Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems
Abstract
1. Introduction
2. Gut Barrier in IBD
3. Traditional Treatments of IBD
3.1. Aminosalicylates
3.2. Corticosteroids
3.3. Immunomodulators
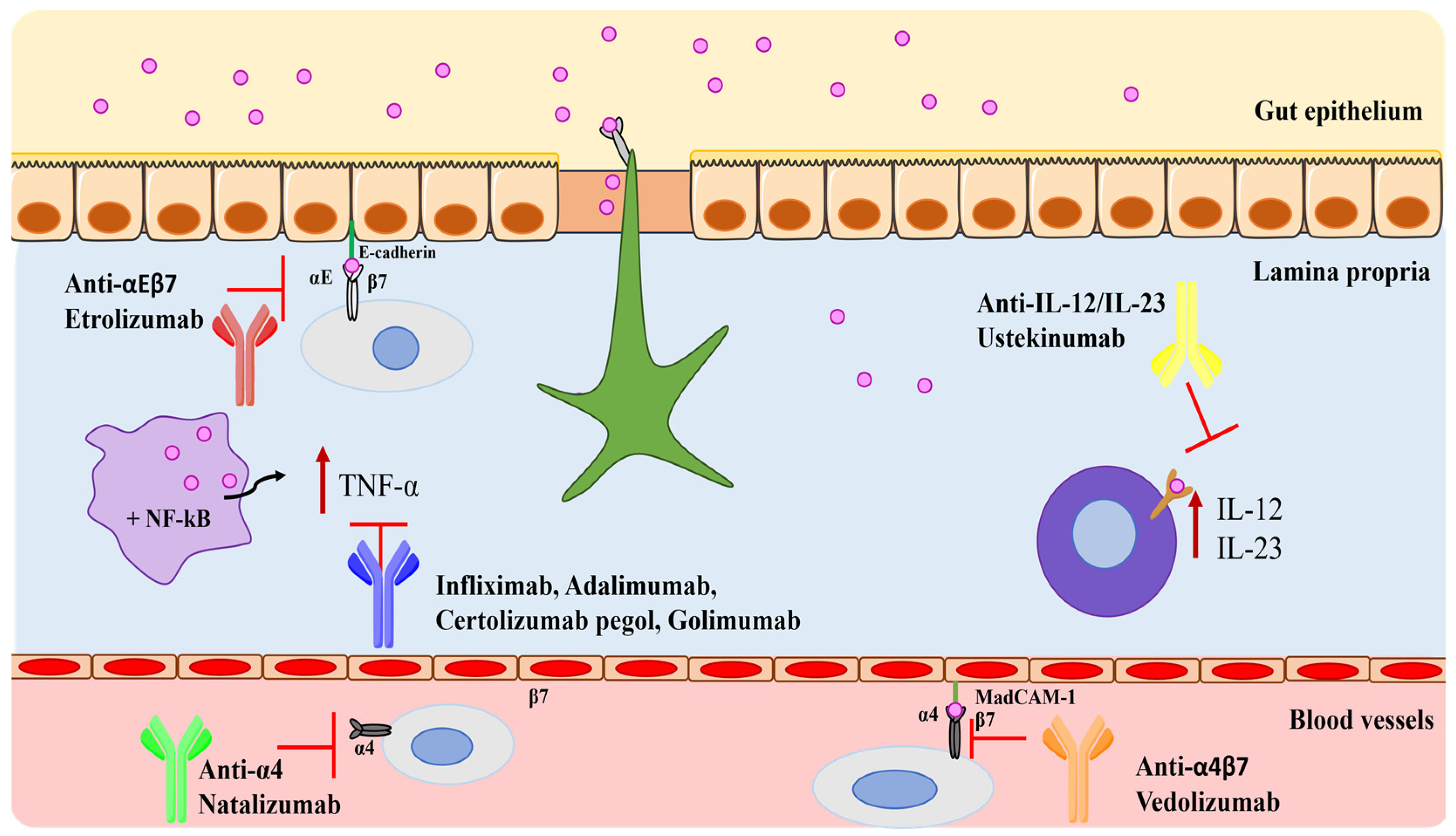
4. Monoclonal Antibodies in IBD
4.1. Tumor Necrosis Factor-Alpha (TNF-α) Blockers
4.2. Integrin Blockers
4.3. Interleukins Blockers
4.4. Monoclonal Antibodies in Development
| Drugs | Mechanism of Action | Administration Route | Doses | Adverse Effects | Ref. |
|---|---|---|---|---|---|
| Infliximab (REMICADE®) | Anti-TNF-α | Intravenous | 5 mg/kg Induction doses: 0, 2, and 6 weeks. Maintenance doses: 8-weekly intervals. | Hypersensitivity reactions. | [44] |
| Infliximab (ZYMFENTRA®) | Anti-TNF-α | Subcutaneous | Maintenance doses only: 120 mg at week 10, 2-weekly intervals. | COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain (for UC). COVID-19, headache, upper respiratory tract infection, injection site reaction, diarrhea, increased blood creatine phosphokinase, arthralgia, increased alanine aminotransferase, hypertension, urinary tract infection, neutropenia, dizziness, and leukopenia (for CD). | [54] |
| Adalimumab (HUMIRA®) | Anti-TNF-α | Intramuscular | Induction doses: 80/160 mg at week 0 and 40/80 mg at week 2 (for CD); 80/160 mg at week 0, 40/80 mg at week 1, and 40/80 mg at week 2 (for UC). Maintenance doses: 20/40 mg every week starting from week 4 (for CD); 40/80 mg every week starting from week 4 or 20/40 mg every week (for UC). | Infections (e.g., upper respiratory, sinusitis), injection site reactions, headache, and rash. | [55] |
| Golimumab (SIMPONI®) | Anti-TNF-α | Subcutaneous | Induction doses: 200 mg week 0 and 100 mg at week 2. Maintenance doses: 50/100 mg 4-weekly intervals. | Paraesthesia, cutaneous infection, pneumonitis, and the recurrence of cervical neoplasia. | [58] |
| Certolizumab pegol (CIMZIA®) | Anti-TNF-α | Subcutaneous | Induction doses: 400 mg at 0, 2, and 4 weeks. Maintenance doses: 400 mg 4-weekly intervals. | Upper respiratory tract infection, rash, and urinary tract infection. | [61] |
| Natalizumab (TYSABRI®) | Integrin blocker | Intravenous | 300 mg 4-weekly intervals. | Headache, the exacerbation of CD, nausea, nasopharyngitis, and progressive multifocal leukoencephalopathy. | [69,70] |
| Vedolizumab (ENTYVIO®) | Integrin blocker | Intravenous and subcutaneous | Induction doses: 300 mg at weeks 0, 2, and 6. Maintenance doses: 300 mg 8-weekly intervals. | Nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, rash, pruritus, sinusitis, oropharyngeal pain, pain in extremities, and injection site reactions with subcutaneous administration. | [72,73] |
