Acai Oil-Based Organogel Containing Hyaluronic Acid for Topical Cosmetic: In Vitro and Ex Vivo Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Physicochemical Characterization of Acai Oil
Determination of Fatty Acid Composition
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
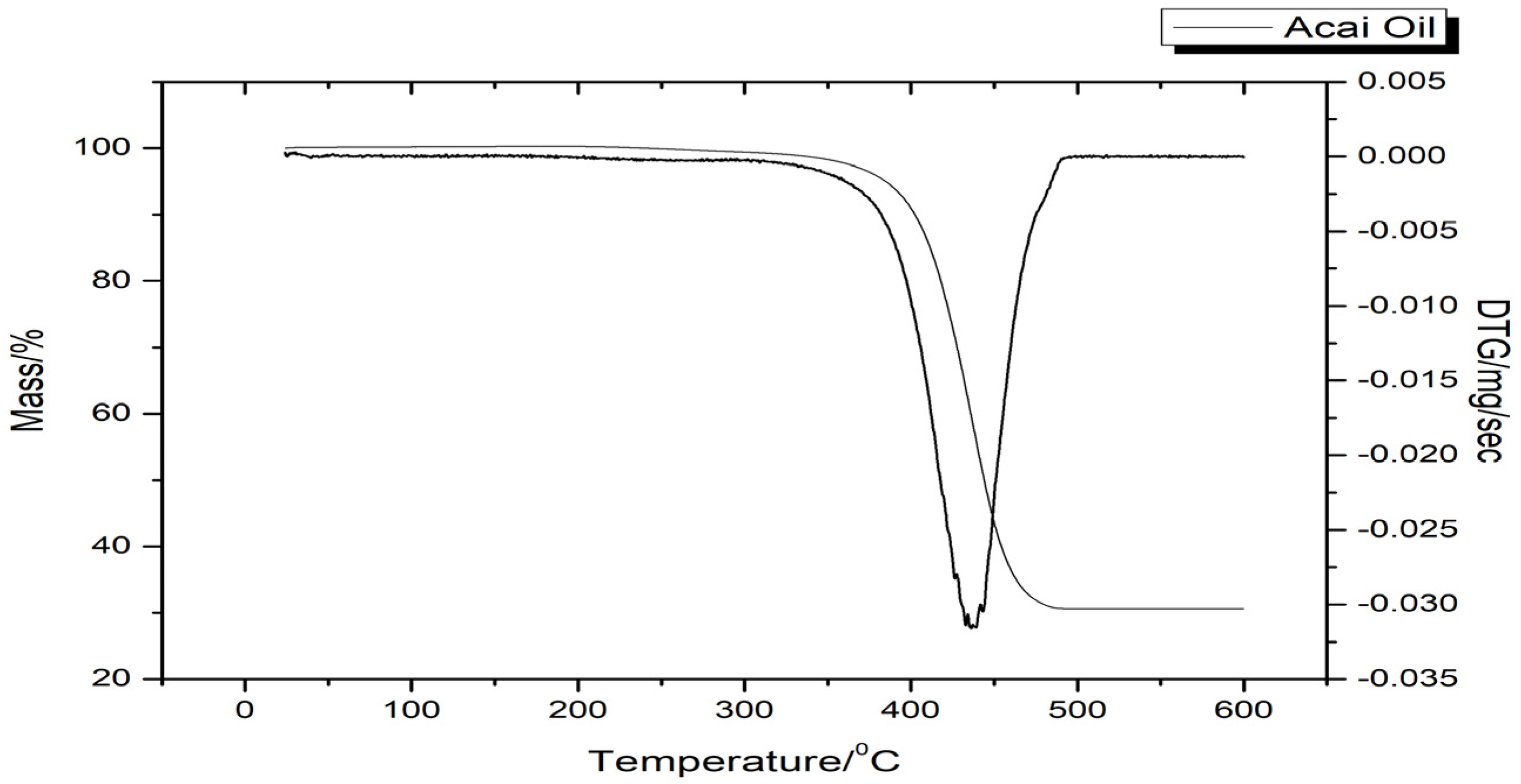
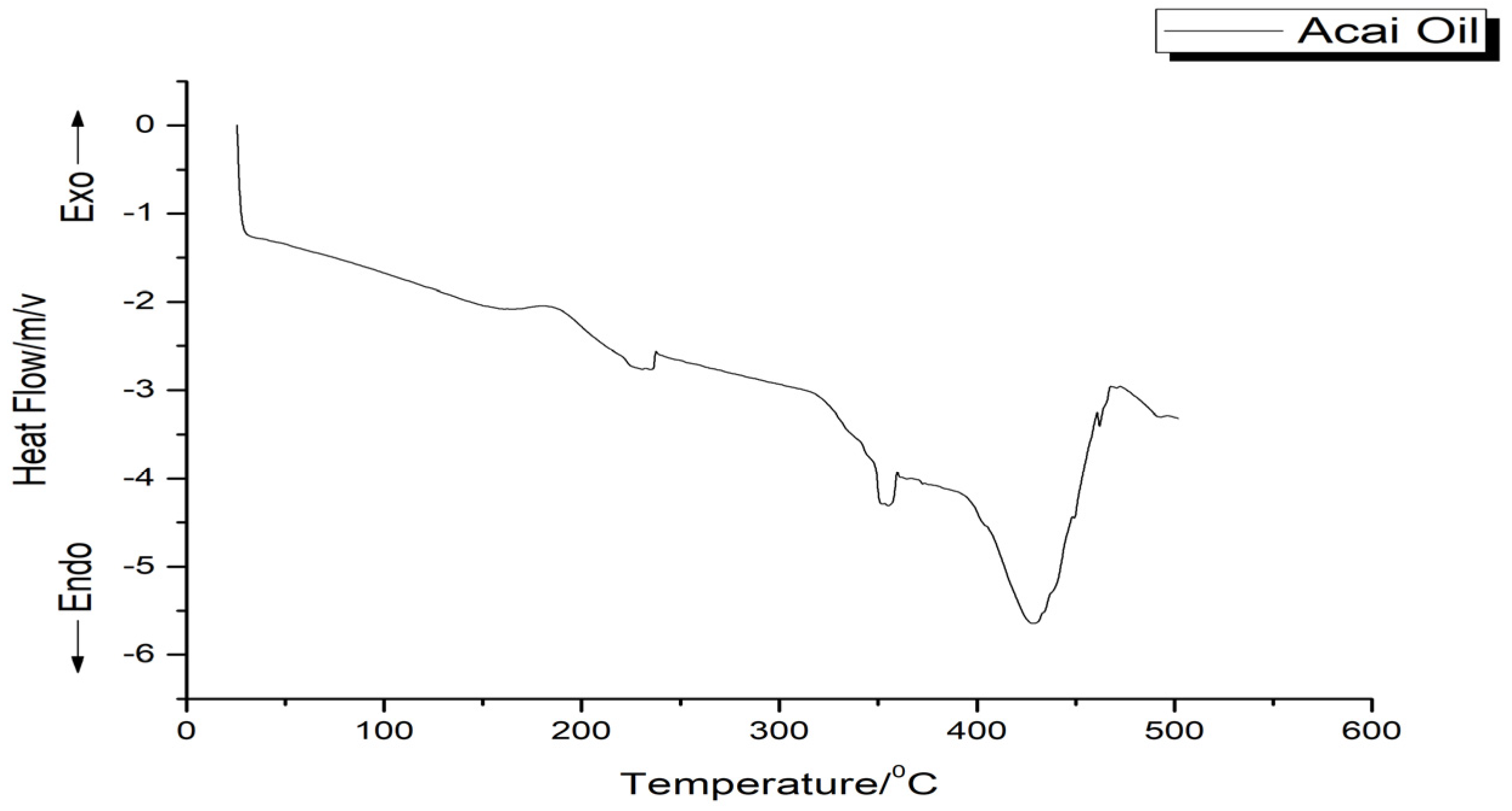
Thermal Analyses
Quantitative Analysis of Total Phenolic Content
Quantitative Analysis of Flavonoids
Total Carotenoids Content
Quantitative Analysis of β-Carotene
ABTS Free-Radical Activity
DPPH Method
β-Carotene/Linolenic Acid System
