Development of Dual-Targeted Mixed Micelles Loaded with Celastrol and Evaluation on Triple-Negative Breast Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
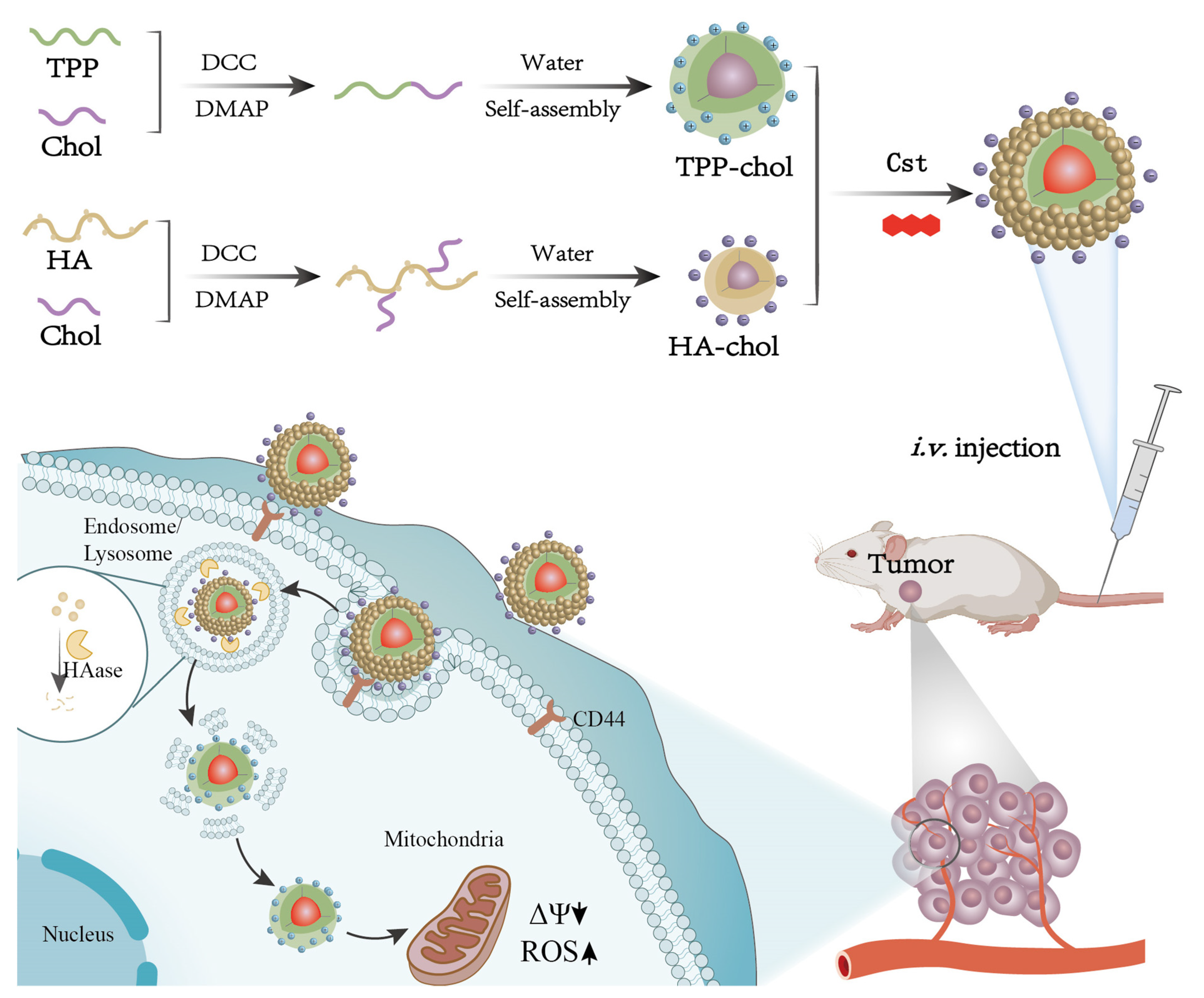
2.2. Synthesis of HA-Chol
2.3. Preparation of Dual-Targeting Mixed Micelles Loaded with Cst (Cst@HA/TPP-M)
2.4. Characterization of Cst@HA/TPP-M
2.5. Stability of the Micelles
2.6. HA Degradation of Cst@HA/TPP-M
2.7. In Vitro Drug Release of the Micelles
2.8. Hemolysis Test
2.9. In Vitro Cellular Uptake
2.10. Endo-Lysosomal Escape and Mitochondrial Localization Study
2.11. ROS Assay
2.12. Mitochondrial Membrane Potential Study
2.13. Cell Apoptosis Assay
2.14. Cell Viability Assay
2.15. In Vivo Imaging Investigation
2.16. In Vivo Antitumor Efficacy
2.17. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of HA-Chol
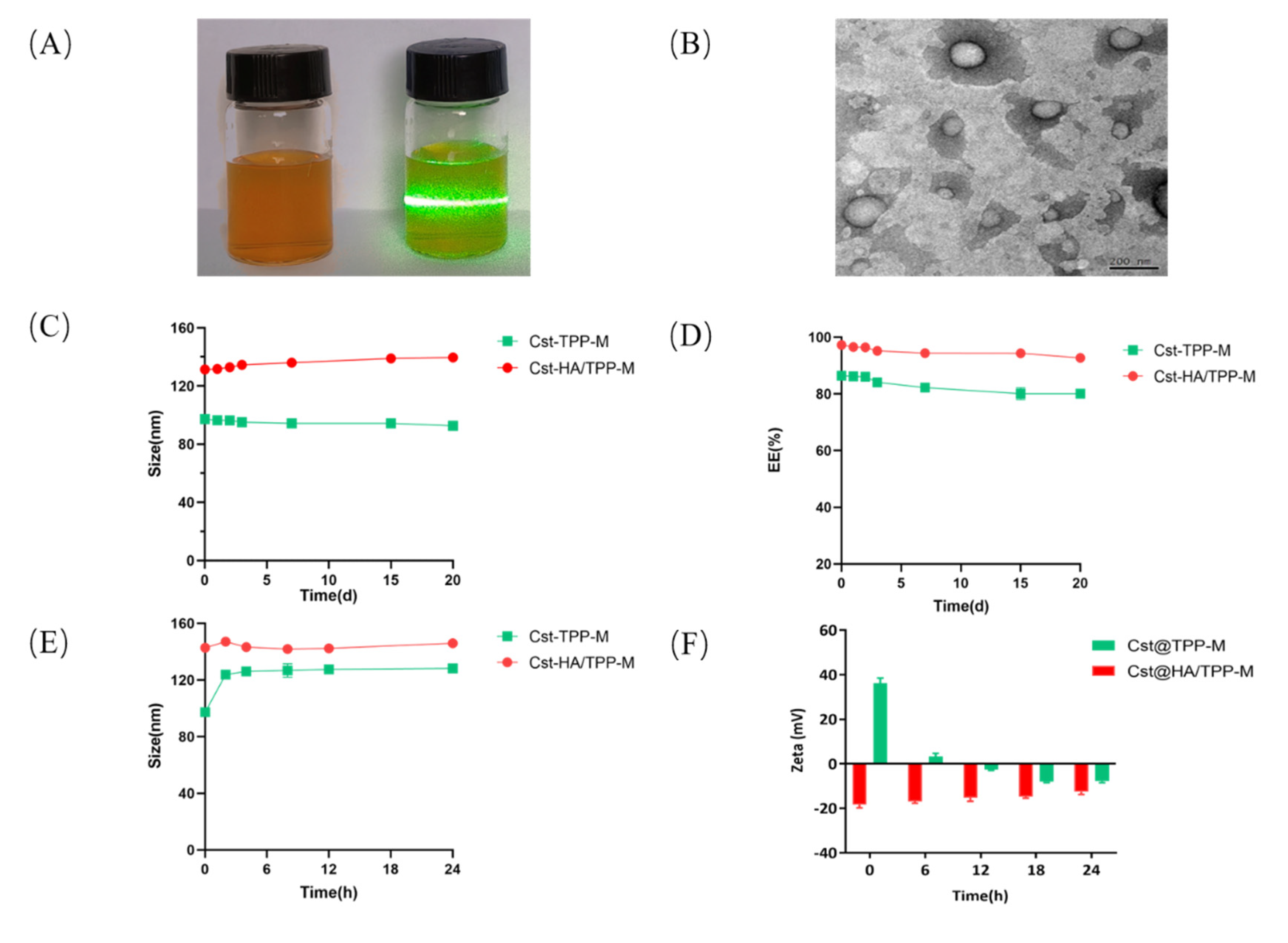
3.2. Preparation and Characterization of Mixed Micelles
3.3. Stability Studies
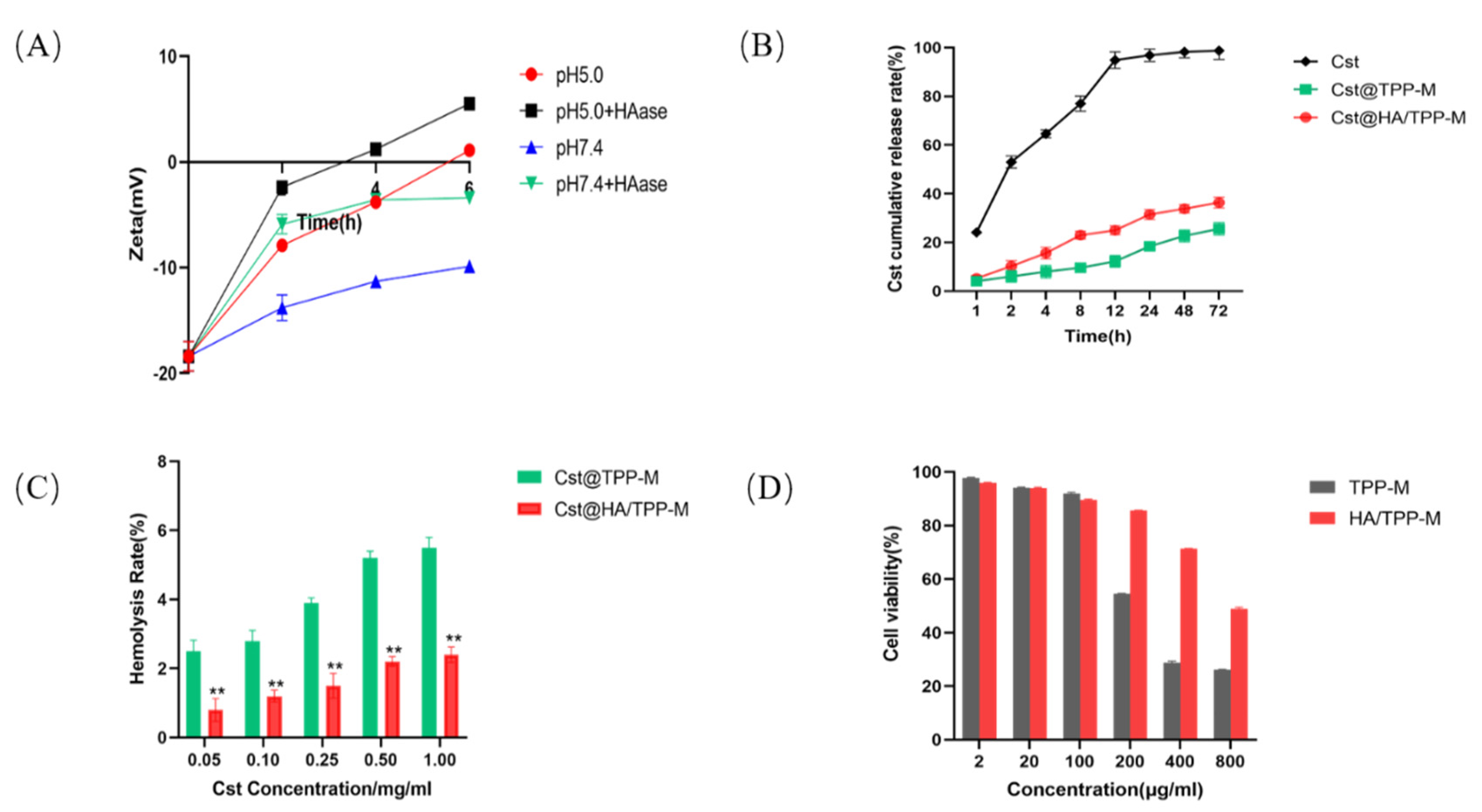
3.4. Degradation of HA
3.5. In Vitro Drug Release
3.6. Safety Evaluation
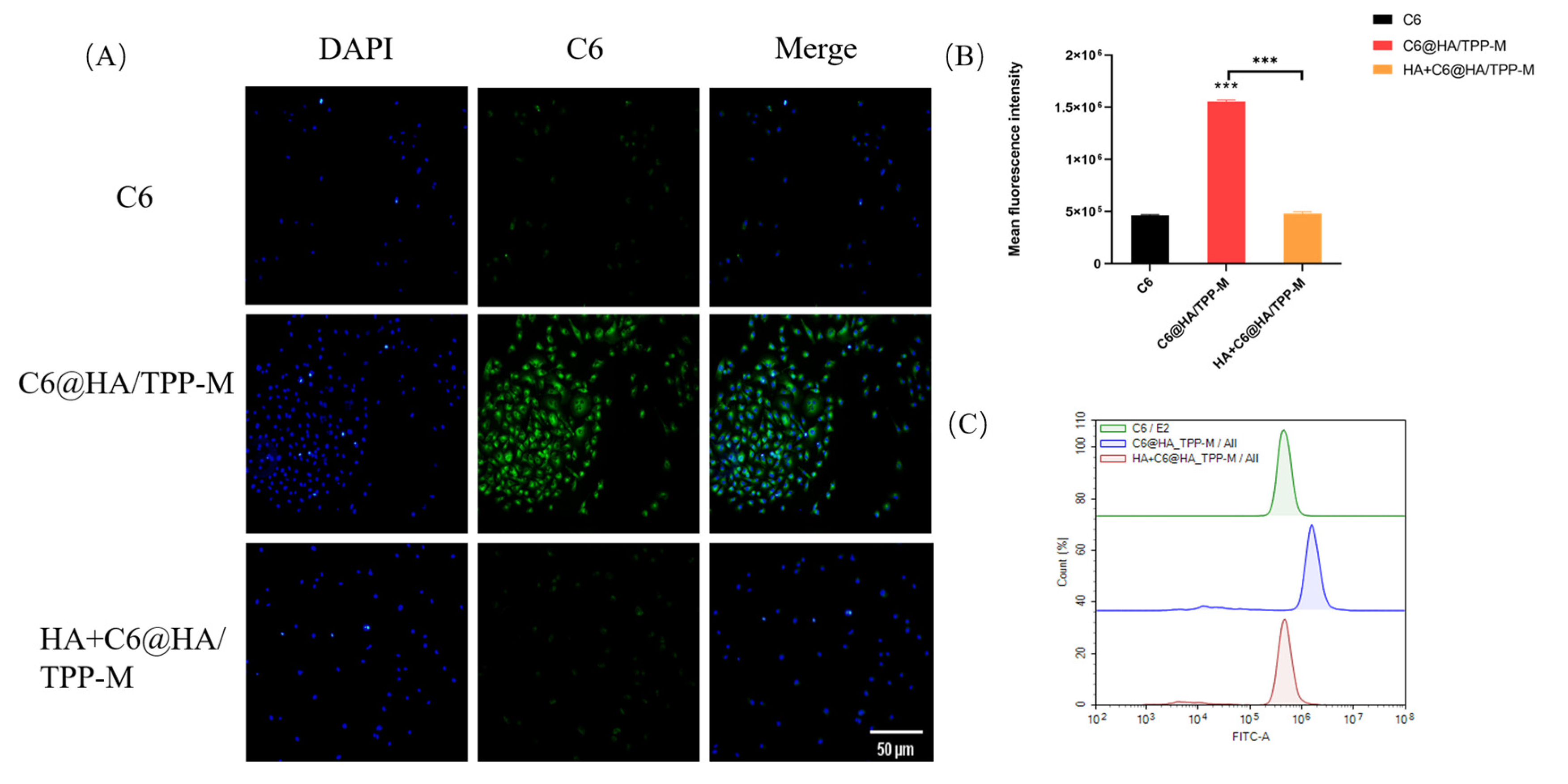
3.7. Cellular Uptake