| Etrolizumab | Integrin blocker | Subcutaneous | 105 mg every 4 weeks for 14 weeks (phase 3 clinical program). | UC flare, appendicitis, and anemia. | [75] |
| Ustekinumab (STELARA®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: depends on the patient’s body weight. Maintenance doses: 90 mg 8–12-weekly intervals. | Headache, nasopharyngitis, inflammation of the nose and throat, and hypersensitivity (allergic reaction). | [79] |
| Mirikizumab (OMVOH®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 300 mg at weeks 0, 4, and 8; 4-weekly intervals. Maintenance doses: 200 mg 4-weekly intervals. | Infections (active tuberculosis). | [83] |
| Briakinumab | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 200, 400, and 700 mg at weeks 0, 4, and 8. Maintenance doses: 200, 400, and 700 mg after 12 weeks (phase 2 clinical program). | Upper respiratory tract infection, nausea, abdominal pain, and headache. | [84] |
| Ontamalimab | Integrin blocker | Subcutaneous | Induction doses: 25/75 mg once 4-weekly intervals to week 12. Maintenance doses: 25/75 mg once at 4-weekly intervals to week 52 (phase 3 clinical program). | Serious infections, gastroenteritis, pelvic abscess, pneumonia, arthralgia, and nasopharyngitis. | [85] |
| Risankizumab (SKYRIZI®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 600 mg at weeks 0, 4, and 8. Maintenance doses: 360 mg at week 12, 8-weekly intervals. | Upper respiratory infection, tinea infection, folliculitis, headache, pruritus, rash, urticaria, fatigue, and injection site reactions. | [86] |
| Brazikumab | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 700 mg at weeks 0 and 4. Maintenance doses: 210 mg from week 12. (Phase 2 clinical program.) | Nasopharyngitis, headache, and abdominal pain. | [86] |
| Guselkumab (TREMFYA®) | Interleukin blocker | Intravenous | Induction doses: 200 mg at weeks 0, 4, and 8 for 12 weeks. | Headache, joint pain, upper respiratory infections, diarrhea, and stomach pain. | [88] |
4.5. Other Treatments
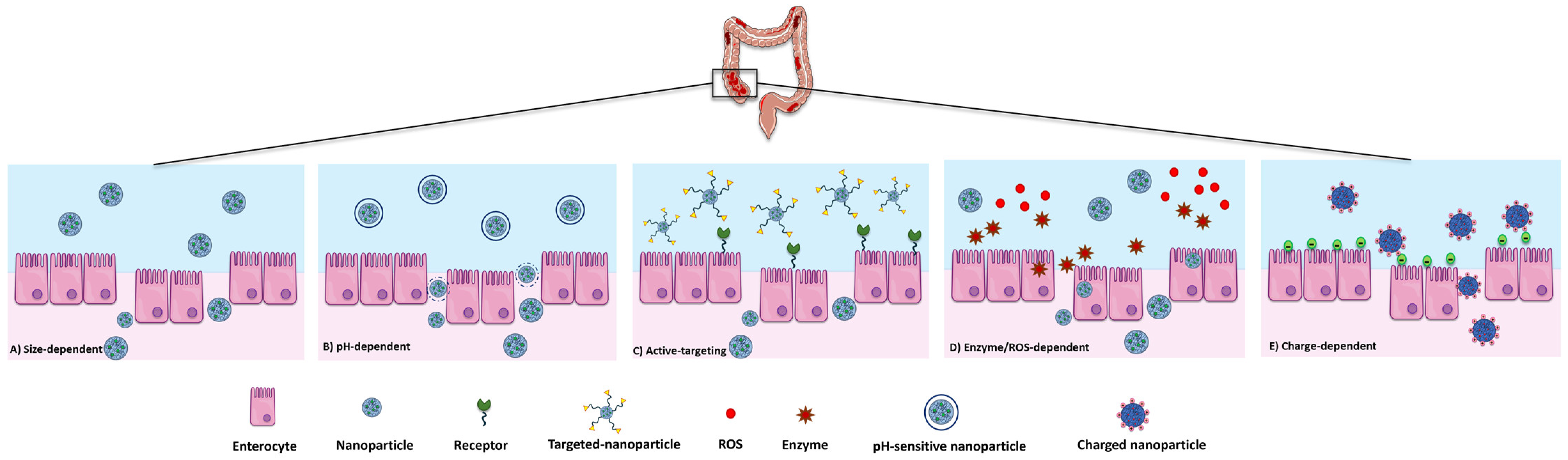
5. Drug Delivery Systems
5.1. General Features of DDSs in the IBD
5.2. Antibody-Loaded DDSs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kontola, K.; Oksanen, P.; Huhtala, H.; Jussila, A. Increasing Incidence of Inflammatory Bowel Disease, with Greatest Change Among the Elderly: A Nationwide Study in Finland, 2000–2020. J. Crohn’s Colitis 2023, 17, 706–711. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Rieder, F.; Kucharzik, T.; Peyrin-Biroulet, L.; Schoepfer, A.M.; Rubin, D.T.; Vavricka, S.R. Emerging treatment options for extraintestinal manifestations in IBD. Gut 2021, 70, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Raj, J.P. Role of biologics and biosimilars in inflammatory bowel disease: Current trends and future perspectives. J. Inflamm. Res. 2018, 11, 215–226. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, W.M.; Guo, Z. Current status of novel biologics and small molecule drugs in the individualized treatment of inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 6888–6899. [Google Scholar] [CrossRef]
- Faris, A.; Cacciatore, I.; Ibrahim, I.M.; Al Mughram, M.H.; Hadni, H.; Tabti, K.; Elhallaoui, M. In silico computational drug discovery: A Monte Carlo approach for developing a novel JAK3 inhibitors. J. Biomol. Struct. Dyn. 2023, 1–23. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.J.; Panaccione, R.; Domènech, E.; Pouillon, L.; Siegmund, B.; Danese, S.; Ghosh, S. Tumour necrosis factor inhibitors in inflammatory bowel disease: The story continues. Therap. Adv. Gastroenterol. 2021, 14, 17562848211059954. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Zhang, D.; Gordon, M.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 9, CD003715. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Kuenzig, M.E.; Hazlewood, G.; Clement, F.; McBrien, K.; Holmes, R.; Panaccione, R.; Ghosh, S.; Seow, C.H.; Rezaie, A.; et al. Comparative Effectiveness of Mesalamine, Sulfasalazine, Corticosteroids, and Budesonide for the Induction of Remission in Crohn’s Disease: A Bayesian Network Meta-analysis. Inflamm. Bowel. Dis. 2017, 23, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Krnjic, A.; Frick, A.; Gmainer, C.; Asboth, M.; Jimenez, K.; Lang, M.; Baumgartner, M.; Evstatiev, R.; Gasche, C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci. Rep. 2019, 9, 2842. [Google Scholar] [CrossRef]
- Mehta, R.S.; Mayers, J.R.; Zhang, Y.; Bhosle, A.; Glasser, N.R.; Nguyen, L.H.; Ma, W.; Bae, S.; Branck, T.; Song, K.; et al. Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease. Nat. Med. 2023, 29, 700–709. [Google Scholar] [CrossRef]
- Gisbert, J.P.; González-Lama, Y.; Maté, J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2007, 13, 629–638. [Google Scholar] [CrossRef]
- Becker, H.E.F.; Demers, K.; Derijks, L.J.J.; Jonkers, D.M.A.E.; Penders, J. Current evidence and clinical relevance of drug-microbiota interactions in inflammatory bowel disease. Front. Microbiol. 2023, 14, 1107976. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.; Saxena, S.; Pollok, R. Using corticosteroids appropriately in inflammatory bowel disease: A guide for primary care. Br. J. Gen. Pract. 2018, 68, 497–498. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef] [PubMed]
