Current Status of Gout Arthritis: Current Approaches to Gout Arthritis Treatment: Nanoparticles Delivery Systems Approach
Abstract
1. Introduction
2. Overview of Gout
2.1. Pathophysiology
2.2. Management of Gout
2.2.1. Diagnostics of Uric Acid Disorders
2.2.2. Pharmacological Management
- 1.
- Curative Treatment
- 2.
- Preventive Treatment
2.2.3. Lifestyle Modifications
2.3. Comorbidities
3. Molecular Mechanisms in the Treatment of Gout
3.1. Hyperuricemia Treatment
3.1.1. Inhibition of Uric Acid Production
3.1.2. Uricosuric Agents
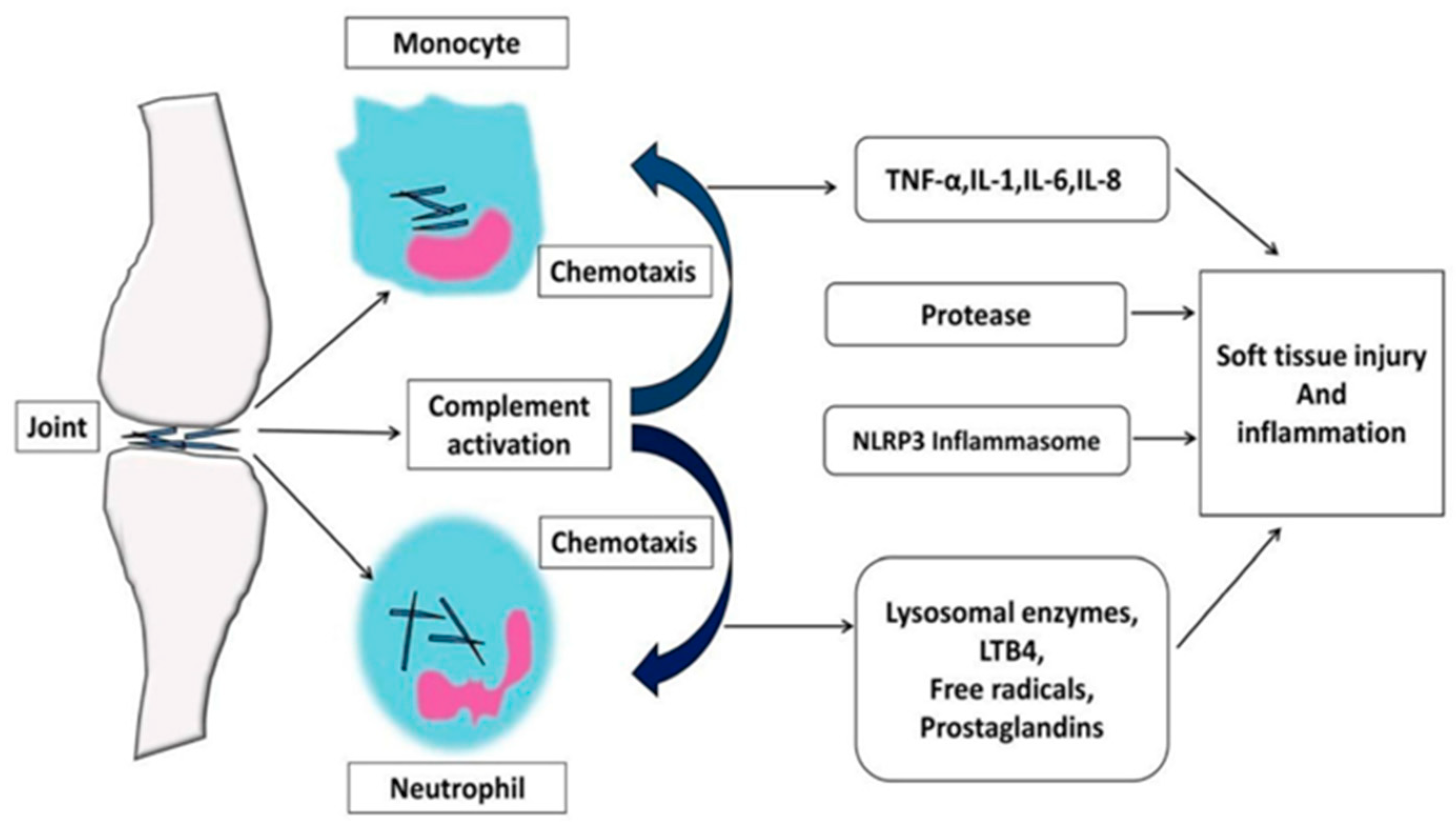
3.2. Modulation of Inflammation
3.3. Erosion of Bone (Stage of Tophi)
3.4. Genetic Variations
4. Nanoparticles in Gout Treatment
- (a)
- Nanoparticle efficacy depends on encapsulated drug properties and carrier material characteristics. These substances were analgesic, had anti-inflammation properties, and controlled oxidative stress. The nanomaterial shell prolonged the biological half-life and improved the pharmacokinetics of encapsulated substances to maximize their therapeutic effect. However, traditional drug treatment strategies show low efficacy and safety due to the (bio)pharmaceutical shortcomings of these drugs, such as poor chemical stability and limited ability to target the pathophysiological pathways [23].
