1. Introduction
Pregnant women affected by epilepsy and HIV often need to take medication. The pharmacokinetics of antiretroviral and anticonvulsant drugs can significantly impact physiological changes during pregnancy and drug-drug interactions (DDIs) [
1,
2]. Adequate exposure to antiretroviral drugs (ARVs) is crucial for achieving and maintaining virologic suppression in patients living with HIV [
3,
4]. However, pregnancy introduces complex changes in drug metabolism, absorption, and elimination, necessitating careful consideration when selecting ARV regimens [
5,
6]. ARVs such as protease inhibitors, non-nucleoside reverse transcriptase inhibitors, and the integrase strand inhibitor elvitegravir are extensively metabolized by the cytochrome P450 (CYP450) enzyme system, leading to clinically relevant DDIs [
7,
8]. For instance, CYP induction by rifampicin decreases ARV exposure [
9], while CYP3A4 inhibition by ritonavir or cobicistat increases it [
10,
11,
12]. These interactions extend beyond CYP metabolism to involve non-CYP pathways, drug transporters, and pharmacokinetic processes.
Additionally, pregnancy alters drug metabolism through changes in gastric pH, intestinal motility, plasma protein concentrations, hepatic blood flow, metabolic enzyme activity, glomerular filtration rate, and renal blood flow [
7,
13]. These physiological changes can modify ARV exposure, rendering it challenging to predict pharmacokinetic outcomes in pregnant women. Consequently, dosing recommendations and contraindications for ARVs are typically based on studies excluding pregnant women, potentially leading to underestimations of DDI significance in this population [
14]. Also, individuals with HIV often have additional comorbidities such as tuberculosis, epilepsy, and other conditions [
9,
15]. In particular, HIV infection combined with epilepsy or bipolar disorder requires careful management. Current guidelines recommend caution when combining antiretrovirals with anticonvulsants to avoid adverse interactions and ensure effective treatment [
16]. Individuals with epilepsy, particularly older adults and those with HIV, have a higher prevalence of comorbid conditions due to both the pathogenic causes of epilepsy and the toxicities of antiepileptic drugs, with HIV-infected patients being especially prone to seizures from various mechanisms and experiencing premature aging-related comorbidities [
17,
18,
19]. The guidelines from the American Academy of Neurology (AAN) and the International League Against Epilepsy (ILAE) focus on selecting antiepileptic drugs (AEDs) for individuals with HIV/AIDS, addressing drug interactions with antiretroviral agents (ARVs). Up to 55% of HIV/AIDS patients on ARVs may need AEDs, as seizure disorders are common [
16]. Specific interactions include adjustments in lopinavir/ritonavir dosage with phenytoin [
20], zidovudine with valproic acid [
21], and lamotrigine (LTG) with ritonavir/atazanavir [
22]. The guidelines recommend avoiding enzyme-inducing AEDs with protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs) to prevent reduced ARV efficacy [
23,
24]. Monitoring through pharmacokinetic assessments is crucial.
Lamotrigine (LTG) and efavirenz (EFV), commonly used for the treatment of epilepsy and HIV, respectively, can suffer altered pharmacokinetics due to increased estrogen levels and genetic polymorphisms, necessitating careful consideration of dose adjustments [
25,
26]. LTG levels can drop significantly during pregnancy, primarily due to enhanced clearance mediated by increased glucuronidation [
27,
28]. Several studies have demonstrated that this reduction, particularly during the second and third trimesters, often requires regular monitoring and dosage adjustments to maintain therapeutic levels [
29]. The rapid return of LTG levels to pre-pregnancy concentrations further underscores the need for dynamic and individualized dosing strategies [
28,
30]. Efavirenz (EFV) is a widely used non-nucleoside reverse transcriptase inhibitor for HIV treatment, particularly in low- and middle-income countries. While historically, there were concerns about its teratogenic potential based on animal studies and isolated case reports of neural tube defects, more recent data have alleviated these concerns. Large-scale studies and meta-analyses have demonstrated that the risk of major birth defects with EFV exposure during pregnancy is not significantly higher than in the general population. For instance, a systematic review found no increased risk of birth abnormalities with EFV exposure during the first trimester, and the overall incidence of birth defects was comparable to the general population [
5]. Genetic polymorphisms influence the pharmacokinetics of EFV during pregnancy in CYP2B6, the enzyme responsible for its metabolism. These polymorphisms can significantly affect drug levels, complicating management during pregnancy. Despite this complexity, there is no consensus on the need for routine dose adjustments of EFV during pregnancy [
31,
32], highlighting the need for further research and careful therapeutic monitoring in this population [
26,
33].
