Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer System Selection and Optimisation
2.3. Rheological Testing
2.4. Fibre Characterisation
2.5. Preparation of Bacteria-Loaded Fibres
2.6. Viability Testing and Imaging
3. Results and Discussion
3.1. Polymer System Selection and Analysis
3.2. Fibre Characterisation
3.3. Bacterial Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2020, 9, e1901287. [Google Scholar] [CrossRef]
- Leena, M.M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Nanofibers in Food Applications. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 634–650. [Google Scholar]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating small molecules or biologics into nanofibers for optimized drug release: A review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef]
- Walsh, S.; Shah, A.; Mond, J. Improved Pharmacokinetics and Reduced Antibody Reactivity of Lysostaphin Conjugated to Polyethylene Glycol. Antimicrob. Agents Chemother. 2003, 47, 554–558. [Google Scholar] [CrossRef]
- Costello, C.M.; Sorna, R.M.; Goh, Y.-L.; Cengic, I.; Jain, N.K.; March, J.C. 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol. Pharm. 2014, 11, 2030–2039. [Google Scholar] [CrossRef]
- González, A.; Sabio, L.; Hurtado, C.; Ramírez-Rodríguez, G.B.; Bansal, V.; Delgado-López, J.M.; Dominguez-Vera, J.M. Entrapping Living Probiotics into Collagen Scaffolds: A New Class of Biomaterials for Antibiotic-Free Therapy of Bacterial Vaginosis. Adv. Mater. Technol. 2020, 5, 2000137. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and encapsulation techniques for probiotics: Current status and future prospects in biomedical applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Li, C. Delivery of probiotics with cellulose-based films and their food applications. Polymers 2024, 16, 794. [Google Scholar] [CrossRef]