- (b)
- Nanocarriers enhance gout treatment by improving drug bioavailability, particularly for non-metallic compounds. These systems provide superior solubility, reduced drug loss, and targeted delivery, optimizing therapeutic efficacy while minimizing side effects.
- (c)
- Nanoparticles offer a promising approach to targeted drug delivery for gout treatment, surpassing traditional medications, like allopurinol, in safety and efficacy. Research indicates that treatments that use nanoparticles affect the kidney and liver less than most other treatments. One example showed how copper sulfate that has been entrapped in nanoparticles could lower UA levels in test animals by about 90%, with less toxicity to the organs than free copper sulfate. In addition to achieving improved targeting and protective encapsulation that ensures high therapeutic outcomes, these nanoparticle-based systems reduce the therapy’s side effects, thereby exhibiting promising strategies for new-era gout treatment, and probably for other chronic diseases. Supercontrol of the biodistribution of drugs results in numerous side effects, which makes the control of gout through drugs a massive challenge. These nanocarrier systems are also employed to overcome the restrictions of today’s drugs, not only the problem of biodistribution but also that of the stability and solubility of the drugs [21].
- (d)
- The application of nanoparticles in gout treatment increases its effectiveness and minimizes the side effects of the drug through better delivery mechanisms. They allow for the extensive control option because they target inflammation and UA production at the same time. Nano could load several therapeutic agents to achieve fast relief from inflammation and decrease UA levels from a single delivering system. This approach promises to provide better gout treatments and diagnoses, thus revolutionizing treatment processes.
5. Future Developments in Gout Treatment
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Liu, H.; Fang, X.M.; Yan, F.; Zhang, Y. Signaling Pathways in Uric Acid Homeostasis and Gout: From Pathogenesis to Therapeutic Interventions. Int. Immunopharmacol. 2024, 132, 111932. [Google Scholar] [CrossRef]
- Kim, S.K. The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; Xue, Y.; He, D. Inflammatory Response to Regulated Cell Death in Gout and Its Functional Implications. Front. Immunol. 2022, 13, 888306. [Google Scholar] [CrossRef]
- Cha, Y.; Lee, J.; Choy, W.; Lee, J.S.; Lee, H.H.; Chae, D.-S. Pathophysiology and Treatment of Gout Arthritis; Including Gout Arthritis of Hip Joint: A Literature Review. Hip Pelvis 2024, 36, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.R.; Wang, J.Q.; Li, J. Role of NLRP3 in the Pathogenesis and Treatment of Gout Arthritis. Front. Immunol. 2023, 14, 1137822. [Google Scholar] [CrossRef]
- Li, C.; Wu, C.; Li, F.; Xu, W.; Zhang, X.; Huang, Y.; Xia, D. Targeting Neutrophil Extracellular Traps in Gouty Arthritis: Insights into Pathogenesis and Therapeutic Potential. J. Inflamm. Res. 2024, 17, 1735–1763. [Google Scholar] [CrossRef]
- Zhu, R.; Niu, Y.; Zhou, W.; Wang, S.; Mao, J.; Guo, Y.; Lei, Y.; Xiong, X.; Li, Y.; Guo, L. Effect of Nanoparticles on Gouty Arthritis: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2023, 24, 124. [Google Scholar] [CrossRef] [PubMed]
- Parisa, N.; Kamaluddin, M.T.; Saleh, M.I.; Sinaga, E. The Inflammation Process of Gout Arthritis and Its Treatment. J. Adv. Pharm. Technol. Res. 2023, 14, 166–170. [Google Scholar] [CrossRef]
- Patil, T.; Soni, A.; Acharya, S. A Brief Review on in Vivo Models for Gouty Arthritis. Metab. Open 2021, 11, 100100. [Google Scholar] [CrossRef]
- Casanova, A.G.; Morales, A.I.; Vicente-Vicente, L.; López-Hernández, F.J. Effect of Uric Acid Reduction on Chronic Kidney Disease. Systematic Review and Meta-Analysis. Front. Pharmacol. 2024, 15, 1373258. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez Lozada, L.G.; Lanaspa, M.A.; Piani, F.; Borghi, C. Uric Acid and Chronic Kidney Disease: Still More to Do. Kidney Int. Rep. 2023, 8, 229–239. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.J.; Wu, C.Y.; Yang, C.W.; Yang, H.Y. Hyperuricemia and Progression of Chronic Kidney Disease: A Review from Physiology and Pathogenesis to the Role of Urate-Lowering Therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sarkar, C.; Rawat, V.S.; Kalita, D.; Deka, S.; Agnihotri, A. Promise of the NLRP3 Inflammasome Inhibitors in In Vivo Disease Models. Molecules 2021, 26, 4996. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, W.; Zhang, B.; Li, W. NLRP3 Inflammasome: A Promising Therapeutic Target for Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Kuwabara, M.; Borghi, C. Therapeutic Strategies for the Treatment of Chronic Hyperuricemia: An Evidence-Based Update. Medicina 2021, 57, 58. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Cincione, R.I.; Tocci, G.; Borghi, C. Clinical Effects of Xanthine Oxidase Inhibitors in Hyperuricemic Patients. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2021, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhai, N.; Chen, Y.; Wang, C.; Wu, F.; Luo, X.; Ju, X.; Liu, H.; Liu, G. A Multiscale Screening Strategy for the Identification of Novel Xanthine Oxidase Inhibitors Based on the Pharmacological Features of Febuxostat Analogues. New J. Chem. 2022, 46, 6549–6559. [Google Scholar] [CrossRef]
- Yadav, S.; Bhosale, M.; Sattigeri, B.; Vimal, S. Pharmacological Overview for Therapy of Gout and Hyperuricemia. Int. J. Health Sci. 2022, 6, 7772–7785. [Google Scholar] [CrossRef]
- Jenkins, C.; Hwang, J.H.; Kopp, J.B.; Winkler, C.A.; Cho, S.K. Review of Urate-Lowering Therapeutics: From the Past to the Future. Front. Pharmacol. 2022, 13, 925219. [Google Scholar] [CrossRef]
- Kiyani, M.M.; Sohail, M.F.; Shahnaz, G.; Rehman, H.; Akhtar, M.F.; Nawaz, I.; Mahmood, T.; Manzoor, M.; Bokhari, S.A.I. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina 2019, 55, 10. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.W.; Mohd Nordin, U.U.; Mahmood, S.; Karusan, N.R.; Khalid, R.; Nordin, N.; Fornaguera, C.; Ahmad, N. Gout Management Using Nanocarrier Systems: A Review. ACS Appl. Nano Mater. 2024, 7, 9816–9846. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Liang, C.; Feng, J.; Yu, C.; Jiang, W.; Cai, K.; Chen, W.; Cai, W.; Zeng, F.; et al. Construction of an Uricase/Catalase/Curcumin-Co-Loaded Drug Delivery System and Its Effect on Hyper-Uric Acid-Induced Kidney Injury. Smart Mater. Med. 2024, 5, 321–335. [Google Scholar] [CrossRef]
- Peng, X.; Li, X.; Xie, B.; Lai, Y.; Sosnik, A.; Boucetta, H.; Chen, Z.; He, W. Gout Therapeutics and Drug Delivery. J. Control. Release 2023, 362, 728–754. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s Frontier in Combatting Infectious and Inflammatory Diseases: Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar]
- Hosseinikhah, S.M.; Barani, M.; Rahdar, A.; Madry, H.; Arshad, R.; Mohammadzadeh, V.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Inflammatory Arthritis. Int. J. Mol. Sci. 2021, 22, 3092. [Google Scholar] [CrossRef] [PubMed]
- Akhileshwar Jha, L.; Imran, M.; Shrestha, J.; Prasad Devkota, H.; Bhattacharya, K.; Alsayari, A.; Wahab, S.; Kumar Jha, S.; Raj Paudel, K.; Kesharwani, P. Effectiveness of Phytoconstituents and Potential of Phyto-Nanomedicines Combination to Treat Osteoarthritis. Eur. Polym. J. 2024, 215, 113243. [Google Scholar] [CrossRef]
- Minekar, A.; Shinde, S.; Chougule, N. Nanoparticles in Pharmaceutical Applications: Current Trends and Future Prospects. Int. J. Pharm. Sci. 2024, 2, 857–867. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Badwaik, C.; Nandgave, M. Gout-a Review on Pathophysiology, Etiology, and Treament. J. Emerg. Technol. Innov. Res. 2022, 9, 688–694. [Google Scholar]
- Luo, Z.; Yang, F.; Hong, S.; Wang, J.; Chen, B.; Li, L.; Yang, J.; Yao, Y.; Yang, C.; Hu, Y.; et al. Role of MicroRNA Alternation in the Pathogenesis of Gouty Arthritis. Front. Endocrinol. 2022, 13, 967769. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and Its Related Diseases: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, D.; Fan, M.; Yuan, J.; Xie, C.; Yuan, H.; Xie, H.; Gao, H. Mechanism of Reactive Oxygen Species-Guided Immune Responses in Gouty Arthritis and Potential Therapeutic Targets. Biomolecules 2024, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Kandav, G.; Bhatt, D.C.; Jindal, D.K.; Singh, S.K. Formulation, Optimization, and Evaluation of Allopurinol-Loaded Bovine Serum Albumin Nanoparticles for Targeting Kidney in Management of Hyperuricemic Nephrolithiasis: Formulation, Optimization, and Evaluation of ABNPs for Kidney Targeting. AAPS PharmSciTech 2020, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Wessig, A.K.; Hoffmeister, L.; Klingberg, A.; Alberts, A.; Pich, A.; Brand, K.; Witte, T.; Neumann, K. Natural Antibodies and CRP Drive Anaphylatoxin Production by Urate Crystals. Sci. Rep. 2022, 12, 4483. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, S.; Deng, Y.; Wang, X.; Wu, S.; Chen, X.; Li, Y.; Ouyang, J.; Lin, D.; Quan, H.; et al. The Dysregulation of Immune Cells Induced by Uric Acid: Mechanisms of Inflammation Associated with Hyperuricemia and Its Complications. Front. Immunol. 2023, 14, 1282890. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.F.; Kos, I.A.; Vargas-Santos, A.B.; da Rocha Castelar Pinheiro, G.; Dos Santos Paiva, E. Benzbromarone in the Treatment of Gout. Adv. Rheumatol. 2019, 59, 37. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Q.; Li, J.; Fu, S.; Yu, W.; Shao, X.; Li, J.; Huang, Y.; Chen, J.; Tang, Y. Effects of Uric Acid-Lowering Therapy (ULT) on Renal Outcomes in CKD Patients with Asymptomatic Hyperuricemia: A Systematic Review and Meta-Analysis. BMC Nephrol. 2024, 25, 63. [Google Scholar] [CrossRef]