Further research and in silico studies are needed to understand better the pharmacokinetics and interactions of these drugs during pregnancy, ensuring safe and effective treatment for pregnant women with epilepsy and HIV. Drug-drug interaction studies indicate that EFV induces UGT1A4 and, therefore, could decrease LTG exposure if given combined. The metabolism of LTG is facilitated by UDP-glucuronosyltransferases, primarily UGT2B7, with transporters SLC22A1 and ABCB1 also playing significant roles. Genetic variations in UGT1A4, UGT2B7, ABCB1, and SLC22A1 can affect the production and activity of these proteins [
34]. Evidence shows that UGT1A4 is up-regulated by 17β-estradiol (E2) through ERα and Sp1, suggesting that elevated E2 levels during pregnancy increase hepatic UGT1A4 expression, altering the metabolism of its substrate drugs [
35]. The CYP primarily metabolizes EFV. However, the glucuronidation of EFV metabolites, such as 8-hydroxyEFV (8-OHEFV) and 8,14-dihydroxyEFV (8,14-diOHEFV), is also carried out by multiple UGT isoforms, including UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A9, UGT1A10, and UGT2B7 [
36]. In vitro studies have shown that EFV can be directly glucuronidase to EFV-N-glucuronide by UGT2B7, although this is a minor pathway following the first dose of EFV [
37]. While the production of 7-hydroxy-EFV-glucuronide, 8-hydroxy-EFV-glucuronide, and 8,14-dihydroxy-EFV-glucuronide is more consistent, the generation rate of EFV-N-glucuronide varies greatly throughout human micro-some samples [
36]. EFV can potentially inhibit the glucuronidation of drugs catalyzed by UGT1A4 and/or UGT1A9. Therefore, potential pharmacokinetic drug interactions due to the inhibition of UGT1A4 and UGT1A9 should be examined in vivo to assess the clinical relevance of the inhibitory interaction of EFV with UGT1A4- and UGT1A9-substrate drugs [
38]. LTG has been found to significantly benefit patients with NRTI-induced neuropathy, a condition that can occur in patients taking certain antiretroviral medications [
39]. The antiretroviral drug most frequently linked to central nervous system toxicity, resulting in sleeplessness, agitation, and vivid nightmares, is the NNRTI. Recent research indicates that individuals with different cytochrome P450 2B6 alleles may be more susceptible to experiencing these negative effects [
40,
41,
42].
Both LTG and EFV fall into the category of narrow therapeutic index (NTI) drugs [
43,
44]. The therapeutic range for LTG is relatively small, and small changes in drug levels can lead to significant changes in therapeutic effects and risk of adverse effects. Due to its narrow therapeutic index and the potential for severe side effects, if levels become too high or too low, Therapeutic Drug Monitoring (TDM) is often recommended for LTG [
45]. The EFV therapeutic window is relatively narrow, and variations in plasma levels can significantly impact both efficacy and toxicity. TDM is less commonly performed for EFV than LTG, but it is still important in certain clinical situations [
43,
46,
47]. Using a virtual approach instead of standard TDM for adjusting anticonvulsant and antiviral drug dosages during pregnancy can offer several significant advantages. A virtual approach can utilize physiologically based pharmacokinetic (PBPK) models to simulate how drugs are metabolized in pregnant women, considering individual variations in physiology and biochemistry. These models can predict optimal drug dosages based on a patient’s specific characteristics (e.g., age, weight, stage of pregnancy), leading to more tailored and potentially more effective treatment plans [
48,
49,
50,
51]. Unlike standard TDM, which requires blood samples at specific intervals, virtual approaches can continuously monitor drug levels and predict the need for dosage adjustments without invasive procedures. Changes in drug metabolism due to physiological changes in pregnancy can be accounted for in real time, providing immediate recommendations for dosage adjustments. This minimizes the need for frequent blood tests and lab analyses. Virtual approaches can be more cost-effective in the long run. Virtual models can dynamically adjust to the rapid physiological changes that occur during pregnancy, such as increased blood volume and altered enzyme activity, ensuring drug dosages remain appropriate throughout the different stages of pregnancy. These approaches can lead to better health outcomes for both the mother and the fetus, making them a promising complement to traditional methods.