2.2.2. Preparation of Organogel Systems
2.2.3. Evaluation of the Efficacy of the Organogel Containing Hyaluronic Acid
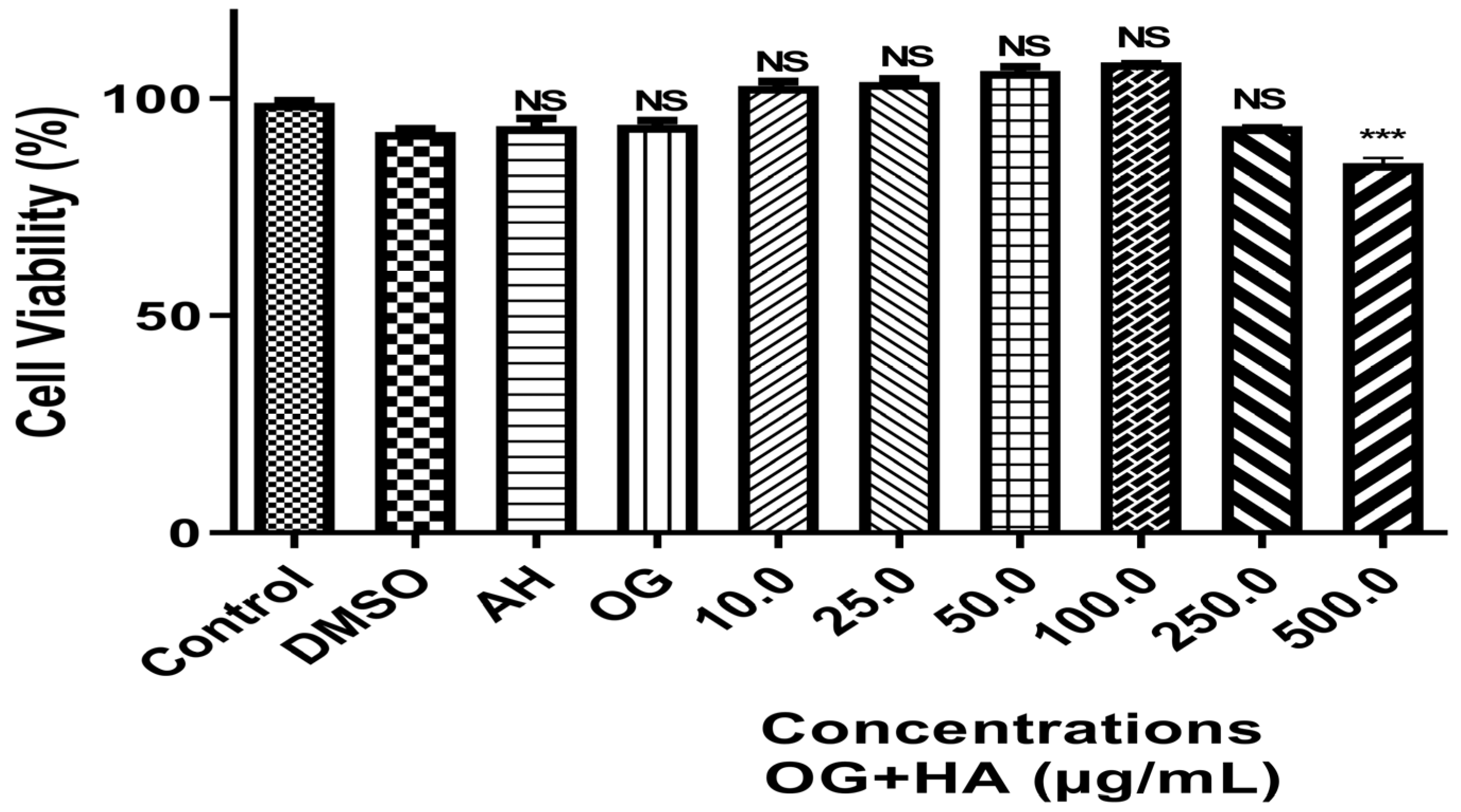
Preliminary Toxicology
- Assessment of Cytotoxicity
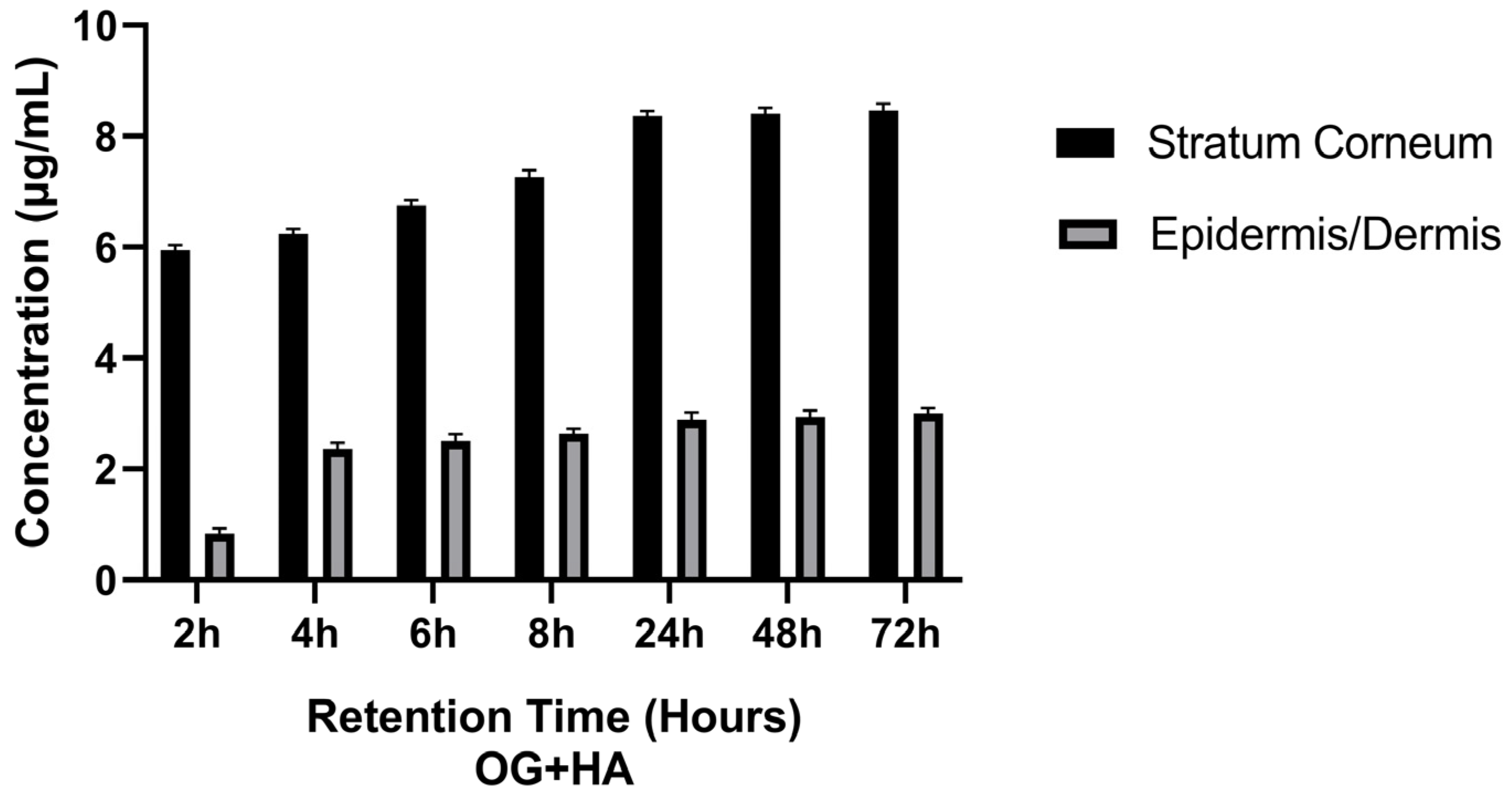
Ex Vivo Skin Permeation and Retention Study
- 1.
