the bioavailability and solubility properties of the substance are altered;
- Enzymes and bioactive substances dissolve in organic solvents or aqueous solutions;
- In vivo, the excretion of PEG-protein conjugate and its circulation time in the blood are prolonged;
- Stabilizes the physiological properties of proteins and bioactive substances;
- Improves the pharmacokinetic properties of various active substances;
- Increases accumulation in tumor tissues [19].
3.3.3. Solid Lipid Nanoparticles (SLN)
Polymer-based nanocarriers offer various advantages due to their versatility in enabling a wide range of modifications, like the creation of block and comb polymers. Solid lipid nanoparticles represent one such type of nanocarrier design that capitalizes on the strengths of colloidal carriers while circumventing their limitations [185,186,187]. SLNs typically consist of approximately 0.1–30% (w/w) solid fat and exhibit an average size ranging from 150 to 300 nm, although their size can extend up to 1000 nm based on the surfactant employed during the manufacturing process. The behavior of SLNs is influenced by their size and the ratio of solid to liquid fat. Some advantages of SLNs include the ability to load both lipophilic and hydrophilic therapeutic agents, high drug loading capacity, easy synthesis in larger quantities, and minimal to no adverse effects on healthy tissue. One of the most common applications of SLNs as nanocarriers is for the oral administration of medications [187,188]. In the study, they developed solid lipid nanoparticles through a heated, high-pressure homogenization process. They conducted a study to evaluate how independent variables, specifically surfactant and lipid ratio, affect the physicochemical properties of SLN, including average particle size (Z-Ave), polydispersity index (PDI), and zeta potential (ZP). ZP measurements were obtained using electrophoretic light scattering, while Z-Ave and PDI were determined using dynamic light scattering. Findings show that the optimal SLN dispersion contains 1 weight percent α-pinene, 4 weight percent solid lipid, and 2.5 weight percent surfactant, resulting in Z-Ave at 136.7 nm, PDI at 0.170, and 0 mV ZP. Additionally, the stability analysis of α-pinene loaded SLN was effectively carried out using LUMISizer® (Berlin, Germany) [189].
3.3.4. Smart Polymeric Nanogels
Polymeric nanovehicles have demonstrated exceptional capabilities for encapsulating and delivering theranostic substances under physiological conditions. They can even be utilized for monitoring therapeutic response. Presently, polymer nanogels are widely recognized as efficient delivery systems for a diverse range of therapeutic and diagnostic compounds [190,191,192,193,194]. Notably, biodegradable and “intelligent” nanogels made from intelligent polymers offer substantial advantages due to their ability to respond to both endogenous and exogenous stimuli, including pH gradients, bioresponsiveness, photoresponsiveness, temperature, and more. Many multifunctional nanogels with excellent targetability and sensitivity have been developed for various theragnostic applications [193,194,195,196,197,198].
The current synthesis techniques for these nanogels can be broadly categorized into four groups [199,200]: (i) homogeneous or heterogeneous monomer polymerization; (ii) appropriate polymer self-assembly; (iii) suitable polymeric architecture cross-linking; and (iv) template-assisted nanogel synthesis. These techniques have found applications in a variety of theragnostic contexts [191]. In the literature study, using a one-step reflux precipitation polymerization process, they formed a series of biodegradable poly(2-methacryloyloxyethyl phosphorylcholine-s-s-vinylimidazole)(PMV) nanogels with uniform spherical shape. These nanogels exhibited specific behaviors in response to pH changes. At physiological pH, the PMV nanogels remained in the zwitterionic state but rapidly switched to a positively charged form at the extracellular pH of tumors. A study focusing on protein stability revealed that DOX-loaded PMV nanogels could resist protein adsorption at pH 7.4 for up to 7 days. However, they readily absorbed proteins at pH 6.5. This behavior highlights the potential of these nanogels for controlled interactions against tumor cells under varying pH conditions (Figure 3F) [201]. In another study, hypoxia degradable zwitterionic phosphorylcholine nanogels called HPMPC were produced as nanoplatforms for chemotherapy treatment of glioblastoma, a malignant tumor. HPMPC nanogels cross-linked with azobenzene were biocompatible and prevented contamination in in vivo studies on mice and showed the ability to circulate in the blood for a long time. Moreover, HPMPC nanogels effectively crossed the blood-brain barriers and provided long-term accumulation in glioblastoma tissue. In conclusion, HPMPC drug nanogels exhibited a positive inhibition effect in the glioblastoma tumor model, making it a potential nanoplatform candidate to treat various hypoxic-related diseases in the central nervous system (Figure 3G) [202].
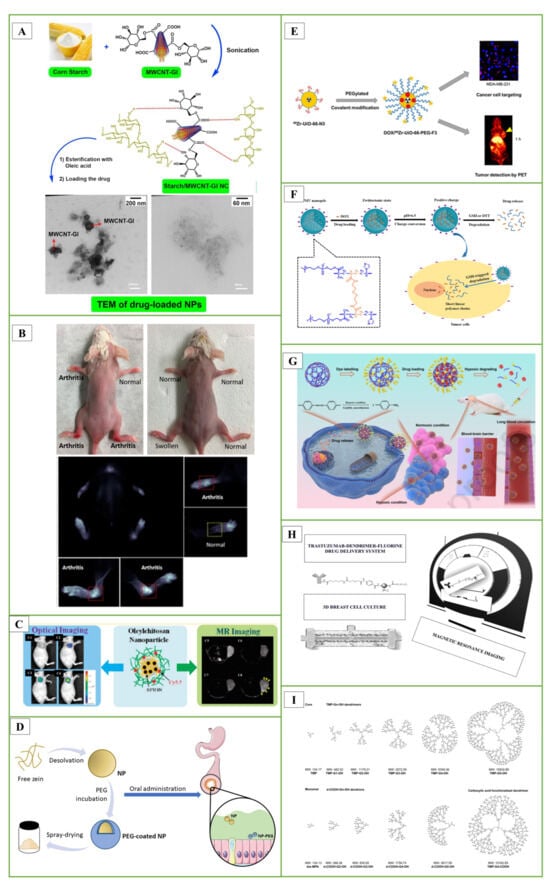
Figure 3.
(A) The sonochemical method was used to prepare starch/MWCNT-Gl NCs nanoparticles for drug delivery Reproduced with permission from [166], Elsevier, 2018. (B) Arthritis imaging of HiPco-cy5.5 in the control group and mice with highlighted arthritis and normal joint Reproduced [167], MDPI, 2021. (C) Oleic acid-conjugated chitosan (oleyl-chitosan) is a powerful platform for encapsulating oleic acid-decorated iron oxide nanoparticles (ION) Reproduced with permission from [178], ACS Publications, 2018. (D) Graphical abstract of PEG-coated Zein nanoparticles Reproduced [183], Elsevier, 2021. (E) Graphical abstract of DOX/Zr-UİO-66-PEG-F3 Reproduced with permission from [184], ACS Publications, 2021. (F) DOX-loaded phosphorylcholine-based zwitterionic polymer nanogels’ charge-conversion ability at tumor extracellular pH Reproduced with permission from [201], Elsevier, 2019. (G) Schematic illustration of the poly(phosphorylcholine)-based (HPMPC) nanogel with long blood circulation, blood-brain barrier (BBB) penetration, and hypoxic controlled drug release for glioblastoma drug delivery Reproduced with permission from [202], Elsevier, 2021. (H) Trastuzumab-dendrimer-fluorine drug delivery system’s efficacy can be evaluated in 3D breast cell culture Reproduced from [203], Elsevier, 2021. (I) Bis-MPA dendrimers and related structures Reproduced with permission from [204], Elsevier, 2012.
Figure 3.
(A) The sonochemical method was used to prepare starch/MWCNT-Gl NCs nanoparticles for drug delivery Reproduced with permission from [166], Elsevier, 2018. (B) Arthritis imaging of HiPco-cy5.5 in the control group and mice with highlighted arthritis and normal joint Reproduced [167], MDPI, 2021. (C) Oleic acid-conjugated chitosan (oleyl-chitosan) is a powerful platform for encapsulating oleic acid-decorated iron oxide nanoparticles (ION) Reproduced with permission from [178], ACS Publications, 2018. (D) Graphical abstract of PEG-coated Zein nanoparticles Reproduced [183], Elsevier, 2021. (E) Graphical abstract of DOX/Zr-UİO-66-PEG-F3 Reproduced with permission from [184], ACS Publications, 2021. (F) DOX-loaded phosphorylcholine-based zwitterionic polymer nanogels’ charge-conversion ability at tumor extracellular pH Reproduced with permission from [201], Elsevier, 2019. (G) Schematic illustration of the poly(phosphorylcholine)-based (HPMPC) nanogel with long blood circulation, blood-brain barrier (BBB) penetration, and hypoxic controlled drug release for glioblastoma drug delivery Reproduced with permission from [202], Elsevier, 2021. (H) Trastuzumab-dendrimer-fluorine drug delivery system’s efficacy can be evaluated in 3D breast cell culture Reproduced from [203], Elsevier, 2021. (I) Bis-MPA dendrimers and related structures Reproduced with permission from [204], Elsevier, 2012.
3.4. Delivery Applications of Dendrimers
Dr. Donald Tomalia’s pioneering research on poly(amidoamine) dendrimers was first published in 1985. Dendrimers were first used as a drug delivery mechanism in the late 1990s [205]. Two methods have been used to use dendrimers for drug delivery: formulation and nanostructure. In the nanostructure approach, drugs are covalently bound onto dendrimers, while in the formulation approach, drugs are physically entrapped within a dendrimer using non-covalent interactions [206,207,208,209].
In the studies, we synthesized and characterized various fluorinated dendrimers and designed a Trastuzumab-dendrimer-fluorine drug delivery system for use in breast cancer. A 3D breast cancer cell culture was grown in a bioreactor device. Using MCF-7 cells with Her-2 overexpression in cell culture, the efficacy of the Trastuzumab-dendrimer-fluorine drug delivery system was studied, and magnetic resonance imaging was used to quantify the efficacy. Results showed that the drug delivery system containing trastuzumab, dendrimer and fluorine was more effective than trastuzumab alone. Amino-functionalized polyester dendrimers, known for their biocompatibility and ability to cleave internal esters, have been described to be used for siRNA to cross the blood-brain barrier (Figure 3H) [203].
In research conducted by Patrik and colleagues, they examined dendrimers composed of 2,2-bis(methylol)propionic acid as nonviral vectors for siRNA delivery. In this study, amino-functional bis-MPA dendrimers effectively facilitated gene transfection in human glioblastoma cells and rat glioma cells, resulting in a 20% reduction in the expression of the target protein (Figure 3I) [204].
4. Nanoparticulate Systems for Brain Delivery of Drugs
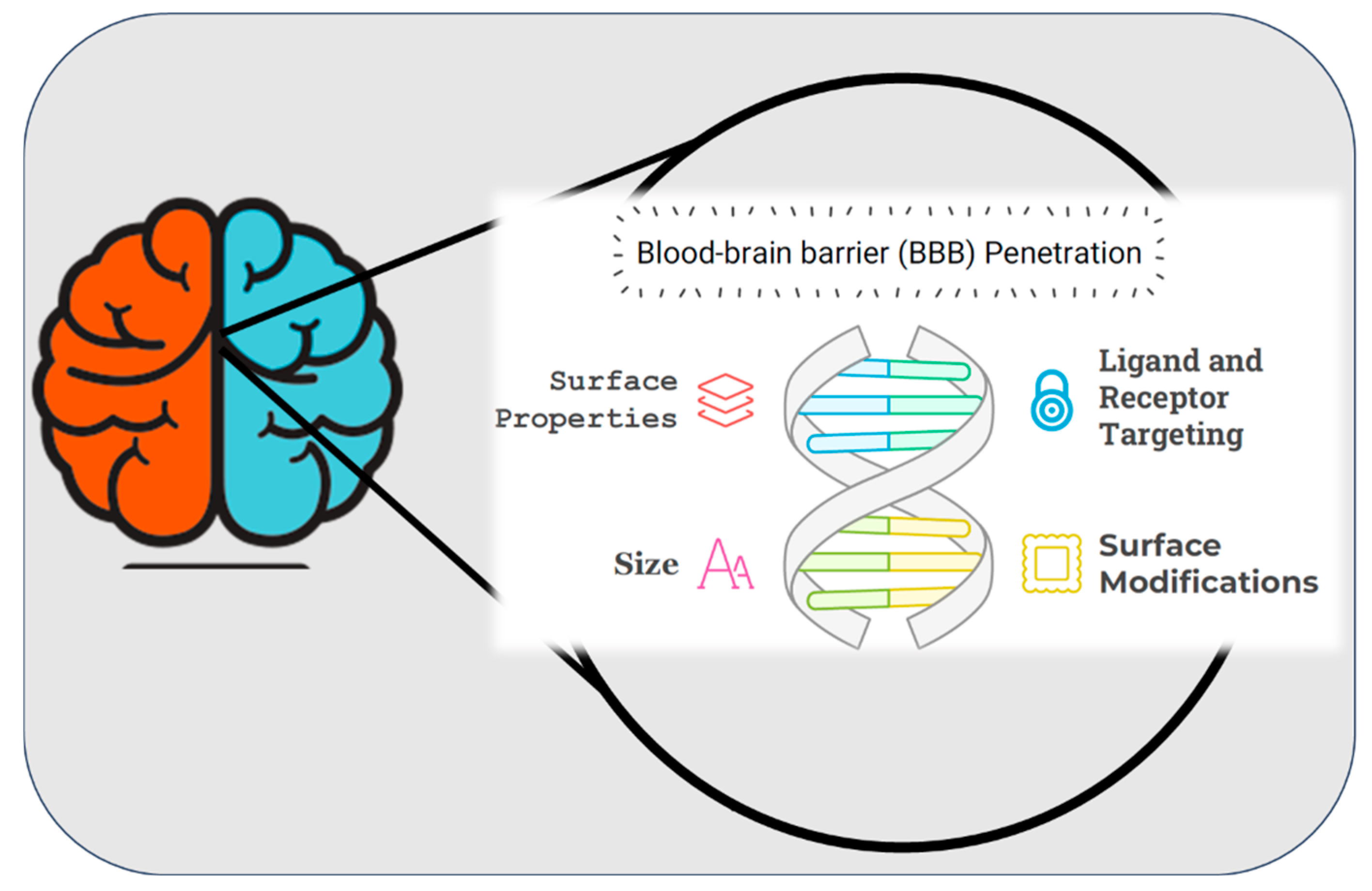
The development of nanoparticle systems for the delivery of drugs to the brain has emerged as a transformative approach in addressing the limitations associated with conventional drug delivery methods. The primary challenge in treating neurological disorders lies in overcoming the highly selective nature of the blood-brain barrier (BBB) (Figure 4), which restricts the passage of most therapeutic agents to the central nervous system (CNS) [210,211,212]. Nanoparticles, owing to their tunable size, surface properties, and biocompatibility, offer a versatile platform to bypass the BBB and enable targeted drug delivery to the brain [213]. Various nanoparticle systems, including polymeric nanoparticles, liposomes, dendrimers, and solid lipid nanoparticles, have demonstrated promising results in preclinical and clinical studies. These systems not only enhance the bioavailability of drugs but also minimize systemic toxicity by providing site-specific delivery [214]. Furthermore, functionalization of nanoparticles with targeting ligands, such as peptides or antibodies, further improves their ability to penetrate the BBB and reach specific neural tissues [215]. This cutting-edge technology has the potential to revolutionize the treatment of neurological disorders, such as Alzheimer’s disease, Parkinson’s disease, and brain tumors, by addressing the unmet need for effective therapeutic delivery. (Neurological disorders and drug distribution methods are shown in Table 2.)