Moreover, given the scarcity of clinical data on DDIs in pregnant women, particularly involving UGT substrate drugs like LTG and EFV, in silico modeling offers a valuable approach to predicting pharmacokinetic changes and potential interactions. This study aimed to model the pharmacokinetics of LTG and EFV in pregnant women using a physiologically based approach to understand better and manage these interactions (
Figure 1) and to understand the difference in comparison with non-pregnant individuals. In silico research can bridge the gap in clinical data by simulating drug metabolism and interaction scenarios, helping to predict changes in drug exposure and efficacy during pregnancy. Understanding these dynamics is crucial for ensuring the safe and effective use of medications in pregnant women living with HIV and other conditions requiring anticonvulsant therapy. This provides insights into the pharmacokinetic profiles of LTG and EFV in pregnant women, contributing to improved clinical management and patient outcomes. By elucidating these dynamics, PBPK models can inform clinical decision-making, ensuring that pregnant women receive safe and effective treatment for epilepsy and HIV. This approach aims to enhance maternal and fetal health outcomes, balancing the mother’s therapeutic needs with the developing fetus’s safety.
4. Discussion
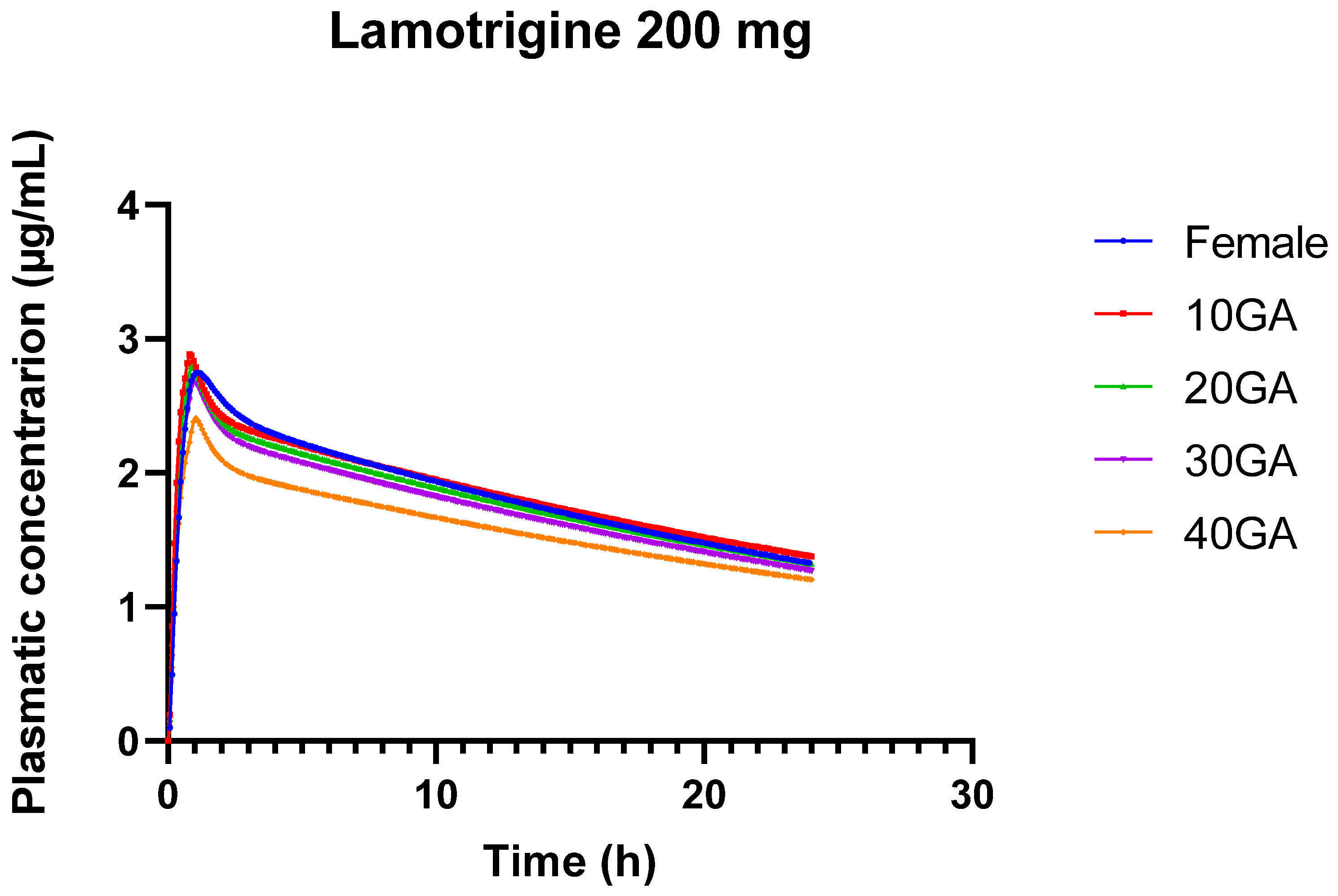
4.1. LTG Model for Pregnant and Non-Pregnant Individuals: Clinical Applicability
Modeling LTG presents significant challenges due to its complex absorption profile, variable bioavailability, and the influence of physiological factors such as gastric transit time, pH levels, and enzyme activity [
53,
54]. These factors are particularly difficult to predict accurately in PK models, especially during pregnancy, when physiological changes further complicate the drug’s behavior in the body. During pregnancy, changes in blood volume, enzyme activity (particularly UGT1A4, which metabolizes LTG), and renal clearance add further complexity. The fact that our model could reasonably predict AUC and Cmax suggests it adequately incorporates these pregnancy-related changes, even though Tmax remains challenging to predict. The difficulty in obtaining reliable clinical data, especially concentration-time profiles (Cp) across different gestational ages, further complicates model validation. This limitation impacts the precision of our model predictions.
Despite these challenges, our model provides valuable insights into LTG´s pharmacokinetics across different stages of pregnancy. The reasonable accuracy in predicting AUC and Cmax is particularly important for dose adjustments, as these parameters are closely related to therapeutic efficacy and safety. The discrepancies in Tmax prediction, while notable, are less likely to impact clinical decision-making, as Tmax is generally less critical for drugs like LTG, where maintaining consistent exposure is more important than the timing of peak concentration. The model’s success in reasonably predicting AUC and Cmax suggests that it is robust enough to inform clinical dosing strategies, especially in the context of pregnancy where physiological changes significantly impact drug pharmacokinetics. Further refinement of the model and acquisition of more comprehensive clinical data could enhance its predictive accuracy.
