Efficacy and Safety of Fluocinolone Acetonide Implant in Diabetic Macular Edema: Practical Guidelines from Reference Center
Abstract
1. Introduction
2. FAc Implant Pharmacokinetics
3. Selection and Monitoring of Adequate Patient Profile for FAc Implant
4. Main Reasons for Switching Patients to the FAc Implant
- (a)
- Reduce the therapeutic burden. The intensive treatment regimen with frequent examinations and repeated intravitreal injections represents a particularly high therapeutic burden for the patient and can be time-consuming for the clinician. Sustained-release implants, such as the FAc implant, can reduce this burden. Real-world data show that one FAc implant is sufficient by itself to control the DME for up to 24 months [34,41,42,43]. While some patients may need additional treatments, the frequency of reinjection is always significantly reduced [44,45], therefore improving the patients’ quality of life [42]. In fact, before switching patients to the FAc implant, DME patients have, on average, one treatment every three months, which is reduced to one treatment every year post-FAc [34,41].
- (b)
- Prevent DME recurrence. The FAc implant can be regarded as a prophylactic foundational treatment that prevents DME recurrence and subsequent vision loss. Similar to migraines, which are often managed with betablockers as a first-line treatment for migraine prevention and with anti-inflammatory drugs and triptans to treat acute attacks [46], administering a FAc implant decreases the overall recurrence of DME, but a DEX-I might still be needed in case of an important relapse [21,22,34]. It is important not to consider this as a failure of the FAc implant, as the overall number of additional treatments and treatment frequency will still be significantly reduced, as described earlier.
- (c)
- Reduce anatomical fluctuations. By sustainably drying the macula over three years and reducing DME recurrences, the FAc implant prevents retinal thickness variations, which directly correlates with better functional outcomes and a reduced supplemental treatment burden [47,48]. In fact, cyclic mechanical stretching of retinal pigment epithelial cells and photoreceptors can cause retinal cell death and inhibit phototransduction [49,50], thereby impacting long-term visual recovery [51]. The FAc implant reduced retinal thickness variations in both prospective and retrospective analyses [51,52,53,54].
5. When to Switch?
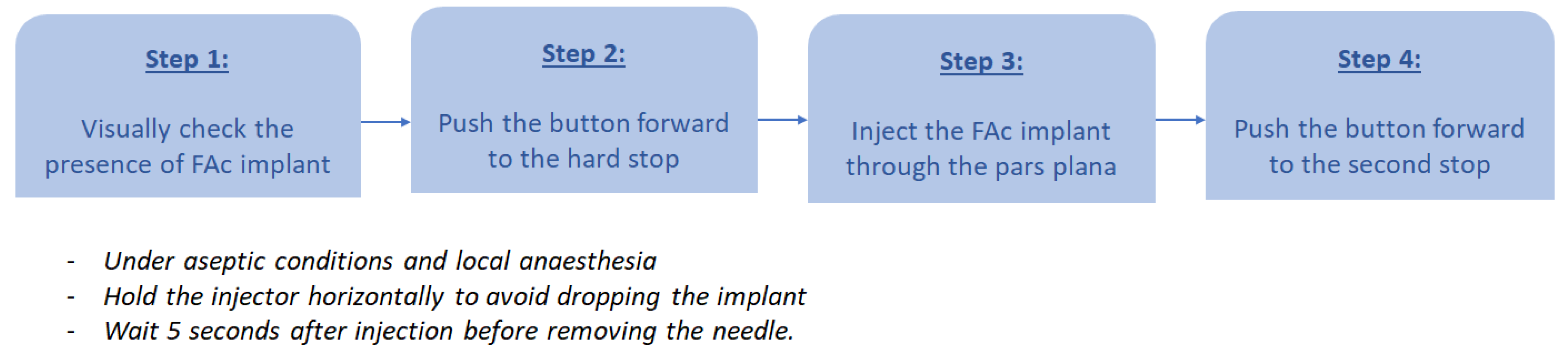
6. Technique of Injection
7. FAc Implant Efficacy
8. FAc Implant Safety
- -
- Switching to FAc following 2 to 3 DEX-I:
- ○
- If IOP remains below 25 mmHg, the FAc implant could be proposed with IOP monitoring every 3 months.
