Abstract
Cancer remains a highly lethal disease globally. The approach centered on REDOX-targeted mitochondrial therapy for cancer has displayed notable benefits. Plant polyphenols exhibit strong REDOX and anticancer properties, particularly by affecting mitochondrial function, yet their structural instability and low bioavailability hinder their utility. To overcome this challenge, researchers have utilized the inherent physical and chemical characteristics of polyphenols and their derivatives to develop innovative nanomedicines for targeting mitochondria. This review examines the construction strategies and anticancer properties of various types of polyphenol-based biological nanomedicine for regulating mitochondria in recent years, such as polyphenol self-assembly, metal–phenol network, polyphenol–protein, polyphenol–hydrogel, polyphenol–chitosan, and polyphenol–liposome. These polyphenolic nanomedicines incorporate enhanced features such as improved solubility, efficient photothermal conversion capability, regulation of mitochondrial homeostasis, and ion adsorption through diverse construction strategies. The focus is on how these polyphenol nanomedicines promote ROS production and their mechanism of targeting mitochondria to inhibit cancer. Furthermore, it delves into the benefits and applications of polyphenolic nanomedicine in cancer treatments, as well as the challenges for future research.
1. Introduction
Cancer remains a significant global health concern, with projections indicating 2,001,140 new cases and 611,720 deaths in the United States in 2024 [1]. While existing cancer treatments such as surgery, chemotherapy, radiotherapy, and immunotherapy have demonstrated some effectiveness, they often lack specificity and can lead to severe side effects [2,3,4]. Researchers are investigating targeted drug delivery systems using nanomaterials to enhance treatment precision [5,6,7,8,9]. The development of tailored nanomedicines, including metal, protein-based, and lipid-based nanoparticles, has shown promise in targeting tumor tissues, cells, and organelles. Despite advancements, challenges persist as many promising nanoparticles in preclinical studies have encountered obstacles in clinical development [5,10].
Various strategies are currently being developed to address the challenges of tumor treatment, with mitochondria emerging as a promising target for anticancer therapies. One of the major reasons is due to the frequent alteration of mitochondrial function in tumors, making it a reliable target [11]. The disruption of REDOX balance and the removal of restrictions on REDOX signaling in mitochondria result in malignant progression and the development of treatment resistance [12]. By enhancing antioxidant capacity to counteract the increase in reactive oxygen species (ROS), mitochondria can generate and tolerate high levels of ROS, which play diverse roles in signaling networks related to tumor proliferation, survival, and metastasis [13,14]. Moreover, multiple biochemical cascade reactions that trigger apoptosis and necrosis converge on mitochondria, with REDOX signals playing a crucial role in activating these cascades [15]. Since mitochondria are the main cellular source of ROS, REDOX-active compounds like polyphenols can target these organelles to regulate reactive oxygen species levels and their REDOX signals. Cancer cells with elevated levels of ROS possess a more significant antioxidant load and a delicate REDOX equilibrium compared to normal cells, rendering them more susceptible to drugs that induce oxidative stress [16,17].
Polyphenols, derived from plants, are well known for their antioxidant properties, but their pro-oxidative activity may actually play a more significant role in cancer treatment. Interestingly, polyphenols have the ability to selectively reduce the vitality of cancer cells in comparison to normal cells [18,19,20]. Studies indicate that polyphenols can rapidly stimulate the formation of ROS in cancer cells while sparing normal cells [19,20]. This imbalance between ROS production and the efficiency of antioxidant defense is a crucial factor. Polyphenols induce differential expression of antioxidant enzymes such as catalase and superoxide dismutase in cancer cells and normal cells, respectively [21]. In cancer cells, there is a decrease in antioxidant levels, increase in free radicals, and a disruption of mitochondrial membrane potential, whereas normal cells show elevated catalase levels and insensitivity to hydrogen peroxide [22]. Furthermore, certain studies have shown that the pro-oxidant effect of polyphenols on cancer cells is concentration-dependent [23,24,25]. Although high concentrations of polyphenols can lead to oxidative stress and harm, lower levels have been shown to protect against H2O2-induced damage in cancer cells. Therefore, maintaining optimal polyphenol levels in vivo is crucial for their anticancer properties. The ROS produced by polyphenols stem from their ortho-di/trihydroxy compound structure, which is prone to self-oxidation or catalytic oxidation by transition metal ions like Cu2+ and Fe3+ in biological systems. Numerous studies have demonstrated that polyphenols induce oxidative stress and apoptosis in various cancer cells by generating a significant amount of ROS [26,27,28]. Subsequent researches have indicated that these ROS primarily target mitochondria, resulting in mitochondrial damage and the activation of the mitochondrial apoptotic pathway. Examples of mitochondrial damage in cancer cells caused by polyphenol-induced ROS include decreased membrane potential, disruption of respiratory chain complexes, mitochondrial DNA damage, and the activation of caspase3 and caspase9 (Table 1).
Polyphenolic compounds have demonstrated potential in combating cancer, but their effectiveness is limited by poor pharmacokinetics, low stability, and restricted water solubility [29,30]. Therefore, the development of new drug delivery systems is crucial to enhance the stability and bioavailability of polyphenols, ensuring their concentration and biological activity in vivo. Recent advancements in nanotechnology have enabled the use of nanocarriers for encapsulating polyphenols [31,32,33,34,35]. These nanocarriers protect the encapsulated polyphenols from external physiological conditions, thereby preventing potential alterations in the composition and structure of bioactive compounds. This review will first discuss common methods for preparing high-performance nanomedicines using polyphenols, followed by an exploration of how these innovative polyphenolic nanomedicines disrupt the mitochondria of tumor cells. Lastly, it will outline potential future opportunities for the development and clinical application of polyphenolic nanomedicines.

Table 1.
Summary of the mitochondrial apoptosis pathway activated via ROS generated by polyphenols.
Table 1.
Summary of the mitochondrial apoptosis pathway activated via ROS generated by polyphenols.