- Ex vivo permeation study
- 2.
- Ex vivo study of retention in the stratum corneum
- 3.
- Ex vivo study of retention in the epidermis and dermis
Assessment of Skin Hydration
Thermal Analysis of Stratum Corneum
2.3. Statistical Analysis
3. Results
3.1. Physical–Chemical Characterization of the Acai Oil
3.1.1. Fatty Acids (FA) Profile
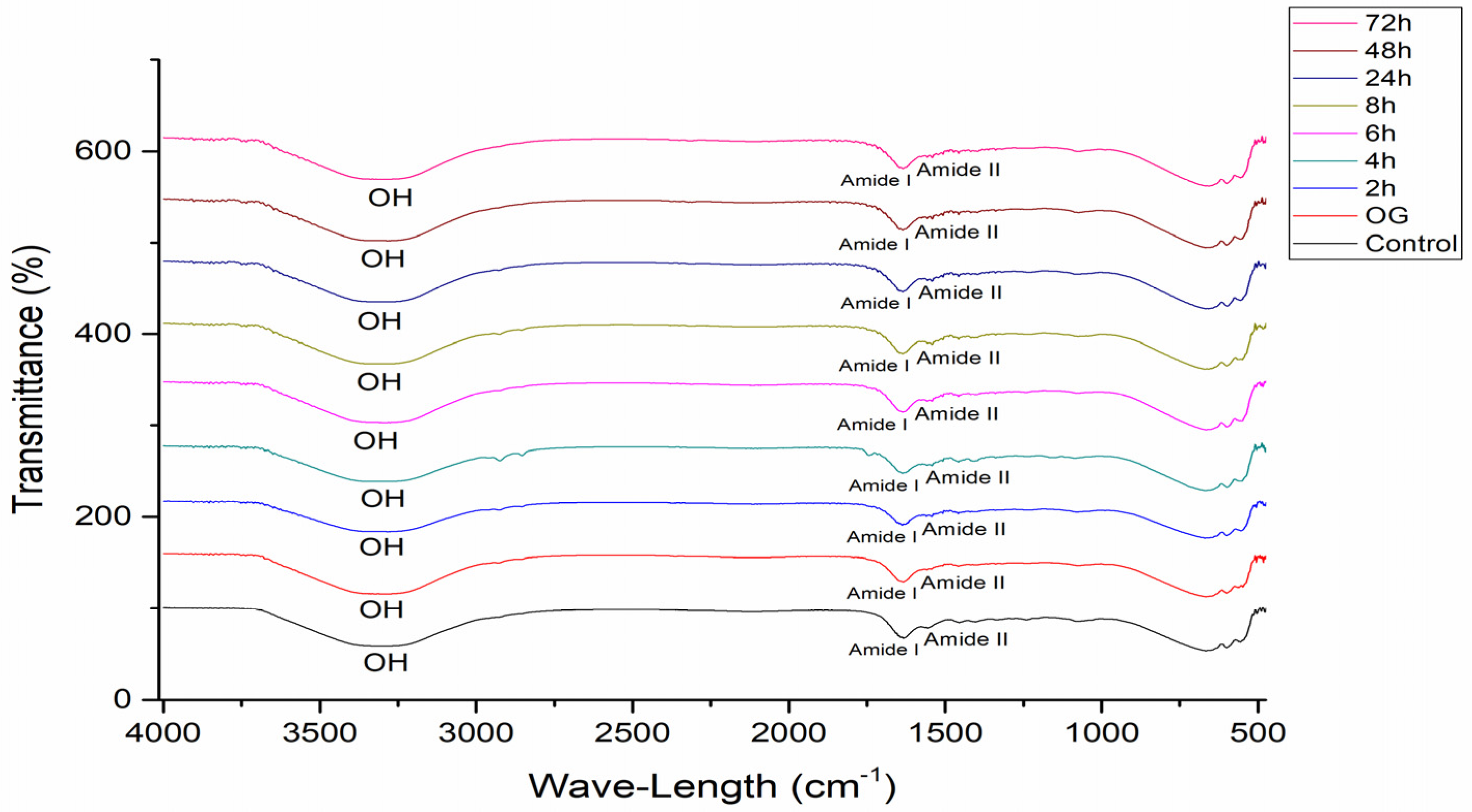
3.1.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
3.1.3. Thermal Behavior
3.1.4. Quantification of Total Phenolic Content, Total Flavonoid Content, Total Carotenoids Content, and β-Carotene Content
3.1.5. Antioxidant Activity
3.2. Evaluation of the Efficacy of Organogel Containing Hyaluronic Acid
3.2.1. Preliminary Toxicity
Cytotoxicity Analysis
3.2.2. Ex Vivo Skin Permeation and Retention Study
3.2.3. Skin Hydration
3.2.4. Thermal Analysis of the Stratum Corneum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Pereira, S.A.; Alves, H.P.; Sousa, C.M.; Costa, G.L.S. Prospecção sobre o conhecimento de espécies amazônicas—Inajá (Maximiliana maripa Aublt.) e bacaba (Oenocarpus bacaba Mart.). Rev. GEINTEC 2013, 3, 110–122. [Google Scholar] [CrossRef]
- da Costa Sanches, S.C.; Silva-Junior, J.O.C.; Ribeiro-Costa, R.M. The use of vegetable oils to prevent skin aging. Res. Soc. Dev. 2021, 10, e44010111941. [Google Scholar]
- Mosquera Narvaez, L.E.; Ferreira, L.M.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef] [PubMed]
- Correa, K.L.; de Carvalho-Guimarães, F.B.; Mourão, E.S.; Oliveira Santos, H.C.; da Costa Sanches, S.C.; Lamarão, M.L.N.; Pereira, R.R.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Converti, A.; et al. Physicochemical and Nutritional Properties of Vegetable Oils from Brazil Diversity and Their Applications in the Food Industry. Foods 2024, 13, 1565. [Google Scholar] [CrossRef]
- da Costa Sanches, S.C.; Ré, M.I.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Organogel of Acai Oil in Cosmetics: Microstructure, Stability, Rheology and Mechanical Properties. Gels 2023, 9, 150. [Google Scholar] [CrossRef]
- Zhou, J.; Han, P.; Liu, M.; Zhou, H.; Zhang, Y.; Jiang, J.; Liu, P.; Wei, Y.; Song, Y.; Yao, X. Self-Healable Organogel Nanocomposite with Angle-Independent Structural Colors. Angew. Chem. Int. 2017, 56, 10462–10466. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Silva, J.A.; Apolinário, A.C.; Souza, M.S.R.; Damasceno, B.P.G.L.; Medeiros, A.C.D. Administração cutânea de fármacos: Desafios e estratégias para o desenvolvimento de formulações transdérmicas. Rev. Ciências Farm. Básica Apl. 2010, 31, 125–131. [Google Scholar]
- Soares, K.C.C.; Moraes, M.V.; Gelfuso, G.M.; Gratieri, T. Bioequivalence of dermatological topical medicines: The Brazilian scenario and the challenges for health surveillance. Ciência Saúde Coletiva 2015, 20, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Correa, N.C.F.; Carvalho Junior, R.; Costa, C.E.F.D.; Moraes, J.D.F.C.; Lannes, S.C.D.S. Quality parameters and thermogravimetric and oxidative profile of Muruci oil (Byrsonima crassifolia L.) obtained by supercritical CO2. Food Sci. Technol. 2018, 38, 1. [Google Scholar] [CrossRef]