- ○
- If OHT > 25 mmHg occurs, the FAc implant is not recommended, and a specialized glaucoma consultation is needed.
- -
- After a FAc implant injection:
- ○
- If IOP remains below 21 mmHg, a yearly assessment of the retinal nerve fiber layer (RNFL) is recommended.
- ○
- If the IOP is between 21 and 25 mmHg, RNFL and visual field testing are recommended at baseline and to be repeated every 6 to 12 months.
- ○
- If the IOP is superior to 25 mmHg, hypotensive treatment should be used, and selective laser trabeculoplasty can be considered. IOP should be controlled at 1 month and RNFL and visual field testing repeated every 6 to 12 months.


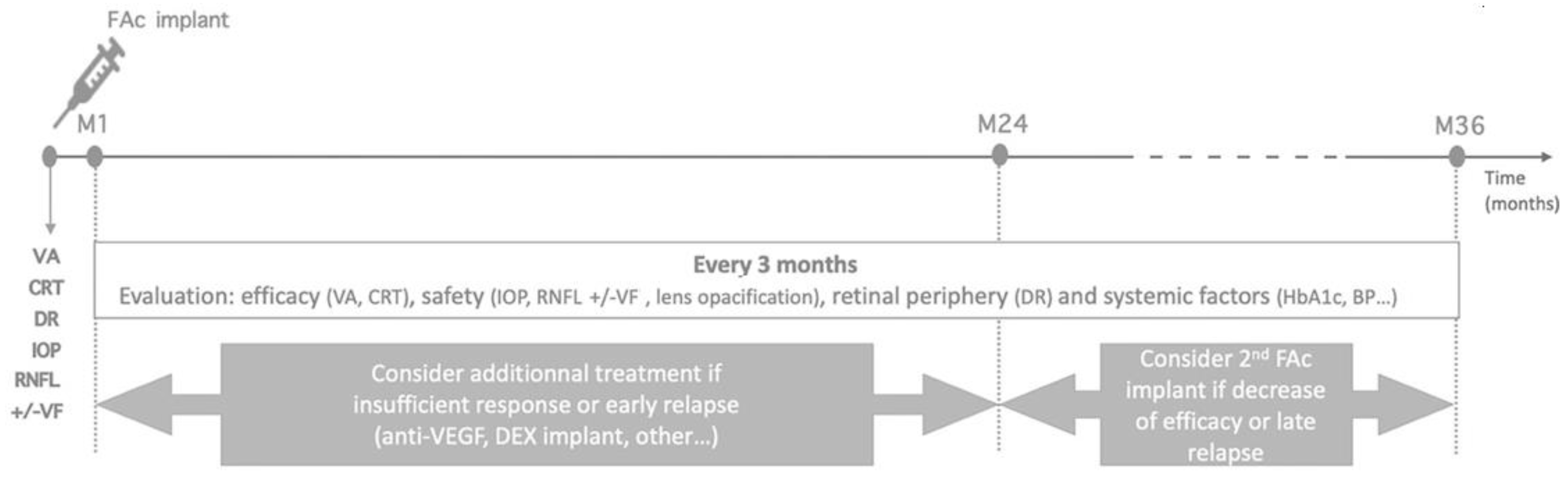
9. How to Manage the Second Injection of FAc?
10. Discussion
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Pascual-Camps, I.; Holz, F.G.; Finger, R.P. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Mieler, W.F.; Grassi, M.; Green, J.L.; Jager, R.D.; Miller, L. Vision-related quality of life in patients with diabetic macular oedema. Br. J. Ophthalmol. 2008, 92, 89–92. [Google Scholar] [CrossRef]
- Klein, R.; Moss, S.E.; Klein, B.E.K.; Gutierrez, P.; Mangione, C.M. The NEI-VFQ-25 in People with Long-term Type 1 Diabetes Mellitus: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch. Ophthalmol. 2001, 119, 733–740. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Oyetunde, S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin. Ophthalmol. 2016, 10, 939–946. [Google Scholar] [CrossRef]
- Fajnkuchen, F.; Delyfer, M.-N.; Conrath, J.; Baillif, S.; Mrejen, S.; Srour, M.; Bellamy, J.-P.; Dupas, B.; Lecleire-Collet, A.; Meillon, C.; et al. Expectations and fears of patients with diabetes and macular edema treated by intravitreal injections. Acta Diabetol. 2020, 57, 1081–1091. [Google Scholar] [CrossRef]
- Ehlken, C.; Helms, M.; Böhringer, D.; Agostini, H.T.; Stahl, A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin. Ophthalmol. 2018, 12, 13–20. [Google Scholar] [CrossRef]
- Garcher, C.P.C.; Massin, P.; Srour, M.; Baudin, F.; Dot, C.; Nghiem-Buffet, S.; Girmens, J.; Collin, C.; Ponthieux, A.; Delcourt, C. Management of diabetic macular oedema in France from 2012 to 2018: The nationwide LANDSCAPE study. Acta Ophthalmol. 2024, 102, e548–e556. [Google Scholar] [CrossRef]
- Vinge, E.; Bro, T. Treatment burden on patients receiving intravitreal anti-VEGF for wet age-related macular degeneration. Acta Ophthalmol. 2024, 102, 478–482. [Google Scholar] [CrossRef]
- Verrecchia, S.; Chiambaretta, F.; Kodjikian, L.; Nakouri, Y.; El Chehab, H.; Mathis, T.; Badri, Y.; Chudzinski, R.; Levron, A.; Chaperon, M.; et al. A prospective multicentre study of intravitreal injections and ocular surface in 219 patients: IVIS study. Acta Ophthalmol. 2021, 99, 877–884. [Google Scholar] [CrossRef]
- Gao, M.; Xia, F.; Wang, P.; Feng, Z.; Wang, X. Influence of serial intravitreal injections on measures of dry eye: A systemic review and meta-analysis. Contact Lens Anterior Eye 2024, 47, 102127. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, H.; Mahmood, S.; McGee, S.; Hubbard, J.; Haque, S.; Paudyal, V.; Denniston, A.K.; Hill, L.J.; Jalal, Z. Non-adherence and non-persistence to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy: A systematic review and meta-analysis. Syst. Rev. 2023, 12, 92. [Google Scholar] [CrossRef]