- Bignardi, P.R.; Ido, D.H.; Garcia, F.A.L.; Braga, L.M.; Delfino, V.D.A. Does Uric Acid-Lowering Treatment Slow the Progression of Chronic Kidney Disease? A Meta-Analysis of Randomized Controlled Trials. Nefrologia 2023, 43, 167–181. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, Y.; Cao, L.; Peng, Q.; Yao, X. Purine Metabolism-Related Genes and Immunization in Thyroid Eye Disease Were Validated Using Bioinformatics and Machine Learning. Sci. Rep. 2023, 13, 18391. [Google Scholar] [CrossRef] [PubMed]
- Nassogne, M.-C.; Marie, S.; Dewulf, J.P. Neurological Presentations of Inborn Errors of Purine and Pyrimidine Metabolism. Eur. J. Paediatr. Neurol. 2024, 48, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, V.; Angeloni, C.; Giovannini, M.; Borghi, C. Purine Metabolism Dysfunctions: Experimental Methods of Detection and Diagnostic Potential. Int. J. Mol. Sci. 2023, 24, 7027. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.D.; Yamaoka, M.; Nishizawa, H.; Fukuda, S.; Fujishima, Y.; Kimura, T.; Kozawa, J.; Kita, S.; Matsuoka, T.A.; Otsuki, M.; et al. Multiple Gouty Tophi with Bone Erosion and Destruction: A Report of an Early-Onset Case in an Obese Patient. Intern. Med. 2017, 56, 1071–1077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, Z.; Huang, Y.; Liang, L.; Lin, P.; Guo, X.; Huang, Q.; Huang, Z.; Chen, S.; Huang, Z.; Wang, B.; et al. Clinical Characteristics and Risk Factors Associated with Bone Erosion in Patients with Tophi. Adv. Rheumatol. 2024, 64, 18. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Pool, B.; Chhana, A.; Gao, R.; Munro, J.T.; Cornish, J.; Dalbeth, N. Factors Secreted by Monosodium Urate Crystal-Stimulated Macrophages Promote a Proinflammatory State in Osteoblasts: A Potential Indirect Mechanism of Bone Erosion in Gout. Arthritis Res. Ther. 2022, 24, 212. [Google Scholar] [CrossRef]
- Lhaglham, P.; Jiramonai, L.; Jia, Y.; Huang, B.; Huang, Y.; Gao, X.; Zhang, J.; Liang, X.J.; Zhu, M. Drug Nanocrystals: Surface Engineering and Its Applications in Targeted Delivery. iScience 2024, 27, 111185. [Google Scholar] [CrossRef]
- Oehler, J.B.; Rajapaksha, W.; Albrecht, H. Emerging Applications of Nanoparticles in the Diagnosis and Treatment of Breast Cancer. J. Pers. Med. 2024, 14, 723. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Chidambaram, V.A.; Choong, M.C.M.; Goud, C.D. Dual-Energy Computed Tomography of the Abdomen: A Reliable Trouble-Shooter. J. Clin. Imaging Sci. 2023, 13, 12. [Google Scholar] [CrossRef]
- Albano, D.; Di Luca, F.; D’Angelo, T.; Booz, C.; Midiri, F.; Gitto, S.; Fusco, S.; Serpi, F.; Messina, C.; Sconfienza, L.M. Dual-Energy CT in Musculoskeletal Imaging: Technical Considerations and Clinical Applications. Radiol. Med. 2024, 129, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lee, J.S.; Chung, M.K.; Ahn, J.K.; Choi, H.-J.; Hong, S.-J.; Yoon, C.-H.; Kim, S.-H.; Jeong, K.-H.; Kim, J.-W.; et al. Korean Guidelines for the Management of Gout. J. Rheum. Dis. 2023, 30, 141–150. [Google Scholar] [CrossRef]
- Giovannuzzi, S. Chapter 10—Molybdenum Enzymes; Supuran, C.T., Donald, W.A.B.T.-M., Eds.; Academic Press: Warsaw, Poland, 2024; pp. 557–580. ISBN 978-0-12-823974-2. [Google Scholar]
- Mehmood, A.; Li, J.; Rehman, A.U.; Kobun, R.; Llah, I.U.; Khan, I.; Althobaiti, F.; Albogami, S.; Usman, M.; Alharthi, F.; et al. Xanthine Oxidase Inhibitory Study of Eight Structurally Diverse Phenolic Compounds. Front. Nutr. 2022, 9, 966557. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Mandell, B.F.; Schlesinger, N.; Mount, D.B.; Botson, J.K.; Abdellatif, A.A.; Rhoades, R.; Singh, J.A. Controversies and Practical Management of Patients with Gout and Chronic Kidney Disease. Kidney Int. 2024, 106, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, M.H.; Mandell, B.F. Therapeutic Approaches in the Treatment of Gout. Semin. Arthritis Rheum. 2020, 50, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; Park, K.; Schlesinger, N. Contentious Issues in Gout Management: The Story so Far. Open Access Rheumatol. Res. Rev. 2021, 13, 111–122. [Google Scholar] [CrossRef]