- Brandão, D.O.; Guimarães, G.P.; Santos, R.L.; Ramos, F.J.L., Jr.; Silva, K.M.A.; Souza, F.S.; Macêdo, R.O. Model Analytical Development for Physical, Chemical, and Biological Characterization of Momordica charantia Vegetable Drug. J. Anal. Methods Chem. 2016, 2016, 7528297. [Google Scholar] [CrossRef]
- AOCS (American Oil Chemists’ Society). Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Codex Stan 210-1999; Standard for Named Vegetable Oils. Codex Alimentarius Commission: Rome, Italy, 2009.
- Silva da Costa, R.; Pinheiro, W.B.S.; Arruda, M.S.P.; Costa, C.E.F.; Converti, A.; Ribeiro Costa, R.M.; Silva Júnior, J.O.C. Thermoanalytical and phytochemical study of the cupuassu (Theobroma grandiflorum Schum.) seed by-product in different processing stages. J. Therm. Anal. Calorim. 2020, 147, 275. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, V.T.; Minh, L.V.; Trieu, L.H.; Cang, M.H.; Bui, L.B.; Le, X.T.; Danh, V.T. Determination of the phytochemical screening, total polyphenols, flavonoids content, and antioxidant activity of soursop leaves (Annona muricata Linn.). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 736. [Google Scholar]
- Ogawa, M.; Maia, E.L.; Fernandes, A.C.; Nunes, M.L.; Oliveira, M.E.B.; Freitas, S.T. Resíduos do beneficiamento do camarão cultivado: Obtenção de pigmentos carotenóides. Food Sci. Technol. 2007, 27, 333–337. [Google Scholar] [CrossRef]
- Cuco, L.R.P.; Cardozo-Filho, C.S. Simultaneous extraction of seed oil and active compounds from peel of pumpkin (Cucurbita maxima) using pressurized carbon dioxide as solvent. J. Supercrit. Fluids 2019, 143, 8–15. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Trolox ASSAY. Int. Antioxid. Res. Centre, Guy’s, King’s St Thomas’ Sch. Biomed. Sci. Kings Coll. Campus London SE1 9RT UK 26. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Silveira, A.C.; Kassuia, Y.S.; Domahovski, R.C.; Lazzarotto, M.; da Silveira, A.C.; Kassuia, Y.S.; Cruz Domahovski, R. Método de DPPH adaptado: Uma ferramenta para analisar atividade antioxidante de polpa de frutos da erva-mate de forma rápida e reprodutível. Embrapa Comun. Técnico 2018, 421, 2. [Google Scholar]
- Duarte-Almeida, J.M.; Dos Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Avaliação da atividade antioxidante utilizando sistema β-caroteno/ácido linoléico e método de seqüestro de radicais DPPH•. Food Sci. Technol. 2006, 26, 446–452. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Hansen, J.A.; Lacis, A.; Prather, M. Greenhouse effect of chlorofluorocarbons and other trace gases. J. Geophys. Res. 1989, 94, 16417–16421. [Google Scholar] [CrossRef]
- SCCP/0970/20006; Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients. European Commission Health & Consumers: Brussels, Belgium, 2006.