- Manguso, F.; Bennato, R.; Lombardi, G.; Riccio, E.; Costantino, G.; Fries, W. Efficacy and Safety of Oral Beclomethasone Dipropionate in Ulcerative Colitis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166455. [Google Scholar] [CrossRef]
- Gabbani, T.; Manetti, N.; Bagnoli, S.; Annese, V. Beclomethasone dipropionate for the treatment of ulcerative colitis. Expert Opin. Orphan Drugs 2015, 3, 87–96. [Google Scholar] [CrossRef]
- Oancea, I.; Movva, R.; Das, I.; Aguirre de Cárcer, D.; Schreiber, V.; Yang, Y.; Purdon, A.; Harrington, B.; Proctor, M.; Wang, R.; et al. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut 2017, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Diall, A.; Dvorsky, R.; Atreya, R.; Henninger, C.; Grün, M.; Hofmann, U.; Schaeffeler, E.; López-Posadas, R.; Daehn, I.; et al. Designer Thiopurine-analogues for Optimised Immunosuppression in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2016, 10, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: A systematic review and meta-analysis of randomized, controlled trials. eClinicalMedicine 2020, 20, 100271. [Google Scholar] [CrossRef]
- Rosh, J.R. The Current Role of Methotrexate in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 43–46. [Google Scholar]
- Weissman, S.; Chris-Olaiya, A.; Mehta, T.I.; Aziz, M.; Alshati, A.; Berry, R.; Fatima, R.; Kolli, S.; Hassan, A.; Sciarra, M.A. A novel player: Cyclosporine therapy in the management of inflammatory bowel disease. Transl. Gastroenterol. Hepatol. 2019, 4, 67. [Google Scholar] [CrossRef]
- Wu, B.; Tong, J.; Ran, Z. Tacrolimus Therapy in Steroid-Refractory Ulcerative Colitis: A Review. Inflamm. Bowel Dis. 2020, 26, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Fujii, T.; Kinoshita, K.; Kawamoto, A.; Hibiya, S.; Takenaka, K.; Saito, E.; Nagahori, M.; Ohtsuka, K.; Watanabe, M.; et al. Intravenous tacrolimus is a superior induction therapy for acute severe ulcerative colitis compared to oral tacrolimus. BMC Gastroenterol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Rehman, M.; Cancarevic, I.; Iskander, B.; Lalani, S.; Malik, B.H. Biologics Targeting in the Treatment of Inflammatory Bowel Disease: A Conundrum. Cureus 2020, 12, e10621. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Billmeier, U.; Dieterich, W.; Neurath, M.F.; Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 9300–9313. [Google Scholar] [CrossRef]
