Abstract
The diversity of cyclodextrins and their derivatives is increasing with continuous research. In addition to monomolecular cyclodextrins with different branched chains, cyclodextrin-based polymers have emerged. The aim of this review is to summarize these innovations, with a special focus on the study of applications of cyclodextrins and their derivatives in nano-delivery systems. The areas covered include nanospheres, nano-sponges, nanogels, cyclodextrin metal–organic frameworks, liposomes, and emulsions, providing a comprehensive and in-depth understanding of the design and development of nano-delivery systems.
1. Introduction
Cyclodextrin (CD) is one of the most widely used excipients produced in nature. It is a cyclic oligosaccharide formed from α-1,4-linked glucose units, where α-CD, β-CD, and γ-CD are the most common natural forms, containing 6, 7, and 8 glucose units, respectively [1]. The abundant hydroxyl groups of CD make it favorable for chemical modification [2], where these groups can be oxidized, esterified, or cross-linked with polymers to prepare CD derivatives [3]. The CD molecule also has an internal three-dimensional hydrophobic cavity, while the outside is hydrophilic [4]. The diversity of CDs and their derivatives are increasing with continuous research, from monomolecular CDs with different branched chains to CD-based polymers, such as CD-based polyrotaxanes [5], polypseudorotaxanes [6], and grafted CD polymers and cross-linked CD polymers [7]. The derivatives of CD are superior to natural CD due to their good biocompatibility, biodegradability, stimulus responsiveness, and the ability to target specific organs, making them an excellent choice as drug delivery systems [8].
CD can form inclusion complexes with hydrophobic molecules, such as paclitaxel, curcumin, catechin, quercetin, and other hydrophobic drugs [9,10,11]. This property makes it widely used in the field of nanotechnology, such as the delivery of drugs and genes [12]. With the continuous development of nanotechnology, CD and its derivatives represent an important class of nanomaterials and have shown remarkable research value in nano-delivery systems, such as micelles, nanoparticles, nanofibers, and nanorods [13].
This review summarizes these innovations, with a special focus on the study of applications of CDs and their derivatives in nano-delivery systems. By covering areas such as nanospheres, nano-sponges, nanogels, CD metal–organic frameworks, liposomes, and emulsions, this review will delve into the properties of these materials as carriers and their potential applications in nano-delivery systems. This comprehensive and in-depth understanding will provide comprehensive support for the design and development of nano-delivery systems and will promote the further innovation and application of nanotechnology in biomedicine, drug delivery, and other fields. In this dynamic area of research, this review focuses on the applications of CDs and their derivatives and the prospects that they bring, providing readers with a starting point to learn more. Figure 1 summarizes the advances of CDs and their derivatives in nano-delivery systems.
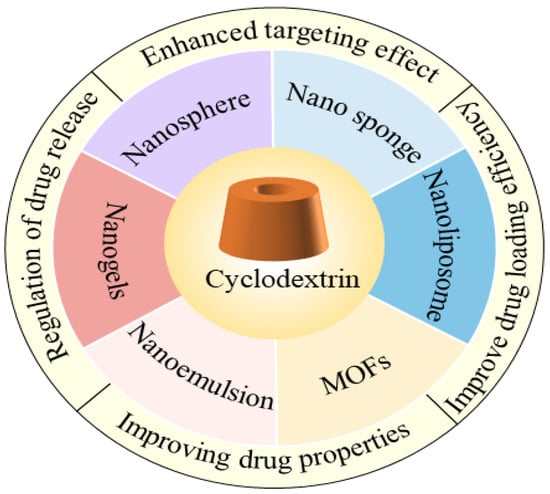
Figure 1.
Advances in CDs and their derivatives in nano-delivery systems.
2. Cyclodextrins (CDs)
2.1. The Types and Characteristics of Natural CDs
The discovery and study of CDs has a history of 130 years. In 1891, Antoine Villiers first reported on α- and β-CDs, conducting pioneering research on their composition and chemical properties [14]. CDs are a type of cyclic oligosaccharide derived from the enzymatic hydrolysis of linear starch and are composed of six or more α-1,4-linked glucose units. The typical natural CDs, namely, α-, β-, and γ-CDs, contain 6, 7, and 8 glucose units, respectively [15]. The inner diameters of the hydrophobic cavities of α-, β-, and γ-CDs are 4.7–5.3, 6.0–6.5, and 7.5–8.3 Å, respectively [16].
β-CD is more accessible, cost-effective, and has a hydrophobic cavity size suitable for encapsulating a large number of drug molecules, making it widely used in various applications [16,17]. However, the hydroxyl groups on the adjacent glucose units of β-CD have strong intermolecular interactions, meaning they do not readily interact with water molecules, leading to the relatively poor aqueous solubility of β-CD. However, α-and γ-CDs are less restricted, with weaker interactions between the hydroxyl groups of adjacent glucose molecules in the ring, which results in a higher solubility than that of β-CD [18].
The surface of CDs is rich in hydroxyl groups, but natural CDs and their complexes have limited solubility in aqueous solutions. Therefore, various derivatives have been developed, for example, by modifying and substituting methyl, ethyl, hydroxypropyl, and sulfobutyl groups, to improve the solubilization and inclusion capacity of natural CDs [16,19,20,21]. Table 1 summarizes the characteristics of three natural CDs.

Table 1.
Characteristics of α-CD, β-CD, and γ-CDs [22,23,24].
2.2. Formation of the CD Inclusion Complex
The most significant feature of CD is the formation of inclusion complexes with various solids, liquids, and gases through host–guest interactions. Since the sizes of the host and guest molecules are compatible, the guest molecules exist within the hydrophobic cavity of the CD [17]. More specifically, CD inclusion complexes are in a dynamic equilibrium, the state of which is determined by the value of the stability constant. The higher the value of this constant, the greater the stability of the inclusion complex [25].
In an aqueous solution, due to non-covalent bonding (such as van der Waals forces and hydrogen bonds), CD forms inclusion complexes with guest molecules at a molar ratio of 1:1, but there are also types involving ratios of 1:2, 2:1, 2:2, and 3:1 [26]. The formation of inclusion complexes between drugs and CDs is dependent on a variety of factors, including the chemical structure of the drug and the selection of the type of CD, solvent, preparation method, and temperature, among others [27]. Eid et al. characterized the phase solubility of the inclusion complexes of thymoquinone (TQ) and sulfobutylether-β-CD (SBE-β-CD) at five different temperatures (293–318 K). The study found that the aqueous solubility of TQ increased linearly with the increase in the molar concentration of SBE-β-CD, indicating the formation of an inclusion complex [28].
2.3. Types and Characteristics of CD Polymers
CD polymers are polymers or networks containing CDs, in which CDs are the main components [7], including polyrotaxane, polypseudorotaxane, and grafted CD polymers. Polyrotaxane is a supermolecular structure obtained by the polymerization of rotaxanes through covalent bonds [16,29]. CDs play an important role in polyrotaxanes, with the most typical type being the polyrotaxane based on α-CD and polyethylene oxide [7]. For example, Yu et al. developed supramolecular nanomedicines using an amphiphilic diblock copolymer as the axle and a primary-amino-containing β-CD (β-CD-NH2) as the wheel, with the system being driven by the host–guest interactions between β-CD-NH2 and segments of poly(ε-caprolactone) [30].