Pregnancy induces significant physiological changes, such as increased renal blood flow and altered metabolism, which can affect drug clearance and distribution. The rapid decline in LTG serum concentrations was primarily due to increased renal blood flow, while later changes were influenced by estradiol-induced glucuronidation [
29,
35]. These dynamic changes over time make it difficult to create a stable model that accurately reflects the pharmacokinetics throughout pregnancy. Our study was conducted exclusively using in silico data, relying on computational models and existing literature to simulate PK parameters. While this approach offers significant advantages, such as the ability to rapidly generate predictions and explore a wide range of scenarios, it also comes with limitations. One key limitation is that we were unable to perform in vitro experiments to directly determine Vmax and Km values for UGT enzymes. As a result, the model adjustments were based solely on available literature data, which may not fully capture the nuances of enzyme activity under various physiological conditions. Additionally, the sample size for certain parameters within the study was relatively small, which could affect the robustness of our statistical analyses and limit the generalizability of our findings. The sample size was constrained by the existing literature, as no new clinical study was initiated to gather more controlled and comprehensive data. Small sample sizes can lead to overfitting in models, where the model may perform well on the data used to create it but may not generalize effectively to a broader population. This can also result in an incomplete representation of variability across different individuals, potentially overlooking rare or outlier responses. The current simulations may not fully capture these dynamics due to limitations in the available data, particularly regarding estrogen conditions and genetic polymorphisms affecting drug metabolism; we can visualize reduced plasma concentrations of LTG. Pregnancy induces numerous physiological changes, such as increased blood volume, enhanced renal clearance, and altered hepatic enzyme activity. These changes can lead to increased drug clearance and reduced plasma concentrations of LTG. Specifically, the activity of enzymes such as UGT1A4 and UGT1A3, which are responsible for the glucuronidation and subsequent clearance of LTG [
35,
79]. Estrogen has a significant impact on the metabolism of LTG through the induction of UGT enzymes, leading to increased clearance and reduced drug levels. Genetic polymorphisms, particularly those affecting the UGT1A4 and UGT1A3 enzymes, can result in interindividual variability in LTG metabolism [
80,
81]. These polymorphisms are not fully integrated into the current simulation models, limiting the accuracy of predictions for different populations. The lack of sufficient empirical data on the pharmacokinetics of LTG under varying hormonal conditions and genetic backgrounds constrains the ability to model these effects accurately. This limitation is a significant factor in the difficulty of modeling this drug. It underscores the need for more extensive pharmacokinetic studies, especially in pregnant populations, incorporating detailed hormonal and genetic data.