- Avedillo-Salas, A.; Corral-Cativiela, S.; Fanlo-Villacampa, A.; Vicente-Romero, J. The Efficacy and Safety of Biologic Drugs in the Treatment of Moderate-Severe Crohn’s Disease: A Systematic Review. Pharmaceuticals 2023, 16, 1581. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Begue, B.; Wajant, H.; Bambou, J.C.; Dubuquoy, L.; Siegmund, D.; Beaulieu, J.F.; Canioni, D.; Berrebi, D.; Brousse, N.; Desreumaux, P.; et al. Implication of TNF-related apoptosis-inducing ligand in inflammatory intestinal epithelial lesions. Gastroenterology 2006, 130, 1962–1974. [Google Scholar] [CrossRef]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, A. Infliximab approved for use in Crohn’s disease: A report on the FDA GI Advisory Committee conference. Inflamm. Bowel Dis. 1998, 4, 328–329. [Google Scholar] [CrossRef]
- de Vries, H.S.; van Oijen, M.G.; Driessen, R.J.; de Jong, E.M.; Creemers, M.C.; Kievit, W.; de Jong, D.J. Appropriate infliximab infusion dosage and monitoring: Results of a panel meeting of rheumatologists, dermatologists and gastroenterologists. Br. J. Clin. Pharmacol. 2011, 71, 7–19. [Google Scholar] [CrossRef] [PubMed]
- ten Hove, T.; van Montfrans, C.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 2002, 50, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Van den Brande, J.M.; Braat, H.; van den Brink, G.R.; Versteeg, H.H.; Bauer, C.A.; Hoedemaeker, I.; van Montfrans, C.; Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 2003, 124, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic. Dis. 2019, 10, 2040622319838443. [Google Scholar] [CrossRef]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’ Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Rosenstein, S.; Yavzori, M.; Dror, Y.; Fudim, E.; Ungar, B.; Kopylov, U.; Picard, O.; Kigel, A.; Ben-Horin, S.; et al. Molecular Landscape of Anti-Drug Antibodies Reveals the Mechanism of the Immune Response Following Treatment with TNFα Antagonists. Front. Immunol. 2019, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Brembilla, N.C. Immunogenicity of biologic therapies: Causes and consequences. Expert. Rev. Clin. Immunol. 2018, 14, 513–523. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Zymfentra (infliximab-dyyb) Subcutaneous Formulation for the Treatment of People with Inflammatory Bowel Disease. Available online: https://www.drugs.com/newdrugs/fda-approves-zymfentra-infliximab-dyyb-subcutaneous-formulation-inflammatory-bowel-6122.html (accessed on 1 December 2023).
- Humira (Adalimumab): Prescribing Information. 2012, pp. 1–73. Available online: http://www.rxabbvie.com/pdf/humira.pdf (accessed on 3 December 2023).
- European Medicines Agency (EMA). Humira Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (accessed on 5 December 2023).