- Müller, S.; Junker, S.; Wilke, T.; Lommatzsch, A.; Schuster, A.K.; Kaymak, H.; Ehlken, C.; Ziemssen, F. Questionnaire for the assessment of adherence barriers of intravitreal therapy: The ABQ-IVT. Int. J. Retin. Vitr. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Bellocq, D.; Mathis, T. Pharmacological Management of Diabetic Macular Edema in Real-Life Observational Studies. BioMed Res. Int. 2018, 2018, 8289253. [Google Scholar] [CrossRef] [PubMed]
- Rezkallah, A.; Mathis, T.; Abukhashabah, A.; Voirin, N.; Malclès, A.; Agard, É.; Lereuil, T.; Denis, P.; Dot, C.; Kodjikian, L. Long-term incidence and risk factors of ocular hypertension following dexamethasone-implant injections the safodex-2 study. Retina 2021, 41, 1438–1445. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Ikeda, T.; Mimura, T.; Eguchi, S.; Hori, S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003, 110, 1690–1696. [Google Scholar] [CrossRef]
- Chang-Lin, J.-E.; Attar, M.; Acheampong, A.A.; Robinson, M.R.; Whitcup, S.M.; Kuppermann, B.D.; Welty, D. Pharmacokinetics and Pharmacodynamics of a Sustained-Release Dexamethasone Intravitreal Implant. Investig. Ophthalmol. Vis. Sci. 2011, 52, 80–86. [Google Scholar] [CrossRef]
- Feldman-Billard, S.; Du Pasquier-Fediaevsky, L.; Héron, E. Hyperglycemia after Repeated Periocular Dexamethasone Injections in Patients with Diabetes. Ophthalmology 2006, 113, 1720–1723. [Google Scholar] [CrossRef]
- Feldman-Billard, S.; Héron, E. Systemic tolerance of corticosteroid therapy in ophthalmology depending on the route of administration. J. Fr. D Ophtalmol. 2008, 31, 1026–1036. [Google Scholar] [CrossRef]
- Malclès, A.; Dot, C.; Voirin, N.; Vié, A.-L.; Agard, É.; Bellocq, D.; Denis, P.; Kodjikian, L. Safety of intravitreal dexamethasone implant (Ozurdex): The SAFODEX study. Incidence and Risk Factors of Ocular Hypertension. Retina 2017, 37, 1352–1359. [Google Scholar] [CrossRef]
- Malclès, A.; Dot, C.; Voirin, N.; Agard, É.; Vié, A.-L.; Bellocq, D.; Denis, P.; Kodjikian, L. Real-life study in diabetic macular edema treated with dexamethasone implant: The reldex study. Retina 2017, 37, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Pinto, M.; Mathis, T.; Massin, P.; Akesbi, J.; Lereuil, T.; Voirin, N.; Matonti, F.; Fajnkuchen, F.; Conrath, J.; Milazzo, S.; et al. Visual Acuity Gain Profiles and Anatomical Prognosis Factors in Patients with Drug-Naive Diabetic Macular Edema Treated with Dexamethasone Implant: The NAVEDEX Study. Pharmaceutics 2021, 13, 194. [Google Scholar] [CrossRef]