| Polyphenols | Mechanisms on REDOXResponsiveness | Mitochondrial Damage | Mitochondrial Apoptosis Pathway | Cancer Cell | Ref. |
|---|---|---|---|---|---|
| EGCG | ROS generation | Altered mitochondrial transmembrane potentials | Altered Bcl-2 family proteins, cytochrome C release, and activation of caspase 3 and caspase 9 | Hepatocarcinoma SMMC7721 cells | [36] |
| Curcumin | superoxide anion O2- production | Mitochondrial DNA damage, disruption of mitochondrial membrane potential | Cytochrome C release into the cytosol | HepG2 hepatocellular carcinoma cells | [37] |
| Dimethoxycurcumin | ROS generation | Reduced mitochondrial membrane potential | Decrease in cellular energy status (ATP/ADP) | Human breast carcinoma MCF7 cells | [38] |
| Resveratrol | ROS generation | Mitochondrial membrane potential loss | Decrease in glutathione levels, along with reduced mRNA expression and activity of superoxide dismutase | T cell leukemia Jurkat cells | [39] |
| trans-Resveratrol | Superoxide anions generation | — | Increasing in caspase 3-like activity | Human colorectal carcinoma HT-29 cells | [40] |
| Resveratrol derivatives | ROS generation | Mitochondrial depolarization | — | Colon cancer CT-26 cell | [41] |
| Quercetin | ROS generation | — | Elevated expression and activity of caspase 9 | Human glioblastoma A172 cell | [42] |
| Oroxylin A | ROS generation | — | Elevated levels of SIRT3 in mitochondria, and the detachment of mitochondrial hexokinase II and the inhibition of glycolysis | Human breast carcinoma cell | [43] |
| Kaempferol | ROS generation | Altered mitochondrial membrane potential | Decreased expression of Bcl-2, elevated active caspase 3 and cleaved poly (ADP-ribose) polymerase expression | Human glioblastoma cells | [44] |
| Flavonoid LW-214 | ROS generation | Mitochondrial membrane potential loss | Increased Bax/Bcl-2 ratio, caspase 9 activation, degradation of poly (ADP-ribose) polymerase (PARP), cytochrome C release and apoptosis-inducing factor transposition | Human breast cancer MCF-7 cells | [45] |
| Novel isoflavone derivative, NV-128 | Superoxide and hydrogen peroxide generation | — | Decreasing in ATP, Cox-I, and Cox-IV levels | CD44+/MyD88+ ovarian cancer stem cells | [46] |
| Hesperidin | ROS generation | Mitochondrial membrane potential loss | Enhanced cytochrome C and apoptosis-inducing factor release from mitochondria, and caspase 3 activation. | HeLa cells | [47] |
| Naringenin | ROS generation | Mitochondrial depolarization | — | human epidermoid carcinoma A431 cells | [48] |
| Apigenin | ROS generation | Disruption of mitochondrial membrane potential | Glutathione depletion, cytosolic release of cytochrome C | Human prostate cancer 22Rv1 cells | [49] |
| luteolin | ROS generation | Mitochondrial membrane potential loss, and mitochondrial swelling | Release of cytochrome C | Hepatocellular carcinoma cells | [50] |
| Hesperetin | ROS generation | Reduced mitochondrial membrane potential | Increase in cytochrome C | Colon adenocarcinoma HT-29 cells | [51] |
| Chrysophanol | ROS generation | Reduced mitochondrial membrane potential | — | A549 human lung cancer cells | [52] |
| Agrimoniin | ROS generation | Disruption of mitochondrial membrane potential | — | Pancreatic cancer cells | [53] |
| 3-deoxysappanchalcone | ROS generation | Mitochondrial membrane potential depolarization | — | Esophageal Squamous cell carcinoma ESCC cells | [54] |
| Calycosin | ROS generation | Reduced mitochondrial membrane potential | Decreased the expression of Bcl-2 and increased the expression of Bax, caspase 3, and poly (ADP ribose) polymerase | HepG2 hepatocellular carcinoma cells | [55] |
| Chlorogenic Acid | Superoxide (O2•-) | Reduced mitochondrial membrane potential, changes in mitochondrial morphology | — | MCF-7, MDA-MB-231, and HCC1419 breast cancer cells | [56] |
| Genistein | ROS generation | Decreased mitochondrial membrane potential, decrease mitochondrial activity | Up-regulated expression cytochrome C and Bax, decreased the expression of Bcl-2 | Non-small lung cancer A549 and 95D cells | [57] |
| Gallic acid | ROS generation | Mitochondrial respiratory inhibition | Reduced ATP levels | Acute myeloid leukemia cells | [58] |
| Tannic acid | ROS generation | Reduced mitochondrial membrane potential | Reduced ATP levels, the activation of the death ligand TRAIL | Human embryonic carcinoma cells | [59] |
| Gossypol | ROS generation | Mitochondrial membrane potential loss, | Release of cytochrome C and apoptosis-inducing factor from mitochondria to the cytoplasm | human colorectal carcinoma cells | [60] |
2. Polyphenolic Nanomedicine
The utilization of polyphenols in nanomaterial synthesis has demonstrated effectiveness in enhancing stability and retention time, while also maintaining their REDOX capabilities. Polyphenols with catechol and gallic groups demonstrated involvement in hydrogen bonding, hydrophobic interactions, and π-π interactions [61]. Several studies have emphasized these attributes of polyphenols as a strong foundation for creating versatile nanomaterials [31]. This article offers a detailed exploration of the potential anticancer properties of polyphenolic nanomaterials specifically engineered to target mitochondria (Figure 1).
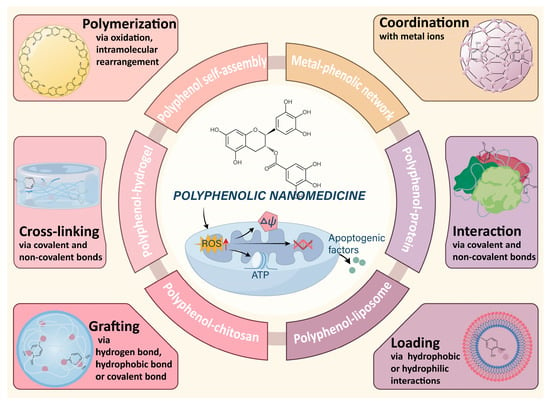
Figure 1.
Schematic diagram of the construction of polyphenolic nanomedicine through various typical chemical materials.
The mechanism by which polyphenols induce cancer cell apoptosis through the production of ROS targeting mitochondria is well understood. However, challenges such as limited water solubility and bioavailability hinder the achievement of optimal concentrations in the blood and at the tumor site for treatment [62]. Polyphenol nanomedicines address these limitations by enhancing water solubility, bioavailability, and pharmacokinetics in vivo [63]. Importantly, polyphenol nanomedicines can accumulate in large amounts at the tumor site [64]. These nanomedicines sustain the REDOX activity of polyphenols upon reaching cancer cells, leading to the generation of significant ROS and mitochondrial impairment [65,66]. Furthermore, the release of Fe3+ or Cu+ from polyphenol nanomedicines can enhance the Fenton reaction in cells, resulting in increased ROS levels [64,67]. Polyphenol nanomedicines with photothermal properties have synergistic therapeutic effects, such as inducing ROS generation in tumors under near-infrared (NIR) laser irradiation [68,69]. In conclusion, the advantages of polyphenol nanomedicines over pure polyphenols include promoting polyphenol accumulation at tumor sites, ultimately inducing cancer cell apoptosis through ROS targeting mitochondria.
2.1. Polyphenol Self-Assembly Nanomedicine
The presence of catechol and/or galloyl groups in polyphenols allows for a variety of covalent and non-covalent interactions to contribute to the formation of polyphenol-based materials [31]. For example, dopamine, containing both catechol and amine groups, readily undergoes oxidation and self-polymerization to create polydopamine (PDA) [70]. Similarly, (-)-Epigallocatechin-3-gallate (EGCG) has been observed to form self-assembled nanoparticles, exhibiting enhanced stability and REDOX activity characteristics compared to native EGCG in both in vitro and in vivo scenarios [71]. Peng et al. [72] also confirmed the existence of self-assembled nanoparticles of polyphenols with pectin, maintaining a stable system in chrysanthemum tea infusion. These self-assembled nanomaterials of polyphenols have been utilized in anticancer therapies due to their REDOX activity, high drug loading capacity for hydrophobic and hydrophilic agents, and photothermal properties [73]. Nieto et al. [74] found that PDA nanoparticles can induce apoptosis in breast cancer cells by stimulating ROS production while maintaining non-toxicity towards normal cells. Moreover, PDA has exhibited efficient conversion of near-infrared light into heat, showcasing its potential as a photothermal agent for eliminating cancer cells both in vitro and in vivo [75,76] (Figure 2A,B). The aromatic rings present in PDA allow for the loading of chemical drugs or dyes on its surface through π-π stacking and/or hydrophobic–hydrophobic interactions. In a study by Meng et al. [77], a mitochondria-targeting nanoparticle was designed to deliver PDA as a photothermal agent and alpha-tocopherol succinate as a chemotherapeutic drug to tumor cell mitochondria, resulting in cellular apoptosis and a synergistic inhibition of tumor cell proliferation. Furthermore, Chen et al. [78] developed a novel aggregation-induced emission (AIE) gen (MeO-TPE-indo, MTi) and incorporated it onto the surface of PDA to create the nanocomposite (PDA-MeO-TPE-indo, PMTi), which could effectively generate ROS for photodynamic therapy and target mitochondria. Moreover, the self-assembly of polyphenols with other substances holds promise in anticancer applications. For example, a nanosystem containing ursolic acid and EGCG demonstrated favorable outcomes in the treatment of hepatocellular carcinoma without causing adverse effects on normal tissues [79] (Figure 2C).