- Yin, J.; Li, Y.; Chen, Y.; Wang, C.; Song, X. Adalimumab for induction of remission in patients with Crohn’s disease: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 190. [Google Scholar] [CrossRef]
- Cunningham, G.; Samaan, M.A.; Irving, P.M. Golimumab in the treatment of ulcerative colitis. Therap. Adv. Gastroenterol. 2019, 12, 1756284818821266. [Google Scholar] [CrossRef]
- Kay, J.; Matteson, E.L.; Dasgupta, B.; Nash, P.; Durez, P.; Hall, S.; Hsia, E.C.; Han, J.; Wagner, C.; Xu, Z.; et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: A randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008, 58, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Cimzia Prescribing Information. Available online: https://www.cimzia.com/themes/custom/cimzia/docs/CIMZIA_full_prescribing_information.pdf (accessed on 5 December 2023).
- Lang, L. FDA approves Cimzia to treat Crohn’s disease. Gastroenterology 2008, 134, 1819. [Google Scholar] [CrossRef]
- Schreiber, S. Certolizumab pegol for the treatment of Crohn’s disease. Therap. Adv. Gastroenterol. 2011, 4, 375–389. [Google Scholar] [CrossRef]
- Okabayashi, S.; Yamazaki, H.; Yamamoto, R.; Anan, K.; Matsuoka, K.; Kobayashi, T.; Shinzaki, S.; Honzawa, Y.; Kataoka, Y.; Tsujimoto, Y.; et al. Certolizumab pegol for maintenance of medically induced remission in Crohn’s disease. Cochrane Database Syst. Rev. 2022, 6, CD013747. [Google Scholar] [CrossRef]
- Shimaoka, M.; Takagi, J.; Springer, T.A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 485–516. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; O’Byrne, S.; Keir, M.E.; Butcher, E.C. Gut-Selective Integrin-Targeted Therapies for Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, S653–S668. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Allez, M.; Danese, S.; Keir, M.; Tole, S.; McBride, J. The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti-integrin therapies. Med. Res. Rev. 2020, 40, 245–262. [Google Scholar] [CrossRef]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.S.; Wang, J.; Buckman, C.A.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef]
- Nelson, S.M.; Nguyen, T.M.; McDonald, J.W.; MacDonald, J.K. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 8, CD006097. [Google Scholar] [CrossRef]
- Van Assche, G.; Van Ranst, M.; Sciot, R.; Dubois, B.; Vermeire, S.; Noman, M.; Verbeeck, J.; Geboes, K.; Robberecht, W.; Rutgeerts, P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 2005, 353, 362–368. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Drug Safety Communication: New Risk Factor for Progressive Multifocal Leukoencephalopathy (PML) Associated with Tysabri (Natalizumab). Available online: http://www.fda.gov/Drugs/DrugSafety/ucm288186.htm (accessed on 18 May 2024).
- Tamilarasan, A.G.; Cunningham, G.; Irving, P.M.; Samaan, M.A. Recent advances in monoclonal antibody therapy in IBD: Practical issues. Frontline Gastroenterol. 2019, 10, 409–416. [Google Scholar] [CrossRef]
- U.S. FDA Approves Subcutaneous Administration of Takeda’s Entyvio (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Ulcerative Colitis. Entyvio (Vedolizumab) FDA Approval History-Drugs.com. Available online: https://www.drugs.com/newdrugs/u-s-fda-approves-subcutaneous-administration-takeda-s-entyvio-vedolizumab-maintenance-therapy-6099.html (accessed on 6 December 2023).
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Hart, A.; Bossuyt, P.; Long, M.; Allez, M.; Juillerat, P.; Armuzzi, A.; Loftus, E.V., Jr.; Ostad-Saffari, E.; Scalori, A.; et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): A phase 3, randomised, controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 128–140. [Google Scholar] [CrossRef]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor. Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Parigi, T.L.; Iacucci, M.; Ghosh, S. Blockade of IL-23: What is in the Pipeline? J. Crohn’s Colitis 2022, 16, ii64–ii72. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Stelara. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/stelara (accessed on 4 December 2023).