Polypseudorotaxanes are very similar to polyrotaxanes, with the difference being that polypseudorotaxanes do not have end-capped macromolecules present [6]. Crivello et al. designed a poly(pseudo)rotaxane supramolecular hydrogel composed of a mixture of α-CD and two customized polyether polyurethanes (PEUs) to encapsulate lignin–cobalt nanoparticles, which was a promising system for treating chronic wounds [31]. CDs can be used to graft various linear, branched, cationic, anionic, copolymer, and cob lock polymers. Linear grafted CDs have the widest range of applications, such as chitosan [32], alginate [33], and cholesterol [34]. For example, Wang et al. used epichlorohydrin (ECH) as a cross-linker to covalently graft chitosan with β-CD (CS-g-β-CD) under mild conditions for the controlled release of the anticancer drug etoposide (VP16), which was stimuli-responsive to pH and temperature [35]. Khodayari et al. grafted hyaluronic acid/β-CD onto FeO magnetic nanoparticles for doxorubicin (DOX) delivery, and the drug release in the simulated cancer fluid (pH = 5.6) reached 92.43% after 48 h, showing a higher DOX release than at pH = 7.4 (77.05%; 48 h) [36].
3. CD-Based Nano-Delivery System
In recent decades, with the continuous development and progress of nanotechnology, nanocarriers have played an important role in drug delivery. They can be used as drug carriers to control drug release or play a targeted role through design or modification [37]. A variety of types of nanocarriers have been applied to drug delivery, including liposomes, nanospheres, nano-sponges, emulsion, nanogels, dendrimers, and metal–organic frameworks [38,39].
3.1. Nanospheres
Nanoparticles are small colloidal particles prepared from biodegradable or non-biodegradable materials, with an average diameter ranging from 10 to 1000 nm. Nanospheres are a specific type of nanoparticle that can uniformly disperse drugs within a matrix [40].
CDs have been widely used to improve the solubility of poorly soluble drugs. Natural CDs and their derivatives form inclusion complexes with these drugs, thereby increasing the drug load of the nanospheres. This allows them to carry a large amount of medication and offers advantages such as enhancing drug permeability, targeting, efficiency, and reducing side effects [41]. Miranko et al. used different CDs to alter the permeability behavior of cetirizine hydrochloride (LC) and found that CDs could significantly enhance its permeability in the nasal cavity, with β-CD notably improving permeability [42]. These researchers developed a star-shaped cationic polymer, CD-OEI, which consisted of a β-CD core and three arm-shaped ethyleneimines (OEIs) for the co-delivery of DOX and the p53 gene. This supramolecular drug and gene co-delivery system exhibited high gene transfection efficiency and effective protein expression in vitro and reduced cell activity and enhanced antitumor effects at low DOX concentrations, showing the promise of combining drugs and genes to treat cancer [43].
Targeting drugs to specific organs can increase the accumulation of the drug, thereby improving therapeutic efficacy and reducing adverse reactions. Chen et al. designed a bio porous nanosphere loaded with sorafenib (SF) using RNA as a nano-scaffold and CD as a binder [44]. The RNA contained Epithelial-cell adhesion-molecule (EpCAM) aptamers for targeted delivery and siRNA sequences for EpCAM gene silencing, and CD could load the first-line drug sorafenib for the targeted therapy of hepatocellular carcinoma through its hydrophobic cavity. With the assistance of nucleic acid aptamers, the drug-loaded porous nano-spheres (PRS@SF) were internalized by hepatocellular carcinoma cells and degraded by the intracellular dicer enzyme to produce siRNA and release SF, which was effective for the co-treatment of hepatocellular carcinoma cells. The porous nucleic acid nanospheres, which contain nucleic acid sequences and CDs, can be used to support different types of gene drugs and small molecule drugs, respectively, providing a new pathway for the targeted delivery of appropriate therapeutic drugs to specific tumors.
3.2. Nano-Sponges
Nanoparticles are drug delivery systems with nanoscale dimensions, characterized by a high surface area-to-volume ratio, which exhibit enhanced permeation and retention effects at tumor sites [45]. Nano-sponges, a special type of nanoparticle delivery system, are known for their strong permeability and good biocompatibility, bioavailability, and stability. They have been widely applied in drug delivery systems and cancer therapy [46].
CD-based nano-sponges possess a sponge-like structure that can form inclusion or non-inclusion complexes with various drugs or active ingredients, serving as an effective delivery vehicle for drugs with low solubility, permeability, and bioavailability. These CD-based nano-sponges combine the highly biocompatible and biodegradable and low toxicity characteristics of CDs with the high thermal stability and insolubility of nano-sponges [47]. CD-based nano-sponges have potential applications in drug delivery systems [48].
Many drugs belong to BCS (biopharmaceutics classification system) II drugs, with low solubility and high permeability. Up to 40% of new drugs are insoluble in water, which greatly limits their clinical applications [49]. CD-based nano-sponges can be used to increase the solubility of insoluble drugs [50]. The complex of the loaded drug with CD will reduce the crystallinity of the drug and thus improve its solubility [51]. Compared with conventional CD complexes, the curcumin solubility of CD nano-sponges was higher, possibly because the capture of curcumin in the nano-sponge reduced the particle size of the drug and significantly enhanced its solubility [52]. For example, Mashaqbeh et al. investigated the complex stability and solubility of curcumin with β-CD and β-CD-based nano-sponges. Compared with the solubility of free drugs, the formation of a β-CD inclusion complex increased the solubility of curcumin by 2.34 times, while when curcumin was loaded in β-CD-based nano-sponges, the solubility increased by 2.95 times. The stability constant of the curcumin nano-sponge is 4972.90 M−1, which was 10 times higher than that of the β-CD complex (487.34 M−1) [53]. Loading active substances in nano-sponges can not only maintain the therapeutic effects but also allows for the design and development of delivery systems for different controlled drugs, which can significantly improve the compliance of patients by reducing the frequency of drug administration [54]. Dai et al. used acryloyl-6-ethylenediamine-6-deoxy-β-CD (β-CD-NH-ACy), acrylic acid (AA), and N, N-bis(acryloyl)-cystamine (BACy) as cross-linking agents, and a valley nano-sponge based on a β-CD-attached highly cross-linked polymer was developed for the controlled release of DOX, which had a high drug-loading rate (22.6%), degradability, and pH response. In vitro release studies showed that the DOX release from nano-sponges was significantly increased (~77.0%) under acidic (pH 5.0) and cytosolic-reducing (10 mM GSH) conditions [55].
3.3. Liposomes
Liposomes are defined as bilayer lipid vesicles with a lipid and aqueous bilayer structure, which allow them to encapsulate both hydrophilic and hydrophobic drugs while maintaining their nanometer size during storage and application [56]. Liposomes offer many advantages in drug delivery and drug targeting [57], such as biocompatibility, reduced toxicity and multidrug resistance [58], and efficient drug delivery [59]. The encapsulation of poorly soluble drugs in lipid bilayers is usually limited by the mass ratio of drug to lipid, so trapping CD inclusion complexes in liposomes can be used to overcome this shortcoming [60].