- Mathis, T.; Lereuil, T.; Abukashabah, A.; Voirin, N.; Sudhalkar, A.; Bilgic, A.; Denis, P.; Dot, C.; Kodjikian, L. Long-term follow-up of diabetic macular edema treated with dexamethasone implant: A real-life study. Acta Diabetol. 2020, 57, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group; Kempen, J.H.; Altaweel, M.M.; Drye, L.T.; Holbrook, J.T.; Jabs, D.A.; Sugar, E.A.; Thorne, J.E. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: Fifty-four–month results of the Multicenter Uveitis Steroid Treatment (MUST) trial and follow-up study. Ophthalmology 2015, 122, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, A.; Chronopoulos, P.; Hattenbach, L.; Ashurov, A.; Schutz, J.S.; Pfeiffer, N.; Korb, C. Intravitreal fluocinolone acetonide implant for chronic postoperative cystoid macular edema—Two years results. Eur. J. Ophthalmol. 2022, 33, 1054–1060. [Google Scholar] [CrossRef]
- Deaner, J.D.; Mammo, D.; Gross, A.; Lee, T.; Sharma, S.; Srivastava, S.K.; Jaffe, G.J.; Grewal, D.S. 0.18 mg fluocinolone acetonide insert for the treatment of chronic postoperative pseudophakic cystoid macular edema. Retina 2023, 43, 897–904. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Cidlowski, J.A.; Csaky, K.G.; Ambati, J. Pharmacology of Corticosteroids for Diabetic Macular Edema. Investig. Opthalmology Vis. Sci. 2018, 59, 1–12. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Hafiz, G.; Shah, S.M.; Bloom, S.; Brown, D.M.; Busquets, M.; Ciulla, T.; Feiner, L.; Sabates, N.; Billman, K.; et al. Sustained Ocular Delivery of Fluocinolone Acetonide by an Intravitreal Insert. Ophthalmology 2010, 117, 1393–1399.e3. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Chen, S.; Boyer, D.; Ruiz-Moreno, J.; Garretson, B.; Gupta, A.; Hariprasad, S.M.; Bailey, C.; et al. Sustained Delivery Fluocinolone Acetonide Vitreous Inserts Provide Benefit for at Least 3 Years in Patients with Diabetic Macular Edema. Ophthalmology 2012, 119, 2125–2132. [Google Scholar] [CrossRef]
- Kane, F.E.; Green, K.E. Ocular Pharmacokinetics of Fluocinolone Acetonide Following Iluvien Implantation in the Vitreous Humor of Rabbits. J. Ocul. Pharmacol. Ther. 2015, 31, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Nguyen, Q.D.; Hafiz, G.; Bloom, S.; Brown, D.M.; Busquets, M.; Ciulla, T.; Feiner, L.; Sabates, N.; Billman, K.; et al. Aqueous Levels of Fluocinolone Acetonide after Administration of Fluocinolone Acetonide Inserts or Fluocinolone Acetonide Implants. Ophthalmology 2013, 120, 583–587. [Google Scholar] [CrossRef] [PubMed]
- E Mansour, S.; Browning, D.J.; Wong, K.; Flynn, H.W., Jr.; Bhavsar, A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef]
- Eaton, A.; Koh, S.S.; Jimenez, J.; Riemann, C.D. The USER Study: A Chart Review of Patients Receiving a 0.2 µg/day Fluocinolone Acetonide Implant for Diabetic Macular Edema. Ophthalmol. Ther. 2019, 8, 51–62. [Google Scholar] [CrossRef]
- Kodjikian, L.; Bandello, F.; de Smet, M.; Dot, C.; Zarranz-Ventura, J.; Loewenstein, A.; Sudhalkar, A.; Bilgic, A.; Cunha-Vaz, J.; Dirven, W.; et al. Fluocinolone acetonide implant in diabetic macular edema: International experts’ panel consensus guidelines and treatment algorithm. Eur. J. Ophthalmol. 2022, 32, 1890–1899. [Google Scholar] [CrossRef]
- Séjournet, L.; Kodjikian, L.; Elbany, S.; Allignet, B.; Agard, E.; Chaperon, M.; Billant, J.; Denis, P.; Mathis, T.; Burillon, C.; et al. Focal Photocoagulation as an Adjunctive Therapy to Reduce the Burden of Intravitreal Injections in Macula Edema Patients, the LyoMAC2 Study. Pharmaceutics 2023, 15, 308. [Google Scholar] [CrossRef]