3.8. Endo-Lysosomal Escape and Mitochondrial Localization
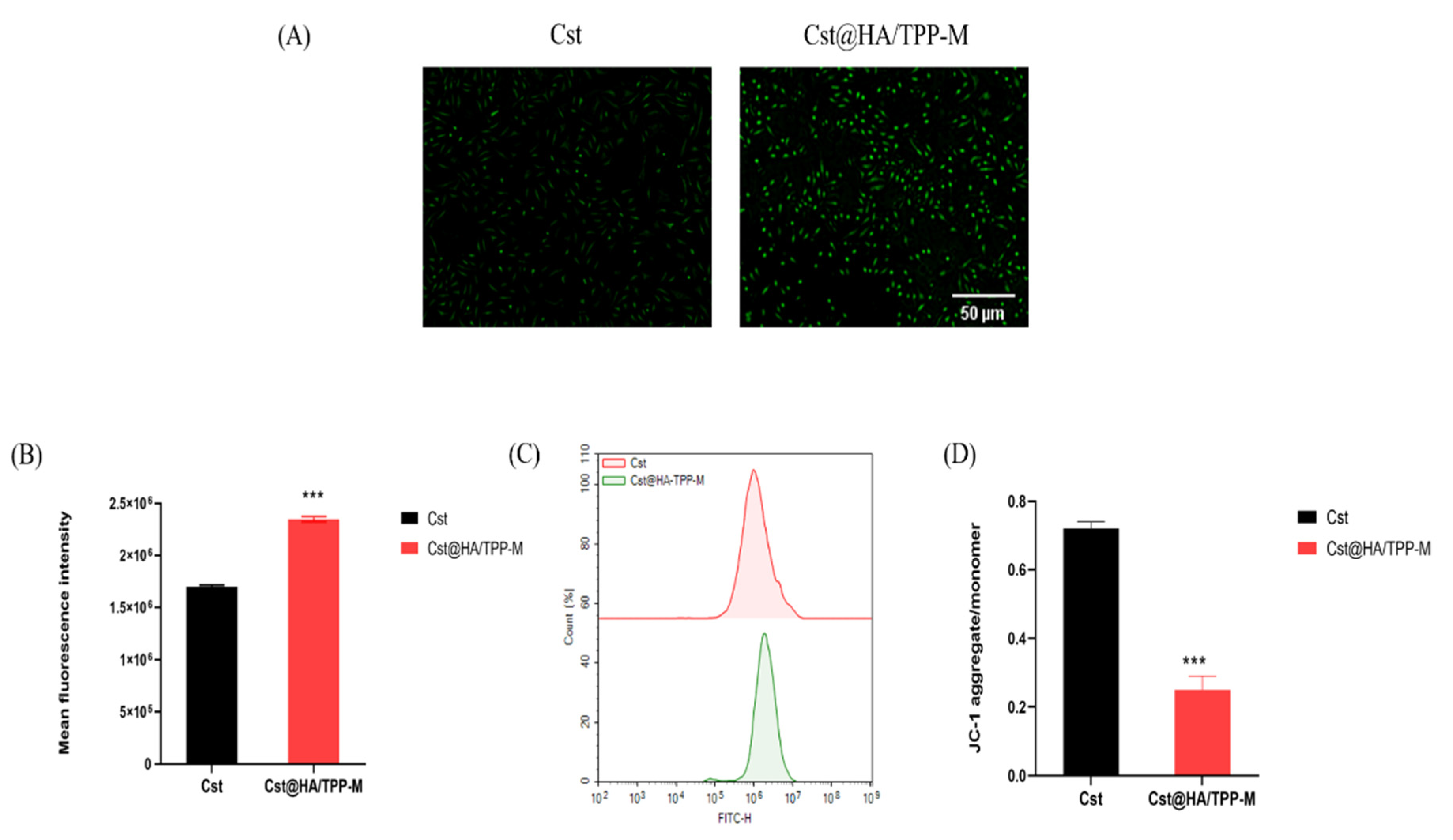
3.9. ROS Level
3.10. Mitochondrial Membrane Potential
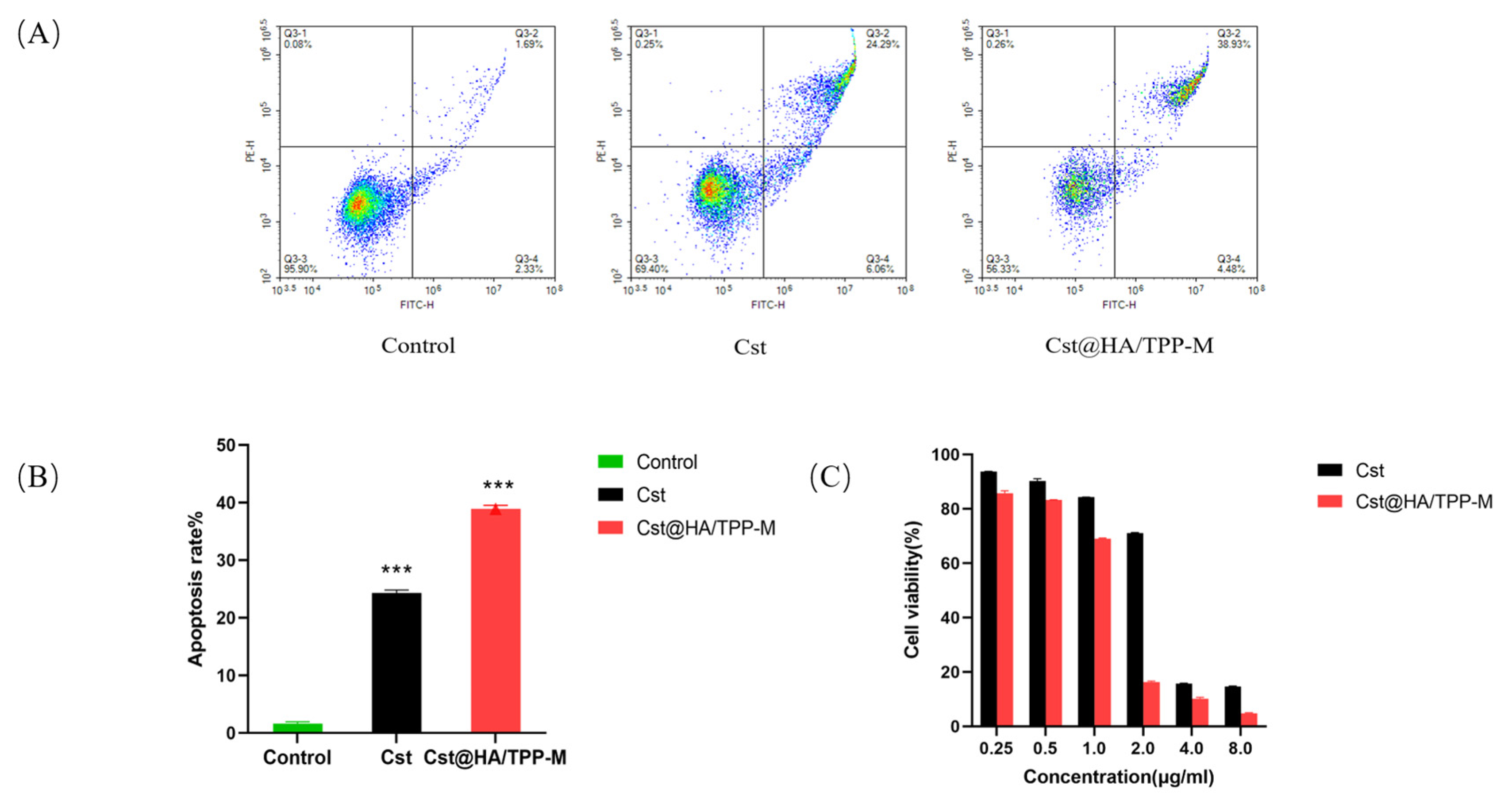
3.11. Cell Apoptosis
3.12. Cell Viability
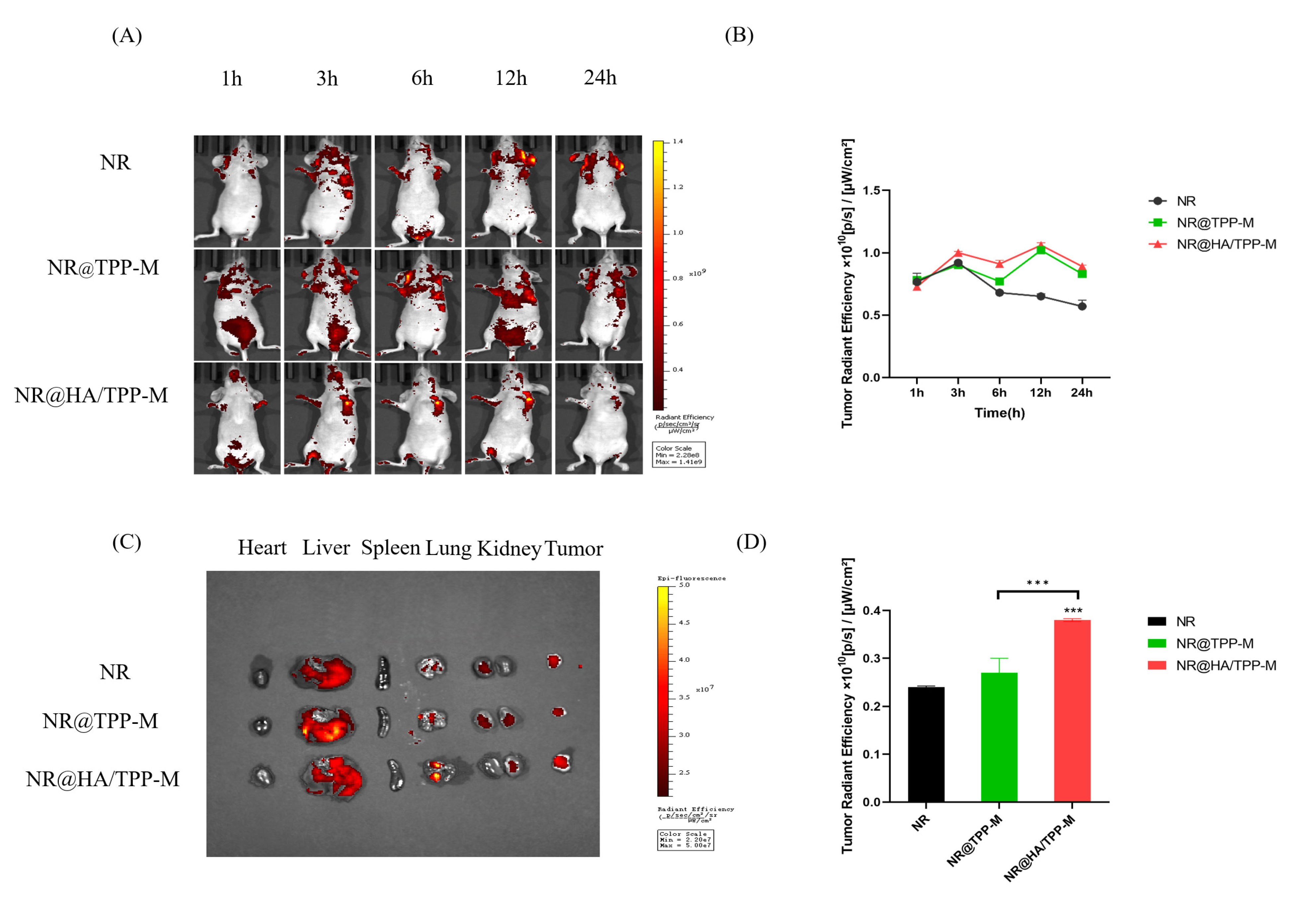
3.13. In Vivo Imaging
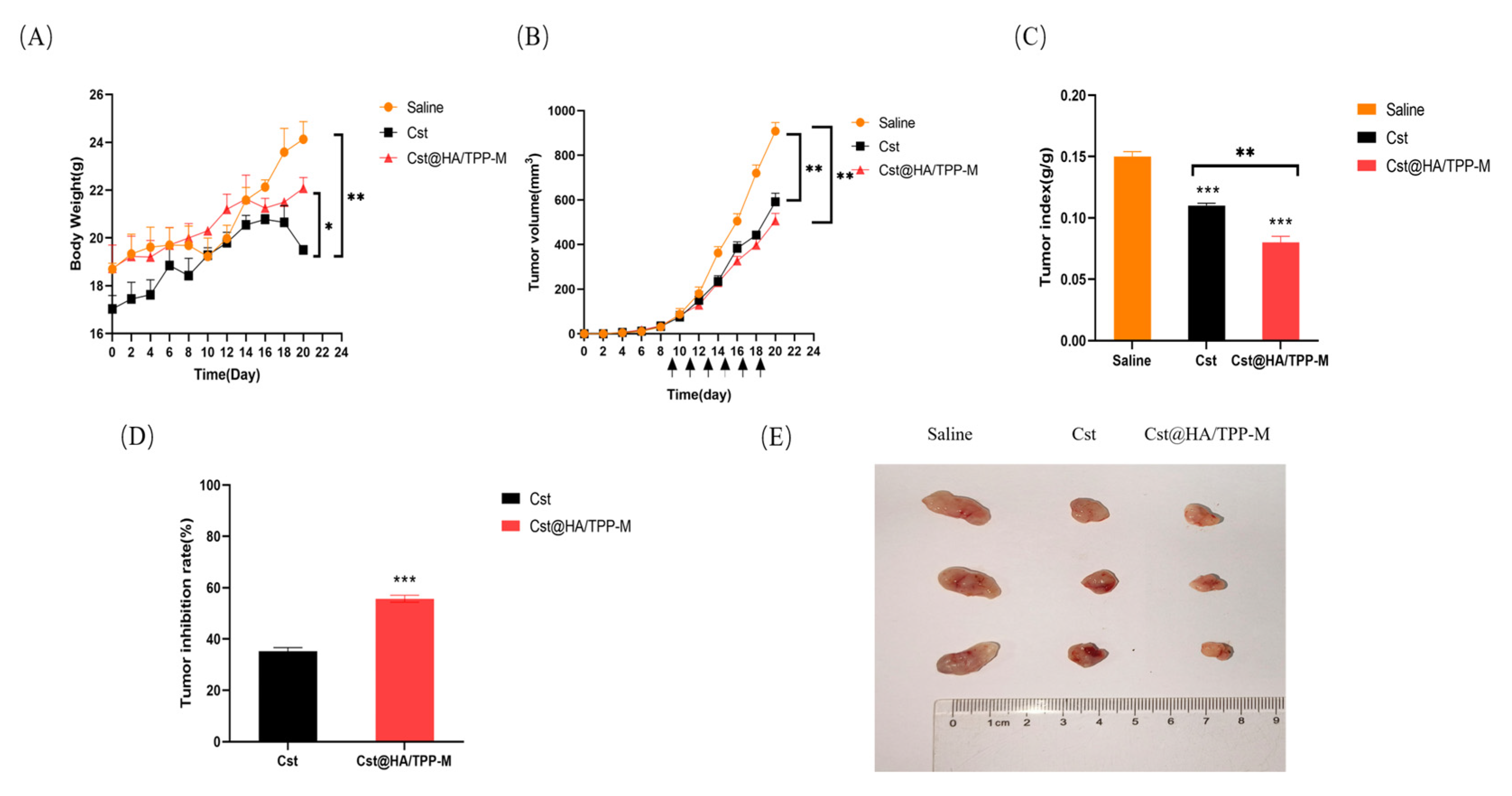
3.14. In Vivo Pharmacodynamics Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, H.Y.; Chu, P.Y. Advances in Understanding Mitochondrial MicroRNAs (mitomiRs) on the Pathogenesis of Triple-Negative Breast Cancer (TNBC). Oxidative Med. Cell. Longev. 2021, 2021, 5517777. [Google Scholar] [CrossRef] [PubMed]
- Won, K.A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jeengar, M.K.; Reddy, V.S.; Reddy, G.B.; Naidu, V.G. Anticancer effect of celastrol on human triple negative breast cancer: Possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp. Mol. Pathol. 2015, 98, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Feng, D.; Lv, J.; Cui, X.; Wang, Y.; Wang, Q.; Zhang, L. Application Prospects of Triphenylphosphine-Based Mitochondria-Targeted Cancer Therapy. Cancers 2023, 15, 666. [Google Scholar] [CrossRef]