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Gasink, C.; Gao, L.L.; Blank, M.A.; Johanns, J.; Guzzo, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Rutgeerts, P.; et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 2012, 367, 1519–1528. [Google Scholar] [CrossRef]
- Sands, B.E.; D’Haens, G.; Clemow, D.B.; Irving, P.M.; Johns, J.T.; Hunter Gibble, T.; Abreu, M.T.; Lee, S.; Hisamatsu, T.; Kobayashi, T.; et al. Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment for Ulcerative Colitis: Results from the LUCENT-3 Open-Label Extension Study. Inflamm. Bowel Dis. 2024, 30, 1044–1045. [Google Scholar] [CrossRef]
- European Medicines Agency. Omvoh. Available online: https://www.ema.europa.eu/en/documents/product-information/omvoh-epar-product-information_en.pdf (accessed on 24 July 2024).
- Panaccione, R.; Sandborn, W.J.; Gordon, G.L.; Lee, S.D.; Safdi, A.; Sedghi, S.; Feagan, B.G.; Hanauer, S.; Reinisch, W.; Valentine, J.F.; et al. Briakinumab for treatment of Crohn’s disease: Results of a randomized trial. Inflamm. Bowel Dis. 2015, 21, 1329–1340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vermeire, S.; Danese, S.; Sandborn, W.J.; Schreiber, S.; Hanauer, S.; D’Haens, G.; Nagy, P.; Thakur, M.; Bliss, C.; Cataldi, F.; et al. Efficacy and Safety of the Anti-mucosal Addressin Cell Adhesion Molecule-1 Antibody Ontamalimab in Patients with Moderate-to-Severe Ulcerative Colitis or Crohn’s Disease. J. Crohn’s Colitis 2024, 18, 708–719. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Skyrizi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi#product-info (accessed on 24 July 2024).
- Danese, S.; Beaton, A.; Duncan, E.A.; Mercier, A.K.; Neisen, J.; Seth, H.; Zetterstrand, S.; Sands, B.E. Long-term safety of brazikumab in the open-label period of a randomized phase 2a study of patients with Crohn’s disease. BMC Gastroenterol. 2023, 23, 451. [Google Scholar] [CrossRef]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results from the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef]
- Dimmito, M.P.; Marinelli, L.; Cacciatore, I.; Valeri, A.L.; Rapino, A.; Di Stefano, A. Self-assembling Peptides (SAPs) as Powerful Tools for the Preparation of Antimicrobial and Wound-Healing Nanostructures. Lett. Drug Des. Discov. 2024, 21, 2232–2247. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Peretz, A.; Saliba, W. Role of antibiotics for treatment of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Docherty, M.J.; Jones, R.C.; Wallace, M.S. Managing pain in inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 592–601. [Google Scholar] [PubMed]
- Gao, J.; Li, J.; Luo, Z.; Wang, H.; Ma, Z. Nanoparticle-Based Drug Delivery Systems for Inflammatory Bowel Disease Treatment. Drug Des. Devel. Ther. 2024, 18, 2921–2949. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef] [PubMed]
- Ben Khalifa, R.; Cacciatore, I.; Dimmito, M.P.; Ciulla, M.; Grande, R.; Puca, V.; Robuffo, I.; De Laurenzi, V.; Chekir-Ghedira, L.; Di Stefano, A.; et al. Multiple lipid nanoparticles as antimicrobial drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 10288. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.M. Budesonide loaded nanoparticles with pHsensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control. Release 2014, 183, 167–177. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Niebel, W.; Walkenbach, K.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J. Control. Release 2012, 160, 659–665. [Google Scholar] [CrossRef]
- Jubeh, T.T.; Barenholz, Y.; Rubinstein, A. Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharm. Res. 2004, 21, 447–453. [Google Scholar] [CrossRef]
- Giron, F.; Pastó, A.; Tasciotti, E.; Abraham, B. Nanotechnology in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Moayedi, S.; Mohajeri, S.; Yadegar, A.; Haririan, I. Targeting pathophysiological changes using biomaterials-based drug delivery systems: A key to managing inflammatory bowel disease. Front. Pharmacol. 2022, 13, 1045575. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef]