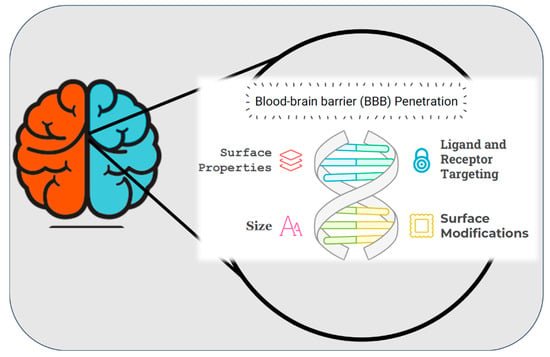
Figure 4.
Schematic representation of surface properties, ligand and receptor targeting, and size characteristics of blood brain barrier penetration. “During the preparation of this manuscript, the author(s) used [Napkin AI, beta-0.10.2] for the purposes of [drawing figure]. The authors have reviewed and edited the output and take full responsibility for the content of this publication”.
Age-related neurodegenerative diseases (NDs) are very common. Although the central nervous system is the part that is most affected, the peripheral nervous system is also affected. The need to manage or control NDs increased the urge to research and create effective alternative techniques because some medications and diagnostics were unavailable [216,217,218]. Sincere attempts are being undertaken to adapt nanotechnology to manage brain cell activity such as deep brain stimulation, implanted stimulation, therapeutic cargo packing, distribution to the brain, and nanomedicine with improved efficacy. These developments can be used to develop treatment plans in the future that take the patient’s neurodegenerative condition into account. Due to recent developments in targeted delivery, improved effectiveness, and fewer adverse effects of nanotechnology-based techniques, they have been advocated as viable and cheap therapeutic options. Recent studies have been dedicated to utilizing poly(lactide-co-glycolic) acid as a foundational material for the development of therapeutic nanoparticles intended for the treatment of brain tumors and Alzheimer’s disease [219]. In vitro tests have demonstrated that the application of polymeric nanoparticles enhances drug delivery to the brain, reducing oxidative stress, inflammation, and plaque accumulation [220,221,222,223,224,225]. This is achieved through improved curcumin delivery for the treatment of Alzheimer’s disease and efficient internalization of doxorubicin into human glioma cells, resulting in a cytotoxic effect on cancer cells [226,227]. Moreover, in vivo experiments have been conducted involving the simultaneous administration of the anti-cancer drug cisplatin and the antioxidant agent boldine, utilizing poly(lactide-co-glycolic) nanocarriers. These experiments have shown promising targeted delivery for therapeutic applications in brain cancer therapy. In another line of research, the focus has been on andrographolide loaded into nanoparticles based on polyethylcyanoacrylate and human serum albumin [228]. These nanoparticles are designed to address inflammation associated with neurodegenerative disorders.
4.1. Blood-Brain Barrier (BBB)
The blood brain barrier (BBB) is created by endothelial cells lining the cerebral microvasculature, and it makes drug targeting to the brain difficult [229,230]. It protects the brain from neurotransmitters and xenobiotics in circulation that might impair neuronal function [210,231]. The BBB is packed with tight connections that block the flow of ions and chemicals. Delivering various drugs to the brain can be challenging due to this reason. Creating a lipid-soluble drug delivery system or utilizing a nanocarrier that can traverse the BBB due to its diminutive size are common noninvasive approaches for administering water-soluble drugs to the brain (Figure 5A) [232].
The BBB is a homeostatic defense mechanism of the brain against pathogens and toxins. It is composed of endothelial cells that form the luminal surface of brain capillaries and are connected by tight junctions. The complex and highly organized BBB maintains the biochemical, physicochemical and structural properties of the substances in its periphery and creates barrier selectivity in the passage of desired molecules into the brain parenchyma. This barrier acts as a selective dynamic filter, preventing the passage of a large number of mostly water-soluble active substances such as antibiotics, antineoplastic agents, peptide-proteins, especially neuropeptides and other oligo- and macro-molecular active substances into the central nervous system [210,233,234]. In order for an active substance to cross the BBB, it must be lipid soluble, non-ionized at physiological pH, have a low molecular weight, and have low binding to serum proteins (Figure 5) [235].
4.2. Crossing the Blood-Brain Barrier
Lipophilic, nonionized at physiological pH, and low molecular weight active substances penetrate the Central Nervous System (CNS) [236,237,238]. However, various transport strategies have been developed for the transport of small molecules with poor fat solubility, hydrogen bonding functional groups, and water-soluble active substances such as peptides and proteins to the CNS. These can be classified into three groups: surgical, pharmacologic and physiologic methods. If we briefly explain the content of these methods: opening of tight junctions with osmotic effect, active substance modifications-use of prodrug, use of special transport systems in the brain (CMT), use of polymeric carriers such as nanoparticles and liposomes [210,239]. In the opening of tight junctions with osmotic pressure effect, endothelial cells shrink and tight junctions in the blood brain barrier are temporarily opened by osmotic fluid exchange using solutions of osmotic agents such as mannitol and arabinose in appropriate concentrations. Although prodrugs formed by preparing lipophilic conjugates of active substances with various chemical substances have advantages such as high lipophilic properties that allow very good penetration, transport to the brain, and easy crossing of the lipophilic endothelial barrier, it is an approach that is not very feasible due to the difficulty and high cost of prodrug design [240,241]. Another strategy, CMT, is a potential route for the transport of circulating nutrients and peptides from the blood to the brain. These transport systems are useful systems that reach saturation, show molecular selectivity, and enable the transport of various active substances (small molecules, peptides, etc.) to the brain. Vectors such as chimeric peptides, modified proteins and peptidomimetic monoclonal antibodies are used for drug targeting to the brain. In these systems, a conjugate of the active substance is prepared by binding it to a peptide or monoclonal antibody to take advantage of facilitated transport. While the active substance in the prepared conjugate maintains its biological activity, the MAb binds to the receptor and enables the drug to pass through the BBB via receptor-mediated transport (RMT). Another method is the design of active substance carrier polymeric systems such as nanoparticles and liposomes. The drug is entrapped in the polymer or adsorbed on the surface. In addition, the nanoparticle/active substance formulation is coated with a surfactant (polysorbate 80). The pharmacological effect of the nanoparticles is related to the formulation of these structures. The surfactants used cause the opening of the BBB [242]. In a study, the efficacy of DOX-loaded polysorbate 80-coated nanoparticles against brain tumors was investigated. As a result of chemotherapy studies on rats with glioblastoma, it was determined that polysorbate 80-coated nanoparticles could cross the blood brain barrier and the doxorubicin they carried could reach therapeutic concentrations in the brain (Figure 5C) [243,244].
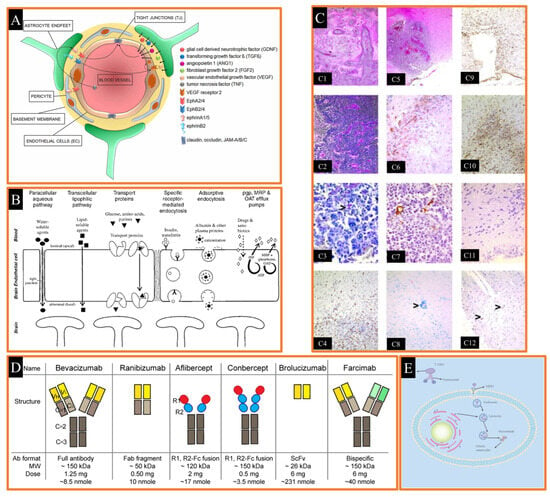
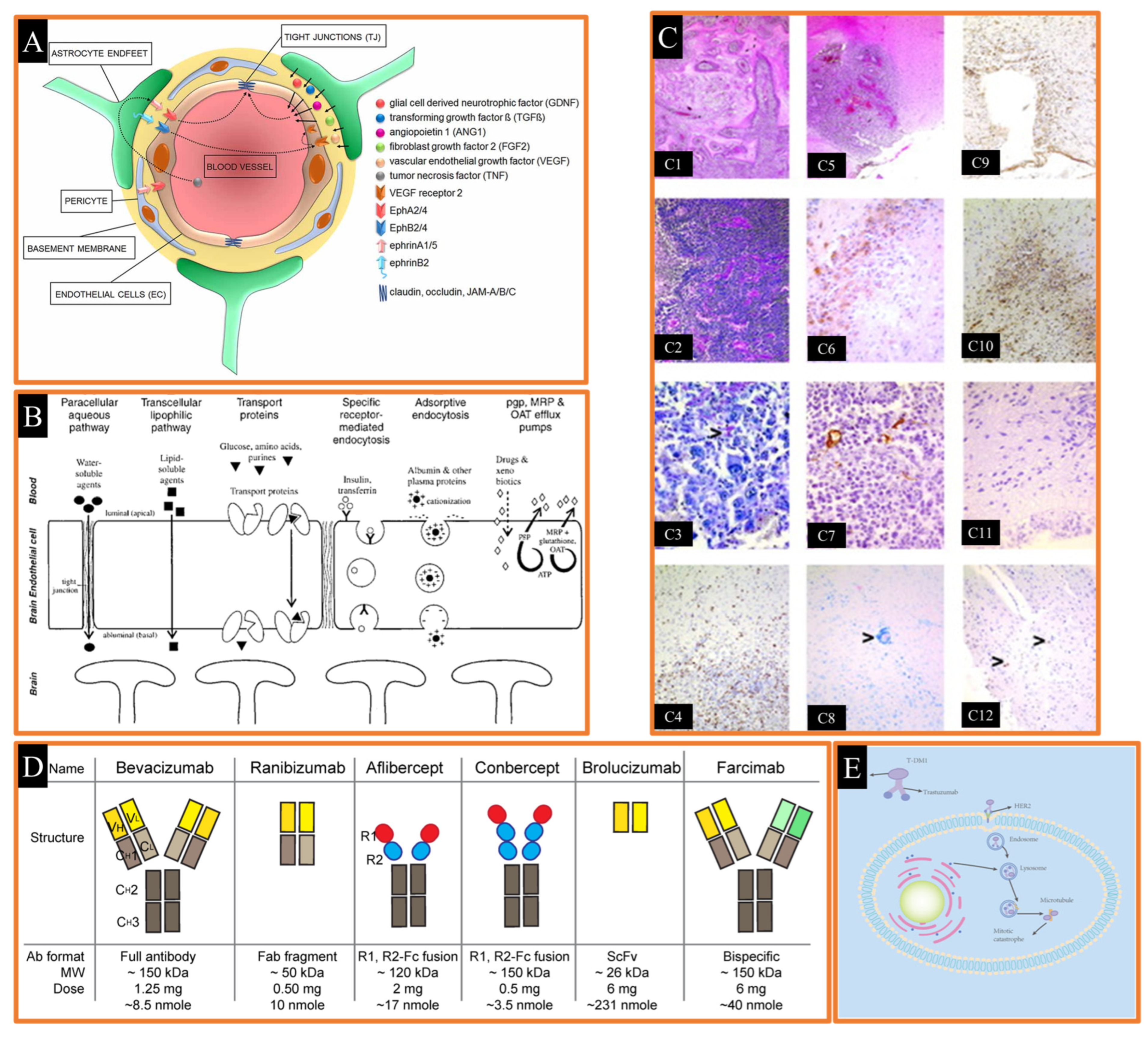
Figure 5.
(A) Cellular and signaling components of the blood–brain barrier (BBB) in activated EphA and EphB receptors and endothelial cell (EC) junctions (TJ) Reproduced from [232], Frontiers, 2018. (B) various crossing mechanisms at the blood brain–barrier Reproduced with permission from [235], Wolters Kluwer, 2004. (C) histology of rats with intracranially implanted 101/8 glioblastoma Reproduced with permission from [244], Wiley Online Library, 2004. (D) graphical abstract of anti-VEGF antibody-derived drugs Reproduced from [245], MDPI, 2023. (E) mechanisms of the action of T-DM1 Reproduced from [243], Frontiers, 2023.
Figure 5.
(A) Cellular and signaling components of the blood–brain barrier (BBB) in activated EphA and EphB receptors and endothelial cell (EC) junctions (TJ) Reproduced from [232], Frontiers, 2018. (B) various crossing mechanisms at the blood brain–barrier Reproduced with permission from [235], Wolters Kluwer, 2004. (C) histology of rats with intracranially implanted 101/8 glioblastoma Reproduced with permission from [244], Wiley Online Library, 2004. (D) graphical abstract of anti-VEGF antibody-derived drugs Reproduced from [245], MDPI, 2023. (E) mechanisms of the action of T-DM1 Reproduced from [243], Frontiers, 2023.
4.3. Cancer Immunotherapy
A diverse array of biomaterials has been employed for the delivery of immunomodulatory substances. In the context of cancer immunotherapy, implantable drug delivery systems can serve two distinct purposes: first, they can be used to administer immunomodulatory agents that target and disrupt checkpoints in the cancer immune cycle, and second, they can facilitate the delivery of cells for adoptive cell transfer (ACT) into cancerous tissue, thereby enhancing the survival and proliferation of these cells. A variety of immunomodulatory drugs, antibodies, antigens, cytokines, and inhibitors have been loaded into implanted biopolymeric drug delivery systems to offer immune-based protection against cancer. Bencherif et al. developed an intelligent DDS in the form of a sponge-like cryogel, where a covalently conjugated RGD peptide was encapsulated alongside cytosine-phosphodiester-guanine oligodeoxynucleotide. This cryogel vaccine was subcutaneously administered to mice and resulted in a potent and sustained antitumor lymphocyte response. In a B16-F10 tumor model, this approach achieved an impressive 80 percent survival rate. Furthermore, the effectiveness of the cryogel vaccines was underscored by the fact that every vaccinated mouse that survived the initial tumor test also withstood a subsequent second test. This highlighted the enduring protective immunity that cryogel vaccinations can provide.