- Pawar, A.; Korake, S.; Pawar, A.; Kamble, R. Delocalized Lipophilic Cation Triphenyl Phosphonium: Promising Molecule for Mitochondria Targeting. Curr. Drug Deliv. 2023, 20, 1217–1223. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Li, W.Q.; Wu, J.Y.; Xiang, D.X.; Luo, S.L.; Hu, X.B.; Tang, T.T.; Sun, T.L.; Liu, X.Y. Micelles Loaded With Puerarin And Modified With Triphenylphosphonium Cation Possess Mitochondrial Targeting And Demonstrate Enhanced Protective Effect Against Isoprenaline-Induced H9c2 Cells Apoptosis. Int. J. Nanomed. 2019, 14, 8345–8360. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, D.; Yan, N.; Li, J.; Zhang, H.; Liu, M.; Tang, X.; Liu, X.; Deng, Y.; Song, Y.; et al. Evasion of the accelerated blood clearance phenomenon by branched PEG lipid derivative coating of nanoemulsions. Int. J. Pharm. 2022, 612, 121365. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, F.; Kang, K.; Xiao, L.; Bi, A.; Chen, Y.; Zhou, Q.; Feng, X.; Chen, Z.; Yin, H.; et al. Precise visualization and ROS-dependent photodynamic therapy of colorectal cancer with a novel mitochondrial viscosity photosensitive fluorescent probe. Biomater. Res. 2023, 27, 112. [Google Scholar] [CrossRef]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting mitochondria for cancer photodynamic therapy. Photodiagn. Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.; Han, H.W.; Lim, Y.R.; Manivasagan, P.; Jang, E.S. Triphenylphosphonium-Functionalized Gold Nanorod/Zinc Oxide Core-Shell Nanocomposites for Mitochondrial-Targeted Phototherapy. Pharmaceutics 2024, 16, 284. [Google Scholar] [CrossRef]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A potential carrier for anti-tumor targeted delivery-hyaluronic acid nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef]
- Li, W.; Yi, X.; Liu, X.; Zhang, Z.; Fu, Y.; Gong, T. Hyaluronic acid ion-pairing nanoparticles for targeted tumor therapy. J. Control. Release 2016, 225, 170–182. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, S.; Yang, X.; Lei, Y.; Chang, M.; Hu, J.; Meng, Y.; Zheng, G.; Chen, X. The development and evaluation of hyaluronic acid coated mitochondrial targeting liposomes for celastrol delivery. Drug Deliv. 2023, 30, 2162156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, K.; Tian, R.; Lu, C. Substrate-Assisted Visualization of Surfactant Micelles via Transmission Electron Microscopy. Front. Chem. 2019, 7, 242. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, L.; Liu, J.; Huang, G. Construction and evaluation in vitro and in vivo of tedizolid phosphate loaded cationic liposomes. J. Liposome Res. 2018, 28, 322–330. [Google Scholar] [CrossRef]
- Bawadud, R.S.; Alkhatib, M.H. Growth and invasion inhibition of T47D ductal carcinoma cells by the association of docetaxel with a bioactive agent in neutral nanosuspension. Bioimpacts 2023, 13, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, Y.; Hao, L.; Yang, Y.; Gao, Y.; Zhang, N.; Zhang, X.; Song, Y. CD44/Folate Dual Targeting Receptor Reductive Response PLGA-Based Micelles for Cancer Therapy. Front. Pharmacol. 2022, 13, 829590. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Kang, R.; Song, M.; Fang, Z.; Liu, K. Nano-composite hydrogels of Cu-Apa micelles for anti-vasculogenic mimicry. J. Drug Target. 2023, 31, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gao, Y.; Yin, S.; Yao, Y.; Yu, H.; Wang, G.; Li, J. Co-delivery of paclitaxel and STAT3 siRNA by a multifunctional nanocomplex for targeted treatment of metastatic breast cancer. Acta Biomater. 2021, 134, 649–663. [Google Scholar] [CrossRef]