- Li, J.X.; Cummins, C.L. Fresh Insights into Glucocorticoid-Induced Diabetes Mellitus and New Therapeutic Directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Noetzlin, S.; Breville, G.; Seebach, J.D.; Gastaldi, G. Short-Term Glucocorticoid-Related Side Effects and Adverse Reactions: A Narrative Review and Practical Approach. Swiss Med. Wkly. 2022, 152, w30088. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Al-Gareeb, A.I.; Saad, H.M.; Al-kuraishy, H.M. COVID-19 and Corticosteroids: A Narrative Review. Inflammopharmacology 2022, 30, 1189–1205. [Google Scholar] [CrossRef]
- Saliba, F.; Mourad, O.; Mina, J.; Haddadin, F.; Aoun, L.; Almardini, S.; Abu-baker, S.; Sangaraju, K.; Di Pietro, G.; Gaballa, D.; et al. Treatment of Gout in Patients with CrCl ≤30 ML/Min and/or on Hemodialysis: A Review. Rheumato 2024, 4, 49–62. [Google Scholar] [CrossRef]
- Beara-Lasic, L.; Pillinger, M.H.; Goldfarb, D.S. Advances in the Management of Gout: Critical Appraisal of Febuxostat in the Control of Hyperuricemia. Int. J. Nephrol. Renovasc. Dis. 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, E.; Chong, Z.P.; Ting, M.N.; Mohd Tajuddin, A.A.; Voon, K.X.; Sasitharan, T.; Tai, K.S.; Yeap, S.S. Relationship of Medication Adherence, Serum Uric Acid Level and Diet to Recurrent Attacks of Gout. Egypt. Rheumatol. 2022, 44, 69–73. [Google Scholar] [CrossRef]
- Qaseem, A.; Harris, R.P.; Forciea, M.A. Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2016, 166, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; He, Q.; Yang, J.; Jia, L.; Xu, G. Relationship between Gout, Hyperuricemia, and Obesity—Does Central Obesity Play a Significant Role?—A Study Based on the NHANES Database. Diabetol. Metab. Syndr. 2024, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xian, W.; Wu, D.; Huo, Z.; Hong, S.; Li, Y.; Xiao, H. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Gout: A Mendelian Randomization Study. Front. Endocrinol. 2022, 13, 917056. [Google Scholar] [CrossRef]
- Ritter, F.; Franzeck, F.; Geisshardt, J.; Walker, U.A.; Osthoff, M. Gout Arthritis During Admission for Decompensated Heart Failure-A Descriptive Analysis of Risk Factors, Treatment and Prognosis. Front. Med. 2022, 9, 789414. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Gul, A. Gout in an Obese Patient with Nonalcoholic Steatohepatitis on a Thiazide Diuretic and Association Between Hyperuricemia and Nonalcoholic Steatohepatitis: A Case Report. Cureus 2023, 15, e39207. [Google Scholar] [CrossRef]
- Karunakaran, S.; Hari, R. Comparative Antioxidant and Anti-Gout Activities of Citrullus Colocynthis Loaded Fruit Silver Nanoparticles with Its Ethanolic Extract. Avicenna J. Med. Biotechnol. 2022, 14, 303–309. [Google Scholar] [CrossRef]
- Fukui, S.; Okada, M.; Rahman, M.; Matsui, H.; Shiraishi, A.; Nakai, T.; Tamaki, H.; Kishimoto, M.; Hasegawa, H.; Matsuda, T.; et al. Differences in the Association Between Alcoholic Beverage Type and Serum Urate Levels Using Standardized Ethanol Content. JAMA Netw. Open 2023, 6, e233398. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.Q.; Miao, M.Y.; Wang, J.M.; Qian, Y.W.; Han, W.W.; Peng, X.Z.; Tao, H.W.; Yang, J.; Chen, J.S.; Qin, L.Q.; et al. Consumption of Total and Specific Alcoholic Beverages and Long-Term Risk of Gout Among Men and Women. JAMA Netw. Open 2024, 7, e2430700. [Google Scholar] [CrossRef] [PubMed]
- Asghari, K.M.; Zahmatyar, M.; Seyedi, F.; Motamedi, A.; Zolfi, M.; Alamdary, S.J.; Fazlollahi, A.; Shamekh, A.; Mousavi, S.E.; Nejadghaderi, S.A.; et al. Gout: Global Epidemiology, Risk Factors, Comorbidities and Complications: A Narrative Review. BMC Musculoskelet. Disord. 2024, 25, 1047. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.; Johnson, R.J.; Shakouri, P.; Athavale, A.; Padnick-Silver, L.; LaMoreaux, B.; Marder, B.A.; Mandayam, S. Prevalence and Influence of Gout in Patients with Advanced Chronic Kidney Disease: Findings of a Large Retrospective Chart Review. Gout Urate Cryst. Depos. Dis. 2024, 2, 77–85. [Google Scholar] [CrossRef]