By using a constant dose (200 mg), we established a baseline to compare how different populations (e.g., females vs. pregnant individuals) process the same amount of medication. This allows for clear observation of how pharmacokinetics differ due to physiological changes rather than differences in dosage. It helps in understanding the inherent variability in drug absorption, distribution, metabolism, and excretion between different populations without the confounding factor of dose variability, and it helps to identify how pregnancy-related physiological changes (e.g., increased blood volume, altered metabolism) impact drug levels and exposure. Since pregnancy can alter drug metabolism and clearance also, simulating varying doses throughout gestational weeks provides insight into how these changes affect drug exposure over time, leading to more accurate dosing recommendations. Understanding how drug exposure varies with different doses at various gestational stages allows for adaptive dosing strategies to maintain therapeutic efficacy and minimize side effects.
The model provides a useful tool for understanding how LTG pharmacokinetics change throughout pregnancy, offering a basis for adjusting doses to maintain therapeutic levels. For example, the model predicts a reduction in LTG plasma concentrations as pregnancy progresses, suggesting the need for dose adjustments to maintain efficacy and prevent suboptimal drug levels. By simulating different doses across gestational stages, the model helps in designing personalized dosing regimens that account for the physiological changes during pregnancy. This can guide clinicians in more accurately monitoring and adjusting doses, potentially improving treatment outcomes and minimizing adverse effects. Given the ethical and logistical challenges of including pregnant women