- Lenormand, H.; Amar-Bacoup, F.; Vincent, J.C. Reaction-complexation coupling between an enzyme and its polyelectrolytic substrate: Determination of the dissociation constant of the hyaluronidase-hyaluronan complex from the hyaluronidase substrate-dependence. Biophys. Chem. 2013, 175–176, 63–70. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Li, G.; Yin, Z.; Fu, B.M. Transcellular Model for Neutral and Charged Nanoparticles Across an In Vitro Blood-Brain Barrier. Cardiovasc. Eng. Technol. 2020, 11, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Zhang, J.P.; Hu, C.Q. A Rapid Assessment Model for Liver Toxicity of Macrolides and an Integrative Evaluation for Azithromycin Impurities. Front. Pharmacol. 2022, 13, 860702. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Reconstituted Lipid Nanoparticles from Cells/Tissues for Drug Delivery in Cancer. Mol. Pharm. 2023, 20, 2891–2898. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, Z.; Lan, T.; Fu, C.; Cheng, P. CD44 and its implication in neoplastic diseases. MedComm 2024, 5, e554. [Google Scholar] [CrossRef]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Geng, N.; Zhu, Y.; Zhang, S.; Liu, Y.; Liu, J. Mitophagy is involved in chromium (VI)-induced mitochondria damage in DF-1 cells. Ecotoxicol. Environ. Saf. 2020, 194, 110414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.; Hu, J.; Qiu, Z.; Yuan, M.; Zheng, G. Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice. Mol. Pharm. 2020, 17, 738–747. [Google Scholar] [CrossRef] [PubMed]

| Micelles | Size in Diameter/nm | Polydispersity Index | Zeta Potential/mV | Encapsulation Efficiency/% | Drug Loading/% |
|---|---|---|---|---|---|
| Cst@TPP-M | 94.6 ± 2.2 | 0.229 ± 0.03 | 38.6 ± 0.6 | 86.42 ± 4.15 | 20.9 ± 0.40 |
| Cst@HA/TPP-M | 131.4 ± 2.4 | 0.156 ± 0.01 | −19.7 ± 0.6 | 97.21 ± 1.27 | 22.8 ± 0.20 |
| C6@HA/TPP-M | 129.8 ± 2.6 | 0.147 ± 0.01 | −20.2 ± 0.6 | 92.37 ± 0.98 | -- |
| NR@HA/TPP-M | 133.5 ± 2.1 | 0.123 ± 0.02 | −18.9 ± 0.6 | 90.65 ± 0.98 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Xiao, S.; Li, X.; Tao, R.; Yang, Z.; Gao, Z.; Hu, J.; Meng, Y.; Zheng, G.; Chen, X. Development