- Billich, A.; Aschauer, H.; Aszódi, A.; Stuetz, A. Percutaneous absorption of drugs used in atopic eczema: Pimecrolimus permeates less through skin than corticosteroids and tacrolimus. Int. J. Pharm. 2004, 269, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.R. In vitro skin permeation techniques. J. Control. Release 1992, 18, 235–248. [Google Scholar] [CrossRef]
- Hardingham, T. Solution properties of hyaluronan. In Chemistry and Biology of Hyaluronan; Garg, H.G., Hales, C.A., Eds.; Elsevier: Oxford, UK, 2004; pp. 1–19. [Google Scholar]
- Milan, A.L.K.; Milão, D.; Souto, A.A.; Corte, T.W.F. Estudo da hidratação da pele por emulsões cosméticas para xerose e sua estabilidade por reologia. Rev. Bras. Ciências Farm. 2007, 43, 4. [Google Scholar] [CrossRef]
- Gloor, M.; Wildebrandt, U.; Thomer, G.; Kupferschmid, W. Water content of the horny layer and skin surface lipids. Arch. Dermatol. Res. 1980, 268, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, J.O.C.; Pereira, N.L. Avaliação da permeação in vitro de gel fitoterápico contendo extrato seco por nebulização de Shymphytum officinale L. Rev. Bras. Ciências Farm. 2009, 90, 3–9. [Google Scholar]
- Ministério da Saúde. Regulamento Técnico para óleos Vegetais, Gorduras Vegetais e creme Vegetal; Resolução RDC nº 270, de 22 de setembro de 2005; Ministério da Saúde: Brasília, Brazil, 2005.
- Agência Nacional da Vigilância Sanitária do Brasil. Regulamento Técnico para Fixação de Identidade e Qualidade de Óleos e Gorduras Vegetais; Resolução RDC nº 482 de 23 de setembro de 1999; Agência Nacional da Vigilância Sanitária do Brasil: Brasília, Brazil, 1999. [Google Scholar]
- ASTM D445, D446; Annual Book of ASTM Standards. ASTM International: West Conshohocken, PE, USA, 2011.
- Sakurai, K.; Shen, C.; Shiraishi, I.; Inamura, N.; Hisatsune, T. Consumption of Oleic Acid on the Preservation of Cognitive Functions in Japanese Elderly Individuals. Nutrient 2021, 13, 284. [Google Scholar] [CrossRef]
- Silva, J.J.M.; Rogez, H. Avaliação da estabilidade oxidativa do óleo bruto de acai (euterpe oleracea) na presença de compostos fenólicos puros ou de extratos vegetais amazônicos. Química Nova 2013, 36, 400–406. [Google Scholar] [CrossRef]
- Loureiro Contente, D.M.; Pereira, R.R.; Rodrigues, A.M.C.; da Silva, E.O.; Ribeiro-Costa, R.M.; Carrera Silva-Junior, J.O. Nanoemulsions of Acai Oil: Physicochemical Characterization for the Topical Delivery of Antifungal Drugs. Chem. Eng. Technol. 2020, 43, 1424. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheykey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: Washington, DC, USA, 2009; p. 473. [Google Scholar]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Gangqiang, G.; Minh, T.N.; Quy, T.N.; Khanh, T.D. An Overview of Chemical Profiles, Antioxidant and Antimicrobial Activities of Commercial Vegetable Edible Oils Marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Senab, C.; Santos, O.V.; da Costa, W.A.; Rodrigues, A.M.C.; Carvalho, R.N., Jr. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Aceites 2018, 69, e246. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Gong, Z.; Li, X.; Jia, C. Formation of fatty acid methyl ester based microemulsion and removal mechanism of PAHs from contaminated soils. J. Hazard Mater. 2021, 413, 125–460. [Google Scholar] [CrossRef]
- de Lima Yamaguchi, K.K.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Cunha, A.L.A.; Freitas, S.P.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Chemical composition and oxidative stability of jussara (Euterpe edulis M.) oil extracted by cold and hot mechanical pressing. Grasas Aceites 2017, 68, e218. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from acai. Food Chem. 2010, 118, 208. [Google Scholar] [CrossRef]
- de Lima Filho, O.C.; Pereira, A.A.M.G.; Da Silva, C.A.D.S.; Da Silva, A.M.; Valadares, M.C.; Cortez, A.P.; Parise, M.R. Avaliação da citotoxicidade do óleo essencial de eremanthus erythropappus sobre células de câncer mamário MCF-7. Braz. J. Health Rev. 2020, 3, 4699–4727. [Google Scholar] [CrossRef]
- Jacobs, J.P. Some comparative characteristics of WI-38 and MRC-5 cells and their suitability for the production of viral vaccines. In Proceedings of the Symposium on Human Diploid Cells, Zagreb, Croatia, 23–24 September 1970; Yugoslav Academy of Sciences and Arts: Zagreb, Croatia, 1970; pp. 43–55. [Google Scholar]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef]
- American Type Culture Collection (ATCC). Available online: https://www.atcc.org/products/ccl-171 (accessed on 10 February 2021).