- Rai, V.; Kyser, A.J.; Goodin, D.A.; Mahmoud, M.Y.; Steinbach-Rankins, J.M.; Frieboes, H.B. Computational modeling of probiotic recovery from 3D-bioprinted scaffolds for localized vaginal application. Ann. 3D Print. Med. 2023, 11, 100120. [Google Scholar] [CrossRef]
- Jiao, J.; Peng, C.; Li, C.; Qi, Z.; Zhan, J.; Pan, S. Dual bio-active factors with adhesion function modified electrospun fibrous scaffold for skin wound and infections therapeutics. Sci. Rep. 2021, 11, 457. [Google Scholar] [CrossRef]
- Garkal, A.; Kulkarni, D.; Musale, S.; Mehta, T.; Giram, P. Electrospinning nanofiber technology: A multifaceted paradigm in biomedical applications. New J. Chem. 2021, 45, 21508–21533. [Google Scholar] [CrossRef]
- Sousa, Â.; Almeida, A.M.; Valente, J.; Queiroz, J.; Sousa, F. Hands-On Laboratory Class for Biopharmaceutical pDNA Quality Control. J. Chem. Educ. 2022, 99, 975–982. [Google Scholar] [CrossRef]
- Heseltine, P.L.; Ahmed, J.; Edirisinghe, M. Developments in Pressurized Gyration for the Mass Production of Polymeric Fibers. Macromol. Mater. Eng. 2018, 303, 1800218. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Lee, R.C. Cell Injury by Electric Forces. Ann. N. Y. Acad. Sci. 2005, 1066, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fatih Canbolat, M.; Tang, C.; Bernacki, S.H.; Pourdeyhimi, B.; Khan, S. Mammalian cell viability in electrospun composite nanofiber structures. Macromol. Biosci. 2011, 11, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical Stimulation of Nerve Cells Using Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Mahalingam, S.; Edirisinghe, M. Forming of Polymer Nanofibers by a Pressurised Gyration Process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Mahalingam, S.; Matharu, R.K.; Edirisinghe, M. Evolution of surface nanopores in pressurised gyrospun polymeric microfibers. Polymers 2017, 9, 508. [Google Scholar] [CrossRef]