in clinical trials, this model provides a strong argument for using observational data to simulate drug effects during pregnancy. Observational studies could enhance the model by providing real-world data on drug safety and efficacy in pregnant women, helping to refine dosing guidelines and improve patient care. Continuous refinement of the model, along with more comprehensive clinical data, will be essential for improving its accuracy and applicability in diverse patient populations.
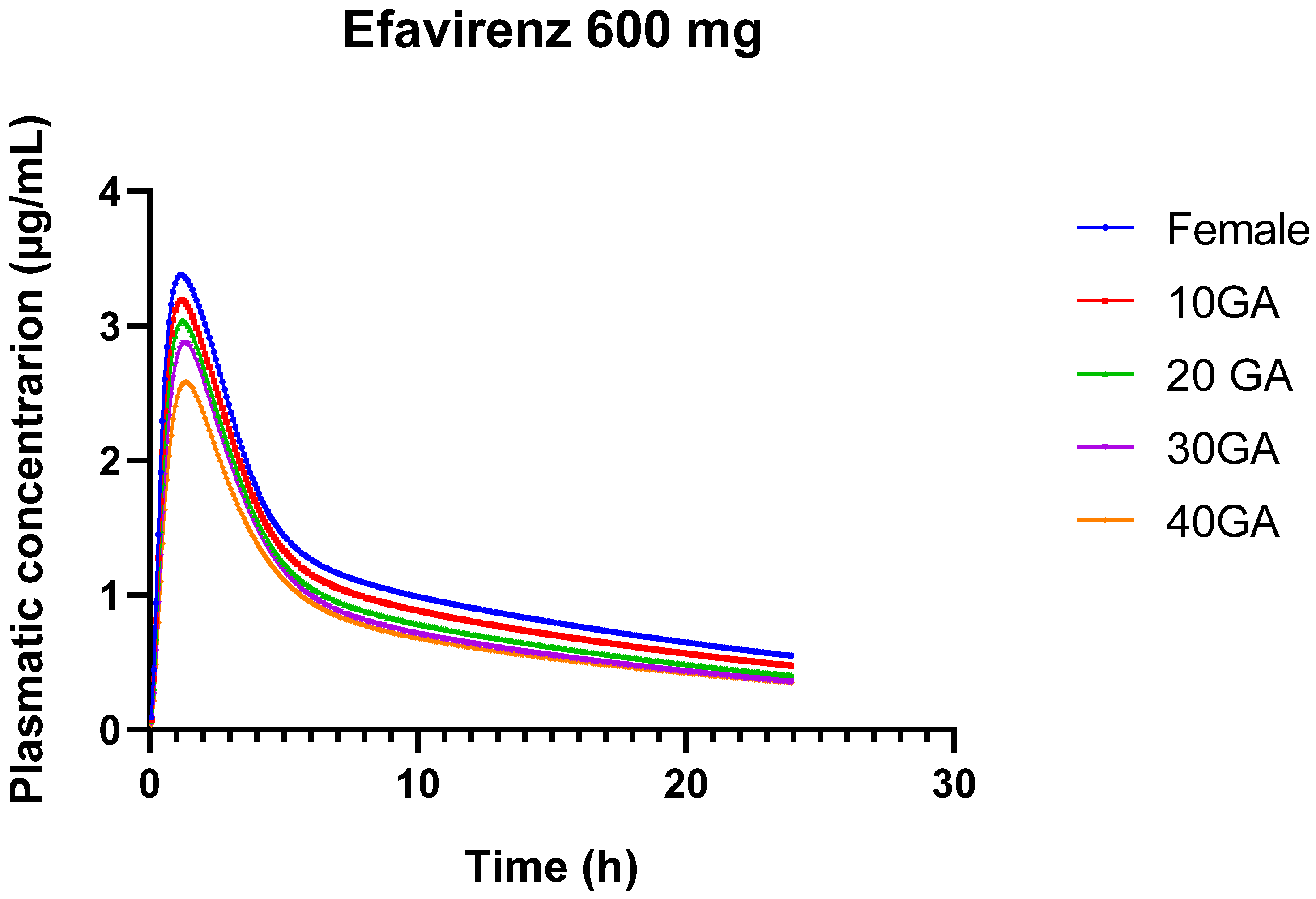
4.2. EFV Model for Pregnant and Non-Pregnant Individuals: Clinical Considerations
The pharmacokinetics of EFV are notoriously variable, heavily influenced by genetic polymorphisms, particularly in the CYP2B6 gene [
82], as well as by other factors such as gender. Drugs like EFV, which are primarily cleared hepatically, present challenges in simulation due to their complex metabolic pathways. Inhibitors or inducers of liver enzymes can interfere with EFV’s metabolism, significantly altering its pharmacokinetic profile. The inability of the current PBPK model to accurately predict the AUC and Cmax in certain populations underscores the complexity of these influencing factors.
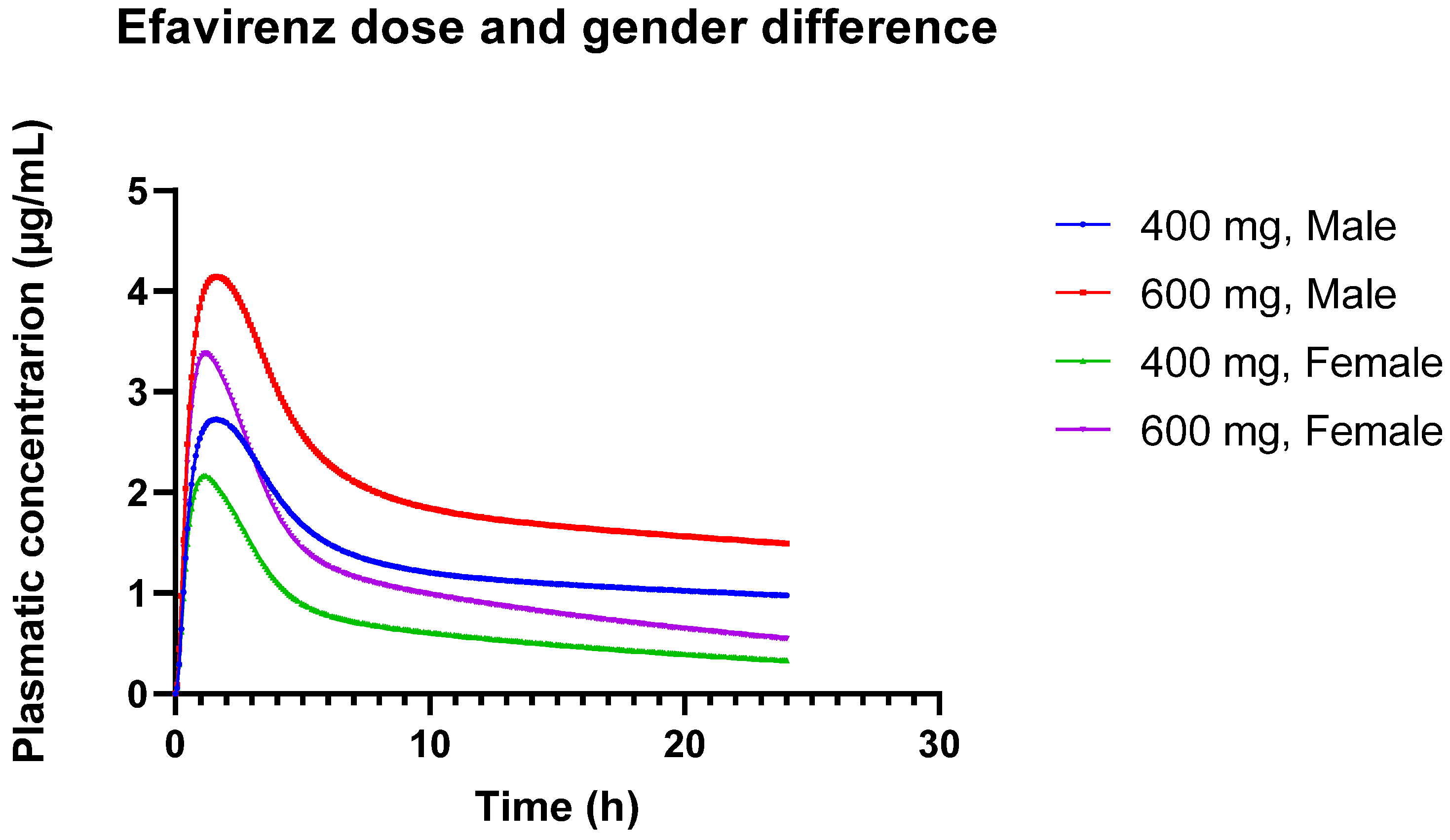
The predicted Cmax values showed a slight overestimation at the 400 mg dose but an underestimation at the 600 mg dose, indicating dose-dependent discrepancies in the model. While these predictions fall within the observed variability reported in the literature, lending partial validity to the model, the significant underprediction at the higher dose highlights its limitations in accurately capturing EFV’s pharmacokinetics across all dosing conditions. Specifically, the model’s underestimation of AUC by 31.42% at the 600 mg dose in the general adult population suggests that, although the model reflects certain aspects of EFV pharmacokinetics, it does not fully account for all sources of variability.