- Malmonge, S.M.; Zavaglia, C.A.C.; Santos JR, A.R.; Wada, M.L.F. Avaliação da citotoxicidade de hidrogéis de polihema: Um estudo in vitro. Rev. Bras. Eng. Biomédica 1999, 15, 49–54. [Google Scholar]
- Frota, R.G.; Da Silva Amorim, Á.; Carneiro, J.K.R.; Oliveira, M.A.S. Citotoxicidade, genotoxicidade e mutagenicidade da infusão de Plectranthus barbatus–Lamiaceae (malva-santa) avaliada pelo sistema teste Allium cepa. Rev. Ciências Médicas Biológicas 2019, 18, 67–72. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Cooney, M.J.; Johns, M.R. Growth and Amino Acid Requirements of Hyaluronic Acid Producing Streptococcus zooepidemicus. Appl. Microbiol. Biotechnol. 1997, 47, 309–312. [Google Scholar] [CrossRef]
- Bernardes, I.N.; Coli, B.A.; Machado, M.G.; Ozolins, B.C.; Silvério, F.R.; Vilela, C.A.; Pereira, L. Preenchimento com ácido hialurônico: Revisão de literatura. Revista Saúde em Foco 2018, 10, 603–612. [Google Scholar]
- Prista, N.L.; Alves, A.C.; Morgado, R. Tecnologia Farmacêutica, 5th ed.; Fundação Caloustre Gulbenkian: Lisbon, Portugal, 1995; pp. 43–44. [Google Scholar]
- Leonardi, G.R.; Maia Campos, P.M.B.G. Estabilidade de formulações cosméticas. Int. J. Pharm. Compd. 2001, 3, 154–156. [Google Scholar]
- Menezes, R.M.R.O.; Costa, L.M.; Tavares, L.R.C.; Bezerra, A.C.D.S.; Aguilar, M.T.P. Efeito do teor de água e tamanho de partícula na decomposição térmica de pastas de cimento moídas. Matéria 2020, 25, 60. [Google Scholar] [CrossRef]
- Rogers, J.; Mayo, A.; Wathinson, A. Skin dryness—What is it? J. Investig. Dermatol. 1993, 100, 510A. [Google Scholar]
- Prasch, T.H.; Knübel, G.; Schimidt-Fonk, K. Infrared spectroscopy of the skin: Influencing the stratum corneum with cosmetics products. Int. J. Cosmet. Sci. 2000, 22, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.W.; Tonge, K.H. Analytical Chemistry by Open Learning: Thermal Methods; ACOL: London, UK, 1987. [Google Scholar]
- Takaoka, M.S.; Baby, A.R.; Prestes, P.S.; Pinto, C.A.S.O.; Silva, V.R.L.; Velasco, M.V.R.; Kawano, Y.; Kaneko, T.M. Avaliação da interação de compostos ativos hidratantes com modelo de biomembrana de Crotalus durissus por meio de calorimetria exploratória diferencial e espectroscopia RAMAN. Rev. Ciências Farm. Básica Apl. 2010, 31, 53–58. [Google Scholar]
- Canevarolo, S.V., Jr. Ciência Dos Polímeros; Artliber Editora: São Paulo, Brazil, 2002; p. 24. [Google Scholar]
- Kempka, A.; Andrade, M.; Mello, F.; Weigelt, W.; Walter, G. Utilização da calorimetria exploratória diferencial (dsc) para avaliação da desnaturação térmica de diferentes colágenos bovinos. Blucher Chem. Eng. Proc. 2015, 1, 4390–4397. [Google Scholar]


| Parameters | Values |
|---|---|
| Refractive index | 1.46 ± 0.01 |
| Relative Density | 0.90 ± 0.01 |
| Kinematic Viscosity (mm2/s) | 43 ± 0.01 |
| Acidity index (mg KOH/g) | 2.31 ± 0.02 |
| Peroxide Index (meq H2O2 kg−1) | 2.08 ± 0.01 |
| Saponification Index (mg KOH/g) | 209.13 ± 0.05 |
| Nomenclature/Symbology | Percentage (%) | Retention Time |
|---|---|---|
| Oleic acid (C18:1) | 59.41 ± 0.05 | 19.800 ± 0.03 |
| Linoleic acid (C18:2) | 10.95 ± 0.03 | 20.160 ± 0.01 |
| Palmitoleic acid (C16:1) | 3.69 ± 0.01 | 17.923 ± 0.02 |
| Palmitic acid (C16) | 23.08 ± 0.04 | 17.698 ± 0.03 |
| Stearic Acid (C18) | 1.74 ± 0.02 | 19.570 ± 0.02 |