- Gao, C.; Yu, S.; Zhang, X.; Dang, Y.; Han, D.D.; Liu, X.; Han, J.; Hui, M. Dual Functional Eudragit® S100/L30D-55 and PLGA Colon-Targeted Nanoparticles of Iridoid Glycoside for Improved Treatment of Induced Ulcerative Colitis. Int. J. Nanomed. 2021, 16, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Turanlı, Y.; Acartürk, F. Preparation and characterization of colon-targeted pH/Time-dependent nanoparticles using anionic and cationic polymethacrylate polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, A.; Marinelli, L.; Di Stefano, A.; Vicaretti, G.; Cacciatore, I. Aptamers-based Strategies for the Treatment of Microbial Infections. Lett. Drug Des. Discov. 2024, 21, 858–865. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Wang, L.; Pan, D.; Xu, Y.; Wang, F.; Sheng, J.; Li, X.; Yang, M. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics 2020, 10, 10808–10822. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.H.; Park, H.J.; Ma, H.W.; Park, I.S.; Son, M.; Ro, S.Y.; Hong, S.; Han, H.K.; Lim, S.J.; et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J. Nanobiotechnology 2020, 18, 133. [Google Scholar] [CrossRef]
- Li, X.; Fang, S.; Yu, Y.; Yang, H.; Rao, Y.; Hong, D.; Lu, C.; Yu, M.; Lu, X.; Yu, C.; et al. Oral administration of inflammatory microenvironment-responsive carrier-free infliximab nanocomplex for the targeted treatment of inflammatory bowel disease. Chem. Eng. J. 2022, 445, 136438. [Google Scholar] [CrossRef]
- Ries, M.; Moulari, B.; Shetab Boushehri, M.A.; Ali, M.E.; Molnar, D.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics 2022, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Xu, Y.; Prévost, J.R.C.; McCartney, F.; Brayden, D.; Frédérick, R.; Beloqui, A.; Préat, V. Impact of PEGylation on an antibody-loaded nanoparticle-based drug delivery system for the treatment of inflammatory bowel disease. Acta Biomater. 2022, 140, 561–572. [Google Scholar] [CrossRef] [PubMed]
| Traditional Drugs | Class of Drugs | Administration Route | Mechanism of Action | Refs. |
|---|---|---|---|---|
| Sulfasalazine | Aminosalicylates | Oral | The inhibition of IL-1, TNF-α production, NF-kB, and lipoxygenase pathways | [21,22,23,24,25,26] |
| Prednisone | Corticosteroids first generation | Oral or parenteral | The inhibition of the immune system | [29] |
| Beclomethasone dipropionate | Corticosteroids second generation | Oral or topical | The inhibition of the immune system | [30,31] |
| Azathioprine | Thiopurines | Oral | The inhibition of T lymphocyte proliferation and activation | [32] |
| Methotrexate | Antimetabolites | Parenteral | Decreasing pro-inflammatory cytokine production and leading to the apoptosis of T cells | [33,34] |
| Cyclosporine | Immunosuppressives | Oral or intravenous | Calcineurin inhibition | [35] |
| Tacrolimus | Immunosuppressives | Oral or intravenous | Calcineurin inhibition | [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rienzo, A.; Marinelli, L.; Dimmito, M.P.; Toto, E.C.; Di Stefano, A.; Cacciatore, I. Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics 2024, 16, 1185. https://doi.org/10.3390/pharmaceutics16091185
Di Rienzo A, Marinelli L, Dimmito MP, Toto EC, Di Stefano A, Cacciatore I. Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics. 2024; 16(9):1185. https://doi.org/10.3390/pharmaceutics16091185
Chicago/Turabian StyleDi Rienzo, Annalisa, Lisa Marinelli, Marilisa Pia Dimmito, Eleonora Chiara Toto, Antonio Di Stefano, and Ivana Cacciatore. 2024. "Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems" Pharmaceutics 16, no. 9: 1185. https://doi.org/10.3390/pharmaceutics16091185
APA StyleDi Rienzo, A., Marinelli, L., Dimmito, M. P., Toto, E. C., Di Stefano, A., & Cacciatore, I. (2024). Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics, 16(9), 1185. https://doi.org/10.3390/pharmaceutics16091185