During pregnancy, significant physiological changes occur that can alter drug metabolism, distribution, and excretion. The current PBPK model’s substantial underprediction of AUC (by −87.43% at 33 weeks’ gestation) and Cmax (by −66.39%) in pregnant women highlights the challenges of modeling EFV pharmacokinetics during pregnancy. These discrepancies likely arise from the model’s failure to fully incorporate the complex changes in enzyme activity, volume of distribution, and other physiological parameters that occur during pregnancy. While the predicted AUC and Cmax values fall within the broad ranges reported in clinical studies, the large percentage of errors indicate that the model does not reliably predict EFV exposure in pregnant populations. This limitation is further emphasized by the model’s performance at 23 weeks’ gestation, where the predicted AUC was −48.22% lower than observed, suggesting that even at earlier stages of pregnancy, the model struggles to accurately predict drug exposure. The slight improvement in Cmax prediction at 23 weeks (with a %PE of −3.22%) suggests that while the model may capture some aspects of peak plasma concentration, it still underestimates the overall drug exposure (AUC). This pattern of underprediction suggests that the model may not be adequately accounting for increased hepatic clearance or other pregnancy-related changes. The fact that the model does not consider genetic polymorphisms, particularly in CYP2B6, which significantly affects EFV metabolism, is a critical limitation. The variability in EFV clearance due to these genetic differences likely contributes to the discrepancies observed between predicted and observed pharmacokinetic parameters [
43,
83].