- Dai, Y.; Ahmed, J.; Edirisinghe, M. Pressurized gyration: Fundamentals, advancements, and future. Macromol. Mater. Eng. 2023, 308, 2300033. [Google Scholar] [CrossRef]
- Dotivala, A.C.; Puthuveetil, K.P.; Tang, C. Shear force fiber spinning: Process parameter and polymer solution property considerations. Polymers 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Porwal, H.; Chen, B.; Ciric, L.; Edirisinghe, M. Viral filtration using carbon-based materials. Med. Devices Sens. 2020, 3, e10107. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenço, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and antibacterial efficacy of graphene oxide nanocomposite fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative study of the antimicrobial effects of tungsten nanoparticles and tungsten nanocomposite fibres on hospital acquired bacterial and viral pathogens. Nanomaterials 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Crabbe-Mann, M.; Brako, F.; Kaur-Matharu, R.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Gunduz, O.; et al. Novel making of bacterial cellulose blended polymeric fiber bandages. Macromol. Mater. Eng. 2018, 303, 1700607. [Google Scholar] [CrossRef]
- Altun, E.; Bayram, C.; Gultekinoglu, M.; Matharu, R.; Delbusso, A.; Homer-Vanniasinkam, S.; Edirisinghe, M. Pressure-Spun Fibrous Surgical Sutures for Localized Antibacterial Delivery: Development, Characterization, and In Vitro Evaluation. ACS Appl. Mater. Interfaces 2023, 15, 45561–45573. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Matharu, R.K.; Shams, T.; Illangakoon, U.E.; Edirisinghe, M. A Comparison of electric-field-driven and pressure-driven fiber generation methods for drug delivery. Macromol. Mater. Eng. 2018, 303, 1700577. [Google Scholar] [CrossRef]