Cancer is one of the most important health problems of our time. In developed countries, new cancer cases are increasing day by day as a result of population growth as well as a Western-style diet, smoking, alcohol consumption and lack of physical activity. Cancer is a cell disease. The human body consists of millions of cells. Our body is constantly producing new cells in order to grow, to replace dead cells, or to repair cells damaged by injury. Cancer, in its shortest definition, is the uncontrolled proliferation of cells. These abnormally proliferating cells invade the tissues and organs where they are located and even far away, causing functional disorders in these areas. The high mortality rate in cancer increases the importance of the subject even more.
For this reason, cancer is one of the most studied topics today, and research continues for more effective and less side-effective treatment. Current methods of cancer treatment include surgery, radiotherapy, chemotherapy and immunotherapy. The most important factor limiting the success of cancer treatment is that anticancer agents used in conventional treatment are not selective for tumor cells and tissues. Almost all chemotherapeutic agents are known to have side effects on normal tissues and organs. Especially anticancer drugs such as cisplatin, cyclophosphamide, methotrexate, mitomycin, and cyclosporine are known to cause severe nephrotoxicity. The main goal of cancer treatment is to destroy the cancer cell without affecting normal tissues. This is possible by selectively targeting the cancer cell. The ideal properties of the drug in tumor targeting can be listed as increasing the localization of the drug in the tumor by active or passive targeting, reducing the accumulation of the drug in non-targeted tissues, minimizing drug leakage from transition zones, protecting the drug from degradation, allowing the drug to remain in the targeted area for the desired period of time, facilitating the uptake of the drug into the cell, and using biocompatible and biodegradable drug carrier system components. For this purpose, interest in different forms of pharmaceutical carrier systems (liposomes, nanoparticles, active substance–polymer conjugates and polymeric micelles, dendrimers) directly binding to the active substance or entrapping the active substance and targeting drugs in this way is increasing day by day. With targeting, conventional, biotechnological and gene-derived drugs can be selectively delivered to specific parts of the body such as organs, tissues and cells. With this selective targeting, undesirable side effects are reduced, the most appropriate therapeutic response is achieved, and substances with toxic effects at high doses can be used safely. New drug carrier systems used for targeting purposes act according to the passive or active targeting principle. Systems such as nanoparticles, microcapsules, microspheres and liposomes, which are prepared using macromolecules such as dextran, albumin, DNA, polyamino acids and other polymers from non-specific carriers, accumulate in the tumor due to environmental characteristics such as tumor size and the EPR effect resulting from the lack of lymphatic drainage around the tumor. In addition, the physicochemical properties of the macromolecule used, such as molecular weight and charge, are also effective in aggregation in the tumor. While these systems can be directed to tumors by passive targeting, they can also be used for active targeting by combining them with molecules such as antibodies, ligands, and peptides.
4.4. Molecular Targeted Therapy
Molecular targeted therapy involves anticancer drugs designed to bind to specific molecular targets. These drugs generally work by specifically binding to proteins that play a critical role in tumor development. This approach is more advantageous than conventional cytotoxic chemotherapy. With targeted therapy, host cell toxicity, which is an important problem in cytotoxic chemotherapy, has been largely eliminated. Molecular targeted therapy can be classified into two categories: small molecules (tyrosine kinase inhibitors) and monoclonal antibodies. Small molecules (tyrosine kinase inhibitors) are organic compounds smaller than 800 daltons [246,247,248]. These molecules can easily penetrate the cell membrane. They inhibit tumor cell proliferation, and antiapoptotic effects, angiogenesis and metastasis caused by the tyrosine kinase enzyme. These molecules are named with the suffix “-ib” due to their inhibitory properties. Examples of tyrosine kinase inhibitors are imatinib, gefitinib, and erlotinib. “Imatinib”, used in the treatment of chronic myeloid leukemia (CML), is one of the successful studies of molecular targeting research. The BCR-ABL mutation related to the Philadelphia chromosome has been detected in patients with CML. Studies have shown that imatinib only binds to myeloid cells containing the BCR-ABL mutation and prevents their proliferation, without harming normal cells. Additionally, fewer side effects were observed compared to cytotoxic chemotherapy [249,250]. Monoclonal antibodies are molecules designed from humanized antibodies that bind to cancer cell-specific antigens. Monoclonal antibodies are named with the suffix “-mab”. Monoclonal antibodies such as bevacizumab, cetuximab, panitumumab, rituximab, ranibizumab and transtuzumab used in the treatment of solid tumors have been approved by the FDA [251,252,253,254]. Bevacizumab and ranibizumab target vascular endothelial growth factor (VEGF) (Figure 5D) [245,255,256]. Bevacizumab is used in colorectal cancer [257], non-small cell lung cancer (NSCLC) [258,259], metastatic renal cancer [260,261], and glioblastoma [262,263,264]. Ranibizumab is used in the treatment of diabetic macular edema. Trastuzumab targets the HER2/neu receptor and is used in HER2-positive metastatic breast cancer. Cetuximab targets the epidermal growth factor receptor (EGFR). It is used in the treatment of colorectal cancer and NSCLC. Rituximab targets CD20 on B cells and is used in non-Hodgkin lymphoma. Another application of monoclonal antibodies is their use as a drug carrier system in the form of an antibody–drug conjugate (ADC). The monoclonal antibody binds to the cancer cell. The antibody–cytotoxic drug conjugate passes through the intracellular membrane of the tumor cell, resulting in cell death. This technology provides a wide therapeutic range by targeting only cancer cells, thereby minimizing the potential side effects of the cytotoxic drug. In 2013, ado-trastuzumab emtansine (T-DM1) was approved by the FDA for HER2-positive metastatic breast cancer. In similar work, an antibody–drug conjugate (ADC) has been developed for the treatment of breast cancer (Figure 5E) [265].

Table 2.
Complex neurological disorders and drug delivery methods.
Table 2.
Complex neurological disorders and drug delivery methods.
| Complex Neurological Disorder | Drug Delivery Methods | Description | Ref. |
|---|---|---|---|
| Epilepsy | Electrophoretic drug delivery | The microfluidic ion pump facilitates the tailored delivery of inhibitory neurotransmitters by detecting seizure activity and transporting ions via the ion exchange membrane via electrophoresis. Mice have been used to test this strategy. | [266,267] |
| Implanted intracerebroventricular delivery system | For patients with epilepsy, the device delivers valproic acid, an anti-seizure medicine, into their cerebrospinal fluid for a protracted course of treatment. | [268] | |
| Microencapsulation of anti-seizure medications | Polymer cores containing lacosamide, an anti-seizure medication, are enveloped by drug-free polymer shells and have been examined in vitro using synthetic cerebrospinal fluid. | [269] | |
| Nanoparticles | Gold nanoparticles coated with glucose are linked to the anti-seizure medication lacosamide, intended for intravenous delivery in rats. | [270] | |
| Stroke | Liposome | ZL006, a neuroprotectant and nNOS/PSD-95 inhibitor, was injected into T7-conjugated PEGylated liposomes in stroke models in living rats and mice. | [271,272] |
| Brain Cancer | Bioresorbable electronic patch | In a mouse model of brain tumor, the patch promotes prolonged drug release and improves drug penetration by modest heat activation | [273] |
| Nanoparticles | Dasatinib, an anti-cancer medication, was administered to a mouse model of glioblastoma using Cornell prime dots conjugated with αv integrin-binding/nontargeting peptides and tagged with PET (positron emission tomography) labels. | [274] | |
| Traumatic Brain Injury | Exosomes | Intravenous delivery of mesenchymal stem cell (MSC)-derived exosomes, which contain physiologically active molecules and reduce inflammation in traumatic brain injury (TBI), has been shown to be effective. Animal studies have shown that these exosomes are capable of crossing the blood-brain barrier. | [275,276] |
| Nanoparticles | Poly(lactic-co-glycolic acid) nanoparticles were used to treat traumatic brain injury (TBI) in vivo in mice by delivering siRNA. These polysorbate 80-coated nanoparticles promoted receptor-mediated transport across the lipoprotein receptor. | [277] | |
| Alzheimer’s Disease | Magnetic resonance-guided low-intensity focused ultrasound | Significantly more of the blood-brain barrier can be reversibly opened when magnetic resonance-guided low-intensity focused ultrasound is applied to the human entorhinal cortex and hippocampal regions. | [278] |
| Parkinson’s Disease | Supramolecular gel | A hydrogel containing the amino acid L-DOPA demonstrates swift drug release upon intranasal delivery in mice. | [279] |
| Nanoparticles | Protocells, carrying both Parkinson’s disease drugs, levodopa and curcumin, had their lipid bilayer modified for brain targeting. This modification was achieved through intraperitoneal injection in a mouse model of Parkinson’s disease. | [280] | |
| Oral and maxillofacial device | A system implanted in the oral or maxillofacial region is specifically engineered to transport drugs to the brain via the respiratory mucosa. This functionality was evaluated through testing in a live rabbit model. | [281] |
5. Advantages of Drug Targeting
Targeted drug delivery systems have revolutionized the pharmacological landscape by enabling the transport of active substances directly to pathological regions or specific cells, thereby reducing the required dosage and minimizing side effects [282,283,284]. These systems allow active compounds to access previously unreachable sites, such as intracellular regions or pathogens like viruses, bacteria, and parasites. By utilizing pharmacological receptors, targeted delivery ensures that the drug remains inert until it reaches the site of action, optimizing dosing frequency and therapeutic speed. Consequently, drug administration protocols are simplified, therapeutic efficiency is enhanced, and treatment costs are reduced. Selective drug transport offers two critical benefits: ensuring optimal drug efficacy by delivering the active compound to the intended site at the desired rate, and reducing systemic distribution to minimize side effects. By restricting the distribution of the drug to target organs, site-specific delivery significantly improves the therapeutic index. This approach holds substantial promise for managing conditions such as uncontrollable intracellular infections (e.g., HIV/AIDS), central nervous system disorders, immune system diseases, cancer, and cardiovascular pathologies [284,285,286,287,288].
The design and development of targeted drug delivery systems presents both opportunities and challenges, necessitating a thorough understanding of four fundamental components: the drug, the target, the disease, and the carrier system. The efficacy of these systems depends on identifying mechanisms to enhance selectivity, which may be biochemical, physiological, or immunological in nature. This requires a multidisciplinary approach, integrating expertise across various scientific domains. Traditional drug administration methods, such as oral or intravenous delivery, often result in widespread distribution of the drug, leading to off-target effects and unwanted side reactions. Targeted drug delivery, by contrast, focuses on directing drug molecules to specific receptors in the desired tissues or regions [3,289,290]. This precision not only enhances the pharmacological response but also offers significant clinical benefits by minimizing adverse effects and maximizing therapeutic outcomes.
6. Drug Market Used in Cancer Nanotechnology
Despite considerable advances in medicine and technology, cancer continues to claim the lives of millions each year [291,292]. Extensive study over decades highlights the disease’s ever-changing character. While treatment choices have improved, the long-term problem is the significant adverse effects of strong chemotherapies [293,294]. The widespread establishment of resistance mechanisms is a key impediment to successful cancer therapies. When the primary cancer-causing pathways are shut down, other signaling pathways are activated, allowing the cancer to adapt and survive [295,296]. This adaptive capability poses a significant challenge in effectively treating cancer.
The approach to cancer therapy must shift from focusing only on novel medicines to improving existing treatments and diagnostics using creative, effective, and practical ways. Pain affects 55% of cancer patients under therapy and 66% of those in late stages [297]. Chemotherapies without specific targeting mechanisms attack both malignant and healthy cells indiscriminately, resulting in systemic toxicity that impairs patients’ quality of life [298,299]. Furthermore, the benefits of early detection are obvious. Early stage cancers have much greater 5-year survival rates, lower total patient expenses, and often require less aggressive treatment options [300,301]. This highlights the crucial role of early identification in improving outcomes and reducing the impact of cancer on patients’ lives. Nanotechnology might provide a viable answer by improving the targeting capabilities of current medicines. It has the potential to improve the effectiveness of localized medication delivery and thereby reduce systemic toxicity. Furthermore, nanotechnology has the potential to increase diagnostic sensitivity, imaging methods, and radiation treatment [302,303]. It has the possibility of changing several areas of cancer treatment and detection by utilizing the unique capabilities of nanotechnology, such as precision targeting and controlled release.
There are many cancer types, and every cancer type possesses different costs for its treatments. Simioa Chen et al. [304] evaluated the global economic cost of different cancer types from 2020 to 2050. According to their study, cancer is expected to cost the world economy $25.2 trillion in international currencies from 2020 to 2050. This amount is comparable to a 0.55% yearly tax on world GDP. Tracheal, bronchus, and lung cancer (15.4%); colon and rectum cancer (10.9%); breast cancer (7.7%); liver cancer (6.5%); and leukemia (6.3%) are the malignancies with the biggest economic expenses. Furthermore, in high-income nations, treatment expenditures contribute more to the overall economic burden of cancer than in low-income countries. This suggests that the costs of cancer treatment have a greater influence on the total economic cost in high-income countries. China and the United States have the greatest economic costs from cancer, accounting for 24.1% and 20.8% of the total worldwide burden, respectively. Despite the fact that low- and middle-income nations account for 75.1% of cancer-related mortality, they account for 49.5% of cancer-related economic losses.