- Bourhis, A.; Girmens, J.-F.; Boni, S.; Pecha, F.; Favard, C.; Sahel, J.-A.; Paques, M. Imaging of macroaneurysms occurring during retinal vein occlusion and diabetic retinopathy by indocyanine green angiography and high resolution optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Hennings, C.; Gillies, M.C.; Nguyen, V.; Campain, A.; Fraser-Bell, S. Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema. Cochrane Database Syst. Rev. 2018, 2018, CD011599. [Google Scholar] [CrossRef]
- Maturi, R.K.; Glassman, A.R.; Liu, D.; Beck, R.W.; Bhavsar, A.R.; Bressler, N.M.; Jampol, L.M.; Melia, M.; Punjabi, O.S.; Salehi-Had, H.; et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: A DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018, 136, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Baillif, S.; Creuzot-Garcher, C.; Delyfer, M.-N.; Matonti, F.; Weber, M.; Mathis, T. Real-World Efficacy and Safety of Fluocinolone Acetonide Implant for Diabetic Macular Edema: A Systematic Review. Pharmaceutics 2021, 13, 72. [Google Scholar] [CrossRef]
- Mathis, T.; Papegaey, M.; Ricard, C.; Rezkallah, A.; Matonti, F.; Sudhalkar, A.; Vartin, C.; Dot, C.; Kodjikian, L. Efficacy and Safety of Intravitreal Fluocinolone Acetonide Implant for Chronic Diabetic Macular Edema Previously Treated in Real-Life Practice: The REALFAc Study. Pharmaceutics 2022, 14, 723. [Google Scholar] [CrossRef]
- Singer, M.A.; Wykoff, C.C.; Grewal, D.S. Effects of Long-Term DME Control With 0.2 µg/Day Fluocinolone Acetonide Implant on Quality of Life: An Exploratory Analysis from the FAME Trial. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Putri, C.; Quhill, H.; Quhill, F. Evaluation of 0.2 µg/day fluocinolone acetonide (ILUVIEN) implant in a cohort of previously treated patients with diabetic macular oedema (DMO): A 36-month follow-up clinical case series. BMJ Open Ophthalmol. 2020, 5, e000484. [Google Scholar] [CrossRef]
- Singer, M.A.; Sheth, V.; Mansour, S.E.; Coughlin, B.; Gonzalez, V.H. Three-Year Safety and Efficacy of the 0.19-mg Fluocinolone Acetonide Intravitreal Implant for Diabetic Macular Edema: The PALADIN Study. Ophthalmology 2022, 129, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Dobler, E.; Mohammed, B.R.; Chavan, R.; Lip, P.L.; Mitra, A.; Mushtaq, B. Clinical efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: Five-year real-world results. Eye 2023, 37, 2310–2315. [Google Scholar] [CrossRef]
- Zobdeh, F.; ben Kraiem, A.; Attwood, M.M.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B.; Mwinyi, J. Pharmacological treatment of migraine: Drug classes, mechanisms of action, clinical trials and new treatments. Br. J. Pharmacol. 2021, 178, 4588–4607. [Google Scholar] [CrossRef]
- Wang, V.Y.; Kuo, B.L.; Chen, A.X.; Wang, K.; Greenlee, T.E.; Conti, T.F.; Singh, R.P. Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents. Eye 2022, 36, 1461–1467. [Google Scholar] [CrossRef]
- Starr, M.R.; Salabati, M.; Mahmoudzadeh, R.; Patel, L.G.; Ammar, M.J.; Hsu, J.; Garg, S.; Ho, A.C.; Kuriyan, A.E. Fluctuations in Central Subfield Thickness Associated with Worse Visual Outcomes in Patients with Diabetic Macular Edema in Clinical Trial Setting. Am. J. Ophthalmol. 2021, 232, 90–97. [Google Scholar] [CrossRef]