- Brako, F.; Luo, C.; Matharu, R.K.; Ciric, L.; Harker, A.; Edirisinghe, M.; Craig, D.Q.M. A Portable device for the generation of drug-loaded three-compartmental fibers containing metronidazole and iodine for topical application. Pharmaceutics 2020, 12, 373. [Google Scholar] [CrossRef]
- Basnett, P.; Matharu, R.K.; Taylor, C.S.; Illangakoon, U.; Dawson, J.I.; Kanczler, J.M.; Behbehani, M.; Humphrey, E.; Majid, Q.; Lukasiewicz, B.; et al. Harnessing polyhydroxyalkanoates and pressurized gyration for hard and soft tissue engineering. ACS Appl. Mater. Interfaces 2021, 13, 32624–32639. [Google Scholar] [CrossRef]
- Alenezi, H.; Cam, M.E.; Edirisinghe, M. Core–sheath polymer nanofiber formation by the simultaneous application of rotation and pressure in a novel purpose-designed vessel. Appl. Phys. Rev. 2021, 8, 041412. [Google Scholar] [CrossRef]
- Majd, H.; Gultekinoglu, M.; Bayram, C.; Karaosmanoğlu, B.; Taşkıran, E.Z.; Kart, D.; Erol, Ö.D.; Harker, A.; Edirisinghe, M. Biomedical Efficacy of Garlic-Extract-Loaded Core-Sheath Plasters for Natural Antimicrobial Wound Care. Macromol. Mater. Eng. 2024, 15, 2400014. [Google Scholar] [CrossRef]
- Qosim, N.; Majd, H.; Huo, S.; Edirisinghe, M.; Williams, G.R. Hydrophilic and hydrophobic drug release from core (polyvinylpyrrolidone)-sheath (ethyl cellulose) pressure-spun fibers. Int. J. Pharm. 2024, 654, 123972. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ahmed, J.; Delbusso, A.; Edirisinghe, M. Nozzle-Pressurized Gyration: A Novel Fiber Manufacturing Process. Macromol. Mater. Eng. 2022, 307, 2200268. [Google Scholar] [CrossRef]
- Harvey, J.L. Title 21—Food and Drugs Chapter I—Food and Drug Administration, Department of Health, Education, and Welfare Subchapter B—Food and Food Products Part 121—Food Additives Definitions and Procedural and Interpretive Regulations. Food Drug Cosmet. Law J. 1959, 14, 269–290. [Google Scholar]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Galai, H.; Touati, F.; Kouass, S. Multifunctional