Solubility is an important factor affecting the achievement of the desired concentration of a drug in systemic circulation to produce the expected pharmacological response. Drugs with low solubility have disadvantages such as low absorption and bioavailability, frequent high-dose administration, and difficulties in development [61]. Silibinin is a natural flavonoid compound used clinically for the treatment of hepatitis. However, its lower aqueous solubility limits its bioavailability [62]. In response to this issue, researchers formed an inclusion complex of silibinin with hydroxypropyl-β-CD (HP-β-CD) and further prepared nanoliposomes. The results show that the maximum release of silibinin from nanoliposomes was 75.40% ± 0.73, while solubility was 73.95 mg/mL, and relative bioavailability was 4.52 [63]. In another study, Aloisio et al. studied the effects of β-CD, methyl-β-CD (M-β-CD), HP-β-CD, and meglumine (MEG) on the low water-soluble drugs sulfamethazine (SMR) and indomethacin (INM) and further prepared them into liposomes. The entrapment efficiency of SMR and INM for MEG and HP-β-CD liposomes were higher (5636.28 and 439.54 mmol/mol), and compared with the ligand-free preparation, the entrapment values of SMR and INM were 18 and 43 times higher, respectively [64].
When poorly soluble drugs are complexed with CD and are encapsulated in the aqueous phase of liposomes, they are provided with dual protection, thereby significantly enhancing the stability of the poorly soluble drugs. Quercetin is a dietary flavonoid with the characteristic of chemical instability. It is easily oxidized and degraded during storage, which greatly limits its application [65]. Azzi et al. encapsulated quercetin with CDs, liposomes, and CD liposomes (DCLs). This phase solubility study showed that the solubility of quercetin was enhanced after complexing with CD, and the quercetin encapsulated in DCLs was better protected from ultraviolet irradiation, and the photostability was further improved [66]. The liposome formed by the catechol–CD complex and soybean lecithin (CCPL) was stable at 4 °C for 15 days but precipitated at 37 °C, indicating that temperature is one of the key parameters affecting liposomes [67]. The stability of liposomes can be enhanced by post-processing techniques such as freeze drying, spray drying, and spray freeze drying [68].
3.4. Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are highly ordered crystalline porous coordination polymers (PCPs), which include organic and inorganic ligands. They are formed by coordination bonds between inorganic ligands (such as metal ions or clusters) and organic ligands (such as carboxylates, phosphonates, and phenolates) to create one-dimensional/two-dimensional/three-dimensional networks of organic frameworks [69,70].
Although MOFs have the characteristics of a high specific surface area, adjustable pore structure, and good thermal stability, their inherent toxicity and moisture sensitivity limit their application [71]. Therefore, the synthesis of linkers and metal ions with good biocompatibility, such as CDs with host–guest interactions; a high encapsulation ability; and hydrophilicity, provides broader prospects for MOFs [71]. Forgan et al. have demonstrated that in the presence of alkali metal salts, γ-CD can be connected to group IA and IAA metal cations through coordination to form an MOF in a manner similar to crown ethers [72]. As a special kind of carbohydrate organic ligand, CD showed -OCCO- motifs on its primary and secondary planes, which improved the probability of forming an extended structure with metal ions. The common low-toxic or non-toxic metal ions that make up Bio-MOFs include K+, Ca2+, Zn2+, Fe3+, Zr2+, Cu2+, and so on. Potassium, as a major element in organisms, is well tolerated by the body. CD-MOF with K+ as the metal ion, due to its excellent drug-loading capacity and high biocompatibility, enables drug targeting and facilitates the improvement of solubility and bioavailability for insoluble drugs, thereby enhancing drug stability.
Drug treatments for acute lung injury (ALI) are unsatisfactory, because drugs cannot specifically target the lungs. Based on the typical characteristics of ALI reactive oxygen species (ROS) excess and acute inflammation. He et al. used CD-MOFs as the template and oxalyl chloride as the cross-linking agent to design a peroxyoxalate bond. The new carrier, OC-COF, was loaded with the natural drug molecule ligustrazine (LIG) to prepare a LIG@OC-COF dry powder for inhalation, which possessed a high deposition rate in the lungs [73]. The researchers used incubation methods to load paeoniflorin (PAE) particles into CD-MOF for inhaled administration to treat acute lung injury. Then, A549 and Caco-2 cell lines were selected to evaluate their cell permeability. The results show that the permeability of PAE-CD-MOF was five times higher than that of free PAE. Lung deposition tests in vivo further showed that CD-MOF loaded with PAE could be effectively transported to the deep lungs instead of being cleared directly in the airway, indicating that CD-MOF can be used as a carrier for inhaled administration [74].
3.5. Nanogels
Nanogels refer to three-dimensional network hydrogels with nanoscale dimensions that are formed by physically or chemically cross-linked polymers [75], with sizes ranging from 100 to 200 nm [76]. Nanogels are composed of various natural polymers, synthetic polymers, or their combinations, which facilitate the encapsulation of small molecule drugs, genes, oligonucleotides, and even proteins [39]. Nanogels, as a type of hydrogel, retain their high degree of hydration and swelling properties [39], and with their three-dimensional cross-linked network structure, they can encapsulate drugs with different chemical structures and properties. Compared to other drug delivery systems, nanogels have the advantages of easy preparation and good biocompatibility, hydrophilicity, and environmental stimulus responsiveness (such as temperature, pH, and light) [77].
Due to CD’s inherent ability to form complexes with various guest molecules and its high biocompatibility, it holds significant importance for nanogels and can be used as pharmaceutical excipients for nanogels [78]. CDs, leveraging their external hydrophilic properties, are incorporated into the polymer network of the nanogel, thereby achieving more effective drug loading and a controlled drug release [79]. For example, a novel hypoxia-sensitive supramolecular nanogel was reported in one study, which was constructed by conjugated azobenzene (Azo) and β-CD on poly (L-glutamic acid)-graft-poly (ethylene glycol) methyl ether (PLG-g-mPEG). The nanogel could efficiently load Ribonuclease A in the mild aqueous phase and achieved drug release through an azobenzene conformational transition triggered by nitroso reductase (NTR) in response to the anoxic environment of tumors [80].
Researchers have been developing innovative nanogel platforms for advanced drug delivery systems. Duan et al. developed a dual-responsive nanogel platform (HPC nanogel) for the delivery of multiple drugs. In this platform, β-CD conjugated with hyaluronic acid (HA-β-CD) and polyethyleneimine (PEI) serve as the skeleton of the nanogel, while cisplatin molecules provide internal linkage through coordination between Pt and the remaining carboxyl groups in HA-β-CD, which also possessed hyaluronidase (HAase) reactivity and glutathione (GSH) reactivity [81]. Building upon this concept, Pooresmaeil et al. designed and constructed a novel dual-responsive (pH and temperature) and photoluminescent nanogel, with carbon quantum dots (CQDs), β-CD, poly(acrylic acid) (PAA), and N-isopropylacrylamide (NIPAAm) (β-CD/NIPAM@AA) being the main components for constructing the nanogel, which was used for the delivery of methotrexate (MTX) and DOX, providing a combined drug delivery for hepatocellular carcinoma [82]. Both platforms demonstrate the versatility and adaptability of nanogels in drug delivery, highlighting progress in the field of responsive and targeted therapeutics.