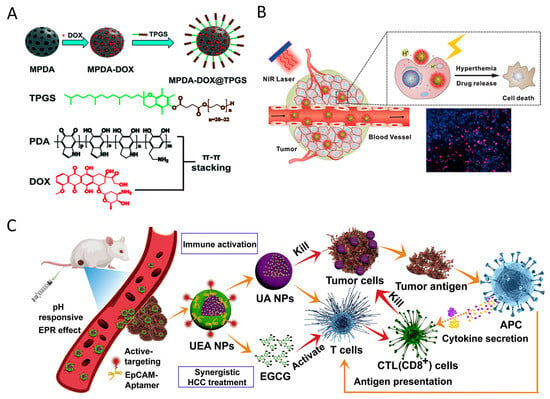
Figure 2.
(A) Doxorubicin hydrochloride (DOX) and d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) were sequentially loaded in the pore space and on the external particle surface of mesoporous polydopamine nanoparticles (MPDA) via π-π stacking and hydrophobic interactions, resulting in a MPDA–DOX@TPGS complex. Reproduced with permission [75]. Copyright 2017, Royal Society of Chemistry. (B) Polydopamine nanoparticles release anticancer drugs upon stimulation by NIR light, pH, and ROS, leading to cancer cell apoptosis. Reproduced with permission [76]. Copyright 2016, Elsevier. (C) Synergistic hepatocellular carcinoma treatment of the “carrier-free” Apt-modified nanodrug based on the ursolic acid and EGCG by activating the innate and acquired immunity. Reproduced with permission [79]. Copyright 2021, Elsevier.
2.2. Metal-Phenolic Network Nanomedicine
Polyphenols are widely present in nature, with over 8000 known organic species that can coordinate with metal ions to form metal–phenolic network nanoparticles (MPNs) [80,81,82]. The rapid chelation between phenolic ligands and metal ions allows for the quick formation of MPNs coatings within minutes [82,83]. By adjusting the pH during the assembly of MPNs, targeted tumor localization can be achieved [84]. MPNs can promptly attach to individual cancer cells [85], leading to long-term antitumor effects by regulating ROS and REDOX processes [86]. These MPNs hold promise in cancer treatment due to the catalytic activity of metal ions and the REDOX activity of polyphenols [87]. Li et al. [88] introduced a combination chemotherapy platform that depletes ATP and enhances ROS by integrating therapeutic samarium (SmIII) ions and (-)-epicatechin (EC) through a metal-phenolic network (SmIII–EC) (Figure 3A). The resulting SmIII–EC nanoparticles not only reduced tumor volume but also did not affect the body weight of mice or normal organs when compared to the clinical anticancer drug 5-fluorouracil for colon cancer treatment. Zhao et al. [67] developed MPNs using copper ions and gallic acid (Cu-GA) to induce apoptosis and cuproptosis for synergistic chemo/chemodynamic therapy. The release of gallic acid significantly reduces intracellular GSH content, making tumor cells unable to scavenge ROS and more susceptible to cuproptosis. Furthermore, the Fenton-like reaction of released Cu+ increases ROS levels within tumor cells, disrupting REDOX homeostasis and leading to apoptosis-related chemodynamic therapy. Liao et al. [89] created lanthanide metal ions (SmIII)–EGCG networks for targeted therapy against metastatic melanoma, and the synergistic effects of SmIII–EGCG networks demonstrate remarkable tumor inhibition properties compared to the clinical anticancer drug, 5-fluorouracil.
MPNs also function as a versatile nanoplatform for enhancing interfacial adhesion and drug loading in cancer therapy. Meng et al. [90] developed a multifunctional nanocomposite (PID@Fe-TA) that combines chemodynamic therapy, photothermal therapy, and chemotherapy to effectively combat breast cancer, offering a promising approach for cancer treatment. Dai et al. [91] created biocompatible MPNs to encapsulate DOX, where DOX coordinates with Fe3+, leading to the formation of DOX@Pt prodrug Fe nanoparticles (DPPF NPs), which act as a ROS enhanced combination chemotherapy platform (Figure 3B). Additionally, EGCG-based MPNs showed potential for incorporating DOX to achieve enhanced synergistic chemotherapy [92,93], due to the EGCG-mediated downregulation of CBR1 protein, which inhibits the generation of doxorubicinol (DOXOL), a reduction product of DOX linked to drug resistance and cardiotoxicity.
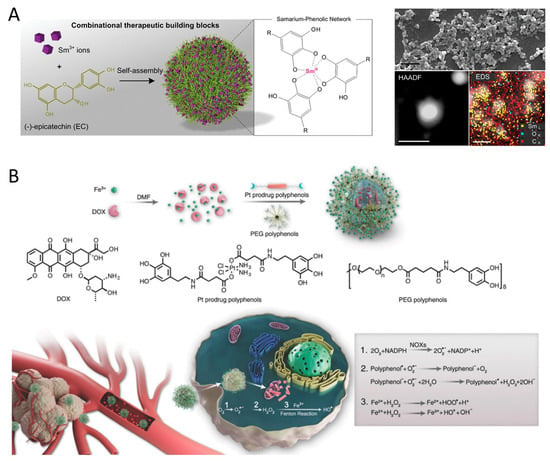
Figure 3.
(A) Self-assembly of the SmIII-EC nanoparticles from two therapeutic building blocks, rare earth Sm3+ ions and phenolic EC molecules. Reproduced with permission [88]. Copyright 2019, WILEY. (B) Formulation of DOX@Pt prodrug Fe nanoparticles (DPPF NPs) and the ROS enhanced chemotherapy mechanism in the tumor cells. NOXs were activated by DOX and platinum drugs and catalyzed the O2 to O2•− SOD-like activity of polyphenols catalyzes H2O2 generation from O2•−. Ferric ions further turn H2O2 into highly toxic HO• by Fenton reaction. Reproduced with permission [91]. Copyright 2018, WILEY.
2.3. Polyphenol–Protein Nanomedicine
Proteins have been extensively studied in cancer therapy, due to their biocompatibility, biodegradability, and non-immunogenicity [94,95]. Although the stability and intracellular delivery efficiency of proteins are limited, custom-designed protein-based nanosystems have shown promise in addressing these challenges. Polyphenols have been shown to interact with proteins through covalent and non-covalent bonds, facilitating cross-linking between proteins and improving delivery efficiency [96] (Figure 4A). For example, Qiao et al. [97] developed an ROS amplifying nanodevice by directly complexing melittin and condensed EGCG, and then prepared pHA-NC by covering it with phenylboronic acid derivatized hyaluronic acid (HA) (Figure 4B). Upon receptor-mediated endocytosis, pHA-NC increased intracellular oxidative stress, resulting in enhanced anticancer efficacy in cancer cells. To enhance the dissolution rate and aqueous solubility of curcumin, Jithan et al. [63] developed curcumin nanoparticle using bovine serum albumin. Curcumin nanoparticle showed superior anticancer effects in MDA-MB-231 cells compared to free curcumin, along with increased bioavailability and enhanced pharmacokinetic properties in rats. To investigate the potential antitumor effects of EGCG nanoparticles, Yang et al. [98] used thermally modified β-lactoglobulin, 3-mercapto-1-hexanol, and EGCG to create stable co-assembled nanocomplexes (MEβ-NPs). These MEβ-NPs showed no toxicity in mice but exhibited a strong inhibitory effect on the growth of implanted human melanoma A375 cell tumors, proving to be twice as effective as free EGCG. Additionally, a more advanced delivery system, micellar nanocomplexes (MNCs), was developed by combining oligomeric EGCG with the anticancer protein Hesetin to create a core, and then combining poly (ethylene glycol)-EGCG to form the shell [99]. The MNCs demonstrated enhanced tumor selectivity, improved growth inhibition, and an extended blood half-life. This EGCG-protein nanomedicine holds promise for phototherapy in cancer treatment. Wang et al. [68] developed nanoparticles S-aPDL1/ICG@NP by combining anti PD-L1 and photosensitizer ICG with EGCG dimer and MMP-2-susceptible blocking polymer PEG-PLGLAG-dEGCG through non-specific hydrophobic and electrostatic interactions. The S-aPDL1/ICG@NP facilitated the generation of intratumoral ROS under NIR laser irradiation. Furthermore, S-aPDL1/ICG@NP enhanced the intratumoral infiltration of cytotoxic T cells, effectively suppressing growth and metastasis.