Roles of PVP as a Versatile Biomaterial in Solid State. In Dosage Forms—Innovation and Future Perspectives; IntechOpen: London, UK, 2021. [Google Scholar]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Brako, F.; Raimi-Abraham, B.; Mahalingam, S.; Craig, D.Q.; Edirisinghe, M. Making nanofibres of mucoadhesive polymer blends for vaginal therapies. Eur. Polym. J. 2015, 70, 186–196. [Google Scholar] [CrossRef]
- Liguori, A.; De Vita, A.; Rossi, G.; Dolci, L.S.; Panzavolta, S.; Gualandi, C.; Mercatali, L.; Ibrahim, T.; Focarete, M.L. A Modular Composite Device of Poly(Ethylene Oxide)/Poly(Butylene Terephthalate) (PEOT/PBT) Nanofibers and Gelatin as a Dual Drug Delivery System for Local Therapy of Soft Tissue Tumors. Int. J. Mol. Sci. 2022, 23, 3239. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Berri, N.; Fares, J.; Fares, Y. Polyethylene Oxide and Silicon-Substituted Hydroxyapatite Composite: A Biomaterial for Hard Tissue Engineering in Orthopedic and Spine Surgery. Adv. Biomed. Res. 2018, 7, 117. [Google Scholar] [CrossRef]
- Graessley, W.W. Polymer chain dimensions and the dependence of viscoelastic properties on concentration, molecular weight and solvent power. Polymer 1980, 21, 258–262. [Google Scholar] [CrossRef]
- Raimi-Abraham, B.T.; Mahalingam, S.; Edirisinghe, M.; Craig, D.Q. Generation of poly(N-vinylpyrrolidone) nanofibres using pressurised gyration. Mater. Sci. Eng. C 2014, 39, 168–176. [Google Scholar] [CrossRef]
- Ober, R.; Paz, L.; Taupin, C.; Pincus, P.; Boileau, S. Study of the surface tension of polymer solutions: Theory and experiments. Good solvent conditions. Macromolecules 1983, 16, 50–55. [Google Scholar] [CrossRef]