3.6. Emulsions
Pickering emulsions are emulsions stabilized by nanosolid particles [83]. Research initiated by Ramsden and Pickering compared these with traditional emulsions and found that Pickering emulsions utilize the surface wettability of the solid particles themselves, irreversibly adsorbing the solid particles at the oil/water interface and forming a stable solid particle film to stabilize the emulsion formed by the droplets [84]. Therefore, they possess characteristics such as low toxicity [85], high security and stability [86], low cost, and environmental protection [87]. CDs can form surface-active complexes at the oil–water interface to stabilize emulsions and have potential applications due to their hydrophobic cavity and host–guest interactions [88].
Drugs encapsulated within the oil phase of oil-in-water emulsions can be protected from hydrolysis and oxidation by emulsions [89]. CDs have a unique hydrophobic cavity structure, and oil molecules and CDs can form complexes. Oil/CD complexes can prevent droplets from moving and colliding with each other (rising of oil droplets and settling of water droplets). The structural characteristics of CDs directly affect the stability of Pickering emulsions [90]. Leclercq et al. proved that natural CDs (α-, β-, or γ-CD) are highly effective in obtaining oil-in-water Pickering emulsions. Pickering emulsions obtained from the inclusion complex of oil and CD through host–guest interactions have high stability [91].
Researchers have compared the impact of HP-β-CD-based emulsions (SNEDDS) and solid dispersions on the solubility and oral bioavailability of dexibuprofen with poor water solubility. The results show that dextrobuprofen showed an amorphous form in both emulsion and solid dispersion, and the solubility of SNEDDS was significantly higher than that of solid dispersion when compared with a free drug powder. The AUC value of solid SNEDDS was significantly increased by 2.1 times and 1.6 times, respectively, leading to a marked improvement in the oral bioavailability of deibuprofen [92]. These findings suggest that CDs act as stabilizers in emulsion systems and can improve the stability and biocompatibility of emulsions.
4. The Application of CDs and Their Derivatives in Nano-Delivery Systems
4.1. Enhanced Targeting Effect
Nanoparticles, due to their specific characteristics, help to target specific areas, thereby enhancing the efficiency of delivering therapeutic drugs [93].
Colorectal cancer (CRC) is the third most common cancer worldwide, and CRC is typically associated with an inflammatory environment [94]. However, the treatment of CRC is limited. To combine therapeutic targeting with tumor microenvironment reprogramming, Bai et al. designed a biocompatible nanoparticle (CNP) that integrated regorafenib (RG) with mannitol-modified γ-CD (M-γ-CD) through host–guest complex formation to form RG@M-γ-CDCNP. The results show that the nanoparticles can reduce inflammation by targeting macrophages. It has also been shown that RG@M-γ-CDCNP exhibited targeting and biocompatibility in colitis-associated cancer and in a CT26 mouse model, which is of practical significance for adjuvant therapy in patients with CRCs [2]. In cancer, non-targeted therapies can cause toxicity and adverse side effects to normal tissues and organs. Based on this, a large amount of targeted cancer research studies are constantly developing to improve treatment efficacy and reduce side effects [95]. Baek et al. designed a kidney-removable zwitterionic CD with a customized structure for selective drug delivery in CRC. Twenty CD derivatives with different charged parts and spacers were synthesized for stability screening, and the biodistribution of five candidates was evaluated. The optimized CD exhibited higher tumor accumulation and could be used for the delivery of DOX and ulixertinib [96]. Varan et al. evaluated the in vivo efficacy and biological distribution of paclitaxel encapsulated in injectable amphiphilic CD nanoparticles with different surface charges. The results show that paclitaxel-loaded amphiphilic CD nanoparticles showed antitumor effects earlier than the drug solution. Furthermore, the trend of tumor-growth inhibition by blank nanoparticles was similar to that of the paclitaxel solution. At 24 h, the biodistribution assessed by in vivo imaging showed no accumulation of nanoparticles in the heart and lungs [97]. The above studies show that CD can be used as a carrier and can be further combined with other carrier materials (such as nanoparticles and liposomes) to develop new drug delivery systems to achieve more efficient drug targeting.
Zhang et al. successfully prepared a nano-delivery-system (Tyr/HA/CD-CS)-loaded baicalein (BA), using chitosan and β-CD (CD-CS) grafted with hyaluronic acid (HA) and D-tyrosine (D-Tyr), which were used as the raw material. The nano-delivery system could enhance the permeability of drugs to biofilms [98]. Building on this, another study developed CD-based chitosan nanospheres (CS NPs) for the nose-to-brain targeting of idebenone (IDE) to improve its low aqueous solubility and first-pass metabolism. The results show that IDE could be released slowly from CS NPs compared to free drugs. An in vitro study of bovine nasal mucosa showed that CSNPs loaded with IDE had higher permeability. The findings suggest that CD-based nanospheres have a potential applicational value in the nasal delivery of IDE for the treatment of neurological diseases [99].
Lung cancer is the tumor with the highest morbidity and mortality worldwide. Lung-targeted drug delivery technology can enrich drugs in the lungs, improve drug efficacy, and reduce side effects [100]. To address the issues of low tissue-targeting efficiency and severe side effects in pulmonary drug delivery, based on the lung-targeting characteristics of cubic cross-linked CD metal–organic framework (CDF) nanoparticles, He et al. utilized RGD-functionalized CDF to deliver low-molecular-weight heparin (LMWH) and DOX to treat lung cancer. The results show that the nanoparticles could effectively target lung tumors after intravenous administration, and accumulation in the lung was 5.8 times higher than that in the liver [101].
4.2. Regulation of Drug Release
Compared with traditional drug delivery systems, CD-based nano-delivery systems can achieve a sustained release of drugs. Matshetshe et al. developed β-CD-modified chitosan nanoparticles (β-CD/CS-NPs) as carriers for cinnamon essential oil (CEO), which is known for its volatility and relatively low stability. The research findings indicate that these nanoparticles were spherical, and they achieved an encapsulation efficiency of 58% for CEO at 55 °C, which was significantly higher than that of CEO-loaded CS nanoparticles (approximately three times as much). Additionally, in vitro release studies demonstrated that the release of CEO from β-CD/CS-NPs was sustained and controllable for over 120 h [102].
CD has the characteristics of low toxicity, good biocompatibility, and ease of modification, so it is widely used in stimulus-responsive systems [103]. For example, Li et al. based the oral delivery of doxorubicin hydrochloride and celastrol (CSL) on mono-(6-pentethylenehexamine)-β-CD (PEHA-β-CD) and sodium dodecylbenzene sulfonate (SDBS) to pH-responsive supramolecular nanoparticles (PEHA-β-CD/SDBS) through electrostatic interactions. These nanoparticles minimally released the drugs in an acidic pH environment (such as the stomach at pH = 1.2) and effectively released them in an alkaline pH environment (such as the intestine at pH = 8.5), making them suitable as carriers for oral drug administration [104]. Unlike normal cells, cancer cells have a higher metabolic rate and a faster proliferation speed, which makes the tumor tissue form a unique microenvironment. The tumor microenvironment has several significant pathological characteristics, such as a lower pH value, EPR effect (enhanced permeability and retention effect), and hypoxia [105]. Mrówczyński et al. functionalized polydopamine-coated DOX magnetic nanoparticles with mono-6-thio-β-CD (SH-β-CD). The encapsulation efficiency of this nano-system could reach up to 90%, and the cumulative release of DOX within 10 h was approximately 9% at a pH of 5.5 and 11% at a pH of 4.5. After nearly 50 h, the release of DOX reached its maximum value. This pH-sensitive release property matches the acidic characteristics of the tumor microenvironment, indicating that this nano-system could serve as an ideal carrier material for cancer treatment [106]. Ramasamy et al. used a CD–dextran complex to coat nickel ferrite nanoparticles and improved their antitumor effect by loading camptothecin, with a loading rate of the nanocarrier reaching 88%. The in vitro release curve showed that the drug was stable and sustained for more than 500 h, and a decrease in pH from 7.4 to 6.0 would cause the drug to be released faster. It was further confirmed that the nanocarrier was suitable for transporting anti-cancer drugs and could respond to pH changes in the tumor microenvironment [107]. These studies indicate that CD-based nanocarriers can achieve a controlled and sustained drug release in the tumor microenvironment through their pH sensitivity, providing effective strategies and materials for cancer treatment.