| MUFA | 59.41 ± 0.01 | - |
| PUFA | 14.64 ± 0.01 | - |
| SFA | 24.82 ± 0.02 | - |
| Bioactive Compounds | Values |
|---|---|
| Total phenolics content (mg GAE/g) | 72.08 ± 0.01 |
| Total flavonoids content (mg RUT/g) | 7.01 ± 0.03 |
| Total carotenoids content (mg/100 g) | 18.48 ± 0.05 |
| β-carotene content (mg/100 g) | 3.45 ± 0.01 |
| Antioxidant Activity | Values |
|---|---|
| ABTS free radical activity (µmol TE mL−1) | 1242.89 ± 0.01 |
| DPPH method (Trolox (TE)/g)) | 378 ± 0.02 |
| β-carotene/linolenic acid system (%) | 45.63 ± 0.03 |
| Time (Hours) | Concentration HA (µg/mL) | Concentration HA (µg/mL) | Concentration HA (%) | |
|---|---|---|---|---|
| Stratum Corneum | Epidermis/Dermis | Stratum Corneum + Epidermis/Dermis | Stratum Corneum + Epidermis/Dermis | |
| 2 | 5.88 ± 0.01 | 0.77 ± 0.05 | 6.65 ± 0.02 | 59.26 ± 0.03 |
| 4 | 6.17 ± 0.01 | 2.28 ± 0.04 | 8.45 ± 0.03 | 75.31 ± 0.02 |
| 6 | 6.69 ± 0.03 | 2.41 ± 0.03 | 9.10 ± 0.01 | 81.1 ± 0.05 |
| 8 | 7.17 ± 0.02 | 2.57 ± 0.01 | 9.74 ± 0.01 | 86.8 ± 0.01 |
| 24 | 8.3 ± 0.05 | 2.79 ± 0.02 | 11.09 ± 0.05 | 98.84 ± 0.04 |
| 48 | 8.33 ± 0.01 | 2.86 ± 0.02 | 11.19 ± 0.04 | 99.73 ± 0.03 |
| 72 | 8.38 ± 0.01 | 2.94 ± 0.01 | 11.32 ± 0.03 | 100 ± 0.02 |
| Formulations | Thermal Event (°C) | Enthalpy (J/g) | Thermal Event (°C) | Enthalpy (J/g) |
|---|---|---|---|---|
| 1st | 2nd | |||
| Control | 28.82–63.09 | 18.72 | 185.86–197.81 | 3.24 |
| OG | 30.25–74.82 | 29.39 | ||
| 2 h | 30.58–55.02 | 68.34 | 192.42–198.95 | 0.48 |
| 4 h | 34.02–54.77 | 14.32 | 162.10–160.96 | 0.26 |
| 6 h | 41.53–94.07 | 95.32 | 190.16–205.29 | 1.84 |
| 8 h | 31.19–77.19 | 41.09 | ||
| 24 h | 29.65–67.69 | 5.26 | ||
| 48 h | 30.25–98.14 | 31.32 | ||
| 72 h | 28.34–62.87 | 17.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanches, S.C.d.C.; Ferreira, L.M.d.M.C.; Pereira, R.R.; Lynch, D.G.; Ramos, I.N.d.F.; Khayat, A.S.; Carrera Silva-Júnior, J.O.; Rossi, A.; Ribeiro-Costa, R.M. Acai Oil-Based Organogel Containing Hyaluronic Acid for Topical Cosmetic: In Vitro and Ex Vivo Assessment. Pharmaceutics 2024, 16, 1195. https://doi.org/10.3390/pharmaceutics16091195
Sanches SCdC, Ferreira LMdMC, Pereira RR, Lynch DG, Ramos INdF, Khayat AS, Carrera Silva-Júnior JO, Rossi A, Ribeiro-Costa RM. Acai Oil-Based Organogel Containing Hyaluronic Acid for Topical Cosmetic: In Vitro and Ex Vivo Assessment. Pharmaceutics. 2024; 16(9):1195. https://doi.org/10.3390/pharmaceutics16091195
Chicago/Turabian StyleSanches, Suellen Christtine da Costa, Lindalva Maria de Meneses Costa Ferreira, Rayanne Rocha Pereira, Desireé Gyles Lynch, Ingryd Nayara de Farias Ramos, André Salim Khayat, José Otávio Carrera Silva-Júnior, Alessandra Rossi, and Roseane Maria Ribeiro-Costa. 2024. "Acai Oil-Based Organogel Containing Hyaluronic Acid for Topical Cosmetic: In Vitro and Ex Vivo Assessment" Pharmaceutics 16, no. 9: 1195. https://doi.org/10.3390/pharmaceutics16091195
APA StyleSanches, S. C. d. C., Ferreira, L. M. d. M. C., Pereira, R. R., Lynch, D. G., Ramos, I. N. d. F., Khayat, A. S., Carrera Silva-Júnior, J. O., Rossi, A., & Ribeiro-Costa, R. M. (2024). Acai Oil-Based Organogel Containing Hyaluronic Acid for Topical Cosmetic: In Vitro and Ex Vivo Assessment. Pharmaceutics, 16(9), 1195. https://doi.org/10.3390/pharmaceutics16091195