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International Tables of the Surface Tension of Water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Gonçalves, F.; Trindade, A.; Costa, C.; Bernardo, J.; Johnson, I.; Fonseca, I.; Ferreira, A. PVT, viscosity, and surface tension of ethanol: New measurements and literature data evaluation. J. Chem. Thermodyn. 2010, 42, 1039–1049. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Errington, J.; Aart, L.T. Microbe Profile: Bacillus subtilis: Model organism for cellular development, and industrial workhorse. Microbiology 2020, 166, 425–427. [Google Scholar] [CrossRef]
- Ingram, L.O. Ethanol Tolerance in Bacteria. Crit. Rev. Biotechnol. 1989, 9, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.B. Effect of alcohol on cellular membranes. Ann. Emerg. Med. 1986, 15, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, W.; Liu, C. Isolation, identification and characterization of novel Bacillus subtilis. J. Vet. Med. Sci. 2018, 80, 427–433. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
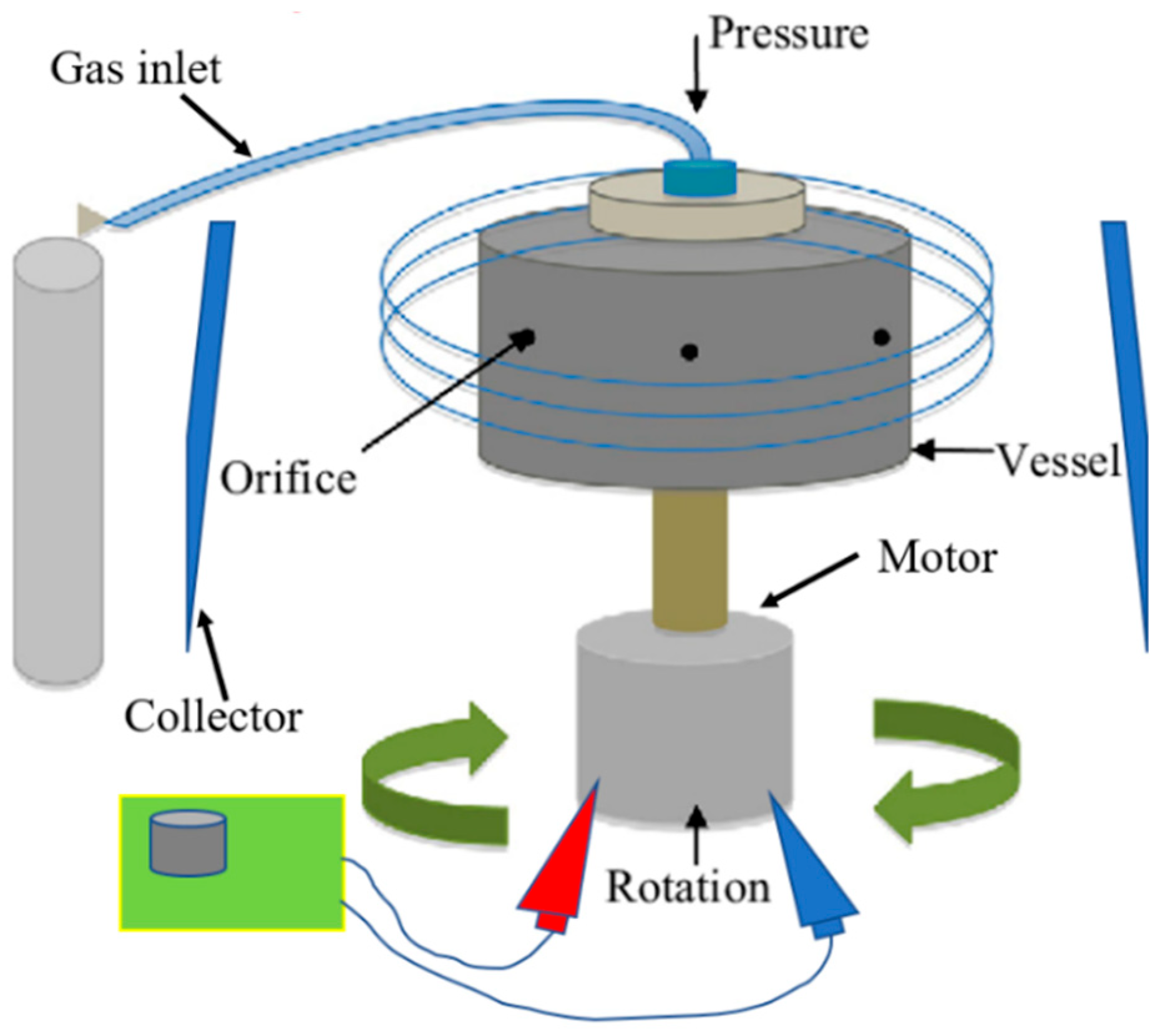
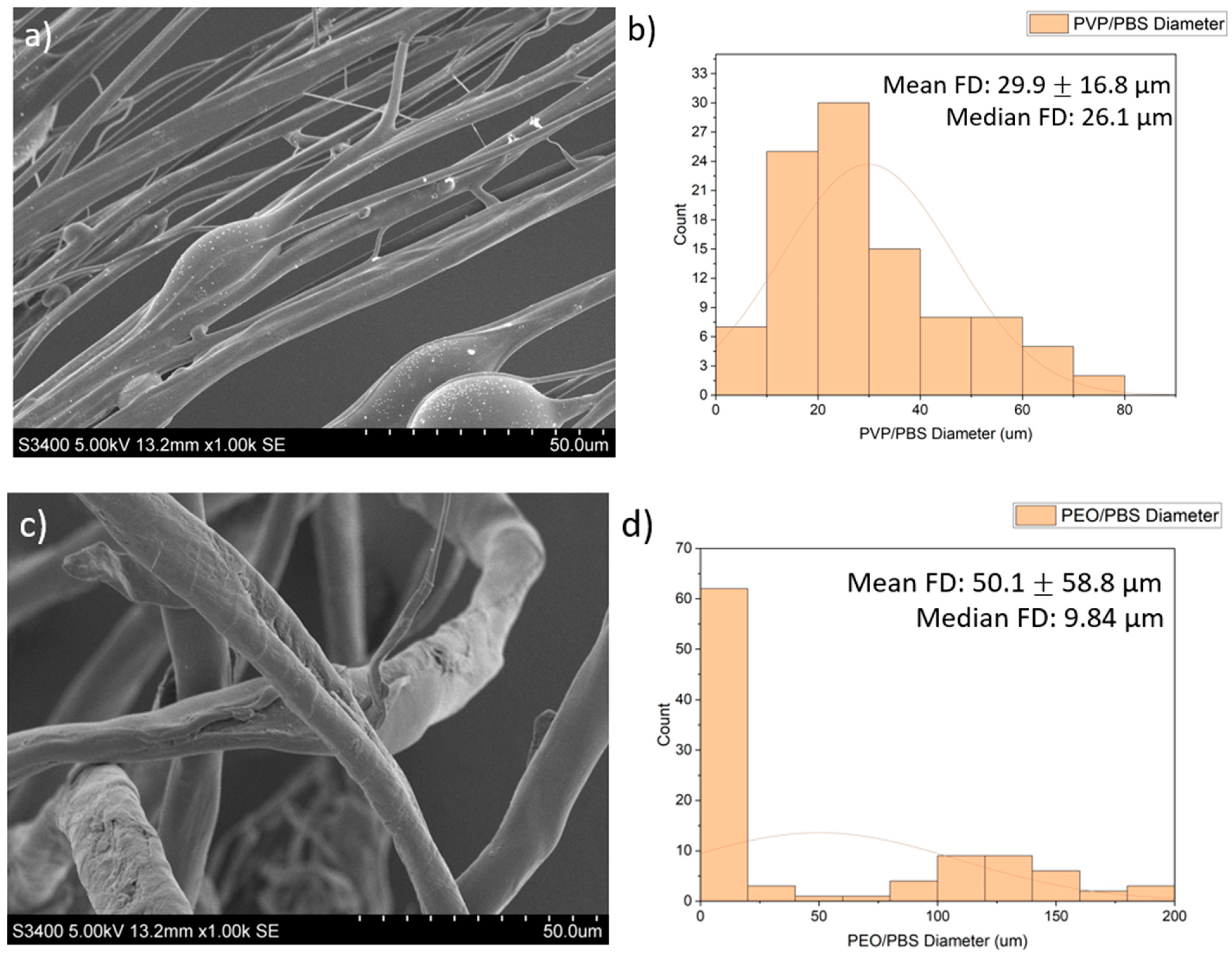
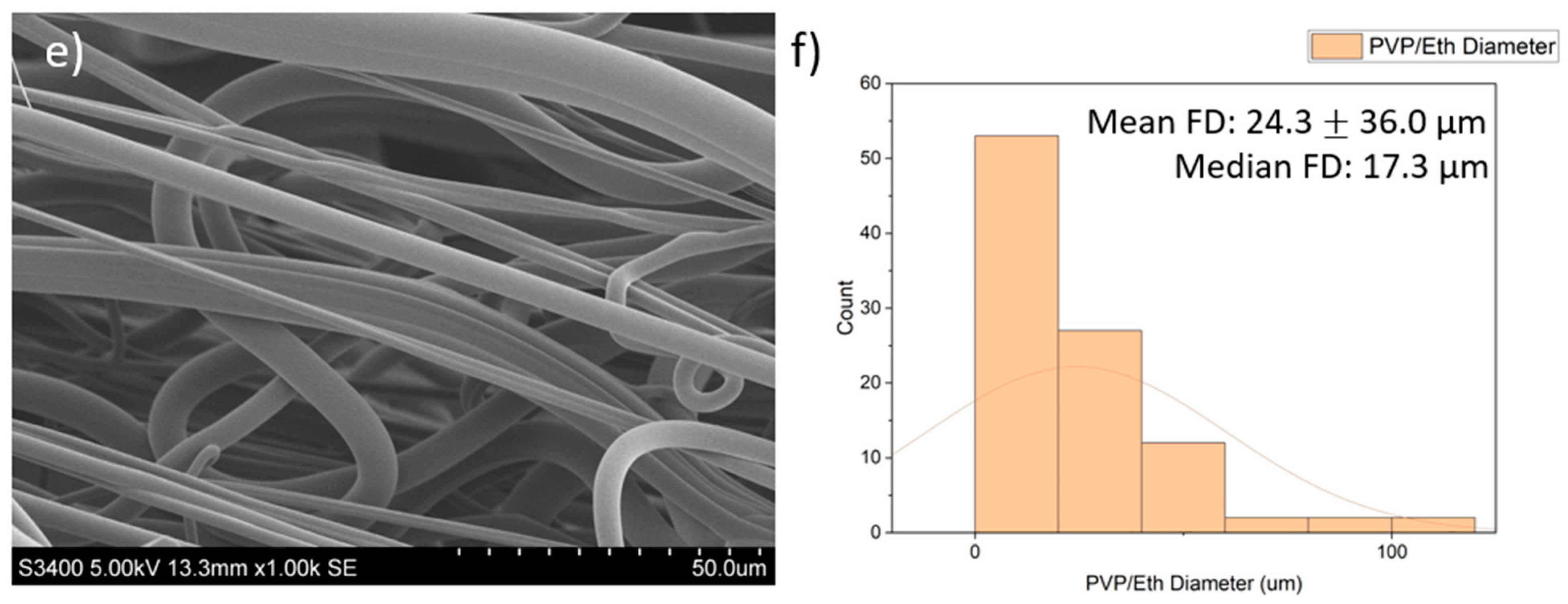
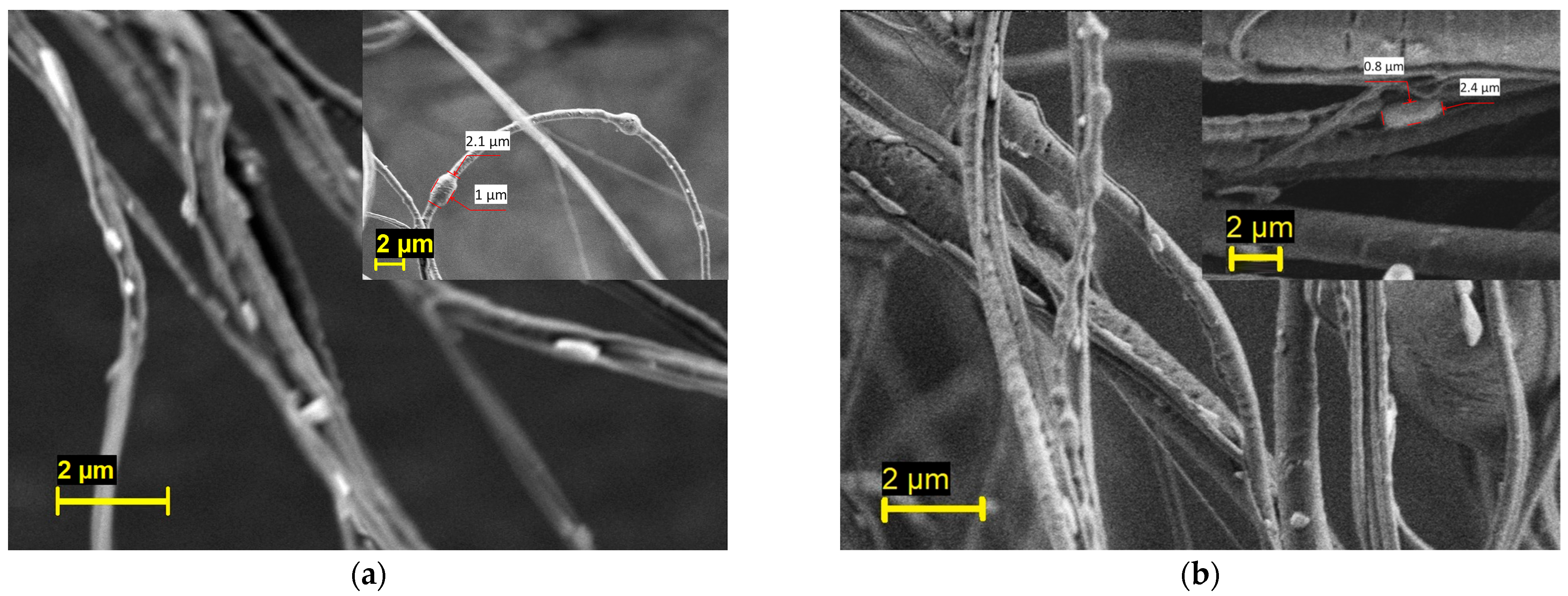

| Polymer/Concentration (w/v) | Molecular Weight (Mw) | Solvent | Fibres Produced |
|---|---|---|---|
| PVP 20% | 1,300,000 | Ethanol | Yes—very strong yield of fibres |
| PVP 20% | 1,300,000 | PBS | No—yield insufficient |
| PVP 30% | 1,300,000 | Ethanol | No—far too viscous |
| PVP 25% | 1,300,000 | PBS | Yes—good yield + wet fibres |
| PVP 30% | 1,300,000 | PBS | Yes—very small yield |
| PVA 20% | 31,000–50,000 | Ethanol | No—did not dissolve at all |
| PVA 20% | 31,000–50,000 | PBS | No—did not dissolve sufficiently |
| PVA 10% | 31,000–50,000 | PBS | No—dissolved but no fibres |
| PVA 10% | 146,000–186,000 | PBS | No—dissolved but no fibres |
| Gelatine 7.5% | Gel strength 300, Type A | PBS | No—stayed jelly-like in pot |
| PEO 20% | 200,000 | PBS | Yes—lots of fibres—quite delicate + stretchy |