4.3. Improving Drug Properties
Improving the properties of drugs includes increasing drug stability, solubility, and bioavailability. As pharmaceutical excipients, CDs are often used to enhance the water solubility of poorly soluble drugs, increase the permeability of drugs through biological membranes, and thus improve the bioavailability of drugs [108].
Due to the susceptibility of intracellular ROS and immunotherapeutic drugs to degradation in vivo, effectively delivering genes or small-molecule drugs to macrophages remains a challenge. Cheng et al. employed star amphiphilic biocompatibility β-CD-graft-(poly(ε-caprolactone)-block-poly-(2-(dimethylamino)ethyl methacrylate)x (β-CD-g-(PCL-b-PDMAEMA))x) copolymers. Through the interaction between genes and cationic PDMAEMA blocks, genes were delivered to macrophages, in which the participation of poly(ε-caprolactone) fragments (PCLs) could enhance the stability of the copolymer by micelle formation, thus significantly improving gene transfection efficiency by up to 10.8% [109].
The water solubility and oral bioavailability of BCS II and IV drugs can be enhanced by complexation with CDs, where Gidwani et al. studied the formation of inclusion complexes, and the results show that the solubility of the inclusion complex was significantly higher than that of the free drug, with the dissolution volume reaching nearly 50% within 45 min. This rapid dissolution was due to the improved wettability of CD and its rapid formation in the solution and its soluble complex ability [110]. To improve the water solubility of resveratrol (RES), Wang et al. prepared RES and a sulfobutyl ether–β-CD complex (CD-RES) and loaded it into polymer nanoparticles. The results show that the nano-carrier increased the water solubility of RES by 66 times. In addition, it also showed a better anticancer effect [111].
Rilpivirine belongs to the BCS II class of drugs and is used to treat HIV infections. Rao et al. studied the use of β-CD-based nano-sponges to improve the solubility of rilpivirine [112]. It has been found that the microwave-assisted synthesis of β-CD nano-sponges can improve the solubility and drug delivery potential of domperidone, thereby improving its oral bioavailability [113].
Nano-sponges have a long stability period when in powder form, which can prevent the degradation of the drug molecules loaded inside [114]. Sharma et al. encapsulated ellagic acid (EA) into CD nano-sponges (CDNSs), and compared to distilled water, the solubilization effect of CDNSs increased by 10 times. In addition, the photostability of EA significantly improved [115].
CD-MOFs can load different drug molecules in their extended framework through co-crystallization [116], and the drug loading of dense metal–organic frameworks (MOFs) can be increased by converting it into a porous form. For example, potassium acetate γ-CD metal–organic frameworks (γ-CD-MOFs) were transformed into a porous form by ethanol to improve their drug-loading capacity [117]. CD-based nanoliposomes have been proven to enhance the bioavailability and stability of drugs such as butylphthalide [118], anethole [119], and linalool [66]. Through these studies, we find that CD and its derivatives have wide applications in improving the water solubility, stability, and bioavailability of drugs, providing a variety of effective strategies in the field of nano-delivery systems.
4.4. Improved Drug-Loading Efficiency
Most nanomedicines have a relatively low drug-loading capacity, which can lead to insufficient drug release and can hinder the clinical translation of nanomedicines. Therefore, it is crucial to improve the drug-loading and encapsulation efficiency of nanomedicines. Not only can this reduce the potential adverse effects of excessive nanomaterials, but it can also lower the manufacturing costs of nanomedicines [120].
As drug carriers, CDs have attracted extensive attention in nano-delivery systems because of their ability to improve the drug encapsulation efficiency and drug load. Gaetano et al. developed sulfobutylether-β-CD-based chitosan nanoparticles (CH/SBE-β-CDNPs) for the ocular delivery of levofloxacin (LVF). Due to the complexation of LVF with SBE-β-CD, both the encapsulation efficiency and drug load were significantly improved compared to free LVF, from 21.53% ± 1.47 and 25.33% ± 1.24 to 41.50% ± 1.19 and 47.83% ± 2.20, respectively, and the bactericidal activity increased by approximately 2 times. The results suggest that CHNPs based on SBE-β-CD may be a potential delivery system for LVF in the treatment of eye infections [121]. In addition, a study by Varan et al. further confirmed the effect of CD type on the drug-loading efficiency of nanosphere. They found that the drug-loading efficiency of nanospheres had a wide range (between 49% and 87%), and the encapsulation efficiency increases in the order of 6OCaproβCD < 6OCaproαCD < PC βCDC6 [122].
The entrapment efficiency of hydrophobic drugs based on liposomes usually depends on the mass ratio of the drug to the liposome. A large number of hydrophobic drug molecules will destroy the integrity and stability of the bilayer structure of liposomes [123]. The water solubility of the drug increases after forming an inclusion complex with the CD, and the complex can be loaded into the liposomes, thus increasing the drug loading of the insoluble drug. Wang et al. encapsulated brinzolamine and hydroxypropyl-β-CD into nanoliposomes (BCLs), achieving an encapsulation efficiency of 92.50 ± 2.1% and showing continuous release [124].
CD-based nano-sponges have been demonstrated to be promising drug carriers, as CD can enhance the drug encapsulation efficiency, thereby improving drug stability and solubility [49]. Dhakar et al. achieved a high drug-loading (19.06%) and -entrapment (95.31%) efficiency using CD nano-sponge-loaded kurenic acid [125]. In addition, Mendes et al. developed a norfloxacin nano-sponge with a high encapsulation efficiency (80%) based on CD to improve its physical and chemical properties and to promote oral absorption, mainly by changing β-CD and the cross-linking agent diphenyl carbonate to improve the encapsulation efficiency [126].
The applications and functions of CDs and their derivatives in nano-delivery systems such as nanospheres, nano-sponges, and CD metal–organic frames are summarized in Table 2.

Table 2.
The role of different CDs in nano-delivery systems.
5. Conclusions and Prospects
CDs are a kind of cyclic oligomer of glucose, which have excellent biocompatibility and low immunogenicity. In host–guest chemistry, CDs can wrap suitable hydrophobic guest molecules or ions in their cavity. Compared with covalent bonds, this non-covalent interaction is cost-effective and environmentally friendly [134], so CD-based polymers have a high specific surface area and good biocompatibility and biodegradability. For example, as a new green material, CD-based eutectic supramolecular polymer plays an important role in the encapsulation and solubilization of compounds [135].