- Sedighi, J.; Luedde, M.; Gaensbacher-Kunzendorf, J.; Sossalla, S.; Kostev, K. The Association between Gout and Subsequent Cardiovascular Events: A Retrospective Cohort Study with 132,000 Using Propensity Score Matching in Primary Care Outpatients in Germany. Clin. Res. Cardiol. 2024. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, N.E.; Yoo, D.M.; Han, K.M.; Hong, J.Y.; Choi, H.G.; Lim, H.; Kim, J.H.; Kim, J.H.; Cho, S.J.; et al. An Elevated Likelihood of Stroke, Ischemic Heart Disease, or Heart Failure in Individuals with Gout: A Longitudinal Follow-up Study Utilizing the National Health Information Database in Korea. Front. Endocrinol. 2023, 14, 1195888. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Curhan, G. Obesity, Weight Change, Hypertension, Diuretic Use, and Risk of Gout in Men: The Health Professionals Follow-up Study. Arch. Intern. Med. 2005, 165, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Gaffo, A. Gout Epidemiology and Comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Yuan, M.; Xu, Y.; Xu, H. Gout and Diet: A Comprehensive Review of Mechanisms and Management. Nutrients 2022, 14, 3525. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Mizuta, E.; Morita, A.; Fujita, T.; Anzai, N.; Hisatome, I. Dysuricemia—A New Concept Encompassing Hyperuricemia and Hypouricemia. Biomedicines 2023, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Dalbeth, N. Combination Urate-Lowering Therapy in the Treatment of Gout: What Is the Evidence? Semin. Arthritis Rheum. 2019, 48, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Henriksen, D.P.; Hallas, J.; Pottegård, A.; Lund, L.C. Sodium–Glucose Cotransporter 2 Inhibitors and Risk of Nephrolithiasis. Diabetologia 2021, 64, 1563–1571. [Google Scholar] [CrossRef]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Jeria-Navarro, S.; Gomez-Gomez, A.; Park, H.S.; Calvo-Aranda, E.; Corominas, H.; Pou, M.A.; Diaz-Torne, C. Effectiveness and Safety of Anakinra in Gouty Arthritis: A Case Series and Review of the Literature. Front. Med. 2023, 9, 1089993. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, N.; De Meulemeester, M.; Pikhlak, A.; Yücel, A.E.; Richard, D.; Murphy, V.; Arulmani, U.; Sallstig, P.; So, A. Canakinumab Relieves Symptoms of Acute Flares and Improves Health-Related Quality of Life in Patients with Difficult-to-Treat Gouty Arthritis by Suppressing Inflammation: Results of a Randomized, Dose-Ranging Study. Arthritis Res. Ther. 2011, 13, R53. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, J.Y.; Wu, H.X.; Zhou, Q.J.; Chen, H.Y.; Lu, Y.F.; Yu, R.S. Relationship between Urate within Tophus and Bone Erosion According to the Anatomic Location of Urate Deposition in Gout: A Quantitative Analysis Using Dual-Energy CT Volume Measurements. Medicine 2019, 98, e18431. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, M.; Gamble, G.D.; Aati, O.; Knight, J.; Horne, A.; Doyle, A.J.; Dalbeth, N. Relationship of Bone Erosion with the Urate and Soft Tissue Components of the Tophus in Gout: A Dual Energy Computed Tomography Study. Rheumatology 2017, 56, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Gravallese, E. Bone Erosion in Rheumatoid Arthritis: Mechanisms, Diagnosis and Treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Hart, T.C. Genetic Factors Associated With Gout and Hyperuricemia. Adv. Chronic Kidney Dis. 2006, 13, 124–130. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal, A.; Ferenc, K.; Motyka, E.; Helma, K.; Filip, R. The Role of Genetic and Epigenetic Regulation in Intestinal Fibrosis in Inflammatory Bowel Disease: A Descending Process or a Programmed Consequence? Genes 2023, 14, 1167. [Google Scholar] [CrossRef]
- Joosten, L.A.B.; Straton, A.R.; Kischkel, B.; Cris, T.O. Epigenomic Reprogramming in Gout. Gout Urate Cryst. Depos. Dis. 2024, 2, 325–338. [Google Scholar]
- Kiyani, M.M.; Butt, M.A.; Rehman, H.; Ali, H.; Hussain, S.A.; Obaid, S.; Arif Hussain, M.; Mahmood, T.; Bokhari, S.A.I. Antioxidant and Anti-Gout Effects of Orally Administered Zinc Oxide Nanoparticles in Gouty Mice. J. Trace Elem. Med. Biol. 2019, 56, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Elzein, B. Nano Revolution: “Tiny Tech, Big Impact: How Nanotechnology Is Driving SDGs Progress”. Heliyon 2024, 10, e31393. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Cao, X.; Wei, Q.; Firempong, C.K.; Guo, M.; Shi, F.; Xu, X.; Deng, W.; Yu, J. Enhanced Oral Bioavailability and Anti-Gout Activity of [6]-Shogaol-Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2018, 550, 24–34. [Google Scholar] [CrossRef]
- Parashar, P.; Mazhar, I.; Kanoujia, J.; Yadav, A.; Kumar, P.; Saraf, S.A.; Saha, S. Appraisal of Anti-Gout Potential of Colchicine-Loaded Chitosan Nanoparticle Gel in Uric Acid-Induced Gout Animal Model. Arch. Physiol. Biochem. 2022, 128, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kandav, G.; Bhatt, D.C.; Jindal, D.K. Formulation and Evaluation of Allopurinol Loaded Chitosan Nanoparticles. Int. J. Appl. Pharm. 2019, 11, 49–52. [Google Scholar] [CrossRef]