The integration of nanotechnology into medicine and pharmacy has profoundly transformed drug development and delivery strategies. Nano-formulated drugs and nano-drug delivery systems represent innovative technologies characterized by their ability to achieve highly precise targeting, enhance drug bioavailability, and minimize adverse effects. These advancements are particularly evident in the treatment of cancer, neurodegenerative disorders, cardiovascular diseases, and infectious diseases, underscoring the significant medical potential of such systems. This article reviews the current state of nano-formulated drugs, highlighting examples from clinical trials and approved products, alongside their applications in various diseases. Nano-formulated drugs are pharmaceutical products engineered at the nanoscale (1–100 nm). These systems offer several distinct advantages, including targeted delivery, improved bioavailability, enhanced permeability and retention (EPR) effects, controlled release, and multifunctional integration. Nano-drug delivery systems leverage diverse carrier platforms, such as liposomes, polymeric nanoparticles, micelles, metallic nanoparticles, and dendrimers. Notable examples of clinically successful nano-drugs include Doxil (doxorubicin-loaded liposomes), Abraxane (paclitaxel-loaded polymeric nanoparticles), and Genexol-PM (paclitaxel-loaded micelles), which have significantly advanced therapeutic efficacy and patient outcomes. Nano-formulated drugs have contributed numerous innovative breakthroughs to medical science and patient care. In oncology, these systems have demonstrated the capacity to precisely target the tumor microenvironment, reducing systemic side effects while improving therapeutic efficacy compared to conventional chemotherapeutics. Furthermore, theranostic applications allow nanoparticles to serve dual roles in diagnosis and therapy. For instance, gold nanoparticles are widely utilized in the imaging and treatment of cancerous tumors. In the field of infectious diseases, nanoformulations provide a highly targeted and effective approach to combat antibiotic-resistant pathogens. Additionally, the development of nano-drugs capable of traversing the blood–brain barrier represents a significant advancement, offering promising therapeutic options for neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. In conclusion, nano-drug delivery systems occupy a critical role in both current clinical practice and research endeavors. Their modular and customizable nature allows for adaptation to a wide array of therapeutic needs, providing innovative solutions for complex medical challenges. Current clinical trials and the availability of approved nano-drug products underscore the transformative potential of nanotechnology in modern medicine, paving the way for future advancements in patient care. Nano-formulated drugs, their stages, and applications in cancer treatment were given in Table 3.

Table 3.
Nano-formulated drugs, their stages, and applications in cancer treatment.
Table 3.
Nano-formulated drugs, their stages, and applications in cancer treatment.
| Product | Type | Indication | Stage | Mechanism/Advantages |
|---|---|---|---|---|
| Doxil [305,306,307] | Liposomal formulation | Ovarian and breast cancer | Marketed | Encapsulates doxorubicin, reduces cardiotoxicity, and enhances tumor targeting through EPR effect. |
| Abraxane [308,309,310] | Polymeric nanoparticles | Pancreatic and breast cancer | Marketed | Albumin-bound paclitaxel improves solubility, bioavailability, and tumor-specific accumulation. |
| Genexol-PM [309,311,312] | Micellar formulation | Breast cancer | Marketed | Paclitaxel-loaded micelles enhance solubility and reduce side effects compared to conventional forms. |
| Onivyde [313,314,315] | Liposomal formulation | Pancreatic cancer | Marketed | Irinotecan encapsulation increases circulation time and targets tumors via passive accumulation. |
| BIND-014 [316,317] | Targeted nanoparticles | Prostate cancer | Clinical trials | Docetaxel-loaded polymeric nanoparticles with ligand-based targeting to PSMA receptors on cancer cells. |
| Nanoxel [309,318,319] | Polymeric nanoparticles | Breast and ovarian cancer | Marketed | Nanoparticle-based paclitaxel improves drug delivery efficiency and reduces hypersensitivity reactions. |
Nano-formulated drugs represent a significant advancement in cancer therapy, offering solutions to overcome the limitations of conventional treatment approaches [320,321,322]. By utilizing the potential of nanotechnology, these drugs are designed to enhance drug bioavailability, improve targeting specificity, and reduce systemic toxicity, thereby optimizing therapeutic outcomes (Table 3). Nano-formulations utilize drug delivery systems that are engineered at the nanoscale to increase the solubility of poorly water-soluble drugs, ensure sustained release, and facilitate targeted drug delivery to cancerous tissues, all while minimizing the impact on healthy cells. This approach is particularly promising in cancer treatment, where precise targeting of tumor cells and the reduction of side effects are critical for improving patient outcomes (Table 4). One of the key features of nano-formulated drugs is their ability to provide controlled drug release, which allows for a more consistent therapeutic effect and reduces the frequency of drug administration. Additionally, nano-carriers, such as liposomes, micelles, and polymeric nanoparticles, play a crucial role in overcoming the challenges associated with drug solubility and stability, thereby enhancing the overall effectiveness of chemotherapy. These formulations also provide the added advantage of reducing the drug’s toxic effects on healthy tissues, which is a common concern with traditional chemotherapy. Among the various nano-formulated drugs, Doxil, a liposomal formulation containing doxorubicin, has gained considerable attention for its ability to deliver the drug to the tumor site more effectively while minimizing cardiotoxicity. Liposomal drug delivery systems enhance the circulation time of the drug and enable more specific targeting of cancer cells, making Doxil a valuable option in the treatment of ovarian and breast cancers [323,324,325]. Similarly, Abraxane, which utilizes polymeric nanoparticles to encapsulate paclitaxel, enhances the drug’s bioavailability and therapeutic efficacy. By overcoming solubility issues and providing a controlled release mechanism, Abraxane has shown promise in the treatment of pancreatic and breast cancers, where conventional paclitaxel formulations often fall short [326,327,328].
Another important nano-formulation, Genexol-PM, employs micellar technology to improve the solubility of paclitaxel and reduce side effects [329,330,331,332]. This formulation has demonstrated improved therapeutic outcomes in the treatment of breast cancer, offering an alternative to traditional paclitaxel formulations that are associated with high toxicity and limited solubility. Onivyde, a liposomal formulation of irinotecan, is specifically designed for the treatment of pancreatic cancer. By encapsulating irinotecan in liposomes, this formulation prolongs the drug’s half-life and allows for more efficient delivery to the tumor, thereby improving treatment efficacy and reducing systemic toxicity [333,334,335,336].
In addition to these formulations, BIND-014, a targeted nanoparticle delivery system encapsulating docetaxel, represents a breakthrough in the treatment of prostate cancer. This drug delivery system is designed to selectively target cancer cells, reducing the exposure of healthy cells to the toxic effects of chemotherapy. The use of targeting ligands allows for the preferential accumulation of the drug in tumor tissues, enhancing therapeutic outcomes while minimizing off-target effects [316,337,338]. Similarly, Nanoxel, another polymeric nanoparticle-based formulation of paclitaxel, has shown efficacy in the treatment of both breast and ovarian cancers. By improving the solubility and bioavailability of paclitaxel, Nanoxel ensures that higher concentrations of the drug reach the cancer cells, thereby increasing the treatment’s effectiveness and reducing side effects [318,319].
These nano-formulations exemplify the significant strides made in the development of targeted cancer therapies. Each drug formulation utilizes different nanocarriers and drug release mechanisms to optimize the delivery of chemotherapy agents to tumor cells while minimizing adverse effects on healthy tissues. The ability to engineer drugs at the nanoscale offers numerous advantages, including improved drug solubility, sustained release, and more precise targeting, all of which contribute to enhanced treatment outcomes and reduced side effects. The clinical stages of these nano-formulated drugs demonstrate their potential to revolutionize cancer therapy. While some of these drugs, such as Doxil, Abraxane, and Nanoxel, have already been successfully marketed and are widely used in clinical practice, others, such as BIND-014, are still undergoing clinical trials. As these therapies continue to evolve, they hold the promise of offering more effective, targeted, and personalized treatment options for cancer patients.
In conclusion, nano-formulated drugs represent a paradigm shift in cancer treatment, offering targeted and controlled drug delivery that enhances therapeutic efficacy while reducing harmful side effects. The development of these innovative therapies has the potential to significantly improve the treatment landscape for various cancer types, providing new hope for patients who may not have responded to traditional treatment options. Continued research and clinical trials are essential to fully realize the potential of nano-formulations in oncology, with the goal of making these advanced therapies accessible to a broader patient population and further advancing the field of cancer treatment.

Table 4.
Drugs used in hydrogel drug delivery systems and their effects.
Table 4.
Drugs used in hydrogel drug delivery systems and their effects.
| Commercial Name of Drug | Loaded Drug | Carrier Polymer of Hydrogel | Results | Limitations | Ref. |
|---|---|---|---|---|---|
| - | Insulin | Chitosan | Generally, 0.5% of orally administered insulin reaches the bloodstream. | Enzymatic barriers and degradation due to the highly acidic environment in the stomach. Onlay a small amount of insulin that is administered reaches the bloodstream due to its hydrophilicity and large size. Also, the structure of intestine is not suitable because of the monolayer of intestinal cells. | [339] |
| Sigma Chem. Co., (St. Louis, MO, USA) | Chlorhexidine gluconate | Chitosan films (partially deacetylated chitin) | A 2% chitosan gel was shown to have a higher viscosity than a 1% gel, making it more appropriate for topical administration without compromising spreadability. Furthermore, over the course of three hours, the 2% gel formulation showed a greater release of chlorhexidine (Chx), according to in vitro release experiments. | As an oral rinse, chlorhexidine (Chx) has been shown to be effective. Gels, on the other hand, have the ability to significantly lengthen their residence duration in the oral cavity, which might boost their therapeutic efficacy in comparison to solutions. In this study, chitosan was used as a carrier to create gel and film formulations that delivered Chx into the oral cavity. Because of its high viscosity and bioadhesive properties, chitosan gel is expected to remain in the oral cavity for an extended period of time, allowing for sustained drug release and improving clinical efficacy. | [340] |
| - | Vascular endothelial growth factor-165 (VEGF) proangiogenic gene | Graphene Oxide-Based Hydrogel | This method has several significant benefits over the widely studied stem cell treatment. The main obstacles to stem cell therapy are frequently occuring immune rejections, difficulties preserving cell viability and retention at the target site, possible teratoma formation risks, and a plethora of ethical, practical, and technical difficulties with cell isolation and culturing. This cooperation of graphene oxide/DNA therapy and tissue engineering can be a novel strategy for regenerative medicine. | The application of intravenous drug delivery of graphene oxide is able to increase the rate of reactive oxygen and induced mutagenesis. In this case, the long-term effects of graphene oxide should be further investigated. | [341] |
| - | Chlorpromazine (antipsychotic drug) | Chitosan/Pectin | This study’s results imply that chitosan/pectin polyelectrolyte complexes can be used to create mucoadhesive nasal inserts with different medication release properties. It was able to modify the inserts’ water absorption behavior as well as the release and penetration of chlorpromazine hydrochloride at the administration site by carefully selecting the chitosan/pectin molar ratio during complex manufacture. | - | [342] |
| GlaxoSmithKline, (London, UK) | Betamethasone-17-valerat | Sodium-Deoxycholate | During the study, sodium deoxycholate gels showed substantially more edema inhibition than a commercial cream. Histology experiments further showed that sodium deoxycholate gel did not cause skin irritation. As a result, topical administration of betamethasone valerate (BMV) in the Na-DOC gel formulation appears to be a viable substitute approach. | There is no specification limit for the hydrogel | [343] |
| - | Dexamethasone | Tyramine-Modified Hyaluronic Acid | In both in vitro and in vivo investigations, the hydrogels made of hyaluronic acid (HA) and tyrosine (Tyr), which encapsulated DMT as a typical anti-inflammatory medication, showed a continuous and extended release of DMT for as long as one month. The study found that the combination of hyaluronic acid (HA) and tyrosine (Tyr) with horseradish peroxidase (HRP) showed some efficacy in the treatment of rheumatoid arthritis (RA). On the other hand, injured cartilage recovered almost entirely when HA–Tyr hydrogels containing DMT were used. | - | [344] |
| Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China) | Curcumin | Pluronic F127 and Poloxamer 188 | A thermosensitive nasal in situ gel containing curcumin was developed in this study. It had desirable qualities such as a short gelation time, prolonged drug release, and safe biological properties. When compared to intravenous (i.v.) treatment, the nasal in situ gel boosted curcumin absorption in the brain. Notably, the fluid-like fluidity of this in situ gel prior to contact with the nasal mucosa is the primary benefit of this in situ gel over a traditional gel. This feature facilitates patient administration and assures correct medicine dose. | - | [345] |
| Riyadh Pharma (Riyadh, Saudi Arabia) | Acyclovir | Polyvinylpyrrolidone (PVP) | The release properties of hydrogels showed that PVP gels had higher release rates, and that this rate increased even more when PEG or glycerol was included. Histopathological analyses confirmed PVP hydrogel’s safety for mucosal administration. | In gel formulations containing PEG, nasal mucosal injury was less severe than in formulations including glycerol. | [346] |
| Janssen-Cilag SpA Co. (Latina, Italy) | Tramadol | Poloxamer | In terms of gelling behavior, drug content, and drug release, the formulation for thermosensitive gelling showed reliable batch-to-batch consistency. When a membrane was added to the immersion cell, the medication released more slowly than when it was not. Moreover, a quicker drug release was connected with a greater paddle rotation speed. Notably, the related drug release efficiency (DE) values and membrane thickness showed a strong and substantial linear connection. | Moreover, an in-depth understanding of the testing parameters that have to be set during dissolving tests is required due to the growing interest in thermosensitive hydrogels for parenteral usage. | [347] |
| - | Calcium phosphate-DNA | Alginate | In this work, transplanted preosteoblasts were given calcium phosphate-DNA (CaP-DNA) via alginate hydrogels to enhance the process of bone formation. Alginate hydrogels are also a flexible biomaterial delivery platform that allows for the modification of the scaffold’s physical and biological characteristics. This involves modifying the mechanical characteristics, degradation profile, and adhesiveness of the cell. | - | [348] |
| GFP-expressing plasmid | Poly(L-lactide)-b-poly(ethylene glycol) (FA-PEG-PLLA) | Activated macrophages internalize FA-PEG-PLLA by means of folate receptor-mediated endocytosis. When their hydrogels release intact 3LM, primary macrophage transfection is facilitated effectively. Because of this, folic acid—3-layer micelles have the potential to be a revolutionary therapeutic for rheumatoid arthritis when developed as an in situ gel. They also show promise as a delivery method for receptor-mediated drug or gene delivery. | - | [349] |
7. Summary
The recent advancements in biopolymer drug delivery applications and nanomedicine have been reviewed. Due to their extensive control over the release of bioactive substances, biopolymeric drug delivery systems have demonstrated tremendous promise in the treatment of numerous diseases. These DDSs can provide passive or active drug delivery and can be constructed from a variety of biopolymers. Additionally, they do not have to be implanted using invasive surgery at the diseased area, as is the case with injectable DDSs, which may simply be injected in the target place to prevent subsequent issues from procedures. But polymeric systems are yet unable to treat complicated diseases. So, they must be utilized in concert with nanoparticulate systems to treat diseases like cancer. The integration of cancer therapy and diagnosis has received a lot of attention in recent years as nanomedicine has advanced. Nanomedicine has already transformed how we locate and use medications in biological systems. Nanomedicine advancements have enabled us to identify diseases and, in some cases, pair therapy with a diagnosis.