- Wu, S.; Lu, Q.; Wang, N.; Zhang, J.; Liu, Q.; Gao, M.; Chen, J.; Liu, W.; Xu, L. Cyclic stretch induced-retinal pigment epithelial cell apoptosis and cytokine changes. BMC Ophthalmol. 2017, 17, 208. [Google Scholar] [CrossRef]
- Križaj, D.; Ryskamp, D.A.; Tian, N.; Tezel, G.; Mitchell, C.H.; Slepak, V.Z.; Shestopalov, V.I. From Mechanosensitivity to Inflammatory Responses: New Players in the Pathology of Glaucoma. Curr. Eye Res. 2014, 39, 105–119. [Google Scholar] [CrossRef]
- Schechet, S.A.; Adams, O.E.; Eichenbaum, D.A.; Hariprasad, S.M. Macular thickness amplitude changes when switching from discontinuous to continuous therapy for diabetic macular oedema. BMJ Open Ophthalmol. 2019, 4, e000271. [Google Scholar] [CrossRef]
- Riemann, C.D.; Eaton, A.M.; Cutino, A. Reduction in Retinal Thickness Fluctuations after Treatment with Fluocinolone Acetonide Implant for DME: A Post-Hoc Analysis of the USER Study. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.S.; Singer, M.; MacCumber, M.; Cutino, A.; Kasper, J.; Coughlin, B.A.; Riemann, C.D. Long-Term Control of Retinal Thickness Variability and Vision Following the 0.19 mg Fluocinolone Acetonide Implant. J. Vitr. Dis. 2023, 7, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.E.; Habib, M.; Currie, C.J. Retinal thickness fluctuations in patients receiving fluocinolone acetonide implant for diabetic macular edema. Curr. Med. Res. Opin. 2020, 36, 959–965. [Google Scholar] [CrossRef]
- Rousseau, N.; Lebreton, O.; Masse, H.; Maucourant, Y.; Pipelart, V.; Clement, M.; Le Lez, M.-L.; Khanna, R.K.; Pepin, M.; Eude, Y.; et al. Fluocinolone Acetonide Implant Injected 1 Month after Dexamethasone Implant for Diabetic Macular Oedema: The ILUVI1MOIS Study. Ophthalmol. Ther. 2023, 12, 2781–2792. [Google Scholar] [CrossRef]
- Baillif, S.; Staccini, P.; Weber, M.; Delyfer, M.-N.; Le Mer, Y.; Gualino, V.; Collot, L.; Merite, P.-Y.; Creuzot-Garcher, C.; Kodjikian, L.; et al. Management of Patients with Diabetic Macular Edema Switched from Dexamethasone Intravitreal Implant to Fluocinolone Acetonide Intravitreal Implant. Pharmaceutics 2022, 14, 2391. [Google Scholar] [CrossRef]
- Khoramnia, R.; Peto, T.; Koch, F.; Taylor, S.R.; de Sousa, J.P.C.; Hill, L.; Bailey, C.; Chakravarthy, U. Safety and effectiveness of the fluocinolone acetonide intravitreal implant (ILUVIEN): 3-year results from the European IRISS registry study. Br. J. Ophthalmol. 2023, 107, 1502–1508. [Google Scholar] [CrossRef]
- Leite, J.; Ferreira, A.; Castro, C.; Coelho, J.; Borges, T.; Correia, N.; Pessoa, B. Retinal changes after fluocinolone acetonide implant (ILUVIEN®) for DME: SD-OCT imaging assessment using ESASO classification. Eur. J. Ophthalmol. 2024, 34, 233–244. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Zollet, P.; Capone, L.; Lattanzio, R.; Bandello, F. Persistent or Recurrent Diabetic Macular Edema after Fluocinolone Acetonide 0.19 mg Implant: Risk Factors and Management. Am. J. Ophthalmol. 2020, 215, 14–24. [Google Scholar] [CrossRef]