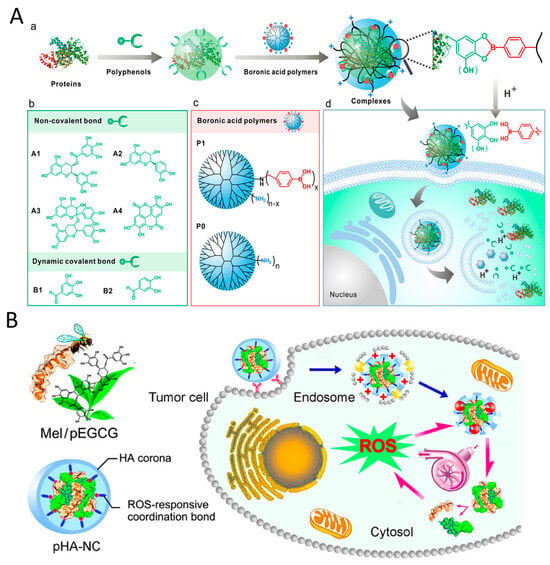
Figure 4.
(A) Polyphenol-mediated cytosolic protein delivery by boronic acid-decorated polymers. (a) Polyphenol facilitates the formation of protein transduction complexes. (b) Structures of the investigated polyphenols. (c) Structures of the boronic acid-decorated polymer P1 and the control polymer PO. (d) Intracellular release of proteins from the nanoparticles triggered by lysosomal acidity. Reproduced with permission. [96] Copyright 2019, ACS Publications. (B) Schematic illustration of a multipronged nanocomplex (pHA-NC) comprising mel and pEGCG surrounded by HA through the coordination bond for ROS self-sufficient oxidation therapy of cancers. Reproduced with permission. [97] Copyright 2018, ACS Publications.
2.4. Polyphenol-Hydrogel Nanomedicine
Hydrogel is a 3D polymer network formed by cross-linking hydrophilic macromolecular chains in an aqueous environment [100]. It provides a promising approach for delivering therapeutic payloads due to its biocompatibility and structural similarity to the natural extracellular matrix [101]. Polyphenol compounds containing groups such as catechol and pyrogallol readily interact with other substances to form 3D hydrogel networks with exceptional properties like adhesion, toughness, photothermal effects, antibacterial, and anticancer properties [102]. Teong et al. [103] synthesized several types of curcumin-hydrogel nanoparticles using curcumin, biopolymeric chitosan, gelatin, and hyaluronan nanoparticles in an electrostatic field system. These curcumin–hydrogel nanoparticles increased apoptosis in A549 cells by stimulating ROS production and reducing mitochondrial membrane potential levels, demonstrating higher anticancer efficacy than curcumin alone. Furthermore, Madeo et al. [104] prepared graphene oxide-loaded curcumin nanosheets encapsulated in hydrogels, cross-linked with Ca2+, to form hybrid hydrogels used as patches for the local treatment of areas affected by squamous cell carcinoma (Figure 5A). The curcumin-loaded systems exhibited potent cytotoxic effects in SCC cancer cells, with sustained release of curcumin (~50% after 96 h). Jeong et al. [105] developed Au ion-crosslinked hydrogels (ICGs) containing indocyanine green and EGCG for photothermal/photodynamic therapy in breast cancer treatment. ICGs not only produced ROS but also induced hyperthermia for photothermal therapy upon exposed to NIR laser, effectively inhibiting the growth of primary breast cancer. He et al. [106] created a palladium single atom nanoenzyme supported by polyphenol modified carbon quantum dots (DA-CQD@Pd SAN), and used it to fabricate a bioadhesive hydrogel for local tumor immunotherapy (Figure 5B). This hydrogel not only locally delivers immune adjuvants but also enhances the antitumor effect by scavenging a significant amount of ROS generated by H2O2 within the tumor.
Polyphenol hydrogels can be enhanced by combining them with chitosan or liposome to improve stability and drug release properties. George et al. [107] used l-histidine coupled chitosan, plant synthesized zinc oxide nanoparticles and dialdehyde cellulose to prepare functional nano hybrid hydrogel (HIS-CHGZ) for delivering naringenin, quercetin, and curcumin. The drug release from HIS-CHGZ-polyphenol followed a non-Fickian diffusion-based mechanism along with polymer erosion, resulting in a significant 15-to-30-fold increase in cytotoxicity of HIS-CHGZ-polyphenol in A431 cells compared to free polyphenols. Li et al. [108] developed a composite hydrogel (Cur-lip/DOX/CSSH) containing thiolated chitosan (CSSH), DOX, and hydrophobic liposome-encapsulated curcumin (Cur-Lip) (Figure 5C). The Cur-Lip/DOX gels exhibit long-term drug release capability and potent anticancer activity. Furthermore, polyphenol hydrogel can be integrated with 3D printing. Zhu et al. [109] synthesized a multifunctional PC@ZIF-8@PDA/SerMA hydrogel using methacrylic anhydride (MA)-modified sericin (SerMA) solution mixed with procyanidin (PC)-loaded PDA-modified ZIF-8 (PC@ZIF-8@PDA) nanoparticles under blue light. PC@ZIF-8@PDA/SerMA hydrogels with personalized shapes fabricated through 3D printing demonstrated the ability to eliminate chondrosarcoma cells through their photothermal activity in conjunction with NIR radiation.
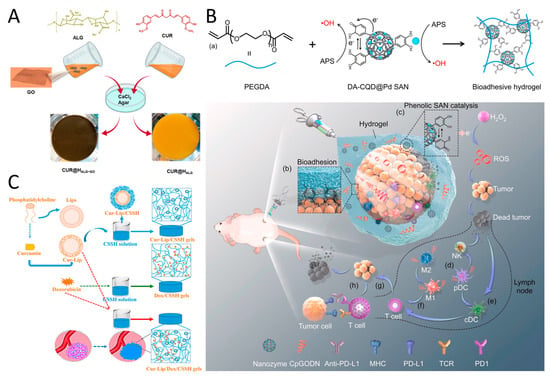
Figure 5.
(A) Curcumin (CUR) was loaded onto graphene oxide (GO) nanosheets, mixed into alginate gel crosslinked by calcium ions, resulting in a hydrogel patch for treating squamous cell carcinoma (SCC). Reproduced with permission [104]. Copyright 2021, Elsevier. (B) DA-CQD@Pd@CpGODN hydrogel induces tumor death by generating ROS and treats distant untreated tumors through catalytic immunotherapy. (a) In situ hydrogel gelation by the SAN catalyzing. (b) Hydrogel was injected and adhered at tumor sites. (c) The catalytic process of the DA-CQD@Pd SAN in the TME. The hydrogel induced activation of (d) pDC, (e) cDC, and (f) T cells in TME. (g) T cells triggered the adaptive immune response. (h) Anti-PD-L1 enhanced the immune response by blocking the binding of PD-L1 to PD1 on T cells. Reproduced with permission [106]. Copyright 2022, Elsevier. (C) A smart, temperature-responsive CSSH hydrogel was designed to facilitate the in situ-coating of solid tumors, repair the leaky vasculature and impaired lymphatic drainage, and fill defects after tumor excision. Reproduced with permission [108]. Copyright 2022, Frontiers Media.