Rapid developments continue to occur in the pharmaceutical industry in the 21st century. Thanks to advances in biotechnology and nanotechnology, new drugs with protein and nucleic acid structures are being produced. These molecules are expected to be more effective in binding to the target receptor, thanks to studies on improving their clinical properties with the help of nanocarriers. Studies are ongoing to selectively deliver conventional, biotechnological and gene-derived drugs to specific parts of the body such as organs, tissues and cells through drug targeting. It is aimed to prevent undesirable effects by applying cytotoxic drugs, especially those used in cancer diagnosis and treatment, in the form of nanocarriers. The use of molecular targeted therapy in cancer treatment provides successful results. Studies initiated under the name “Cancer Genome Atlas Project” aiming to find personalized drug targets are continuing. With this project, people’s gene maps can be created, individual differences in cancer risk and tumor structure can be detected, so that specific drugs can be developed. In the treatment of central nervous system diseases, nanocarriers are being developed to cross the blood-brain barrier, increase the bioavailability of the drug and be effective in the desired region. In the treatment of cardiovascular diseases, targeting studies are carried out for the factors that cause the disease. In this way, it is expected to prevent myocardial infarction, which is the leading cause of death in the world. In order to prevent the undesirable effects of drugs used in the treatment of rheumatoid arthritis, especially glucocorticoids and methotrexate, studies are being carried out to administer these drugs with the help of nanocarriers, aiming for the drug to act only in the inflamed area (pannus). Additionally, drugs that can specifically bind to the receptors of molecules that cause rheumatoid arthritis are being developed. The acceleration of developments in drug targeting studies is promising for the treatment of important uncontrolled diseases. As the molecular and cellular biology of diseases is better understood; Thanks to the discovery of new targets and new ligands, it is expected that the drug will be transported to the targeted area more effectively and cause fewer side effects. Thus, the treatment of diseases can be done more rationally and effectively.
Author Contributions
Conceptualization, F.C., A.C.Ö. and İ.C.K.; methodology, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G.; investigation, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G.; data curation, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G.; writing—original draft, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G.; writing—review and editing, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G.; visualization, F.C., A.C.Ö., İ.C.K., A.Y., O.T. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Victor, R.d.S.; Santos, A.M.d.C.; de Sousa, B.V.; Neves, G.d.A.; Santana, L.N.d.L.; Menezes, R.R. A review on Chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S.V. Nanostructured lipid carriers: A promising drug carrier for targeting brain tumours. Futur. J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Ezegbe, C.; Umeh, O.; Ofoefule, S. Drug Carriers. J. Curr. Biomed. Res. 2022, 2, 77–105. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bansal, V.; Madhavan, A.; Kumar, M.; Sindhu, R.; Awasthi, M.K.; Binod, P.; Saran, S. Active pharmaceutical ingredient (API) chemicals: A critical review of current biotechnological approaches. Bioengineered 2022, 13, 4309–4327. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Zhang, S.; Zhou, Y.; Xiao, X.; Cui, P.; Xu, B.; Zhao, Q.; Kong, S.; Dai, Y. Emerging biotechnology applications in natural product and synthetic pharmaceutical analyses. Acta Pharm. Sin. B 2022, 12, 4075–4097. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, N.; Golchin, H.; Sadri, Z.; Tabari, Y.; Borhanifar, F.; Makani, S. Nano-biotechnology, an applicable approach for sustainable future. 3 Biotech 2022, 12, 65. [Google Scholar] [CrossRef]
- Celedón, R.S.; Díaz, L.B. Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms 2021, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Saini, K.C.; Bast, F.; Varjani, S.; Mehariya, S.; Bhatia, S.K.; Sharma, N.; Funk, C. A review on microbial products and their perspective application as antimicrobial agents. Biomolecules 2021, 11, 1860. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-based drug-delivery systems in biotechnological applications: Recent advances and perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Burnouf, P.A.; Chew, C.H.; Burnouf, T. Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol. 2019, 37, 707–729. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Zhao, Q.; Pan, T.; Zhong, H.; Wang, W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics 2021, 13, 1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, Y.; Sun, S.; Huang, C.; Yi, Y.; Wang, J.; Deng, Y.; Wu, M. Exosome-based drug delivery systems in cancer therapy. Chin. Chem. Lett. 2023, 34, 107508. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Bravo-Díaz, C.; Sova, M.; Kristl, J.; Saso, L. Nanotechnology-based drug delivery to improve the therapeutic benefits of NRF2 modulators in cancer therapy. Antioxidants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- MacHtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kirar, M.; Bindeliya, M.; Sen, L.; Soni, M.; Shan, M.; Purohit, A.; Jain, P.K. Novel Drug Delivery Systems: An Overview. Asian J. Dent. Health Sci. 2022, 2, 33–39. [Google Scholar] [CrossRef]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming barriers to patient adherence: The case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef]
- Allen, L.V.; Popovich, N.G.; Ansel, H.C. Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, A.; Mandal, U.K.; Thakur, A.; Bhatia, A. Foam-Based Drug Delivery: A Newer Approach for Pharmaceutical Dosage Form. AAPS PharmSciTech 2022, 23, 244. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.I.; Narang, A.S. Pharmaceutical Dosage Forms and Drug Delivery; CRC: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of smart drug-delivery systems based on magnetic molecularly imprinted polymers for targeted cancer therapy: A short review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, A.; Pooja, R.; Suchithra, V.; Ravikumar, A.; Kumar Gupta, P.; Niranjan, V. Smart Drug Delivery Systems for Cancer Treatment Using Nanomaterials. Mater. Today Proc. 2018, 5, 21047–21054. [Google Scholar] [CrossRef]
- Darvin, P.; Chandrasekharan, A.; Santhosh Kumar, T.R. Introduction to smart drug delivery systems. Biomim. Nanoeng. Mater. Adv. Drug Deliv. 2019, 1–9. [Google Scholar] [CrossRef]
- Kleinstreuer, C. Drug-targeting methodologies with applications: A review. World J. Clin. Cases 2014, 2, 742. [Google Scholar] [CrossRef]
- Patel, J. Liposomal doxorubicin: Doxil®. J. Oncol. Pharm. Pract. 1996, 2, 201–210. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel nano-delivery systems: A comprehensive review. J. Nanomedicine Nanotechnol. 2013, 4, 6. [Google Scholar] [CrossRef]
- Blackledge, G.; Averbuch, S. Gefitinib (“Iressa”, ZD1839) and new epidermal growth factor receptor inhibitors. Br. J. Cancer 2004, 90, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Bou-Assaly, W.; Mukherji, S. Cetuximab (Erbitux). Am. J. Neuroradiol. 2010, 31, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, M.V.; Yarosh, I.V.; Sanarova, E.V.; Lantsova, A.V.; Orlova, O.L. The Construction of Immunoliposomes (Review). Drug Dev. Regist. 2022, 11, 97–112. [Google Scholar] [CrossRef]
- Lapka, M. Trastuzumab-induced cardiotoxicity: A review. Klin. Farmakol. a Farm. 2023, 37, 64–67. [Google Scholar] [CrossRef]
- Linev, A.J.; Ivanov, H.J.; Zhelyazkov, I.G.; Ivanova, H.; Goranova-Marinova, V.S.; Stoyanova, V.K. Mutations Associated with Imatinib Mesylate Resistance—Review. Folia Med. (Plovdiv.) 2018, 60, 617–623. [Google Scholar] [CrossRef]
- Tang, P.A.; Tsao, M.S.; Moore, M.J. A review of erlotinib and its clinical use. Expert Opin. Pharmacother. 2006, 7, 177–193. [Google Scholar] [CrossRef]
- Fan, G.; Wei, X.; Xu, X. Is the era of sorafenib over? A review of the literature. Ther. Adv. Med. Oncol. 2020, 12, 1758835920927602. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Sorafenib: A Review in Hepatocellular Carcinoma. Target. Oncol. 2017, 12, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Vallina, C.; Ramírez, L.; Torres, J.; Casañas, E.; Hernández, G.; López-Pintor, R.M. Osteonecrosis of the jaws produced by sunitinib: A systematic review. Med. Oral Patol. Oral Y Cirugía Bucal 2019, 24, e326–e338. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent advancements in stimuli responsive drug delivery platforms for active and passive cancer targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Handb. Exp. Pharmacol. 2010, 197, 3–53. [Google Scholar] [CrossRef]
- Nagpure, G.; RB Singh, K.; Singh, J.; Singh, R.P. Passive and active targeted drug delivery strategies. In Nanotechnology for Drug Delivery and Pharmaceuticals; Academic Press: Cambridge, MA, USA, 2023; pp. 225–234. [Google Scholar] [CrossRef]
- Zhai, B.T.; Sun, J.; Shi, Y.J.; Zhang, X.F.; Zou, J.B.; Cheng, J.X.; Fan, Y.; Guo, D.Y.; Tian, H. Review targeted drug delivery systems for norcantharidin in cancer therapy. J. Nanobiotechnol. 2022, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.R.; Wang, Y.H.; Xiong, Z.Z.; Wang, Y.M. Research progress of nanomaterials in tumor-targeted drug delivery and imaging therapy. OpenNano 2023, 14, 100184. [Google Scholar] [CrossRef]
- Jabir, N.R.; Anwar, K.; Firoz, C.K.; Oves, M.; Kamal, M.A.; Tabrez, S. An overview on the current status of cancer nanomedicines. Curr. Med. Res. Opin. 2018, 34, 911–921. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Mucker, E.M.; Karmali, P.P.; Vega, J.; Kwilas, S.A.; Wu, H.; Joselyn, M.; Ballantyne, J.; Sampey, D.; Mukthavaram, R.; Sullivan, E.; et al. Lipid Nanoparticle Formulation Increases Efficiency of DNA-Vectored Vaccines/Immunoprophylaxis in Animals Including Transchromosomic Bovines. Sci. Rep. 2020, 10, 8764. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, F.; Feng, L.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef]
- Ferreira-Silva, M.; Faria-Silva, C.; Baptista, P.V.; Fernandes, E.; Fernandes, A.R.; Corvo, M.L. Drug delivery nanosystems targeted to hepatic ischemia and reperfusion injury. Drug Deliv. Transl. Res. 2021, 11, 397–410. [Google Scholar] [CrossRef]
- Raucher, D.; Dragojevic, S.; Ryu, J. Macromolecular drug carriers for targeted glioblastoma therapy: Preclinical studies, challenges, and future perspectives. Front. Oncol. 2018, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Mukherjee, B. Nanosize drug delivery system. Curr. Pharm. Biotechnol. 2013, 14, 1221. [Google Scholar] [CrossRef]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef]
- Ye, W.L.; Du, J.B.; Zhang, B.L.; Na, R.; Song, Y.F.; Mei, Q.B.; Zhao, M.G.; Zhou, S.Y. Cellular uptake and antitumor activity of DOX-hyd-PEG-FA nanoparticles. PLoS ONE 2014, 9, e97358. [Google Scholar] [CrossRef]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Zhu, S.; Jiang, Y.; Tang, G.; Sun, C.; Fang, C.; Shi, B.; Pei, Y. Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles. J. Control. Release 2010, 141, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Fan, C.; Gao, W.; Chen, Z.; Fan, H.; Li, M.; Deng, F.; Chen, Z. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int. J. Pharm. 2011, 404, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Szoka, F.C. Antiviral activity and pharmacokinetics of liposome-encapsulated phosphonoformate in rauscher murine leukemia virus-infected mice. J. Liposome Res. 1992, 2, 67–92. [Google Scholar] [CrossRef]
- Chrastina, A.; Massey, K.A.; Schnitzer, J.E. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, J.; Yuan, Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J. Mater. Chem. B 2020, 8, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Hirsjarvi, S.; Passirani, C.; Benoit, J.-P. Passive and Active Tumour Targeting with Nanocarriers. Curr. Drug Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Lee, J.Y.; Kim, D.D.; Yoon, I.S.; Cho, H.J. Recent progress in the development of poly(lactic-co-glycolic acid)-based nanostructures for cancer imaging and therapy. Pharmaceutics 2019, 11, 280. [Google Scholar] [CrossRef]
- Islam, W.; Niidome, T.; Sawa, T. Enhanced Permeability and Retention Effect as a Ubiquitous and Epoch-Making Phenomenon for the Selective Drug Targeting of Solid Tumors. J. Pers. Med. 2022, 12, 1964. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Liao, X.; Yang, B. Targeted drug delivery systems based on cyclodextrins. Prog. Chem. 2014, 26, 1039–1049. [Google Scholar] [CrossRef]
- He, Y.F.; Fan, Q. Research progress of active targeting modification of nanoparticles in tumor therapy. J. Dalian Med. Univ. 2012, 34, 617–621. [Google Scholar]
- Wu, J. The enhanced permeability and retention (Epr) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wan, D.; Chen, Z.; Pan, J.; Liang, X.J. A micelle-based stage-by-stage impelled system for efficient doxorubicin delivery. Bioact. Mater. 2023, 25, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Sat-Klopsch, Y.N.; Burger, A.M.; Bibby, M.C.; Fiebig, H.H.; Sausville, E.A. Validation of tumour models for use in anticancer nanomedicine evaluation: The EPR effect and cathepsin B-mediated drug release rate. Cancer Chemother. Pharmacol. 2013, 72, 417–427. [Google Scholar] [CrossRef]
- Shim, M.K.; Park, J.; Yoon, H.Y.; Lee, S.; Um, W.; Kim, J.H.; Kang, S.W.; Seo, J.W.; Hyun, S.W.; Park, J.H.; et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J. Control. Release 2019, 294, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.Y. Development of a polysaccharide-based hydrogel drug delivery system (DDS): An update. Gels 2021, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Tronci, G.; Neffe, A.T.; Pierce, B.F.; Lendlein, A. An entropy-elastic gelatin-based hydrogel system. J. Mater. Chem. 2010, 20, 8875–8884. [Google Scholar] [CrossRef]