- Kandav, G.; Bhatt, D.C.; Singh, S.K. Effect of Different Molecular Weights of Chitosan on Formulation and Evaluation of Allopurinol-Loaded Nanoparticles for Kidney Targeting and in Management of Hyperuricemic Nephrolithiasis. AAPS PharmSciTech 2022, 23, 144. [Google Scholar] [CrossRef]
- Kandav, G.; Bhatt, D.C.; Jindal, D.K. Targeting Kidneys by Superparamagnetic Allopurinol Loaded Chitosan Coated Nanoparticles for the Treatment of Hyperuricemic Nephrolithiasis. DARU J. Pharm. Sci. 2019, 27, 661–671. [Google Scholar] [CrossRef]
- Zhang, M.; Hussain, A.; Hu, B.; Yang, H.; Li, C.; Guo, S.; Han, X.; Li, B.; Dai, Y.; Cao, Y.; et al. Atavistic Strategy for the Treatment of Hyperuricemia via Ionizable Liposomal MRNA. Nat. Commun. 2024, 15, 6463. [Google Scholar] [CrossRef]
- Adewale, O.B.; Anadozie, S.O.; Davids, H.; Roux, S. Chapter 7—Potential Therapeutic Role of Gold Nanoparticles in Inflammatory Diseases; Kesharwani, P., Ed.; Academic Press: Warsaw, Poland, 2024; pp. 197–225. ISBN 978-0-443-19061-2. [Google Scholar]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-K.; Liu, T.-W.; Hsu, S.-J.; Huynh, Q.-D.T.; Thi Duong, T.-L.; Chu, M.-H.; Wang, Y.-H.; Vo, T.-H.; Lee, C.-K. Xanthine Oxidase Inhibition Study of Isolated Secondary Metabolites from Dolichandrone Spathacea (Bignoniaceae): In Vitro and in Silico Approach. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2024, 32, 101980. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.N.N.; Ho, L.B.N.; Nguyen, K.N.N.; Dong, T.A.D.; Le, T.H.A. The Inhibition Activity on Tyrosinase, Xanthine Oxidase, and Lipase of Musa Balbisiana Parts Grown in Vietnam. Food Sci. Nutr. 2024, 12, 7428–7437. [Google Scholar] [CrossRef]
| No | Drug | Materials/Route of Administration | Characteristic | Physicochemical Enhancement | Pharmacological Enhancement | Ref |
|---|---|---|---|---|---|---|
| 1 | Shogaol | SLN preparation utilizes pressure homogenization techniques, combining triglyceride-monostearate lipid matrix with a dual emulsifier system (span/tween 80). SLN was administered intragastrically in Male Sprague Dawley (SD) rats. | The SLNs produced were small (<100 nm), spherical, and smooth, with negative zeta potential (−15.2 mV), high encapsulation efficiency (87.67%), and acceptable polydispersity. | The SLNs demonstrated improved in vitro release profiles and enhanced oral bioavailability compared to the free drug. | In hyperuricemic models, SLNs exhibited enhanced UA-lowering effects through enzyme inhibition and cytokine reduction, surpassing free drug performance. | [92] |
| 2 | Colchicine | Chitosan nanocarriers containing colchicine were synthesized via the spontaneous emulsion method. NP gel was administered topically in albino rabbits. | Optimized CHNPs demonstrated 294 ± 3.75 nm diameter, with 92.89 ± 1.1% entrapment and 83.45 ± 2.5% drug loading. | In vitro release: 89.34 ± 2.90% over 24 h. | Colchicine-loaded CHNPgel outperformed plain colchicine, showing potential as an efficient gout treatment delivery system. | [93] |
| 3 | Turmeric | Solvent evaporation or nano-precipitation technique. NPs were orally administered. | T-NPs: ~46 nm size, +29.55 ± 3.44 zeta potential, 0.264 PDI. | T-NPs are designed for enhanced oral solubility. | T-NPs significantly reduced UA levels, showing superior potential for gout management. | [20] |
| 4 | Zinc oxide | Precipitation method. NPs were orally administered in Balb/C mice. | SEM analysis showed zinc oxide nanoparticles: ~37 nm, amorphous morphology. | ZnO-NPs significantly reduced urea, creatinine, and UA in gout-induced mice (p < 0.001). | ZnO nanoparticles (10, 20 ppm) significantly reduced serum UA (p < 0.001), in treating GA. They decreased ROS and Thiobarbituric Acid Reactive Substances (TBARS) and improved blood count and Liver Function Tests (LFTs), indicating reduced hyperuricemia. Histopathology showed no changes in liver, kidney, or muscle tissues. | [90] |
| 5 | Allopurinol | Allopurinol-BSA nanoparticles (ABNPsopt) prepared via desolvation. NPs were intravenously administered in Swiss albino mice. | The 13 ABNP batches showed particle sizes ranging from 220.1 to 358.6 nm, with PDI values between 0.155 and 0.499. The zeta potential varied from −34.1 to −21.7 mV, while encapsulation efficiency ranged from 10.2% to 67.5%. | ABNPsopt demonstrated superior kidney targeting, achieving 21.26-fold higher drug levels than serum, whereas free drug showed no tissue retention after two h. | ABNPsopt enhanced renal allopurinol uptake through cubilin/megalin receptor recognition of albumin carrier, offering improved therapeutic strategy for hyperuricemic nephrolithiasis. | [33] |