The observed differences in EFV pharmacokinetics between males and females, with higher AUC and Cmax values in males, align with reports in the literature that suggest sex-based differences in drug metabolism and exposure [
84]. However, the inconsistency of these findings across studies suggests that more controlled research is needed to fully understand the influence of sex on EFV pharmacokinetics. Naidoo et al. [
84] emphasize the need for more controlled studies to conclusively determine the influence of sex on EFV pharmacokinetics, due to mixed findings and potential confounding factors. For example, most women in the studies were of African origin, a population more likely to carry CYP2B6 alleles with reduced activity, and no genotyping was performed. Smith et al. [
85] demonstrated the clinical implications of sex differences in treatment outcomes, particularly noting that women had a higher risk of virologic failure when treated with ATV/r compared to EFV. These findings suggest that sex-specific considerations are crucial in optimizing antiretroviral therapy, especially for women of childbearing potential, to ensure efficacy and minimize adverse effects. This underscores the need for gender-specific dosing guidelines to account for these differences.
The limitations of the current PBPK model are clear. It does not fully account for the complex physiological changes during pregnancy, including increased hepatic clearance, altered enzyme activity, and changes in the volume of distribution. Additionally, the model’s inability to incorporate genetic polymorphisms limits its predictive accuracy across diverse populations. The use of digitized data from published studies, while necessary, may introduce additional variability that could further complicate model accuracy, though this factor alone is unlikely to account for the large discrepancies observed.
Moving forward, it is imperative to refine the PBPK model to better account for pregnancy-specific physiological changes and genetic variability. This could involve incorporating more detailed patient-specific data, such as genotyping for CYP2B6, or using a population-based modeling approach that can better handle the wide variability seen in EFV pharmacokinetics. In vitro inhibition studies, particularly for enzymes like UGT2B7, are also needed to provide valuable insights into EFV metabolism and support more effective dosing strategies. Moreover, updating the GastroPlus model or developing new models that incorporate the complexities of pregnancy and genetic differences is crucial. This will enhance the clinical utility of these models, allowing for more accurate predictions of drug exposure and more personalized dosing strategies, particularly for vulnerable populations such as pregnant women.
4.3. Reduction in EFV’s Exposure Due to the Interaction with LTG
The observed reduction in Cmax and AUC for EFV under DDI conditions indicates that the interaction between these is minimal; the change in the total effect of EFV or LTG with co-administration was very small (
Table 15 and
Table 16). This suggests that the interaction might not be significant in terms of clinical outcomes. However, the study still emphasizes the importance of individualized dosing because even minimal interactions can be clinically relevant, especially in complex situations like HIV treatment and pregnancy.
EFV’s Cmax and AUC (0-inf) values slightly decrease when co-administered with LTG, and this reduction becomes more pronounced as gestational age progresses. However, the reduction is relatively modest and consistent across different stages of pregnancy. This suggests that while LTG might slightly enhance the metabolism or clearance of EFV, particularly in the later stages of pregnancy, the impact on EFV’s effectiveness may not be substantial enough to warrant significant clinical concern. These findings could play a supportive role in ensuring that EFV levels remain sufficient for therapeutic efficacy, particularly in clinical settings where EFV is a key component of treatment regimens for conditions like HIV. The interaction between LTG and EFV results in a slight but consistent increase in LTG’s Cmax and AUC across all gestational ages, indicating a modest enhancement in LTG exposure due to EFV co-administration. However, this increase is stable across different stages of pregnancy, suggesting that it is unlikely to require dose adjustments for LTG. The stability of LTG levels despite co-administration with EFV underscores the robustness of LTG dosing during pregnancy.
Further validation with clinical data is recommended to confirm these findings and guide appropriate dosing strategies, particularly in pregnant patients and those with different metabolic capacities (e.g., CYP and UGT metabolism). Additionally, exploring the limitations of the EFV PBPK model, especially regarding higher doses (600 mg), is warranted to ensure the model’s accuracy and reliability across various dosing scenarios.