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.S.; Bae, H. Nanogels derived from fish gelatin: Application to drug delivery system. Mar. Drugs 2019, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; O’connell, C.D.; Cometta, S.; Hutmacher, D.W.; Meinert, C.; Bock, N. Gelatin methacryloyl hydrogels for the localized delivery of cefazolin. Polymers 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, K.S.; Srinivasan, V.; Pandita, D. Extracellular matrix extraction techniques and applications in biomedical engineering. Regen. Med. 2021, 16, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeong, W.; Kang, H.W. Liver dECM–Gelatin Composite Bioink for Precise 3D Printing of Highly Functional Liver Tissues. J. Funct. Biomater. 2023, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- González-Callejo, P.; Vázquez-Aristizabal, P.; García-Astrain, C.; Jimenez de Aberasturi, D.; Henriksen-Lacey, M.; Izeta, A.; Liz-Marzán, L.M. 3D bioprinted breast tumor-stroma models for pre-clinical drug testing. Mater. Today Bio 2023, 23, 100826. [Google Scholar] [CrossRef] [PubMed]
- Lysáková, K.; Hlináková, K.; Kutálková, K.; Chaloupková, R.; Žídek, J.; Brtníková, J.; Vojtová, L. A novel approach in control release monitoring of protein-based bioactive substances from injectable PLGA-PEG-PLGA hydrogel. Express Polym. Lett. 2022, 16, 798–811. [Google Scholar] [CrossRef]
- Baldwin, A.; Hartl, M.; Tschaikowsky, M.; Balzer, B.N.; Booth, B.W. Degradation and release of tannic acid from an injectable tissue regeneration bead matrix in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Samanta, H.S.; Bhattacharjee, C. Hydrogel: Polymeric smart material in drug delivery. Mater. Sci. Forum 2016, 875, 45–62. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo-Sensitive Poly (N-isopropylacrylamide-co-polyacrylamide) Hydrogel for pH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2021, 413, 127413. [Google Scholar] [CrossRef]
- Akduman, C.; Özgüney, I.; Kumbasar, E.P.A. Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater. Sci. Eng. C 2016, 64, 383–390. [Google Scholar] [CrossRef]
- Juster, H.; van der Aar, B.; de Brouwer, H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A versatile family of polymers for hot melt extrusion and 3D printing processes in pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C. Preparation of poly(aspartic acid) based fiber hydrogel and its drug release behavior. Fangzhi Xuebao/Journal Text. Res. 2020, 41, 20–25. [Google Scholar] [CrossRef]
- Hernandez, E.D.D.; Reyes-Romero, J.R. Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Vitta, S.; Kannapiran, S.; Shanmugam, K.; Rao, G.; Balachandran, M.; Sridhar, T.M.; Ranganathan, B.; Rajagopal, V.; Ramakrishnan, P. Personalized nanomedicine for breast cancer therapy using poly (lactic-co-glycolic acid) nanoscaffolds: A perspective. Trends Biomater. Artif. Organs 2021, 35, 287–295. [Google Scholar]
- Wu, J.; Zhang, L.; Yang, G.D.; Lin, X.C.; Ji, R.; Wang, C.H.; Lou, W.J.; Wang, X.B. The mechanisms of 5-FU-PLA-O-CMC-NPS-mediated inhibition of the proliferation of colorectal cancer cell line SW480. Tumor Biol. 2014, 35, 6095–6103. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.H.; Kim, S.; Khetan, R.; Afinjuomo, F.; Song, Y.; Garg, S. The development of 5-fluorouracil biodegradable implants: A comparative study of PCL/PLGA blends. J. Drug Deliv. Sci. Technol. 2023, 81, 104300. [Google Scholar] [CrossRef]
- Ashour, A.E.; Badran, M.M.; Kumar, A.; Rishi, A.K.; Yassin, A.E. Di-Block PLCL and Tri-Block PLCLG matrix polymeric nanoparticles enhanced the anticancer activity of loaded 5-fluorouracil. IEEE Trans. Nanobioscience 2016, 15, 739–747. [Google Scholar] [CrossRef]
- Penhasi, A.; Gertler, A.; Baluashvili, I.; Elzinaty, O.; Shalev, D.E. High modulus thermoplastic segmented polyurethane/poly(L-lactide) blends as potential candidates for structural implantable drug delivery systems: I. Structure-properties relationship study. J. Appl. Polym. Sci. 2020, 137, 49517. [Google Scholar] [CrossRef]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shimada, Y.; Olsthoorn, R.C.L.; Snaar-Jagalska, B.E.; Spaink, H.P.; Kros, A. Application of Coiled Coil Peptides in Liposomal Anticancer Drug Delivery Using a Zebrafish Xenograft Model. ACS Nano 2016, 10, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.J.; Kjeldsen, R.B.; Christfort, J.F.; Vila, E.M.; Alstrøm, T.S.; Zór, K.; Hwu, E.T.; Nielsen, L.H.; Boisen, A. 3D-Printed Radiopaque Microdevices with Enhanced Mucoadhesive Geometry for Oral Drug Delivery. Adv. Healthc. Mater. 2023, 12, 2201897. [Google Scholar] [CrossRef] [PubMed]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. Eur. J. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, F. Release kinetics modelling and in vivo-vitro, shelf-life study of resveratrol added composite transdermal scaffolds. Int. J. Biol. Macromol. 2023, 235, 123769. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Finbloom, J.A.; Mofidfar, M.; Diltemiz, S.E.; Nasrollahi, F.; Davoodi, E.; Hosseini, V.; Mylonaki, I.; Sangabathuni, S.; Montazerian, H.; et al. Micro and nanoscale technologies in oral drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 37–62. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.T.; Gopal, A.; Starr, K.; Zhang, X.; Thomsen, S.; Tunnell, J.W.; Welch, A.J.; Rylander, H.G. Micro-patterned drug delivery device for light-activated drug release. Lasers Surg. Med. 2012, 44, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Moradi, E.; Moghadas, H. Micro/nanofluidic devices for drug delivery. Prog. Mol. Biol. Transl. Sci. 2022, 187, 9–39. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, P. Micro-electro-mechanical system-based drug delivery devices. In Bioelectronics and Medical Devices: From Materials to Devices—Fabrication, Applications and Reliability; Woodhead Publishing: Cambridge, UK, 2019; pp. 183–210. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tang, J.H.; Chan, P.C.; Li, J.J.; Lin, M.H.; Shen, C.C.; Liu, R.S.; Wang, H.E. Monitoring Tumor Response after Liposomal Doxorubicin in Combination with Liposomal Vinorelbine Treatment Using 3′-Deoxy-3′-[18F]Fluorothymidine PET. Mol. Imaging Biol. 2017, 19, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Masi, B.C.; Tyler, B.M.; Bow, H.; Wicks, R.T.; Xue, Y.; Brem, H.; Langer, R.; Cima, M.J. Intracranial MEMS based temozolomide delivery in a 9L rat gliosarcoma model. Biomaterials 2012, 33, 5768–5775. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Melero, A.; Keller, S.S.; Jacobsen, J.; Garrigues, T.; Rades, T.; Müllertz, A.; Boisen, A. Polymeric microcontainers improve oral bioavailability of furosemide. Int. J. Pharm. 2016, 504, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Patel, M.V.; Patel, K.N.; Patel, B.A.; Patel, P.A. Transdermal Patches: A Complete Review on Transdermal Drug Delivery System. Int. J. Pharm. Res. Sch. 2012, 1, 62–78. [Google Scholar] [CrossRef]
- Sadab, S.; Sahu, S.; Patel, S.; Khan, R.; Khare, B.; Thakur, B.S.; Jain, A.; Jain, P.K. A Comprehensive Review: Transdermal Drug Delivery System: A Tool For Novel Drug Delivery System. Asian J. Dent. Health Sci. 2022, 2, 40–47. [Google Scholar] [CrossRef]
- Galge, A.G.; Pagire, D. A Review on Transdermal Patches. Int. J. Res. Publ. Rev. 2022, 3, 1810–1824. [Google Scholar] [CrossRef]
- Tiwari, C.; Choudhary, M.; Malik, P.; JAISWAL, P.K.; Chauhan, R. Transdermal Patch: A Novel Approach for Transdermal Drug Delivery. J. Drug Deliv. Ther. 2022, 12, 179–188. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Ganji, F.; Taghizadeh, S.M.; Vasheghani-Farahani, E.; Mohiti-Asli, M. Drug in adhesive transdermal patch containing antibiotic-loaded solid lipid nanoparticles. J. Biosci. Bioeng. 2022, 134, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Martins, J.P.; das Neves, J.; de la Fuente, M.; Celia, C.; Florindo, H.; Günday-Türeli, N.; Popat, A.; Santos, J.L.; Sousa, F.; Schmid, R.; et al. The solid progress of nanomedicine. Drug Deliv. Transl. Res. 2020, 10, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.P.; Huber, S.D.; Willy, N.M.; Aygün, E.; Goker, S.; Atabey, T.; Kural, C. Mechanoregulation of clathrin-mediated endocytosis. J. Cell Sci. 2017, 130, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Drubin, D.G.; Sun, Y. Clathrin-mediated endocytosis in budding yeast at a glance. J. Cell Sci. 2016, 129, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, D.; Zhao, Y.; Zhang, L.; Chen, R.; Liu, W.; Wen, Y.; Liao, Y.; Wen, Y.; Wu, R.; et al. Porcine Deltacoronavirus (PDCoV) Entry into PK-15 Cells by Caveolae-Mediated Endocytosis. Viruses 2022, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.H.; Shen, Y.; Roshdy, M.; Cheng, X.; Wang, G.; Yang, X. Polystyrene nanoplastics exposure caused defective neural tube morphogenesis through caveolae-mediated endocytosis and faulty apoptosis. Nanotoxicology 2021, 15, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Kay, R.R. The origins and evolution of macropinocytosis. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180158. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in different cell types: Similarities and differences. Membranes 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Palm, W. Metabolic functions of macropinocytosis. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180285. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Shen, S.; Pang, Z.; Jiang, X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci. Rep. 2013, 3, 2534. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yu, C.; Xu, T.; Yao, D.; Zhu, L.; Shen, Z.; Huang, X. Self-assembly of DNA nanostructure containing cell-specific aptamer as a precise drug delivery system for cancer therapy in non-small cell lung cancer. J. Nanobiotechnol. 2022, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Tang, F.M.A.; Li, J.J.; Ong, C.; Yung, L.L.Y.; Bay, B.H. Clathrin-mediated endocytosis of gold nanoparticles in vitro. Anat. Rec. 2015, 298, 418–427. [Google Scholar] [CrossRef]
- Pan, L.L.; Chen, Q.F.; Zhao, J.J.; Guo, T.; Wang, X.W.; Hariton-Shalev, A.; Czosnek, H.; Liu, S.S. Clathrin-mediated endocytosis is involved in Tomato yellow leaf curl virus transport across the midgut barrier of its whitefly vector. Virology 2017, 502, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Phuc, L.T.M.; Taniguchi, A. Epidermal growth factor enhances cellular uptake of polystyrene nanoparticles by clathrin-mediated endocytosis. Int. J. Mol. Sci. 2017, 18, 1301. [Google Scholar] [CrossRef] [PubMed]
- Fliri, A.F.; Kajiji, S. Functional characterization of nutraceuticals using spectral clustering: Centrality of caveolae-mediated endocytosis for management of nitric oxide and vitamin D deficiencies and atherosclerosis. Front. Nutr. 2022, 9, 885364. [Google Scholar] [CrossRef] [PubMed]
- Park, T.E.; Kang, B.; Kim, Y.K.; Zhang, Q.; Lee, W.S.; Islam, M.A.; Kang, S.K.; Cho, M.H.; Choi, Y.J.; Cho, C.S. Selective stimulation of caveolae-mediated endocytosis by an osmotic polymannitol-based gene transporter. Biomaterials 2012, 33, 7272–7281. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Twomey, M.; Machado, C.; Gomez, G.; Doshi, M.; Gesquiere, A.J.; Moon, J.H. Caveolae-Mediated Endocytosis of Conjugated Polymer Nanoparticles. Macromol. Biosci. 2013, 13, 913–920. [Google Scholar] [CrossRef]
- Shajahan, A.N.; Timblin, B.K.; Sandoval, R.; Tiruppathi, C.; Malik, A.B.; Minshall, R.D. Role of Src-induced Dynamin-2 Phosphorylation in Caveolae-mediated Endocytosis in Endothelial Cells. J. Biol. Chem. 2004, 279, 20392–20400. [Google Scholar] [CrossRef]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.R. Macropinocytosis: Biology and mechanisms. Cells Dev. 2021, 168, 203713. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Liu, W.; Zhu, Q.; Ke, K.; Zhu, Q.; Jin, W.; Yu, S.; Yang, Z.; Li, L.; Sun, X.; et al. The Role and Therapeutic Potential of Macropinocytosis in Cancer. Front. Pharmacol. 2022, 13, 919819. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.D.; Bidlingmaier, S.M.; Liu, B. Macropinocytosis exploitation by cancers and cancer therapeutics. Front. Physiol. 2016, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Pacitto, R.; Inoki, K.; Swanson, J. Macropinocytosis, mTORC1 and cellular growth control. Cell. Mol. Life Sci. 2018, 75, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Aspects Med. 2022, 83, 100993. [Google Scholar] [CrossRef]
- Wong, N.K.Y.; Shenoi, R.A.; Abbina, S.; Chafeeva, I.; Kizhakkedathu, J.N.; Khan, M.K. Nontransformed and Cancer Cells Can Utilize Different Endocytic Pathways to Internalize Dendritic Nanoparticle Variants: Implications on Nanocarrier Design. Biomacromolecules 2017, 18, 2427–2438. [Google Scholar] [CrossRef]
- Sifniotis, V.; Cruz, E.; Eroglu, B.; Kayser, V. Current advancements in addressing key challenges of therapeutic antibody design, manufacture, and formulation. Antibodies 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Jahanban-Esfahlan, R.; Samadian, H.; Hamidi, M.; Seidi, K.; Dolatshahi-Pirouz, A.; Yazdi, A.A.; Shavandi, A.; Laurent, S.; Be Omide Hagh, M.; et al. Multifunctional nanostructures: Intelligent design to overcome biological barriers. Mater. Today Bio 2023, 20, 100672. [Google Scholar] [CrossRef] [PubMed]