of Dual-Targeted Mixed Micelles Loaded with Celastrol and Evaluation on Triple-Negative Breast Cancer Therapy. Pharmaceutics 2024, 16, 1174. https://doi.org/10.3390/pharmaceutics16091174
Huang S, Xiao S, Li X, Tao R, Yang Z, Gao Z, Hu J, Meng Y, Zheng G, Chen X. Development of Dual-Targeted Mixed Micelles Loaded with Celastrol and Evaluation on Triple-Negative Breast Cancer Therapy. Pharmaceutics. 2024; 16(9):1174. https://doi.org/10.3390/pharmaceutics16091174
Chicago/Turabian StyleHuang, Siying, Simeng Xiao, Xuehao Li, Ranran Tao, Zhangwei Yang, Ziwei Gao, Junjie Hu, Yan Meng, Guohua Zheng, and Xinyan Chen. 2024. "Development of Dual-Targeted Mixed Micelles Loaded with Celastrol and Evaluation on Triple-Negative Breast Cancer Therapy" Pharmaceutics 16, no. 9: 1174. https://doi.org/10.3390/pharmaceutics16091174
APA StyleHuang, S., Xiao, S., Li, X., Tao, R., Yang, Z., Gao, Z., Hu, J., Meng, Y., Zheng, G., & Chen, X. (2024). Development of Dual-Targeted Mixed Micelles Loaded with Celastrol and Evaluation on Triple-Negative Breast Cancer Therapy. Pharmaceutics, 16(9), 1174. https://doi.org/10.3390/pharmaceutics16091174