2.5. Polyphenol–Chitosan Nanomedicine
Chitosan, a naturally derived polymer produced through alkaline deacetylation of chitin, is recognized for its non-toxic, biocompatible, and biodegradable properties [110]. Due to its abundant functional amino and hydroxyl groups, chitosan readily binds to other active molecules [111,112]. Polyphenols can form stable conjugates with chitosan through mechanisms such as hydrogen bonding, hydrophobic interactions, and van der Waals forces [113]. Various types of polyphenol–chitosan conjugates have demonstrated enhanced solubility, sustained release, and improved bioavailability [114,115] (Figure 6A). For example, Mariadoss et al. [116] developed chitosan nanoparticles loaded with phloretin (PhCsPs) to investigate their potential in oral cancer treatment. These nanoparticles enhanced the mitochondrial-mediated apoptotic mechanism by inducing ROS generation in KB cells. Similarly, Tsai et al. [117] created nanoparticles (CENP) containing curcumin by crosslinking chitosan and tripolyphosphate for photodynamic therapy (Figure 6B). The CENP nanoparticles exhibited enhanced photodynamic therapy in cancer cells, producing significantly more 1O2, resulting in an approximately fourfold decrease in the IC50 value. Khan et al. [118] synthesized an oral formulation of chitosan-based nanoparticles loaded with EGCG for the sustainable and controlled release of polyphenol to combat prostate cancer. The EGCG–chitosan significantly suppressed tumor growth and prostate-specific antigen levels compared with free EGCG in 22Rν1 tumor xenografts implanted in athymic nude mice. The enhanced anticancer efficacy of EGCG–chitosan is attributed to its prolonged release, as encapsulated EGCG exhibited slow discharge from the nanoparticles.
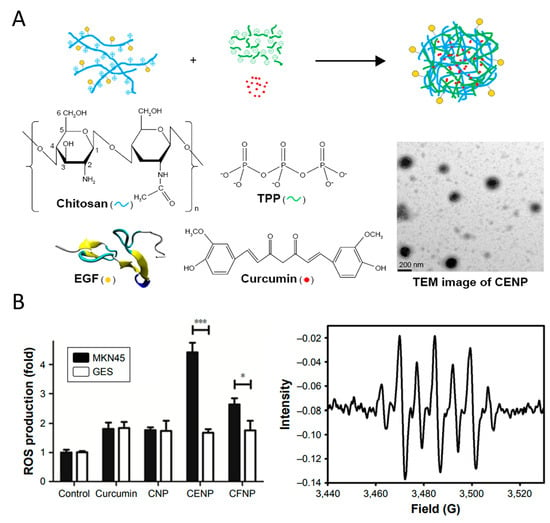
Figure 6.
(A) A schematic diagram for the nanoparticle assembly of curcumin-encapsulated and EGF-conjugated chitosan/TPP nanoparticles (CENP) from EGF-conjugated chitosan, TPP, and curcumin. (B) ROS generated in photodynamic therapy treated with various nanoparticles include the production of free radicals and 1O2, * p < 0.05, *** p < 0.001. Reproduced with permission [117]. Copyright 2017, Dove Medical Press Ltd.
Polyphenol–chitosan has demonstrated potential not only as an anticancer agent, but also as a drug delivery system for other active ingredients. Hu et al. [119] synthesized polymer nanoparticles (GA-g-CS-CPP) made from gallic acid grafted chitosan and caseinophosphopeptides to enhance the delivery efficiency and resistance to degradation of EGCG nanoparticles. Moreover, polyphenol–chitosan has been utilized for delivering chemotherapy drugs. Wang et al. [120] developed an amphiphilic carboxymethyl chitosan–quercetin (CQ) to improve oral bioavailability of paclitaxel (PTX). The PTX-loaded CQ exhibited sustained release in simulated gastrointestinal fluid and enhanced paclitaxel permeability in intestinal absorption experiments. Compared to Taxol (®) and Taxol (®) combined with verapamil, PTX-loaded CQ showed potent antitumor efficacy in tumor xenograft models. Similarly, a novel pH-responsive nanomicelle (QT-CA-CS) was designed using chitosan, quercetin, and citraconic anhydride to encapsulate the anticancer drug DOX [121]. The QT-CA-CS-DOX nanomicelles improved the cellular uptake of DOX in drug-resistant MCF-7/ADR cells by 8.62 times compared to free DOX. Notably, these nanomicelles could evade lysosomes, leading to the rapid release of DOX and quercetin in the cytoplasm, resulting in a stronger inhibitory effect on tumor cells. Recently, Mu et al. [122] introduced a delivery system, Cabazitaxel (Cab)@MPN/CS, comprising metal–polyphenol and chitosan, for melanoma therapy. (Cab)@MPN/CS exhibited prolonged retention in tumor tissue and effective tumor suppression.
2.6. Polyphenol–Liposome Nanomedicine
Liposomes, nanoscale artificial vesicles composed of lipid bilayers encapsulating an aqueous core [123], mimic natural cell membranes and possess biocompatible and biodegradable properties, making them ideal carriers for drug delivery. One notable characteristic of liposomes is their ability to encapsulate hydrophobic drugs in the lipid layer and hydrophilic drugs in the internal water core, protecting them from degradation and metabolism in the body’s circulation [124,125]. Given this feature, various plant polyphenols have been loaded into liposomes to create polyphenol–liposome complexes with enhanced solubility and chemical stability [126]. Caddeo et al. [65] developed eudragit-coated liposomes to safely transport resveratrol and artemisinin through the gastrointestinal tract, targeting the intestine. These vesicles exhibited pro-oxidant effects in intestinal adenocarcinoma cells, resulting in significant cell death by inducing mitochondrial ROS production. To achieve extended blood circulation and improve permeability and retention, Kang and Ko [66] constructed mitochondria-targeting liposomes loaded with resveratrol by surface modification with TPP-PEG or DQA-PEG. These resveratrol–liposomes exhibited improved cellular uptake and specific accumulation in the mitochondria. Furthermore, they induced cancer cell cytotoxicity through ROS generation and dissipation of mitochondrial membrane potential. Additionally, in a cisplatin-resistant human ovarian tumor xenograft model, quercetin–liposome and honokiol–liposome exhibited significant suppression of tumor growth compared to free polyphenols [127,128].
Polyphenol liposomes can be combined with chitosan or hydrogel to enhance the bioavailability of polyphenols. Ezzat et al. [129] detailed the creation of catechin-loaded chitosan-tethered liposomes (CHS) through the ethanol injection technique to enhance the oral bioavailability of catechin. Pharmacokinetic studies in rats demonstrated that catechin liposomes increased the levels and duration of catechins in plasma compared to free catechins. Li et al. [130] developed curcumin liposome (CurLip), and coated it with CSSH to create liposomal hydrogels (CSSH/CurLip gel), which improved the solubility and bioavailability of curcumin. The CSSH/Cur-Lip gel inhibited breast cancer recurrence post-tumor resection and promoted tissue repair of the defect. Wu et al. [64] recently introduced an inhalable gallic acid–Fe MPN hybrid liposome (LDG) to enhance the intracellular Fenton reaction (Figure 7). LDG demonstrated excellent nebulization capability, significantly increasing lung accumulation. Furthermore, LDG initiated a strong Fenton response, resulting in intracellular ROS generation, and displaying significant antitumor efficacy in an orthotopic lung tumor model.
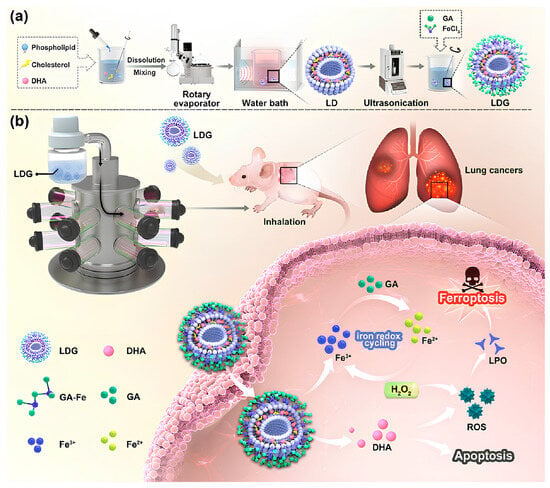
Figure 7.