- Raval, J.P.; Joshi, P.; Chejara, D.R. Carbon nanotube for targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Cambridge, UK, 2018; pp. 203–216. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon nanotubes: Current perspectives on diverse applications in targeted drug delivery and therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Beg, S.; Rizwan, M.; Sheikh, A.M.; Hasnain, M.S.; Anwer, K.; Kohli, K. Advancement in carbon nanotubes: Basics, biomedical applications and toxicity. J. Pharm. Pharmacol. 2011, 63, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Khodadadzadeh, L. Ultrasonic-assisted fabrication of starch/MWCNT-glucose nanocomposites for drug delivery. Ultrason. Sonochem. 2018, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.K.; Khatri, S.; Hansen, J.; Slott, S.; Parvathaneni, R.P.; Mendes, A.C.; Chronakis, I.S.; Hung, S.C.; Rajasekaran, N.; Ma, Z.; et al. Carbon nanotubes—Potent carriers for targeted drug delivery in rheumatoid arthritis. Pharmaceutics 2021, 13, 453. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Faraji, F.; Torghabeh, F.M.; Faraji, F.; Mansouri, K.; Abam, F.; Shohaimi, S.; Akbari, H.; Mohammadi, M. Polymer-based drug delivery systems for anticancer drugs: A systematic review. Cancer Treat. Res. Commun. 2022, 32, 100605. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Rangari, A.T. Polymeric Nanoparticles Based Topical Drug Delivery: An Overview. Asian J. Biomed. Pharm. Sci. 2015, 5, 5–12. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, S.; Lamberti, G.; Barba, A.A. Polymer–lipid pharmaceutical nanocarriers: Innovations by new formulations and production technologies. Pharmaceutics 2021, 13, 198. [Google Scholar] [CrossRef]
- Ryu, S.; Park, S.; Lee, H.Y.; Lee, H.; Cho, C.W.; Baek, J.S. Biodegradable nanoparticles-loaded plga microcapsule for the enhanced encapsulation efficiency and controlled release of hydrophilic drug. Int. J. Mol. Sci. 2021, 22, 2792. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Y.; Zhang, P.; Yang, Z.; Deng, W.; Sun, Z.; Yang, M.; Li, X.; Ma, G.; Deng, G.; et al. Immunocyte membrane-coated nanoparticles for cancer immunotherapy. Cancers 2021, 13, 77. [Google Scholar] [CrossRef]
- Bansal, K.K.; Mishra, D.K.; Rosling, A.; Rosenholm, J.M. Therapeutic potential of polymer-coated mesoporous silica nanoparticles. Appl. Sci. 2020, 10, 289. [Google Scholar] [CrossRef]
- Lee, C.M.; Jang, D.; Kim, J.; Cheong, S.J.; Kim, E.M.; Jeong, M.H.; Kim, S.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; et al. Oleyl-Chitosan nanoparticles based on a dual probe for optical/MR imaging in vivo. Bioconjug. Chem. 2011, 22, 186–192. [Google Scholar] [CrossRef]
- Park, J.H.; Jiang, Y.; Zhou, J.; Gong, H.; Mohapatra, A.; Heo, J.; Gao, W.; Fang, R.H.; Zhang, L. Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021, 7, eabf7820. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Sanchez Armengol, E.; Unterweger, A.; Laffleur, F. PEGylated drug delivery systems in the pharmaceutical field: Past, present and future perspective. Drug Dev. Ind. Pharm. 2022, 48, 129–139. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Fee, C. Protein PEGylation: An overview of chemistry and process considerations. Eur. Pharm. Rev. 2010, 15, 18–26. [Google Scholar]
- Reboredo, C.; González-Navarro, C.J.; Martínez-Oharriz, C.; Martínez-López, A.L.; Irache, J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120287. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xiao, J.; Cong, Y.; Liu, J.; He, Y.; Ross, B.D.; Xu, H.; Yin, Y.; Hong, H.; Xu, W. PEGylated Nanoscale Metal-Organic Frameworks for Targeted Cancer Imaging and Drug Delivery. Bioconjug. Chem. 2021, 32, 2195–2204. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. Perspectives and Prospective on Solid Lipid Nanoparticles as Drug Delivery Systems. Molecules 2022, 27, 1543. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.C.; Lu, X. Solid Lipid Nanoparticles for Drug Delivery. Methods Mol. Biol. 2023, 2622, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN). Pharm. Dev. Technol. 2020, 25, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Preman, N.K.; Jain, S.; Johnson, R.P. “smart” Polymer Nanogels as Pharmaceutical Carriers: A Versatile Platform for Programmed Delivery and Diagnostics. ACS Omega 2021, 6, 5075–5090. [Google Scholar] [CrossRef]
- Zhao, Q.; Yue, X.; Miaomiao, L.; Yanming, W.; Wu, G. Nano-injectable pH/NIR-responsive hydrogel for chemo-photothermal synergistic drug delivery. J. Biomater. Appl. 2023, 38, 614–628. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Bhattacharjee, S. Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol. 2023, 226, 535–553. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.; Yang, J.; Baek, S.Y.; Kim, D. Hydrogel nanoparticle as a functional coating layer in biosensing, tissue engineering, and drug delivery. Coatings 2020, 10, 663. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D.; Cuggino, J.C.; Calderon, M. Functional Nanogels for Biomedical Applications. Curr. Med. Chem. 2012, 19, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Vakar; Poonia, N.; Chaudhary, G.; Jain, G.K.; Lather, V.; Khar, R.K. pH-sensitive polymeric nanocarriers for enhanced intracellular drug delivery. In Smart Polymeric Nano-Constructs in Drug Delivery; Academic Press: Cambridge, MA, USA, 2023; pp. 65–107. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Cirne, M.F.R.; Immich, M.F.; Poletto, F. Stimuli-responsive polymeric hydrogels and nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers; Woodhead Publishing: Cambridge, UK, 2018; pp. 343–374. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, B.; Behl, G. A Comprehensive Outlook of Synthetic Strategies and Applications of Redox-Responsive Nanogels in Drug Delivery. Macromol. Biosci. 2019, 19, e1900071. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Peng, S.; Men, Y.; Xie, R.; Tian, Y.; Yang, W. Biodegradable phosphorylcholine-based zwitterionic polymer nanogels with smart charge-conversion ability for efficient inhibition of tumor cells. J. Colloid Interface Sci. 2019, 539, 19–29. [Google Scholar] [CrossRef] [PubMed]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Chrzanowski, G.; Bober, Z.; Aebisher, D. An analytical study of Trastuzumab-dendrimer-fluorine drug delivery system in breast cancer therapy in vitro. Biomed. Pharmacother. 2021, 133, 111053. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Walter, M.V.; Montañez, M.I.; Kunzmann, A.; Hult, A.; Nyström, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanoparticle Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C 2018, 90, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo De Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 2014, 507273. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Bickel, U. How to measure drug transport across the blood-brain barrier. NeuroRx 2005, 2, 15–26. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, L.; Li, W.; Zhang, Y.; Wu, Y.; Zhi, D.; Wang, H.; Wang, L.; Kong, D.; Zhu, M. Construction of vascular graft with circumferentially oriented microchannels for improving artery regeneration. Biomaterials 2020, 242, 119922. [Google Scholar] [CrossRef] [PubMed]
- DuRoss, A.N.; Neufeld, M.J.; Rana, S.; Thomas, C.R.; Sun, C. Integrating nanomedicine into clinical radiotherapy regimens. Adv. Drug Deliv. Rev. 2019, 144, 35–56. [Google Scholar] [CrossRef]
- Cassano, R.; Servidio, C.; Trombino, S. Biomaterials for drugs nose–brain transport: A new therapeutic approach for neurological diseases. Materials 2021, 14, 1802. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Poka, M.S.; Demana, P.H.; Matafwali, S.K.; Melamane, S.; Khamanga, S.M.M.; Makoni, P.A. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics 2022, 14, 836. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Musumeci, T.; Di Benedetto, G.; Carbone, C.; Bonaccorso, A.; Amato, G.; Lo Faro, M.J.; Burgaletto, C.; Puglisi, G.; Bernardini, R.; Cantarella, G. Intranasal Administration of a TRAIL Neutralizing Monoclonal Antibody Adsorbed in PLGA Nanoparticles and NLC Nanosystems: An In Vivo Study on a Mouse Model of Alzheimer’s Disease. Biomedicines 2022, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Annu; Sartaj, A.; Qamar, Z.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front. Bioeng. Biotechnol. 2022, 10, 788128. [CrossRef]
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of Various Types of Nanomaterials for the Treatment of Neurological Disorders. Nanomaterials 2022, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, K.; Narayan, J. Nanoparticle: Significance as smart material in therapeutic strategies in drug delivery in biological systems. Appl. Biomed. Eng. Neurosci. 2019, 327–339. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Duan, L.; Li, X.; Ji, R.; Hao, Z.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers 2023, 15, 2196. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Ayhan, H.; Ozyayla, V.F.; Denkbas, E.B.; Kaitian, X.; Cicek, H.; Tuncel, A.; Piskin, E. Phagocytosis of biodegradable polymeric particles. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994, 22, A3. [Google Scholar]
- Dingezweni, S. The blood–brain barrier. S. Afr. J. Anaesth. Analg. 2020, 26, S32–S34. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Zhao, F.; Zhong, L.; Luo, Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci. Ther. 2021, 27, 26–35. [Google Scholar] [CrossRef]
- Malik, V.A.; Di Benedetto, B. The blood-brain barrier and the ephr/ephrin system: Perspectives on a link between neurovascular and neuropsychiatric disorders. Front. Mol. Neurosci. 2018, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Greig, N.H.; Raffel, C.; Amar, A.P.; Apuzzo, M.L.J.; Antel, J.P.; Rosenberg, G.A. Mechanisms of Disease: The Blood-Brain Barrier. Neurosurgery 2004, 54, 131–142. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Kim, D.H.; Oh, B.K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- René, C.A.; Parks, R.J. Delivery of therapeutic agents to the central nervous system and the promise of extracellular vesicles. Pharmaceutics 2021, 13, 492. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier endogenous transporters as therapeutic targets: A new model for small molecule CNS drug discovery. Expert Opin. Ther. Targets 2015, 19, 1059–1072. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood cells and endothelial barrier function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Mei, D.; Huo, Y.; Mao, S. Effect of polysorbate 80 on the intranasal absorption and brain distribution of tetramethylpyrazine phosphate in rats. Drug Deliv. Transl. Res. 2019, 9, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zou, J.; Sun, C.; Peng, F.; Peng, C. Ado-tratuzumab emtansine beyond breast cancer: Therapeutic role of targeting other HER2-positive cancers. Front. Mol. Biosci. 2023, 10, 1165781. [Google Scholar] [CrossRef]
- Steiniger, S.C.J.; Kreuter, J.; Khalansky, A.S.; Skidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; Uhl, R.; Kock, M.; Geiger, K.D.; et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer 2004, 109, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.H.; Kim, Y.; Kim, S.Y. Twenty Years of Anti-Vascular Endothelial Growth Factor Therapeutics in Neovascular Age-Related Macular Degeneration Treatment. Int. J. Mol. Sci. 2023, 24, 13004. [Google Scholar] [CrossRef] [PubMed]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef]
- Saha, T.; Lukong, K.E. Breast Cancer Stem-Like Cells in Drug Resistance: A Review of Mechanisms and Novel Therapeutic Strategies to Overcome Drug Resistance. Front. Oncol. 2022, 12, 856974. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Taron, M. Pharmacogenomic biomarkers for personalized cancer treatment. J. Intern. Med. 2015, 277, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.N.; Goldman, J.M.; Melo, J.V. The molecular biology of chronic myeloid leukemia. Blood 2000, 96, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y. Molecular biology of chronic myeloid leukemia. Cancer Sci. 2012, 103, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Kinetics of blood–brain barrier transport of monoclonal antibodies targeting the insulin receptor and the transferrin receptor. Pharmaceuticals 2022, 15, 3. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.N.; Do Carmo Pereira, M.; Rocha, S. Targeting nanoparticles across the blood-brain barrier with monoclonal antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Boado, R.J.; Patrick, D.J.; Hui, E.K.W.; Lu, J.Z. Blood-Brain Barrier Transport, Plasma Pharmacokinetics, and Neuropathology Following Chronic Treatment of the Rhesus Monkey with a Brain Penetrating Humanized Monoclonal Antibody Against the Human Transferrin Receptor. Mol. Pharm. 2018, 15, 5207–5216. [Google Scholar] [CrossRef]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for enhancing the permeation of cns-active drugs through the blood-brain barrier: A review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef]
- ElSheikh, R.H.; Chauhan, M.Z.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Neovascular Age-Related Macular Degeneration. Biomolecules 2022, 12, 1629. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, T.E.; Kertes, P.J. Ranibizumab in neovascular age-related macular degeneration. Clin. Interv. Aging 2006, 1, 451–466. [Google Scholar] [CrossRef]
- Kristin, E.; Endarti, D.; Khoe, L.C.; Taroeno-Hariadi, K.W.; Trijayanti, C.; Armansyah, A.; Sastroasmoro, S. Economic Evaluation of Adding Bevacizumab to Chemotherapy for Metastatic Colorectal Cancer (mCRC) Patients in Indonesia. Asian Pac. J. Cancer Prev. 2021, 22, 1921–1926. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Sandler, A. Bevacizumab in non-small cell lung cancer. Clin. Cancer Res. 2007, 13, 4613s–4616s. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A Randomized Trial of Bevacizumab, an Anti–Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N. Engl. J. Med. 2003, 349, 427–434. [Google Scholar] [CrossRef]
- De Gramont, A.; Van Cutsem, E. Investigating the potential of bevacizumab in other indications: Metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology 2005, 69, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, Y.; Hata, N.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Fujioka, Y.; Takigawa, K.; Mizoguchi, M. Update on chemotherapeutic approaches and management of bevacizumab usage for glioblastoma. Pharmaceuticals 2020, 13, 470. [Google Scholar] [CrossRef]
- Field, K.M.; Jordan, J.T.; Wen, P.Y.; Rosenthal, M.A.; Reardon, D.A. Bevacizumab and glioblastoma: Scientific review, newly reported updates, and ongoing controversies. Cancer 2015, 121, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y. Bevacizumab for glioblastoma. Ther. Clin. Risk Manag. 2015, 11, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Chari, R.V.J. Ado-trastuzumab emtansine (T-DM1): An antibody-drug conjugate (ADC) for HER2-positive breast cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.M.; Slézia, A.; Kaszas, A.; Ghestem, A.; del Agua, I.; Pappa, A.M.; Bernard, C.; Williamson, A.; Malliaras, G.G. Electrophoretic drug delivery for seizure control. Sci. Adv. 2018, 4, eaau1291. [Google Scholar] [CrossRef] [PubMed]