This review summarized the main research methods involved in novel drug delivery systems based on CDs and their derivatives as nanocarriers, including nanospheres, nano-sponges, CD metal–organic frameworks, nanogels, liposomes, and emulsions. CDs can form host–guest complexes with a variety of hydrophobic guest molecules. This unique structural property gives CDs and their derivatives a broad applicational prospect in drug delivery systems, playing an important role in targeted drug delivery, regulating drug release, and improving drug delivery characteristics and bioavailability. They are expected to play an even more significant role in future drug delivery applications. Although CDs have great potential in nano-delivery systems, there are also some challenges, such as the solubility, stability, and biodegradability of CDs, as well as their interactions with drug molecules, and the long-term stability, safety, and efficacy of these formulations still require further clinical trials for validation. Therefore, future research needs to further optimize the chemical structure and functionality of CDs to achieve a more efficient and safer drug delivery. At the same time, the combination of two or more nano-delivery systems may fully exert the advantages of each drug delivery system, forming an excellent composite drug delivery system, further improving the physicochemical properties of drugs, and thereby better exerting the clinical therapeutic effect.
Funding
This research was funded by [National Institutes for Food and Drug Control, and National Key R&D Program of China] grant number [2023YFC3403200].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.U.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of starch, cellulose, and their derivatives in the development of microparticle drug-delivery systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host–guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2022, 454, 214352. [Google Scholar] [CrossRef]
- Taharabaru, T.; Kihara, T.; Obata, A.; Onodera, R.; Wen, Y.; Li, J.; Motoyama, K.; Higashi, T. Cyclodextrin-based tailored polyrotaxanes for highly efficient delivery of the genome-editing molecule. Carbohydr. Polym. 2024, 323, 121443. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Motoyama, K.; Arima, H. Cyclodextrin-based polyrotaxanes and polypseudorotaxanes as drug delivery carriers. J. Drug Deliv. Sci. Technol. 2013, 23, 523–529. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Yakupova, L.R.; Skuredina, A.A.; Kopnova, T.Y.; Kudryashova, E.V. In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria. Polysaccharides 2023, 4, 343–357. [Google Scholar] [CrossRef]
- Ma, P.; Huang, J. Nanoformulation of Paclitaxel: Exploring the Cyclodextrin/PLGA Nano Delivery Carrier to Slow Down Paclitaxel Release, Enhance Accumulation in Vivo. J. Cancer 2023, 14, 759–769. [Google Scholar] [CrossRef]
- Arya, P.; Raghav, N. In-vitro studies of Curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Kim, J.S. Study of Flavonoid/Hydroxypropyl-beta-Cyclodextrin Inclusion Complexes by UV-Vis, FT-IR, DSC, and X-Ray Diffraction Analysis. Prev. Nutr. Food Sci. 2020, 25, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Villiers, A. Sur la fermentation de la fécule par l’action du ferment butyrique. Compt. Rend. Acad. Sci. 1891, 112, 536–538. [Google Scholar]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.J.; Hedges, A.R. Properties and Applications of Cyclodextrins. J. Macromol. Sci. Part A 1996, 33, 673–683. [Google Scholar] [CrossRef]
- da Silva Júnior, W.F.; de Oliveira Pinheiro, J.G.; Moreira, C.D.; de Souza, F.J.; de Lima, Á.A. Alternative technologies to improve solubility and stability of poorly water-soluble drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–305. [Google Scholar]
- Liu, J.Y.; Zhang, X.; Tian, B. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. gamma-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Sabadini, E.; Cosgrove, T.; Egidio Fdo, C. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: A comparative study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y. Multicharged cyclodextrin supramolecular assemblies. Chem. Soc. Rev. 2022, 51, 4786–4827. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Niculescu, A.-G.; Bolocan, A.; Rădulescu, M.; Grumezescu, A.M.; Beuran, M.; Gaspar, B.S. Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers. Pharmaceutics 2023, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Eid, E.E.; Almaiman, A.A.; Alshehade, S.A.; Alsalemi, W.; Kamran, S.; Suliman, F.O.; Alshawsh, M.A. Characterization of thymoquinone-sulfobutylether-β-cyclodextrin inclusion complex for anticancer applications. Molecules 2023, 28, 4096. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, Y.; Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Crivello, G.; Orlandini, G.; Morena, A.G.; Torchio, A.; Mattu, C.; Boffito, M.; Tzanov, T.; Ciardelli, G. Lignin–cobalt nano-enabled poly (pseudo) rotaxane supramolecular hydrogel for treating chronic wounds. Pharmaceutics 2023, 15, 1717. [Google Scholar] [CrossRef]
- Evangelista, T.F.; Andrade, G.R.; Nascimento, K.N.; Dos Santos, S.B.; Santos, M.D.F.C.; D’Oca, C.D.R.M.; Estevam, C.D.S.; Gimenez, I.F.; Almeida, L.E. Supramolecular polyelectrolyte complexes based on cyclodextrin-grafted chitosan and carrageenan for controlled drug release. Carbohydr. Polym. 2020, 245, 116592. [Google Scholar] [CrossRef]
- Omtvedt, L.A.; Dalheim, M.Ø.; Nielsen, T.T.; Larsen, K.L.; Strand, B.L.; Aachmann, F.L. Efficient grafting of cyclodextrin to alginate and performance of the hydrogel for release of model drug. Sci. Rep. 2019, 9, 9325. [Google Scholar] [CrossRef]
- Osman, S.K.; Brandl, F.P.; Zayed, G.M.; Teßmar, J.K.; Göpferich, A.M. Cyclodextrin based hydrogels: Inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer 2011, 52, 4806–4812. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Xiong, J.; Wu, D.; Li, S.; Tao, Y.; Qin, Y.; Kong, Y. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, H.; Heydarinasab, A.; Moniri, E.; Miralinaghi, M. Synthesis and characterization of magnetic nanoparticles-grafted-hyaluronic acid/β-cyclodextrin as a novel pH-sensetive nanocarrier for targeted delivery of doxorubicin. Inorg. Chem. Commun. 2023, 148, 110366. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Decher, G. Self-assembled smart nanocarriers for targeted drug delivery. Adv. Mater. 2016, 28, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier drug delivery systems: Characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166. [Google Scholar] [CrossRef]
- Pandey, D.; Panwar, V.S.; Mishra, H.; Adhikari, L.; Pandey, M.; Semalty, M. Cyclodextrin Based Nanoparticles For Improved Solubility and Drug Delivery. J. Mt. Res. 2021, 16, 187–199. [Google Scholar] [CrossRef]
- Mirankó, M.; Tóth, J.; Bartos, C.; Ambrus, R.; Feczkó, T. Nano-spray-dried levocetirizine dihydrochloride with mucoadhesive carriers and cyclodextrins for nasal administration. Pharmaceutics 2023, 15, 317. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.; Zhu, J.; Song, X.; Li, J. Codelivery of Doxorubicin and p53 Gene by β-Cyclodextrin-Based Supramolecular Nanoparticles Formed via Host–Guest Complexation and Electrostatic Interaction. Biomacromolecules 2024, 25, 2980–2989. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Zhang, L.; Wang, Z.; Zhou, Q.; Huang, T.; Ge, C.; Xu, H.; Zhu, M.; Zhao, F.; et al. Cyclodextrin-mediated formation of porous RNA nanospheres and their application in synergistic targeted therapeutics of hepatocellular carcinoma. Biomaterials 2020, 261, 120304. [Google Scholar] [CrossRef]