MPN hybrid liposomes for lung cancer ferroptosis-apoptosis synergetic therapy. (a) Gallic acid–Fe MPN hybrid liposome (LDG) liposomes were prepared by thin film ultrasonic dispersion. (b) Nebulization delivered antitumor strategy of LDG based on iron REDOX cycling. Reproduced with permission [64]. Copyright 2024, Tsinghua University Press.
2.7. Other Polyphenolic Nanomedicine
Other nano carriers for polyphenol delivery, such as nanoemulsions and nanogels, have also been developed to cater different anticancer strategies. Nanoemulsion, a distinctive structure consisting of an oil phase, water phase, and surfactant or emulsifier, is regarded as a delivery system for to enhance the effectiveness of hydrophobic antitumor drugs [131]. It can improve drug dosage efficacy, stability, and demonstrate slow release and targeting effects. For example, quercetin-nanoemulsion has shown improved encapsulation efficiency, and enhanced antitumor effects compared to free quercetin [132]. Nanogel has also emerged as a promising nano carrier for targeted delivery of therapeutic agents in cancer treatment [133]. With its porous structure and large surface-to-volume ratio, nanogel can enhance drug permeability and retention at the tumor site, thereby improving therapeutic outcomes. A curcumin-loaded nanogel has achieved sustained drug release, resulting in enhanced suppression of cancer cells and tumor growth compared to free curcumin [134].
3. Conclusions and Outlooks
This review summarizes recent advancements in utilizing polyphenol molecules as the fundamental components for developing a range of biocompatible nanomaterials. The study indicates that these biocompatible nanomaterials, formed through self-assembly, MPN formation, covalent bonding, and hydrophobic/hydrogen bonding interactions, exhibit anticancer properties with targeting mitochondria. Furthermore, these polyphenol nanomedicines have also acted as nanocarriers for delivering various therapeutic drugs to tumors, including different metal ions, chemotherapy drugs, and photothermal agents.
Polyphenol nanomedicines are typically prepared by combining different materials to enhance their functionality. However, the intricate nature of nanomedicines poses challenges in terms of body metabolism. Future research on polyphenol nanomedicines should concentrate on developing simple and versatile preparation techniques to achieve rational design and customizable synthesis. The self-assembly of polyphenolic metals and chemical grafting techniques may provide promising avenues for further exploration. Furthermore, combination therapy appears to better reflect the therapeutic characteristics of polyphenol nanomedicines compared to single therapies. Understanding how polyphenols, chemotherapy, phototherapy, and other drugs interact within in cancer cells can help in leveraging the characteristics of polyphenolic nanomedicines. Polyphenol nanoparticles, with their photothermal properties and the ability to overcome drug resistance, hold significant promise when combined with treatments like radiotherapy and immunotherapy. It is essential to integrate nanotechnology with other scientific disciplines to broaden treatment options such as transdermal agents, inhalable drugs, and photothermal therapy, to meet the specific needs of patients. Future research on polyphenolic nanomedicines should prioritize their REDOX activity for precise targeting of tumor cell mitochondria.
Author Contributions
All authors contributed to the article preparation. Conceptualization, X.Z., J.Y., G.L. and H.C.; writing-original draft, M.Y., Y.H., Q.N. and X.W.; writing-review and editing, X.Z. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Research and Development Program of Zhejiang (2023C02040), National Natural Science Foundation of China (32372757), the Anhui Provincial Natural Science Foundation (2308085QC113), the Innovative Program of Chinese Academy of Agricultural Sciences (CAASASTIP-2021-TRI), and the Agriculture Research System of China of MOF and MARA (CARS-19).
Data Availability Statement
Data derived from public domain resources. The data presented in this study are available in reference numbers [64,75,76,79,88,91,96,97,104,106,108,117].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C.; et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Pei, Z.; Jiang, C.; Cheng, L. Recent progress of metal-based nanomaterials with anti-tumor biological effects for enhanced cancer therapy. Exploration 2023, 3, 20220001. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Cao, J.; Cao, F.; Chen, X. Manipulating calcium homeostasis with nanoplatforms for enhanced cancer therapy. Exploration 2023, 4, 20230019. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol. 2008, 18, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Raut, G.K.; Chakrabarti, M.; Pamarthy, D.; Bhadra, M.P. Glucose starvation-induced oxidative stress causes mitochondrial dysfunction and apoptosis via Prohibitin 1 upregulation in human breast cancer cells. Free Radic. Biol. Med. 2019, 145, 428–441. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Giannoni, E.; Chiarugi, P.; Parri, M. Mitochondrial Redox Hubs as Promising Targets for Anticancer Therapy. Front. Oncol. 2020, 10, 256. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Dong, L.F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; Ouari, O.; Lopez, M.; McAllister, D.; Boyle, K.; Barrios, C.S.; Weber, J.J.; Johnson, B.D.; Hardy, M.; et al. Mitochondria-Targeted Analogues of Metformin Exhibit Enhanced Antiproliferative and Radiosensitizing Effects in Pancreatic Cancer Cells. Cancer Res. 2016, 76, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2014, 58, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Takemura, Y.; Hamada, H.; Sekido, Y.; Kubota, S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int. 2013, 13, 19. [Google Scholar] [CrossRef]
- Hsu, S.; Bollag, W.B.; Lewis, J.; Huang, Q.; Singh, B.; Sharawy, M.; Yamamoto, T.; Schuster, G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2003, 306, 29–34. [Google Scholar] [CrossRef]
- Tao, L.; Park, J.Y.; Lambert, J.D. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol. Nutr. Food Res. 2015, 59, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hsu, S.; Lewis, J.; Wataha, J.; Dickinson, D.; Singh, B.; Bollag, W.B.; Lockwood, P.; Ueta, E.; Osaki, T.; et al. Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. J. Pharmacol. Exp. Ther. 2003, 307, 230–236. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Liang, W.Z.; Lu, C.H. Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci. 2012, 90, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Bilgin, M.G.; Ergün, İ.S.; Dadak, A. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Sang, S.; You, H.; Lee, M.J.; Hong, J.; Chin, K.V.; Yang, C.S. Mechanism of action of (-)-epigallocatechin-3-gallate: Auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Wei, X.L.; Fang, Y.P.; Gan, R.Y.; Wang, M.; Ge, Y.Y.; Zhang, D.; Cheng, L.Z.; Corke, H. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 2020, 60, 1243–1264. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Gámez-Meza, N.; Molina-Domínguez, C.C.; Hayano-Kanashiro, C.; Medina-Juárez, L.A. Simulated Gastrointestinal Digestion, Bioaccessibility and Antioxidant Capacity of Polyphenols from Red Chiltepin (Capsicum annuum L. Var. glabriusculum) Grown in Northwest Mexico. Plant Foods Hum. Nutr. 2018, 73, 116–121. [Google Scholar] [CrossRef]
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-Based Particles for Theranostics. Theranostics 2019, 9, 3170–3190. [Google Scholar] [CrossRef]
- Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, J.; Qiao, L.; Wang, C.; Wang, Y.; Yu, R.; Xu, W.; Wang, F.; Yang, S.; Zhang, X.; et al. Multifunctional Dual Network Hydrogel Loaded with Novel Tea Polyphenol Magnesium Nanoparticles Accelerates Wound Repair of MRSA Infected Diabetes. Adv. Funct. Mater. 2024. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Hu, Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Exploration 2022, 2, 20210106. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Li, W.; Nie, S.; Yu, Q.; Xie, M. (-)-Epigallocatechin-3-gallate induces apoptosis of human hepatoma cells by mitochondrial pathways related to reactive oxygen species. J. Agric. Food Chem. 2009, 57, 6685–6691. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Jia, L.; Zhou, H.M.; Kong, Y.; Yang, G.; Jiang, L.P.; Li, Q.J.; Zhong, L.F. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic. Biol. Med. 2007, 43, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Jayakumar, S.; Srivastava, A.K.; Priyadarsini, K.I. Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: Evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch. Toxicol. 2012, 86, 603–614. [Google Scholar] [CrossRef]
- Kucinska, M.; Piotrowska, H.; Luczak, M.W.; Mikula-Pietrasik, J.; Ksiazek, K.; Wozniak, M.; Wierzchowski, M.; Dudka, J.; Jäger, W.; Murias, M. Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line: Prooxidative potential of hydroxylated resveratrol analogs. Chem. Biol. Interact. 2014, 209, 96–110. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agric. Food Chem. 2008, 56, 4813–4818. [Google Scholar] [CrossRef]
- Sassi, N.; Mattarei, A.; Azzolini, M.; Bernardi, P.; Szabo, I.; Paradisi, C.; Zoratti, M.; Biasutto, L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr. Pharm. Des. 2014, 20, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, Y.; Dai, Q.; Qiao, C.; Zhao, L.; Hui, H.; Lu, N.; Guo, Q.L. Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis. 2013, 4, e601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef]
- Pan, D.; Li, W.; Miao, H.; Yao, J.; Li, Z.; Wei, L.; Zhao, L.; Guo, Q. LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem. Pharmacol. 2014, 87, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Alvero, A.B.; Montagna, M.K.; Holmberg, J.C.; Craveiro, V.; Brown, D.; Mor, G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol. Cancer Ther. 2011, 10, 1385–1393. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef]
- Sivagami, G.; Vinothkumar, R.; Bernini, R.; Preethy, C.P.; Riyasdeen, A.; Akbarsha, M.A.; Menon, V.P.; Nalini, N. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line—A comparative study. Food Chem. Toxicol. 2012, 50, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.H.; Yu, C.S.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Chen, P.Y.; Wu, S.H.; Ip, S.W.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol. 2014, 29, 740–749. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine 2022, 96, 153807. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.W.; Lee, M.J.; Lee, M.H.; Yoon, G.; Cho, S.S.; Chae, J.I.; Shim, J.H. The 3-deoxysappanchalcone induces ROS-mediated apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway in human esophageal cancer cells. Phytomedicine 2021, 86, 153564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. Vitro 2021, 70, 105052. [Google Scholar] [CrossRef]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Pang, Y.; Wang, Y.; Zhu, D.; Taledaohan, A.; Jia, Y.; Zhao, L.; Wang, W. Genistein-induced mitochondrial dysfunction and FOXO3a/PUMA expression in non-small lung cancer cells. Pharm. Biol. 2022, 60, 1876–1883. [Google Scholar] [CrossRef]
- Gu, R.; Zhang, M.; Meng, H.; Xu, D.; Xie, Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed. Pharmacother. 2018, 105, 491–497. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Jo, E.S.; Rugamba, A.; Kim, W.S.; Park, Y.M.; Hwang, D.Y.; Yoo, J.S.; Liu, Q.; Jang, K.J.; et al. Tannic Acid Promotes TRAIL-Induced Extrinsic Apoptosis by Regulating Mitochondrial ROS in Human Embryonic Carcinoma Cells. Cells 2020, 9, 282. [Google Scholar] [CrossRef]
- Ko, C.H.; Shen, S.C.; Yang, L.Y.; Lin, C.W.; Chen, Y.C. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int. J. Cancer 2007, 121, 1670–1679. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Z.; Chen, M.; Feng, L. Phenolic molecules constructed nanomedicine for innovative cancer treatment. Coord. Chem. Rev. 2021, 439, 213912. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol. Res. 2011, 64, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jithan, A.; Madhavi, K.; Madhavi, M.; Prabhakar, K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int. J. Pharm Investig. 2011, 1, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, W.; Guo, M.; Fu, F.; Wang, W.; Sung, T.; Zhang, M.; Zhong, Z.; Wu, C.; Pan, X.; et al. Inhalable iron redox cycling powered nanoreactor for amplified ferroptosis-apoptosis synergetic therapy of lung cancer. Nano Res. 2024, 17, 5435–5451. [Google Scholar] [CrossRef]
- Caddeo, C.; Gabriele, M.; Nácher, A.; Fernàndez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and artemisinin eudragit-coated liposomes: A strategy to tackle intestinal tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yu, H.; Liang, L.; Wang, C.; Shi, D.; Zhang, X.; Ying, Y.; Cai, W.; Li, W.; Li, J.; et al. Redox Homeostasis Disruptors Based on Metal-Phenolic Network Nanoparticles for Chemo/Chemodynamic Synergistic Tumor Therapy through Activating Apoptosis and Cuproptosis. Adv. Healthc. Mater. 2023, 12, e2301346. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K. Recent Advances in Synergistic Effect of Nanoparticles and Its Biomedical Application. Int. J. Mol. Sci. 2024, 25, 3266. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wang, D.; Wang, Z.; Yang, M.; Yang, L.; Wang, F.; Wang, W.; Zhang, X. Formation of EGCG oxidation self-assembled nanoparticles and their antioxidant activity in vitro and hepatic REDOX regulation activity in vivo. Food Funct. 2024, 15, 2181–2196. [Google Scholar] [CrossRef]
- Peng, A.; Lin, L.; Zhao, M. Chemical basis and self-assembly mechanism of submicroparticles forming in chrysanthemum tea infusion. Food Chem. 2023, 427, 136745. [Google Scholar] [CrossRef]
- Bigaj-Józefowska, M.J.; Coy, E.; Załęski, K.; Zalewski, T.; Grabowska, M.; Jaskot, K.; Perrigue, P.; Mrówczyński, R.; Grześkowiak, B.F. Biomimetic theranostic nanoparticles for effective anticancer therapy and MRI imaging. J. Photochem. Photobiol. B. 2023, 249, 112813. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín Del Valle, E.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, B.; Liu, Y.; Wan, Y.; Liu, Q.; Xu, H.; Liang, R.; Shi, Y.; Tu, P.; Wu, H.; et al. Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer. Regen Biomater. 2022, 9, rbac051. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, W.; Guo, X.; Li, Y.; Ma, Y.; Chen, L.; Zhang, H.; Wang, T.; Zhang, X.; Wang, Z. Mitochondria-Targeted Polydopamine Nanocomposite with AIE Photosensitizer for Image-Guided Photodynamic and Photothermal Tumor Ablation. Small 2019, 15, e1902352. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, J.; Wu, P.; Zou, J.; Le, J.; Lin, J.; Li, C.; Luo, B.; Zhang, Y.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, T.; Gao, W.; Iffelsberger, C.; Kong, B.; Pumera, M. Multi-Wavelength Light-Responsive Metal-Phenolic Network-Based Microrobots for Reactive Species Scavenging. Adv. Mater. 2023, 35, e2210994. [Google Scholar] [CrossRef]