- Merrill, P.T.; Holekamp, N.; Roth, D.; Kasper, J.; Grigorian, R.; PALADIN Study Group. The 0.19-mg Fluocinolone Acetonide Intravitreal Implant Reduces Treatment Burden in Diabetic Macular Edema. Am. J. Ophthalmol. 2023, 248, 16–23. [Google Scholar] [CrossRef]
- Bailey, C.; Chakravarthy, U.; Lotery, A.; Menon, G.; Talks, J.; Medisoft Audit Group. Extended real-world experience with the ILUVIEN® (fluocinolone acetonide) implant in the United Kingdom: 3-year results from the Medisoft® audit study. Eye 2022, 36, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Pessoa, B.; Ruão, M.; Sousa, J.P.C.; Penas, S.; Silva, R.; Carneiro, Â.; Meireles, A. ILUVIEN® in diabetic macular edema that persists or recurs despite treatment: Results from the Retina.pt® RIVER audit. Eur. J. Ophthalmol. 2024, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Lebrize, S.; Arnould, L.; Bourredjem, A.; Busch, C.; Rehak, M.; Massin, P.; Barbosa-Breda, J.; Lupidi, M.; Mariotti, C.; Hamza, M.; et al. Intraocular Pressure Changes after Intravitreal Fluocinolone Acetonide Implant: Results from Four European Countries. Ophthalmol. Ther. 2022, 11, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Dot, C.; Poli, M.; Aptel, F.; Labbe, A.; Kodjikian, L.; Baillif, S.; Bodaghi, B.; Denis, P. Intraocular pressure elevation and intravitreal steroid implant injection: State of the art in 2023. Recommendations of the French Glaucoma Society and French Ophthalmology Society [French version]. J. Fr. D’ophtalmologie 2023, 46, 803–810. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Luo, C.; Almeida, D.R.; Cutino, A.; Coughlin, B.; Kasper, J.; Kiernan, D.F. Better baseline vision leads to better outcomes after the 0.19-mg fluocinolone acetonide intravitreal implant in diabetic macular edema. Retina 2023, 43, 1301–1307. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejournet, L.; Mathis, T.; Vermot-Desroches, V.; Serra, R.; Fenniri, I.; Denis, P.; Kodjikian, L. Efficacy and Safety of Fluocinolone Acetonide Implant in Diabetic Macular Edema: Practical Guidelines from Reference Center. Pharmaceutics 2024, 16, 1183. https://doi.org/10.3390/pharmaceutics16091183
Sejournet L, Mathis T, Vermot-Desroches V, Serra R, Fenniri I, Denis P, Kodjikian L. Efficacy and Safety of Fluocinolone Acetonide Implant in Diabetic Macular Edema: Practical Guidelines from Reference Center. Pharmaceutics. 2024; 16(9):1183. https://doi.org/10.3390/pharmaceutics16091183
Chicago/Turabian StyleSejournet, Lucas, Thibaud Mathis, Victor Vermot-Desroches, Rita Serra, Ines Fenniri, Philippe Denis, and Laurent Kodjikian. 2024. "Efficacy and Safety of Fluocinolone Acetonide Implant in Diabetic Macular Edema: Practical Guidelines from Reference Center" Pharmaceutics 16, no. 9: 1183. https://doi.org/10.3390/pharmaceutics16091183
APA StyleSejournet, L., Mathis, T., Vermot-Desroches, V., Serra, R., Fenniri, I., Denis, P., & Kodjikian, L. (2024). Efficacy and Safety of Fluocinolone Acetonide Implant in Diabetic Macular Edema: Practical Guidelines from Reference Center. Pharmaceutics, 16(9), 1183. https://doi.org/10.3390/pharmaceutics16091183