| 6 | Allopurinol | Allopurinol-loaded chitosan nanoparticles (A-CNPs). NPs were intra-venously administered in Swiss albino mice. | A-CNPs showed a size of 375.3 ± 10.1 nm, PDI of 0.362 ± 0.01, and ZP of 32.5 ± 2.7 mV. The low PDI and High positive ZP indicates a stable, monodisperse formulation. | Drug release from A-CNPs fits the Higuchi model (R2 = 0.9916) with a release exponent (n) of 0.59. This indicates diffusion-controlled, non-Fickian release kinetics. | Low molecular weight chitosan demonstrated enhanced renal targeting through megalin receptor-mediated uptake and increased solubility, optimizing drug delivery for hyperuricemic nephrolithiasis treatment. | [94,95] |
| 7 | Allopurinol-loaded chitosan-coated magnetic nanoparticles (A-MNPs) | Magnetic iron oxide nanoparticles synthesized through co-precipitation technique. NPs were intra-venous administered in Swiss albino mice. | All magnetic nanoparticles were below 250 nm with low PDI, indicating homogeneous dispersions. High zeta potential values suggested good stability. Size increases in C-MNPs and A-MNPs confirmed successful polymer coating and drug loading. A-MNPs showed 57.55 ± 0.05% entrapment and 27.35 ± 0.02% loading efficiency. | Drug release from A-MNPs best fits the Higuchi model, with a release exponent (n) of 0.67, indicating diffusion-controlled, non-Fickian release through the swollen polymer matrix. This pattern matches previous findings with chitosan nanoparticles. | A-MNPs were developed using chitosan coating to protect from early clearance and enable kidney targeting. The formulation achieved 19.07-fold higher drug concentration in kidneys than serum, with sustained release demonstrated through in vivo and histological studies for treating hyperuricemic nephrolithiasis. | [96] |
| 8 | Ethanolic fruit extract of Citrullus colocynthis (C. colocynthis) (EFECC) and synthesized silver nanoparticles (CC-AgNPs) | C. colocynthis fruit extract mediated AgNP synthesis. NPs activity tested in vitro. | Microscopy revealed spherical CC-AgNPs (10–45 nm), crystallinity confirmed via XRD analysis. FTIR indicated phenolic compounds and metabolite functionalization. | Synthesized nanoparticles exhibited enhanced antioxidant activity versus crude extract while demonstrating multi-target anti-gout effects through membrane protection, enzyme inhibition, and protein stabilization. | CC-AgNPs exhibited superior antioxidant capacity and anti-arthritic properties versus fruit extract, demonstrating enhanced inhibition of xanthine oxidase, protein denaturation, and membrane damage. | [68] |
| 9 | Urate oxidase mRNA encapsulated in ionizable lipid nanocarriers (mUox@iLAND). | Ionizable lipid carriers loaded with dual nucleic acids: luciferase mRNA and fluorescent siRNA. Intravenously administered to HU mice. | Nanocarrier analysis revealed size ~169 nm, uniformity index 0.148, surface charge −1.22 mV, and mRNA loading of 94.2%. | iLAND internalization occurs via multiple routes: caveolae, clathrin-dependent endocytosis, macropinocytosis, and independent mechanisms. | The Nanocarrier system employs receptor-mediated internalization and endosomal release, providing sustained therapeutic effects. Unlike allopurinol’s acute response, mUox@iLAND achieved prolonged UA reduction through controlled delivery. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdiana, Y.; Wardhana, Y.W.; Kurniawansyah, I.S.; Gozali, D.; Wathoni, N.; Sofian, F.F. Current Status of Gout Arthritis: Current Approaches to Gout Arthritis Treatment: Nanoparticles Delivery Systems Approach. Pharmaceutics 2025, 17, 102. https://doi.org/10.3390/pharmaceutics17010102
Herdiana Y, Wardhana YW, Kurniawansyah IS, Gozali D, Wathoni N, Sofian FF. Current Status of Gout Arthritis: Current Approaches to Gout Arthritis Treatment: Nanoparticles Delivery Systems Approach. Pharmaceutics. 2025; 17(1):102. https://doi.org/10.3390/pharmaceutics17010102
Chicago/Turabian StyleHerdiana, Yedi, Yoga Windhu Wardhana, Insan Sunan Kurniawansyah, Dolih Gozali, Nasrul Wathoni, and Ferry Ferdiansyah Sofian. 2025. "Current Status of Gout Arthritis: Current Approaches to Gout Arthritis Treatment: Nanoparticles Delivery Systems Approach" Pharmaceutics 17, no. 1: 102. https://doi.org/10.3390/pharmaceutics17010102
APA StyleHerdiana, Y., Wardhana, Y. W., Kurniawansyah, I. S., Gozali, D., Wathoni, N., & Sofian, F. F. (2025). Current Status of Gout Arthritis: Current Approaches to Gout Arthritis Treatment: Nanoparticles Delivery Systems Approach. Pharmaceutics, 17(1), 102. https://doi.org/10.3390/pharmaceutics17010102