- Mattos, B.D.; Zhu, Y.; Tardy, B.L.; Beaumont, M.; Ribeiro, A.C.R.; Missio, A.L.; Otoni, C.G.; Rojas, O.J. Versatile Assembly of Metal-Phenolic Network Foams Enabled by Tannin-Cellulose Nanofibers. Adv. Mater. 2023, 35, e2209685. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly for Particle Engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Penna, M.; Pan, S.; Ju, Y.; Li, S.; Han, Y.; Chen, J.; Lin, G.; Richardson, J.J.; et al. Particle engineering enabled by polyphenol-mediated supramolecular networks. Nat. Commun. 2020, 11, 4804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Jiang, L.; Gao, H.; Liu, Z.; Mäkilä, E.; Wang, S.; Saiding, Q.; Xiang, L.; Tang, X.; Shi, M.; et al. A pH-Responsive Cluster Metal-Organic Framework Nanoparticle for Enhanced Tumor Accumulation and Antitumor Effect. Adv. Mater. 2022, 34, e2203915. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gong, G.; Chen, M.; Zhang, H.; Zhang, Y.; Richardson, J.J.; Chan, W.Y.; He, Y.; Guo, J. Metal-Phenolic Nanocloaks on Cancer Cells Potentiate STING Pathway Activation for Synergistic Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314501. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, Z.; Pan, S.; Chen, J.; Wang, T.; Cortez-Jugo, C.; Caruso, F. Direct Assembly of Metal-Phenolic Network Nanoparticles for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2023, 62, e202312925. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Yu, J.; Zhang, Y.; Zhou, Y. Applications of metal-phenolic networks in nanomedicine: A review. Biomater. Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Li, K.; Dai, Y.; Chen, W.; Yu, K.; Xiao, G.; Richardson, J.J.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Self-Assembled Metal-Phenolic Nanoparticles for Enhanced Synergistic Combination Therapy against Colon Cancer. Adv. Biosyst. 2019, 3, e1800241. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiao, G.; Richardson, J.J.; Tardy, B.L.; Ejima, H.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Targeted Therapy against Metastatic Melanoma Based on Self-Assembled Metal-Phenolic Nanocomplexes Comprised of Green Tea Catechin. Adv. Sci. 2019, 6, 1801688. [Google Scholar] [CrossRef]
- Meng, X.; Chen, L.; Lv, R.; Liu, M.; He, N.; Wang, Z. A metal-phenolic network-based multifunctional nanocomposite with pH-responsive ROS generation and drug release for synergistic chemodynamic/photothermal/chemo-therapy. J. Mater. Chem. B 2020, 8, 2177–2188. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, Z.; Cheng, S.; Wang, Z.; Zhang, R.; Zhu, G.; Wang, Z.; Yung, B.C.; Tian, R.; Jacobson, O.; et al. Toxic Reactive Oxygen Species Enhanced Synergistic Combination Therapy by Self-Assembled Metal-Phenolic Network Nanoparticles. Adv. Mater. 2018, 30, 1704877. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, J.X.; Zheng, D.W.; Liu, F.; Zeng, X.; Yan, G.P.; Zhang, X.Z. A multi-functional drug delivery system based on polyphenols for efficient tumor inhibition and metastasis prevention. Biomater. Sci. 2020, 8, 702–711. [Google Scholar] [CrossRef]
- Shan, L.; Gao, G.; Wang, W.; Tang, W.; Wang, Z.; Yang, Z.; Fan, W.; Zhu, G.; Zhai, K.; Jacobson, O.; et al. Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials 2019, 210, 62–69. [Google Scholar] [CrossRef]
- Zhang, N.; Mei, K.; Guan, P.; Hu, X.; Zhao, Y. Protein-Based Artificial Nanosystems in Cancer Therapy. Small 2020, 16, e1907256. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Liu, C.; Shen, W.; Li, B.; Li, T.; Chang, H.; Cheng, Y. Natural Polyphenols Augment Cytosolic Protein Delivery by a Functional Polymer. Chem. Mater. 2019, 31, 1956–1965. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Zhang, L.; Gu, X.; Lu, Y.; Sun, M.; Sun, C.; Ping, Q.; Li, J.; Chen, Z.; et al. Nanostructured Peptidotoxins as Natural Pro-Oxidants Induced Cancer Cell Death via Amplification of Oxidative Stress. ACS Appl. Mater. Interfaces 2018, 10, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, P.; Zhang, X.; Ravichandran, N.; Ying, H.; Yu, C.; Ying, H.; Xu, Y.; Yin, J.; Wang, K.; et al. New epigallocatechin gallate (EGCG) nanocomplexes co-assembled with 3-mercapto-1-hexanol and β-lactoglobulin for improvement of antitumor activity. J. Biomed. Nanotechnol. 2017, 13, 805–814. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.K.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Lin, C.Y.; Chang, S.J.; Niu, G.C.; Yao, C.H.; Chen, I.F.; Kuo, S.M. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J. Mater. Sci. Mater. Med. 2015, 26, 5357. [Google Scholar] [CrossRef] [PubMed]
- Madeo, L.F.; Sarogni, P.; Cirillo, G.; Vittorio, O.; Voliani, V.; Curcio, M.; Shai-Hee, T.; Büchner, B.; Mertig, M.; Hampel, S. Curcumin and Graphene Oxide Incorporated into Alginate Hydrogels as Versatile Devices for the Local Treatment of Squamous Cell Carcinoma. Materials 2022, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.I.; Kim, H.J.; Lee, S.Y.; Kim, S.; Huh, J.W.; Ahn, J.H.; Karmakar, M.; Kim, H.J.; Lee, K.; Lee, J.; et al. Hydrogel design to overcome thermal resistance and ROS detoxification in photothermal and photodynamic therapy of cancer. J. Control. Release 2024, 366, 142–159. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fei, Z.; Guo, T.; Hou, Y.; Li, D.; Wang, K.; Ren, F.; Fan, K.; Zhou, D.; Xie, C.; et al. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 2022, 280, 121272. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J.; et al. Temperature- and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Z.; Huang, Y.; Liu, H.; He, N.; Zhu, X.; Han, X.; Zhou, D.; Duan, X.; Chen, X.; et al. A versatile 3D-printable hydrogel for antichondrosarcoma, antibacterial, and tissue repair. J. Mater. Sci. Technol. 2023, 136, 200–211. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Muthukumar, S.P.; Vallikanan, B. Biodegradable chitosan-sodium alginate-oleic acid nanocarrier promotes bioavailability and target delivery of lutein in rat model with no toxicity. Food Chem. 2020, 330, 127195. [Google Scholar] [CrossRef] [PubMed]
- Anisiei, A.; Rosca, I.; Sandu, A.I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers-A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of tea catechins with chitosan nanoparticles. Food Hydrocoll. 2018, 84, 561–570. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Y.; Xie, M.; Hu, G.; Ma, F.; Zeng, X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J. Funct. Foods 2015, 15, 593–603. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liang, X.; Chuan, D.; Zhao, S.; Yu, W.; Fan, R.; Tong, A.; Zhao, N.; Han, B.; Guo, G. Chitosan coated pH-responsive metal-polyphenol delivery platform for melanoma chemotherapy. Carbohydr. Polym. 2021, 264, 118000. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Zeng, S.; Wang, Y.; Hou, J.; Yang, D.; Zhou, S. A multifunctional liposomal nanoplatform co-delivering hydrophobic and hydrophilic doxorubicin for complete eradication of xenografted tumors. Nanoscale 2019, 11, 17759–17772. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, W.; Xia, Q.; Zhou, W.; Yao, C.; Li, X. Thioether Phosphatidylcholine Liposomes: A Novel ROS-Responsive Platform for Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 37411–37420. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Xiel, Y.; Huang, Y.; Wu, Q.; Zhang, H.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of apoptosis and inhibition of angiogenesis by PEGylated liposomal quercetin in both cisplatin-sensitive and cisplatin-resistant ovarian cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhong, Q.; Chen, L.J.; Qi, X.R.; Fu, A.F.; Yang, H.S.; Yang, F.; Lin, H.G.; Wei, Y.Q.; Zhao, X. Liposomal honokiol, a promising agent for treatment of cisplatin-resistant human ovarian cancer. J. Cancer Res. Clin. Oncol. 2008, 134, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-Grade Quercetin-Loaded Nanoemulsion Ameliorates Effects Associated with Parkinson’s Disease and Cancer: Studies Employing a Transgenic C. elegans Model and Human Cancer Cell Lines. Antioxidants 2022, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.J.; Liu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.J.; Xu, Z. Bioengineered nanogels for cancer immunotherapy. Chem. Soc. Rev. 2022, 51, 5136–5174. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yu, S.; Wang, Z.; Huang, P.; Wang, W.; Xing, J. Nanogels loading curcumin in situ through microemulsion photopolymerization for enhancement of antitumor effects. J. Mater. Chem. B 2022, 10, 3293–3302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).