| PEO 20% | 1,000,000 | PBS | No—did not dissolve (too viscous) |
| Suspension | Avg. Absorbance 600 nm | McFarland Standard of Solvent Suspension | Approximate Cell Density (1 × 108 CFU/mL) | Volume Added to Polymer System (mL) | CFU Added to Polymer System |
|---|---|---|---|---|---|
| 1 (PVP/PBS 25%) | 0.2830 | 1 | 3.0 | 2.0 | |
| 2 (PEO/PBS 20%) | 0.2785 | 1 | 3.0 | 2.0 | |
| 3 (PVP/Eth 20%) | 0.0994 | 0.5 | 1.5 | 2.0 |
| Polymer System (w/v) | Surface Tension (mN/m) | Viscosity (mPa·s) |
|---|---|---|
| PVP/PBS 25% | 73.4 ± 0.5 | 3659.0 ± 82.4 |
| PEO/PBS 20% | 73.9 ± 0.4 | 1513.0 ± 60.5 |
| PVP/Ethanol 20% | 22.6 ± 0.1 | 1132.0 ± 55.4 |
| Polymer System | Yield of Collected Fibres (%) | Starting Microbial Concentration in Scaffold (CFU/mL) | Microbial Concentration after 24 h (CFU/mL) | |
|---|---|---|---|---|
| PVP/PBS 25% | 1.2 | 60 ± 2 | ||
| PEO/PBS 20% | 3.1 | 47 ± 4 | ||
| PVP/Ethanol 20% | 4.5 | 0 | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.M.; Qosim, N.; Williams, G.; Edirisinghe, M.; Matharu, R.K. Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome. Pharmaceutics 2024, 16, 1066. https://doi.org/10.3390/pharmaceutics16081066
Klein AM, Qosim N, Williams G, Edirisinghe M, Matharu RK. Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome. Pharmaceutics. 2024; 16(8):1066. https://doi.org/10.3390/pharmaceutics16081066
Chicago/Turabian StyleKlein, Anne Marie, Nanang Qosim, Gareth Williams, Mohan Edirisinghe, and Rupy Kaur Matharu. 2024. "Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome" Pharmaceutics 16, no. 8: 1066. https://doi.org/10.3390/pharmaceutics16081066
APA StyleKlein, A. M., Qosim, N., Williams, G., Edirisinghe, M., & Matharu, R. K. (2024). Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome. Pharmaceutics, 16(8), 1066. https://doi.org/10.3390/pharmaceutics16081066