- Slezia, A.; Proctor, C.M.; Kaszas, A.; Malliaras, G.G.; Williamson, A. Electrophoretic delivery of γ-aminobutyric acid (GABA) into epileptic focus prevents seizures in mice. J. Vis. Exp. 2019, 2019, e59268. [Google Scholar] [CrossRef]
- Cook, M.; Murphy, M.; Bulluss, K.; D’Souza, W.; Plummer, C.; Priest, E.; Williams, C.; Sharan, A.; Fisher, R.; Pincus, S.; et al. Anti-seizure therapy with a long-term, implanted intra-cerebroventricular delivery system for drug-resistant epilepsy: A first-in-man study. EClinicalMedicine 2020, 22, 100326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yue, Z.; Moulton, S.E.; Hayes, P.; Cook, M.J.; Wallace, G.G. A simple and versatile method for microencapsulation of anti-epileptic drugs for focal therapy of epilepsy. J. Mater. Chem. B 2015, 3, 7255–7261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ugur Yilmaz, C.; Emik, S.; Orhan, N.; Temizyurek, A.; Atis, M.; Akcan, U.; Khodadust, R.; Arican, N.; Kucuk, M.; Gurses, C.; et al. Targeted delivery of lacosamide-conjugated gold nanoparticles into the brain in temporal lobe epilepsy in rats. Life Sci. 2020, 257, 118081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Jiang, Y.; Lv, W.; Wu, L.; Wang, B.; Lv, L.; Xu, Q.; Xin, H. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci. Rep. 2015, 5, 12651. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Jasim, D.; Ahmad, S.S.; Wong, R.; Haley, M.; Coutts, G.; Schiessl, I.; Allan, S.M.; Kostarelos, K. Selective Liposomal Transport through Blood Brain Barrier Disruption in Ischemic Stroke Reveals Two Distinct Therapeutic Opportunities. ACS Nano 2019, 13, 12470–12486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef] [PubMed]
- Juthani, R.; Madajewski, B.; Yoo, B.; Zhang, L.; Chen, P.M.; Chen, F.; Turker, M.Z.; Ma, K.; Overholtzer, M.; Longo, V.A.; et al. Ultrasmall core-shell silica nanoparticles for precision drug delivery in a high-grade malignant brain tumor model. Clin. Cancer Res. 2020, 26, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, B.; Corey, S.; Shear, A.; Heyck, M.; Cozene, B.; Sadanandan, N.; Zhang, H.; Gonzales-Portillo, B.; Sheyner, M.; Borlongan, C.V. Mesenchymal stem cell therapy alleviates the neuroinflammation associated with acquired brain injury. CNS Neurosci. Ther. 2020, 26, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiu, J.; Li, X.L.; Aday, S.; Zhang, J.; Conley, G.; Xu, J.; Joseph, J.; Lan, H.; Langer, R.; et al. BBB pathophysiology–independent delivery of siRNA in traumatic brain injury. Sci. Adv. 2021, 7, eabd6889. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.W.; Rodrigo, A.C.; Patterson, A.K.; Hawkins, K.; Aly, M.M.S.; Sun, J.; Al Jamal, K.T.; Smith, D.K. Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Adv. Sci. 2021, 8, 2101058. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.F.; Knol, M.J.; Schiffelers, R.M.; Caiazzo, M.; Fens, M.H. Levodopa-loaded nanoparticles for the treatment of Parkinson's disease. J. Control. Release 2023, 360, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Anoop, U.R.; Verma, K. New technique and device for controlled and continuous drug delivery into the brain: A proof-of-concept study. BMJ Innov. 2021, 7, 470–477. [Google Scholar] [CrossRef]
- Pereira, K.V.; Giacomeli, R.; Gomes de Gomes, M.; Haas, S.E. The challenge of using nanotherapy during pregnancy: Technological aspects and biomedical implications. Placenta 2020, 100, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Prabahar, K.; Alanazi, Z.; Qushawy, M. Targeted drug delivery system: Advantages, carriers and strategies. Indian J. Pharm. Educ. Res. 2021, 55, 346–353. [Google Scholar] [CrossRef]
- Dhilip Kumar, S.S.; Abrahamse, H. Recent advances in the development of biocompatible nanocarriers and their cancer cell targeting efficiency in photodynamic therapy. Front. Chem. 2022, 10, 969809. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Deng, W.Q.; Fan, S.R.; Chen, M.F.; Qi, M.; Lyu, W.Y.; Qi, Q.; Tiwari, A.K.; Chen, J.X.; Zhang, D.M.; et al. m6A modification: Recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer 2022, 21, 52. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.; Rodrigues, A.S. Cancer drug resistance: A brief overview from a genetic viewpoint. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1395, pp. 1–18. [Google Scholar]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Lorusso, D.; Bria, E.; Costantini, A.; Di Maio, M.; Rosti, G.; Mancuso, A. Patients’ perception of chemotherapy side effects: Expectations, doctor–patient communication and impact on quality of life—An Italian survey. Eur. J. Cancer Care 2017, 26, e12618. [Google Scholar] [CrossRef] [PubMed]
- Moghimi-Dehkordi, B. An overview of colorectal cancer survival rates and prognosis in Asia. World J. Gastrointest. Oncol. 2012, 4, 71. [Google Scholar] [CrossRef]
- Welch, H.G.; Schwartz, L.M.; Woloshin, S. Are increasing 5-year survival rates evidence of success against cancer? JAMA 2000, 283, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am. Health Drug Benefits 2016, 9, 23–31. [Google Scholar]
- Caracciolo, G.; Vali, H.; Moore, A.; Mahmoudi, M. Challenges in molecular diagnostic research in cancer nanotechnology. Nano Today 2019, 27, 6–10. [Google Scholar] [CrossRef]
- Goel, S.; Ni, D.; Cai, W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano 2017, 11, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Makwana, V.; Karanjia, J.; Haselhorst, T.; Anoopkumar-Dukie, S.; Rudrawar, S. Liposomal doxorubicin as targeted delivery platform: Current trends in surface functionalization. Int. J. Pharm. 2021, 593, 120117. [Google Scholar] [CrossRef]
- van der Meel, R.; Vehmeijer, L.J.C.; Kok, R.J.; Storm, G.; van Gaal, E.V.B. Ligand-targeted Particulate Nanomedicines Undergoing Clinical Evaluation: Current Status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Lee, Y.M.; Park, D.; Im, S.; Song, E.H.; Kim, W.J. Self-assembled nanocomplex between polymerized phenylboronic acid and doxorubicin for efficient tumor-targeted chemotherapy. Acta Pharmacol. Sin. 2017, 38, 848–858. [Google Scholar] [CrossRef]
- Gawde, K.A.; Kesharwani, P.; Sau, S.; Sarkar, F.H.; Padhye, S.; Kashaw, S.K.; Iyer, A.K. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J. Colloid Interface Sci. 2017, 496, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; De Matos, M.B.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Boulikas, T.; Tsogas, I. Microtubule-targeted antitumor drugs: Chemistry, mechanisms and nanoparticle formulations. Gene Ther. Mol. Biol. 2008, 12, 313–358. [Google Scholar]
- Kim, J.H.; Kim, Y.; Bae, K.H.; Park, T.G.; Lee, J.H.; Park, K. Tumor-targeted delivery of paclitaxel using low density lipoprotein-mimetic solid lipid nanoparticles. Mol. Pharm. 2015, 12, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Lavasanifar, A. Effects of block copolymer micelles on the pharmacokinetics of encapsulated drugs. In Nanoarchitectonics in Biomedicine; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 507–546. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, D.; Wu, X.; Wang, Y.; Lin, X.; Xu, Z.; Liu, H.; Sun, J.; He, Z.; Wang, Y. Tyrosine modified irinotecan-loaded liposomes capable of simultaneously targeting LAT1 and ATB0,+ for efficient tumor therapy. J. Control. Release 2019, 316, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Nagy, J.; Triche, T. Abstract 3705: Targeted anticancer drug delivery to Ewing’s sarcoma using human anti-CD99 targeted hybrid polymerization liposomal nanoparticles. Cancer Res. 2018, 78, 3705. [Google Scholar] [CrossRef]
- Kang, H.; Nagy, J.; Mitra, S.; Triche, T. Abstract 2875: Targeted therapy of Ewing’s sarcoma by human anti CD99 targeted hybrid polymerized liposomal nanoparticles (HPLNs) encapsulating anticancer agents. Cancer Res. 2019, 79, 2875. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Karthiga, A.; Tripathi, S.K.; Shanmugam, R.; Suryanarayanan, V.; Singh, S.K. Targeting the cyclin-binding groove site to inhibit the catalytic activity of CDK2/cyclin A complex using p27KIP1-derived peptidomimetic inhibitors. J. Chem. Biol. 2015, 8, 11–24. [Google Scholar] [CrossRef][Green Version]
- Madaan, A.; Singh, P.; Awasthi, A.; Verma, R.; Singh, A.T.; Jaggi, M.; Mishra, S.K.; Kulkarni, S.; Kulkarni, H. Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system, NanoxelTM. Clin. Transl. Oncol. 2013, 15, 26–32. [Google Scholar] [CrossRef]
- Wotman, M.; El-Naggar, A.; Ferrarotto, R. Targeting human EGFR 2 (HER2) in salivary gland carcinoma. Expert Rev. Anticancer Ther. 2023, 23, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.; Simbo, D.A.; Mursalin, M.; Mishra, V.; Bashary, R.; Khatik, G.L. Drug Delivery Approaches for Doxorubicin in the Management of Cancers. Curr. Cancer Ther. Rev. 2019, 16, 320–331. [Google Scholar] [CrossRef]
- Sharma, S. Nanoemulsions for brain drug delivery systems. J. Pharm. Sci. Res. 2020, 12, 1112–1118. [Google Scholar]
- Das, P.; Panda, J.R.; Patro, C.N.; Sahu, B.; Patnaik, S.S. A Comprehensive Review of Nanoemulsion Applications and their Recent Advancements. Curr. Nanomater. 2022, 8, 209–223. [Google Scholar] [CrossRef]
- Khan, M.S.; Baskoy, S.A.; Yang, C.; Hong, J.; Chae, J.; Ha, H.; Lee, S.; Tanaka, M.; Choi, Y.; Choi, J. Lipid-based colloidal nanoparticles for applications in targeted vaccine delivery. Nanoscale Adv. 2023, 5, 1853–1869. [Google Scholar] [CrossRef]
- Gajera, K.; Patel, A. An Overview of FDA Approved Liposome Formulations for Cancer Therapy. J. Adv. Med. Pharm. Sci. 2022, 24, 1–7. [Google Scholar] [CrossRef]
- Arshad, R.; Kiani, M.H.; Rahdar, A.; Sargazi, S.; Barani, M.; Shojaei, S.; Bilal, M.; Kumar, D.; Pandey, S. Nano-Based Theranostic Platforms for Breast Cancer: A Review of Latest Advancements. Bioengineering 2022, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Thiruvengadam, M.; Farooqui, Z.; Rajakumar, G.; Sajid Jamal, Q.M.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Chung, I.M.; Al-Suhaimi, E.A. Nanotechnology, in silico and endocrine-based strategy for delivering paclitaxel and miRNA: Prospects for the therapeutic management of breast cancer. Semin. Cancer Biol. 2021, 69, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Miao, D.; Zhou, M.; Wang, L.; Zhou, H.; Su, G. Bio-inspired protein-based nanoformulations for cancer theranostics. Front. Pharmacol. 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, S.Y.; Kim, H.K.; Kim, S.W.; Shin, S.W.; Kim, J.S.; Park, K.; Lee, M.Y.; Heo, D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007, 18, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.T.; Tan, E.H.; Toh, C.K.; Hee, S.W.; Leong, S.S.; Ang, P.C.S.; Wong, N.S.; Chowbay, B. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol®-PM) in patients with solid tumors. Ann. Oncol. 2009, 21, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Jung, M.; Sym, S.J.; Shin, D.B.; Kang, S.M.; Kyung, S.Y.; Park, J.W.; Jeong, S.H.; Cho, E.K. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2014, 74, 277–282. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Xu, W.; Jia, L.; Chi, D.; Yu, J.; Wang, J.; He, Z.; Liu, X.; Wang, Y. Irinotecan and berberine co-delivery liposomes showed improved efficacy and reduced intestinal toxicity compared with Onivyde for pancreatic cancer. Drug Deliv. Transl. Res. 2021, 11, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- In brief: Liposomal irinotecan (Onivyde) for pancreatic cancer. Med. Lett. Drugs Ther. 2016, 58, e76.
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; Lorusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Mita, M.; Eisenberg, P.; LoRusso, P.; Weiss, G.; Sachdev, J.; Mita, A.; Low, S.; Hrkach, J.; Summa, J.; et al. Abstract LB-203: A phase I study of BIND-014, a PSMA-targeted nanoparticle containing docetaxel, in patients with refractory solid tumors. Cancer Res. 2013, 73, LB-203. [Google Scholar] [CrossRef]
- Glasmacher, B.; Evertz, F.; Bernemann, I.; Sun, H.; Pogozhikh, D.; Spindler, R.; Hofmann, N. New Cryopreservation Strategies: A View from Biothermal and Biomedical Process Technology. In Proceedings of the 7th International Conference of Boar Semen Preservation, Bonn, Germany, 14–17 August 2011. [Google Scholar]
- Şenel, S.; Ikinci, G.; Kaş, S.; Yousefi-Rad, A.; Sargon, M.F.; Hincal, A.A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 2000, 193, 197–203. [Google Scholar] [CrossRef]
- Paul, A.; Hasan, A.; Kindi, H.A.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Şenyiit, T.; Tekmen, I.; Sönmez, Ü.; Santi, P.; Özer, Ö. Deoxycholate hydrogels of betamethasone-17-valerate intended for topical use: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 403, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, S.J.; Yang, J.A.; Jeon, J.H.; Bhang, S.H.; Kim, B.S.; Hahn, S.K. Injectable hyaluronic acid-tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Alsarra, I.A.; Hamed, A.Y.; Mahrous, G.M.; El Maghraby, G.M.; Al-Robayan, A.A.; Alanazi, F.K. Mucoadhesive polymeric hydrogels for nasal delivery of acyclovir. Drug Dev. Ind. Pharm. 2009, 35, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Bisharat, L.; Perinelli, D.R.; Berardi, A.; Bonacucina, G.; Logrippo, S.; Darwish Elhajji, F.W.; Cespi, M.; Palmieri, G.F. Influence of Testing Parameters on In Vitro Tramadol Release from Poloxamer Thermogels using the Immersion Cell Method. AAPS PharmSciTech 2017, 18, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.D.; Salter, E.; Chen, E.; Sutter, K.A.; Alsberg, E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J. Biomed. Mater. Res. Part A 2010, 92, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Li, Y.; Abebe, D.G.; Xie, Y.; Kandil, R.; Kraus, T.; Gomez-Lopez, N.; Fujiwara, T.; Merkel, O.M. Folate receptor targeted three-layered micelles and hydrogels for gene delivery to activated macrophages. J. Control. Release 2016, 244, 269–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).