- Khairnar, P.; Kolipaka, T.; Pandey, G.; Phatale, V.; Shah, S.; Srinivasarao, D.A.; Saraf, S.; Srivastava, S. Nanosponge-mediated oligonucleotide delivery: A cutting-edge technology towards cancer management. J. Drug Deliv. Sci. Technol. 2023, 91, 105226. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for drug delivery and cancer therapy: Recent advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.; Murtinho, D.; Valente, A.J. Cyclodextrin-based nanosponges: Overview and opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X.; et al. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 5995–6015. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 315–322. [Google Scholar] [CrossRef]
- Duchene, D.; Bochot, A. Thirty years with cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-beta-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Kumar, S.; Dalal, P.; Rao, R. Cyclodextrin nanosponges: A promising approach for modulating drug delivery. Colloid Sci. Pharm. Nanotechnol. 2020, 79. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Q.; Zhang, S.; Shi, S.; Li, Y.; Zhao, X.; Zhou, L.; Wang, X.; Zhu, Y.; Li, W. Smart GSH/pH dual-bioresponsive degradable nanosponges based on β-CD-appended hyper-cross-linked polymer for triggered intracellular anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102650. [Google Scholar] [CrossRef]
- Aguilar-Perez, K.M.; Aviles-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef]
- Hashemi, M.; Ghadyani, F.; Hasani, S.; Olyaee, Y.; Raei, B.; Khodadadi, M.; Ziyarani, M.F.; Basti, F.A.; Tavakolpournegari, A.; Matinahmadi, A.; et al. Nanoliposomes for doxorubicin delivery: Reversing drug resistance, stimuli-responsive carriers and clinical translation. J. Drug Deliv. Sci. Technol. 2023, 80, 104112. [Google Scholar] [CrossRef]
- Yang, J.; Wen, C.; Pan, C.; Guo, H.; Zhao, W.; Zhang, J.; Zhu, Y.; Zhang, Y.; Zhang, L. Nanoliposomal multi-drug delivery system with reduced toxicity and multi-drug resistance. J. Mater. Sci. 2019, 54, 9718–9728. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, H.; Cai, Y.; Sheng, T.; Wang, P.; Li, Z.; Yang, F.; Gu, N. Magnet-activatable nanoliposomes as intracellular bubble microreactors to enhance drug delivery efficacy and burst cancer cells. Nanoscale 2019, 11, 18854–18865. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-cyclodextrin-in-liposome: The effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. Photobiol. B Biol. 2021, 216, 112147. [Google Scholar] [CrossRef] [PubMed]
- Takke, A.; Shende, P. Potential of cyclodextrin in hybrid liposomes for improving the solubility, bioavailability and stability of silibinin. Chem. Pap. 2022, 76, 6579–6589. [Google Scholar] [CrossRef]
- Aloisio, C.; Antimisiaris, S.G.; Longhi, M.R. Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J. Mol. Liq. 2017, 229, 106–113. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocoll. 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Development and characterization of catechin-in-cyclodextrin-in-phospholipid liposome to eradicate MRSA-mediated surgical site infection: Investigation of their anti-infective efficacy through in vitro and in vivo studies. Int. J. Pharm. 2021, 609, 121130. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar]
- Fatima, S.F.; Sabouni, R.; Garg, R.; Gomaa, H. Recent advances in Metal-Organic Frameworks as nanocarriers for triggered release of anticancer drugs: Brief history, biomedical applications, challenges and future perspective. Colloids Surf. B Biointerfaces 2023, 225, 113266. [Google Scholar] [CrossRef]
- Dummert, S.V.; Saini, H.; Hussain, M.Z.; Yadava, K.; Jayaramulu, K.; Casini, A.; Fischer, R.A. Cyclodextrin metal–organic frameworks and derivatives: Recent developments and applications. Chem. Soc. Rev. 2022, 51, 5175–5213. [Google Scholar] [CrossRef]
- Forgan, R.S.; Smaldone, R.A.; Gassensmith, J.J.; Furukawa, H.; Cordes, D.B.; Li, Q.; Wilmer, C.E.; Botros, Y.Y.; Snurr, R.Q.; Slawin, A.M.; et al. Nanoporous carbohydrate metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 406–417. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Sun, H.; Wu, D.; Wang, C.; Ren, X.; Shao, Q.; York, P.; Tong, J.; Zhu, J.; et al. Antioxidant Biodegradable Covalent Cyclodextrin Frameworks as Particulate Carriers for Inhalation Therapy against Acute Lung Injury. ACS Appl. Mater. Interfaces 2022, 14, 38421–38435. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Wang, C.; Qin, W.; Hu, X.; Tong, J.; Yu, L.; Zhang, G.; Ren, X.; Li, Z.; et al. Paeonol loaded cyclodextrin metal-organic framework particles for treatment of acute lung injury via inhalation. Int. J. Pharm. 2020, 587, 119649. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rangaraj, N.; Laxmikeshav, K.; Sampathi, S. Nanogels as drug carriers–Introduction, chemical aspects, release mechanisms and potential applications. Int. J. Pharm. 2020, 581, 119268. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef]
- Kubeil, M.; Suzuki, Y.; Casulli, M.A.; Kamal, R.; Hashimoto, T.; Bachmann, M.; Hayashita, T.; Stephan, H. Exploring the Potential of Nanogels: From Drug Carriers to Radiopharmaceutical Agents. Adv. Healthc. Mater. 2024, 13, 2301404. [Google Scholar] [CrossRef]
- Si, X.; Ma, S.; Xu, Y.; Zhang, D.; Shen, N.; Yu, H.; Zhang, Y.; Song, W.; Tang, Z.; Chen, X. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J. Control. Release 2020, 320, 83–95. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Guo, Y.; Zhang, X.; Gu, R.; Li, C.; Wu, F.G. Platinum-Coordinated Dual-Responsive Nanogels for Universal Drug Delivery and Combination Cancer Therapy. Small 2022, 18, 2203260. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H.; Salehi, R. Dual anticancer drug delivery of D-galactose-functionalized stimuli-responsive nanogels for targeted therapy of the liver hepatocellular carcinoma. Eur. Polym. J. 2022, 167, 111061. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Regenstein, J. Slow-release and nontoxic Pickering emulsion platform for antimicrobial peptide. J. Agric. Food Chem. 2020, 68, 7453–7466. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Ming, L.; Wu, H.; Liu, A.; Naeem, A.; Dong, Z.; Fan, Q.; Zhang, G.; Liu, H.; Li, Z. Evolution and critical roles of particle properties in Pickering emulsion: A review. J. Mol. Liq. 2023, 388, 122775. [Google Scholar] [CrossRef]
- Wu, L.; Liao, Z.; Liu, M.; Yin, X.; Li, X.; Wang, M.; Lu, X.; Lv, N.; Singh, V.; He, Z.; et al. Fabrication of non-spherical Pickering emulsion droplets by cyclodextrins mediated molecular self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 163–172. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent. Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, C.; Cui, B. Pickering Emulsions Stabilized by Cyclodextrin Nanoparticles: A Review. Starch-Stärke 2021, 73, 2100077. [Google Scholar] [CrossRef]
- Leclercq, L.; Dechézelles, J.-F.; Rauwel, G.; Nardello-Rataj, V. In vitro study of versatile drug formulations based on α-cyclodextrin and polyethylene glycol using colloidal tectonics. J. Drug Deliv. Sci. Technol. 2020, 59, 101913. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-beta-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Velhal, K.; Barage, S.; Roy, A.; Lakkakula, J.; Yamgar, R.; Alqahtani, M.S.; Yadav, K.K.; Ahn, Y.; Jeon, B.-H. A promising review on cyclodextrin conjugated paclitaxel nanoparticles for cancer treatment. Polymers 2022, 14, 3162. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Kara, G.; Calin, G.A.; Ozpolat, B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv. Rev. 2022, 182, 114113. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.J.; Nguyen, D.T.; Kim, D.; Yoo, S.Y.; Lee, S.M.; Lee, J.Y.; Kim, D.D. Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Nat. Nanotechnol. 2023, 18, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Varan, C.; Ozturk, S.C.; Benito, J.M.; Esendagli, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Zou, L.; Tang, J.; Zheng, J.; Luo, M.; Wang, G.; Liang, D.; Li, Y.; Chen, B.; et al. Preparation, Characterization, and Staphylococcus aureus Biofilm Elimination Effect of Baicalein-Loaded beta-Cyclodextrin-Grafted Chitosan Nanoparticles. Int. J. Nanomed. 2022, 17, 5287–5302. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- He, Y.; Xiong, T.; He, S.; Sun, H.; Huang, C.; Ren, X.; Wu, L.; Patterson, L.H.; Zhang, J. Pulmonary targeting crosslinked cyclodextrin metal–organic frameworks for lung cancer therapy. Adv. Funct. Mater. 2021, 31, 2004550. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of beta-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-L.; Zhang, J.; Jin, W.; Chen, X.-Y.; Yang, J.-M.; Chi, S.-M.; Ruan, Q.; Zhao, Y. Oral administration of pH-responsive polyamine modified cyclodextrin nanoparticles for controlled release of anti-tumor drugs. React. Funct. Polym. 2022, 172, 105175. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Jedrzak, A.; Szutkowski, K.; Grzeskowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Enoch, I.V.M.V.; Rex Jeya Rajkumar, S. Polymeric cyclodextrin-dextran spooled nickel ferrite nanoparticles: Expanded anticancer efficacy of loaded camptothecin. Mater. Lett. 2020, 261, 127114. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, X.; Wu, C.; Wang, X.; Wang, L.J.; Loh, X.J.; Li, Z.; Wu, Y.L. Cyclodextrin-Based Star-Like Amphiphilic Cationic Polymer as a Potential Pharmaceutical Carrier in Macrophages. Macromol. Rapid Commun. 2019, 40, e1800207. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Pharmacokinetic study of solid-lipid-nanoparticles of altretamine complexed epichlorohydrin-beta-cyclodextrin for enhanced solubility and oral bioavailability. Int. J. Biol. Macromol. 2017, 101, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-invasive Pulmonary Delivery of Resveratrol-Applications in Non-small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 183. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech 2018, 19, 2358–2369. [Google Scholar] [CrossRef]
- Vij, M.; Dand, N.; Kumar, L.; Wadhwa, P.; Wani, S.U.D.; Mahdi, W.A.; Alshehri, S.; Alam, P.; Shakeel, F. Optimisation of a Greener-Approach for the Synthesis of Cyclodextrin-Based Nanosponges for the Solubility Enhancement of Domperidone, a BCS Class II Drug. Pharmaceuticals 2023, 16, 567. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Sharma, K.; Kadian, V.; Kumar, A.; Mahant, S.; Rao, R. Evaluation of solubility, photostability and antioxidant activity of ellagic acid cyclodextrin nanosponges fabricated by melt method and microwave-assisted synthesis. J. Food Sci. Technol. 2022, 59, 898–908. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal-Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Ding, H.; Wu, L.; Guo, T.; Zhang, Z.; Garba, B.M.; Gao, G.; He, S.; Zhang, W.; Chen, Y.; Lin, Y.; et al. CD-MOFs Crystal Transformation from Dense to Highly Porous Form for Efficient Drug Loading. Cryst. Growth Des. 2019, 19, 3888–3894. [Google Scholar] [CrossRef]
- Lin, E.Y.; Chen, Y.S.; Li, Y.S.; Chen, S.R.; Lee, C.H.; Huang, M.H.; Chuang, H.M.; Harn, H.J.; Yang, H.H.; Lin, S.Z.; et al. Liposome Consolidated with Cyclodextrin Provides Prolonged Drug Retention Resulting in Increased Drug Bioavailability in Brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-cyclodextrin-in-liposomes as a carrier system for volatile essential oil components: Application to anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of high-drug-loading nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistara, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Akkin, S.; Demirturk, N.; Benito, J.M.; Bilensoy, E. Erlotinib entrapped in cholesterol-depleting cyclodextrin nanoparticles shows improved antitumoral efficacy in 3D spheroid tumors of the lung and the liver. J. Drug Target. 2021, 29, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.P.; Cai, B.C.; Yang, X.X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, X.; Fang, A.; Li, H.; Zhou, Y.; Liu, Y.; Jiang, C.; Wu, J.; Song, X. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-beta-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug-Delivery System. Front. Pharmacol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.S.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Yakupova, L.R.; Skuredina, A.A.; Markov, P.O.; Le-Deygen, I.M.; Kudryashova, E.V. Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Appl. Sci. 2023, 13, 3608. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Usman Minhas, M. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Rizvi, S.S.B.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels 2022, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Duggi Vamshidhar Reddy, A.S.R. Development And Evaluation Of Nanosponges Based Controlled Release Tapentadol Tablets By Box-Behnken Design. Nveo-Nat. Volatiles Essent. Oils J. 2021, 8, 5000–5016. [Google Scholar]
- He, Y.; Hou, X.; Guo, J.; He, Z.; Guo, T.; Liu, Y.; Zhang, Y.; Zhang, J.; Feng, N. Activation of a gamma-cyclodextrin-based metal-organic framework using supercritical carbon dioxide for high-efficient delivery of honokiol. Carbohydr. Polym. 2020, 235, 115935. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Alsotari, S.; Buqaien, R.; Ismail, S.; Awidi, A.; Al Bawab, A. Remote loading of curcumin-in-modified beta-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv. 2019, 9, 37148–37161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Sang, S.; Julian McClements, D.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Preparation, characterization and in vitro digestive behaviors of emulsions synergistically stabilized by gamma-cyclodextrin/sodium caseinate/alginate. Food Res. Int. 2022, 160, 111634. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhou, Y.; Xu, Y.; Chen, Y.; Yudintceva, N.; Shevtsov, M.; Gao, H. Supramolecular nanomedicines based on host–guest interactions of cyclodextrins. Exploration 2023, 3, 20210111. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, L.; Li, S.; Li, S.; Wu, Y.; Li, Z.; Qiu, H. Green materials with promising applications: Cyclodextrin-based deep eutectic supramolecular polymers. Green Chem. 2023, 25, 4180–4195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).